Abstract
Purpose:
Sleep disturbance may be an overlooked modifiable risk factor for health disparities among African American breast cancer survivors (AABCS). This study aimed to identify the prevalence of and risk factors for sleep disturbance in a cohort of AABCS.
Methods:
The study was conducted among participants in the Women’s Circle of Health Follow-up Study, a longitudinal study of breast cancer in 10 counties in New Jersey. Cases were identified shortly after diagnosis by the New Jersey State Cancer Registry. Self-reported sleep disturbance (Pittsburgh Sleep Quality Index) and other factors (e.g., socioeconomic status, menopausal status) were assessed at pre-diagnosis (n=637), 10 months post-diagnosis (n=261), and 24 months post-diagnosis (n=632). Clinical data were obtained via medical record abstraction, and height and weight were measured by study staff.
Results:
Most AABCS (57%) reported clinically-significant sleep disturbance before diagnosis, and this rate remained largely unchanged at 10 months (53%) and 24 months post-diagnosis (61%). Average sleep disturbance scores indicated clinically-significant disturbance at all three assessments (M range=6.67–7.57). Most reported sleeping fewer than the recommended 7 hours per night at each assessment (range 57%–65%). Risk factors for sleep disturbance were identified at each assessment, including pre-diagnosis (less education), 10 months post-diagnosis (lack of insurance, treatment with chemotherapy), and 24 months post-diagnosis (younger age, less education, lower income, obesity, and lymphedema). Treatment with endocrine therapy was a protective factor at 10 months post-diagnosis.
Conclusion:
Most AABCS report clinically-significant sleep disturbance from before diagnosis through 24 months post-diagnosis. These rates appear indicate AABCS experience significant sleep-related disparities.
Keywords: Cancer, Oncology, Sleep, Quality of Life, African American or African American Cancer Survivors, Breast Neoplasms, Survivorship
Background
Recent attention has focused on understanding disparities in outcomes among African American breast cancer survivors (AABCS). Recent attention is due in part because of higher breast cancer mortality and worse quality of life (QoL).1 among African American women compared to white women.2 Sleep disturbance is an often-overlooked and potentially modifiable risk factor that may contribute to poor survival, QoL, and medication adherence. We define sleep disturbance in this study as difficulty falling or staying asleep. Although evidence is mixed, sleep disturbance has predicted survival among cancer survivors in some studies.3,4 Although there is a lack of consensus on how to define “short” sleep duration, the literature among the general population5–7 and among cancer survivors8,9 has generally shown that sleeping fewer than the consensus recommendation of 7 hours per night10 is associated with worse survival. Data from chronically ill patients provide evidence that sleep disturbance may account for some disparities in treatment adherence.11–14 Moreover, sleep disturbance is strongly linked with impaired QoL in cancer patients. One study of women receiving breast cancer treatment found sleep disturbance was associated with worse physical and mental quality of life.15 Another study of women with ovarian cancer found a temporal relationship, such that improvements in sleep quality from diagnosis to one year later were associated with improvements in QoL.16 This relationship remained after adjusting for disease stage, menopausal status at diagnosis, and use of medications for sleep, depression, anxiety, and pain.
Studies among white breast cancer survivors have demonstrated high rates of sleep disturbance. A cohort study of mostly white breast cancer patients in Canada found that rates of sleep disturbance as long as 18 months after diagnosis remained as high as 42%.17 In another US study, breast cancer survivors reported significantly worse sleep disturbance than matched controls in both white and African American patients.18 Similarly, a study comparing 62 African-American breast cancer survivors (AABCS) to 78 African American women with no history of cancer found AABCS to report significantly worse sleep disturbance than controls.19 However, little is known about the prevalence and longevity of sleep disturbance among AABCS. One study with AABCS that assessed sleep disturbance using a single-item measure found that 50% reported sleep disturbances over four years after diagnosis.20 Only one study has assessed the prevalence of sleep disturbance in an African American cancer patients using a validated scale. This cross-sectional sample of AABCS ranging from 1 – 18 years (mean = 7 years) post-diagnosis found that 43% reported clinically-significant sleep disturbance.21 Overall, the current literature suggests that sleep disturbance is a major issue in AABCS, but few studies have evaluated the prevalence or risk factors for sleep disturbance among African Americans using validated measures.
This study aimed to examine risk factors and change over time in sleep disturbance spanning the time prior to breast cancer diagnosis through 2 years post-diagnosis in a longitudinal cohort of AABCS. Based on previous research showing elevated rates of sleep disturbance among breast cancer survivors and higher rates among AA women compared to white women, we hypothesize that a majority of AABCS will report clinically significant sleep disturbance at 10 months after diagnosis. We also hypothesize that the percentage of AABCS who report clinically significant sleep disturbance will decrease from 10 to 24 months post-diagnosis.
Methods
Participants and Procedure
This study used data from two assessments conducted approximately 10 months (i.e., the baseline assessment) and 24 months after diagnosis in the Women’s Circle of Health Follow-up Study, an ongoing population-based longitudinal study of AABCS. The study has been described in detailed elsewhere.22 In brief, the study was built upon the Women’s Circle of Health Study, which was conducted between 2006 and 2014 and aimed to evaluate risk factors for early onset and aggressive breast cancer in African American women.23–27 Potentially eligible cases were identified using rapid case ascertainment through the New Jersey State Cancer Registry. Eligibility criteria included self-identification as African American, diagnosis of histologically confirmed invasive or ductal carcinoma in situ breast cancer, residence in one of ten counties in New Jersey, age 20–75 years, no previous history of cancer other than non-melanomatous skin cancer, and ability to speak English. In 2014, with additional funding we continued to recruit newly diagnosed African American breast cancer cases using the same criteria and expanded the study to evaluate breast cancer survivorship and outcomes by collecting data on QoL and risk factors related to prognosis, including treatment, comorbidities, patient-related symptoms, and patient-reported outcomes. Medical records data were verified by medical record abstraction and data linkage with the New Jersey State Cancer Registry. The study protocol (ID # Pro2017000069) was approved by the Institutional Review Board at Rutgers, The State University of New Jersey and Roswell Park Comprehensive Cancer Center, and all participants provided written informed consent before participating.
Measures
Demographic and Clinical Factors.
Participants completed an in-person home interview with a trained interviewer who collected information on sociodemographic, reproductive, lifestyle, and medical history one year before diagnosis, as well as clinical factors. Pre-diagnostic self-reported height and weight were collected and participants’ body measurements at the time of the interview were collected by trained interviewers using a standardized protocol.25,28 Medical records, including pathology reports, were obtained and abstracted to assess tumor stage, diagnosis and treatment information, and other clinical factors.
Sleep Disturbance.
Sleep disturbance was assessed using the Pittsburgh Sleep Quality Index (PSQI).29 This 19-item scale assesses 7 components of sleep quality: subjective sleep quality, sleep latency, sleep duration, habitual sleep disturbances, use of sleep medication, and daytime dysfunction over the past month. Items ask respondents to indicate “how long (in minutes) has it usually taken you to fall asleep each night” and “how would you rate your sleep quality overall”? A sleep efficiency score is calculated as a percentage of the time spent in bed that is spent asleep. Global sleep disturbance scores of ≥ 5 indicate clinically-significant sleep disturbance,29 as does a sleep efficiency score <85%.30,31 This scale has been validated, demonstrated to be reliable, and is widely used in studies of cancer patients.32,33 In this sample, the internal consistency reliability of the PSQI measure was adequate with Cronbach’s alpha values at each assessment ranging from .70 to .73.
The PSQI was administered at the baseline assessment to solicit data on sleep disturbance in the year prior to diagnosis as well as on sleep disturbance in the month prior to the baseline assessment (~10 months after diagnosis). At the 24-month assessment sleep disturbance was again assessed for the prior month. The assessment of sleep disturbance in the month prior to the baseline interview was later removed from the protocol to reduce participant burden; thus, fewer participants reported data at this assessment than at the other two assessments.
Statistical Analyses
Analyses included participants recruited between October, 2012 through March, 2019 who provided sleep disturbance data retrospectively for the time prior to diagnosis (n=637), at 10 months after diagnosis (n=261), or at 24 months after diagnosis (n=632). Descriptive statistics were used to describe the sample on demographic and clinical characteristics. Next, we used descriptive statistics to describe sleep disturbance at each assessment.
We used two sets of analyses to identify risk factors for sleep disturbance. First, we conducted chi-square tests to identify risk factors for sleep disturbance in bivariate analyses. Second, we fitted age-adjusted and fully adjusted (adjusting for age, menopausal status at diagnosis, education, and BMI) logistic regression models to identify additional risk factors that were independently associated with sleep disturbance above and beyond the effects of these covariates that are commonly associated with sleep disturbance in the literature. We then conducted multivariate logistic regression analyses including only those risk factors that were associated with sleep disturbance in any of the previous bivariate, age-adjusted, or fully adjusted models.
Lastly, we plotted change in sleep disturbance over time among the subset of AABCS who provided sleep data at all three assessments (n=138). Analyses were conducted with SAS 9.4 (SAS Institute, Cary, NC) using an alpha level of .05.
Results
Participants
The left-most column of Table 1 provides demographic and clinical characteristics who provided data on pre-diagnostic sleep disturbance. Participants were on average 54.99 years of age (SD = 10.71), 67% had completed at least some college, and 38% were married. Table 1 presents results of chi-square analyses identifying demographic and clinical factors associated with clinically-significant sleep disturbance at each assessment. At pre-diagnosis, less education was a risk factor for sleep disturbance (p < .01). At 10 months post-diagnosis, risk factors for sleep disturbance included lack of health insurance and treatment with chemotherapy (ps ≤ .03); treatment with endocrine therapy was a protective at 10 months post-diagnosis (p = .02). At 24 months post-diagnosis, risk factors for sleep disturbance included younger age at diagnosis, less education, less household income, greater post-diagnosis BMI, and lymphedema at 24 months (ps ≤ .03).
Table 1.
Demographic and clinical characteristics as well as associations with sleep disturbance (WCHSF, 2012 – 2019).
| Participant Characteristics (n=637) |
Number (%) with Clinically-Significant Sleep Disturbance | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre-Diagnosis (n=637) |
10 Months post-Dx (n=261) |
24 Months post-Dx (n=632) |
||||||||
| n (%) | Yes | No | P | Yes | No | P | Yes | No | P | |
| Age (years) | .19 | .63 | <.01 | |||||||
| <50 | 191 (30) | 117(32) | 74 (27) | 47 (34) | 35 (29) | 128(33) | 68 (27) | |||
| 50–60 | 192(30) | 114(31) | 78 (29) | 42 (30) | 37 (30) | 138(36) | 68 (27) | |||
| >60 | 254 (40) | 135(37) | 119(44) | 50 (36) | 50(41) | 119(31) | 111 (45) | |||
| Education | <.01 | .10 | 054 | |||||||
| < High School | 205 (32) | 117(32) | 88 (33) | 48 (35) | 28 (23) | 146 (38) | 75 (30) | |||
| Some college | 220 (35) | 146 (40) | 74 (27) | 49 (35) | 46 (38) | 131 (34) | 82 (33) | |||
| College or Grad Degree | 212(33) | 103(28) | 109(40) | 42 (30) | 48 (39) | 108(28) | 90 (37) | |||
| Marital Status | .36 | .31 | .09 | |||||||
| Not married | 393 (62) | 231 (63) | 162 (60) | 88 (63) | 69 (57) | 261 (68) | 151 (61) | |||
| Married | 242 (38) | 133(37) | 109(40) | 51 (37) | 53 (43) | 124(32) | 96 (39) | |||
| Annual Household Income | .10 | .89 | .03 | |||||||
| < $25,000 | 176(30) | 112(33) | 64 (26) | 37 (28) | 30 (27) | 136(37) | 63 (27) | |||
| $25,000 – $69,000 | 214(37) | 113(33) | 101 (41) | 48 (36) | 44 (39) | 120(33) | 89 (38) | |||
| > $70,000 | 194(33) | 113(33) | 81 (33) | 49 (36) | 39 (34) | 110(30) | 84 (35) | |||
| Insurance Status | .52 | .03 | .08 | |||||||
| None | 61 (10) | 35(10) | 26(10) | 23(17) | 9(7) | 44(11) | 18(7) | |||
| Medicaid | 76(12) | 49(13) | 27(10) | 10(7) | 18(15) | 48(13) | 20(8) | |||
| Medicare | 119(19) | 64(18) | 55 (20) | 20 (14) | 24 (20) | 66(17) | 48 (20) | |||
| Private/Other | 374 (59) | 212 (59) | 162 (60) | 85 (62) | 71 (58) | 224 (59) | 160(65) | |||
| Smoking Status | .15 | .92 | .17 | |||||||
| Never | 372 (58) | 209 (57) | 163 (60) | 87 (63) | 74(61) | 237 (62) | 140(57) | |||
| Former | 162 (26) | 89 (24) | 73 (27) | 35 (25) | 31 (25) | 85 (22) | 71 (29) | |||
| Current | 103(16) | 68(19) | 35(13) | 17(12) | 17(14) | 63(16) | 36 (14) | |||
| Menopausal Status | .35 | .61 | .07 | |||||||
| Premenopausal | 220 (35) | 132(36) | 88 (33) | 53 (38) | 42 (34) | 141 (37) | 73 (30) | |||
| Postmenopausal | 413(65) | 231 (64) | 182(67) | 86 (62) | 80 (66) | 242 (63) | 174(70) | |||
| Pre-Diagnostic BMI (Kg/m2) | .13 | .14 | .09 | |||||||
| <25 | 86(13) | 44 (12) | 42 (16) | 14(10) | 21 (17) | 57(15) | 40(16) | |||
| 25–29 | 189(30) | 102(28) | 87 (32) | 41 (30) | 40 (33) | 94 (24) | 78 (32) | |||
| >30 | 360 (57) | 219(60) | 141 (52) | 84 (60) | 61 (50) | 233(61) | 129(52) | |||
| 24 Months Post-Diagnosis BMI | .51 | .46 | .03 | |||||||
| <25 | 47(14) | 25 (12) | 22(16) | 8(10) | 12(18) | 51 (13) | 36(15) | |||
| 25–29 | 90 (27) | 53 (26) | 37 (28) | 22 (28) | 18(26) | 94 (25) | 82 (33) | |||
| >30 | 201 (59) | 125 (62) | 76 (56) | 48 (62) | 38 (56) | 237 (62) | 128(52) | |||
| Pre-Diagnostic Type 2 Diabetes | .70 | .88 | >.99 | |||||||
| No | 495 (78) | 282 (77) | 213(79) | 111 (80) | 99(81) | 298 (78) | 192(78) | |||
| Yes | 142(22) | 84 (23) | 58 (21) | 28 (20) | 23(19) | 86 (22) | 55 (22) | |||
| Pre-Diagnostic Hypertension | .51 | >.99 | .80 | |||||||
| No | 243 (38) | 144 (39) | 99 (37) | 86 (62) | 75(61) | 155(40) | 102(41) | |||
| Yes | 394 (62) | 222(61) | 172(63) | 53 (38) | 47 (39) | 230 (60) | 145(59) | |||
| AJCC Stage | ||||||||||
| 0 | 120(23) | 72 (24) | 48 (22) | .85 | 25 (20) | 32 (28) | .26 | 71 (20) | 58 (26) | .19 |
| I/II | 363 (70) | 206 (69) | 157(71) | 90 (70) | 75 (65) | 256(71) | 152(67) | |||
| III/IV | 35(7) | 20(7) | 15(7) | 13(10) | 8(7) | 32(9) | 15(7) | |||
| Surgery Type | .76 | .10 | .09 | |||||||
| None | 46(7) | 28(8) | 18(7) | 14(10) | 10(8) | 24(6) | 19(8) | |||
| Lumpectomy | 421 (66) | 237 (65) | 184(68) | 83 (60) | 88 (72) | 240 (62) | 169(69) | |||
| Mastectomy | 168(27) | 99 (27) | 69 (25) | 42 (30) | 24 (20) | 121 (32) | 58 (23) | |||
| Treated with Chemotherapy | .63 | .01 | .06 | |||||||
| No | 311 (49) | 175(48) | 136(50) | 58 (42) | 72 (59) | 180(47) | 134(54) | |||
| Yes | 324(51) | 189(52) | 135(50) | 81 (58) | 50(41) | 205 (53) | 112(46) | |||
| Treated with Radiotherapy | .80 | .53 | .19 | |||||||
| No | 223 (35) | 126(35) | 97 (36) | 59 (42) | 46 (38) | 171 (44) | 95 (39) | |||
| Yes | 411 (65) | 237 (65) | 174(64) | 80 (58) | 75 (62) | 214(56) | 150(61) | |||
| Treated with Endocrine Therapy | .57 | .02 | .33 | |||||||
| No | 295 (47) | 173(48) | 122(45) | 81 (59) | 53 (44) | 188(49) | 110(45) | |||
| Yes | 338 (53) | 190(52) | 148 (55) | 57(41) | 68 (56) | 196(51) | 135(55) | |||
| .Lymphedema at 24 Months | .26 | .39 | <.01 | |||||||
| No | 616(97) | 351 (96) | 265 (98) | 131 (94) | 118(97) | 348 (90) | 240 (97) | |||
| Yes | 21(3) | 15(4) | 6(2) | 8(6) | 4(3) | 37(10) | 7(3) | |||
Note: Some categories do not add up to N=637, n=261, or n=632 due to missing data. Clinically-significant sleep disturbance was defined as having a Pittsburgh Sleep Quality Index score of ≥ 5.29 Treatment information is based on participant self-report.
Sleep Disturbance
Descriptive statistics for sleep disturbance are presented in Table 2. Average Pittsburgh Sleep Quality Index scores at all timepoints (range=6.67 to 7.57) were above 5, the level indicating clinically-significant sleep disturbance. The majority of AABCS reported clinically-significant sleep disturbance prior to diagnosis (57%), 10 months after diagnosis (53%), and 24 months after diagnosis (61%). Average sleep efficiency was approximately 78% and 79% at each assessment, below the accepted cutoff of 85%. Participants reported sleeping approximately 6 hours per night on average at each assessment, below the recommended 7 hours. Most (57% – 65%) reported sleeping fewer than 7 hours per night. The proportion of participants reporting sleep efficiency below the cutoff of 85% ranged from 54% to 56%.
Table 2.
Sleep quality in the year before diagnosis as well as 10 months and 24 months post-diagnosis (WCHFS, 2012 – 2019).
| Sleep Outcome | 10 Pre-Diagnosis (n=637) |
Months Post-Diagnosis (n=261) |
24 Months Post-Diagnosis (n=632) |
|---|---|---|---|
| PSQI Score, Mean (SD) | 6.84 (4.29) | 6.67 (4.03) | 7.57 (4.53) |
| Clinically-Significant Sleep Disturbance1 | |||
| No, N (%) | 271 (42.54%) | 122(46.74%) | 247 (39.08%) |
| Yes, N (%) | 366 (57.46%) | 139(53.26%) | 385 (60.92%) |
| Sleep Efficiency, Mean (SD) | 79.20(18.54) | 79.11 (18.66) | 77.69(19.55) |
| Sleep Efficiency < 85% | |||
| No, N (%) | 296 (46.47%) | 119(45.59%) | 279(44.15%) |
| Yes, N (%) | 341 (53.53%) | 142(54.41%) | 353 (55.85%) |
| Hours of Overnight Sleep, Mean (SD) | 5.88(1.56) | 6.08(1.54) | 5.92(1.54) |
| Sleep < 7 hours Per Night | |||
| No, N (%) | 229 (35.95%) | 112(42.91%) | 221 (34.97%) |
| Yes, N (%) | 408 (64.05%) | 149(57.09%) | 411 (65.03%) |
Note: PSQI = Pittsburgh Sleep Quality Index; SD = standard deviation;
Clinically-significant sleep disturbance was defined as a Pittsburgh Sleep Quality Index (PSQI) score of ≥ 5.29
Change Over Time in Sleep Disturbance
The course of sleep disturbance is presented in Figure 1 among patients with data at all three assessments (n=138), and similar trends are demonstrated in Supplementary Figure 1 for the n=317 participants who reported sleep disturbance data at pre-diagnosis and 24 months post-diagnosis. Most (60%) of these 138 participants reported clinically-significant sleep disturbance at pre-diagnosis. Of these, most (60%) sustained clinically-significant sleep disturbance at the 10-month and 24-month assessments. An additional 18% temporarily remitted at the 10-month assessment but returned to clinically-significant levels at the 24-month assessment. Sleep disturbance had resolved by the 24-month assessment in only 22% of women reporting sleep disturbance at pre-diagnosis.
Figure 1:
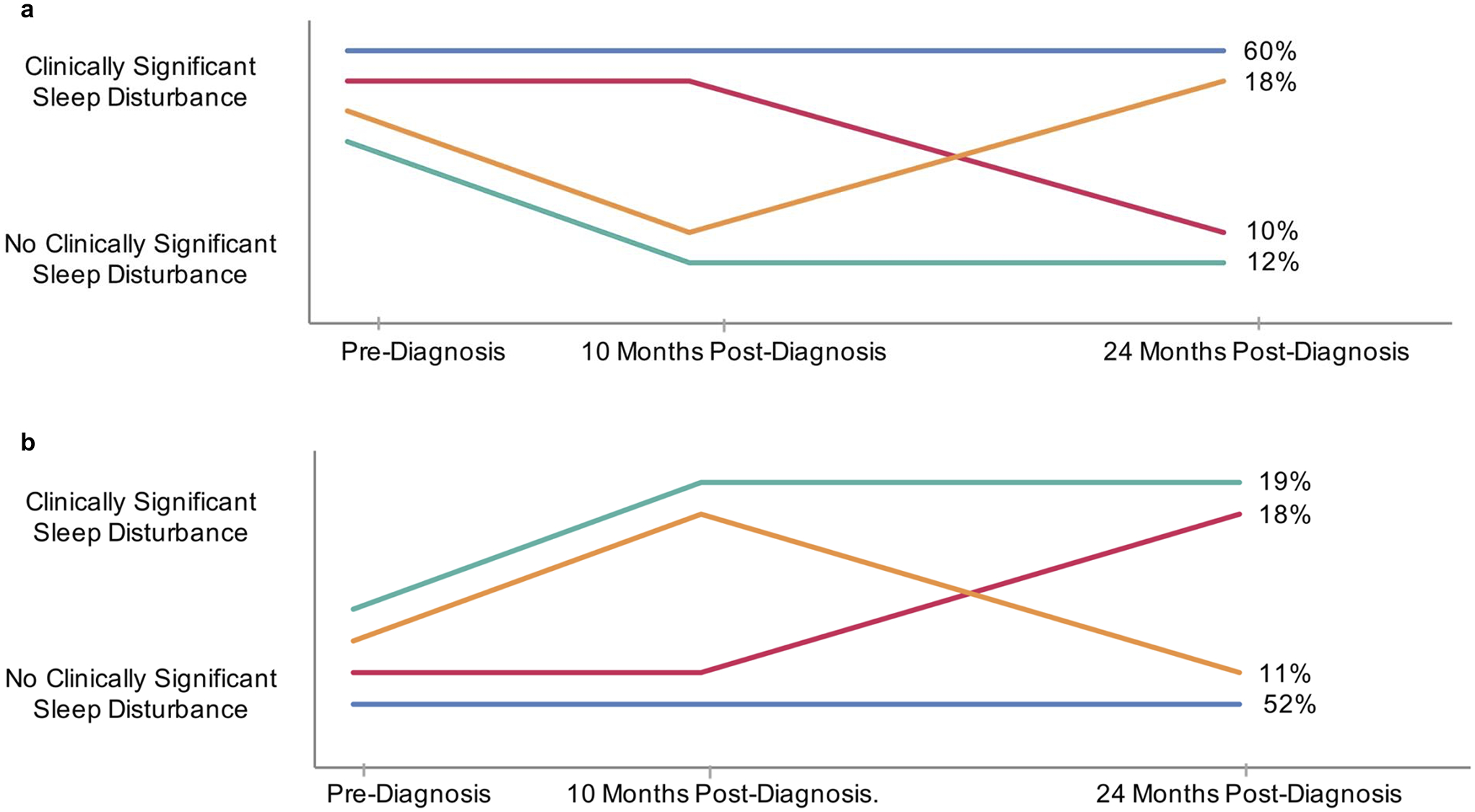
Change over time in sleep disturbance for patients with clinically-significant sleep disturbance at pre-diagnosis (panel A, n=82) and without sleep disturbance at pre-diagnosis (panel B, n=56) (WCHFS, 2012 – 2019).
Of the 40% whose sleep disturbance score was not above the cutoff for clinical significance at pre-diagnosis, over a third (37%) went on to develop clinically-significant sleep disturbance by the 24-month assessment. An additional 11% temporarily developed clinically-significant levels at the 10-month assessment but resolved at the 24-month assessment. Most (52%) continued to report sub-clinical levels of sleep disturbance at the 10-month and 24-month assessments.
Demographic and Clinical Risk Factors for Sleep Disturbance
Results from age-adjusted and fully-adjusted (e.g., adjusted for age, menopausal status, and BMI) logistic regression models predicting clinically-significant sleep disturbance from sociodemographic factors are presented in Supplementary Table 1 and from clinical factors are presented in Supplementary Table 2. At pre-diagnosis, less education was a risk factor for sleep disturbance in age- and fully adjusted models (ps ≤ .01). At 10 months post-diagnosis, treatment with chemotherapy was associated with higher risk of sleep disturbance, whereas patients with health insurance and who were treated with endocrine therapy were less likely to have sleep disturbance in the age- and fully adjusted models (ps ≤ .03). At 24 months post-diagnosis, younger age, less education, lower income, obesity and lymphedema at 24 months were significant risk factors for sleep disturbance in the age-adjusted model (ps ≤ .03). Younger age, less education, and lymphedema remained significant risk factors for sleep disturbance at 24 months in the fully adjusted model (ps ≤ .03).
Table 3 presents results from multivariate models to identify which of those risk factors identified in previous models remained independently associated with sleep disturbance. We included only those risk factors that emerged as statistically significant in bivariate chi-square analyses, presented in Table 1, or multivariate logistic regression analyses, presented in Supplementary Tables 1 and 2. Thus, at pre-diagnosis the only risk factor included in the model was education because it was the only risk factor associated with pre-diagnostic sleep disturbance in previous analyses. Less education remained significantly associated with higher risk of sleep disturbance (p < .01), with patients who had attended some college being more likely to report significant sleep disturbance before diagnosis than patients with ≤ high school education (OR = 1.48, 95%CI = 1.001, 2.20). At 10 months post-diagnosis, lack of health insurance and treatment with chemotherapy were independently associated with higher risk of sleep disturbance (ps = .04). At 24 months post-chemotherapy, younger age, higher BMI at 24 months post-diagnosis, and lymphedema at 24 months post-diagnosis were independently associated with higher risk of sleep disturbance at 24 months post-diagnosis (ps ≤ .02).
Table 3.
Multivariate models identifying risk factors for sleep disturbance at pre-diagnosis as well as 10 and 24 months post-diagnosis (WCHFS, 2012 – 2019)
| Pre-Diagnosis (n=637) |
10 Months Post-Diagnosis (n=261) |
24 Months Post-Diagnosis (n=632) |
||||
|---|---|---|---|---|---|---|
| Predictor/Variable | ||||||
| Estimate (95% Cl) | p | Estimate (95% Cl) | p | Estimate (95% Cl) | p | |
| Education | <.01 | .32 | ||||
| < High school | Referent | Referent | ||||
| Some college | 1.48(1.001–2.20) | 0.80(0.52–1.22) | ||||
| College or grad degree | 0.71 (0.48–1.05) | 0.70(0.44–1.13) | ||||
| Insurance Status | .04 | |||||
| None | Referent | |||||
| Medicaid | 0.21 (0.07–0.65) | |||||
| Medicare | 0.39(0.14–1.07) | |||||
| Private/Other | 0.55(0.24–1.30) | |||||
| Treated with Chemotherapy | .04 | |||||
| No | Referent | |||||
| Yes | 1.76(1.03–3.03) | |||||
| Treated with Endocrine Therapy | .13 | |||||
| No | Referent | |||||
| Yes | 0.67(0.39–1.13) | |||||
| Age (years) | «.01 | |||||
| <50 | Referent | |||||
| 50–60 | 1.03(0.67–1.60) | |||||
| >60 | 0.53 (0.35–0.80) | |||||
| Annual Household Income | .26 | |||||
| < $25,000 | Referent | |||||
| $25,000 - $69,000 | 0.71 (0.46–1.10) | |||||
| > $70,000 | 0.71 (0.44–1.15) | |||||
| BMI at 24 Months Post-Diagnosis | .03 | |||||
| <25 | Referent | |||||
| 25–29 | 0.85(0.49–1.48) | |||||
| >30 | 1.44(0.87–2.40) | |||||
| Lymphedema at 24 Months | <.01 | |||||
| No | Referent | |||||
| Yes | 3.13(1.34–7.30) | |||||
Note: BMI calculated as (Kg/m2). Risk factors were included if they were associated with sleep disturbance at the respective assessment.
Discussion
This study is the largest to our knowledge to characterize sleep disturbance in African American cancer survivors and the first to longitudinally examine sleep quality. We examined changes occurring from the time of diagnosis to 2 years post diagnosis. As we hypothesized, most AABCS reported clinically-significant sleep disturbance 10 months after diagnosis. Contrary to hypotheses, the rate of clinically-significant sleep disturbance did not decline at 24 months after diagnosis. On average, participants also reported sleeping approximately six hours of sleep per night, below the recommended seven hours per night. Risk factors for sleep disturbance included less education (likely a proxy for socioeconomic status), younger age, greater BMI, treatment with chemotherapy, and lymphedema. Protective factors included higher income, having medical insurance, and treatment with endocrine therapy.
More than half of the AABCS in this study experienced clinically-significant sleep disturbance before diagnosis and at 10 and 24 months after diagnosis. This rate of clinically-significant sleep disturbance (57% – 61%) is higher than the rate observed in a longitudinal study among predominantly white Canadian breast cancer survivors at 18 months after diagnosis (42%).17 These discrepant findings may be partly attributed to the previous study’s use of a semi-structured clinical interview to assess sleep disturbance,17 which may have resulted in lower rates of sleep disturbance than the self-report measure used in the present study. Additionally, participants in the current sample may have had lower socioeconomic status, as evidenced by lower levels of education (33% had ≥ college degree) than the previous sample (51% had ≥ college degree).34 Socioeconomic status is consistently associated with greater sleep disturbance;35 however, previous research has demonstrated that racial disparities in sleep disturbance persist independent of socioeconomic status.36 Thus, in the context of existing literature, our findings suggest that AABCS experience high rates of sleep disturbance before diagnosis and perhaps higher rates than white breast cancer survivors well into the survivorship phase.17 This difference may reflect an important disparity between white breast cancer survivors and AABCS that is similar to that observed in individuals without cancer.37–39
The finding that the rate of sleep disturbance did not change over time after diagnosis is striking. We hypothesized that, as observed in the study of Canadian breast cancer survivors,17 AABCS would report a gradual reduction in clinically-significant sleep disturbance over time. Instead, the rate remained stable with over 60% reporting clinically-significant sleep disturbance approximately 24 months post-diagnosis. Similarly, longitudinal findings demonstrated that the vast majority of participants who reported clinically-significant sleep disturbance before their diagnosis continued to have difficulty sleeping throughout the assessment period. There was also a high rate of development of new sleep disturbance among those who did not report sleep disturbance prior to diagnosis. In the subset of participants who reported sleep disturbance data at all three assessments, most who reported clinically-significant sleep disturbance before diagnosis continued to report elevated sleep disturbance 24 months after diagnosis, suggesting sleep disturbance may be intractable in this cohort. Conversely, over a third of patients who reported sleeping well at pre-diagnosis went on to develop clinically-significant sleep disturbance by the 24-month assessment. This increase may be attributed to the many stress-related and cancer treatment-related risk factors for sleep disturbance reported here and in previous research.
Survivors’ average overnight sleep duration of approximately 6 hours per night may also increase risk of adverse outcomes, given epidemiologic studies in the general population finding that individuals with short overnight sleep duration, generally defined as < 7 hours per night,10 have higher risk of mortality.5,40 As with sleep disturbance, sleep duration remained largely unchanged over time in this study. The prevalence of insufficient sleep in this sample of AABCS (57% – 65%) was higher than the 47% prevalence observed in a nation-wide cohort of 3,750 African American adults.41 Additional research is needed to examine whether lower sleep duration represents a significant factor contributing to cancer health disparities among AABCS. However, research in non-cancer populations has consistently shown that African Americans are more likely to report lower overnight sleep duration than their white counterparts and that this disparity has worsened over time.6,42
We identified several risk factors associated with worse sleep disturbance among AABCS. African American breast cancer survivors who were less educated were more likely to report sleep disturbance. These findings are consistent with findings in the non-cancer and cancer literature.42,43 We also found that obesity was associated with sleep disturbance. This may be attributed to the greater risk of obstructive sleep apnea among overweight and obese individuals.44 Future studies should aim to disentangle the prevalence in AABCS of various sleep disorders, such as sleep apnea and insomnia.
A novel finding is that persistent lymphedema was associated with worse sleep disturbance. We are unaware of any studies reporting this association among African American population. A previous qualitative study found that breast cancer survivors with lymphedema commonly reported sleep disturbance,45 and a randomized trial of a lymphatic drainage intervention showed improvements in sleep.46 Lymphedema may disturb survivors’ sleep due to pain experienced while putting pressure on one’s swollen arm. Attempting to address this barrier to sleep was reportedly difficult in previous studies. In the qualitative study, survivors reported taking steps to avoid the pain associated with lymphedema while sleeping, such as using pillows to elevate their arms while in bed.45 Those who slept on their sides tried sleeping only on the side without swelling. Future intervention studies should aim to target lymphedema as a source of sleep disturbance in breast cancer survivors.
Strengths of the study include the examination of sleep disturbance at clinically important assessment points, using a validated scale assessing sleep disturbance, and the simultaneous collection of risk factor data obtained during in-person interviews. However, some limitations should be noted. Pre-diagnostic sleep quality was retrospectively assessed by asking participants to recall their sleeping habits one year before diagnosis, which may have introduced some degree of recall bias. Another limitation is that not all participants completed the measure of sleep disturbance at each assessment because measure was included in the study assessments after recruitment had begun. However, distributions for major variables of interest for the subset of participants with the three assessments and those with only two assessments were similar (Supplementary Table 3). Lastly, we assessed sleep disturbance using a validated self-report scale rather than a clinical interview and did not employ objective tools that would permit distinguishing between distinct sleep disorders (e.g., insomnia, obstructive sleep apnea, restless leg syndrome). Future research on this topic would be strengthened by measuring sleep disturbance closer to the time of diagnosis, collecting complete sleep disturbance data on all participants at each assessment, and combining objective and subjective measures.
In conclusion, these findings suggest AABCS may be particularly vulnerable to sleep disturbance and suggest risk factors for worse sleep disturbance at various points along the survivorship continuum. Given the adverse consequences of chronic sleep disturbance in cancer survivors, clinicians may wish to inquire about sleep quality in AABCS, particularly those with the risk factors identified in this study. Future studies should aim to replicate and extend these findings and examine biological and psychological mechanisms of these risk factors and the consequences of sleep disturbance in AABCS.
Supplementary Material
Funding:
This work was supported by National Cancer Institute grants K01 CA211789 (Gonzalez), R25 HL105444 (Jean-Louis, Ogedegbe), K01 CA193527 (Llanos), K99 MD013300 (Qin), R01 CA185623 (Bandera, Demissie, Hong), and R01 CA185623S1 (Bandera, Demissie, Hong), P01 CA151135 (Ambrosone, Palmer, Olshan), P30 CA072720-5929 (Delnevo, Bandera), R25 CA090314 (Brandon), P30 CA016056 (Ambrosone), and the Breast Cancer Research Foundation (Ambrosone). The New Jersey State Cancer Registry, Cancer Epidemiology Services, New Jersey Department of Health, is funded by the Surveillance, Epidemiology and End Results (SEER) Program of the National Cancer Institute under contract HHSN261201300021I and control No. N01-PC-2013-00021, the National Program of Cancer Registries (NPCR), Centers for Disease Control and Prevention under grant NU5U58DP003931-05-00 as well as the State of New Jersey and the Rutgers Cancer Institute of New Jersey. We are grateful to the study participants and staff of the Women Circle of Health Follow-up Study for their contribution to this study.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflicts of Interest: HJ: Red Hill Biopharma, Janssen Scientific Affairs. BG: SureMed Compliance, Elly Health.
Ethics Approval: The study protocol (ID # Pro2017000069) was approved by the Institutional Review Board at Rutgers, The State University of New Jersey and Roswell Park Comprehensive Cancer Center.
Availability of Data and Material: The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Code Availability: The code that support the findings of this study are available on request from the corresponding author.
References
- 1.Merluzzi TV, Philip EJ, Zhang Z, Sullivan C (2015) Perceived discrimination, coping, and quality of life for African-American and Caucasian persons with cancer. Cultur Divers Ethnic Minor Psychol 21(3):337–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeSantis CE, Siegel RL, Sauer AG, et al. : Cancer statistics for African Americans, 2016: Progress and opportunities in reducing racial disparities. CA Cancer J Clin 66:290–308, 2016 [DOI] [PubMed] [Google Scholar]
- 3.Palesh O, Aldridge-Gerry A, Zeitzer JM, et al. : Actigraphy-Measured Sleep Disruption as a Predictor of Survival among Women with Advanced Breast Cancer. Sleep 37:837–842, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Innominato PF, Spiegel D, Ulusakarya A, et al. : Subjective sleep and overall survival in chemotherapy-naïve patients with metastatic colorectal cancer. Sleep medicine 16:391–398, 2015 [DOI] [PubMed] [Google Scholar]
- 5.Gallicchio L, Kalesan B: Sleep duration and mortality: a systematic review and meta-analysis. Journal of sleep research 18:148–158, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Grandner MA, Patel NP, Gehrman PR, et al. : Problems associated with short sleep: bridging the gap between laboratory and epidemiological studies. Sleep medicine reviews 14:239–247, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grandner MA, Hale L, Moore M, et al. : Mortality associated with short sleep duration: the evidence, the possible mechanisms, and the future. Sleep medicine reviews 14:191–203, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins KP, Geller DA, Antoni M, et al. : Sleep duration is associated with survival in advanced cancer patients. Sleep medicine 32:208–212, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steel JL, Terhorst L, Collins KP, et al. : Prospective analyses of cytokine mediation of sleep and survival in the context of advanced cancer. Psychosomatic medicine 80:483–491, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Panel CC, Watson NF, Badr MS, et al. : Recommended amount of sleep for a healthy adult: a joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society. Journal of Clinical Sleep Medicine 11:591–592, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phillips KD, Moneyham L, Murdaugh C, et al. : Sleep disturbance and depression as barriers to adherence. Clinical nursing research 14:273–293, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Chasens ER, Olshansky E: The experience of being sleepy while managing type 2 diabetes. Journal of the American Psychiatric Nurses Association 12:272–278, 2006 [Google Scholar]
- 13.Babson KA, Heinz AJ, Bonn-Miller MO: HIV medication adherence and HIV symptom severity: the roles of sleep quality and memory. AIDS Patient Care STDS 27:544–52, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Williams NJ, Jean-Louis G, Pandey A, et al. : Excessive daytime sleepiness and adherence to antihypertensive medications among Blacks: analysis of the counseling African Americans to control hypertension (CAATCH) trial. Patient Prefer Adherence 8:283–7, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu L, Fiorentino L, Rissling M, et al. : Decreased health-related quality of life in women with breast cancer is associated with poor sleep. Behavioral sleep medicine 11:189–206, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clevenger L, Schrepf A, DeGeest K, et al. : Sleep disturbance, distress, and quality of life in ovarian cancer patients during the first year after diagnosis. Cancer 119:3234–3241, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Savard J, Ivers H, Villa J, et al. : Natural course of insomnia comorbid with cancer: an 18-month longitudinal study. J Clin Oncol 29:3580–6, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Otte JL, Carpenter JS, Russell KM, et al. : Prevalence, severity, and correlates of sleep-wake disturbances in long-term breast cancer survivors. Journal of pain and symptom management 39:535–547, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Von Ah DM, Russell KM, Carpenter J, et al. : Health-related quality of life of African American breast cancer survivors compared to healthy African American women. Cancer nursing 35:337, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Northouse LL, Caffey M, Deichelbohrer L, et al. : The quality of life of African American women with breast cancer. Res Nurs Health 22:449–60, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Taylor TR, Huntley ED, Makambi K, et al. : Understanding sleep disturbances in African-American breast cancer survivors: a pilot study. Psycho‐Oncology 21:896–902, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bandera EV, Demissie K, Qin B, et al. : The Women’s Circle of Health Follow-Up Study: a population-based longitudinal study of Black breast cancer survivors in New Jersey. Journal of Cancer Survivorship, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ambrosone CB, Ciupak GL, Bandera EV, et al. : Conducting molecular epidemiological research in the age of HIPAA: A multi-institutional case-control study of breast cancer in African-American and European-American women. Journal of oncology 2009, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bandera EV, Chandran U, Zirpoli G, et al. : Body size in early life and breast cancer risk in African American and European American women. Cancer Causes & Control 24:2231–2243, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bandera EV, Chandran U, Zirpoli G, et al. : Body fatness and breast cancer risk in women of African ancestry. BMC Cancer 13:475, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ambrosone CB, Zirpoli GR, Bovbjerg DH, Shankar J, Hong CC, McCann SE, Ruszczyk M, Khoury T, Yao S, Ciupak GL, Jandorf L, Pawlish KS, Bandera EV (2014) Associations between estrogen receptor–negative breast cancer and timing of reproductive events differ between African American and European American women. Cancer Epidemiol Prev Biomarkers 23(6):1115–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gong Z, Ambrosone CB, McCann SE, et al. : Associations of dietary folate, Vitamins B6 and B12 and methionine intake with risk of breast cancer among African American and European American women. International Journal of Cancer 134:1422–1435, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qin B, Llanos AA, Lin Y, et al. : Validity of self-reported weight, height, and body mass index among African American breast cancer survivors. Journal of Cancer Survivorship 12:460–468, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buysse DJ, Reynolds CF, Monk TH, et al. : The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 28:193–213, 1989 [DOI] [PubMed] [Google Scholar]
- 30.Lichstein K, Durrence H, Taylor D, et al. : Quantitative criteria for insomnia. Behaviour research and therapy 41:427–445, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Levenson JC, Troxel WM, Begley A, et al. : A quantitative approach to distinguishing older adults with insomnia from good sleeper controls. Journal of Clinical Sleep Medicine 9:125–131, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carpenter JS, Andrykowski MA: Psychometric evaluation of the Pittsburgh Sleep Quality Index. J Psychosom Res 45:5–13, 1998 [DOI] [PubMed] [Google Scholar]
- 33.Beck SL, Schwartz AL, Towsley G, et al. : Psychometric evaluation of the Pittsburgh Sleep Quality Index in cancer patients. J Pain Symptom Manage 27:140–8, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Savard J, Villa J, Ivers H, et al. : Prevalence, natural course, and risk factors of insomnia comorbid with cancer over a 2-month period. J Clin Oncol 27:5233–9, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Williams N, Jean-Louis G, Blanc J, et al. : Race, socioeconomic position and sleep, Sleep and Health, Elsevier, 2019, pp 57–76 [Google Scholar]
- 36.Mezick EJ, Matthews KA, Hall M, et al. : Influence of race and socioeconomic status on sleep: Pittsburgh Sleep SCORE project. Psychosomatic medicine 70:410, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Redline S, Tishler PV, Hans MG, et al. : Racial differences in sleep-disordered breathing in African-Americans and Caucasians. Am J Respir Crit Care Med 155:186–92, 1997 [DOI] [PubMed] [Google Scholar]
- 38.Nunes J, Jean-Louis G, Zizi F, et al. : Sleep duration among black and white Americans: Results of the National Health Interview Survey. Journal of the National Medical Association 100:317–322, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Hall MH, Matthews KA, Kravitz HM, et al. : Race and financial strain are independent correlates of sleep in midlife women: the SWAN sleep study. Sleep 32:73–82, 2009 [PMC free article] [PubMed] [Google Scholar]
- 40.Cappuccio FP, D’Elia L, Strazzullo P, et al. : Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep 33:585–592, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Williams NJ, Grandner MA, Wallace DM, et al. : Social and behavioral predictors of insufficient sleep among African Americans and Caucasians. Sleep medicine 18:103–107, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stamatakis KA, Kaplan GA, Roberts RE: Short sleep duration across income, education, and race/ethnic groups: population prevalence and growing disparities during 34 years of follow-up. Annals of epidemiology 17:948–955, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patel NP, Grandner MA, Xie D, et al. : “ Sleep disparity” in the population: poor sleep quality is strongly associated with poverty and ethnicity. BMC Public Health 10:475, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Romero-Corral A, Caples SM, Lopez-Jimenez F, et al. : Interactions between obesity and obstructive sleep apnea: implications for treatment. Chest 137:711–719, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ridner SH: Quality of life and a symptom cluster associated with breast cancer treatment-related lymphedema. Supportive Care in Cancer 13:904–911, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Williams A, Vadgama A, Franks PJ, et al. : A randomized controlled crossover study of manual lymphatic drainage therapy in women with breast cancer-related lymphoedema. European journal of cancer care 11:254–261, 2002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


