Abstract
Background
Stress in pregnancy predicts adverse birth outcomes. Stressors occurring prior to conception may also pose risk for the mother and child. The few published studies on preconception stress test a single stress measure and examine only linear associations with birth outcomes.
Purpose
Guided by findings in the prenatal stress literature, the current study aimed to (i) identify latent factors from a set of preconception stress measures and (ii) examine linear and curvilinear associations between these stress factors and length of gestation.
Methods
Study 1 utilized a sample of 2,637 racially/ethnically diverse women to develop a measurement model of maternal stress from assessments of seven acute and chronic stress measures. Factor analysis revealed three latent factors representing stressors (life events, financial strain, interpersonal violence, discrimination), stress appraisals (perceived stress, parenting stress), and chronic relationship stress (family, partner stress). Study 2 examined the associations of these three latent preconception stress factors with the length of gestation of a subsequent pregnancy in the subset of 360 women who became pregnant within 4.5 years.
Results
Controlling for prenatal medical risks, there was a significant linear effect of stress appraisals on the length of gestation such that more perceived stress was associated with shorter gestation. There was a curvilinear effect of stressors on the length of gestation with moderate levels associated with longer gestation.
Conclusions
These results have implications for research on intergenerational origins of developmental adversities and may guide preconception prevention efforts. Findings also inform approaches to the study of stress as a multidimensional construct.
Keywords: Preconception, Stress, Gestational length, Preterm birth
Greater perceived stress prior to pregnancy is associated with earlier births, but exposure to moderate levels of environmental stressors preconception is associated with the longest gestation.
Introduction
A considerable body of research implicates maternal stress during pregnancy as a potent influence in fetal development, adverse birth outcomes, and many infant and child developmental adversities. For example, prenatal maternal stress is prospectively associated with shorter gestational length and preterm birth [1–3]. Preterm birth in turn increases the risk for postpartum maternal complications and infant mortality [4], as well as infant developmental delays and behavioral and mental health problems in offspring [5–7]. Emerging evidence suggests that the biological and health conditions involved in the etiological pathways to adverse birth outcomes originate prior to pregnancy; that is, these conditions exist before conception [8]. In fact, studies comparing the effects of preconception stressors to prenatal stressors show higher relative risk for preterm birth, low birthweight, and infant mortality when the event occurred before conception [9–11]. The explosion of research on early life adversity documenting powerful lifelong health effects of major stressors occurring in childhood [12] further supports the need to examine stress processes during the months and years before conception. Moreover, this approach may identify modifiable stressors early enough to intervene more effectively and improve maternal and child outcomes [13].
A life course perspective posits that stressors occurring prior to conception contribute to a mother’s health capital, which in turn affects her body’s ability to cope with future stressors, including pregnancy [14]. The accumulation of all preconception stressors, no matter when they occurred in a woman’s life or their nature or origin, are thought to increase the likelihood of adverse birth outcomes. Others theorize that stressors occurring 6 months to 1 year prior, or just proximal to conception, can influence embryonic implantation and placental and fetal development [15]. This proximal preconception period (6–12 months before conception) has been the most closely studied in both the animal and human literature. Animal models indicate that offspring of rats randomly assigned to stress exposures such as overcrowding or isolation, intermittent pain, or food/water deprivation prior to pregnancy have higher risk for adverse neurodevelopmental outcomes at birth compared with those not exposed, and the effects last into adulthood [15]. Moreover, population-based studies of humans in the United States, Denmark, and Sweden have shown that exposure to stressful life events such as divorce or the death or illness of a close relative or loved one in the year prior to conception increases the risk for preterm birth (delivery at less than 37 weeks gestation) [9], low birthweight [10, 16, 17], and infant mortality [11]. Taken together, these studies point to 1 year prior to conception as a sensitive period when maternal stress may increase the risk for a range of adverse birth and offspring outcomes. Yet, prior studies have typically included only one preconception stress measure, most often an inventory of negative life events, in order to test associations with birth outcomes [18]. Thus, the associations of different types of maternal preconception stress with birth outcomes, and their independent contributions have not been tested.
Furthermore, the possibility of non-linear associations between maternal preconception stress and birth outcomes has not been addressed in this body of work, despite a small literature suggesting that a small or moderate degree of stress may be adaptive for the fetus [19]. Some researchers posit that the link between stress and child outcomes may mirror the U-shaped relation between arousal and performance (moderate arousal leading to optimal performance) previously demonstrated in the psychological literature [20]. In other words, low and high stress may be detrimental, whereas moderate stress may prepare the fetus for the postnatal environment [21]. A few studies show that moderate levels of anxiety, depression, pregnancy-specific stress, and general stress occurring during pregnancy predict better motor and cognitive outcomes in infants and children [22, 23] but this relationship has not been tested with respect to pre-pregnancy or preconception stress. It is plausible given some continuity of preconception and prenatal stress in women that curvilinear relationships also apply to preconception stress.
Types of Stress
Researchers continue to debate the concept of stress, emphasizing that it is not unidimensional in nature [24]. Rather, the stress process includes exposures to environmental stressors, events, or conditions that are observable in the social or physical environment as well as subjective responses involving individuals’ perceptions or appraisals of their ability to cope with those stressors [24–29]. Researchers also differentiate between episodic or acute stressors, which are characterized as discrete or time-limited, and chronic stressors, which are longer-lasting challenges [24, 30, 31]. Studies examining stress in the prenatal period have identified multiple stress constructs including stress exposures, chronic stressful conditions, and perceptions of stress [2]. During pregnancy, acute or episodic stress exposures such as negative life events or major catastrophes have been more consistently linked with the risk for preterm birth, whereas chronic stressors and emotional distress have more often been found to increase risk for low birth weight [18]. Although the type of stressor a woman encounters may have different implications for birth outcomes, multiple measures of stress have rarely been included in the same study during pregnancy [32–34], let alone studied before conception.
The current study has two aims: (i) To differentiate distinct and conceptually interpretable latent preconception stress factors (i.e., a measurement model) from a fairly comprehensive set of stress measures administered by interview to a large and racially/ethnically diverse sample of women in the United States and (ii) To test the independent associations of preconception stress factors with the length of gestation within a subset of women who became pregnant again and had live births. Both linear and curvilinear effects were tested. The terms “preconception” and “interconception” are used interchangeably and variably in the literature. Sometimes “preconception” refers to the period before conception of a first child, but more often it is now used to refer to the period before conception of any child, which is how it is used here. In Study 1, we expected to find latent preconception stress factors representing stressors and stress appraisals, consistent with theoretical stress frameworks [27, 28]. For Study 2, we hypothesized that episodic forms of stress before conception would be associated with shorter gestational length based on prior evidence [35–37]. It is known that every day in the womb is of benefit to fetal development especially toward the end of pregnancy; thus, gestational length in weeks is an important health outcome [38, 39]. The current study’s comprehensive measurement of stressors and stress appraisals prior to conception, tests for independent associations between latent factors of stress and birth outcomes, and exploratory analyses of curvilinear associations are all novel, and results may advance the understanding of the biological and etiological pathways that lead to adverse birth outcomes even before a pregnancy begins.
Materials and Methods
Participants and Procedure
Participants for Study 1 were 2,637 mothers from the five sites in the Community Child Health Research Network (CCHN) study: Washington, DC, Baltimore, Maryland, Los Angeles County, California, Lake County, Illinois, and seven counties in eastern North Carolina. The CCHN is a multi-site research network funded by the Eunice Kennedy Shriver National Institutes of Child Health and Human Development (NICHD) to investigate health disparities in pregnancy outcomes and biopsychosocial factors that affect maternal and child health [40]. The CCHN cohort included African-American/Black (53.4%), Latina (25.6%), and non-Hispanic White (20.8%) women who were recruited immediately after the birth of a child in predominantly low-income and diverse areas of the United States. As such, this sample includes more women of color and families living below or near the poverty level than many studies of prenatal health and child development. As the focus of CCHN was on maternal child health disparities, the study provides a rare opportunity to examine stress among those in a population with highest exposures.
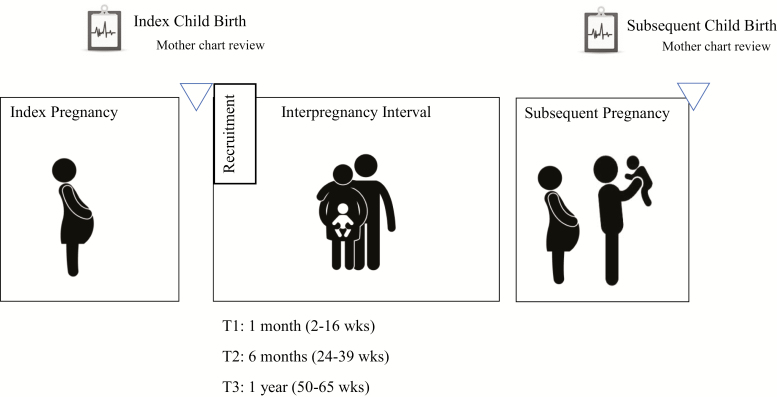
The Institutional Review Board at each participating site approved the study. Participants were interviewed by trained community members in their language of preference (English or Spanish) in their homes using structured interviews. The current study uses data collected from mothers 1 (Time 1), 6 (Time 2), and 12 (Time 3) months after the birth of a child (see Fig. 1 for a diagram of the study design). The majority of this sample (71%) were living at or below 200% of the federal poverty level. The average per capita household income, adjusted for cost of living, was $12,425.21 (SD = $20,542.39). Mothers were between 17 and 42 years old at Time 1 (M = 25.56, SD = 5.63 years) and had completed an average 12.91 years of education (SD = 2.78). Approximately one-third (31.6%) of the sample was married, 28.1% were cohabitating but not married, and 40.4% were not married or cohabitating.
Fig. 1.
Diagram of Community Child Health Network (CCHN) and subsequent child follow-up study design.
The sample for Study 2 includes 360 women who became pregnant again during the study period and participated in follow-up assessments after the birth of their subsequent child (interpregnancy interval ranged from 1.71 to 55.39 months, M (SD) = 15.85 (10.33) months and was highly negatively skewed). Of the women who became pregnant again and were included in the follow-up sample, 67% were pregnant within 18 months of giving birth. Data about birth outcomes and pregnancy risk from these subsequent pregnancies were obtained from medical records and supplemented with self-report from structured interviews 1 month after the birth. In this Study 2 sub-sample, 43.8% identified as Black or African-American, 29.6% as Latina or Hispanic, and 26.6% as non-Hispanic White. The majority of this sub-sample (72%) had incomes at or below 200% of the federal poverty level. The average per capita household income, adjusted for cost of living, was $13, 275.57 (SD = $20,233.62). Mothers were on average 25.22 years old at Time 1 (SD = 5.10 years) and received on average 12.62 years of education (SD = 2.75). Approximately one-third of this sub-sample was married (35.6%), 30.1% were cohabitating, and 34.4% were not married or cohabitating. Participants in Study 2 were more likely to be married (χ 2 (2) = 6.98, p < .05) and less likely to be Black (χ 2 (2) = 17.2, p < .001) compared with participants in the Study 1 sample. There were no other significant differences between the Study 1 and Study 2 samples on other demographic variables.
Measures
Maternal Preconception Stress
Maternal stress measures were administered at one of the three timepoints across one year of study, all occurring prior to conception of a child for those included in Study 2. Chronic Stress was measured using a CCHN adaptation of the UCLA Life Stress Interview [41] at Time 2. This semi-structured interview assesses chronic demands on three life domains (family, partner, and co-parenting relationship). Trained interviews assigned objective scores in the three domains based on the content of the interviews. Discrimination was assessed using the Everyday Discrimination Scale [42] at Time 1 (α = .89 in English α = .88 in Spanish). Scores on this measure were highly skewed and log-transformed prior to analyses. Financial Stress including Food Insecurity was measured using CCHN-developed survey items at Time 1 (α = .69 in English α = .68 in Spanish). Interpersonal Violence (IPV) was measured using the HITS [43, 44] at Time 1 (α = .74 in English and Spanish). Data were highly skewed and log-transformed prior to analyses. Parenting Stress, which assessed stress related to parenting the child from their pregnancy before enrolling in the study, was measured with the Parenting Stress Index [45, 46] at Time 3 (α = .92 in English α = .94 in Spanish). Perceived Stress was measured using the Perceived Stress Scale [47] at Times 1-3 (Mean over three timepoints: α = .88 in English and α = .73 Spanish). Stressful Life Events in the past year was assessed using the Life Events Inventory [48] at Time 1 and Time 3. Given the theorized difference between exposure to the adverse events and the subjective response to them [24], and the equally potent predictive value of total number of events [49], total count of stressful life events was used.
Most of the measures of stress in the current paper have previously been used in Spanish in perinatal research evidencing strong psychometrics where appropriate. The PSS is the most validated of the set with research in English and Spanish worldwide [this study, [50]. Life events inventories similar to this one have been translated and used as well in these prior papers and in many others. The Parenting Stress Index was used in the CCHN Study in Spanish and showed alpha coefficients over .90 for both English and Spanish (this study). Everyday discrimination has been used less often in Spanish speaking populations, but recent work has investigated the measures properties in Spanish [51]. The HITS for measuring IPV has been used in both English and Spanish previously [52] and is relatively brief and straight forward. Finally, the UCLA Life Stress Interview is relatively new and well-validated in English but not Spanish as yet. It is an interviewer-completed measure based on a semi-structured interview, however, and not one based on self-ratings. Interviewers who administered interviews in Spanish in the present study spoke Spanish fluently and rated interviews in English.
Length of Gestation
Gestational length (weeks) was extracted from birth records/medical charts by trained research staff. Gestational length in weeks was treated as a continuous outcome. Eleven percent of the sample had preterm birth, defined as gestational length of less than 37 weeks. Birth weight was not examined in the current paper given its high correlation with gestational length (r = .60, p < .001) and interest in having an independent outcome, as well as a lower incidence of low birth weight babies.
Pregnancy Risk
Information regarding infections, hypertension, vascular and diabetes-related, and previous pregnancy conditions that increase risk for preterm birth was extracted from the participants’ medical charts (see Hobel et al. [53] for the full list of conditions examined). Conditions that significantly related to gestational length in the current sample (i.e., preeclampsia, hypertension, and history of preterm birth) were combined to create a binary variable coded as low risk (88%) or high risk (12%). Given that all pregnancies in the study were at least the mother’s second pregnancy, parity was coded using a binary variable (0 = second birth, 50%; 1 = greater than second birth, 50%) and interpregnancy interval was calculated in months. Type of delivery, coded as spontaneous vaginal delivery (62%) versus other (i.e., induced vaginal delivery, or scheduled or emergency C-section; 38%), was obtained from the medical chart. All pregnancy risk variables were examined as possible covariates. Only medical risk (low/high) was significantly related to gestational length (r = -.23, p < .01) and was therefore included in Study 2 models. Note that participant demographic characteristics (i.e., age, income, education, race/ethnicity) were not related to gestational length (all p-values > .17).
Analyses
Study 1: measurement model
Using the larger CCHN sample, we first examined factors in the maternal stress data using exploratory factor analyses (EFA), allowing for up to four factors to emerge. The exploratory factor analytic model used maximum likelihood with oblique rotation, which allowed for correlations among factors. Good (acceptable) model fit was determined based on recommendations from simulation studies that minimize Type I and Type II error rates: SRMR ≤ .05 (.08); RMSEA ≤ .05 (.08); CFI ≥ .95 (.90) [54, 55]. We replicated the best EFA solution with a confirmatory factor analysis (CFA) using two random halves of the sample. In the CFA, the unstandardized parameter estimate for the first predictor of each factor was set to 1 and other factor loadings were freely estimated. In the full sample, we used modification indices to guide the inclusion of correlated residuals among maternal stress variables that were measured at the same time point. A likelihood ratio test then compared the model fit between the original measurement model and model with correlated residuals within time-points. Further, we compared the parameter estimates and correlations among factors between the two measurement models to assure that estimates were not changing substantially by adding correlated residuals.
Study 2: prediction of birth outcomes
Using the sub-sample of mothers who became pregnant with a subsequent child, a structural equation model (SEM) examined the associations between the identified maternal preconception stress latent factors from Study 1 and gestational length in weeks, controlling for medical risk during the pregnancy (see Supplementary Table 1 for descriptive statistics on study variables and Supplementary Table 2 for bivariate correlations among stress indicators and study variables). Linear and quadratic relations between the preconception stress factors and gestational length were examined. Due to the smaller sample size with the SEM analyses (n = 360), only one quadratic predictor of maternal preconception stress could be tested at a time. As a result, three models were examined, each with one quadratic predictor and three linear predictors of gestational length, controlling for mothers’ medical risk during her pregnancy. All analyses were run using Mplus software [56], which employs full information maximum likelihood estimation for handling missing data.
Results
Study 1
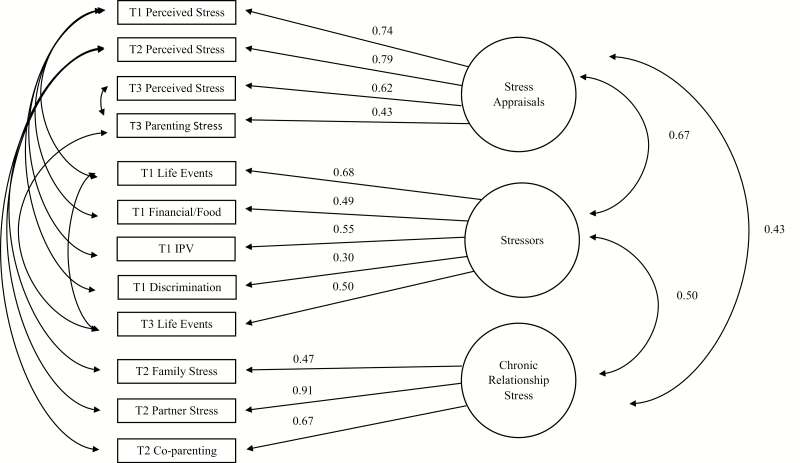
EFA results indicated that a three-factor solution fit the data best. The three-factor solution was significantly better than the one-factor (χ 2difference = 786.00, p < .001) and two-factor solutions (χ 2difference = 497.23, p < .001) and the four-factor solution did not converge. Each factor included measures that clearly loaded most strongly on that particular factor (no cross-factor loadings were above .25). Discrimination had the lowest factor loading (β = .30). Analyses were run with and without the discrimination variable and results from Study 1 and Study 2 remained the same. The three-factor model had acceptable fit: SRMR = .05; RMSEA = .06; CFI = .91. CFA replicated the three-factor model (see Table 1 for factor loadings and fit indices in the full sample). Inclusion of correlated residuals between variables measured at the same time-point, significantly improved the model fit (χ 2difference = 326.75, p < .001) and model fit indices increased from acceptable to good ranges (Table 1). The largest parameter change was .10 (T3 Negative Life Events). All other changes were less than .10, including inter-factor correlations (see Table 1 for model fit and parameter estimate comparisons and Fig. 2). The three latent factors were labeled: stressors, stress appraisals, and chronic relationship stress. Measures that loaded onto the Stressors factor included exposure to stressful life events, financial stress and food insecurity, interpersonal violence, and discrimination. The Stress appraisals factor included general perceived stress and parenting stress. The Chronic relationship stress factor included assessment of family, partner, and co-parenting stress.
Table 1.
Model fit and standardized factor loadings in original measurement model and model with correlated residuals within variables measured at the same time-point (N = 2,637)
| Fit | CFI model | CFI model with correlated residuals |
|---|---|---|
| χ 2 = 530, p < .001; CFI = .91; RMSEA = .06; SRMR = .047 | χ 2 = 203, p < .001; CFI = .97; RMSEA = .038; SRMR = .036 | |
| Stress appraisals | ||
| T1 perceived stress | .72 | .74 |
| T2 perceived stress | .79 | .78 |
| T3 perceived stress | .68 | .62 |
| T3 parenting stress | .49 | .43 |
| Stressors | ||
| T1 negative life events | .71 | .68 |
| T1 financial stress | .49 | .49 |
| T1 IPV | .54 | .55 |
| T1 discrimination | .29 | .30 |
| T3 negative life events | .60 | .50 |
| Chronic Relationship Stress | ||
| T2 family stress | .48 | .47 |
| T2 partner stress | .91 | .91 |
| T2 co-parenting stress | .67 | .67 |
Fit of the uncorrelated 3-factor solution was superior to the uncorrelated 1-factor solution (χ 2 = 1,316, p < .001; CFI = .75; RMSEA = .09; SRMR = .076; χ 2difference = 786, p < .001).
Fig. 2.
Maternal stress measurement model (N = 2,637).
Study 2
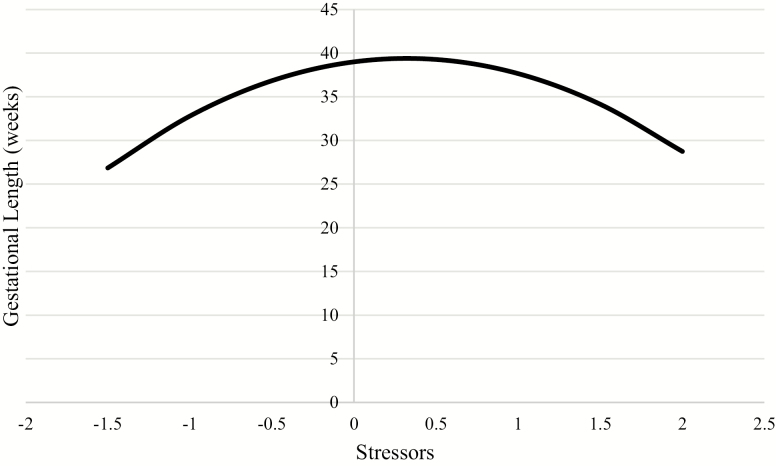
When testing the three SEM models that each included one quadratic effect, only the quadratic effect of stressors was significant. The final predictive model presented shows the linear effects of stressors, stress appraisals, and chronic relationship stress on gestational length and the quadratic effect only for stressors, controlling for mothers’ medical risk during her pregnancy (Table 2). Higher stress appraisal before conception was significantly associated with shorter gestational length (linear effect). There were significant quadratic effects of preconception stressors on gestational length and, therefore, the linear effect cannot be interpreted. Interpretation of the quadratic effect indicates that low levels of stressors and high levels of stressors both relate to shorter gestational length, with longer gestational length associated with moderate levels of stressors prior to conception (Fig. 3). This quadratic effect remained significant when the other latent preconception stress predictors were removed from the model. Preconception chronic relationship stress was not significantly associated with gestational length.
Table 2.
Results from structural equation model of the linear and quadratic associations between preconception maternal stress and gestational length (n = 360)
| Gestational Length | |
|---|---|
| β (SE) | |
| Stress appraisals (linear) | -.19 (.06)** |
| Stressors (linear) | .59 (.05)** |
| Stressors (quadratic) | -.52 (.03)** |
| Chronic relationship stress (linear) | .05 (.06) |
| Medical risk | -.09 |
*p < .01
Fig. 3.
Curvilinear (quadratic) association between preconception exposure to stressors and gestational length (n = 360). Range of graph is restricted to the range of gestational length in the current sample (23–42 weeks).
Discussion
This prospective longitudinal study measured many forms of stress in a large, diverse sample of predominantly low-income women and derived three latent stress factors from the set of 12 stress measures. For those women who later became pregnant, we tested whether the derived stress factors independently predicted length of gestation in a linear or curvilinear manner. Results of both studies inform our understanding of maternal stress processes and of preconception stress and birth outcomes.
Consistent with the literature which distinguishes stress exposures from subjective responses [24, 25, 27, 28, 30], Study 1 found support for two distinct stress factors representing stress appraisals (perceived stress and parenting stress) and stressors (negative life events, financial stress/food insecurity, interpersonal violence, discrimination). The stress appraisals factor captures the common definition of stress as perceived inability to cope with demands and feeling overwhelmed by ongoing stressors. The second latent factor, labeled stressors, represents more objective stressors and stress exposures. Notably, the stressors assessed in this study were mostly chronic ones, except for negative life events which includes both chronic and episodic stressors that may often have chronic sequalae (such as losing one’s job). We also found a third latent factor reflecting close relationship stress which included stress in family and partner relationships and is of interest given that interpersonal stress is among the most potent stressors for health and wellbeing [57, 58].
Results from Study 2 showed that higher stress appraisals before conception were linearly associated with shorter gestational length, controlling for medical risk and other types of stressors. Levels of stressors before conception, on the other hand, showed a curvilinear effect, independent of stress appraisals, chronic relationship stress, and other covariates. Chronic relationship stress was not significantly associated with gestational length. The association between the latent stressors factor and gestational length reflects a moderate effect size (see Fig. 2 for associations of stressors with the number of weeks in utero), while the association between stress appraisals and gestational length reflects a small effect size (for each one unit increase on the stress appraisals factor, time in utero decreased by 1 week). Given that every additional day in utero benefits the fetus [38, 39], even small effects on gestational length have important implications for health and development.
These findings echo the conclusions from prior pregnancy research showing that the type of stress matters with respect to how it predicts specific maternal and child outcomes (e.g., gestational length vs. birth weight) [1]. These results extend our knowledge to stress before conception, providing information on even earlier predictors of gestational length. Notably, these findings may or may not apply to birth weight despite strong correlation between gestational length and birthweight in the current sample. Previous epidemiological studies during the preconception period have shown significant associations between negative life events [9–11,16] or mental health measures [59] and adverse birth outcomes, but did not examine extensive measures of stress like those in the current study. Further, past work has not considered whether associations with birth outcomes are linear or not. The present findings indicate that the nature of the association of preconception stress with gestational length depends on the type of stress and that the association may not be linear for stressors.
The linear effect of stress appraisals on the length of gestation is consistent with widespread theory that stress during pregnancy is not healthy for mothers and fetuses, and leads to more adverse outcomes. However, this work extends that to the time before a woman conceives. High stress appraisals can adversely affect a pregnancy, particularly during embryonic implantation and placental and fetal development [15]. Greater appraisals of stress before conception may be consistent with a woman’s stress appraisal tendencies during pregnancy, suggesting that this risk factor that can be targeted for earlier intervention. One likely mechanism of this association is cardiovascular risk, which would likely remain stable from preconception through the prenatal period, and has been linked with high stress appraisal and risk for preterm birth [60, 61]. On the other hand, the linear effect of stress appraisals may also be specific to the preconception period.
Stress during the preconception period is thought to increase the risk for adverse birth outcomes via hormonal mechanisms that when dysregulated can set a stage for a less optimal pregnancy. Women with high appraised stress levels are also likely to have dysregulated stress hormones [62–64]. Prior to pregnancy, these dysregulated stress hormones could contribute to the maternal physiological milieu in which conception occurs and thus affect the placenta and/or fetal HPA axis, and mediate negative birth effects [65]. Consistent with this proposed mechanism, earlier work with this cohort demonstrated that maternal preconception cortisol was a stronger predictor of birth outcomes than prenatal cortisol [40]. In addition, behavioral mechanisms may be implicated. For example, behavioral preparations, like good nutrition, good sleep, moderate physical activity, or taking vitamins (e.g., folic acid) before a pregnancy, may be suboptimal under stress and negatively impact the fetus [66].
The curvilinear effect of exposure to stressors supports the view that not all stress is “bad stress” [20] and is consistent with previous suggestions that some stress in mothers may even be adaptive for the developing fetus [19, 21]. Our results show that a moderate amount of stressors was associated with the longest gestational length. As discussed in the stress literature and shown in our factors, exposure to stressors is separate from an individual’s appraisals of such stressors which also reflect their perception of their ability, or inability, to cope [24, 27], and also distinct from their actual coping strategies which were not measured in the current study. Therefore, while the current findings highlight the unique contribution of stressors, independently from how they are appraised, it is less clear how these two stress factors may interact. Women who have been exposed to a moderate level of stressors such as financial strain or negative life events may have developed adaptive coping strategies that serve them well, thus mitigating the adverse influence of stressors at moderate levels on pregnancy outcomes. Future studies with adequate power should examine how appraisal of stressors and coping may buffer against the adverse effects of stressors on birth outcomes. When stressors become more severe and possibly traumatic, as with IPV or more lifelong and chronic discrimination, a woman’s ability to cope effectively may be overtaxed and her physiology adversely affected, thus posing risk for her baby. It is not clear why women who have low levels of stressors before conception also have shorter gestations. Low stress may be confounded with other factors such as social isolation that account for the findings as noted in earlier work [29]. Given the novelty of this curvilinear analysis and finding, further research regarding women who report very low stress is warranted.
The longitudinal design of this study is a strength in that it enabled us to measure stressors before pregnancy and predict the outcome of a subsequent pregnancy. Another strength is that the study had measurements of multiple types of stress assessed with standardized measures by home interviews in a community sample. However, analyses did not adjust for stress in the prenatal period, precluding us from making firm statements about the unique associations with preconception stress based on these findings. It is possible that these types of stressors would continue to have impact during the prenatal period. We suggest that more confidence can be placed in the conclusion that preconception stressors are potent given that previous studies, including our own, have found stronger effects in the year prior to conception compared with pregnancy [10, 11, 16, 40]. Indeed some studies have found increased risk associated with preconception factors occurring as early as adolescence [67]. In this study, the timing of preconception measures ranged from 1 month to 4 years prior to conception which extends the preconception period beyond that typically examined in the literature which is no more than 1 year. Nevertheless, further investment in the study of preconception and prenatal stress effects and pathways to birth outcomes is warranted and may widen the window for effective preventive intervention to improve maternal and child health. For example, if health and psychosocial factors as early as adolescence are influential in future outcomes of pregnancy, then interventions at that time of life might be the most optimal for improving maternal and child health outcomes [68, 69].
Recognizing these strengths, the findings of the current study should be considered in light of limitations. First, although diversity typically enhances generalizability, the racial, ethnic, and socioeconomic composition of the study sample may limit the generalizability of the findings to populations not sampled in this study. For example, participants in this study were predominantly low-income and may have increased stress exposure compared with higher-income populations. In addition, the preconception stress measured in the current study was assessed following a previous pregnancy and may reflect the outcomes of that pregnancy and the demands of parenting an infant. It is possible that the effects of stress on birth outcomes may differ as a function of parity. Further, the preconception stress variables were measured at different times in the study over the course of 1 year in the women’s lives. It is possible that the relationship stress factor was not associated with gestational length because it was the only factor measured at just one time point (T2) and may be a less reliable indicator. Finally, as noted, there was variability in the length of the interpregnancy interval and, therefore, variability in the timing of the measurement of the preconception stress relative to the subsequent pregnancy. This limits the ability to pinpoint sensitive periods during which stress exposure may be most potent in the preconception period.
Current findings argue against conceptualizing stress as a unidimensional construct to study health at any time in life. Stress takes many forms, as is well known, yet conceptualization remains a topic of discussion [24, 70]. Systematic investigation of multiple stress constructs and examination of underlying stress processes promises more clarity in terms of which type of stress matters for which health outcomes, for which populations, and when in the lifespan. This study points to preconception as a highly sensitive period when exposure to stressors and appraisal of these stressors can increase risk for shorter gestational length and demonstrates that the forms of these associations may differ depending on the type of stress. Curvilinear results suggest that exposure to some stressors before conception may not increase the risk for preterm birth, while greater stress appraisals is consistently associated with shorter gestational length across the full range of values. In summary, this study contributes to our understanding of the biopsychosocial etiological pathways that increase risk for adverse birth outcomes even before a pregnancy begins.
Supplementary Material
Acknowledgments
This paper is based on data collected by the Child Community Health Network (CCHN), supported through cooperative agreements with the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD; U HD44207, U HD44219, U HD44226, U HD44245, U HD44253, U HD54791, U HD54019, U HD44226-05S1, U HD44245-06S1, R03 HD59584) and the National Institute for Nursing Research (U NR008929). N.E.M. was supported by the National Institute of Mental Health (5 T32 MH015750; C.D.S.) and the NICHD (5R01HD072021-05).
Members of Each Site
Baltimore, MD: Baltimore City Healthy Start, Johns Hopkins University
Community PI: M. Vance
Academic PI: C. S. Minkovitz; Co-Invs: P. O’Campo, P. Schafer
Project Coordinators: N. Sankofa, K. Walton
Lake County, IL: Lake County Health Department and Community Health Center, the North Shore University Health System
Community PI: K. Wagenaar
Academic PI: M. Shalowitz
Co-Invs: E. Adam, G. Duncan*, A. Schoua-Glusberg, C. McKinney, T. McDade, C. Simon
Project Coordinator: E. Clark-Kauffman
Los Angeles, CA: Healthy African American Families, Cedars-Sinai Medical Center, University of California, Los Angeles
Community PI: L. Jones
Academic PI: C. Hobel; Co-PIs: C. Dunkel Schetter, M. C. Lu
Project Coordinators: F. Jones, D. Serafin, D. Young
North Carolina: East Carolina University, NC Division of Public Health, NC Eastern Baby Love Plus Consortium, University of North Carolina, Chapel Hill
Community PIs: S. Evans, J. Ruffin, R. Woolard
Academic PI: J. Thorp; Co-Is J. DeClerque, C. Dolbier, C. Lorenz
Project Coordinators: L. S. Sahadeo, K. Salisbury
Washington, DC: Virginia Tech Carilion Research Institute, Virginia Tech, Washington Hospital Center, Developing Families Center
Community PI: L. Patchen; Academic PI: S. L. Ramey; Academic Co-PI R.Gaines Lanzi
Co-Invs: L. V. Klerman, M. Miodovnik, C. T. Ramey, L. Randolph
Project Coordinator: N. Timraz
Community Coordinator: R. German
Data Coordination and Analysis Center DCAC (Pennsylvania State University)
PI: V. M. Chinchilli
Co-Invs: R, Belue, G. Brown Faulkner*, M, Hillemeier, I. Paul, M. L. Shaffer
Project Coordinator: G. Snyder
Biostatisticians: E. Lehman, C. Stetter
Data Managers: J. Schmidt, K. Cerullo, S. Whisler
Programmers: J. Fisher, J, Boyer, M. Payton
NIH Program Scientists: V. J. Evans and T. N.K. Raju, Eunice Kennedy Shriver National Institute of Child Health and Human Development; L. Weglicki, National Institute of Nursing Research, Program Officers: M. Spittel* and M. Willinger, NICHD; Y. Bryan,* NINR.
Steering Committee Chairs: M. Phillippe (University of Vermont) and E. Fuentes-Afflick* (University of California–San Francisco School of Medicine)
*Indicates those who participated in only the planning phase of CCHN.
Compliance with Ethical Standards
Authors’ Statement of Conflict of Interest and Adherence to Ethical Standards The authors declare that they have no conflict of interest.
Authors’ Contributions C.D.S. was a CoPI of the CCHN LA site and C.H. was PI. C.D.S. was PI of the subsequent child follow-up study ("Preconception Study"). C.M.G. and N.E.M. were postdoctoral fellows and project coordinators and were involved in study design, data collection, and analyses. N.E.M. had primary responsibility for the writing. C.D.S. was involved in all phases.
Ethical Approval The institutional review boards at each site approved the study.
Informed Consent Individual participants provided consent to participate in the CCHN study and the subsequent child follow-up study.
References
- 1. Dunkel Schetter C. Psychological science on pregnancy: Stress processes, biopsychosocial models, and emerging research issues. Annu Rev Psychol. 2011;62:531–558. [DOI] [PubMed] [Google Scholar]
- 2. Lobel M, Dunkel Schetter C. Pregnancy and prenatal stress. In: Friedman HS, ed. Encyclopedia of Mental Health. 2nd ed. Waltham, MA: Academic Press; 2016:318–329. [Google Scholar]
- 3. Littleton HL, Bye K, Buck K, et al. Psychosocial stress during pregnancy and perinatal outcomes : A meta-analytic review. J Psychosom Obstet Gynecol. 2010;31:219–222. [DOI] [PubMed] [Google Scholar]
- 4. Heron MP. Deaths : Leading Causes for 2013. Hyattsville, MD: National Center for Health Statistics; 2016. [PubMed] [Google Scholar]
- 5. Bhutta AT, Cleves MA, Casey PH, Cradock MM, Anand KJ. Cognitive and behavioral outcomes of school-aged children who were born preterm: A meta-analysis. JAMA. 2002;288:728–737. [DOI] [PubMed] [Google Scholar]
- 6. McGowan JE, Alderdice FA, Holmes VA, Johnston L. Early childhood development of late-preterm infants: A systematic review. Pediatrics. 2011;127:1111–1124. [DOI] [PubMed] [Google Scholar]
- 7. Vohr BR, Wright LL, Dusick AM, et al. Neurodevelopmental and functional outcomes of extremely low birth weight infants in the National Institute of Child Health and Human Development Neonatal Research Network, 1993–1994. Pediatrics. 2000;105:1216–1226. [DOI] [PubMed] [Google Scholar]
- 8. Weisman CS, Hillemeier MM, Chase GA, et al. Preconceptional health: Risks of adverse pregnancy outcomes by reproductive life stage in the Central Pennsylvania Women’s Health Study (CePAWHS). Womens Health Issues. 2006;16:216–224. [DOI] [PubMed] [Google Scholar]
- 9. Khashan AS, McNamee R, Abel KM, et al. Rates of preterm birth following antenatal maternal exposure to severe life events: A population-based cohort study. Hum Reprod. 2009;24:429–437. [DOI] [PubMed] [Google Scholar]
- 10. Precht DH, Andersen PK, Olsen J. Severe life events and impaired fetal growth: A nation-wide study with complete follow-up. Acta Obstet Gynecol Scand. 2007;86:266–275. [DOI] [PubMed] [Google Scholar]
- 11. Class QA, Khashan AS, Lichtenstein P, Långström N, D’Onofrio BM. Maternal stress and infant mortality: The importance of the preconception period. Psychol Sci. 2013;24:1309–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shonkfoff JP, Garner AS. The lifelong effects of early childhood adversity and toxic stress. Pediatrics. 2012;36:102–108. [Google Scholar]
- 13. Misra DP, Guyer B, Allston A. Integrated perinatal health framework. A multiple determinants model with a life span approach. Am J Prev Med. 2003;25:65–75. [DOI] [PubMed] [Google Scholar]
- 14. Witt WP, Litzelman K, Cheng ER, Wakeel F, Barker ES. Measuring stress before and during pregnancy: A review of population-based studies of obstetric outcomes. Matern Child Health J. 2014;18:52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Keenan K, Hipwell AE, Class QA, Mbayiwa K. Extending the developmental origins of disease model: Impact of preconception stress exposure on offspring neurodevelopment. Dev Psychobiol. 2018;60:753–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Khashan AS, McNamee R, Abel KM, et al. Reduced infant birthweight consequent upon maternal exposure to severe life events. Psychosom Med. 2008;70:688–694. [DOI] [PubMed] [Google Scholar]
- 17. Witt WP, Mandell KC, Wisk LE, et al. Infant birthweight in the US: The role of preconception stressful life events and substance use. Arch Womens Ment Health. 2016;19:529–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dunkel Schetter C, Tanner L. Anxiety, depression and stress in pregnancy: Implications for mothers, children, research, and practice. Curr Opin Psychiatry. 2012;25:141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. DiPietro JA. Maternal influences on the developing fetus. In: Maternal Influences on Fetal Neurodevelopment. New York, NY: Springer; 2010;19–32. [Google Scholar]
- 20. Sapolsky RM. Stress and the brain: Individual variability and the inverted-U. Nat Neurosci. 2015;18:1344–1346. [DOI] [PubMed] [Google Scholar]
- 21. Dipietro JA. The role of prenatal maternal stress in child development. Sage. 2004;13:71–74. [Google Scholar]
- 22. DiPietro JA, Ghera MM, Costigan K, Hawkins M. Measuring the ups and downs of pregnancy stress. J Psychosom Obstet Gynaecol. 2004;25:189–201. [DOI] [PubMed] [Google Scholar]
- 23. DiPietro JA, Novak MF, Costigan KA, Atella LD, Reusing SP. Maternal psychological distress during pregnancy in relation to child development at age two. Child Dev. 2006;77:573–587. [DOI] [PubMed] [Google Scholar]
- 24. Epel ES, Crosswell AD, Mayer SE, et al. More than a feeling: A unified view of stress measurement for population science. Front Neuroendocrinol. 2018;49:146–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cohen S, Kessler RC, Gordon LU. Measuring Stress: A Guide for Health and Social Scientists. New York: Oxford University Press on Demand, 1997. [Google Scholar]
- 26. Lazarus RS. Theory-based stress measurement. Psychol Inq. 1990;1:3–13. [Google Scholar]
- 27. Lazarus RS, Folkman S. Stress, Appraisal, and Coping. New York: Springer.1984. [Google Scholar]
- 28. Lobel M, Dunkel-schetter C. Conceptualizing stress to study effects on health: Environmental, perceptual, and emotional components. Anxiety Res. 1990;3:213–230. [Google Scholar]
- 29. Lobel M. Conceptualizations, measurement, and effects of prenatal maternal stress on birth outcomes. J Behav Med. 1994;17:225–272. [DOI] [PubMed] [Google Scholar]
- 30. Hammen C, Kim EY, Eberhart NK, Brennan PA. Chronic and acute stress and the prediction of major depression in women. Depress Anxiety. 2009;26:718–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lepore SJ. Social–environmental influences on the chronic stress process. In: Gottlieb BH, ed. Coping with Chronic Stress. Boston, MA: Springer; 1997:133–160. [Google Scholar]
- 32. Kramer MR, Hogue CJ, Dunlop AL, Menon R. Preconceptional stress and racial disparities in preterm birth: An overview. Acta Obstet Gynecol Scand. 2011;90:1307–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lobel M, Dunkel-Schetter C, Scrimshaw SC. Prenatal maternal stress and prematurity: A prospective study of socioeconomically disadvantaged women. Health Psychol. 1992;11:32–40. [DOI] [PubMed] [Google Scholar]
- 34. Roesch SC, Schetter CD, Woo G, Hobel CJ. Modeling the types and timing of stress in pregnancy. Stress Coping. 2004;17:87–102. [Google Scholar]
- 35. Dunkel Schetter C, Glynn LM. Stress in pregnancy: Empirical evidence and theoretical issues to guide interdisciplinary research. In: Contrada R, Baum A, eds. The Handbook of Stress Science Biology, Psychology and Health. New York: Springer Publishing Company; 2011:321–343. [Google Scholar]
- 36. Dole N, Savitz DA, Hertz-Picciotto I, Siega-Riz AM, McMahon MJ, Buekens P. Maternal stress and preterm birth. Am J Epidemiol. 2003;157:14–24. [DOI] [PubMed] [Google Scholar]
- 37. Hedegaard M, Henriksen TB, Secher NJ, Hatch MC, Sabroe S. Do stressful life events affect duration of gestation and risk of preterm delivery? Epidemiology. 1996;7:339–345. [DOI] [PubMed] [Google Scholar]
- 38. Behrman RE, Butler AS. Preterm birth: Causes, Consequences, and prevention. 2007. [PubMed] [Google Scholar]
- 39. Bouyssi-Kobar M, Du Plessis AJ, McCarter R, et al. Third trimester brain growth in preterm infants compared with in utero healthy fetuses. Obstet Gynecol Surv. Vol. 772. Washington, DC: National academies press; 2017;72:145–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Guardino CM, Schetter CD, Saxbe DE, Adam EK, Ramey SL, Shalowitz MU; Community Child Health Network . Diurnal salivary cortisol patterns prior to pregnancy predict infant birth weight. Health Psychol. 2016;35:625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tanner Stapleton LR, Dunkel Schetter C, Dooley LN, et al. ; Community Child Health Network . The community child health network life stress interview: A brief chronic stress measure for community health research. Anxiety Stress Coping. 2016;29:352–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Williams DR, Yan Yu, Jackson JS, Anderson NB. Racial differences in physical and mental health: Socio-economic status, stress and discrimination. J Health Psychol. 1997;2:335–351. [DOI] [PubMed] [Google Scholar]
- 43. O’Campo P, Caughy MO, Nettles SM. Partner abuse or violence, parenting and neighborhood influences on children’s behavioral problems. Soc Sci Med. 2010;70:1404–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sherin KM, Sinacore JM, Li XQ, Zitter RE, Shakil A. HITS: A short domestic violence screening tool for use in a family practice setting. Fam Med. 1998;30:508–512. [PubMed] [Google Scholar]
- 45. Abidin RR. Parenting Stress Index (PSI). Charlottseville: Pediatric Psychology Press; 1990. [Google Scholar]
- 46. Abidin RR, Brunner JF. Development of a parenting alliance inventory. J Clin Child Psychol. 1995;24:31–40. [Google Scholar]
- 47. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 48. Dominguez TP, Schetter CD, Mancuso R, Rini CM, Hobel C. Stress in African American pregnancies: Testing the roles of various stress concepts in prediction of birth outcomes. Ann Behav Med. 2005;29:12–21. [DOI] [PubMed] [Google Scholar]
- 49. Turner RJ, Wheaton B. Checklist measurement of stressful life events. In: Measuring Stress: A guide for health and social scientists. 1995: 29–58. [Google Scholar]
- 50. Zambrana RE, Scrimshaw SC, Collins N, Dunkel-Schetter C. Prenatal health behaviors and psychosocial risk factors in pregnant women of Mexican origin: The role of acculturation. Am J Public Health. 1997;87:1022–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kim G, Sellbom M, Ford KL. Race/ethnicity and measurement equivalence of the Everyday Discrimination Scale. Psychol Assess. 2014;26:892–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rabin RF, Jennings JM, Campbell JC, Bair-Merritt MH. Intimate partner violence screening tools: A systematic review. Am J Prev Med. 2009;36:439–445.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hobel CJ, Youkeles L, Forsythe A. Prenatal and intrapartum high-risk screening. II. Risk factors reassessed. Am J Obstet Gynecol. 1979;135:1051–1056. [DOI] [PubMed] [Google Scholar]
- 54. Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct Equ Model A Multidiscip J. 1999; 6:1–55. [Google Scholar]
- 55. Kline RB. Principles and Practice of Structural Equation Modelling. 2nd ed. New York: The Guilford Press; 2005. [Google Scholar]
- 56. Muthen LK, Muthen BO. Statistical Analysis with latent variables using Mplus. Los Angeles: no date. [Google Scholar]
- 57. Cohen S. Psychological stress, immunity, and physical disease. In: Sternberg R, Fiske F, Foss D, eds. Scientists Making a Difference: The Greatest Living Behavioral and Brain Scientists Talk About Their Most Important Contributions. Cambridge: Cambridge University Press; 2016:419–423. [Google Scholar]
- 58. Rook KS. Investigating the positive and negative sides of personal relationships: through a glass darkly? In: Spitzberg B, Cupach W, eds. The Dark Side of Close Relationships. Mahwah, NJ: Lawrence Erlbaum; 1998:369–393. [Google Scholar]
- 59. Wisk LE, Witt WP, Hampton JM, Cheng ER, Hagen EW. Preconception mental health predicts pregnancy complications and adverse birth outcomes: A national population-based study. Matern Child Health J. 2011;16: 1525–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hobel CJ, Dolan SM, Hindoyan NA, Zhong N, Menon R. History of the establishment of the Preterm Birth International Collaborative (PREBIC). Placenta. 2019;79:3–20. [DOI] [PubMed] [Google Scholar]
- 61. Tanz LJ, Stuart JJ, Williams PL, et al. Preterm delivery and maternal cardiovascular disease in young and middle-aged adult women. Circulation. 2017;135:578–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130:355–391. [DOI] [PubMed] [Google Scholar]
- 63. Sandman CA, Wadhwa PD, Dunkel-Schetter C, et al. Psychobiological influences of stress and HPA regulation on the human fetus and infant birth outcomes. Ann N Y Acad Sci. 1994;739:198–210. [DOI] [PubMed] [Google Scholar]
- 64. Wadhwa PD, Dunkel-Schetter C, Chicz-DeMet A, Porto M, Sandman CA. Prenatal psychosocial factors and the neuroendocrine axis in human pregnancy. Psychosom Med. 1996; 58:432–446. [DOI] [PubMed] [Google Scholar]
- 65. van Montfoort N, Finken MJ, le Cessie S, Dekker FW, Wit JM. Could cortisol explain the association between birth weight and cardiovascular disease in later life? A meta-analysis. Eur J Endocrinol. 2005;153:811–817. [DOI] [PubMed] [Google Scholar]
- 66. Berghella V, Buchanan E, Pereira L, Baxter JK. Preconception care. Obstet Gynecol Surv. 2010;65:119–131. [DOI] [PubMed] [Google Scholar]
- 67. Harville EW, Boynton-Jarrett R, Power C, Hyppönen E. Childhood hardship, maternal smoking, and birth outcomes: A prospective cohort study. Arch Pediatr Adolesc Med. 2010;164:533–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lu MC, Kotelchuck M, Culhane JF, Hobel CJ, Klerman LV, Thorp JM Jr. Preconception care between pregnancies: The content of internatal care. Matern Child Health J. 2006;10:S107–S122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lu MC. Recommendations for preconception care. Am Fam Physician. 2007;76:397–400. [PubMed] [Google Scholar]
- 70. Cohen S, Murphy MLM, Prather AA. Ten surprising facts about stressful life events and disease risk. Annu Rev Psychol. 2019;70:577–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.