Abstract
Objectives:
The aim of this in vitro study was to incorporate two anti-caries agents, Apigenin and tt-Farnesol, to resin composite and resin cement to reduce the virulence of Streptococcus mutans around dental restorations.
Methods:
Apigenin (Api, 5mM) and tt-Farnesol (Far, 5mM) were added alone, together, and combined with fluoride (F). Biofilm of S. mutans was grown on composite discs, and the dry-weight, bacterial viability, and the polysaccharides (alkali-soluble, intracellular and water-soluble) were quantified. CLSM images of the S. mutans biofilm were obtained after three years of water-storage. The effect of the additions on the physicochemical properties and the composite colorimetric parameters were also analyzed.
Results:
The additions did not affect bacterial viability. Api alone and combined with Far or combined with Far and F decreased the bacterial dry-weight, alkali-soluble and intracellular polysaccharides. After three years, the composites containing the additions presented a greater EPS matrix on the top of biofilm. Statistical difference was obtained for the degree of conversion; however, the maximum polymerization rate and curing kinetics were unaffected by the additions. No difference was observed for the water-soluble polysaccharides, flexural strength, and elastic modulus. Api increased the yellowness of the composites.
Keywords: dental caries, Streptococcus mutans, resin composite, resin cement, apigenin, tt-farnesol
1. INTRODUCTION
The initiation and development of dental caries are well recognized, from clinical and laboratory studies, to be associated with the oral pathogen Streptococcus mutans [1–3]. This bacterium can proliferate inside tooth pit and fissures, between teeth (interproximal areas) and around prosthetic materials [4]. Therefore, restorative materials containing antibacterial agents could yield benefits in terms of enhanced durability of dental restorations [5–7]. However, some antibacterial agents, with broad antimicrobial spectra, have been added to materials without concern regarding their effect on the health of resident oral bacteria and the promotion of bacterial resistance, which can produce undesirable outcomes [8, 9]. Therefore, the development of specific-targeted therapies, focusing on attenuating microbial virulence is more desirable and may contribute more effectively to the reduction of dental caries [10, 11].
S. mutans virulence is associated with its ability to form biofilms on tooth surfaces via the synthesis of extracellular polysaccharides (EPS) and ultimately the production of weak acids from the metabolism of simple sugars, while having the ability to adapt to fluctuations in pH, oxygen tension and nutrient availability [1, 3]. EPS are the main constituents in the biofilm matrix, being composed mostly of glucans synthesized by microbial glycosyltransferases (Gtfs), and are recognized as critical virulence factors associated with the initiation of a cariogenic biofilm [12–15]. The EPS matrix acts as a scaffold for the biofilm, limiting diffusion of antimicrobial compounds, providing primary binding sites for bacteria, acting as a source of fermentable sugars, and creating an acidic environment, which leads to demineralization of adjacent tooth structure and ultimately to clinical cavitation [10, 14, 16].
Apigenin and tt-Farnesol, two compounds derived from Brazilian propolis, are described in the literature as potential anti-caries agents and have been considered for use in a new approach to antimicrobial therapy that does not exert a significant effect on the viability of the oral microbiota [15, 17, 18]. Propolis is a nontoxic natural beehive product, and both compounds have shown in vitro and in vivo to be non-mutagenic and nontoxic [18, 19]. According to Koo et al. (2003 and 2009), Apigenin, a flavone (4’,5,7-trihydroxyflavone), is a potent inhibitor of Gtf enzymes in solution and on surfaces, particularly GtfB and GtfC; and has virtually no antibacterial activity against S. mutans [11, 18]. tt-Farnesol is a natural sesquiterpene (3,7,11-trimethyl-2,6,10-dodecatrien-1-ol) that disrupts the proton permeability of the S. mutans membrane, affecting the production and secretion of Gtfs and acidurance [15, 18]. Besides, when combined with fluoride, both compounds showed a reduction of S. mutans virulence [17]. The combination of these compounds with fluoride could enhance their anti-cariogenic properties, since fluoride can modify the ΔpH across the cell membrane, interfering with bacterial glycolysis and affecting the production-secretion of Gtfs [11]. This combination, which is based on their interconnected biological activity described above, may modulate the virulence of S. mutans associated both with EPS matrix formation and the acid production [11, 17]. Though such compounds may be applied directly, a parallel approach to their use is the incorporation of these compounds into dental materials with the possibility of longer-term effects in the oral environment.
Therefore, the aim of this in vitro study was to incorporate two anti-caries agents, Apigenin and tt-Farnesol, alone, together, and combined with fluoride, into resin composite and resin cement to analyze their effect on the virulence of S. mutans biofilms, as quantified by polysaccharide production. In addition, the influence of the additions on the physical-chemical properties of these restorative materials was assessed.
2. MATERIALS AND METHODS
2.1. Anti-caries agents addition
Two anti-caries agents found in Brazilian propolis, Apigenin and tt-Farnesol, were incorporated within a resin composite and a resin cement. Apigenin and tt-Farnesol are commercially available synthesized by Sigma-Aldrich and verified as a standard procedure performed by the company for purity (100% and 98.4%, respectively). The same blend was used for both materials, with only the amount of filler varying between the two (Table 1). The concentration of the anti-caries agents was determined from a preliminary study (concentration able to reduced bacteria biomass without interfering with flexural strength and flexural modulus of the resin composite) [20] and added to the resin composite and resin cement alone, together and combined with fluoride (F, in the form of sodium fluoride). A total of ten materials were produced: (1) resin composite with no additions; (2) resin composite with Apigenin (Api); (3) resin composite with tt-Farnesol (Far); (4) resin composite with Api and Far; (5) resin composite with Api, Far and fluoride; (6) resin cement with no addition; (7) resin cement with Api; (8) resin cement with Far; (9) resin cement with Api and Far and (10) resin cement with Api, Far and fluoride.
Table 1.
Restorative materials composition, additions, manufacturer and lot number
| Manufacturer (lot number) | Resin composite | Resin cement | |
|---|---|---|---|
| BisGMA | Esstech, Inc., Essington, Pennsylvania, USA (688–51) | 15 wt% | 20 wt% |
| TEGDMA | Esstech, Inc., Essington, Pennsylvania, USA (736–51-04) | 15 wt% | 20 wt% |
| Camphorquinone | Sigma-Aldrich, St. Louis, Missouri, United States (10726H0) | 0.6 wt% | 0.6 wt% |
| EDMAB | Across Organics- Thermo Fisher Scientific Inc., Waltham, MA, USA (A0237872) | 1.2 wt% | 1.2 wt% |
| BHT | Sigma-Aldrich, St. Louis, Missouri, United States (37H0294) | 0.05 wt% | 0.05 wt% |
| 0.7 silanated μm barium borosilicate glass | Esstech, Inc., Essington, Pennsylvania, USA (845–09) | 65 wt% | 55 wt% |
| 50 nm silanated fumed silica | Aerosil OX-50. Sun. Medical Co., Ltd – Japan (1228059 3B) | 5 wt% | 5 wt% |
| Addition: Apigenin | Sigma-Aldrich, St. Louis, Missouri, United States (SLBL4733V) | 5 mM | 5 mM |
| Addition: tt-Farnesol | Sigma-Aldrich, St. Louis, Missouri, United States (MKBQ3298V) | 5 mM | 5 mM |
| Addition: Sodium fluoride | J.T. Baker - Thermo Fisher Scientific Inc., Waltham, MA, USA (38716) | 250 ppm | 250 ppm |
2.2. Streptococcus mutans biofilm
A single organism biofilm of S. mutans was used. Disks of resin composites and resin cements were produced (n=6) within a silicon mold (10 mm diameter x 2 mm thick) and polymerized between two glass slides (20 s each side) using a curing unit (Valo, Ultradent Products Inc., South Jordan, UT) placed directly on the glass slide with radiant exposure of 30 J/cm2 (1480 mW/cm2). The surface roughness of each specimen was standardized by uniformly grinding each specimen on #600 silicon carbide paper. The surface roughness (Ra) was measured using a surface roughness tester (TR200, TIME Group, Pittsburgh, PA, USA), and found to be 0.6–0.7 μm for the resin composite and 0.5–0.6 μm for the resin cement. This standardization allowed to place the disks in a vertical position into well-plates for biofilm growth and to avoid the biofilm deposition by gravity on both sides of the disk.
The disks were sterilized with UV light radiation (mercury vapor lamp with >90% radiation at 253.7 nm) for 1 min on both sides (manipulating the disks with sterile materials). This time of sterilization was chosen based on a preliminary study that showed that it had the least deleterious effects on the resin composite [21]. Human whole saliva was collected from one donor, clarified by centrifugation (10000 g, 4°C, 10 min), sterilized and diluted (1:1) in adsorption buffer, and supplemented with the protease inhibitor phenylmethylsulfonyl-fluoride (PMSF) at a final concentration of 1 mmol/L. The disks were placed in a vertical position in 24-well plates, left in clarified saliva for 30 min, and then inoculated with approximately 2 × 106 CFU/mL S. mutans (UA159) in low molecular weight media (buffered ultrafiltered - 10 kDa cutoff membrane; Prep/Scale; Millipore, MA, USA, of 2.5% tryptone and 1.5% yeast extract (with the addition of 4.35 g l−1 of potassium phosphate and 1 g l−1 of MgSO4·7H2O), pH 7.0, and 1% (w/v) sucrose), at 37°C and 5% CO2. The biofilms were grown undisturbed for 24 hours, and then the culture medium was replaced daily during the 5 days of each experiment (total of 115 h of growth). After 5 days, the biofilm was washed three times in sterile saline (0.9%NaCl) to remove non-adhered cells. For biochemical collection data, the biofilms were removed by scraping and subject to ultrasound bath and sonication (20s pulse; output 7W). The biofilm was then processed to count the viable cells (colony forming units CFU/mL), to calculate the dry-weight (biomass), and the amount of polysaccharides (alkali-soluble, intracellular and water-soluble) using colorimetric assays detailed elsewhere [18]. The alkali-soluble and water-soluble polysaccharides were quantified by the phenolsulfuric method using glucose as standard [22], while intracellular polysaccharides were quantified using 0.2% I2/2% KI solution and glycogen as standard [23]. Additional disks were analyzed using scanning electron microscopy (Quanta 200, FEI Company, Hillsboro, OR, USA). The 115 h old biofilms were washed in sterile 0.9% NaCl then fixed with a 4% glutaraldehyde solution (v/v, in phosphate buffered saline PBS, pH 7.4) for 24 h. Biofilms then were dehydrated in ascending ethanol concentration (50, 70, 90, and 100%), dried for 24 h, and sputter-coated with gold-palladium (Denton Vacuum Inc., Moorestown, NJ, USA). Biofilms were analyzed using magnifications of 20x (that covered almost the whole disk) and 5000x.
2.3. CLSM analysis for S. mutans biofilm after three years of water storage
Resin composite and resin cement disks were stored for three years in water to analyze the long-term effect of the incorporations on S. mutans biofilm. Biofilm was grown on top of the disk for 48h, as previously described, and analyzed by a confocal laser scanning microscope (CLSM - Zeiss LSM 780-NLO, Carl Zeiss Microscopy GmbH, Jena, Germany). The EPS was identified via the daily incorporation of Alexa Fluor 647 dextran conjugate (Life Technologies, Carlsbad, CA, USA) to the culture media. In contrast, bacterial cells were identified by incorporation of SYTO 9 (Life Technologies, Carlsbad, CA, USA) to the media 30 min before confocal imaging (absorbance/fluorescence emission maxima of 647/668nm and 485/498 nm, respectively). The biofilm was maintained intact and analyzed using CLSM equipped with an EC Plan-Neofluar 20x water immersion objective lens. Each disk was scanned at five randomly selected points, and a 3D confocal image was generated by obtaining serial images in depth (z stack - 4 μm intervals). The confocal images were analyzed with Zen lite 2012 (Carl Zeiss Microscopy GmbH, Jena, Germany), and the fluorescence intensity was obtained and calculated as a percentage.
2.4. Flexural strength and elastic modulus
For each group, 10 bar-shaped specimens (25 mm x 2 mm x 2 mm) were made using a split stainless-steel mold according to ISO 4049. The bars were polymerized between glass slides with three overlapping exposures (20 s each), to cover the entire specimen, using a curing unit (Valo) with radiant exposure of 30 J/cm2. Specimens were stored dry for 24 h in the dark at room temperature (20°C). The flexural strength and elastic modulus of the samples were tested in 3-point bending on a universal test machine (Q-test, MTS, Eden Prairie, WI) at a crosshead speed of 0.5 mm/min. The flexural strength (FS) in MPa was calculated as FS(σ) = 3Fl/2bh2, where F is the load at fracture (N), l is the span length (20 mm), and b and h are the width and thickness of the specimens in mm, respectively. The elastic modulus (E) was determined from the slope of the initial linear part of the stress-strain curve, calculated as E = FI3/4bh3d, where F is the load at some point on the linear region of the stress-strain curve; d is the slack compensated deflection at load F; and l, b, and h are as defined above.
2.5. Degree of conversion, maximum rate of polymerization and kinetics of conversion by near-IR and extraction of natural products into solution
The addition of any new compound to a material may have detrimental effects on the quality of that material by interfering with certain aspects of the polymerization process. Therefore, it is essential to characterize the extent of polymerization. The experimental dental resin composites and resin cements were placed in a silicon rubber mold (n=3; 5 mm in diameter × 2 mm in thickness for composites and 5 mm x 0.5 mm for cements) sandwiched between two glass slides and then clamped in a holder inside the IR chamber (Nicolet Nexus 6700, Thermo Scientific). The peak area correspondent to the vinyl stretching absorption in the near-IR spectrum (6165 cm−1) [24] was followed during continuous irradiation with the light-curing unit (Valo, Ultradent) for 1 min. The degree of conversion was calculated as a function of the area of the vinyl peak (DC = (1 - final peak area/initial peak area) x 100%). The rate of polymerization was calculated as the first derivative of the “conversion x time” curve, and the maximum value was recorded (Rpmax). The polymerization kinetics were defined by the degree of “conversion x time” curve for 5 min.
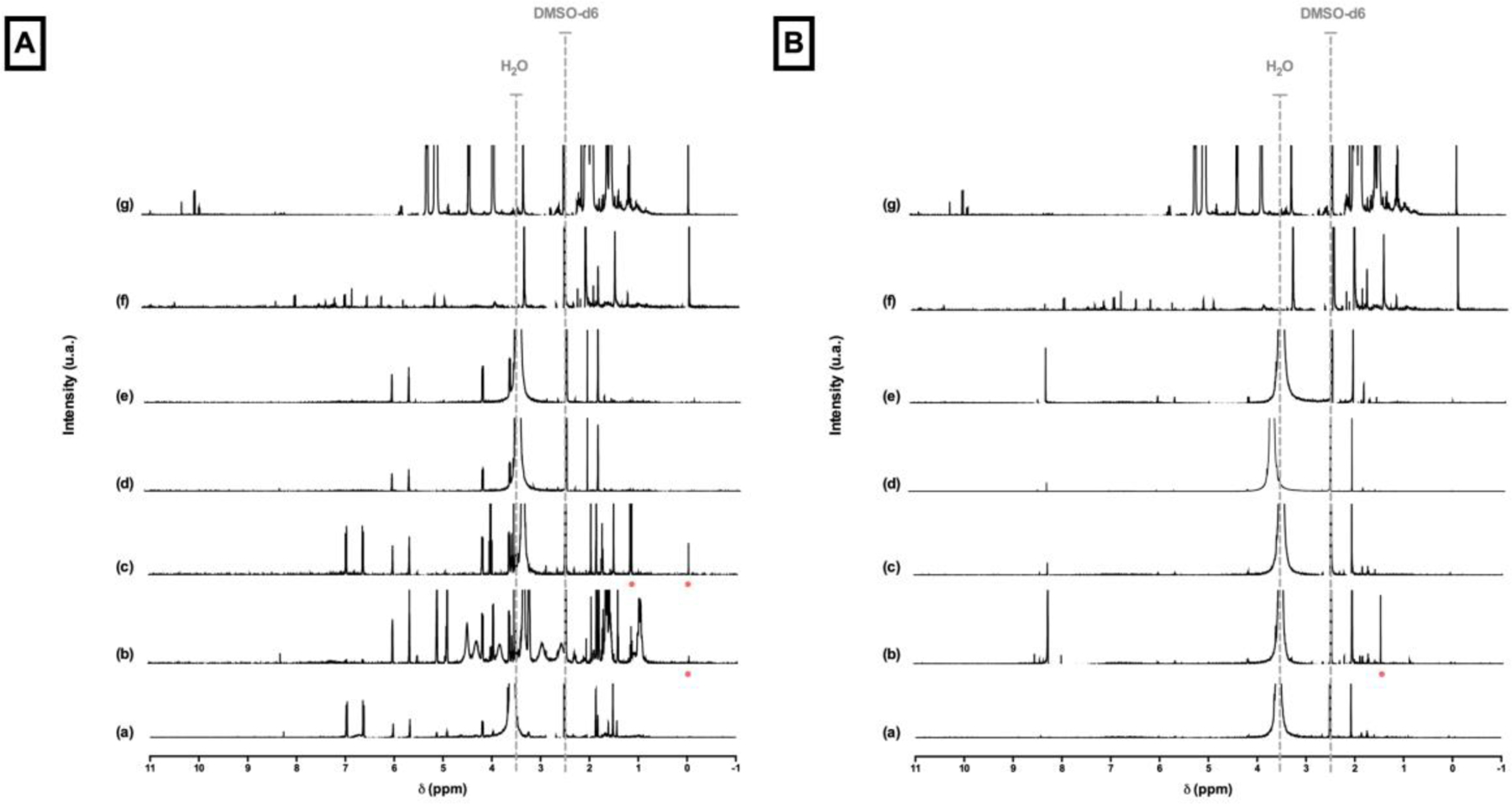
The ability of the compounds to be extracted into water was assessed by NMR (nuclear magnetic resonance). After determining the conversion, the specimens were immersed in 8 mL of deionized water for seven days. The water from the extraction solutions was removed under rotary evaporation to constant mass, DMSO-d6 was added, and 1H-NMR (600 MHz, Varian, Palo Alto, CA) spectra were obtained from the extracts to determine the composition of the sol fraction and to identify both compounds. A 1H-NMR spectrum of Api and Far in DMSO-d6 were also obtained to compare it with extraction solutions spectra.
2.6. Colorimetric evaluation
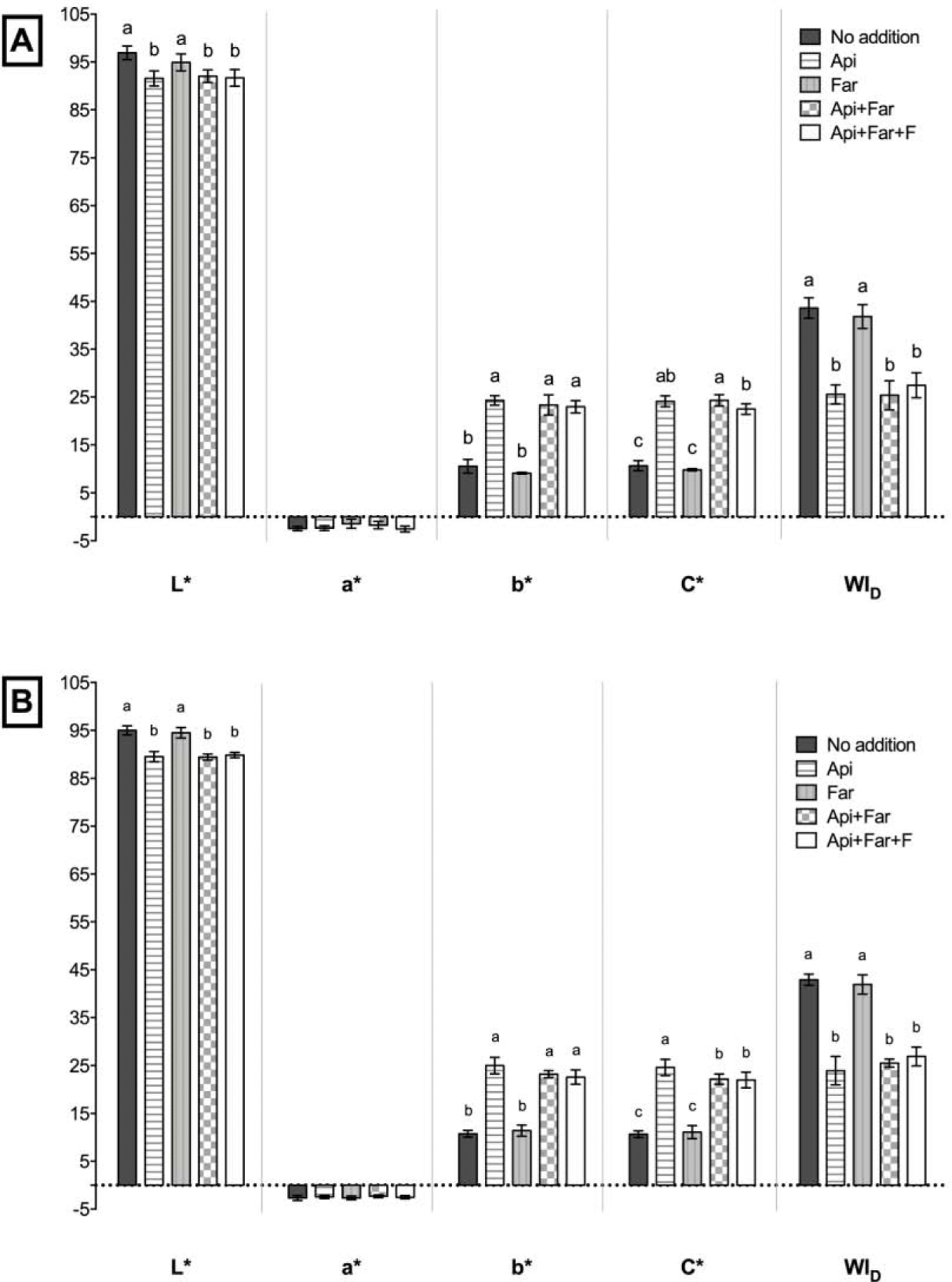
Disks of resin composites and resin cements were produced (n=6) within a silicon mold (10 mm diameter x 2 mm thick) and polymerized between two glass slides (20 s each side), as described above. Four color-measurements were performed using a digital spectrophotometer (EasyShade, Vita Zahnfabrik, Bad Säckingen, Germany) on each sample, and an average was obtained. A white ceramic background was used for color measurements. The L* (black-white axis), a* (red-green axis), b* (yellow-blue axis), and C* (chroma) color parameters were obtained for each group, and the whiteness index for dentistry (WID) was calculated according to the literature [25].
2.7. Statistical Analysis
Statistical analyses were performed by one-way ANOVA followed by Tukey’s multiple-comparison test for bacterial viability (transformed by log), dry-weight, alkali-soluble polysaccharides, intracellular polysaccharides, water-soluble polysaccharides, degree of conversion, maximum polymerization rate, and colorimetric parameters (L*, a*, b*, and WID), for the resin composites and the resin cements separately (α ≤ 0.05). A two-way ANOVA followed by Tukey’s multiple-comparison test was performed for flexural strength and flexural modulus (α ≤ 0.05). Fluorescence intensity was analyzed by generalized linear models. Qualitative analyses were performed for SEM images, curing kinetics, and 1H-NMR spectra.
3. RESULTS
3.1. Streptococcus mutans biofilm
Analyses of the S. mutans biofilms on the resin composite and the resin cement are shown in Figures 1 and 2, respectively. No difference in bacterial viability was observed for the different formulations of the resin composite and resin cement (Figures 1A–I and 2A–I). A decrease of the total amount of biomass (dry weight) was shown with the additions of Api, Api+Far, and Api+Far+F to both the composite (Figure 1A–II) and cement (Figure 1B–II). The additions of Api, Api+F, and Api+Far+F decreased the alkali-soluble (insoluble) polysaccharides, and the intracellular polysaccharides for the composite (Figure 1A–III and 1A–IV) and the cement (Figure 2A–III and 2A–IV). The additions did not interfere with the total amount of soluble polysaccharides produced (Figures 1A–V and 2A–V). Representative images of the biofilm are shown in Figures 1B and 2B. The smoothest biofilm was observed on the top of the disks for the control groups (Figures 1B–I and 2B–I), indicating that the additions promoted a morphological modification to the biofilm growth. At higher magnifications, the compound addition groups presented a more sparse bacterial biofilm, with a higher EPS production on the top of the biofilm (Figures 1B–II and 2B–II).
Figure 1.
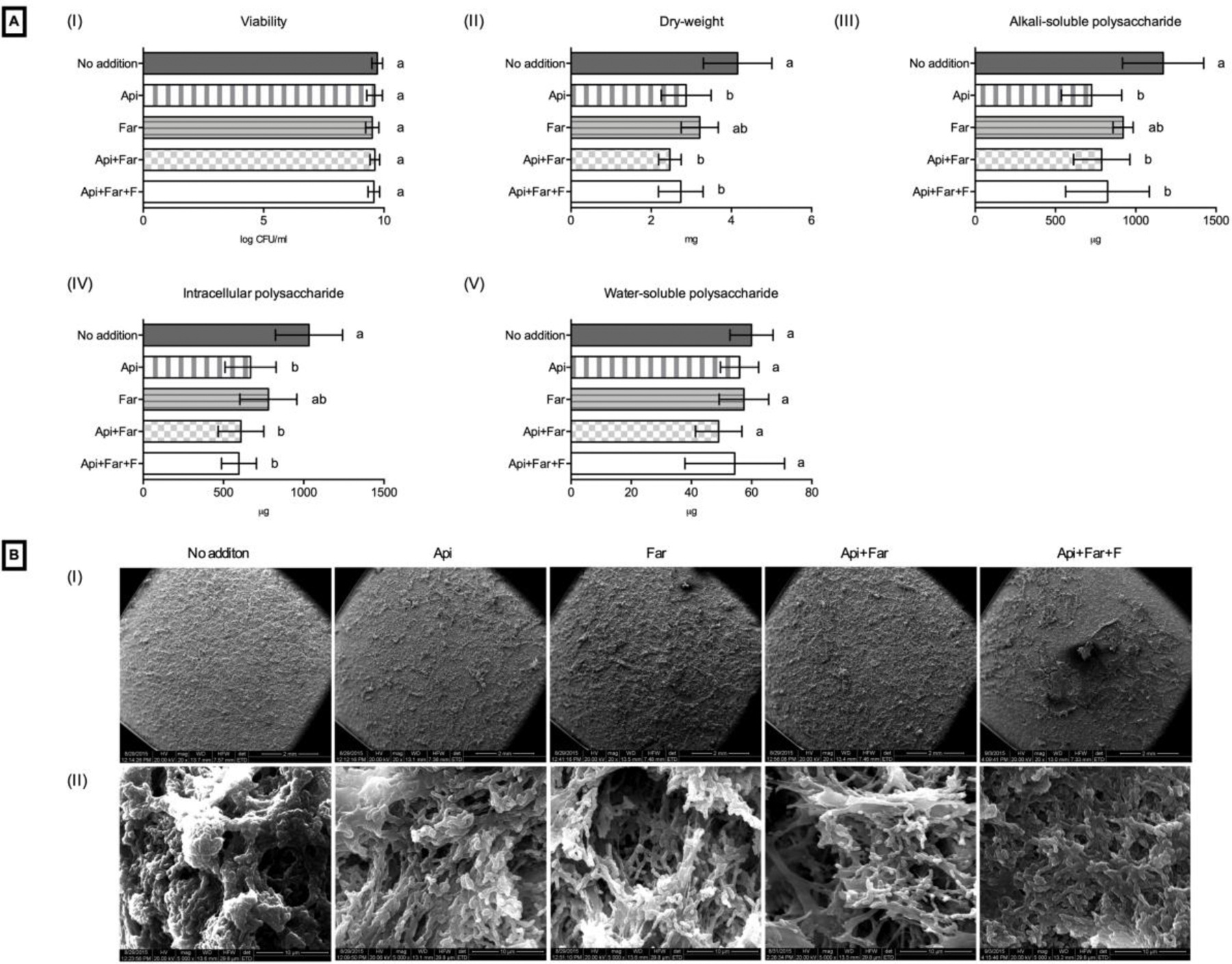
Effect of the addition of test compounds on S. mutans biofilms grown on resin composite disks. (A) Biochemical data: (I) bacterial viability; (II) dry weight; (III) alkali-soluble polysaccharide; (IV) intracellular polysaccharide; (V) water-soluble polysaccharide. Different letters indicate a significant difference (p<0.05). (B) Representative SEM images of biofilm of S. mutans: (I) 20x and (II) 5000x.
Figure 2.
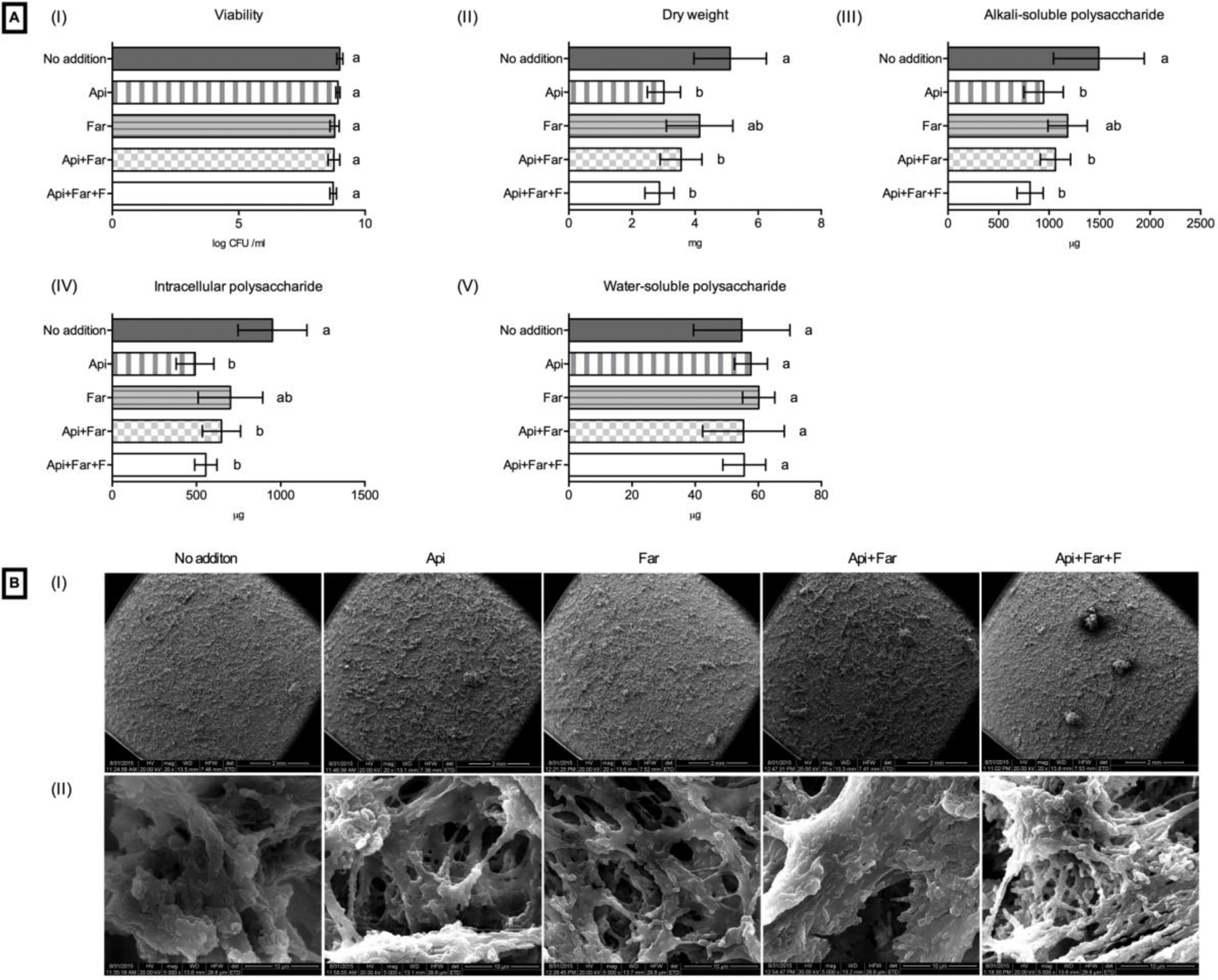
Effect of the addition of test compounds on S. mutans biofilms grown on resin ciments disks. (A) Biochemical data: (I) bacterial viability; (II) dry weight; (III) alkali-soluble polysaccharide; (IV) intracellular polysaccharide; (V) water-soluble polysaccharide. Different letters indicate a significant difference (p<0.05). (B) Representative SEM images of biofilm of S. mutans: (I) 20x and (II) 5000x.
3.2. CLSM analysis for S. mutans biofilm after three years of water storage
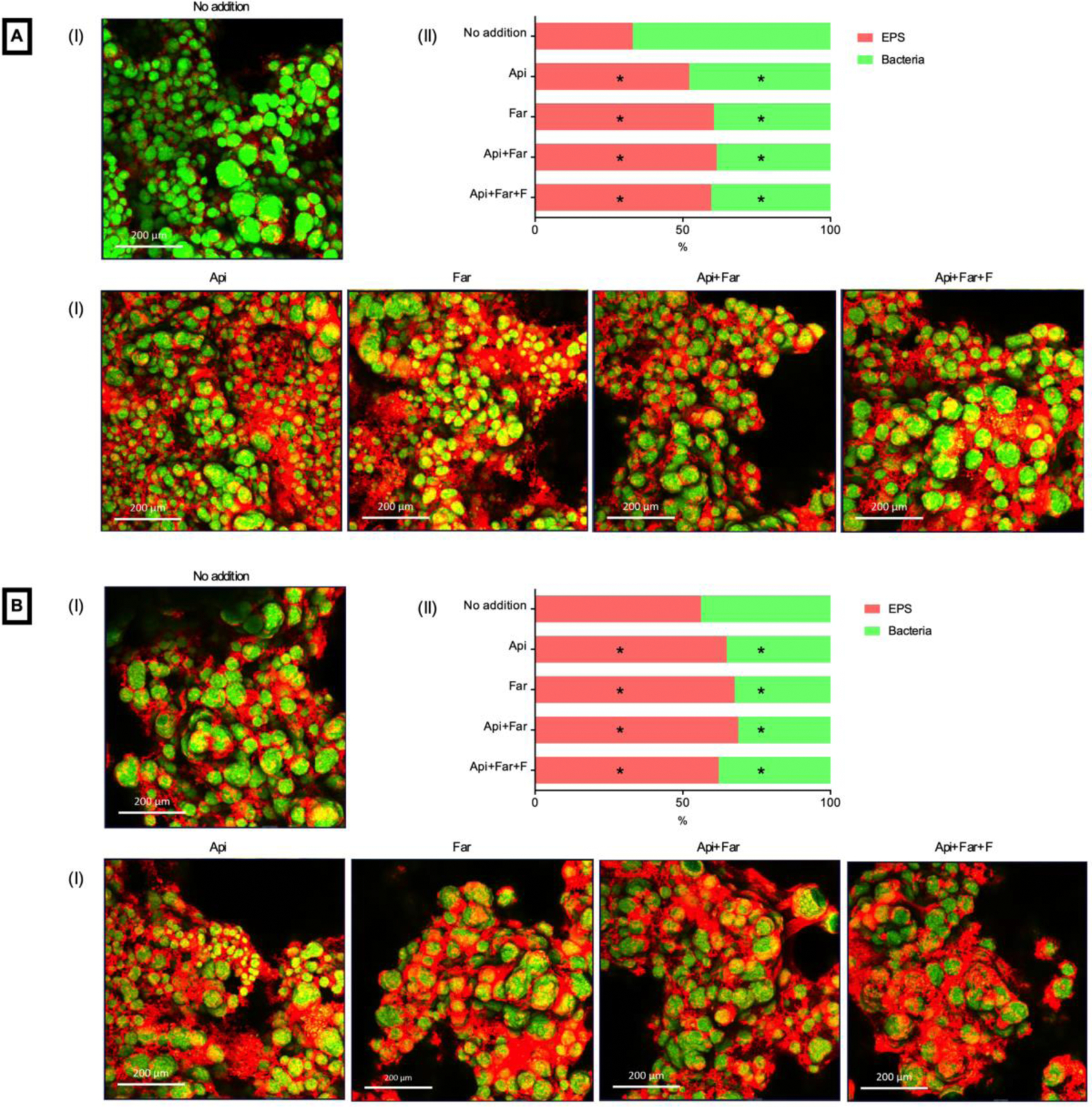
Figures 3A (resin composite) and 3B (resin cement) present representative images in 3D maximum projection (I) and depicts the average fluorescence intensity of EPS and bacteria in percentage for each group tested (II). Images were obtained in a ranging depth of 220 to 350 μm. Due to the high density of biofilm, the biofilm in contact if the composites were not detected. All groups containing the additions, for both materials, presented an increase in EPS on the top of the biofilm after three years of water storage. This EPS difference was more obvious for resin composite materials. The statistical analysis showed significant differences for EPS and bacteria fluorescence for the additions groups compared to no addition group, for both materials (Figure 3A and B - II). However, the reduced bacteria fluorescence does not indicate an antibacterial effect; it only confirms the increased EPS production.
Figure 3.
Curing kinetics. (A) Resin composite. (B) Resin cement.
3.3. Flexural strength and flexural modulus
No decrease in flexural strength or flexural modulus was observed when the compounds were added to the resin composite or resin cement (Table 2). The flexure strength of the composites and cements were similar, except when Far was added to resin cement. The resin cement, which had lower filler concentration, had a lower flexural modulus.
Table 2.
Flexural strength (MPa) and Flexural modulus (GPa) of resin composite and resin cement.
| Resin Composite | Resin Cement | |||
|---|---|---|---|---|
| Groups | Flexural strength | Elastic modulus | Flexural strength | Elastic modulus |
| No addition | 87.5 (9.5) a | 7.9 (0.3) a | 86.2 (9.4) a | 5.5 (0.4) a* |
| Api | 89.3 (12.5) a | 7.8 (0.5) a | 85.6 (8.8) a | 5.5 (0.4) a* |
| Far | 98.3 (15.9) a | 7.9 (0.6) a | 87.4 (14.4) a* | 5.7 (0.5) a* |
| Api+Far | 90.0 (15.0) a | 7.7 (0.6) a | 91.4 (10.0) a | 5.8 (0.5) a* |
| Api+Far+F | 92.7 (12.6) a | 7.5 (0.4) a | 86.4 (11.7) a | 5.6 (0.3) a* |
Means having similar letters are not significantly different (p<0.05). Lower case letters compare groups within the same test and material (column). Asterisk indicates a significant difference compared to resin composite (same test and group).
3.4. Degree of conversion, maximum rate of polymerization and kinetics of conversion by near-IR and extraction of natural products into solution
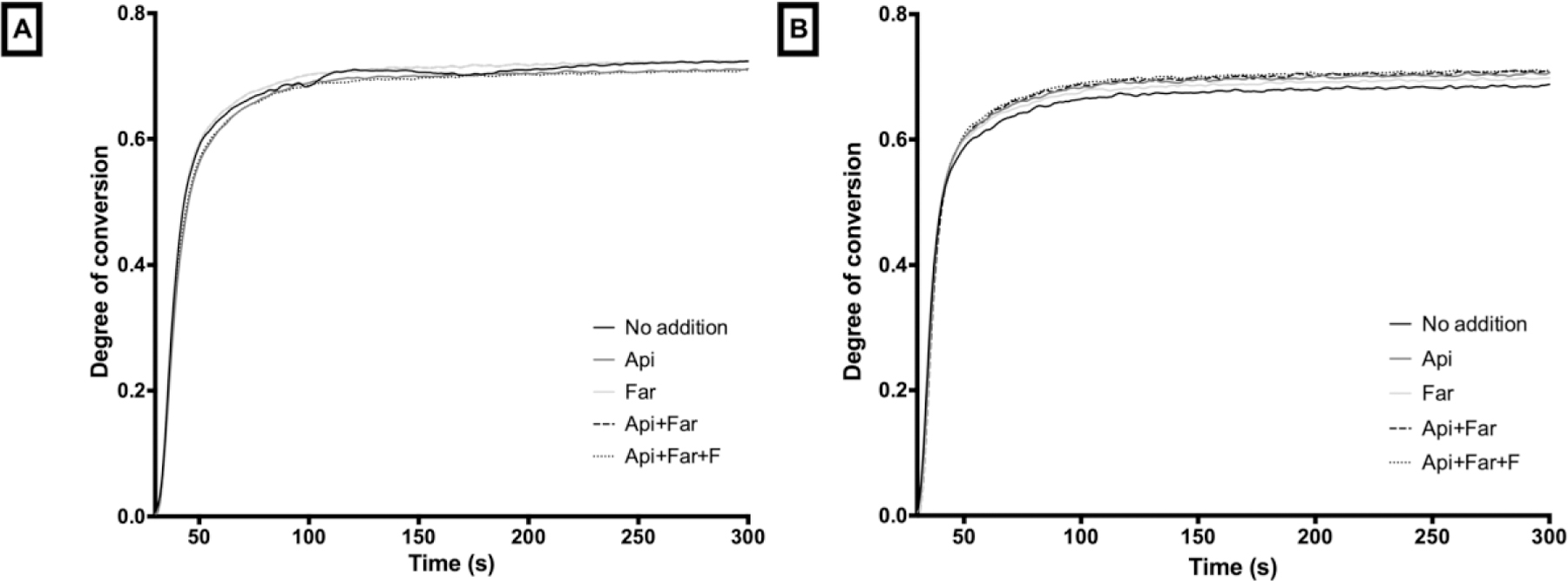
Table 3 depicts the degree of conversion (DC) and the maximum rate of polymerization (Rpmax). Small but statistical differences were obtained for the degree of conversion when Api alone and combined with Far were added into the resin composite, and when Api or Far were added alone and combined into the resin cement. However, no difference was observed for the maximum rate of polymerization and curing kinetics (Figure 4) for both materials, independently of the compound incorporation.
Table 3.
Overall degree of conversion (DC) and maximum rate of polymerization (Rpmax) of resin composites and resin cements.
| Resin composite | Resin cement | |||
|---|---|---|---|---|
| DC (%) | Rpmax (% s−1) | DC (%) | Rpmax (% s−1) | |
| No addition | 71.7 (0.6) a | 11.5 (0.9) a | 68.3 (0.1) b | 11.1 (0.9) a |
| Api | 69.9 (0.3) b | 12.7 (0.8) a | 69.8 (0.5) a | 11.3 (0.9) a |
| Far | 70.6 (0.5) ab | 11.9 (0.5) a | 69.8 (0.2) a | 11.6 (1.4) a |
| Api+Far | 69.9 (0.3) b | 12.6 (2.5) a | 69.0 (0.2) b | 10.3 (1.5) a |
| Api+Far+F | 70.5 (0.6) ab | 13.0 (0.8) a | 69.9 (0.2) a | 11.5 (1.1) a |
Means having similar letters are not significantly different (p<0.05).
Figure 4.
1H-NMR spectra in DMSO-d6. (A) Resin composite. (B) Resin cement. (a) No addition. (b) Api addition. (c) Far addition. (d) Api+Far addition. (e) Api+Far+F addition. (f) Api spectrum. (g) Far spectrum. 2.5 ppm: DMSO-d6 peak. Around 3.5: remaining water peak. Red dots: matching peaks with Api or Far spectra.
1H-NMR spectra are presented in Figure 5. A few peaks can be related to Api or Far extracted in water only for Api and Api+Far additions (Figure 5A - b and c) of resin composite material (red dots) and Api addition (Figure 5B – b) of resin cement material. No extraction of either compound was observed after 7 days in water. The other peaks presented may be related to unreacted monomer extracted in the water.
Figure 5.
Colorimetric results. (A) Resin composite. (B) resin cement. Different letters indicate a significant difference (p<0.05).
3.5. Colorimetric evaluation
Colorimetric parameters were reported in Figure 6A and B. For both materials tested, the incorporation of Api (alone or combined), decreased the L* and WID while increased the b*and C* colorimetric parameters; indicating that Api can increase the yellowness of the composites.
Figure 6.
CLSM of biofilm of S. mutans. (A) Resin composite. (B) Resin cement. (I) Representative maximum projection of the 3D biofilm images. (II) Fluorescence intensity percentage of each group. Asterisk: indicate significant statistical difference compared to the no addition group.
4. DISCUSSION
EPS and the acidification of the biofilm matrix are considered primary targets for a highly specific chemotherapeutic intervention to reduce cariogenic biofilms [11]. Therapeutic agents that act on the virulence properties of the pathogens without necessarily killing the target organism may avoid alterations of the resident microbiota and may offer reduced selection pressure for drug resistance [11, 26]. Apigenin and tt-Farnesol, two naturally occurring compounds, have been described as anti-caries agents that may alter the virulence of S. mutans, thus providing a new approach to antimicrobial therapy for dental caries [15, 17, 18].
In this study, a decrease in bacterial viability was found only for the resin cement when both compounds were combined with fluoride (Api+Far+F). In previous studies, this combination also statistically decreased the viability of S. mutans [17]. According to Koo et al. (2002), Apigenin has virtually no antibacterial activity, and tt-Farnesol shows bactericidal activity against planktonic cells of mutans streptococci, but in biofilms, its effect is less evident. Also, to disrupt the streptococcus cell membrane, a higher concentration would be necessary (>10 mM) [27]. Therefore, it can be considered that the agents do not have direct bactericidal activity, as evidenced by the minimal effect on bacterial viability in the biofilm.
On the other hand, Api, Api+Far, and Api+Far+F diminished the further accumulation of S. mutans biofilms compared to the control group, for both restorative materials (31%, 41% and 34% reduction of biomass, respectively, for resin composite; and 41%, 30% and 44% for the resin cement). As has been shown in the literature, when in solution, the compounds together and when combined with fluoride caused a decrease in biofilm biomass of 40.7% and 50.6%, respectively [17]. Though the results shown in this study are slightly lower, likely due to the incorporation of the compounds into the polymeric resin, requiring them to be released by diffusion or to be in direct contact with biofilm, these anti-caries agents were able to show a significant effect on the quality of the biofilm. The decrease of biomass without reducing the bacterial viability suggests a modification of the biofilm structure, most likely a reduction in EPS composition, which was confirmed by the chemical analysis of specific polysaccharides.
For both restorative materials, the addition of Api, Api+Far, and Api+Far+F reduced the amount of insoluble polysaccharides and intracellular polysaccharides. S. mutans produce at least three Gtfs: GtfB that synthesizes mostly insoluble glucans, GtfC that synthesizes a mixture of insoluble and soluble glucan, and GtfD that synthesizes mostly soluble glucans [19, 28, 29]. The insoluble glucans synthesized by surface-adsorbed GtfB and GtfC provide binding sites for the establishment of S. mutans on the solid surface and facilitate its coadherence with other bacterial cells, mediating the transition from initial cell attachment to microcolony and multi-microcolony aggregates, in the presence of sucrose [14, 30]. The reduction in insoluble polysaccharides could influence the pathogenesis of the bacteria by reducing their accumulation and the binding of the microorganisms (mutans streptococci and lactobacilli) to the tooth surface, disrupting the integrity, stability, and diffusion properties of the biofilm [15, 16, 29].
Besides, the decrease of intracellular polysaccharides can reduce the exposure of the tooth surface to the organic acids. The intracellular polysaccharide is an endogenous source of carbohydrate that can be metabolized when exogenous sources are depleted in the oral cavity. As a result, tooth surfaces experience prolonged exposure to the acidic production and acidification within the biofilm, even in the short-term absence of an external nutrient source, leading to enamel dissolution and initiating the pathogeneses of dental caries [15, 28, 31]. The reduction of both insoluble and intracellular polysaccharides could affect the ability of S. mutans to colonize the tooth surface, thus reducing its chances of becoming the dominant bacteria and expressing its virulence [15]. Other beneficial effects from the reduction of polysaccharides could be the facilitation of mechanical detachment of the bacteria since the exopolysaccharides are critical determinants for the mechanical stability of biofilms [32, 33].
An alteration on the top of the biofilms was also observed in lower magnifications of SEM images. However, in higher magnifications, the EPS production seems to be greater than in the no additions groups. These SEM results suggest that the compounds can reduce the virulence of S. mutans only on the base of the biofilm (confirmed by biochemical analysis for Api addition). Moreover, the lack of water-extraction of the compounds in NMR spectra may indicate that the compounds’ effect is limited to the biofilm directly in contact with the composite surfaces. Also, the low amount of extract of the compounds could interfere with the metabolic activity on the top of the biofilm. Dong L et al. (2012) demonstrated that the expression of gtfB, luxS, comD, and comE in S. mutans biofilm was significantly upregulated when treated with sub-MICs of antimicrobial agents (chlorhexidine, tea polyphenols, and sodium fluoride) compared to a control group [34]. We suggest that the compounds in lower concentrations may also upregulate gtfB (responsible for synthesizing insoluble glucans) and increase the amount of EPS on the top of the biofilm.
According to Koo et al. (2009), the combinations of these compounds could affect the pathogenicity of S. mutans within the biofilm by: (1) reducing the activity of GtfB and GtfC and consequently inhibition of insoluble glucan synthesis; (2) modulating the gtfBC gene expression at the transcriptional level; (3) disrupting ΔpH across the cell membrane and inducing starvation stress; and (4) inhibiting synthesis-accumulation of IPS [11]. However, in this study, the combinations of the compounds and the combination with fluoride did not increase the anti-caries effect, no difference was observed in the amount of polysaccharides between the groups. Also, tt-Farnesol alone showed no anti-caries effect compared to the control group, and it can be suggested that tt-Farnesol can be trapped inside the polymeric structure and is difficult to be diffused out, thus leaving it with only a slight effect on S. mutans virulence. In a previous study, the addition of Apigenin alone to a dental adhesive also showed better results in reducing the virulence of S. mutans [35].
However, after three years of water-storage, this reduction in the EPS matrix was not observed on the top of S. mutans biofilm grown on composites disks. Considering the higher EPS matrix in CFLM images compared to the no addition groups, it can be suggested that both compounds still promote some effect on the biofilm formation, due to the similarity in the pattern observed in the SEM images (no water storage). Although the lack of virulence reduction by the compounds after long-term water storage, the EPS quantification in contact with the composite disks, and the biochemical quantification of total EPS are still necessary.
Besides the beneficial effect demonstrated by incorporating anti-caries agents into the composite and cement materials, no negative effect was observed for their flexural strength and elastic modulus, degree of conversion, and maximum polymerization rate. The resin cement showed lower elastic modulus compared to the resin composite due to the lower filler amount (70% and 60%, respectively) [36]. A preliminary study showed a decrease in elastic modulus when tt-Farnesol was added into a resin composite in a concentration of 50 mM [20]. The statistical difference for the degree of conversion is correlated to the low standard deviation obtained; besides, the maximum polymerization rate and curing kinetics were similar between groups, indicating that the compounds do not diminish the polymerization mechanism. Also, the cross-linking density of the polymer formed is more important for the mechanical properties than the degree of conversion itself [37, 38].
The incorporation of antibacterial agents to composites raises concerns regarding water degradation [39]. Although both compounds are poorly water-soluble [40, 41], water degradation can still affect the resin-matrix over time, and the presence of non-copolymerized compounds may increase the composite degradation. We suggest that the combination of both compounds and fluoride may enhance the water-degradation in the long-term. As no additional effect regarding the reduction of S. mutans biofilm virulence was obtained, this combined formulation should be discouraged. However, little is known if the lower amount of Api added (5 mM) alone is able to reduce the physicochemical properties over time. Furthermore, the increase in yellowness when Api is incorporated into the composites, over the perceptibility and acceptability thresholds [42], might only affect anterior restorations where maximum esthetics is important. Thus, it can be suggested that the small concentrations of the compounds were not sufficient to impair the immediate physicochemical properties of the restorative materials tested. The shelf-life of the proposed formulations, the Api and Far kinetics release, water sorption, water solubility, and its effect on physicochemical properties after a long-term water storage awaits further evaluation.
CONCLUSION
Within the limitations of this in vitro study, it can be concluded that: (1) the addition of Apigenin and tt-Farnesol to resin-based composite and cement materials can significantly decrease the amount of biomass and polysaccharides of an S. mutans biofilm, acting on the main virulence factors of S. mutans without killing the target organism; (2) better results regarding the reduction of virulence of S. mutans were found with the addition of Api, Api+Far, and Api+Far+F to both materials; (3) the addition of fluoride or the combination of the compounds did not increase the reduction in EPS matrix, compared to Apigenin alone, for both materials; (4) after three years of water-storage the compounds incorporated into composites promoted S. mutans biofilm morphological alterations; (5) the additions did not jeopardize the physicochemical properties of the composite tested; however, Api increase the composite yellowness.
Significance:
Api, alone and combined, reduced the expression of virulence of S. mutans without jeopardizing the physicochemical properties of the composites.
Highlights.
Apigenin added to composites decreases S. mutans biofilm virulence.
Apigenin containing composites reduced S. mutans biofilm biomass, insoluble polysaccharides, and intracellular polysaccharides.
There was no adverse effect of apigenin and tt-farnesol addition on composites physicochemical properties.
ACKNOWLEDGMENTS
This research was supported by NIH/NIDCR K02 DE025280 and Brazilian Financial Agencies: CNPq: 140698/2013–2, 310522/2015–3, and 150813/2017–1, FAPESP: 2013/22823–9 and 2014/15543–0, and CAPES 001. The authors would like to thank Dr. Kristen Lampi and Satin Salehi (in memoriam) for assistance with the bacterial experiments and shared knowledge.
Abbreviations
- EPS
extracellular polysaccharides
- Gtf
glucosyltransferase
- BisGMA
bisphenol A-glycidyl methacrylate
- TEGDMA
triethylene glycol dimethacrylate
- EDMAB
Ethyl-4-dimethylamino benzoate
- BHT
butylatedhydroxytoluene
- Api
apigenin
- Far
tt-farnesol
- F
fluoride
- CFU
colony forming units
- SEM
scanning electron microscope
- CLSM
confocal laser scanning microscopy
- FS
flexural strength
- E
elastic modulus
- DC
degree of conversion
- Rpmax
maximum rate of polymerization
- NMR
nuclear magnetic resonance
- DMSO-d6
dimethyl sulfoxide deuterated
- WID
whiteness index for dentistry
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interest
None.
The authors declare no conflict of interest.
REFERENCES
- [1].Lemos JA, Abranches J, Burne RA. Responses of cariogenic streptococci to environmental stresses. Curr Issues Mol Biol, 2005;7:95–107. [PubMed] [Google Scholar]
- [2].Takahashi N, Nyvad B. The role of bacteria in the caries process: ecological perspectives. J Dent Res, 2011;90:294–303. 10.1177/0022034510379602 [DOI] [PubMed] [Google Scholar]
- [3].Galvao LC, Rosalen PL, Rivera-Ramos I, Franco GC, Kajfasz JK, Abranches J, et al. Inactivation of the spxA1 or spxA2 gene of Streptococcus mutans decreases virulence in the rat caries model. Mol Oral Microbiol, 2016;32:142–53. 10.1111/omi.12160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Nakamura M, Oyane A, Shimizu Y, Miyata S, Saeki A, Miyaji H. Physicochemical fabrication of antibacterial calcium phosphate submicrospheres with dispersed silver nanoparticles via coprecipitation and photoreduction under laser irradiation. Acta Biomater, 2016;46:299–307. 10.1016/j.actbio.2016.09.015 [DOI] [PubMed] [Google Scholar]
- [5].Andre CB, Gomes BP, Duque TM, Stipp RN, Chan DC, Ambrosano GM, et al. Dentine bond strength and antimicrobial activity evaluation of adhesive systems. J Dent, 2015;43:466–75. 10.1016/j.jdent.2015.01.004 [DOI] [PubMed] [Google Scholar]
- [6].Cocco AR, Rosa WL, Silva AF, Lund RG, Piva E. A systematic review about antibacterial monomers used in dental adhesive systems: Current status and further prospects. Dent Mater, 2015;31:1345–62. 10.1016/j.dental.2015.08.155 [DOI] [PubMed] [Google Scholar]
- [7].André CB, Gomes BPFA, Duque TM, Rosalen PL, Chan DCN, Ambrosano GMB, et al. Antimicrobial activity, effects on Streptococcus mutans biofilm and interfacial bonding of adhesive systems with and without antibacterial agent. Inter J Adhes Adhes, 2017;72:123–9. 10.1016/j.ijadhadh.2016.10.011 [DOI] [Google Scholar]
- [8].Falsetta ML, Klein MI, Lemos JA, Silva BB, Agidi S, Scott-Anne KK, et al. Novel antibiofilm chemotherapy targets exopolysaccharide synthesis and stress tolerance in Streptococcus mutans to modulate virulence expression in vivo. Antimicrob Agents Chemother, 2012;56:6201–11. 10.1128/AAC.01381-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].McDonnell G, Russell AD. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev, 1999;12:147–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Freires IA, Bueno-Silva B, Galvao LC, Duarte MC, Sartoratto A, Figueira GM, et al. The Effect of Essential Oils and Bioactive Fractions on Streptococcus mutans and Candida albicans Biofilms: A Confocal Analysis. Evid Based Complement Alternat Med, 2015;2015:871316. 10.1155/2015/871316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Koo H, Jeon JG. Naturally occurring molecules as alternative therapeutic agents against cariogenic biofilms. Adv Dent Res, 2009;21:63–8. 10.1177/0895937409335629 [DOI] [PubMed] [Google Scholar]
- [12].Kim D, Hwang G, Liu Y, Wang Y, Singh AP, Vorsa N, et al. Cranberry Flavonoids Modulate Cariogenic Properties of Mixed-Species Biofilm through Exopolysaccharides-Matrix Disruption. PLoS One, 2015;10:e0145844. 10.1371/journal.pone.0145844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bowen WH, Koo H. Biology of Streptococcus mutans-derived glucosyltransferases: role in extracellular matrix formation of cariogenic biofilms. Caries Res, 2011;45:69–86. 10.1159/000324598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Koo H, Falsetta ML, Klein MI. The Exopolysaccharide Matrix: A Virulence Determinant of Cariogenic Biofilm. J Dent Res, 2013;92:1065–73. 10.1177/0022034513504218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Jeon JG, Klein MI, Xiao J, Gregoire S, Rosalen PL, Koo H. Influences of naturally occurring agents in combination with fluoride on gene expression and structural organization of Streptococcus mutans in biofilms. BMC Microbiol, 2009;9:228. 10.1186/1471-2180-9-228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lei L, Yang Y, Mao M, Li H, Li M, Yang Y, et al. Modulation of Biofilm Exopolysaccharides by the Streptococcus mutans vicX Gene. Front Microbiol, 2015;6:1432. 10.3389/fmicb.2015.01432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Koo H, Schobel B, Scott-Anne K, Watson G, Bowen WH, Cury JA, et al. Apigenin and tt-farnesol with fluoride effects on S. mutans biofilms and dental caries. J Dent Res, 2005;84:1016–20. 10.1177/154405910508401109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Koo H, Hayacibara MF, Schobel BD, Cury JA, Rosalen PL, Park YK, et al. Inhibition of Streptococcus mutans biofilm accumulation and polysaccharide production by apigenin and tt-farnesol. J Antimicrob Chemother, 2003;52:782–9. 10.1093/jac/dkg449 [DOI] [PubMed] [Google Scholar]
- [19].Koo H, Rosalen PL, Cury JA, Park YK, Bowen WH. Effects of compounds found in propolis on Streptococcus mutans growth and on glucosyltransferase activity. Antimicrob Agents Chemother, 2002;46:1302–9. 10.1128/AAC.46.5.1302-1309.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Andre CB, Rosalen PL, Pfeifer C, Giannini M, Ferracane JL. Properties of resin composites containing natural antimicrobial components. Dent Mater, 2015;31:e29. [Google Scholar]
- [21].Andre CB, Dos Santos A, Pfeifer CS, Giannini M, Girotto EM, Ferracane JL. Evaluation of three different decontamination techniques on biofilm formation, and on physical and chemical properties of resin composites. J Biomed Mater Res B Appl Biomater, 2018;106:945–53. 10.1002/jbm.b.33907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Dubois M, Gilles KA, Hamilton JK, Rebers Pt, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem, 1956;28:350–6. [Google Scholar]
- [23].DiPersio JR, Mattingly SJ, Higgins ML, Shockman GD. Measurement of intracellular iodophilic polysaccharide in two cariogenic strains of Streptococcus mutans by cytochemical and chemical methods. Infect Immun, 1974;10:597–604. 10.1128/IAI.10.3.597-604.1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Stansbury JW, Dickens SH. Determination of double bond conversion in dental resins by near infrared spectroscopy. Dent Mater, 2001;17:71–9. 10.1016/s0109-5641(00)00062-2 [DOI] [PubMed] [Google Scholar]
- [25].Perez Mdel M, Ghinea R, Rivas MJ, Yebra A, Ionescu AM, Paravina RD, et al. Development of a customized whiteness index for dentistry based on CIELAB color space. Dent Mater, 2016;32:461–7. 10.1016/j.dental.2015.12.008 [DOI] [PubMed] [Google Scholar]
- [26].Cegelski L, Marshall GR, Eldridge GR, Hultgren SJ. The biology and future prospects of antivirulence therapies. Nat Rev Microbiol, 2008;6:17–27. 10.1038/nrmicro1818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Koo H, Pearson SK, Scott-Anne K, Abranches J, Cury JA, Rosalen PL, et al. Effects of apigenin and tt-farnesol on glucosyltransferase activity, biofilm viability and caries development in rats. Oral Microbiol Immunol, 2002;17:337–43. 10.1034/j.1399-302x.2002.170602.x [DOI] [PubMed] [Google Scholar]
- [28].Koo H, Xiao J, Klein MI, Jeon JG. Exopolysaccharides produced by Streptococcus mutans glucosyltransferases modulate the establishment of microcolonies within multispecies biofilms. J Bacteriol, 2010;192:3024–32. 10.1128/JB.01649-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Krzysciak W, Jurczak A, Koscielniak D, Bystrowska B, Skalniak A. The virulence of Streptococcus mutans and the ability to form biofilms. Eur J Clin Microbiol Infect Dis, 2014;33:499–515. 10.1007/s10096-013-1993-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Guo L, McLean JS, Lux R, He X, Shi W. The well-coordinated linkage between acidogenicity and aciduricity via insoluble glucans on the surface of Streptococcus mutans. Sci Rep, 2015;5:18015. 10.1038/srep18015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Murata RM, Branco-de-Almeida LS, Franco EM, Yatsuda R, dos Santos MH, de Alencar SM, et al. Inhibition of Streptococcus mutans biofilm accumulation and development of dental caries in vivo by 7-epiclusianone and fluoride. Biofouling, 2010;26:865–72. 10.1080/08927014.2010.527435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hwang G, Klein MI, Koo H. Analysis of the mechanical stability and surface detachment of mature Streptococcus mutans biofilms by applying a range of external shear forces. Biofouling, 2014;30:1079–91. 10.1080/08927014.2014.969249 [DOI] [PubMed] [Google Scholar]
- [33].Hwang G, Liu Y, Kim D, Sun V, Aviles-Reyes A, Kajfasz JK, et al. Simultaneous spatiotemporal mapping of in situ pH and bacterial activity within an intact 3D microcolony structure. Sci Rep, 2016;6:32841. 10.1038/srep32841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Dong L, Tong Z, Linghu D, Lin Y, Tao R, Liu J, et al. Effects of sub-minimum inhibitory concentrations of antimicrobial agents on Streptococcus mutans biofilm formation. Int J Antimicrob Agents, 2012;39:390–5. 10.1016/j.ijantimicag.2012.01.009 [DOI] [PubMed] [Google Scholar]
- [35].Andre CB, Rosalen PL, Galvao LCC, Fronza BM, Ambrosano GMB, Ferracane JL, et al. Modulation of Streptococcus mutans virulence by dental adhesives containing anti-caries agents. Dent Mater, 2017;33:1084–92. 10.1016/j.dental.2017.07.006 [DOI] [PubMed] [Google Scholar]
- [36].Lohbauer U, Belli R, Ferracane JL. Factors involved in mechanical fatigue degradation of dental resin composites. J Dent Res, 2013;92:584–91. 10.1177/0022034513490734 [DOI] [PubMed] [Google Scholar]
- [37].Dauvillier BS, Feilzer AJ, De Gee AJ, Davidson CL. Visco-elastic parameters of dental restorative materials during setting. J Dent Res, 2000;79:818–23. 10.1177/00220345000790030601 [DOI] [PubMed] [Google Scholar]
- [38].Freitas PH, André CB, Fronza BM, Giannini M, Rosalen PL, Consani S, et al. Physicochemical properties, metalloproteinases inhibition, and antibiofilm activity of doxycycline-doped dental adhesive. J Dent, 2020:103550. 10.1016/j.jdent.2020.103550 [DOI] [PubMed] [Google Scholar]
- [39].Xu X, Wang Y, Liao S, Wen ZT, Fan Y. Synthesis and characterization of antibacterial dental monomers and composites. J Biomed Mater Res B Appl Biomater, 2012;100:1151–62. 10.1002/jbm.b.32683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Zhang J, Liu D, Huang Y, Gao Y, Qian S. Biopharmaceutics classification and intestinal absorption study of apigenin. Int J Pharm, 2012;436:311–7. 10.1016/j.ijpharm.2012.07.002 [DOI] [PubMed] [Google Scholar]
- [41].Wang Z, Chen HT, Roa W, Finlay W. Farnesol for aerosol inhalation: nebulization and activity against human lung cancer cells. J Pharm Pharm Sci, 2003;6:95–100. [PubMed] [Google Scholar]
- [42].Paravina RD, Perez MM, Ghinea R. Acceptability and perceptibility thresholds in dentistry: A comprehensive review of clinical and research applications. J Esthet Restor Dent, 2019;31:103–12. 10.1111/jerd.12465 [DOI] [PubMed] [Google Scholar]


