Abstract
Background
It has been reported that patients with inflammatory bowel disease (IBD) are more susceptible to periodontitis. However, data regarding the risk of periodontitis in IBD patients are scarce, and results from individual studies remain controversial. The aim of this study is to investigate the risk of periodontitis in IBD patients.
Methods
Web of Science, PubMed, and Embase were searched for studies investigating the risk of periodontitis in the IBD patient population from Jan. 2000 to Nov. 2020. Articles were included if they contained the number of people with IBD diagnosed with periodontitis (or periodontal disease parameters) compared with a control group. Case reports, reviews, animal studies, and articles without available abstracts were excluded. A pooled odds ratio (OR) and 95% confidence interval (CI) were calculated to assess the association between periodontitis and IBD.
Results
Six studies were included in the meta-analysis. The overall risk of periodontitis was significantly higher in IBD patients than controls (OR: 2.10, 95% CI: 1.60-2.74; I2 = 27%). In particular, Crohn's disease (CD) and ulcerative colitis (UC) were both linked to an increased risk of periodontitis (OR: 1.72, 95% CI: 1.36-2.19; I2 = 0% for CD vs. OR:2.39, 95% CI: 1.19-4.80; I2 = 85% for UC).
Conclusions
IBD patients are at higher risk of periodontitis than controls. After subgroup analysis, the elevated risk remained significant when analyzing CD or UC alone. UC patients were at higher risk of developing periodontitis than CD patients.
1. Introduction
Inflammatory bowel disease (IBD) is a chronic inflammatory disorder of the gut, which includes Crohn's disease (CD) and ulcerative colitis (UC) [1]. UC mainly affects the colon and rectum, with inflammation restricted to the mucosal layer [2], whereas CD affects the entire gastrointestinal tract and involves transmural inflammation [2]. Although both diseases are characterized by inflammation of the gut, certain extraintestinal manifestations could occur in the wake of systemic inflammation triggered by the disease [3, 4]. Although the pathogenesis of IBD remains unknown, disturbed host-microbiota interactions and aberrant activation of the host immune system are thought to be critical factors [5].
Up to 9% of patients with IBD present with oral manifestations [6, 7], of which periodontitis is closely related to IBD pathogenesis. Periodontitis is a chronic inflammatory disease involving the supporting structures of the teeth [8]. Periodontitis is common worldwide, with prevalence ranging from 4% to 76% in developed countries and 50% to 90% in developing countries [9]. The pathogenesis of periodontitis mirrors that of IBD and mainly involves interactions between the host and oral pathogens. Consequently, the host inflammatory response against the pathogens leads to the destruction of soft and hard periodontal tissues [10].
Several studies have found that IBD is often associated with a higher prevalence of periodontitis. Brito et al. have shown that the prevalence of periodontitis is higher in patients with IBD than in healthy subjects [11]. In addition, periodontal lesions in IBD patients are more serious and extensive compared with those of control patients [12, 13]. In contrast, a case-control study argued that IBD was not associated with worsened periodontal conditions [14], indicating that IBD did not necessarily enhance susceptibility to periodontitis. Another study reported that poor oral hygiene, which is often linked to higher incidences of periodontitis, was inversely correlated with IBD [15]. Data about the prevalence of periodontitis in IBD patients are limited and controversial. Therefore, the aim of this meta-analysis was to systematically evaluate the risk of periodontitis in IBD patients following the PICO principle (P: human subjects; I: IBD; C: No IBD; O: periodontitis).
2. Materials and Methods
This meta-analysis was reported according to the instructions of the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) statement [16] and conducted according to the Cochrane Handbook [17].
2.1. Search Strategy
Web of Science, PubMed, and Embase were extensively searched from Jan. 2000 to Nov. 2020. The following medical subject heading terms were used: “inflammatory bowel disease,” “Crohn's disease,” “ulcerative colitis,” “periodontal disease,” “periodontitis,” and “gingivitis.” All items were searched as key words or medical subject headings (MESH) where available. The electronic search strategy for PubMed, for example, was as follows: (“inflammatory bowel disease” or “Crohn's disease” or “ulcerative colitis”) and (“periodontal disease” or “periodontitis” or “gingivitis”). The search was performed in English. Based on titles and abstracts, the records were screened, and relevant studies were selected for full-text assessments. References of the eligible studies were also checked for studies not identified by the primary search strategy. The inclusion and exclusion criteria for published studies are shown in Table 1.
Table 1.
Inclusion and exclusion criteria for published studies.
| Inclusion criteria | Exclusion criteria |
|---|---|
| a) Studies on human subjects b) Cohort or case-control studies including patients with known IBD diagnoses and no IBD c) Studies using periodontitis as a primary observation d) Studies reporting an estimated measure of effect size ([RR], [HR], or [OR]) and its associated 95% confidence interval (CI) or those providing calculable data |
a) Case reports b) Reviews c) Animal studies d) Articles without available abstracts e) Duplicated studies |
2.2. Data Extraction
A study was deemed eligible if it met all the inclusion criteria and none of the exclusion criteria. From each study enrolled, three authors (Y.Z., D.Q., and R.C.) extracted information related to the name of the journal, names of authors, year of publication, location of study, study design, study population, sample size, and periodontal manifestations. Disagreement or uncertainty was resolved by discussion among the authors. In these studies, periodontitis was assessed using different markers and indices, such as the plaque index, gingival index, bleeding on probing, pocket depth, clinical attachment loss, gingival recession, and periodontal index. If the information provided in the study was insufficient, the corresponding author of the article was contacted for the missing data. However, all studies provided sufficient information about outcomes.
2.3. Quality Assessment
The Newcastle-Ottawa Scale (range, 0–9 stars) was used to assess the quality of the enrolled studies [18]. Briefly, a maximum of 9 stars were given after comprehensive evaluation on 9 aspects (e.g., selection of cases and controls, comparability, and outcomes). Studies possessing 5 or more stars were deemed as moderate or high methodological quality.
2.4. Statistical Analysis
The association between periodontitis and IBD was calculated using odds ratios (ORs) extracted from individual studies. A random-effects model was used to obtain the pooled ORs with the 95% confidence interval (CI). Heterogeneity was evaluated using the Cochrane I2 statistic, with I2 > 50% indicating substantial heterogeneity [19]. Subgroup analyses were performed in the CD and UC groups. Sensitivity analyses were conducted by sequential removal of single studies to investigate if a single study was driving the results. Statistical analysis was conducted using the R packages meta [20] and metafor (version 3.6.3; Linux; R Core Team) [21]. A two-sided p value < 0.05 was considered statistically significant.
3. Results
3.1. Study Characteristics
The literature search process is summarized in Figure 1. Briefly, 467 articles were retrieved by an initial database search, including exclusion of duplications. Four hundred and fifty-nine publications were excluded after screening the abstracts. Two relevant publications were excluded because they did not include the prevalence of periodontitis as a separate observation [7, 22]. Finally, a total of 6 publications were pooled for analysis with a total of 3711 patients [11, 12, 14, 23–25]. The included studies were published between 2004 and 2020, reporting data from Greece, Germany, Brazil, Sweden, Jordan, and China. The characteristics of these studies are shown in Table 2.
Figure 1.
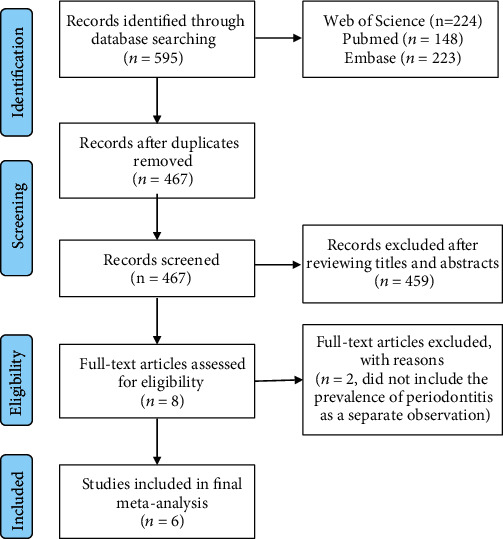
Flow chart demonstrating the study selection process.
Table 2.
Characteristics of the included studies.
| Author, year of publication, and country | Study design | Study size | IBD patients | Control | Rate, IBD/control | Periodontal manifestations | Definition of periodontitis | Reference |
|---|---|---|---|---|---|---|---|---|
| Zervou et al. (2004), Greece | Case-control | 77 | 30 | 47 | 0.06/0 | Periodontitis; gingivitis; gingival bleeding | Not described | [24] |
| Grössner et al. (2006), Germany | Case-control | 121 | 62 | 59 | 0.81/0.64 | %PI; %BOP; PPD; %CAL ≥ 4 mm; %CAL ≥ 5 mm | ≥1 sites with CAL ≥ 4 | [14] |
| Brito et al. (2008), Brazil | Case-control | 253 | 179 | 74 | 0.85/0.67 | Periodontitis (at least 4 × CAL ≥ 3 mm); %PI; %BOP; PPD; CAL; %CAL ≥ 3 mm | ≥4 sites in different teeth with CAL ≥ 3 mm | [11] |
| Rikardsson et al. (2009), Sweden | Case-control | 2346 | 1598 | 748 | 0.7/0.51 | Periodontitis; gingival bleeding | Self-reported | [23] |
| Habashneh et al. (2012), Jordan | Case-control | 260 | 160 | 100 | 0.58/0.4 | Periodontitis; PI; GI; PPD; CAL; GR; %BOP; %PPD ≥ 3; %PPD ≥ 4; %CAL ≥ 3; %CAL ≥ 4; %CAL ≥ 5 | ≥4 teeth with one site or more having PD ≥ 4 mm and CAL ≥ 3 mm | [12] |
| Zhang et al. (2020), China | Case-control | 654 | 389 | 265 | 0.38/0.19 | PD; CAL; GR; GI; PI; %PD ≥ 4; %PD ≥ 5; %CAL ≥ 3; %CAL ≥ 4; %GR ≥ 1; %GR ≥ 2; %BOP; %CI | ≥2 interproximal sites with CAL ≥ 3 mm, and ≥2 interproximal sites with PD ≥ 4 mm (not on the same tooth), or ≥1 site with PD ≥ 5 mm | [25] |
PI: plaque index; BOP: bleeding on probing; PPD: probing pocket depth; CAL: clinical attachment loss; GI: gingival index; GR: gingival recession; CI: calculus index.
3.2. Study Quality
All 6 studies ranked between 7 and 9 stars according to the Newcastle-Ottawa Scale (Table 3); they were all of reasonably moderate or high quality with clear definitions of cases, representativeness of the cases, and comparability based on design or analysis.
Table 3.
Methodological quality of case-control studies included in the meta-analysis.
| Study | Selection | Comparability | Outcomes | Total scores | |||||
|---|---|---|---|---|---|---|---|---|---|
| Case definition adequate | Representative-ness of the cases | Selection of controls | Definition of controls | Comparability based on design or analysis | Ascertainment of exposure | Same method of ascertainment for cases and controls | Nonresponse rate | ||
| Zervou et al. (2004) [24] | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 7 | |
| Grössner et al. (2006) [14] | ☆ | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | ☆ | 9 |
| Brito et al. (2008) [11] | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | ☆ | 8 | |
| Rikardsson et al. (2009) [23] | ☆ | ☆ | ☆ | ☆ | ☆ | ☆ | 6 | ||
| Habashneh et al. (2012) [12] | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | 7 | ||
| Zhang et al. (2020) [25] | ☆ | ☆ | ☆ | ☆☆ | ☆ | ☆ | ☆ | 8 | |
3.3. Overall Risk of Periodontitis
The data of the 6 studies were pooled to assess the influence of IBD diagnosis on the development of periodontitis. In all, 556 cases of periodontitis were identified in 2418 IBD patients, while 217 cases were identified in 1293 controls. IBD was associated with a 2.10-fold risk for periodontitis (OR: 2.10, 95% CI: 1.60-2.74; I2 = 27%) (Figure 2). Because CD and UC involve different pathogeneses and disease behaviors, we further investigated the risk of periodontitis in CD and UC separately. In particular, 5 studies had accessible data on CD-related periodontitis and 4 had data on UC-related periodontitis. A pooled analysis showed the OR of periodontitis for CD patients was 1.72 (95% CI: 1.36-2.19; I2 = 0%) (Figure 3), whereas that for UC patients was 2.39 (95% CI: 1.19-4.80; I2 = 85%) (Figure 4).
Figure 2.
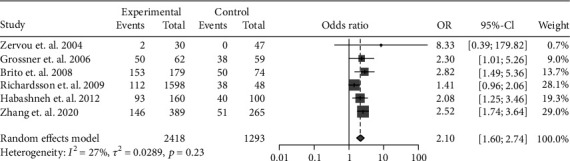
Forest plot demonstrating the association between the risk of periodontitis and IBD (p < 0.001).
Figure 3.
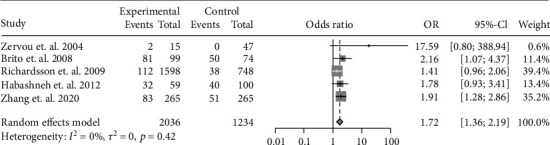
Forest plot demonstrating the association between the risk of periodontitis and CD (p < 0.001).
Figure 4.
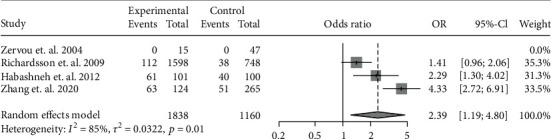
Forest plot demonstrating the association between the risk of periodontitis and UC (p = 0.0145).
3.4. Sensitivity Analysis
Heterogeneity analysis showed that the I2 statistic was highest when analyzing the UC subgroup (I2 = 27%; p = 0.23 for IBD vs. I2 = 0%; p = 0.42 for CD and I2 = 85%; p < 0.01 for UC). The potential effects of any single study on heterogeneity were investigated by sensitivity analysis. Briefly, each study was removed sequentially to obtain the OR. When analyzing the remaining studies, we found that the heterogeneity across studies significantly decreased after removing the study by Zhang et al. [25] (I2 = 49%, p = 0.16), suggesting it was the source of the heterogeneity. The OR of periodontitis for UC after exclusion of the Zhang et al. study was 1.71 (95% CI: 1.07-2.73; I2 = 49%) (Figure 5).
Figure 5.
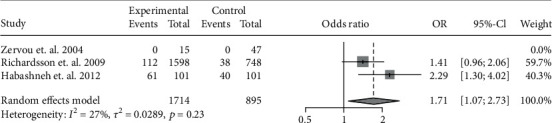
Forest plot demonstrating the association between the risk of periodontitis and UC after excluding the study conducted by Zhang et al. [25] (p = 0.0239).
4. Discussion
In recent decades, the association between IBD and periodontitis has been recognized on account of their similar etiologies. Both diseases involve dysbiotic microbiota, deregulation of the immune response, and chronic inflammation in genetically susceptible individuals [26–28]. Our study found that IBD patients had a higher risk of periodontitis than controls (OR: 2.10, 95% CI: 1.60-2.74; I2 = 27%), which was in agreement with previous publications [29–32]. Notably, the OR was higher in the UC subgroup than in the CD subgroup according to our analysis (OR: 1.72, 95% CI: 1.36-2.19; I2 = 0% for CD vs. OR: 2.39, 95% CI: 1.19-4.80; I2 = 85% for UC).
The number of publications included in the current meta-analysis is relatively small because of limited relevant research and the strict inclusion criteria. Because this study is aimed at calculating the risk of periodontitis in the IBD population, only publications with clear diagnosis of periodontitis were selected. Two studies that investigated the community periodontal index of treatment needs (CPITN) and loss of attachment at sites with maximal periodontal pocket depth (LA-PPD) were not included in this study [7, 22]. Despite the small number of eligible publications, this meta-analysis included 2418 cases of IBD and 1293 controls; overall, they indicated a higher risk of periodontitis in IBD patients than controls.
Microbiota play important roles in the pathogenesis of IBD and periodontitis [33]. A dysbiotic microbial community initiates nonresolving, chronic inflammation, leading to disruption of periodontal tissue or intestinal mucosa. Previous studies have shown significant differences in salivary microbiota compositions between IBD patients and controls [34]. It was found that overall diversity decreased significantly in the oral microbiome of pediatric CD patients [35]. Lira-Junior et al. suggested that certain species might damage host-microbe interactions in patients with untreated periodontal disease and IBD [36]. In a recent study, a distinct saliva microbiota dysbiosis in IBD was observed using 16S rRNA gene sequencing [37]. The results showed that some oral biofilm-forming bacteria, including Absconditabacteria (SR1), Saccharibacteria (TM7), Leptotrichia, Prevotella, Bulleidia, and Atopobium, were significantly increased [37]. However, the subgingival microbiota in IBD, which are closely related to periodontitis, are less well characterized. Periodontitis could lead to dysbiotic oral microbiota and potentially alter the gut microbiota [38]. Every day, more than 1012 oral bacteria in swallowed saliva can enter the gut and affect the gut's microbial composition [39, 40], which subsequently decreases the expression of tight-junction proteins and increases gut bacterial translocation and systemic inflammation [41]. Future large-sample studies using in-depth sequencing techniques are warranted to delineate the microbial link between IBD and periodontitis.
The aberrant immune response during IBD could cause inflammation of the oral cavity. IBD is an autoimmune disease, whereas poor oral health is associated with an overly aggressive immune response in local periodontal tissues [42]. Elevated cytokines may be released systemically in the processes of IBD. Figueredo et al. reported that higher IL-18 levels were detected in serum from patients with IBD and periodontitis [43]. In addition, increased levels of proinflammatory cytokines have been found in saliva from IBD patients. Higher levels of salivary TNF-α, IL-1β, and IL-6 were found in patients with active CD, and elevated salivary TNF-α and IL-6 correlate with specific oral lesions [44]. TNF inhibitors have been used to treat IBD and could reduce inflammation and stop disease progression [45]. Similarly, anti-TNF treatment has shown promising results in periodontitis. In periodontitis animal models, anti-TNF treatment can reduce inflammatory cell recruitment and bone loss [46, 47]. This evidence indicates that IBD and periodontitis share similar immunological etiologies.
Despite their similar etiologies, it is likely that IBD and periodontitis could trigger one another. That is, periodontitis, as one of the extraintestinal manifestations of IBD, could present before or after the onset of intestinal symptoms. There were limited studies that evaluated the risk of IBD in patients with periodontitis [48, 49]. A cohort study reported a 1.56-fold significantly higher risk of UC, but not CD, in patients with periodontal disease [48]. Similarly, it was found that the risk of developing UC increased significantly in patients with periodontitis in a recent retrospective study involving 1 million subjects [49]. In this meta-analysis, it was found that patients with UC had a higher risk for developing periodontitis than CD patients (OR:2.39 vs. OR: 1.72). This evidence suggests periodontitis is more correlated with UC than with CD.
Certain limitations must be considered when interpreting the results of this study. First, there were some differences in the definition of periodontitis in the included studies, which may have caused some bias. Furthermore, the use of studies including self-reported periodontitis could have introduced measurement error. The risk of developing periodontitis in IBD subjects may be higher in fact. Second, the risk of developing periodontitis among patients with IBD was not adjusted for relevant factors, especially medications and smoking habits. The use of antibiotics, immunomodulatory drugs, and corticosteroids are possible confounders for evaluating the risk of periodontitis in IBD patients. Smoking is a risk for periodontitis [50], whereas individuals who smoke have a higher risk of CD but a lower risk of UC [51]. Smoking habits could influence the development of both periodontitis and IBD. Third, all the included studies were case-control studies. Well-designed prospective cohort studies of patients with/without IBD and periodontitis are needed to determine the causal relationship. Lastly, publication bias was not evaluated by funnel plots because the number of included studies was too small.
5. Conclusions
This meta-analysis showed that IBD patients are at higher risk of developing periodontitis than controls. After subgroup analysis, the increased risk remained significant when analyzing CD or UC alone. UC patients were at higher risk of developing periodontitis than CD patients. Additional large-scale, prospective studies incorporating professional dental care and IBD centers are essential to clarify the relationship between periodontitis and IBD.
Acknowledgments
This work was supported by the National Natural Science Foundation Project (81970939), the Natural Science Foundation of Jiangsu Province (BK20190133), and the Nanjing Clinical Research Center for Oral Diseases (2019060009).
Data Availability
The datasets generated or analyzed during the current study are available from the corresponding author on reasonable request.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflicts of Interest
The authors declare no conflict of interests.
References
- 1.Khor B., Gardet A., Xavier R. J. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474(7351):307–317. doi: 10.1038/nature10209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xavier R. J., Podolsky D. K. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448(7152):427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 3.Greuter T., Bertoldo F., Rechner R., et al. Extraintestinal manifestations of pediatric inflammatory bowel disease: prevalence, presentation, and anti-TNF treatment. Journal of Pediatric Gastroenterology and Nutrition. 2017;65(2):200–206. doi: 10.1097/MPG.0000000000001455. [DOI] [PubMed] [Google Scholar]
- 4.Zippi M., Corrado C., Pica R., et al. Extraintestinal manifestations in a large series of Italian inflammatory bowel disease patients. World journal of gastroenterology: WJG. 2014;20(46):17463–17467. doi: 10.3748/wjg.v20.i46.17463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jostins L., Ripke S., Weersma R. K., et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491(7422):119–124. doi: 10.1038/nature11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lisciandrano D., Sardella A., Campanini M. C., et al. Prevalence of oral lesions in inflammatory bowel disease. American Journal of Gastroenterology. 1996;91(1):7–10. [PubMed] [Google Scholar]
- 7.Vavricka S. R., Manser C. N., Hediger S., et al. Periodontitis and gingivitis in inflammatory bowel disease: a case–control study. Inflammatory Bowel Diseases. 2013;19(13):2768–2777. doi: 10.1097/01.MIB.0000438356.84263.3b. [DOI] [PubMed] [Google Scholar]
- 8.Kinane D. F., Stathopoulou P. G., Papapanou P. N. Periodontal diseases. Nature Reviews. Disease Primers. 2017;3(1, article 17038) doi: 10.1038/nrdp.2017.38. [DOI] [PubMed] [Google Scholar]
- 9.Jiao J., Jing W., Si Y., Feng X., Tai B., Hu D., et al. The prevalence and severity of periodontal disease in Mainland China: data from the Fourth National Oral Health Survey (2015-2016) Journal of Clinical Periodontology. 2020 doi: 10.1111/jcpe.13396. [DOI] [PubMed] [Google Scholar]
- 10.Graves D. Cytokines that promote periodontal tissue destruction. Journal of Periodontology. 2008;79(8s):1585–1591. doi: 10.1902/jop.2008.080183. [DOI] [PubMed] [Google Scholar]
- 11.Brito F., Barros F. C., Zaltman C., et al. Prevalence of periodontitis and DMFT index in patients with Crohn's disease and ulcerative colitis. Journal of Clinical Periodontology. 2008;35(6):555–560. doi: 10.1111/j.1600-051X.2008.01231.x. [DOI] [PubMed] [Google Scholar]
- 12.Habashneh R., Khader Y., Alhumouz M., Jadallah K., Ajlouni Y. The association between inflammatory bowel disease and periodontitis among Jordanians: a case–control study. Journal of Periodontal Research. 2012;47(3):293–298. doi: 10.1111/j.1600-0765.2011.01431.x. [DOI] [PubMed] [Google Scholar]
- 13.Lauritano D., Boccalari E., Di Stasio D., et al. Prevalence of oral lesions and correlation with intestinal symptoms of inflammatory bowel disease: a systematic review. Diagnostics. 2019;9(3):p. 77. doi: 10.3390/diagnostics9030077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grössner-Schreiber B., Fetter T., Hedderich J., Kocher T., Schreiber S., Jepsen S. Prevalence of dental caries and periodontal disease in patients with inflammatory bowel disease: a case–control study. Journal of Clinical Periodontology. 2006;33(7):478–484. doi: 10.1111/j.1600-051X.2006.00942.x. [DOI] [PubMed] [Google Scholar]
- 15.Yin W., Ludvigsson J. F., Liu Z., Roosaar A., Axell T., Ye W. Inverse association between poor oral health and inflammatory bowel diseases. Clinical Gastroenterology and Hepatology. 2017;15(4):525–531. doi: 10.1016/j.cgh.2016.06.024. [DOI] [PubMed] [Google Scholar]
- 16.Moher D., Liberati A., Tetzlaff J., Altman D. G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. International Journal of Surgery. 2010;8(5):336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 17.Higgins J. P., Thomas J., Chandler J., et al., editors. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons; 2019. [Google Scholar]
- 18.Wells G. The Newcastle-Ottawa Scale (NOS) for assessing the quality of non randomised studies in meta-analyses. 2001, http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 19.Higgins J. P., Thompson S. G. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 20.Balduzzi S., Rücker G., Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evidence-Based Mental Health. 2019;22(4):153–160. doi: 10.1136/ebmental-2019-300117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Viechtbauer W. Conducting meta-analyses in R with the metafor package. Journal of Statistical Software. 2010;36:1–48. [Google Scholar]
- 22.Koutsochristou V., Zellos A., Dimakou K., et al. Dental caries and periodontal disease in children and adolescents with inflammatory bowel disease: a case–control study. Inflammatory Bowel Diseases. 2015;21(8):1839–1846. doi: 10.1097/MIB.0000000000000452. [DOI] [PubMed] [Google Scholar]
- 23.Rikardsson S., Jonsson J., Hultin M., Gustafsson A., Johannsen A. Perceived oral health in patients with Crohn's disease. Oral Health & Preventive Dentistry. 2009;7:p. 277. [PubMed] [Google Scholar]
- 24.Zervou F., Gikas A., Merikas E., et al. Oral manifestations of patients with inflammatory bowel disease. Annals of Gastroenterology. 2004;17:395–401. [Google Scholar]
- 25.Zhang L., Gao X., Zhou J., et al. Increased risks of dental caries and periodontal disease in Chinese patients with inflammatory bowel disease. International Dental Journal. 2020;70(3):227–236. doi: 10.1111/idj.12542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bouma G., Strober W. The immunological and genetic basis of inflammatory bowel disease. Nature Reviews Immunology. 2003;3(7):521–533. doi: 10.1038/nri1132. [DOI] [PubMed] [Google Scholar]
- 27.Eckburg P. B., Relman D. A. The role of microbes in Crohn's disease. Clinical Infectious Diseases. 2007;44(2):256–262. doi: 10.1086/510385. [DOI] [PubMed] [Google Scholar]
- 28.Indriolo A., Greco S., Ravelli P., Fagiuoli S. What can we learn about biofilm/host interactions from the study of inflammatory bowel disease. Journal of Clinical Periodontology. 2011;38:36–43. doi: 10.1111/j.1600-051X.2010.01680.x. [DOI] [PubMed] [Google Scholar]
- 29.Flemmig T. F., Shanahan F., Miyasaki K. T. Prevalence and severity of periodontal disease in patients with inflammatory bowel disease. Journal of Clinical Periodontology. 1991;18(9):690–697. doi: 10.1111/j.1600-051X.1991.tb00111.x. [DOI] [PubMed] [Google Scholar]
- 30.Stein J. M., Lammert F., Zimmer V., et al. Clinical periodontal and microbiologic parameters in patients with Crohn's disease with consideration of the CARD15 genotype. Journal of Periodontology. 2010;81(4):535–545. doi: 10.1902/jop.2009.090563. [DOI] [PubMed] [Google Scholar]
- 31.Papageorgiou S. N., Hagner M., Nogueira A. V. B., Franke A., Jaeger A., Deschner J. Inflammatory bowel disease and oral health: systematic review and a meta-analysis. Journal of Clinical Periodontology. 2017;44(4):382–393. doi: 10.1111/jcpe.12698. [DOI] [PubMed] [Google Scholar]
- 32.She Y.-Y., Kong X. B., Ge Y. P., et al. Periodontitis and inflammatory bowel disease: a meta-analysis. BMC Oral Health. 2020;20(1):p. 67. doi: 10.1186/s12903-020-1053-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Dyke T., Dowell V., Offenbacher S., Snyder W., Hersh T. Potential role of microorganisms isolated from periodontal lesions in the pathogenesis of inflammatory bowel disease. Infection and Immunity. 1986;53(3):671–677. doi: 10.1128/IAI.53.3.671-677.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Said H. S., Suda W., Nakagome S., et al. Dysbiosis of salivary microbiota in inflammatory bowel disease and its association with oral immunological biomarkers. DNA Research. 2014;21(1):15–25. doi: 10.1093/dnares/dst037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Docktor M. J., Paster B. J., Abramowicz S., et al. Alterations in diversity of the oral microbiome in pediatric inflammatory bowel disease. Inflammatory Bowel Diseases. 2011;18(5):935–942. doi: 10.1002/ibd.21874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lira-Junior R., Figueredo C. M. Periodontal and inflammatory bowel diseases: is there evidence of complex pathogenic interactions? World Journal of Gastroenterology. 2016;22(35):p. 7963. doi: 10.3748/wjg.v22.i35.7963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qi Y., Zang S.-Q., Wei J., Yu H.-C., Yang Z., Wu H.-M., et al. High-throughput sequencing provides insights into oral microbiota dysbiosis in association with inflammatory bowel disease. Genomics. 2020 doi: 10.1016/j.ygeno.2020.09.063. [DOI] [PubMed] [Google Scholar]
- 38.Seymour G., Ford P., Cullinan M., Leishman S., Yamazaki K. Relationship between periodontal infections and systemic disease. Clinical Microbiology and Infection. 2007;13:3–10. doi: 10.1111/j.1469-0691.2007.01798.x. [DOI] [PubMed] [Google Scholar]
- 39.Han P., Sun D., Yang J. Interaction between periodontitis and liver diseases. Biomedical Reports. 2016;5(3):267–276. doi: 10.3892/br.2016.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakajima M., Arimatsu K., Kato T., et al. Oral administration of P. gingivalis induces dysbiosis of gut microbiota and impaired barrier function leading to dissemination of enterobacteria to the liver. PloS One. 2015;10(7, article e0134234) doi: 10.1371/journal.pone.0134234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Furusho H., Miyauchi M., Hyogo H., et al. Dental infection of Porphyromonas gingivalis exacerbates high fat diet-induced steatohepatitis in mice. Journal of Gastroenterology. 2013;48(11):1259–1270. doi: 10.1007/s00535-012-0738-1. [DOI] [PubMed] [Google Scholar]
- 42.Blasco-Baque V., Garidou L., Pomié C., et al. Periodontitis induced byPorphyromonas gingivalisdrives periodontal microbiota dysbiosis and insulin resistance via an impaired adaptive immune response. Gut. 2017;66(5):872–885. doi: 10.1136/gutjnl-2015-309897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Figueredo C., Brito F., Barros F., et al. Expression of cytokines in the gingival crevicular fluid and serum from patients with inflammatory bowel disease and untreated chronic periodontitis. Journal of Periodontal Research. 2011;46(1):141–146. doi: 10.1111/j.1600-0765.2010.01303.x. [DOI] [PubMed] [Google Scholar]
- 44.Szczeklik K., Owczarek D., Pytko-Polończyk J., Kęsek B., Mach T. H. Proinflammatory cytokines in the saliva of patients with active and non-active Crohn's disease. Polskie Archiwum Medycyny Wewnętrznej. 2012;122(5):200–208. doi: 10.20452/pamw.1256. [DOI] [PubMed] [Google Scholar]
- 45.Colombel J.-F., Narula N., Peyrin-Biroulet L. Management strategies to improve outcomes of patients with inflammatory bowel diseases. Gastroenterology. 2017;152(2):351–361.e5. doi: 10.1053/j.gastro.2016.09.046. [DOI] [PubMed] [Google Scholar]
- 46.Assuma R., Oates T., Cochran D., Amar S., Graves D. IL-1 and TNF antagonists inhibit the inflammatory response and bone loss in experimental periodontitis. The Journal of Immunology. 1998;160(1):403–409. [PubMed] [Google Scholar]
- 47.Delima A., Oates T., Assuma R., et al. Soluble antagonists to interleukin-1 (IL-1) and tumor necrosis factor (TNF) inhibits loss of tissue attachment in experimental periodontitis. Journal of Clinical Periodontology. 2001;28(3):233–240. doi: 10.1034/j.1600-051x.2001.028003233.x. [DOI] [PubMed] [Google Scholar]
- 48.Lin C.-Y., Tseng K.-S., Liu J.-M., et al. Increased risk of ulcerative colitis in patients with periodontal disease: a nationwide population-based cohort study. International Journal of Environmental Research and Public Health. 2018;15(11):p. 2602. doi: 10.3390/ijerph15112602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kang E. A., Chun J., Kim J. H., et al. Periodontitis combined with smoking increases risk of the ulcerative colitis: a national cohort study. World Journal of Gastroenterology. 2020;26(37):5661–5672. doi: 10.3748/wjg.v26.i37.5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tomar S. L., Asma S. Smoking-attributable periodontitis in the United States: findings from NHANES III. Journal of Periodontology. 2000;71(5):743–751. doi: 10.1902/jop.2000.71.5.743. [DOI] [PubMed] [Google Scholar]
- 51.Parkes G. C., Whelan K., Lindsay J. O. Smoking in inflammatory bowel disease: impact on disease course and insights into the aetiology of its effect. Journal of Crohns & Colitis. 2014;8(8):717–725. doi: 10.1016/j.crohns.2014.02.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated or analyzed during the current study are available from the corresponding author on reasonable request.


