Abstract
Lyme disease results from infection of humans with the spirochete Borrelia burgdorferi. The first and most common clinical manifestation is the circular, inflamed skin lesion referred to as erythema migrans; later manifestations result from infections of other body sites. Laboratory diagnosis of Lyme disease can be challenging in patients with erythema migrans because of the time delay in the development of specific diagnostic antibodies against Borrelia. Reliable blood biomarkers for the early diagnosis of Lyme disease in patients with erythema migrans are needed. Here, we performed selected reaction monitoring, a targeted mass spectrometry-based approach, to measure selected proteins that 1) are known to be predominantly expressed in one organ (i.e., organ-specific blood proteins) and whose blood concentrations may change as a result of Lyme disease, or 2) are involved in acute immune responses. In a longitudinal cohort of 40 Lyme disease patients and 20 healthy controls, we identified 10 proteins with significantly altered serum levels in patients at the time of diagnosis, and we also developed a 10-protein panel identified through multivariate analysis. In an independent cohort of patients with erythema migrans, six of these proteins, APOA4, C9, CRP, CST6, PGLYRP2 and S100A9, were confirmed to show significantly altered serum levels in patients at time of presentation. Nine of the 10 proteins from the multivariate panel were also verified in the second cohort. These proteins, primarily innate immune response proteins or proteins specific to liver, skin or white blood cells, may serve as candidate blood biomarkers requiring further validation to aid in the laboratory diagnosis of early Lyme disease.
Keywords: Lyme disease, organ-specific protein, acute phase proteins, proteomics, selective reaction monitoring, SRM, tick-borne disease
Graphical Abstract
INTRODUCTION
Lyme disease (LD), or Lyme borreliosis, is the most common tick-borne infectious disease in North America. According to estimates from the Centers for Disease Control and Prevention (CDC), LD accounted for 82% of all tick-borne disease reports during 2004–2016 1. New cases of LD in the United States exceed 300,000 per year 2–4, and the geographic range of LD has been expanding recently in this country as well 1. The most common (70–90% of cases) and earliest clinical manifestation of LD is the skin lesion referred to as erythema migrans (EM), which typically occurs at the tick bite site at which the etiologic agent Borrelia burgdorferi was inoculated into the skin. B. burgdorferi may spread hematogenously from the initial site to other skin sites resulting in additional EM skin lesions, and/or resulting in the infection of other organs including the joints, nervous system and heart 5, 6. About 27–40% of LD patients with EM have abnormal liver function tests, but the cause of these abnormalities has not been determined 7–9.
Early diagnosis and treatment of patients with EM skin lesions is critical for preventing the development of extracutaneous manifestations of LD, such as Lyme arthritis and Lyme neuroborreliosis 6, 10. Due to a delay in the development of specific antibodies, laboratory diagnosis of LD in patients with or without EM often lacks optimal sensitivity during the first few weeks of infection—the time at which broad spectrum antibiotic treatment should be initiated 11. Direct diagnostic testing by culture or polymerase chain reaction (PCR) have several limitations including lower than ideal sensitivity, false negative test results (e.g., due to mutations in the sites of PCR amplification) and absence of effective assays that have been approved by the United States Food and Drug Administration (FDA).
There are currently over 40 diagnostic tests for LD in humans, which are available either commercially or from private laboratories 11. All of them fall into two main categories; indirect methods that detect the host immune responses against the pathogen, mainly the detection of IgM/IgG antibodies against B. burgdorferi, and direct methods to detect the pathogen itself.
The CDC-recommended two-step serological testing algorithm—which detects the presence of IgG and/or IgM antibodies against B. burgdorferi using first an enzyme immunoassay (typically an ELISA) and then Western blotting—is currently the most widely applied indirect approach to test for LD 12, 13. It performs well in later-stage disseminated LD but is not a sensitive test for early, localized LD, since IgM antibodies usually take 2 to 3 weeks to develop to detectable levels 11, 14. In addition, prompt antibiotic treatment of some patients with EM may prevent the development of diagnostic levels of anti-B. burgdorferi antibodies during the convalescent phase. Furthermore, both IgG and IgM antibodies specific for B. burgdorferi may persist long after symptoms have disappeared; therefore, their presence may not distinguish active current disease from historic earlier exposures 11. Since the 1990s, the specificity of first-tier serologic tests has improved slightly with the introduction of the C6 test 15 and the OspC7 ELISA 16. However, these newer tests still target IgG and/or IgM antibodies and do not improve the sensitivity or specificity to a satisfactory level for the early stage of disease 11, 17.
The main direct detection modalities in use are B. burgdorferi spirochete cultures and detection of bacterial DNA by PCR, but both of these methods can suffer from low sensitivity. Although the isolation and culturing of B. burgdorferi spirochetes is the gold standard to confirm diagnosis, culture is not a routine laboratory diagnostic test for LD due to its low success rate (40–60%), long incubation time (up to 8–12 weeks), susceptibility to contamination, and the level of expertise required 18, 19. Direct detection of outer surface membrane proteins of B. burgdorferi, e.g., OspA, by mass spectrometry has been reported in serum, but thus far only from three untreated LD patients 20.
Currently, no PCR-based assay has been cleared by the FDA for the diagnosis of LD. In patients with EM and a short duration of disease (<14 days), a B. burgdorferi-16S ribosomal RNA-based PCR on clinical specimens may be a useful diagnostic supplement 21. The blood-borne, i.e., bacteremic phase of B. burgdorferi is relatively brief and the concentration of spirochetes is generally quite low, therefore directly detecting the presence of B. burgdorferi DNA by PCR in blood from LD patients can be challenging 22. An assay employing a larger volume of blood coupled with a pre-enrichment step for B. burgdorferi DNA improved net sensitivity from 34% to only 62% 23. The bottom line is that none of these assays are suitable for the effective early detection of acute Lyme disease.
In this study, we employed a different strategy by targeting two classes of host proteins in blood whose blood concentration changes may reflect early Lyme infection: 1) acute-phase proteins (APP) involved in innate immune responses as the first line of defense against microorganisms; and 2) proteins synthesized specifically from organs known to be affected by B. burgdorferi infection (i.e., organ-specific proteins from liver and skin). APPs constitute part of the innate immune system that can respond within a few hours after bacteria enter the skin. Examples of APPs are C-reactive protein (CRP), the complement factors, ferritin, ceruloplasmin, serum amyloid A and haptoglobin. In response to injury or infection, local inflammatory cells secrete cytokines into the bloodstream, stimulating liver cells to increase (or decrease) the production of APPs that help the host to clear or inhibit the growth of pathogens.
Organ-specific transcripts are transcripts highly enriched in only one organ or expressed at least 10-fold over their expression levels in all other organs 24. Most proteins translated from organ-specific transcripts are involved in important organ-related functions and a few of these will be secreted into the blood through classic secretion, apoptosis, or shedding from the cell membrane by enzymatic cleavage. These blood organ-specific proteins may change their concentration levels in the blood when their cognate biological networks become disease perturbed—thus identifying the organ that is disease perturbed and pointing towards relevant pathology through the identification of their cognate biological networks. Thus, the levels of organ-specific blood proteins can be fingerprints of the health status of their corresponding organs. We have created a comprehensive list of organ-specific proteins that includes >2,500 proteins from 23 organs by mining more than 20 RNA and protein expression datasets for their expression patterns of these proteins (https://github.com/caballero/GeneAtlas). We have applied the highly sensitive and accurate targeted proteomics approach of selective reaction monitoring (LC-MS-SRM) 25 to measure the concentration of organ-specific proteins in blood and used this strategy successfully to identify candidate biomarkers for chronic and acute liver injury and other diseases 26, 27.
In this study, we measured the concentration of selected innate immune response and organ-specific proteins by LC-MS-SRM in LD samples in blood from the SLICE study (Study of Lyme Disease Immunology and Clinical Events) at Johns Hopkins University School of Medicine, and in an independent cohort from New York Medical College. We identified and verified serum proteins with altered abundances in patients during the early stage of B. burgdorferi infection, relative to healthy controls.
MATERIALS AND METHODS
ETHICS STATEMENT
This study was approved by the Institutional Review Boards of Johns Hopkins University and New York Medical College, as well as Western Institutional Review Board (WIRB), which approved the work performed at Institute for Systems Biology. It was conducted according to the principles of the Declaration of Helsinki. All patients provided written informed consent to participate.
PATIENT CHARACTERISTICS
1. DISCOVERY COHORT (SLICE)
The discovery cohort was obtained from Johns Hopkins University School of Medicine. These banked serum samples were previously collected under the project “Study of Lyme Disease Immunology and Clinical Events” (SLICE). This longitudinal sample set contained 40 participants with an EM skin lesion (greater than 5 cm in diameter); all patients included had either multiple EM skin lesions (n = 13) or a single lesion plus one or more systemic symptoms (n = 27). Patients were excluded if they had a history of LD, had received a LD vaccine, had a concomitant, objective extracutaneous manifestation of LD (such as neuro, cardiac, or arthritic complications), had an autoimmune condition, cancer, or a condition associated with non-specific fatigue and pain such as chronic fatigue syndrome, fibromyalgia, or major depression. All patients were untreated at the time of enrollment and were treated with antibiotic regimens consistent with current guidelines 5. The mean duration of illness prior to the first study visit was 9.5 ± 8.7 days.
The sample set included in the current study also comprised sera from 20 healthy controls. All controls were negative for prior LD by both self-report and two-tier serology performed at study entry; they were screened for the same exclusionary conditions as the cases with LD. The patients with LD and the healthy controls did not differ significantly in age or gender (Table 1). We analyzed serum samples collected from patients with LD at four time points: 1) pre-treatment (diagnosis/baseline), 2) 4 weeks post-diagnosis upon completion of antimicrobial treatment, 3) 6 months post-treatment, and 4) 12 months post-treatment, and from the control subjects we obtained samples at two time points (at the initial visit and 6 months later). Additional information on the study subjects can be found in Table S1. Serum was prepared according to standard protocol including clotting for 15–30 min at room temp before spinning at 3,000 RPM for 10 min, and storage at −80°C until analysis. Two-tier antibody testing, including a whole cell sonicate-based first-tier enzyme immunoassay, was performed by QUEST Diagnostics (Secaucus, NJ), a large commercial laboratory, following CDC interpretation guidelines at both the baseline and the 4 weeks post-treatment study visits.
Table 1.
Demographic and clinical characteristics of patients with Lyme disease (n=40 in SLICE-JHU cohort, and n=30 in NYMC cohort) and healthy controls (n=20 for each of the cohorts) in the discovery and verification cohorts.
| n | Age, years | Female | Acute seropositive2 at first visit | Ever seropositive2 (acute/convalescent) | Multiple erythema migrans | |
|---|---|---|---|---|---|---|
| SLICE-JHU | ||||||
| Control | 20 | 54.9 ± 15.2 | 10 (50.0%) | |||
| Lyme | 40 | 48.9 ± 13.9 | 21 (52.5%) | 17 (42.5%) | 30 (76.9%) | 13 (32.5%) |
| p-value1 | 0.132 | 0.855 | ||||
| NYMC | ||||||
| Control | 20 | 49.1 ± 11.3 | 10 (50.0%) | |||
| Lyme | 30 | 49.1±10.9 | 18 (60.0%) | 16 (53.3%) | 20 (66.7%) | 10 (33.3%) |
| p-value1 | 0.995 | 0.143 | ||||
Group comparisons for the SLICE-JHU, and separately for the NYMC cohorts, were performed using chi-squared test or Fisher’s exact test for categorical variables, and t-test or Wilcoxon rank sum test of medians, where appropriate.
Based on two-tier antibody testing conducted by large commercial laboratory following CDC interpretation of results for the SLICE-JHU cohort; based on a first-tier enzyme immunoassay performed at NYMC for the NYMC cohort.
2. VERIFICATION COHORT (NYMC)
A similar sized LD patient cohort from the “Culture Confirmed Lyme Disease” and “Outcome of Lyme Disease” studies at New York Medical College (NYMC) was used to validate altered abundances of serum proteins in the early stage of LD. This verification cohort included serum samples from 30 patients with systemic LD symptoms in addition to the EM skin lesion at diagnosis (Table 1). Patients with concomitant, objective extracutaneous manifestations of LD were not included. Blood was drawn from all LD patients at 4 time points: 1) baseline (diagnosis), 2) convalescence (11.9 ± 5.9 days after antibiotic treatment started), 3) one-year post-treatment, and 4) 4–6 years post-treatment; blood was obtained from 20 healthy controls at 2 time points: baseline and one year later. No information on the duration of illness prior to the baseline visit was available. Blood was drawn into serum separator tubes (SST) and allowed to clot for 15–30 min at room temp. Tubes were spun at 3,000 RPM for 10 min, and serum poured off into tubes before being stored at −80°C until analysis. A whole cell sonicate-based first-tier enzyme immunoassay (EIA) was performed at NYMC for all of the patients at both the baseline and convalescence time points 28. A C6 test was performed for the controls (Immunetics, Inc., now Oxford Immunotec USA, Marlborough, MA).
All patients from NYMC were untreated at the time of enrollment and were then treated with antibiotic regimens consistent with current guidelines 5. Selected subject demographic and clinical characteristics can be found in Table S1. The sample distributions in the two LD cohorts are summarized in Table S2.
SERUM SAMPLE PREPARATION FOR SRM ANALYSIS
Each serum sample was diluted 1:4 (v/v) in phosphate-buffered saline and spun through a .22 μm filter (Costar® Spin-X® centrifuge tube, Corning) at 10,000 × g to remove debris before being transferred to a clean polypropylene tube and stored at −80°C. To reduce the complexity of the serum proteome, an immuno-affinity depletion LC column (Multiple Affinity Removal Column Human 14 column, 4.6 × 100 mm, Agilent, Santa Clara, CA) was used to remove the 14 most abundant serum proteins. This procedure generally results in a 20-fold enrichment of low-abundance proteins. Samples were processed in random order. A reference pooled serum sample was added after every 50 sample runs.
Aliquots of 100 μL of 1:4 (v/v) diluted and filtered serum samples were loaded on the depletion column. Manufacturer recommended LC program was applied. The flow-through fractions (1.2 mL) were collected and concentrated to 200 μL using Amicon Ultra Centrifugal Filters (4 mL 3K MWCO, MilliporeSigma, Burlington, MA). BCA protein assay was performed to measure protein concentrations before and after depletion. Proteins were denatured in 50% (v/v) TFE (2,2,2-Trifluoroethanol) at 37°C for 30 min before reduction (TCEP), alkylation (iodoacetamide) and digestion with trypsin (1:25 trypsin/protein ratio) for 14 hours. After desalting using 1 cc C18 and MCX cartridges (Waters, Milford, MA), the proper amounts of stable C13N15 isotope-labeled (SIL) synthetic standards for target proteins were spiked into experimental samples before analysis in Agilent 6490 triple-quadrupole mass spectrometers (Agilent, Santa Clara, CA). A yeast protein (enolase 1, P00924) was spiked in as internal standard before depletion. All chemical reagents were obtained from Sigma-Aldrich (St. Louis, MO).
TARGET PROTEIN LIST FOR SRM ANALYSIS
We have developed Gene Atlas Interface, a discovery system that facilitates the identification of transcripts/genes that are highly expressed in one particular organ or tissue. Currently, this system stores 21 publicly available microarray and RNA-seq gene expression datasets for more than 30 human and mouse tissues (body atlas). Microarray data were processed from the raw data (CEL files) with the R:Bioconductor “affy” and “limma” packages. All samples were normalized using the Robust Multi-Array Average (RMA) method and gene expression values were stored in individual tables. RNA-seq data were processed from raw reads (FASTQ files), after removing low-quality reads, repeats and ribosomal sequences. The reads were mapped to the human reference genome GRCh37 with an optimized version of Blat, then converted to BAM and transcript quantification was achieved with Cufflinks using the gene models of Ensembl r64 as described in the following references 26, 29.
Our pipeline allows comparison of expression levels of all transcripts in one or more tissues/organs of interest with all other organs in the same dataset, providing two methods to evaluate significance: 1) normalized value of the log-enriched-ratio multiplied by the normalized expression level, and 2) the lower bound of the Wilson score confidence interval for a Bernoulli parameter (95% confidence). Both methods can use the maximal value observed or an average of values. Candidate transcripts from a specific organ or tissue are compared with all other organs/tissues in the same dataset, retaining only those showing > 10-fold enrichment over all other organs summed and P value < 0.005. The transcript lists thus generated from each dataset are then compared. Because each transcriptional dataset may discover a different group of “organ-specific transcripts” that overlap to varying extents, we determined that, in general, a gene is most likely organ-specific if four or more out of the 21 independent datasets support the call. Genes (transcripts) identified by three or fewer datasets were evaluated by their log-enriched ratio, agreement of mRNA expression data with protein expression data as available, and manual inspection (for example, determining if it is a known protein with organ-specific function or alternatively a product of a housekeeping gene for basic cell functions).
The GeneAtlas pipeline, including software and datasets, is publicly available at https://github.com/caballero/GeneAtlas.
SRM ASSAYS
Serum samples were analyzed in a triple-quadrupole mass spectrometer (Agilent 6490, Agilent, Santa Clara, CA) integrated with a nanospray ion source and Chip Cube nano-HPLC. Three to four pairs of light (endogenous) and heavy (stable C13N15 isotope-labeled (SIL) synthetic standards) transitions were monitored for each target peptide. A 90-min gradient of acetonitrile from 3% to 40% was used to elute peptides from a high-capacity nano-HPLC Chip (160 nL, 150 mm × 75 μm ID, Agilent, Santa Clara, CA) as described elsewhere 26. Other settings included operating the nano-HPLC separation chip at 0.3 μL/min nano pump flow rate, 2 μg peptide loading amount, 1820 V capillary voltage and Dynamic MRM with 200 Delta EMV (+). Duplicate runs were performed for each sample. All SRM parameters and results are deposited in the SRM chromatographic repository at ISB and are publicly available (http://www.srmatlas.org and http://www.peptideatlas.org/passel/, PASS01453). The final SRM methods used for 174 proteotypic peptides are summarized in Supplement Table 3 in PeptideAtlas SRM Experiment Library (PASSEL) format.
DATA ANALYSIS
Raw SRM mass spectrometry data were processed using the Skyline targeted proteomics environment 30. For each peptide, the total peak area and light/heavy (L/H) ratio of all monitored transitions were calculated and manually checked before export. The quantifier transition(s) were selected and used in further calculations in cases of high background and overlapping peaks from co-eluted peptides. Although the same amount of total peptide (2 μg) from each depleted sample was loaded into the mass spectrometer, the L/H ratios were adjusted by serum volume such that the relative peptide abundances from the same volume of serum were compared. The peptide L/H ratios were then log2-transformed for a more normal-like distribution. The peptide levels were normalized against the mean of the 20 control samples from two time points since there was no significant difference in controls between the two time points. Significantly differentially expressed proteins were identified by both Student’s t-test and multivariate analysis (MVA).
T-tests (Benjamini-Hochberg adjusted p-values) were used to identify differentially expressed proteins 31. The area under the curve (AUC) for the receiver operating characteristics (ROC) curve were generated in Prism 6.0 (GraphPad Software). Unsupervised principal component analysis (PCA) was performed in Perseus (1.6) 32 and hierarchical cluster analysis (HCA) heatmaps were generated in MultiExperiment Viewer (MeV, 4.9.0) 33.
Partial AUC (pAUC):
Partial AUC was applied to summarize a portion of the AUC curve over the pre-specified range of interest (leading to a low False Discovery Rate) such that only a portion of cases would be identified but with high confidence (>90% specificity) 34.
Combined AUC:
Logistic regression modeling was applied to establish a prediction model with multiple individual peptides identified by Student’s t-test. The AUCs for both whole-set and in 10-fold cross-validations (CV) were then calculated to assess the effectiveness of this model.
Multivariate analysis (MVA):
In addition to the t-test, a multivariate algorithm similar to that used for an earlier study in a proteomic characterization of pulmonary nodules 35 was developed to identify the most informative proteins. In the discovery SLICE set, Monte Carlo Cross-Validation (MCCV) was performed on 106 panels with 5-fold cross-validation. Each panel was composed of 10 features randomly selected from all measured peptides and fitted to a logistic regression model, using a 20% holdout rate and 100 sample permutations. In the verification NYMC set, the same model from the discovery SLICE set was applied to calculate the ROC curve.
WESTERN BLOT ANALYSIS OF PATIENT SERUM
Aliquots of 0.5 μL of serum (not depleted) from each sample were loaded on 4–12% SDS-PAGE Bis-Tris gels (Life Technologies, Grand Island, NY USA). Following separation, the proteins were transferred to PVDF membranes using an iBlot® dry blotting system from Life Technologies (Thermo Fisher Scientific, Carlsbad, CA). The membrane was blocked for non-specific binding sites in 5% non-fat milk, 0.1% (v/v) Tween 20 in PBS at room temperature for 1 hour, then incubated with primary antibody overnight at 4ºC. The membrane was washed three times in 0.1% Tween in PBS for 5 minutes each time and then incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies for 1 hour at room temperature. Detection was carried out by SuperSignal West Dura enhanced Duration substrate (Thermo Scientific, cat # 34076, Rockford, IL) with FluorchemE (ProteinSimple, Santa Clara, CA). The images were analyzed using ImageJ 36. Commercial rabbit polyclonal antibodies against ALDOB (Cat # 18065–1-AP, 1:1000), CRP (Cat # 24175–1-AP, 1:500), CST6 (Cat # 17076–1-AP, 1:200), and FBP1 (Cat # 12842–1-AP, 1:500) were obtained from ProteinTech (Rosemont, IL).
RESULTS AND DISCUSSION
The objective of this study was to identify blood proteins with altered abundances in patients with early stage Lyme disease as compared to healthy controls. Such proteins are candidate biomarkers requiring further validation that may ultimately lead to the development of improved diagnostic tests for early Lyme disease, especially in patients with a negative antibody-based serologic test. We used the targeted LC-MS-SRM proteomics technique to monitor selected proteins that fall within two categories: 1) key innate immunity regulators including acute phase proteins, reflecting the fact that LD is an acute inflammatory disease; and 2) organ-specific blood proteins predominantly expressed in organs and tissues known to be affected by B. burgdorferi, i.e., liver, heart, brain and skin 5, 37. We hypothesize that at least a subset of these organ-specific proteins may serve as surrogate indicators of the health status of their cognate organs (i.e., when networks are disease-perturbed, blood levels of their individual component proteins frequently change).
We designed SRM assays for 456 proteins with 2–3 proteotypic peptides per protein, using the SRMAtlas, a database containing SRM assays for more than 99.7% of the human proteome 38. Two pooled sera, one from the discovery LD sample set of 40 patients at the baseline time point and the other from the healthy controls, were used to pre-screen the detectability of all selected peptides in LC-MS-SRM. In all, 174 peptides (representing 124 human proteins) were detected and selected to be measured in individual serum samples. In the discovery sample set of 40 LD patients (each with 4 time points) and 20 healthy controls (each with 2 time points) from baseline to 12 months post-treatment, 90 proteotypic peptides from 73 targeted proteins were successfully detected and quantified.
1. Protein Changes Associated with Early Stage Lyme Disease
1a. Proteins Identified by Student’s t-test
In the discovery cohort, we first looked for proteins with significantly different serum levels in LD patients at the baseline visit (i.e., time of diagnosis) relative to healthy controls by t-test (two-tailed distribution, homoscedastic) with a Benjamini–Hochberg adjusted P value cutoff < 0.05 to adjust for multiple hypothesis comparisons, corresponding to roughly an uncorrected t-test P < 0.005. At this level of significance, we identified 10 proteins (represented by 13 proteotypic peptides) with serum levels significantly elevated (ALDOB, C9, CRP, FBP1 and S100A9) or reduced (AFM, APOA4, CST6, ITIH2 and PGLYRP2) in LD patients (Figure 1A and Table 2). We refer to these proteins as the t-Test Set. Before treatment, in response to inflammatory stimuli by B. burgdorferi infection, CRP serum levels increased the most, from 3 to 276-fold in 30 of the 40 LD patients (75%), a finding consistent with other studies 39, 40.
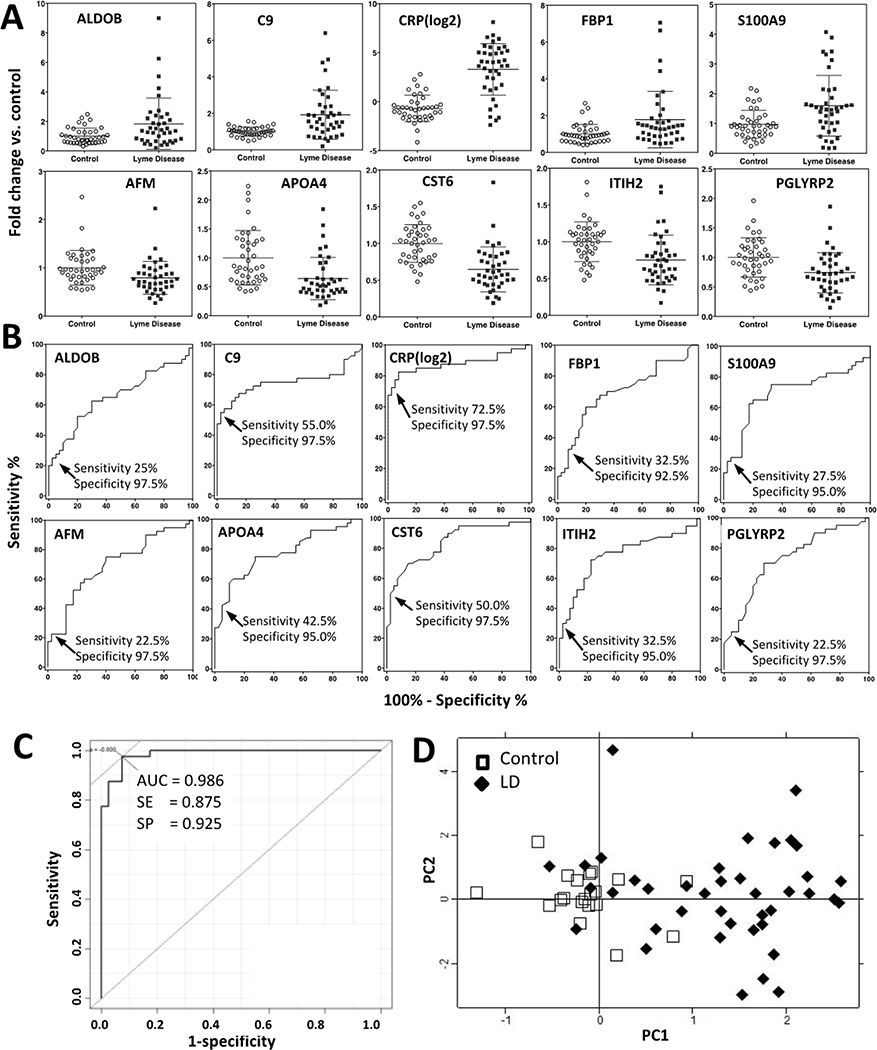
Figure 1.
The 10 early Lyme disease-associated proteins identified by t-test in the discovery cohort (t-Test Set).
1A, Dot plots showing the distribution and mean of relative serum levels with standard deviation for 10 proteins with altered serum levels associated with LD by t-test (Benjamini–Hochberg P < 0.05), at the baseline time point (i.e., at LD diagnosis). The average of control = 1. 1B, Individual AUCs. Cutoff points are shown where low false positive rates (2.5% - 7.5%) are achieved with variable sensitivities. 1C, Combined AUC (whole set) of the 10 proteins to distinguish LD patients from healthy controls. 1D, Biplots of PC1 and PC2 scores of 40 LD patients and 20 healthy controls from unsupervised PCA analyses, computed using the 10 t-Test Set proteins (13 proteotypic peptides). Filled diamonds and open squares represent PC scores for LD patients and controls, respectively.
Table 2.
Summarized information of the 16 LD-associated blood proteins discovered in the discovery SLICE cohort.
| Gene Symbol | UniProt | Protein name | Pepetide sequence | Organ origin | Subcellular location | LD vs. Control | t test p value | Adjusted p value * | In 10-prot MVA panel |
|---|---|---|---|---|---|---|---|---|---|
| AFM | P43652 | Afamin | LPNNVLQEK | Liver | extracellular | Down | 4.43E-03 | 4.30E-02 | |
| ALDOB | P05062 | Aldolase B, fructose-bisphosphate | ALQASALAAWGGK | Liver | cytosol | Up | 1.75E-03 | 4.03E-02 | YES |
| APOA4 | P06727 | Apolipoprotein A-IV | LGPHAGDVEGHLSFLEK | Liver | cytosol/extracellular | Down | 1.41E-04 | 1.34E-02 | |
| APOB | P04114 | Apolipoprotein B | GFEPTLEALFGK | Liver | cytosol/extracellular | Up | YES | ||
| C9 | P02748 | Complement component 9 | LSPIYNLVPVK | Liver | extracellular | Up | 2.59E-05 | 1.08E-02 | YES |
| CFHR1 | Q03591 | Complement factor H related 1 | ITCTEEGWSPTPK | Liver | extracellular | Up | YES | ||
| CRP | P02741 | C-reactive protein, pentraxin-related | ESDTSYVSLK | Liver | extracellular | Up | 1.44E-04 | 1.88E-02 | YES |
| CST6 | Q15828 | Cystatin E/M | AQSQLVAGIK | Skin | extracellular | Down | 3.9E-03 | 5.38E-03 | YES |
| F9 | P00740 | Coagulation factor IX | SCEPAVPFPCGR | Liver | extracellular | Down | YES | ||
| FBP1 | P09467 | Fructose-1,6-bisphosphatase 1 | EAVLDVIPTDIHQR | Liver | cytosol | Up | 1.58E-03 | 3.49E-02 | |
| GC | P02774 | Vitamin D-binding protein-macrophage activating factor | ELSSFIDK | Liver | extracellular | Down | YES | ||
| ITIH2 | P19823 | Inter-alpha (globulin) inhibitor H2 | VQFELHYQEVK | Liver | extracellular | Down | 2.56E-04 | 2.42E-02 | |
| ITIH4 | Q14624 | Inter-Alpha-Trypsin Inhibitor 4 | LGVYELLLK | Liver | extracellular | Up | YES | ||
| PF4 | P02776 | Platelet factor 4 | ICLDLQAPLYK | Platelet | extracellular | Up | YES | ||
| PGLYRP2 | Q96PD5 | Peptidoglycan recognition protein 2 | EFTEAFLGCPAIHPR | Liver | extracellular | Down | 4.60E-04 | 2.96E-02 | |
| S100A9 | P06702 | S100 calcium binding protein A9 | LGHPDTLNQGEFK | White blood cells | extracellular | Up | 3.8E-04 | 2.69E-02 | |
Benjamini-Hochberg adjusted t-test P value (LD at the baseline vs control).
We computed the area under the curve (AUC) for the receiver operating characteristics (ROC) curve for each of the 10 proteins (Figure 1B). Then we computed partial AUCs to identify the cut-off points of each protein in serum to reach a high specificity, i.e., between a 2.5% and 7.5% false positive rate in the ROC curve, with variable sensitivities (22.5% to 72.5%) in all 40 patients with LD (Figure 1B).
At these cutoff levels with high specificity but relatively low sensitivity, only a variable subset of LD patients could be classified by each of the 10 proteins. However, when all 10 t-Test Set proteins were combined, all 40 patients with LD had at least one protein exceeding the cutoff threshold (right column in Table 3). When considering patients as LD positive by at least two of the 10 proteins, 36 out of the 40 patients still could be identified at baseline, i.e., a sensitivity of 90%. The combined AUC of 10 proteins, when combined into a single prediction model using logistic regression, was 0.938 in 10-fold CV (0.986 whole set), with 0.85 in sensitivity and 0.93 in specificity (Figure 1C). To visualize the performance of these 10 proteins (represented by 13 proteotypic peptides) in identifying patients with LD, we also performed unsupervised principal component analysis (PCA). The distinction between LD patients and healthy controls was apparent at baseline (Figure 1D).
Table 3.
LD patients identified at baseline by the 10 proteins as described in Figures 1 and 2. Each of the 10 proteins identifies patients with various sensitivity at the indicated cutoff level of fold change compared to average of healthy controls as shown in Figure 1B, with low false positive rates (2.5–7.5%). A combination of 10 proteins identified all 40 LD patients at the indicated cutoff protein levels. In the righthand column, the number of proteins with serum level exceeding the cutoff fold change is listed for every LD patient.
| Patient ID | AFM | ALDOB | APOA4 | C9 | CRP | CST6 | FBP1 | ITIH2 | PGLYRP2 | S100A9 | # of “+” proteins |
|---|---|---|---|---|---|---|---|---|---|---|---|
| < 0.55* | > 2.2 | < 0.46 | > 1.45 | > 2.7 | < 0.58 | > 1.6 | < 0.60 | < 0.58 | >1.8 | ||
| LD01 | + | + | 2 | ||||||||
| LD02 | + | + | 2 | ||||||||
| LD03 | + | + | + | + | + | + | + | 7 | |||
| LD04 | + | + | + | + | + | 5 | |||||
| LD05 | + | + | + | 3 | |||||||
| LD06 | + | + | + | + | + | 5 | |||||
| LD07 | + | + | + | + | 4 | ||||||
| LD08 | + | + | + | 3 | |||||||
| LD09 | + | + | + | 3 | |||||||
| LD10 | + | + | + | 3 | |||||||
| LD11 | + | + | 2 | ||||||||
| LD12 | + | + | + | 3 | |||||||
| LD13 | + | + | + | 3 | |||||||
| LD14 | + | + | 2 | ||||||||
| LD15 | + | + | + | 3 | |||||||
| LD16 | + | + | 2 | ||||||||
| LD17 | + | + | + | 3 | |||||||
| LD18 | + | + | 2 | ||||||||
| LD19 | + | 1 | |||||||||
| LD20 | + | + | 2 | ||||||||
| LD21 | + | + | + | + | 4 | ||||||
| LD22 | + | + | 2 | ||||||||
| LD23 | + | + | + | 3 | |||||||
| LD24 | + | + | 2 | ||||||||
| LD25 | + | + | + | + | + | + | 6 | ||||
| LD26 | + | 1 | |||||||||
| LD27 | + | + | + | + | + | 5 | |||||
| LD28 | + | + | + | + | + | + | 6 | ||||
| LD29 | + | + | + | + | + | 5 | |||||
| LD30 | + | + | + | + | + | 5 | |||||
| LD31 | + | + | + | + | + | 5 | |||||
| LD32 | + | + | + | + | + | 5 | |||||
| LD33 | + | + | + | + | + | + | 6 | ||||
| LD34 | + | + | + | + | + | 5 | |||||
| LD35 | + | + | + | + | + | 5 | |||||
| LD36 | + | + | + | 3 | |||||||
| LD37 | + | + | + | + | + | 5 | |||||
| LD38 | + | + | + | + | + | + | + | 7 | |||
| LD39 | + | + | + | + | + | + | + | + | + | 9 | |
| LD40 | + | + | + | + | + | 5 | |||||
| # LD patient | 9 | 11 | 18 | 23 | 31 | 21 | 13 | 15 | 13 | 11 | |
| % LD patient | 22.5% | 27.5% | 45.0% | 57.5% | 77.5% | 52.5% | 32.5% | 37.5% | 32.5% | 27.5% | |
the cut-off fold change of serum concentration compared to average of healthy controls.
+: patient identified by each of the 10 proteins with relative serum concentrations beyond the cut-off protein values.
1b. Proteins Identified by Multivariate Analysis (MVA)
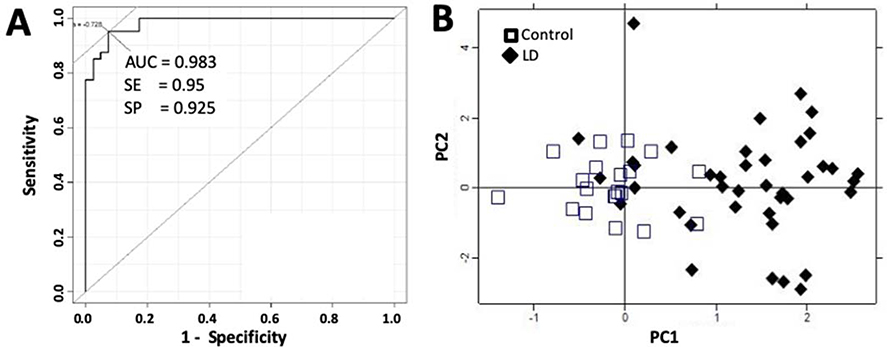
An alternative way to discover disease-associated molecules with changing concentration levels is to identify features that have synergistic effects 35, 41. In this approach we started with the 90 peptides quantified by LC-MS-SRM and randomly generated one million panels of 10 features. We assessed each of these panels for their ability to distinguish LD from healthy controls. We then defined a protein as “cooperative” if it was found more frequently in best performing panels than expected by chance alone. To identify the cooperative proteins, we implemented a multivariate algorithm similar to that used in a proteomic blood diagnosis to distinguish benign from neoplastic pulmonary nodules 35. The MVA analysis revealed 10 proteins (utilizing 11 proteotypic peptides) that were cooperative in distinguishing LD patients at baseline from healthy controls: ALDOB, APOB, C9, CFHR, CRP, CST6, GC, F9, ITIH4 and PF4 (Figure 2 and Table 2). The AUC of this 10-protein panel (the MVA Panel) was computed using logistic regression modeling, which reached 0.983 in distinguishing LD from controls at diagnosis, with 0.95 in sensitivity and 0.925 in specificity, respectively (Figure 2A). Using 2*104 Monte Carlo cross-validation (MCCV) permutations with a 33% holdout rate, we assessed the performance of this MVA Panel and got an average AUC of 0.944. In fact, a combination of 4 proteins (ALDOB, CST6, FBP1 and PGLYRP2) from this 10-protein panel performed at a relatively high accuracy of 0.83 (0.85 sensitivity, 0.80 specificity) with an AUC = 0.92.
Figure 2.
Performance of a group of 10 proteins discovered by multivariate analysis in the discovery cohort to identify patients with LD at baseline (MVA Panel).
2A, AUC, and 2B, Biplots of PC1 and PC2 scores of samples (40 LD patients and 20 healthy controls) from unsupervised PCA analyses, computed using the 10 MVA Panel proteins (11 proteotypic peptides). Filled diamonds and open squares represent PC scores for LD patients and controls, respectively.
Interestingly, four of these MVA Panel proteins (ALDOB, C9, CRP and CST6) overlapped with the t-Test Set of proteins, although the most cooperative proteins may not necessarily be the proteins with best individual performance identified by t-test 35. In unsupervised PCA analysis, similar to the 10 t-Test Set proteins, the PC scores of these 10 MVA Panel proteins presented a distinctive distribution between LD patients at baseline and healthy controls (Figure 2B).
Figure S1A illustrates the relative serum abundances of these 16 LD-associated proteins (10 t-Test Set proteins and 10 proteins in MVA Panel, with 4 overlapping), in LD individuals at baseline and in healthy controls.
2. Seronegative versus Seropositive Samples in Early Stage Lyme Disease
Next, we analyzed the performance of the 10 individual proteins of the t-Test Set, as well as the MVA Panel, in stratifying LD patients in seronegative (Sero-, n = 23) and seropositive (Sero+, n = 17) subgroups at the baseline visit.
Interestingly, all 10 individual proteins (13 proteotypic peptides) presented significant serum level changes in the Sero- subgroup versus controls (t-test P < 0.05, Table S4), with no significant difference between the 2 LD subgroups (t-test P > 0.05). The MVA Panel also returned similar AUC scores from multivariate analysis in both Sero- and Sero+ subgroups versus healthy controls (Table S5), performing slightly better in Sero- (AUC 0.911) compared to the Sero+ subgroup (AUC 0.865).
These findings suggest that these LD-associated serum proteins and the protein panel recognize acute LD in both seronegative and seropositive patients, when compared to healthy controls. If further validated, these proteins could potentially be used to aid the diagnosis of LD in patients with a negative serologic test result at the early stage of infection.
3. Protein Level Changes After Antimicrobial Treatment
At 4 weeks post-antimicrobial treatment, the perturbed serum levels of these 16 proteins returned to normal (fold change < 0.5 and P > 0.05 vs. healthy controls) in more than 80% of patients (Figure S1B), indicating diminished host immune responses and the reversibly acute effects in B. burgdorferi affected organs. This observation is consistent with multiple reports indicating that LD-associated hepatic dysfunction usually resolves within 2–3 weeks after starting treatment 7–9.
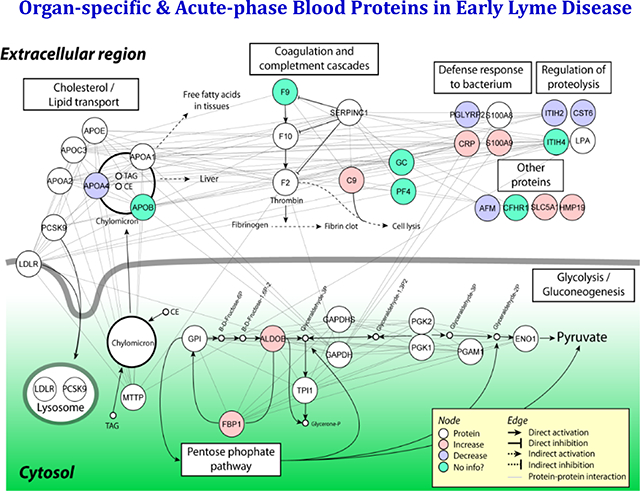
4. Network Analysis of Proteins Associated with Lyme Disease
We investigated the functional relationships among the 16 proteins discovered in this study and their potential involvement in response to B. burgdorferi infection and LD progression, using Gene Ontology (DAVID) and pathway-based (KEGG) analyses. As illustrated in Figure S2, many of the 16 proteins are involved in multiple extracellular pathways, including proteolysis, the immune response, and defense responses to bacteria. The remaining organ-specific proteins, for example, ALDOB (aldolase, fructose-bisphosphate B) and FBP1 (fructose-bisphosphatase 1), are members of intracellular metabolic pathways.
Pathways and functions related to innate immune reaction and acute phase response to pathogen infections are highly enriched (Figure S2). At least five proteins are directly involved in host responses to B. burgdorferi infection. For example, CRP (C-reactive protein), an acute reactant protein produced and secreted by hepatocytes, is a component of first line innate host defense, and serves as a sensitive signal of inflammation or infection 42. Two other proteins, C9 (complement component 9) and CFHR1 (complement factor H-related protein 1), are members of complement component cascades. C9, which can polymerize into complexes with C5, C6, C7, C8 or itself to neutralize a pathogen’s plasma membrane 43, also plays a key role in the adaptive immune response by enhancing the ability of antibodies and phagocytic cells to clear microbes. CFHR1 is a complement regulator and a member of factor H-related proteins that B. burgdorferi can bind to via outer surface proteins like Erp—an interaction that allows spirochetes to evade host complement during the initial stages of mammalian infection 44. PGLYRP2 (peptidoglycan recognition protein 2) belongs to the peptidoglycan recognition protein (PGRP) family, which recognizes and binds to peptidoglycan, an essential cell wall component of bacteria 45. It hydrolyzes the link between N-acetylmuramoyl residues and L-amino acid residues in peptidoglycan and may play a scavenger role by digesting biologically active peptidoglycan into biologically inactive fragments. S100A9 (S100 calcium-binding protein A9), a damage-associated molecular pattern molecule, is expressed specifically in monocytes and granulocytes. In association with its dimerization partner S100A8, S100A9 plays a prominent role in the regulation of inflammatory processes and immune response. Its differential expression has been associated with acute and chronic inflammation, and in sterile inflammatory conditions and cancers 46.
Whether the altered pattern of acute-phase related proteins discovered in this study is unique to B. burgdorferi infection, or may also reflect other types of infections, remains to be determined. We have observed that serum levels of complement factors C9 and CFHR1 changed significantly in LD at baseline, while some others, e.g., C4BPB, C5, C6, C8A/B/G, CFB, CFH, and CFP did not. The explanation for this behavior is not clear.
5. Organ-Specific Proteins Associated with Lyme Disease
Among the 16 LD-associated blood proteins, 13 are expressed predominantly in the liver. These 13 liver proteins consist of seven acute immune response proteins (C9, CFHR1, CRP, GC, ITIH2, ITIH4 and PGLYRP2), and four secreted proteins (AFM, APOA4, APOB and F9) that are involved in protein/lipid/polysaccharide transport or coagulation in the blood. The remaining two proteins (ALDOB and FBP1) are intracellular proteins involved in hepatocyte glycolysis/gluconeogenesis metabolism. Intracellular liver proteins are normally present in blood at low concentrations due to leakage. Blood levels of secreted liver proteins may decline under disease conditions due to disturbed protein production and secretion caused by organ injuries or infection. We have found that the elevation of intracellular proteins and the reduction of extracellular proteins in the blood may serve as sensitive markers of tissue injury or disease-perturbed tissue function in specific organs 25.
CST6 (Cystatin E/M) is the only skin-specific protein in our acute LD-associated protein list. It is secreted specifically from differentiated epidermal keratinocytes and serves as a skin-specific proteinase inhibitor. Cystatins and cystatin-like molecules belong to a new category of immunomodulatory molecules that act as inhibitors of lysosomal cysteine proteases, i.e., the cathepsins. Cathepsins are involved in processing and presentation of antigens, and have been associated with pathological conditions such as inflammation and cancers 47. An imbalance between cathepsins and cystatins may attenuate immune cell functions and thus facilitate tumor cell invasion 48. Decreased CST6 levels have been observed in inflamed skin 49, 50, which may reflect inflammatory processes in the skin area affected by EM in early LD patients.
We wish to emphasize the power of monitoring the concentration of organ-specific proteins in the blood to identify organs that have been perturbed in the course of a disease. We envision a time when at least 10 or more organ-specific proteins for the 25 most common organs will be in-hand—thus affording the ability, for a given disease, to determine which organs have been affected throughout the course of that disease. Examination of the disease-perturbed networks related to these sets of organ-specific proteins also provides insights into the pathophysiology of the disease. A key to increasing the number of detectable organ-specific blood proteins may lie in the ability to enrich and hence quantify low-abundance blood proteins (see discussion below).
6. Verification of SRM Results by Western Blotting
To verify the SRM results, we performed Western blot analysis with antibodies against four candidate proteins: ALDOB, CRP, CST6, and FBP1. For each protein, we measured serum levels in four randomly chosen LD patients (with 4 time points) and four healthy controls (2 time points) from the same cohort. Characteristic bands for ALDOB and CRP were observed, whereas antibodies against CST6 and FBP1 did not work well presumably due to their low antigen abundance in serum. Western blot results of both ALDOB and CRP agreed with SRM results, with Pearson’s correlation coefficient r = 0.83 for CRP and r = 0.80 for ALDOB (representative Western blot results are shown in Figure S3).
7. Verification of LD-associated Proteins in an Independent LD Cohort
We further evaluated the levels of these 16 LD-associated proteins by SRM in an independent LD cohort from NYMC, as described in Materials and Methods. This verification cohort included 30 patients with symptoms in addition to EM rash at diagnosis. Demographic and clinical characteristics of the NYMC LD patients are similar to those of the discovery SLICE cohort (Table S1).
Sample preparation, the SRM runs and data analyses in the verification cohort were exactly the same as for the discovery cohort. Fourteen proteins (18 peptides) out of the 16 LD-associated proteins were successfully detected and measured. The abundances of the remaining two proteins, ALDOB and FBP1, were too low to be reliably quantified in most samples, and were consequently removed from statistical analysis. Coagulation Factor IX (F9), a protein involved in the blood coagulation cascade, was also eliminated from further statistical analysis due to its abnormally low serum concentrations (~50% lower) in all LD samples compared to healthy controls. A heatmap of the 16 quantified proteins in LD patients over the 4 time points is presented in Figure S4.
Six early LD-associated serum proteins were verified
Six of the eight quantified proteins (APOA4, C9, CRP, CST6, PGLYRP2 and S100A9) in the t-Test Set were successfully verified in the second verification patient cohort (Student’s t-test P < 0.05). Similar cut-off levels were identified by pAUC, with similar sensitivities and specificities as in the discovery cohort (Table 4). Two proteins, AFM and ITIH2, had no power for discriminating LD from healthy controls and could not be verified.
Table 4:
LD patients identified at baseline by the six individual proteins in the verification cohort.
| Protein | APOA4 | C9 | CRP | CST6 | PGLYRP2 | S100A9 |
|---|---|---|---|---|---|---|
| Peptide | LGPHAGDVEGHLSFLEK | LSPIYNLVPVK | ESDTSYVSLK | AQSQLVAGIK | EFTEAFLGCPAIHPR | LGHPDTLNQGEFK |
| P value (t-test) | 2.5E-05** | 3.0E-03** | 5.0E-04** | 4.0E-02* | 7.0E-03* | 4.0E-03** |
| FC cutoff | < 0.33 | > 1.6 | > 4.5 | < 0.60 | < 0.35 | > 2.2 |
| Partial AUC at cutoff | ||||||
| sensitivity | 40.0% | 41.2% | 64.7% | 24.3% | 22.9% | 27.0% |
| specificity | 92.5% | 92.5% | 97.5% | 90.0% | 97.5% | 92.5% |
| # of patients detected at cutoff | ||||||
| Lyme (n=30) | 8 (26.7%) | 12 (40.0%) | 22 (73.3%) | 8 (26.7%) | 6 (20.0%) | 11 (36.7%) |
| Average FC to controls | ||||||
| Sero − | 0.42 ± 0.29 | 1.61 ± 0.98 | 24.47 ± 36.86 | 0.81 ± 0.37 | 0.78 ± 0.31 | 1.69 ± 0.95 |
| Sero + | 0.57 ± 0.47 | 1.59 ± 0.73 | 14.00 ± 15.74 | 0.83 ± 0.4 | 0.81 ± 0.38 | 1.76 ± 1.36 |
| Healthy control | 1 ± 0.6 | 1 ± 0.43 | 1 ± 1.17 | 1 ± 0.32 | 1 ± 0.41 | 1 ± 0.75 |
| t-test P value | ||||||
| Sero − vs. Control | 1.4E-05** | 3.2E-02* | 2.2E-02* | 7.8E-02* | 4.3E-02* | 1.6E-02* |
| Sero + vs. Control | 9.2E-03* | 1.1E-02* | 4.7E-03** | 1.4E-01 | 1.1E-01 | 5.4E-02 |
| Sero + vs. Sero − | 3.3E-01 | 9.4E-01 | 3.1E-01 | 8.7E-01 | 7.9E-01 | 8.7E-01 |
cutoff value is the fold change to average of healthy controls in the same sample set.
Sero−: LD patient group with negative first-tier serology test (EIA) at diagnosis
Sero+: LD patient group with positive serology test at diagnosis.
t-test P < 0.05
P < 0.005.
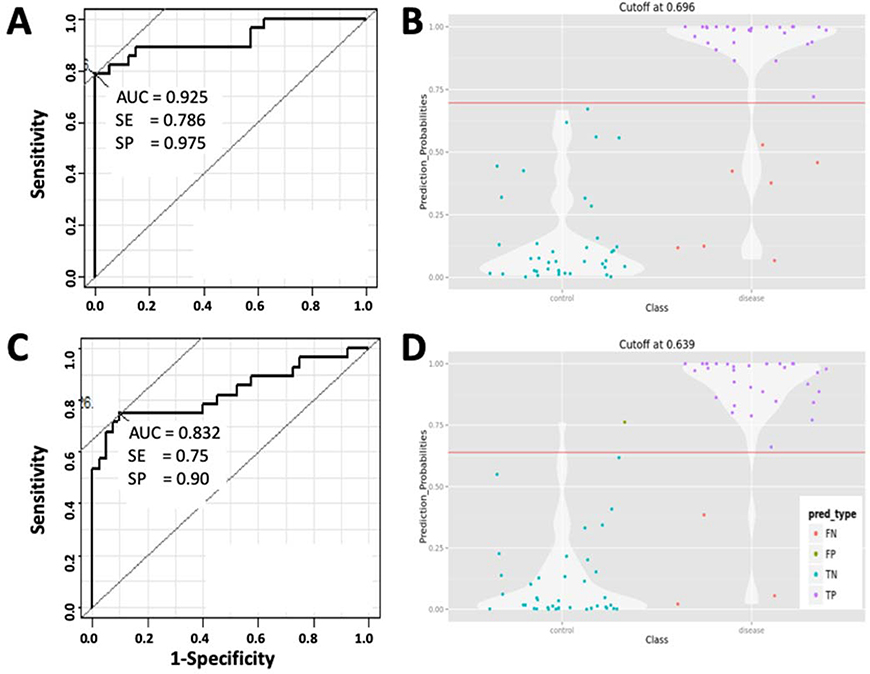
The combined ROC of these six verified proteins was calculated using logistic regression modeling, which reached an AUC = 0.893 in 10-fold CV (whole set = 0.925), with 0.88 in sensitivity, 0.83 in specificity and 0.90 in accuracy, all scores slightly lower than in the discovery cohort (Figure 3A). At the prediction probability cutoff value of 0.696, the combination of these six proteins correctly predicted 23 out of the 30 LD patients in the verification cohort (Figure 3B).
Figure 3.
Serum levels of early Lyme disease associated proteins in the verification cohort.
3A, Combined AUC (whole set), sensitivity and specificity of the 6 verified blood proteins showing statistically altered serum levels in LD at the baseline time point. 3B, Violin plot combines a box plot and a kernel density plot, showing the probability density to classify LD patients by the six proteins at the indicated cutoff value of AUC. The four possible categories: False Negative (FN); False Positives (FP); True Negative (TN); True Positive (TP). 3C, AUC, sensitivity and specificity of the 9-protein panel identified by multivariate analysis. 3D, Violin plot showing the probability density to classify LD patients by the 9-protein panel at the indicated cutoff level of AUC.
The 10-protein panel from multivariate analysis performs well in verification cohort
The 10-protein MVA Panel discovered by MVA was also verified. As discussed in the previous section, F9 was removed because of its abnormally low concentrations in LD samples which seemed likely to be technical error, thus artificially increasing accuracy as a result. Subsequently, the ROC of the 9-protein panel was computed using the model “locked-down” from the discovery cohort, except the contributions of F9 were set as zero in the formula. This model resulted in an AUC = 0.832 (sensitivity 0.75, specificity 0.9 and accuracy 0.84) (Figure 3C). Interestingly, serum levels of two proteins, APOB (p < 0.001) and CFHR1 (p = 0.05), were also significantly increased in this verification cohort, in contrast to results in the discovery cohort. As illustrated in Figure 3D, at a cutoff value of 0.639, this 9-protein panel correctly classified 27 out of the 30 LD patients in the verification cohort with one false-positive among the 20 controls.
Seronegative versus seropositive samples
As in the first cohort, all six verified individual proteins from the t-Test Set demonstrated significant serum level changes in Sero- patients (n=16) versus healthy controls (n=20, t test P < 0.05, Table 4), with no significant difference between the 2 LD subgroups (t test P > 0.05, Table 4). The MVA Panel (nine proteins) also reached similar AUC scores from multivariate analysis in both Sero- and Sero+ (n=14) subgroups versus healthy controls (Table S5), performing slightly better in Sero- (AUC 0.904) compared to the Sero+ subgroup (AUC 0.842).
As shown in Figure S4, after an average of 11.9 ± 5.9 days of antimicrobial treatment, the perturbed serum levels of these proteins returned to normal, similar to the discovery samples.
It should be noted that, as most of the patient samples in this cohort were collected in the 1990s, some of the candidate proteins could have degraded during storage, which may explain why they were apparently below the level of detection in this verification sample set.
8. Limitations of the Study
The major limitation of this study is the relatively small sample size (70 LD and 40 healthy controls) from two cohorts. Thus, findings herein should not be considered as biomarkers, but as candidates that will require further validation in appropriately controlled studies. Also, the possibility of misdiagnosed patients in those without microbiologic confirmation, and the different levels of serology tests performed in the two cohorts, could impact the observed outcomes. We therefore plan to validate these acute LD-associated blood proteins in additional sample sets, ultimately aiming to develop a diagnostic protein panel for detection of early LD, prediction of disease progression, and assessment of the overall health of specific organ systems in patients who have persistent symptoms seemingly related to LD. We also need to evaluate our candidate proteins in blood from patients that have other types of infections as well to determine how specific these markers are for LD. That said, in geographic areas with high rates of LD, these blood proteins, if validated, could prove useful in informing early treatment with antibiotics in seronegative patients without an EM rash.
Another limitation of this study is that most organ-specific proteins that can be detected and quantified in depleted serum by LC-MS-SRM are from several large organs like the liver. It is still challenging to detect proteins originating from small organs (thus presumably contributing lower levels of proteins to the blood) or organs isolated from systemic blood flow, e.g., via the blood brain barrier. These less detectable proteins could be very informative to the study of many diseases. One approach to investigating these very low-abundance organ-specific proteins in the blood is to specifically capture them using a multiplex affinity column of antibodies or other types of protein or peptide binding reagents. In theory, such reusable positive enrichment methods could improve our ability to study dozens of target proteins present at very low levels in the blood from specific organs that are involved in early or late manifestations of B. burgdorferi infections. Equalizing approaches to reduce high-abundance proteins and enrich low-abundance proteins in biological liquids, for example, ProteoMiner hexapeptide beads, could also enhance our ability to detect and measure low-abundant proteins in blood secreted from particularly small organs 51, 52. We plan to quantify and assess additional organ-specific proteins, especially proteins enriched in other B. burgdorferi-affected organs such as the bladder, brain, heart, and muscle, with improved sample enrichment procedures.
CONCLUSIONS
In this study, we focused on specific blood proteins drawn from two key categories related to B. burgdorferi infection: 1) proteins involved in the host innate immune response during the acute phase of infection and 2) proteins specifically originating from single organs that are possible targets of the infection, for example, liver, brain, heart and skin. With this focused approach we aimed to identify serum proteins whose altered blood abundances, relative to healthy controls, could identify individuals infected with B. burgdorferi at the earliest stage possible.
In two independent LD cohorts, we identified a set of proteins that may fulfill that aim:
In the discovery cohort, we identified 10 proteins by t-test (ALDOB, AFM, APOA4, C9, CRP, CST6, FBP1, ITIH2, PGLYRP2 and S100A9), each of which has significantly altered protein levels in serum and is capable of distinguishing LD patients from healthy controls at the time of diagnosis. We have also identified an MVA Panel of 10 proteins (ALDOB, APOB, C9, CFHR, CRP, CST6, GC, F9, ITIH4 and PF4) by multivariate analysis that distinguished LD patients from healthy controls with high accuracy.
In an independent LD cohort, we verified i) 6 proteins from the t-Test Set of 10 proteins, and ii) 9 of the 10 proteins in the MVA Panel, with similar sensitivity and specificity as in the discovery cohort.
These LD-associated serum proteins (t-Test Set) and protein panel (MVA Panel) identified acute LD in both seronegative and seropositive patients.
We plan to further validate these early LD-associated serum proteins by testing additional independent LD and healthy control cohorts, along with other appropriate disease control cohorts.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by Bay Area Lyme Foundation (www.bayarealyme.org), The Steven and Alexandra Cohen Foundation (http://www.steveandalex.org), NIH grant P30AR05350 (to JNA and MJS), P50GM076547 (LH and RLM), the National Institute of Allergy and Infectious Diseases under Award Number R21AI133335 (RLM), and The Wilke Family Foundation. GSO acknowledges NIH grants P30ES017885 and U24CA210967. The authors would like to thank the individuals with LD and healthy controls who participated in this study. We thank our colleagues at ISB: Dr. Simon Evans who helpfully read and commented on the manuscript, and Dr. Max Robinson for helpful discussions on the statistics and analytical aspects of this study.
FUNDING SOURCES:
Bay Area Lyme Foundation (www.bayarealyme.org)
The Steven and Alexandra Cohen Foundation (http://www.steveandalex.org)
The Wilke Family Foundation
NIH P50GM076547 (LH and RLM)
NIH grant P30AR05350 (to JNA and MJS)
the National Institute of Allergy and Infectious Diseases R21AI133335 (RLM)
NIH grants P30ES017885 and U24CA210967 GSO acknowledges
CONFLICT OF INTEREST
Disclosures: Dr. Wormser reports receiving research grants from Immunetics, Inc., Institute for Systems Biology, Rarecyte, Inc., and Quidel Corporation. He owns equity in Abbott/AbbVie; has been an expert witness in malpractice cases involving Lyme disease; and is an unpaid board member of the American Lyme Disease Foundation. Other authors have no disclosures.
ABBREVIATIONS
- AFM
afamin
- ALDOB
aldolase B
- AUC
area under the curve
- C9
complement component 9
- CFHR1
complement factor H-related protein 1
- CRP
C-reactive protein
- CST6
cystatin E/M
- F9
Coagulation Factor IX
- FBP1
fructose-bisphosphate 1
- GC
vitamin D-binding protein-macrophage activating factor
- ITIH2
inter-alpha (globulin) inhibitor H2
- ITIH4
inter-alpha-trypsin inhibitor 4
- LD
Lyme disease
- MVA
multivariate analysis
- MS
mass spectrometry
- pAUC
partial AUC
- PF4
platelet factor 4
- PGLYRP2
peptidoglycan recognition protein 2
- ROC curve
Receiver operating characteristic curve
- SRM
selective reaction monitoring
REFERENCES
- 1.Rosenberg R, Lindsey NP, Fischer M, Gregory CJ, Hinckley AF, Mead PS, et al. Vital Signs: Trends in Reported Vectorborne Disease Cases - United States and Territories, 2004–2016. MMWR Morb Mortal Wkly Rep 2018; 67:496–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shapiro ED. Clinical practice. Lyme disease. N Engl J Med 2014; 370:1724–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuehn BM. Insect Borne Disease Threat Grows. JAMA : the journal of the American Medical Association. 2018; 319:2471. [DOI] [PubMed] [Google Scholar]
- 4.Nelson CA, Saha S, Kugeler KJ, Delorey MJ, Shankar MB, Hinckley AF, et al. Incidence of Clinician-Diagnosed Lyme Disease, United States, 2005–2010. Emerg Infect Dis 2015; 21:1625–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wormser GP, Dattwyler RJ, Shapiro ED, Halperin JJ, Steere AC, Klempner MS, et al. The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2006; 43:1089–134. [DOI] [PubMed] [Google Scholar]
- 6.Steere AC, Strle F, Wormser GP, Hu LT, Branda JA, Hovius JW, et al. Lyme borreliosis. Nat Rev Dis Primers. 2016; 2:16090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horowitz HW, Dworkin B, Forseter G, Nadelman RB, Connolly C, Luciano BB, et al. Liver function in early Lyme disease. Hepatology. 1996; 23:1412–7. [DOI] [PubMed] [Google Scholar]
- 8.Kazakoff MA, Sinusas K, Macchia C. Liver function test abnormalities in early Lyme disease. Arch Fam Med 1993; 2:409–13. [DOI] [PubMed] [Google Scholar]
- 9.Zaidi SA, Singer C. Gastrointestinal and hepatic manifestations of tickborne diseases in the United States. Clin Infect Dis 2002; 34:1206–12. [DOI] [PubMed] [Google Scholar]
- 10.Koedel U, Fingerle V, Pfister HW. Lyme neuroborreliosis-epidemiology, diagnosis and management. Nat Rev Neurol 2015; 11:446–56. [DOI] [PubMed] [Google Scholar]
- 11.Waddell LA, Greig J, Mascarenhas M, Harding S, Lindsay R, Ogden N. The Accuracy of Diagnostic Tests for Lyme Disease in Humans, A Systematic Review and Meta-Analysis of North American Research. PLoS One. 2016; 11:e0168613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liang FT, Steere AC, Marques AR, Johnson BJ, Miller JN, Philipp MT. Sensitive and specific serodiagnosis of Lyme disease by enzyme-linked immunosorbent assay with a peptide based on an immunodominant conserved region of Borrelia burgdorferi vlsE. J Clin Microbiol 1999; 37:3990–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prevention CfDCa. Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. JAMA : the journal of the American Medical Association. 1995; 274:937.7674514 [Google Scholar]
- 14.Aguero-Rosenfeld ME, Wang G, Schwartz I, Wormser GP. Diagnosis of lyme borreliosis. Clin Microbiol Rev 2005; 18:484–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bacon RM, Biggerstaff BJ, Schriefer ME, Gilmore RD Jr., Philipp MT, Steere AC, et al. Serodiagnosis of Lyme disease by kinetic enzyme-linked immunosorbent assay using recombinant VlsE1 or peptide antigens of Borrelia burgdorferi compared with 2-tiered testing using whole-cell lysates. J Infect Dis 2003; 187:1187–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jobe DA, Lovrich SD, Asp KE, Mathiason MA, Albrecht SE, Schell RF, et al. Significantly improved accuracy of diagnosis of early Lyme disease by peptide enzyme-linked immunosorbent assay based on the borreliacidal antibody epitope of Borrelia burgdorferi OspC. Clin Vaccine Immunol 2008; 15:981–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goossens HA, van den Bogaard AE, Nohlmans MK. Evaluation of fifteen commercially available serological tests for diagnosis of Lyme borreliosis. Eur J Clin Microbiol Infect Dis 1999; 18:551–60. [DOI] [PubMed] [Google Scholar]
- 18.Marques AR. Laboratory diagnosis of Lyme disease: advances and challenges. Infect Dis Clin North Am 2015; 29:295–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wormser GP, Nowakowski J, Nadelman RB, Bittker S, Cooper D, Pavia C. Improving the yield of blood cultures for patients with early Lyme disease. J Clin Microbiol 1998; 36:296–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheung CS, Anderson KW, Benitez KY, Soloski MJ, Aucott JN, Phinney KW, et al. Quantification of Borrelia burgdorferi Membrane Proteins in Human Serum: A New Concept for Detection of Bacterial Infection. Anal Chem 2015; 87:11383–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lebech AM, Hansen K, Brandrup F, Clemmensen O, Halkier-Sorensen L. Diagnostic value of PCR for detection of Borrelia burgdorferi DNA in clinical specimens from patients with erythema migrans and Lyme neuroborreliosis. Mol Diagn 2000; 5:139–50. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz I, Wormser GP, Schwartz JJ, Cooper D, Weissensee P, Gazumyan A, et al. Diagnosis of early Lyme disease by polymerase chain reaction amplification and culture of skin biopsies from erythema migrans lesions. J Clin Microbiol 1992; 30:3082–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eshoo MW, Crowder CC, Rebman AW, Rounds MA, Matthews HE, Picuri JM, et al. Direct molecular detection and genotyping of Borrelia burgdorferi from whole blood of patients with early Lyme disease. PLoS One. 2012; 7:e36825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Breschi A, Djebali S, Gillis J, Pervouchine DD, Dobin A, Davis CA, et al. Gene-specific patterns of expression variation across organs and species. Genome Biol 2016; 17:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Picotti P, Aebersold R. Selected reaction monitoring-based proteomics: workflows, potential, pitfalls and future directions. Nat Methods. 2012; 9:555–66. [DOI] [PubMed] [Google Scholar]
- 26.Qin S, Zhou Y, Gray L, Kusebauch U, McEvoy L, Antoine DJ, et al. Identification of Organ-Enriched Protein Biomarkers of Acute Liver Injury by Targeted Quantitative Proteomics of Blood in Acetaminophen- and Carbon-Tetrachloride-Treated Mouse Models and Acetaminophen Overdose Patients. J Proteome Res 2016; 15:3724–40. [DOI] [PubMed] [Google Scholar]
- 27.Qin S, Zhou Y, Lok AS, Tsodikov A, Yan X, Gray L, et al. SRM targeted proteomics in search for biomarkers of HCV-induced progression of fibrosis to cirrhosis in HALT-C patients. Proteomics. 2012; 12:1244–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wormser GP, Schriefer M, Aguero-Rosenfeld ME, Levin A, Steere AC, Nadelman RB, et al. Single-tier testing with the C6 peptide ELISA kit compared with two-tier testing for Lyme disease. Diagn Microbiol Infect Dis 2013; 75:9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glusman G, Caballero J, Robinson M, Kutlu B, Hood L. Optimal scaling of digital transcriptomes. PLoS One. 2013; 8:e77885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.MacLean B, Tomazela DM, Shulman N, Chambers M, Finney GL, Frewen B, et al. Skyline: an open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics. 2010; 26:966–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behavioural brain research. 2001; 125:279–84. [DOI] [PubMed] [Google Scholar]
- 32.Tyanova S, Temu T, Sinitcyn P, Carlson A, Hein MY, Geiger T, et al. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat Methods. 2016; 13:731–40. [DOI] [PubMed] [Google Scholar]
- 33.Saeed AI, Sharov V, White J, Li J, Liang W, Bhagabati N, et al. TM4: a free, open-source system for microarray data management and analysis. BioTechniques. 2003; 34:374–8. [DOI] [PubMed] [Google Scholar]
- 34.Wallstrom G, Anderson KS, LaBaer J. Biomarker discovery for heterogeneous diseases. Cancer Epidemiol Biomarkers Prev 2013; 22:747–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li XJ, Hayward C, Fong PY, Dominguez M, Hunsucker SW, Lee LW, et al. A blood-based proteomic classifier for the molecular characterization of pulmonary nodules. Science translational medicine. 2013; 5:207ra142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012; 9:671–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kern A, Schnell G, Bernard Q, Boeuf A, Jaulhac B, Collin E, et al. Heterogeneity of Borrelia burgdorferi Sensu Stricto Population and Its Involvement in Borrelia Pathogenicity: Study on Murine Model with Specific Emphasis on the Skin Interface. PLoS One. 2015; 10:e0133195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kusebauch U, Campbell DS, Deutsch EW, Chu CS, Spicer DA, Brusniak MY, et al. Human SRMAtlas: A Resource of Targeted Assays to Quantify the Complete Human Proteome. Cell. 2016; 166:766–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soloski MJ, Crowder LA, Lahey LJ, Wagner CA, Robinson WH, Aucott JN. Serum inflammatory mediators as markers of human Lyme disease activity. PLoS One. 2014; 9:e93243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uhde M, Ajamian M, Li X, Wormser GP, Marques A, Alaedini A. Expression of C-Reactive Protein and Serum Amyloid A in Early to Late Manifestations of Lyme Disease. Clin Infect Dis 2016; 63:1399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grimnes S, Martinsen ÿG. Data and Models. In: Sverre Grimnes ØGM, editor. Bioimpedance and bioelectricity basics 3rd edition ed: Academic Press; 2015. p. 329–404. [Google Scholar]
- 42.Volanakis JE. Human C-reactive protein: expression, structure, and function. Mol Immunol 2001; 38:189–97. [DOI] [PubMed] [Google Scholar]
- 43.Tschopp J, Muller-Eberhard HJ, Podack ER. Formation of transmembrane tubules by spontaneous polymerization of the hydrophilic complement protein C9. Nature. 1982; 298:534–8. [DOI] [PubMed] [Google Scholar]
- 44.Brissette CA, Cooley AE, Burns LH, Riley SP, Verma A, Woodman ME, et al. Lyme borreliosis spirochete Erp proteins, their known host ligands, and potential roles in mammalian infection. Int J Med Microbiol 2008; 298 Suppl 1:257–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu C, Xu Z, Gupta D, Dziarski R. Peptidoglycan recognition proteins: a novel family of four human innate immunity pattern recognition molecules. J Biol Chem 2001; 276:34686–94. [DOI] [PubMed] [Google Scholar]
- 46.Shabani F, Farasat A, Mahdavi M, Gheibi N. Calprotectin (S100A8/S100A9): a key protein between inflammation and cancer. Inflamm Res 2018; 67:801–12. [DOI] [PubMed] [Google Scholar]
- 47.Kopitar-Jerala N The role of cystatins in cells of the immune system. FEBS letters. 2006; 580:6295–301. [DOI] [PubMed] [Google Scholar]
- 48.Magister S, Kos J. Cystatins in immune system. J Cancer. 2013; 4:45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cheng T, Tjabringa GS, van Vlijmen-Willems IM, Hitomi K, van Erp PE, Schalkwijk J, et al. The cystatin M/E-controlled pathway of skin barrier formation: expression of its key components in psoriasis and atopic dermatitis. Br J Dermatol 2009; 161:253–64. [DOI] [PubMed] [Google Scholar]
- 50.Zeeuwen PL, van Vlijmen-Willems IM, Egami H, Schalkwijk J. Cystatin M / E expression in inflammatory and neoplastic skin disorders. Br J Dermatol 2002; 147:87–94. [DOI] [PubMed] [Google Scholar]
- 51.Li S, He Y, Lin Z, Xu S, Zhou R, Liang F, et al. Digging More Missing Proteins Using an Enrichment Approach with ProteoMiner. J Proteome Res 2017; 16:4330–9. [DOI] [PubMed] [Google Scholar]
- 52.Righetti PG, Boschetti E. Sherlock Holmes and the proteome--a detective story. The FEBS journal. 2007; 274:897–905. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.