Abstract
Objective
18F-labeled prostate-specific membrane antigen (PSMA) ligand, [18F]PSMA-1007, has the benefit of a higher synthetic yield and minimal excretion in the urine. High detection efficacy was reported in biochemical recurrence (BCR) of prostate cancer after radical prostatectomy. Thus, we evaluated the preliminary diagnostic utility of [18F]PSMA-1007 PET in patients with prostate cancer, focusing on the BCR which is not detected on conventional imaging.
Methods
We enrolled a total of 28 patients (age 51–79 years) with BCR of prostate cancer. BCR was defined as a continuous increase in PSA after radical prostatectomy or radiation therapy without any apparent recurrent lesions on conventional diagnostic imaging (CT and bone scintigraphy). PSMA-PET scanning was performed approximately 60 min after intravenous injection of [18F]PSMA-1007 (259 ± 37 MBq). PSMA-PET images were evaluated for lesion detection as well as its relation to PSA values and location.
Results
Abnormal uptake, which was suspected to be recurrence or metastasis, was detected in 92.9% (26/28) of patients with BCR. The SUVmax was 8.4 ± 6.4 in local recurrence, 11.5 ± 11.8 in pelvic lymph nodes (LN), and 4.1 ± 1.6 in bone metastasis. The detection rates were 66.7% in the PSA group-1 (0.1–0.5 ng/mL), 85.7% in the PSA group-2 (0.5–1.0 ng/mL), and 100% in the PSA group-3 (above 1.0 ng/mL). Among the PET-positive BCR patients (n = 26), local recurrence was detected in 57.7% (15/26), pelvic LN in 42.3% (11/26), and bone metastasis in 15.4% (4/26). In 53% (8/15) of BCR patients who were suspected of local recurrence, focal uptake was detected adjacent to the bladder on [18F]PSMA-1007 PET. This suggested the significant advantage of having minimal physiological urine excretion.
Conclusions
[18F]PSMA-1007 PET showed a high detection rate in recurrent and metastatic lesions. In patients with BCR, its high detection led to suitable treatment strategies, such as salvage radiation therapy or surgical removal of recurrent lymph nodes.
Trial registration
(UMIN Clinical Trials Registry) UMIN000037697.
Keywords: PSMA, PET, Prostate cancer, Biochemical recurrence, Diagnosis
Introduction
Prostate-specific membrane antigen (PSMA) is a cell membrane-bound protein that is highly expressed in prostate cancer cells and the neovasculature of other tumors [1]. Expression of PSMA was observed in most prostate cancer patients and was positively correlated with Gleason score, a histological marker for degree of malignancy [2, 3]. PSMA expression is preserved or increased after androgen deprivation therapy (ADT) in patients with metastatic CRPC (castration-resistant prostate cancer) [4]. Thus, PSMA-PET can be used for a wide variety of purposes in prostate cancer patients, from initial staging and detection of recurrence, to pre-treatment evaluation of PSMA-targeted radionuclide therapy using [177Lu]Lu-/[225Ac]Ac-PSMA-617.
[68 Ga]Ga-PSMA-11 PET has been mainly used for PET imaging targeting PSMA and was recently approved by the FDA as the 1st PSMA PET probe. Meanwhile, the 18F-labeled PSMA ligand, [18F]PSMA-1007, has the advantages of the abundant availability of 18F and higher synthetic yield. It also showed favorable biodistribution in humans, with minimal excretion in the urine [5]. High detection efficacy has been reported in biochemical recurrence (BCR) of prostate cancer after radical prostatectomy using [18F]PSMA-1007 PET [6]. Its diagnostic accuracy was also confirmed for lymph node staging of prostate carcinoma in primary and biochemical recurrence compared to histological findings [7]. In this study, we evaluated the preliminary diagnostic utility of [18F]PSMA-1007 PET in patients with prostate cancer, focusing on the BCR, as an interim report.
Materials and methods
The study protocol and patient population
A total of 28 patients (age 51–79 years) with prostate cancer were included in this prospective study. Inclusion criteria were patients diagnosed with prostate cancer and who underwent CT and bone scintigraphy (BS) and showed continuously increased PSA after local treatment with radical prostatectomy or radiation therapy, with no apparent recurrent lesion on CT and BS. Patient characteristics are summarized in Table 1. The study protocol was approved by the institutional review board of Osaka University Hospital and the study has, therefore, been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. Written informed consent was obtained from all patients prior to their inclusion in the study.
Table 1.
Clinical characteristics of the patients
| Characteristics | |
|---|---|
| Age at PET [median (range)] | 67.5 (51–79) year |
| PSA at PET [median (range)] | 2.39 (0.12–39.78) ng/mL |
| Gleason score | |
| 6 | 5 |
| 7 | 12 |
| 8 | 4 |
| 9 | 7 |
Imaging protocol
[18F]PSMA-1007 solution was synthesized using MPS200 (Sumitomo Heavy Industries) according to a previous study [8, 9]. After at least 2 h of fasting, PSMA-PET scanning was performed approximately 60 min (57.7 ± 4.9 min, range 47.5–65.5) after intravenous injection of [18F]PSMA-1007 (259 ± 37 MBq). PET/CT images were acquired using Discovery 710 (GE, Milwaukee, United States) in 3-D mode (matrix 192 × 192, pixel size 3.65 mm) with 2 min per bed position. PET images were reconstructed using an ordered subset expectation maximization (OSEM) algorithm with three iterations per eight subsets, and Gauss-filtered to a transaxial resolution of 4 mm at full-width at half-maximum (FWHM). Attenuation correction was performed using the unenhanced low-dose CT data (tube current 100 mA). The CT-scans were reconstructed to a slice thickness of 3.75 mm with an increment of 3.27 mm. CT and BS were performed according to the institutional standard protocol as routine clinical practice.
Data analysis
PSMA-PET images were analyzed for detection of lesions, as well as their relation to PSA values and location in patients with BCR. All PET images were interpreted by two physicians with board certifications. When there were differences in the interpretation of a result, it was judged by the senior nuclear medicine physician who has enough knowledge about PSMA-PET imaging. Detection rate was defined as the image-based positive finding which was suspected recurrence on PSMA-PET. True positivity was defined based on the histopathological confirmation (n = 5) or clinical course [PSA decline after the radiation therapy to PSMA-positive lesions (n = 11), decreased uptake on PSMA-PET after systemic therapy along with PSA decline or increase in size on CT (n = 4)]. In other cases without sufficient evidence (n = 6), apparent abnormal uptakes were judged as positive in potentially recurrent or metastatic regions. The impact on patient management was also evaluated after PSMA-PET in patients with BCR.
Results
Abnormal uptake, which was suspected to be recurrence or metastasis, was detected in 92.9% (26/28) of the patients with BCR. The SUVmax was 8.4 ± 6.4 in local recurrence, 11.5 ± 11.8 in pelvic lymph nodes (LN), and 4.1 ± 1.6 in bone metastasis (Fig. 1). The detection rates were 66.7% in the PSA group-1 (0.1–0.5 ng/mL), 85.7% in the PSA group-2 (0.5–1.0 ng/mL), and 100% in the PSA group-3 (above 1.0 ng/mL) (Fig. 2a). Among the PET-positive BCR patients (n = 26), local recurrence was detected in 57.7% (15/26), pelvic LN in 42.3% (11/26), and bone metastasis in 15.4% (4/26) (Fig. 2b). Representative cases are shown in Fig. 3. In patient with BCR after 125I-seed implantation, PSMA-PET showed a local recurrence with focal uptakes which was confirmed by biopsy. As shown in Fig. 4, PSMA-PET detected bone lesions without sclerotic lesions on CT, indicating the early stage of bone metastasis. Follow-up CT (11 months after PSMA-PET) showed osteosclerotic lesion in the PSMA-positive lesion on the left ischium, compatible with bone metastasis.
Fig. 1.
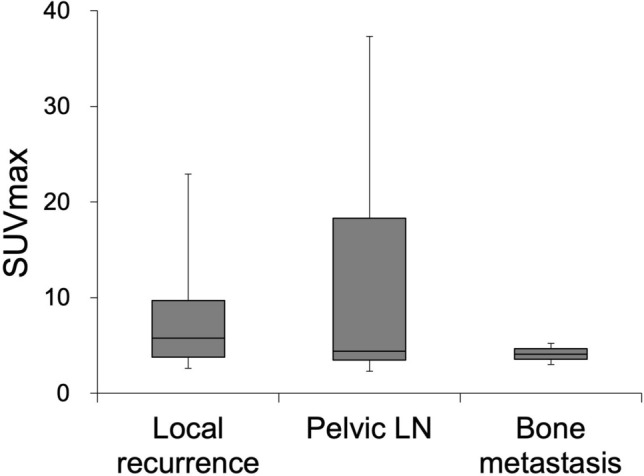
Uptake in recurrent lesions on [18F]PSMA-1007 PET (Pelvic LN: pelvic lymph nodes)
Fig. 2.
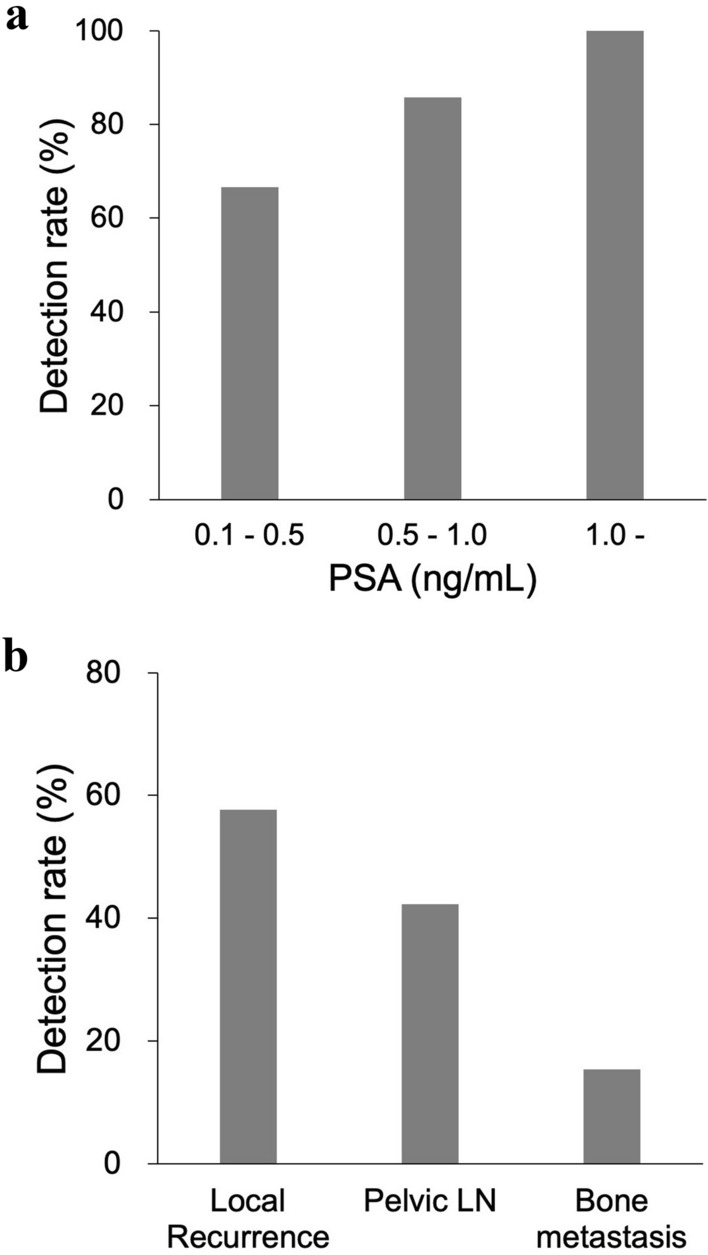
a Detection rates and their relation to PSA values and b location in BCR patients
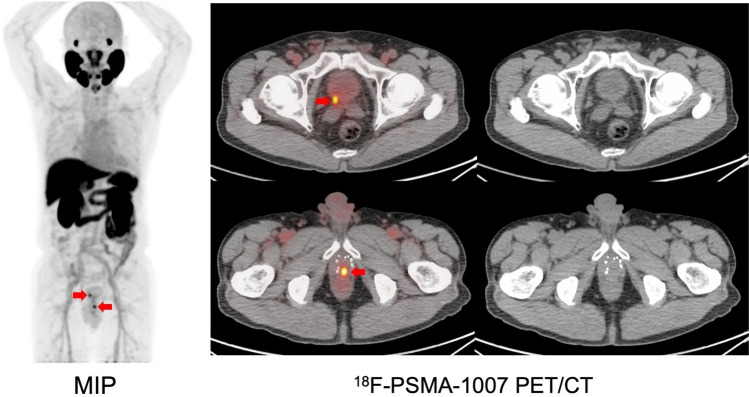
Fig. 3.
[18F]PSMA-1007 PET in BCR patient after 125I-seed implantation (PSA at PET: 3.32 ng/mL) (MIP: maximum intensity projection). Focal uptakes are observed on PSMA-PET (red arrows) and biopsy on the caudal lesion revealed a recurrence. Radiation therapy (cyber-knife) is performed targeting the two lesions and PSA value shows a decrease (PSA at 7 months after cyber-knife: 0.61 ng/mL)
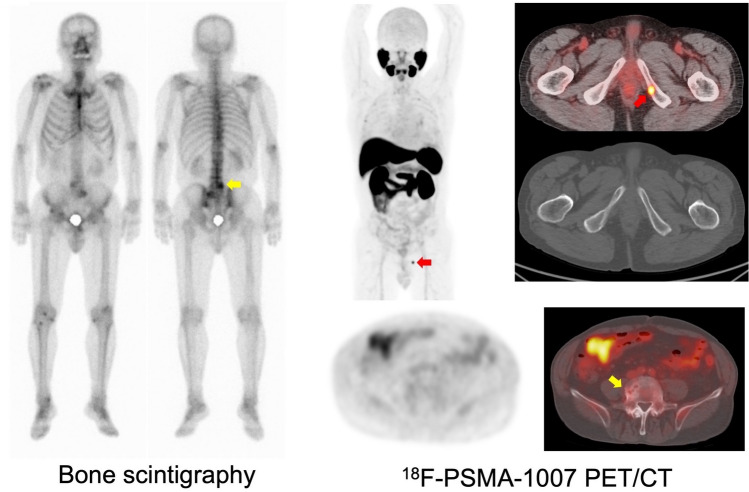
Fig. 4.
BCR patient after radical prostatectomy (PSA at PET: 1.7 ng/mL). Bone scintigraphy shows abnormal uptakes (yellow arrow) on the lower lumbar spine, which is considered to be a degenerative change. PSMA-PET shows focal uptake (red arrows) on the left ischium; whereas, it shows no significant uptake on the lower lumbar spine
The location of the detected recurrence and its impact on patient management in the patients with BCR are shown in Table 2. The treatment strategy was finalized based on the results of PSMA-PET in 78.6% of BCR patients.
Table 2.
(a) Location of the detected recurrence on [18F]PSMA-1007 PET and its relation to the treatment history in BCR patients (n = 28). Radiation therapy included 125I-seed implantation therapy (n = 2). (b) Impact on patient management after [18F]PSMA-1007 PET in BCR patients
| Treatment history | Number of patients | |
|---|---|---|
| Local recurrence | Metastasis | |
| Radiation therapy (RT) | 8/9 (88.9%) | 1/9 (11.1%) |
| Radical prostatectomy | 5/11 (45.5%) | 4/11 (36.4%) |
| Radical prostatectomy and salvage RT | 1/8 (12.5%) | 7/8 (87.5%) |
| Impact on management | Number of patients |
|---|---|
| The treatment strategy was properly decided from PSMA-PET | 22 / 28 (78.6%) |
| The decision by PSMA-PET was correct in patients treated with RT when evaluated from PSA response | 11/11 (100%) |
Discussion
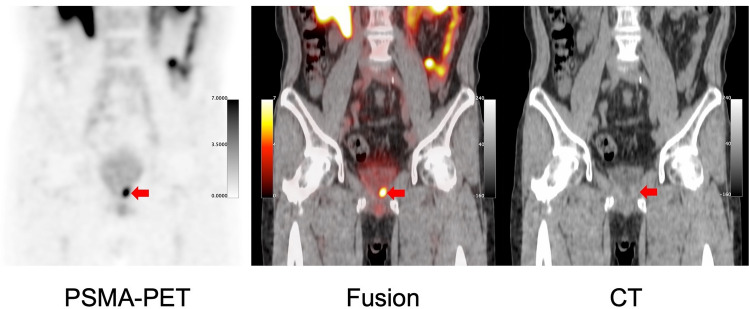
In this study, [18F]PSMA-1007 PET had a high detection rate in patients with BCR. Since urine excretion is minimal in [18F]PSMA-1007 PET, it detects local recurrence more clearly than other PSMA-PET probes. In 53% (8/15) of BCR patients, who were suspected of local recurrence, focal uptake was detected adjacent to the bladder on [18F]PSMA-1007 PET. This suggested its significant advantage of less physiological urine excretion (Fig. 5). In addition, its high detection rate led to determining the proper treatment strategy, particularly local salvage radiation therapy, and boosting PSMA-positive lesions. These findings were consistent with those of previous reports [6, 11].
Fig. 5.
BCR patient after radical prostatectomy. Focal uptakes (red arrows) are detected at the edge of the bladder on [18F]PSMA-1007 PET (PSA at PET: 0.19 ng/mL). The patient is treated by radiation therapy targeting the PSMA-positive lesion and PSA values return to normal level (< 0.01 ng/mL)
In BCR patients who had negative findings on conventional CT or BS, the PSMA-positive lesions were mainly smaller lymph nodes or nodules undetectable by CT. In comparison to BS, PSMA-PET was more effective in detecting small lesions with minimal osteosclerosis or excluded degenerative change although BS is sufficiently sensitive to detect small bone metastasis in most of the recurrent cases (data not shown). However, caution is advised when interpreting bone uptake of [18F]PSMA-1007, especially in the ribs, considering false-positive findings [12]. In addition, PSMA is not specific to prostate cancer. Some cancer lesions showed high PSMA uptake, mimicking metastasis of prostate cancer on PSMA-PET [13].
Conclusions
[18F]PSMA-1007 PET showed a high detection rate in recurrent and metastatic lesions. In patients with BCR, its high detection led to proper treatment strategies, such as salvage radiation therapy or surgical removal of recurrent lymph nodes.
Acknowledgements
We would like to thank all the members of the Department of Nuclear Medicine and the Department of Radiology at Osaka University Hospital for preparing the PET probes and performing PET acquisition. This study was funded by the QiSS program of the OPERA (Grant Number: JPMJOP1721) from the Japan Science and Technology Agency (JST) and Innovative Cancer Medical Practical Research Project from AMED (Japan Agency for Medical Research and Development). FLG and JC: patent application of PSMA-1007. FLG is also an advisor at ABX Radeberg, SOFIE Biosciences and Telix pharmaceutical.
Funding
This study was funded by the QiSS program of the OPERA (Grant Number: JPMJOP1721) from the Japan Science and Technology Agency (JST) and Innovative Cancer Medical Practical Research Project from AMED (Japan Agency for Medical Research and Development).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Evans JC, Malhotra M, Cryan JF, O’Driscoll CM. The therapeutic and diagnostic potential of the prostate specific membrane antigen/glutamate carboxypeptidase II (PSMA/GCPII) in cancer and neurological disease. Br J Pharmacol. 2016;173(21):3041–3079. doi: 10.1111/bph.13576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kasperzyk JL, Finn SP, Flavin R, Fiorentino M, Lis R, Hendrickson WK, et al. Prostate-specific membrane antigen protein expression in tumor tissue and risk of lethal prostate cancer. Cancer Epidemiol Biomarkers Prev. 2013;22(12):2354–2363. doi: 10.1158/1055-9965.EPI-13-0668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koerber SA, Utzinger MT, Kratochwil C, Kesch C, Haefner MF, Katayama S, et al. 68Ga-PSMA-11 PET/CT in newly diagnosed carcinoma of the prostate: correlation of intraprostatic PSMA uptake with several clinical parameters. J Nucl Med. 2017;58(12):1943–1948. doi: 10.2967/jnumed.117.190314. [DOI] [PubMed] [Google Scholar]
- 4.Emmett L, Yin C, Crumbaker M, Hruby G, Kneebone A, Epstein R, et al. Rapid modulation of PSMA expression by androgen deprivation: serial 68Ga-PSMA-11 PET in men with hormone-sensitive and castrate-resistant prostate cancer commencing androgen blockade. J Nucl Med. 2019;60(7):950–954. doi: 10.2967/jnumed.118.223099. [DOI] [PubMed] [Google Scholar]
- 5.Giesel FL, Hadaschik B, Cardinale J, Radtke J, Vinsensia M, Lehnert W, et al. F-18 labelled PSMA-1007: biodistribution, radiation dosimetry and histopathological validation of tumor lesions in prostate cancer patients. Eur J Nucl Med Mol Imaging. 2017;44(4):678–688. doi: 10.1007/s00259-016-3573-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giesel FL, Knorr K, Spohn F, Will L, Maurer T, Flechsig P, et al. Detection efficacy of 18F-PSMA-1007 PET/CT in 251 patients with biochemical recurrence of prostate cancer after radical prostatectomy. J Nucl Med. 2019;60(3):362–368. doi: 10.2967/jnumed.118.212233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sprute K, Kramer V, Koerber S, Meneses M, Fernandez R, Soza-Ried C, et al. Diagnostic accuracy of 18F-PSMA-1007-PET/CT imaging for lymph node staging of prostate carcinoma in primary and biochemical recurrence. J Nucl Med. 2021;62(2):208–213. doi: 10.2967/jnumed.120.246363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cardinale J, Schäfer M, Benešová M, Bauder-Wüst U, Leotta K, Eder M, et al. Preclinical evaluation of 18F-PSMA-1007, a new prostate-specific membrane antigen ligand for prostate cancer imaging. J Nucl Med. 2017;58(3):425–431. doi: 10.2967/jnumed.116.181768. [DOI] [PubMed] [Google Scholar]
- 9.Naka S, Watabe T, Kurimoto K, Uemura M, Soeda F, Neels OC, et al. Automated [18F]PSMA-1007 production by a single use cassette-type synthesizer for clinical examination. EJNMMI Radiopharm Chem. 2020;5(1):18. doi: 10.1186/s41181-020-00101-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Afshar-Oromieh A, Holland-Letz T, Giesel FL, Kratochwil C, Mier W, Haufe S, et al. Diagnostic performance of 68Ga-PSMA-11 (HBED-CC) PET/CT in patients with recurrent prostate cancer: evaluation in 1007 patients. Eur J Nucl Med Mol Imaging. 2017;44(8):1258–1268. doi: 10.1007/s00259-017-3711-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koerber SA, Will L, Kratochwil C, Haefner MF, Rathke H, Kremer C, et al. 68Ga-PSMA-11 PET/CT in primary and recurrent prostate carcinoma: Implications for radiotherapeutic management in 121 patients. J Nucl Med. 2018;60(2):234–40. doi: 10.2967/jnumed.118.211086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rauscher I, Krönke M, König M, Gafita A, Maurer T, Horn T, et al. Matched-pair comparison of 68Ga-PSMA-11 PET/CT and 18F-PSMA-1007 PET/CT: frequency of pitfalls and detection efficacy in biochemical recurrence after radical prostatectomy. J Nucl Med. 2020;61(1):51–57. doi: 10.2967/jnumed.119.229187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soeda F, Watabe T, Kato H, Uemura M, Nonomura N. Duodenal adenocarcinoma mimicking metastasis of prostate cancer on 18F-prostate-specific membrane Antigen-1007 PET/CT. Clin Nucl Med. 2021;46(1):49–51. doi: 10.1097/RLU.0000000000003400. [DOI] [PMC free article] [PubMed] [Google Scholar]