Abstract
Background
High stress prenatally contributes to poor maternal and infant well-being. The coronavirus disease 2019 (COVID-19) pandemic has created substantial stress for pregnant women.
Purpose
To understand whether stress experienced by women pregnant at the beginning of the pandemic was associated with a greater prevalence of adverse perinatal outcomes.
Methods
Pregnant women across the USA aged ≥18 years old enrolled in a prospective cohort study during the pandemic onset (T1) in April–May 2020. This report focuses on the 1,367 participants who gave birth prior to July–August 2020 (T2). Hierarchical logistic regression models predicted preterm birth, small for gestational age infants, and unplanned operative delivery from T1 stress, sociodemographic, and medical factors.
Results
After controlling for sociodemographic and medical factors, preterm birth was predicted by high prenatal maternal stress, delivering an infant small for gestational age was predicted by interpersonal violence and by stress related to being unprepared for birth due to the pandemic, and unplanned cesarean or operative vaginal delivery was predicted by prenatal appointment alterations, experiencing a major stressful life event, and by stress related to being unprepared for birth due to the pandemic. Independent of these associations, African American women were more likely than other groups to deliver preterm.
Conclusion
Pregnant women who are experiencing high stress during the COVID-19 pandemic are at risk of poorer perinatal outcomes. A longitudinal investigation is critical to determine whether prenatal maternal stress and resulting outcomes have longer-term consequences for the health and well-being of children born in the midst of the current pandemic.
Keywords: Pregnancy, Birth, Prenatal maternal stress, Adverse perinatal outcomes, COVID-19 pandemic, Pandemic-related stress, Behavioral medicine
Pregnant women who experienced greater stress during the COVID-19 pandemic onset were more likely to have a preterm birth, to deliver a small-for-gestational-age infant, and to have an unplanned operative delivery.
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has changed the daily lives of people across the globe, causing tremendous psychological stress for parents and children [1, 2]. High levels of stress and anxiety were documented among women pregnant at the start of the pandemic [3–7]. Although the effects of SARS-CoV-2 infection on perinatal outcomes have not yet been definitively determined [8–12], there is a large body of existing literature demonstrating harmful effects of maternal psychological stress on adverse maternal and neonatal outcomes including preterm birth, low birth weight, small for gestational age, and unplanned operative delivery [13–16]. There is also evidence regarding the harmful effects of maternal psychological stress resulting from exposure to natural disasters on such outcomes [17–19]. Adverse perinatal outcomes subsequently predict a higher risk of neonatal mortality and morbidity as well as chronic illnesses, temperamental difficulties, emotional dysregulation, and developmental delays in infancy and childhood [20, 21]. Therefore, it is critical to investigate whether maternal stress brought on by the COVID-19 pandemic is affecting infants born in its midst [22, 23].
Psychological stress is a multidimensional factor that can be defined and measured by three dimensions: stressful conditions or events, individual perceptions of stress, and emotions such as anxiety [24, 25]. All three of these stress dimensions were present at the onset of the COVID-19 pandemic and were evident in the experiences of pregnant women. Stressful conditions were produced by policies to reduce transmission by sheltering in place, social distancing caused disruptions to prenatal care and childbirth procedures [26–28], and high rates of pandemic-related economic strain [29, 30] and intimate partner violence were documented [31–33]. Pregnant women also reported experiencing high stress related to the potential impact of SARS-CoV-2 on the fetus and to their lack of preparation for the birth due to the pandemic [34]. Furthermore, significant elevations of depression and anxiety symptoms have been reported among pregnant women across the globe [3–7, 35]. The presence of these stressful conditions, perceptions, and emotions during this unprecedented and prolonged emergency raise the specter of serious population health consequences [36] for mothers and their offspring. Additionally, known social determinants of health may be putting particular groups of pregnant women at greater risk during the pandemic and exacerbating already stressful conditions. Reports indicate that pregnant African American women, and other women of color, who were under regular circumstances experience barriers to prenatal care and disproportionately high rates of adverse perinatal outcomes [37–39], have been particularly burdened by pandemic restrictions, and have higher rates of COVID-19 infection than other groups of pregnant women [40, 41].
To identify stress-related impacts of the COVID-19 pandemic on children born in its midst, there is a need to rigorously examine the potential contribution of prenatal maternal stress to adverse maternal and neonatal outcomes. The rigorous examination requires isolating the prospective prediction of adverse outcomes by all three prenatal stress dimensions, independent of other variables that are also associated with elevated risk for these outcomes, including medical factors and sociodemographic characteristics such as African American identity [42]. The current study presents such an investigation among women who gave birth in the midst of the pandemic.
Methods
Procedure and Participants
Data for this study are from the first and second timepoint of the COVID-19 Pregnancy Experiences (COPE) Study. Between April 24 and May 15, 2020, pregnant women across the USA at least 18 years of age were recruited to participate in the COPE Study through targeted Facebook advertisements (95% of participants) and invitations posted on pregnancy-related social media groups and pages (Facebook, Instagram, and Reddit). Advertisements and invitations included photos of pregnant women with varying racial and ethnic features and types of clothing to encourage wide participation. After reviewing study purposes and requirements and providing informed consent, participants completed an online questionnaire containing psychometrically rigorous instruments to assess stress, sociodemographic, and medical factors. In Summer 2020, 2,897 participants (65.5% retention rate) completed the second study questionnaire (T2; July 14 to August 21, 2020). The current analysis focuses on 1,367 COPE Study participants who reported a live birth at T2. Participants were entered into a raffle with a 1/100 chance to win a $100 gift card for each completed questionnaire. The study was approved by the Institutional Review Board of Stony Brook University.
Measures
Sociodemographic and medical factors.
Well-demonstrated risk factors for the adverse perinatal outcomes examined in this study were assessed with relevant items [42]. Sociodemographic characteristics were assessed at T1 and included maternal age, race, and perceived financial status (below average/average/above average). Obstetric characteristics were assessed at T1 and included parity, gestational age, self-reported pregnancy risk (Yes/Unsure/No), and multiple gestations. Health behaviors included tobacco use and general healthy prenatal activities. Participants who endorsed tobacco use at either timepoint were considered tobacco users. General healthy prenatal behaviors were assessed at T1 by asking: “To what extent are you involved in healthy activities (eat well, take vitamins, exercise, sleep enough, etc.)” scored from 1 = Very Little to 5 = Very Much. COVID-19 infection was reported by participants at T1 and T2. Participants who endorsed positive results of antibody or viral testing at T1 or T2 were considered as having COVID-19 prenatally.
Stressful conditions.
COVID-19 related income loss was assessed at T1 using the item: “Have you, or someone you rely on, lost income due to COVID-19?.” COVID-19 related prenatal appointment alteration was assessed at T1 by asking: “Have you had prenatal care appointments canceled or rescheduled due to the current pandemic?.” Discrimination was assessed at T2 using the item: “During your pregnancy, have you experienced discrimination or harassment because of your race, sexuality, gender, or body size?.” Interpersonal violence was assessed at T2. Women were asked if they felt physically unsafe or emotionally unsafe in their home in the last 2 months. A participant who answered Yes to either situation was considered exposed to interpersonal violence. Major life events during pregnancy were assessed at T1 with the item: “Did any major negative or stressful life events (e.g., break-up, moving, death of someone close) happen in your life since you became pregnant?.”
Stress perceptions.
Pandemic-Related Pregnancy Stress was assessed at T1 using the Pandemic-Related Pregnancy Stress Scale (PREPS) [43]. It includes two internally consistent, pandemic-specific prenatal stress factors: Preparedness Stress (PREPS-Preparedness, seven items, α = 0.81) and Perinatal Infection Stress (PREPS-Infection, five items, α = 0.86). PREPS-Preparedness reflects concerns related to feeling unprepared for birth or postpartum due to the pandemic, with items such as: “I am worried that the pandemic could ruin my birth plans,” “I am worried I will not be prepared for the birth due to the pandemic restrictions,” and “I am concerned that I won’t get the prenatal care I need because of COVID-19.” PREPS-Infection reflects concerns about COVID-19 infection of oneself or one’s infant/fetus, with items such as: “I am worried that my baby could get COVID-19 at the hospital after birth,” “I am concerned that a COVID-19 infection could harm my pregnancy (such as miscarriage or preterm birth),” and “I am concerned about going to prenatal care appointments due to COVID-19.” Scores for each PREPS scale are calculated as mean item response on a scale from 1 = Very Little to 5 = Very Much. Prenatal Maternal Stress was assessed at T1 using the Revised Prenatal Distress Questionnaire (NuPDQ) [44]. Respondents rate the extent to which they are “feeling bothered, upset, or worried” about 17 pregnancy-related stressors (e.g., “changes in your weight and body shape,” “what will happen during labor and delivery,” “whether you might have an unhealthy baby”) on a scale from 0 = Not at All to 2 = Very Much. Scores were calculated as the mean response of items. The NuPDQ was internally consistent (α = 0.80).
Stress emotions.
General Anxiety Symptoms were assessed at T1 using the Generalized Anxiety Disorder-7 (GAD-7) [44], self-report measure of anxiety symptoms. Respondents report the frequency of symptoms over the last 2 weeks on a scale ranging from 0 = Not at All to 3 = Nearly Every Day. Scores were calculated as the sum of item responses with the following clinical cut-offs for anxiety severity: 0–4 minimal and 5–9 mild versus 10–14 moderate and 15–21 severe [44]. The instrument was internally consistent (α = 0.91).
Adverse Perinatal Outcomes were reported at T2. Preterm birth: Participants who reported gestational age at delivery <37 weeks were classified as preterm. Small for gestational age (SGA): Based on participant-reported birth weight and gestational weeks at delivery, participants whose newborn weighed less than the 10th percentile for gestational age at delivery according to World Health Organization standards [45] were categorized as delivering an SGA infant. Unplanned cesarean delivery or operative vaginal delivery (hereafter, unplanned operative delivery): Participants who reported delivering via unplanned (including emergency) cesarean birth or assisted vaginal delivery were considered to have had an unplanned operative delivery.
Statistical Analysis
Statistical analyses were conducted using SPSS version 26.0. Univariate analyses were performed to examine associations among study variables. Thereafter, we conducted multivariate hierarchical binary logistic regression, separately predicting preterm birth, SGA, and unplanned operative delivery. Predictors in the first step of each regression analysis included sociodemographic variables, the second step added medical factors, and the third step added stress variables. This approach isolates the unique predictive value of stress variables beyond known sociodemographic and medical risk factors. Adjusted odds ratios and 95% confidence intervals were calculated for each predictor variable and p values < .05 were considered significant. Missing data for study variables was minimal, ranging from 0.0% to 0.1% per variable, and was missing completely at random (Little’s Missing Completely at Random Test p >.05).
Results
Sample Characteristics and Perinatal Outcomes
The average age of the 1,367 study participants was 31.5 ± 4.4 years, average gestational age at T1 survey completion was 34 weeks. 47.7% (n = 651) were nulliparas, and 1.7% (n = 23) had been diagnosed with SARS-CoV-2. On average, participants were 40 ± 21 days post-delivery at T2. Fewer than 3% identified themselves as African American. Additional sociodemographic and medical sample characteristics can be found in Table 1.
Table 1.
Sociodemographic and Medical Characteristics and Univariate Associations with Adverse Perinatal Outcomes n(%)
| Preterm birth | Small for gestational age | Unplanned operative delivery | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | No n = 1270 (92.9%) |
Yes n = 97 (7.1%) |
χ 2 | No n = 1228 (91.4%) |
Yes n = 115 (8.6%) |
χ 2 | No n = 1092 (81.2%) |
Yes n = 252 (18.8%) |
χ 2 | |
| Maternal age | 0.36 | 0.82 | 0.76 | |||||||
| <35 years | 1034 (75.6) | 963 (93.1) | 71 (6.9) | 932 (91.8) | 83 (8.2) | 820 (80.7) | 196 (19.3) | |||
| ≥35 years | 332 (24.3) | 306 (92.2) | 26 (7.8) | 295 (90.2) | 32 (9.8) | 271 (82.9) | 56 (17.1) | |||
| African American | 10.96 ** | 5.21 * | 0.16 | |||||||
| No | 1328 (97.1) | 1239 (93.3) | 89 (6.7) | 1198 (91.7) | 108 (8.3) | 1061 (81.2) | 246 (18.8) | |||
| Yes | 39 (2.9) | 31 (79.5) | 8 (20.5) | 30 (81.1) | 7 (18.9) | 31 (83.8) | 6 (16.2) | |||
| Financial Status | 6.23 * | 3.72 | 3.68 | |||||||
| Above or average | 1193 (87.3) | 1117 (93.6) | 76 (6.4) | 1077 (91.7) | 98 (8.3) | 948 (80.5) | 229 (19.5) | |||
| Lower | 173 (12.7) | 153 (88.4) | 20 (11.6) | 150 (89.8) | 17 (10.2) | 144 (86.7) | 22 (13.3) | |||
| Parity | 8.44 ** | 16.03 *** | 124.34 *** | |||||||
| Nullipara | 651 (47.7) | 591 (90.8) | 60 (9.2) | 561 (88.2) | 75 (11.8) | 437 (68.7) | 199 (31.3) | |||
| Multipara | 715 (52.3) | 678 (94.8) | 37 (5.2) | 666 (94.3) | 40 (5.7) | 654 (92.5) | 53 (7.5) | |||
| Pregnancy risk | 43.49 *** | 5.09 * | 9.16 ** | |||||||
| Low risk | 898 (65.7) | 864 (96.2) | 34 (3.8) | 822 (92.7) | 65 (7.3) | 742 (83.6) | 146 (16.4) | |||
| High risk/ unsure | 469 (34.3) | 406 (86.6) | 63 (13.4) | 406 (89.0) | 11.0 (50) | 350 (76.8) | 106 (23.2) | |||
| Multiple gestations | 119.17 *** | 0.68 | 0.36 | |||||||
| No | 1341 (98.1) | 1260 (94.0) | 81 (6.0) | 1209 (91.5) | 113 (8.5) | 1076 (81.3) | 247 (18.7) | |||
| Yes | 26 (1.9) | 10 (38.5) | 16 (61.5) | 20 (90.5) | 2 (9.5) | 16 (76.2) | 5 (23.8) | |||
| Tobacco use | 3.33 | 4.72 * | 0.08 | |||||||
| No | 1334 (97.6) | 1242 (93.1) | 92 (6.9) | 1203 (91.7) | 109 (8.3) | 1067 (81.3) | 246 (18.7) | |||
| Yes | 33 (2.4) | 28 (84.8) | 5 (15.2) | 25 (80.6) | 6 (19.4) | 25 (80.6) | 6 (19.4) | |||
| COVID-19 Diagnosis | 1.26 | 10.00 *** | 0.38 | |||||||
| No | 1344 (98.3) | 1250 (93.0) | 94 (7.0) | 1212 (91.7) | 109 (8.3) | 1073 (81.2) | 249 (18.8) | |||
| Yes | 23 (1.7) | 20 (87.0) | 3 (13.0) | 16 (72.7) | 6 (27.3) | 19 (86.4) | 3 (13.6) |
* p < .05, **p < .01, ***p < .001.
Study participants reported an array of stressful conditions, including 58.0% who had prenatal appointments canceled or altered due to the pandemic, 40.5% who experienced COVID-19 pandemic-related income loss, and 30.3% who experienced major life events during their pregnancy. Prevalence of stressful conditions, stress perceptions, and anxiety symptoms are presented in Table 2.
Table 2.
Stress at the Pandemic Onset and Univariate Associations with Adverse Perinatal Outcomes n(%)
| Preterm birth | Small for Gestational Age | Unplanned operative delivery | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | No n = 1270 (92.9%) |
Yes n = 97 (7.1%) |
No n = 1228 (91.4%) |
Yes n = 115 (8.6%) |
No n = 1092 (81.2%) |
Yes n = 252 (18.8%) |
||||
| Stress conditions | χ 2 | χ 2 | χ 2 | |||||||
| COVID-19-related income loss | 0.03 | 0.21 | 0.00 | |||||||
| No | 814 (59.6) | 757 (93.0) | 57 (7.0) | 732 (91.7) | 66 (8.3) | 650 (81.3) | 150 (18.8) | |||
| Yes | 553 (40.5) | 513 (92.8) | 40 (7.2) | 496 (91.0) | 49 (9.0) | 442 (81.3) | 102 (18.8) | |||
| Prenatal care alterations | 0.24 | 1.80 | 6.32 * | |||||||
| No | 574 (42.0) | 531 (92.5) | 43 (7.5) | 508 (90.2) | 55 (9.8) | 476 (84.4) | 88 (15.6) | |||
| Yes | 793 (58.0) | 739 (93.2) | 54 (6.8) | 720 (92.3) | 60 (7.7) | 616 (79.0) | 64 (21.0) | |||
| Discrimination | 0.07 | 0.00 | 0.14 | |||||||
| No | 1277 (93.4) | 1187 (93.0) | 90 (7.0) | 1147 (91.5) | 107 (8.5) | 1021 (81.4) | 34(18.6) | |||
| Yes | 90 (6.6) | 83 (92.2) | 7 (7.8) | 81 (91.0) | 8 (9.0) | 71 (79.8) | 18 (20.2) | |||
| Interpersonal violence | 0.15 | 6.12 * | 2.09 | |||||||
| No | 1294 (94.7) | 1203 (93.0) | 7.0 (91) | 1167 (91.9) | 103 (8.1) | 1028 (80.9) | 43 (19.1) | |||
| Yes | 73 (5.3) | 67 (91.8) | 8.2 (6) | 61 (83.6) | 12 (16.4) | 64 (87.7) | 9 (12.3) | |||
| Major life event | 4.87 * | 0.02 | 4.45 * | |||||||
| No | 953 (69.7) | 895 (93.9) | 58 (6.1) | 862 (91.5) | 80 (8.5) | 780 (82.7) | 163 (17.3) | |||
| Yes | 414 (30.3) | 375 (90.6) | 9 (9.4) | 366 (91.3) | 35 (8.7) | 312 (77.8) | 89 (22.2) | |||
| Stress perceptions M ± SD | t | t | t | |||||||
| PREPS-Preparedness | 3.43 ± 0.85 | 3.43 ± 0.85 | 3.50 ± 0.80 | 0.88 | 3.41 ± 0.85 | 3.65 ± 0.77 | 2.98 * | 3.39 ± 0.85 | 3.61 ± 0.82 | 3.42 ** |
| PREPS-Infection | 3.21 ± 0.98 | 3.21 ± 0.97 | 3.20 ± 1.05 | 0.14 | 3.21 ± 0.97 | 3.25 ± 0.99 | 0.39 | 3.20 ± 0.97 | 3.26 ± 1.03 | 0.71 |
| Prenatal Maternal Stress | 0.82 ± 0.33 | 0.81 ± 0.33 | 0.91 ± 0.31 | 3.06 ** | 0.81 ± 0.32 | 0.85 ± 0.33 | 1.28 | 0.80 ± 0.32 | 0.87 ± 0.32 | 2.90 ** |
| Stress emotions | χ 2 | χ 2 | χ 2 | |||||||
| Anxiety Symptoms | 0.22 | 0.13 | 1.06 | |||||||
| None or Mild | 891 (65.2) | 826 (92.7) | 65 (7.3) | 802 (91.2) | 77 (8.8) | 708 (80.5) | 172 (19.5) | |||
| Moderate or severe | 476 (34.8) | 437 (93.3) | 32 (6.7) | 426 (91.8) | 38 (8.2) | 384 (82.8) | 80 (17.2) |
* p < 0.05, **p < 0.01, ***p < 0.001. Notes:PREPS - Preparedness Pandemic-Related Prenatal Stress.
The rate of preterm delivery among study participants was 7.1% (n = 97) and 8.6% (n = 115) delivered an SGA infant. Close to a fifth (18.8%, n = 252) reported an unplanned operative delivery. Most of these were cesarean deliveries (15.1%, n = 202); the remainder were assisted vaginal deliveries (3.7%, n = 50).
Univariate Analyses
Participants who delivered preterm and those who had an unplanned operative delivery experienced greater Prenatal Maternal Stress and were more likely to report that they had experienced a major life event during pregnancy than participants who did not experience either of these adverse outcomes (Table 2). Unplanned operative delivery was also significantly more common among participants who reported alterations in their prenatal care and those who experienced greater Preparedness Stress. Participants who delivered an SGA infant were more likely to report interpersonal violence and experienced greater Preparedness Stress than those who did not deliver an SGA infant. Outcomes were also significantly related to SARS-CoV-2 diagnosis and to known sociodemographic and medical risk factors: that is, some outcomes were more common among participants who were African American, nulliparas, tobacco users, and those with high-risk pregnancy status (Table 1). Anxiety symptoms, Infection Stress, and other stress conditions (i.e., discrimination, income loss) were unrelated to adverse outcomes in the univariate analyses (Table 2).
Multivariate Analyses
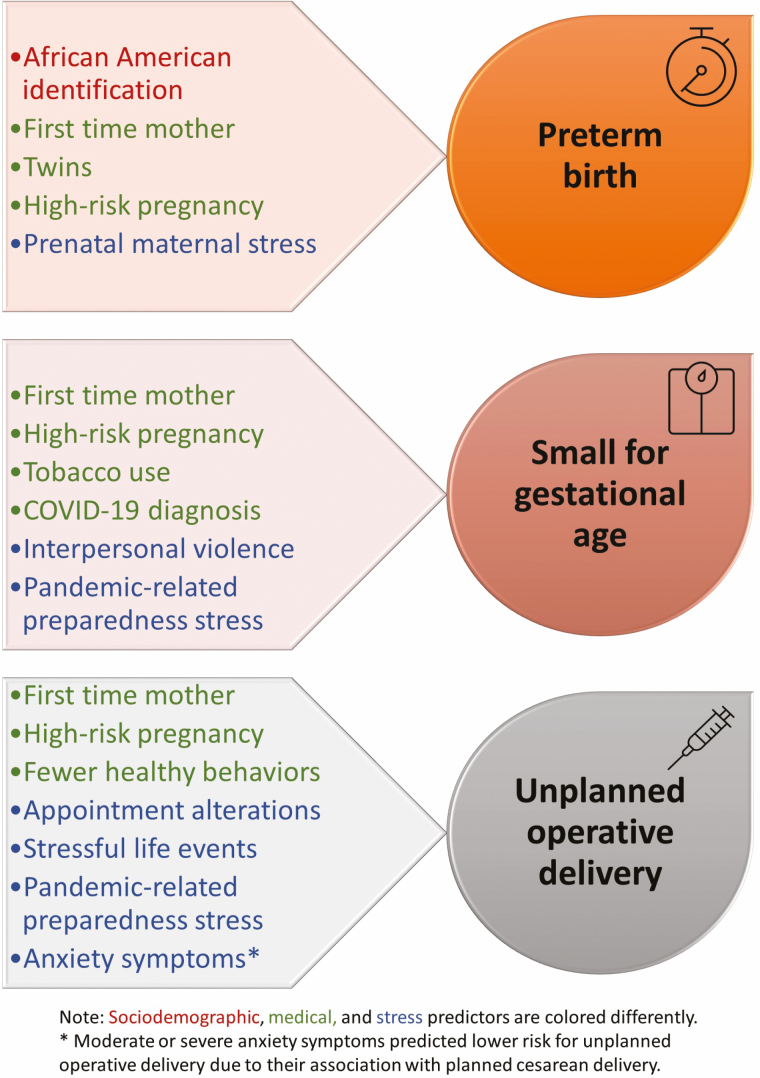
Medical variables were significant and strong predictors of each of the adverse outcomes, yet stress variables that were entered in the third and final step of the regressions were independent predictors above and beyond both sociodemographic and medical variables. As displayed in Table 3, preterm birth was predicted by elevated Prenatal Maternal Stress. This type of stress increased the risk of preterm birth by 40% and overall, explained 1% of the variance in preterm birth, over and above maternal sociodemographic (i.e., African American racial identification) and medical characteristics (i.e., nulliparity, multiple gestation, and high-risk pregnancy) that also predicted preterm birth. As displayed in Table 4, stress explained 3% of the variance in SGA. Specifically, risk of SGA was approximately twice as great for women who experienced interpersonal violence, and 66% greater for those who reported higher Preparedness Stress. SGA was also more than five times greater for those who reported a SARS-CoV-2 diagnosis during pregnancy, and SGA was more prevalent among nulliparas, women with a high-risk pregnancy, and those who used tobacco. Finally, as seen in Table 5, stress explained 3% of the variance in an unplanned operative delivery. The odds of unplanned operative delivery increased by 51% and by for participants who reported alterations to prenatal appointments due to the pandemic and for those who experienced a major life event during pregnancy, respectively. Higher Preparedness Stress increased the odds of unplanned operative delivery by 32%, whereas moderate or severe anxiety symptoms decreased the odds of this outcome by 39%. These associations of stress variables with unplanned operative delivery were independent of the greater likelihood of unplanned operative delivery for nulliparas and those with high-risk pregnancy status. A summary of predictors for adverse perinatal outcomes can be seen in Fig. 1.
Table 3.
Hierarchical Logistic Regression Predicting Preterm Birth from Sociodemographic, Medical, and Prenatal Stress Variables (n = 1,363)
| Step 1 | Step 2 | Step 3 | ||||
|---|---|---|---|---|---|---|
| AOR | 95% CI | AOR | 95% CI | AOR | 95% CI | |
| Below average income | 1.72 * | (1.01, 2.94) | 1.71 | (0.93, 3.13) | 1.65 | (0.85, 3.19) |
| African American | 3.41 ** | (1.51, 7.69) | 2.92 * | (1.22, 6.95) | 2.72 * | (1.10, 6.71) |
| Older maternal age | 0.90 | (0.52, 1.53) | 0.89 | (0.52, 1.53) | ||
| Nulliparity | 2.07 ** | (1.29, 3.32) | 1.84 * | (1.13, 2.99) | ||
| Multiple gestations | 15.05 *** | (6.24, 36.32) | 14.52 *** | (5.90, 35.73) | ||
| High-risk pregnancy | 3.42 *** | (2.10, 5.58) | 3.41 *** | (2.07, 5.63) | ||
| Healthy behaviors | 1.13 | (0.86, 1.47) | 1.13 | (0.86, 1.49) | ||
| Tobacco use | 1.54 | (0.50, 4.79) | 1.33 | (0.42, 4.26) | ||
| Diagnosed COVID-19 | 1.84 | (0.45, 7.53) | 1.81 | (0.43, 7.55) | ||
| Discrimination | 0.73 | (0.29, 1.82) | ||||
| Interpersonal violence | 0.95 | (0.35, 2.56) | ||||
| Appointment alteration | 0.93 | (0.58, 1.47) | ||||
| COVID-19 related income loss | 0.91 | (0.56, 1.48) | ||||
| Major life event | 1.42 | (0.88, 2.29) | ||||
| PREPS-Preparedness | 0.98 | (0.71, 1.35) | ||||
| PREPS-Infection | 0.90 | (0.67, 1.20) | ||||
| Prenatal Maternal Stress | 1.40* | (1.03, 1.90) | ||||
| Moderate/ severe anxiety symptoms | 0.68 | (0.39, 1.20) | ||||
| ΔR 2 = 0.02 | ΔR 2 = 0.16 | ΔR 2 = 0.01 | ||||
| R 2=0.02 | R 2=0.18 | R 2=0.19 |
* p < 0.05, **p < 0.01, ***p < 0.001
Notes:AOR Adjusted Odds Ratio; PREPS Preparedness Pandemic-Related Prenatal Stress.
Table 4.
Hierarchical Logistic Regression Predicting Small for Gestational Age Infant from Sociodemographic, Medical, and Prenatal Stress Variables (n = 1,339)
| Step 1 | Step 2 | Step 3 | ||||
|---|---|---|---|---|---|---|
| AOR | 95% CI | AOR | 95% CI | AOR | 95% CI | |
| Below average income | 1.20 | (0.70–2.08) | 1.15 | (0.64–2.04) | 1.02 | (0.55–1.91) |
| African American | 2.52 * | (1.08–5.89) | 2.25 | (0.94–5.39) | 2.14 | (0.87–5.29) |
| Older maternal age | 1.26 | (0.78–2.01) | 1.27 | (0.79–2.06) | ||
| Nulliparity | 2.36 *** | (1.55–3.59) | 2.27 *** | (1.47–3.50) | ||
| Multiple gestations | 0.84 | (0.19–3.74) | 0.77 | (0.17–3.54) | ||
| High-risk pregnancy | 1.55 * | (1.01–2.38) | 1.57 * | (1.01–2.44) | ||
| Healthy behaviors | 1.18 | (0.93–1.49) | 1.19 | (0.93–1.52) | ||
| Tobacco use | 2.90 * | (1.10–7.66) | 3.11 * | (1.14–8.49) | ||
| Diagnosed COVID-19 | 4.75 ** | (1.76–12.82) | 5.56 ** | (2.04–15.18) | ||
| Discrimination | 0.85 | (0.38–1.90) | ||||
| Interpersonal violence | 2.31 * | (1.15–4.63) | ||||
| Appointment alteration | 0.69 | (0.46–1.03) | ||||
| COVID-19 related income loss | 0.95 | (0.62–1.46) | ||||
| Major life event | 0.92 | (0.59–1.44) | ||||
| PREPS-Preparedness | 1.66 ** | (1.23–2.23) | ||||
| PREPS-Infection | 0.85 | (0.66–1.09) | ||||
| Prenatal Maternal Stress | 0.92 | (0.70–1.19) | ||||
| Moderate/ severe anxiety symptoms | 0.78 | (0.48–1.26) | ||||
| ΔR2 = 0.01 | ΔR2 = 0.06 | ΔR2 = 0.03 | ||||
| R 2 = 0.01 | R 2 = 0.07 | R 2 = 0.10 |
* p < 0.05, **p < 0.01, ***p < 0.001
Notes:AOR Adjusted Odds Ratio; PREPS - Preparedness Pandemic-Related Prenatal Stress.
Table 5.
Hierarchical Logistic Regression Predicting Unplanned Operative Delivery from Sociodemographic, Medical, and Prenatal Stress Variables (n = 1,328)
| Step 1 | Step 2 | Step 3 | ||||
|---|---|---|---|---|---|---|
| AOR | 95% CI | AOR | 95% CI | AOR | 95% CI | |
| Below average income | 0.73 | (0.45–1.20) | 0.71 | (0.42–1.21) | 0.70 | (0.40–1.24) |
| African American | 0.68 | (0.24–1.93) | 0.46 | (0.15–1.36) | 0.43 | (0.14–1.33) |
| Older maternal age | 0.91 | (0.61–1.37) | 0.88 | (0.58–1.33) | ||
| Nulliparity | 6.31 *** | (4.34–9.17) | 5.89 *** | (4.01–8.65) | ||
| Multiple gestations | 0.92 | (0.28–3.00) | 0.96 | (0.29–3.17) | ||
| High-risk pregnancy | 1.93 *** | (1.37–2.72) | 1.98 *** | (1.39–2.83) | ||
| Healthy behaviors | 0.82 * | (0.68–0.98) | 0.82 * | (0.68–1.00) | ||
| Tobacco use | 1.60 | (0.59–4.29) | 1.57 | (0.58–4.22) | ||
| Diagnosed COVID-19 | 0.97 | (0.27–3.54) | 0.86 | (0.23–3.19) | ||
| Discrimination | 0.65 | (0.33–1.30) | ||||
| Interpersonal violence | 0.82 | (0.37–1.85) | ||||
| Appointment alteration | 1.51 * | (1.08–2.12) | ||||
| COVID-19 related income loss | 1.05 | (0.75–1.48) | ||||
| Major life event | 1.45 * | (1.03–2.05) | ||||
| PREPS-Preparedness | 1.32 * | (1.05–1.67) | ||||
| PREPS-Infection | 0.93 | (0.76–1.14) | ||||
| Prenatal Maternal Stress | 1.02 | (0.82–1.27) | ||||
| Moderate/ severe anxiety symptoms | 0.61 * | (0.41–0.91) | ||||
| ΔR2 = 0.00 | ΔR2 = 0.16 | ΔR2 = 0.03 | ||||
| R 2 = 0.00 | R 2 = 0.16 | R 2 = 0.19 |
* p < 0.05, **p < 0.01, ***p < 0.001
Notes:AOR Adjusted Odds Ratio; PREPS Preparedness Pandemic-Related Prenatal Stress.
Figure 1.
Predictors of perinatal outcomes during the COVID-19 pandemic (N = 1,367).
Discussion
Results of the present study demonstrate that the disruptive impact of the COVID-19 pandemic on prenatal care at the beginning of the pandemic, exposure to interpersonal violence, and other types of stress experienced by pregnant women during this period increased their risks of adverse perinatal outcomes. Findings from the hierarchical regressions indicate that beyond known medical and sociodemographic predictors, pandemic-related and general stressful conditions and perceptions of stress during pregnancy were associated with a greater likelihood of preterm birth, delivering an SGA infant, and unplanned operative delivery.
Of the stress dimensions examined in this study, prenatal maternal stress was the only stress variable that independently predicted preterm birth in the multivariate regression model. Prenatal maternal stress encompasses an array of pregnancy-specific concerns or worries that women may have about their physical changes and symptoms, the health of their fetus, impending labor and delivery, caring for a newborn, and changes in their interpersonal relationships [46]. The average level of prenatal maternal stress in this study was higher than that reported across numerous prior studies using the same instrument [47]. We presume that this increase is attributable in part to the stressful circumstances that women likely experienced at the pandemic outbreak, including uncertainty about the impact of the virus on their and their infant’s health, pandemic-related financial and employment strains, and the loss and closure of many childcare options, to name a few examples. As evidence of such associations, the correlation of the prenatal maternal stress variable (NuPDQ score) with each of the pandemic-related stress variables (PREPS-Preparation and PREPS-Infection) was r = .55, p < .001 and r = .42, p = .42, respectively, indicating that these types of stress did co-occur, although a portion of women’s pregnancy-specific stress was unrelated to pandemic stressors. Prior studies have linked pregnancy-specific stress to preterm birth via unhealthful prenatal behaviors and inflammatory stress responses in pregnancy [16, 47–50] and there is evidence that this type of stress is a particularly strong predictor of preterm birth compared to other types of prenatal stress (see reviews by [47, 51]).
Despite the association of prenatal maternal stress with preterm birth, the overall rate of preterm birth in this sample (7.1%) was lower than the 2019 U.S. population average (10.2%) and lower than the White Non-Hispanic population average (9.3%), which may be a more fitting comparison given the composition of our study sample [52]. The lower prevalence of preterm birth could be attributable to the exclusion of minors from the study since youth is a risk factor for preterm birth, although some reports suggest that rates of preterm birth and SGA during the COVID-19 pandemic are lower than usual [53–55]. African American women in our study were more than twice as likely to deliver preterm than other study participants; their rate of preterm birth (20.5%) was higher than prepandemic U.S. population rates for African American women (14.4%) [52], although this should be interpreted with caution due to the small number of African American women in our sample (n = 39). Emerging evidence suggests that pregnant African American women and other women of color are being disproportionately affected during the COVID-19 pandemic [40], including evidence that SARS-CoV-2 [10] and pandemic-related restrictions on prenatal care have exacerbated longstanding racial inequities [41]. There is also growing recognition that pregnant Black women in the U.S. experience high levels of stress, including a potent type of stress that is unique to their intersecting gender, race, and pregnant status (sometimes labeled gendered racism in pregnancy), and that stress elevates African American women’s risk of adverse outcomes—including preterm birth [37–39]. Comparison of the odds ratio from Step 1 to Step 3 (see Table 3) raises the possibility that the greater prevalence of preterm birth among African American women was attributable to some degree, although not entirely, to higher stress and to medical risk variables.
Two stress variables predicted a higher likelihood of delivering an SGA infant: higher stress about being unprepared for birth due to pandemic restrictions, and experiencing interpersonal violence, a known predictor of adverse perinatal outcomes [56, 57]. During the pandemic, women are at greater risk than ever when sheltering in place with a violent partner [31–33]. Being infected with SARS-CoV-2 during pregnancy was also a strong independent predictor of SGA, which may reflect medical consequences of the virus, as well as risk attributable to the stress that women infected with SARS-CoV-2 during pregnancy are likely to experience.
Women who experienced a major life event during pregnancy, those who had prenatal appointments altered, and those who perceived greater stress because they felt unprepared for birth due to the pandemic were more likely to have an unplanned cesarean or operative vaginal delivery. A separate study of this cohort found that women with greater pandemic-related stress associated with feeling unprepared were more likely to prefer an in-hospital birth (vs. home birth or free-standing birth center) [58]. Ironically, hospital restrictions instituted during the pandemic to protect childbearing women may have increased the likelihood of operative deliveries. That is, women less informed about childbirth due to canceled prenatal appointments may have arrived at hospitals less prepared, more emotionally anxious, or in latent labor, which increases the likelihood of labor augmentation and unplanned cesarean delivery [59, 60]. Altered appointments may also represent missed opportunities to identify pregnancy complications that resulted in emergency deliveries [61]. These findings underscore the importance of ensuring that prenatal care is not disrupted, even when local SARS-CoV-2 infection rates are high. Recent findings indicate that in-person prenatal care appointments do not expose pregnant women to greater infection risk [62].
Somewhat surprisingly, women with moderate or severe anxiety symptoms were less likely to have an unplanned operative delivery than women with minimal or mild anxiety symptoms. However, this finding may be attributable to an apparent association of anxiety with electing to have a scheduled cesarean delivery: 13.3% of women with minimal or mild anxiety symptoms had an elective cesarean delivery versus 19.4% of those who had moderate or severe anxiety symptoms.
The current study is the first in-depth examination of multiple dimensions of stress among women pregnant at the onset of the COVID-19 pandemic and it provides a unique, prospective investigation of their impact on perinatal outcomes. The study does, however, have several limitations related to its reliance on a self-selected cohort who were targeted via social media; the sample is also not racially and socioeconomically diverse and did not include minors. These recruitment and data collection methods were necessitated by pandemic-related constraints preventing face-to-face research recruitment and participation. Further large studies with more diverse samples are needed to validate study findings, as well as research employing more direct measurement of variables, such as through medical record data extraction (e.g., including medical factors such as obesity, substance use). Although there is evidence that women have a good recollection of birth events [63, 64] and pregnant women provide accurate information about their health status and behaviors (e.g., pregnancy risk, tobacco use) [65–67], the possibility of memory bias or distortion due to social desirability concerns cannot be ruled out in a study such as this.
Finally, results of the present study provide a strong foundation for further research investigating why particular outcomes are associated with some types of stress but not others. It is notable, for example, that preterm birth was predicted by nonpandemic, pregnancy-specific stress, that delivering an SGA infant was predicted by interpersonal violence and by pandemic-related pregnancy stress, and that unplanned operative delivery was predicted by a combination of pandemic-related and -unrelated, pregnancy-related and -unrelated stress factors. Although behavioral and biological pathways (including maternal neuroendocrine, immune, metabolic, and cardiovascular systems) have been shown to explain the association between prenatal stress and perinatal outcomes [15], existing evidence is not sufficiently advanced to link types of stress with the pathways that explain their impact on specific outcomes. This is an important avenue for future work.
Conclusions
Study findings offer evidence that particular groups of women, namely those experiencing stress surrounding specific aspects of being pregnant or who feel unprepared for birth, those who experience interpersonal violence, women who have experienced a major life event or disruptions to their prenatal care, those who were infected with COVID-19 during pregnancy, and women who identify as African American are at risk for adverse maternal and neonatal outcomes. Prenatal stress may have additional, undetected effects, as well. We do not know the potential impact on the future health and development of children born to mothers who experienced stress prenatally during the COVID-19 pandemic. Results of this study should be a clarion call for long-term, in-depth investigation and follow-up of these potentially vulnerable children.
Study findings demonstrate that appropriate care, attention, and resources that mitigate pregnant women’s stress are likely to reduce their vulnerability to adverse outcomes. These findings have implications that will outlast the current pandemic and are relevant not merely in times of crisis, but for all pregnant women in all types of life circumstances. To promote population health, it is imperative to systematically identify, address, and resolve the stressful conditions experienced by women in the perinatal period, including those that further elevate the likelihood of adverse outcomes for African American women. Access to a safe living environment and the provision of high-quality medical care, including mental health and prenatal care, are critical for all pregnant women. Additionally, measures to identify [68] and help those experiencing high levels of stress—such as Screening, Brief Intervention and Referral to Treatment (SBIRT), psychoeducation, and other forms of mental health promotion such as the Collaborative Care model—may reduce women’s risks [57, 69, 70]. During the COVID-19 pandemic, telehealth services for mental health treatment and prenatal care have expanded tremendously. Relevant technologies have been adapted to ensure high-quality, evidence-based, remote care [71, 72]. The acceptability of online care delivery platforms by clients, providers, and insurers has opened-up new possibilities for treatment and care in the perinatal period. For women seeking behavioral treatments, access to care has in the past often been impeded by stigma, financial strain, limited mobility, lack of childcare, and conflicts with work and other responsibilities [73, 74]. The possibility of receiving high-quality, evidence-based, effective individual or group care to reduce perinatal stress remotely [75] may be a silver lining of the COVID-19 pandemic, but only if existing racial and socioeconomic disparities in access to technology and facility with online platforms can be overcome [72, 76]. Findings of the current study demonstrate the urgency of such interventions to ensure the health and well-being of pregnant women and children born during the COVID-19 pandemic, but these exigencies will continue long beyond.
Acknowledgements
Funding for this study was provided by a Stony Brook University Office of the Vice President for Research and Institute for Engineering-Driven Medicine COVID-19 Seed Grant. The authors would like to thank the wonderful research assistants who helped in carrying out this project: Emily Rehbein, Donna Wilcox, Ilka St. Denis, Francine Chirico, Lucero Molina, and Katya Potkin. We thank all the women who participated in this study for their time and their willingness to share their experiences from being pregnant during the COVID- 19 Pandemic.
Compliance with Ethical Standards
Authors’ Statement of Conflict of Interest and Adherence to Ethical Standards Brittain Mahaffey received support from the National Institutes of Health Grant K23HD092888 during the preparation of this article. Heidi Preis received support from National Institutes of Health Grant R21DA049827 during the preparation of this article.
Primary Data The authors have full control of all primary data and it will be shared upon reasonable request.
Authors’ Contributions H.P.: conceptualization; data curation; formal analysis; investigation; methodology; project administration; resources; software; visualization; writing- original draft; supervision. B.M.: resources; conceptualization; writing-review & editing. S.P.: conceptualization; methodology; writing-review & editing. C.H.: conceptualization; methodology; writing-review & editing. M.L.: conceptualization; investigation; methodology; resources; writing-review & editing, supervision. Authors whose names appear on the submission have contributed sufficiently to the scientific work and therefore share collective responsibility and accountability for the results.
Ethical Approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent Informed consent was obtained from all individual participants included in the study.
References
- 1. Gassman-Pines A, Ananat EO, Fitz-Henley J. COVID-19 and parent-child psychological well-being. Pediatrics. 2020;146:e2020007294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Patrick SW, Henkhaus LE, Zickafoose JS, et al. Well-being of parents and children during the COVID-19 pandemic: a national survey. Pediatrics. 2020;146:e2020016824. [DOI] [PubMed] [Google Scholar]
- 3. Saccone G, Florio A, Aiello F, et al. Psychological impact of COVID-19 in pregnant women. Am J Obstet Gynecol. 2020;223:293–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lebel C, MacKinnon A, Bagshawe M, Tomfohr-Madsen L, Giesbrecht G. Elevated depression and anxiety symptoms among pregnant individuals during the COVID-19 pandemic. J Affect Disord. 2020;277:5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Taubman – Ben-Ari O, Chasson M, Abu Sharkia S, Weiss E. Distress and anxiety associated with COVID-19 among Jewish and Arab pregnant women in Israel. J Reprod Infant Psychol. 2020; 38(3): 340– 348. [DOI] [PubMed] [Google Scholar]
- 6. Preis H, Mahaffey B, Heiselman C, Lobel M. Pandemic-related pregnancy stress and anxiety among women pregnant during the COVID-19 pandemic. Am J Obstet Gynecol MFM. 2020;2:100155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moyer CA, Compton SD, Kaselitz E, Muzik M. Pregnancy-related anxiety during COVID-19: a nationwide survey of 2740 pregnant women. Arch Womens Ment Health. 2020:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Verma S, Bradshaw C, Auyeung NSF, et al. Outcomes of maternal-newborn dyads after maternal SARS-CoV-2. Pediatrics. 2020;23: 757– 765. [DOI] [PubMed] [Google Scholar]
- 9. Allotey J, Stallings E, Bonet M, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Delahoy MJ, Whitaker M, O’Halloran A, et al. Characteristics and maternal and birth outcomes of hospitalized pregnant women with laboratory-confirmed COVID-19. MMWR Morb Mortal Wkly Rep. 2020;69:1347–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huntley BJF, Huntley ES, Di Mascio D, et al. Rates of maternal and perinatal mortality and vertical transmission in pregnancies complicated by severe acute respiratory syndrome Coronavirus 2 (SARS-Co-V-2) infection: a systematic review. Obstet Gynecol. 2020;136:303–312. [DOI] [PubMed] [Google Scholar]
- 12. Adhikari EH, Moreno W, Zofkie AC, et al. Pregnancy outcomes among women with and without severe acute respiratory syndrome Coronavirus 2 infection. JAMA Netw Open. 2020;3:e2029256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grigoriadis S, Graves L, Peer M, et al. Maternal anxiety during pregnancy and the association with adverse perinatal outcomes: systematic review and meta-analysis. J Clin Psychiatry. 2018;79:17r12011. [DOI] [PubMed] [Google Scholar]
- 14. Bussières E-L, Tarabulsy GM, Pearson J, et al. Maternal prenatal stress and infant birth weight and gestational age: a meta-analysis of prospective studies. Dev Rev. 2015;36: 179–199. [Google Scholar]
- 15. Lobel M, Dunkel Schetter C. Pregnancy and prenatal stress. In: Friedman HS, ed. Encyclopedia of Mental Health. Oxford: Academic Press; 2016:318–329. [Google Scholar]
- 16. Mahrer NE, Guardino CM, Hobel C, Dunkel Schetter C. Maternal stress before conception is associated with shorter gestation. Ann Behav Med. 2020:047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim B, Carruthers CK, Harris MC. Maternal stress and birth outcomes: evidence from the 1994 Northridge earthquake. J Econ Behav Organ. 2017;140:354–373. [Google Scholar]
- 18. Harville E, Xiong X, Buekens P. Disasters and perinatal health: a systematic review. Obstet Gynecol Surv. 2010;65:713–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Buthmann J, Ham J, Davey K, et al. Infant temperament: repercussions of superstorm sandy-related maternal stress. Child Psychiatry Hum Dev. 2019;50:150–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moster D, Lie RT, Markestad T. Long-term medical and social consequences of preterm birth. N Engl J Med. 2008;359:262–273. [DOI] [PubMed] [Google Scholar]
- 21. Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. 2008;371:261–269. [DOI] [PubMed] [Google Scholar]
- 22. Hu YJ, Wake M, Saffery R. Clarifying the sweeping consequences of COVID-19 in pregnant women, newborns, and children with existing cohorts. JAMA pediatrics. 2021; 175: 117– 118. [DOI] [PubMed] [Google Scholar]
- 23. Abdoli A, Falahi S, Kenarkoohi A, Shams M, Mir H, Jahromi MAM. The COVID-19 pandemic, psychological stress during pregnancy, and risk of neurodevelopmental disorders in offspring: A neglected consequence. J Psychosom Obstet Gynaecol. 2020;41:247–248. [DOI] [PubMed] [Google Scholar]
- 24. Lazarus RS, Folkman S. Stress, Appraisal, and Coping. New York, NY: Springer; 1984. [Google Scholar]
- 25. Lobel M. Conceptualizations, measurement, and effects of prenatal maternal stress on birth outcomes. J Behav Med. 1994;17:225–272. [DOI] [PubMed] [Google Scholar]
- 26. Dethier D, Abernathy A. Maintaining certainty in the most uncertain of times. Birth. 2020;47:257–258. [DOI] [PubMed] [Google Scholar]
- 27. Ecker JL, Minkoff HL. Laboring alone? Brief thoughts on ethics and practical answers during the coronavirus disease 2019 pandemic. Am J Obstet Gynecol MFM. 2020; 2:100141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Justman N, Shahak G, Gutzeit O, et al. Lockdown with a price: the impact of the COVID-19 pandemic on prenatal care and perinatal outcomes in a tertiary care center. Isr Med Assoc J. 2020;9:467–471. [PubMed] [Google Scholar]
- 29. Thayer ZM, Gildner TE. COVID-19-related financial stress associated with higher likelihood of depression among pregnant women living in the United States. Am J Hum Biol. 2020:e23508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bureau of Labor Statistics U.S. Department of Labor. The Employment Situation- September 2020. Washington DC: Department of Labor; 2020. [Google Scholar]
- 31. Campbell AM. An increasing risk of family violence during the COVID-19 pandemic: strengthening community collaborations to save lives. Forensic Sci Int. 2020; 2:100089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Froimson JR, Bryan DS, Bryan AF, Zakrison TL. COVID-19, Home confinement, and the fallacy of “Safest at Home”. Am J Public Health. 2020;110:960–961. [Google Scholar]
- 33. Usher K, Bhullar N, Durkin J, Gyamfi N, Jackson D. Family violence and COVID-19: increased vulnerability and reduced options for support. Int J Ment Health Nurs. 2020;29:549–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Preis H, Mahaffey B, Heiselman C, Lobel M. Vulnerability and resilience to pandemic-related stress among U.S. women pregnant at the start of the COVID-19 pandemic. Soc Sci Med. 2020;266:113348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wu Y, Zhang C, Liu H, et al. Perinatal depressive and anxiety symptoms of pregnant women along with COVID-19 outbreak in China. Am J Obstet Gynecol. 2020;223:240e.1–240e.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ruiz JM, Revenson TA. Behavioral medicine in the COVID-19 era: dawn of the golden age. Ann Behav Med. 2020;54:541–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rosenthal L, Lobel M. Explaining racial disparities in adverse birth outcomes: unique sources of stress for Black American women. Soc Sci Med. 2011;72:977–983. [DOI] [PubMed] [Google Scholar]
- 38. Dominguez TP, Schetter CD, Mancuso R, Rini CM, Hobel C. Stress in African American pregnancies: testing the roles of various stress concepts in prediction of birth outcomes. Ann Behav Med. 2005;29:12–21. [DOI] [PubMed] [Google Scholar]
- 39. Earnshaw VA, Rosenthal L, Lewis JB, et al. Maternal experiences with everyday discrimination and infant birth weight: a test of mediators and noderators among young, urban women of color. Ann Behav Med. 2012;45:13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gur RE, White LK, Waller R, et al. The disproportionate burden of the COVID-19 pandemic among pregnant black women. Psychiatry Res. 2020;293:113475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Onwuzurike C, Meadows AR, Nour NM. Examining inequities associated with changes in obstetric and gynecologic care delivery during the Coronavirus Disease 2019 (COVID-19) pandemic. Obstet Gynecol. 2020; 136: 37– 41. [DOI] [PubMed] [Google Scholar]
- 42. Behrman RE, Butler AS. Preterm Birth: Causes, Consequences, and Prevention. Washington D.C.: National Academies Press; 2007. [PubMed] [Google Scholar]
- 43. Preis H, Mahaffey B, Lobel M. Psychometric properties of the Pandemic-Related Pregnancy Stress Scale (PREPS). J Psychosom Obstet Gynaecol. 2020;41:191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch Intern Med. 2006;166:1092–1097. [DOI] [PubMed] [Google Scholar]
- 45. Kiserud T, Piaggio G, Carroli G, et al. The World Health Organization fetal growth charts: a multinational longitudinal study of ultrasound biometric measurements and estimated fetal weight. PLOS Med. 2017;14:e1002220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yali AM, Lobel M. Coping and distress in pregnancy: an investigation of medically high risk women. J Psychosom Obstet Gynaecol. 1999;20:39–52. [DOI] [PubMed] [Google Scholar]
- 47. Ibrahim SM, Lobel M. Conceptualization, measurement, and effects of pregnancy-specific stress: review of research using the original and revised prenatal distress questionnaire. J Behav Med. 2020;43:16–33. [DOI] [PubMed] [Google Scholar]
- 48. Auerbach MV, Nicoloro-SantaBarbara J, Rosenthal L, et al. Psychometric properties of the prenatal health behavior scale in mid- and late pregnancy. J Psychosom Obstet Gynaecol. 2017;38:143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lobel M, Cannella DL, Graham JE, et al. Pregnancy-specific stress, prenatal health behaviors, and birth outcomes. Health Psychol. 2008;27:604. [DOI] [PubMed] [Google Scholar]
- 50. Coussons-Read ME, Lobel M, Carey JC, et al. The occurrence of preterm delivery is linked to pregnancy-specific distress and elevated inflammatory markers across gestation. Brain Behav Immun. 2012;26:650–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Alderdice F, Lynn F, Lobel M. A review and psychometric evaluation of pregnancy-specific stress measures. J Psychosom Obstet Gynaecol. 2012;33:62–77. [DOI] [PubMed] [Google Scholar]
- 52. Martin JA, Hamilton BE, Osterman MJK. Births in the United States, 2019. Hyattsville, MD: National Center for Health Statistics; 2020. [Google Scholar]
- 53. Khalil A, Von Dadelszen P, Draycott T, et al. Change in the incidence of stillbirth and preterm delivery during the COVID-19 pandemic. JAMA. 2020;324:705–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Caniglia EC, Magosi LE, Zash R, et al. Modest reduction in adverse birth outcomes following the COVID-19 lockdown. Am J Obstet Gynecol. 2020. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Been JV, Burgos Ochoa L, Bertens LCM, et al. Impact of COVID-19 mitigation measures on the incidence of preterm birth: a national quasi-experimental study. Lancet Public Health. 2020;5:e604–e611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shah PS, Shah J. Maternal exposure to domestic violence and pregnancy and birth outcomes: a systematic review and meta-analyses. J Womens Health. 2010;19:2017–2031. [DOI] [PubMed] [Google Scholar]
- 57. Alhusen JL, Ray E, Sharps P, Bullock L. Intimate partner violence during pregnancy: maternal and neonatal outcomes. J Womens Health. 2015;24:100–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Preis H, Mahaffey B, Lobel M. The role of pandemic-related pregnancy stress in preference for community birth during the beginning of the COVID-19 pandemic in the U.S. Birth. 2021. In Press [DOI] [PMC free article] [PubMed]
- 59. Bailit JL, Dierker L, Blanchard MH, Mercer BM. Outcomes of women presenting in active versus latent phase of spontaneous labor. Obstet Gynecol. 2005;105:77–79. [DOI] [PubMed] [Google Scholar]
- 60. Neal JL, Lamp JM, Buck JS, et al. Outcomes of nulliparous women with spontaneous labor onset admitted to hospitals in preactive versus active labor. J Midwifery Womens Health. 2014;59:28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Goyal M, Singh P, Singh K, et al. The effect of the COVID-19 pandemic on maternal health due to delay in seeking health care: experience from a tertiary center. Int J Gynaecol Obstet. 2021; 152: 231– 235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Reale SC, Fields KG, Lumbreras-Marquez MI, et al. Association between number of in-person health care visits and SARS-CoV-2 infection in obstetrical patients. JAMA. 2020;324:1210–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bat-Erdene U, Metcalfe A, McDonald SW, Tough SC. Validation of Canadian mothers’ recall of events in labour and delivery with electronic health records. BMC Pregnancy Childbirth. 2013;13Suppl 1:S3-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gartland D, Lansakara N, Flood M, Brown SJ. Assessing obstetric risk factors for maternal morbidity: Congruity between medical records and mothers’ reports of obstetric exposures. Am J Obstet Gynecol. 2012;206:152.e1–152.10. [DOI] [PubMed] [Google Scholar]
- 65. McDonald SD, Perkins SL, Walker MC. Correlation between self-reported smoking status and serum cotinine during pregnancy. Addict Behav. 2005;30:853–857. [DOI] [PubMed] [Google Scholar]
- 66. Timperio A, Salmon J, Crawford D. Validity and reliability of a physical activity recall instrument among overweight and non-overweight men and women. J Sci Med Sport. 2003;6:477–491. [DOI] [PubMed] [Google Scholar]
- 67. Cannella D, Auerbach M, Lobel M. Predicting birth outcomes: Together, mother and health care provider know best. J Psychosom Res. 2013;75:299–304. [DOI] [PubMed] [Google Scholar]
- 68. Preis H, Garry DJ, Herrera K, Garretto DJ, Lobel M. Improving assessment, treatment, and understanding of pregnant women with opioid use disorder: the importance of life context. Womens Reprod Health. 2020;7:153–163. [Google Scholar]
- 69. Traylor CS, Johnson JD, Kimmel MC, Manuck TA. Effects of psychological stress on adverse pregnancy outcomes and nonpharmacologic approaches for reduction: An expert review. Am J Obstet Gynecol MFM. 2020;2:100229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Grote NK, Katon WJ, Russo JE, et al. Collaborative care for perinatal depression in socioeconomically disadvantaged women: a randomized trial. Depress Anxiety. 2015;32:821–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gülmezoglu AM, Ammerdorffer A, Narasimhan M, et al. Self-care and remote care during pregnancy: A new paradigm? Health Res Policy Syst. 2020;18:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Futterman I, Rosenfeld E, Toaff M, et al. Addressing disparities in prenatal care via telehealth during COVID-19: prenatal satisfaction survey in east Harlem. Am J Perinatol. 2021;38:88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Poleshuck EL, Cerrito B, Leshoure N, Finocan-Kaag G, Kearney MH. Underserved women in a women’s health clinic describe their experiences of depressive symptoms and why they have low uptake of psychotherapy. Community Ment Health J. 2013;49:50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. O’Mahen HA, Woodford J, McGinley J, et al. Internet-based behavioral activation–treatment for postnatal depression (Netmums): a randomized controlled trial. J Affect Disord. 2013;150:814–822. [DOI] [PubMed] [Google Scholar]
- 75. Aksoy Derya Y, Altiparmak S, AkÇa E, GÖkbulut N, Yilmaz AN. Pregnancy and birth planning during COVID-19: the effects of tele-education offered to pregnant women on prenatal distress and pregnancy-related anxiety. Midwifery. 2021;92:102877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Limaye MA, Lantigua-Martinez M, Trostle ME, et al. Differential uptake of telehealth for prenatal care in a large New York City academic obstetrical practice during the COVID-19 pandemic. Am J Perinatol. 2020; 38:304–306. [DOI] [PubMed] [Google Scholar]