Abstract
Fatigue is considered a key symptom of major depressive disorder (MDD), yet the term lacks specificity. It can denote a state of increased sleepiness and lack of drive (i.e., downregulated arousal) as well as a state of high inner tension and inhibition of drive with long sleep onset latencies (i.e., upregulated arousal), the latter typically found in depression. It has been proposed to differentiate fatigue along the dimension of brain arousal. We investigated whether such stratification within a group of MDD patients would reveal a subgroup with distinct clinical features. Using an automatic classification of EEG vigilance stages, an arousal stability score was calculated for 15-min resting EEGs of 102 MDD patients with fatigue. 23.5% of the patients showed signs of hypoarousal with EEG patterns indicating drowsiness or sleep; this hypoaroused subgroup was compared with remaining patients (non-hypoaroused subgroup) concerning self-rated measures of depressive symptoms, sleepiness, and sleep. The hypoaroused subgroup scored higher on the Beck Depression Inventory items “loss of energy” (Z = − 2.13, p = 0.033; ɳ2 = 0.044, 90% CI 0.003–0.128) and “concentration difficulty” (Z = − 2.40, p = 0.017; ɳ2 = 0.056, 90% CI 0.009–0.139), and reported higher trait and state sleepiness (p < 0.05) as compared to the non-hypoaroused group. The non-hypoaroused subgroup, in contrast, reported more frequently the presence of suicidal ideation (Chi2 = 3.81, p = 0.051; ɳ2 = 0.037, 90% CI 0.0008–0.126). In this study, we found some evidence that stratifying fatigued MDD patients by arousal may lead to subgroups that are pathophysiologically and clinically more homogeneous. Brain arousal may be a worth while target in clinical research for better understanding the mechanisms underlying suicidal tendencies and to improve treatment response.
Electronic supplementary material
The online version of this article (10.1007/s00406-020-01216-w) contains supplementary material, which is available to authorized users.
Keywords: Fatigue, Depression, EEG, Brain arousal regulation, Neurophysiology
Introduction
The World Health Organization estimates that major depressive disorder (MDD) is the leading cause of the global burden of disease [1]. To date, a major shortcoming of the classification systems for MDD is the lack of methods to identify pathophysiologically and clinically more homogeneous subgroups. Further, there is a lack of semantic clarity regarding its key symptoms [2] which is particularly true when considering fatigue—a core symptom of depression according to ICD-10 and one of the diagnostic criteria in DSM-5. Over 90% of depressed patients complain about fatigue; it is a highly prevalent prodromal and residual symptom of MDD, which has significant effects on functional outcomes (see [3] for review). However, fatigue is also commonly reported in the context of a variety of inflammatory and immunological processes (e.g., [4]).
It has been proposed to differentiate two subtypes of fatigue, namely hyper- and hypoaroused fatigue, based on clinical phenomenology (see Table 1; [5, 6] for detailed explanation). According to this model, fatigue in the context of typical depression is often associated with inner tension and inhibition of drive—a state with an upregulated brain arousal. This is supported by clinical studies demonstrating hyperarousal in depression, as evidenced by hypothalamic–pituitary–adrenal (HPA) axis hyperactivity [7, 8], prolonged sleep onset latencies [9, 10], or increases in heart rate and skin conductance [11]. In contrast, fatigue in the context of inflammatory and immunological disorders or cancer is, according to the model, a state with downregulated arousal. This is corroborated by the finding of an underactive HPA axis in some disorders associated with fatigue (e.g., cancer-related fatigue [12]; for review, see [13]), increased daytime sleepiness (e.g., cancer-related fatigue [14]; multiple sclerosis [15]) and short sleep onset latencies [15]. However, there is first evidence that hyperarousal in these conditions is associated with a higher depression score [16].
Table 1.
Proposed features distinguishing between two types of fatigue according to the regulation of brain arousal
| Hypoaroused fatigue | Hyperaroused fatigue | |
|---|---|---|
| Drive | Lack of drive/anergic state [17] | Inhibition of drive/retardation [18] |
| Daytime wakefulness |
Excessive daytime sleepiness [14] short sleep latency in the Multiple Sleep Latency Test [19] |
Not sleepy, long sleep latency in the Multiple leep atency Test [9] |
| Wakefulness regulation | Unstable [43], short sleep latency | Hyperstable [37], prolonged sleep latency |
| Hypothalamic–pituitary–adrenal axis activity | Decreased, blunted [12,20] | Increased [7, 19, 20] |
| Sleep disturbances | Predict fatigue severity [21] | Not associated with fatigue severity [21] |
| Positive treatment response |
Psychostimulants [22] |
Antidepressants [46] |
A modified version of this table was first published in [5]
The suggested distinction assumes differences in the underlying pathophysiology that may have implications for treatment—fatigue syndromes with an unstable arousal regulation might respond to drugs with wakefulness stabilizing properties like psychostimulants, whereas antidepressants might be more effective for conditions with a hyperstable arousal regulation. Moreover, the suggested heterogeneity may at least partly explain the inconsistent effect of the pharmacological treatment on fatigue which has been reported for MDD [3], multiple sclerosis [22] or cancer [25].
The term brain arousal used herein denotes global functional states of the central nervous system (CNS). At the behavioral level, brain arousal levels are associated with different degrees of wakefulness, ranging from alert wakefulness to deep sleep [26]. A successful situational adaptation to changing environmental conditions requires an adequate regulation of arousal; arousal increases in threatening situations and decreases under rest. Besides environmental conditions substantially affecting the level of arousal, stable interindividual differences are known [27, 28], and a genetic basis has been proposed [29, 30]. A growing body of evidence suggests that brain arousal regulation is a fundamental neurophysiological process (for review see [26, 31]). Furthermore, it is one of the five domains to consider for creating meaningful clinical subgroups in psychiatric research proposed by the US-American National Institute of Mental Health (NIMH) in their Research Domain Criteria Project (RDoC; [32]).
The level and the regulation of brain arousal can be assessed using an electroencephalogram (EEG) under resting condition with closed eyes. To objectively assess arousal regulation, an algorithm was developed that allows the reliable classification of EEG vigilance stages [27] within multichannel resting EEG recordings (Vigilance Algorithm Leipzig (VIGALL 2.1); for a detailed description, see [33]). VIGALL has been validated with simultaneous EEG–PET and EEG–fMRI studies (for review, see [34]) and by relating EEG vigilance stages to parameters of the autonomic nervous system [28, 35, 36]. Using this approach, arousal dysregulations have been found in patients with affective disorders and ADHD [10, 37–40]. In patients with MDD an upregulated arousal regulation, compared to healthy controls during a resting state EEG, is a robust finding [37, 41, 42]. In contrast, patients with cancer–related fatigue have been shown to have a downregulation of arousal, with more rapid declines to lower arousal levels than healthy controls during a resting-state EEG [43].
Besides frequently found hyperarousal in MDD, signs of hypoarousal (e.g., excessive daytime sleepiness, hypersomnia) have also been reported (e.g., [44]). In the present study, we set out to examine whether stratifying MDD patients with fatigue by brain arousal would reveal subgroups which differ concerning depressive symptomatology, daytime trait and state sleepiness, and sleep characteristics.
Materials and methods
Subjects
Archival records of depressed in- and outpatients admitted to the Psychiatric Department at the University Hospital Leipzig between 08/2012 and 12/2014 were screened. Included were subjects with age ≥ 18, a diagnosis of MDD with a current depressive episode, who had filled out the multidimensional fatigue inventory (MFI-20 [47]; only patients admitted to our department for the first time filled out the MFI-20). From the 141 initially eligible patients, 132 (93.6%) fulfilled the MFI cut-off score criteria (see below for the cut-off definition). Thereof, we excluded patients with comorbid psychiatric (DSM-IV axis I), major somatic or neurological disorders (diagnoses had been confirmed by a senior physician); diseases associated with fatigue (e.g., obesity (BMI ≥ 30 kg/m2), HIV, rheumatic disorders, advanced cancer); current z-hypnotics or benzodiazepine treatment; a history of head injury with loss of consciousness over 1 h; alcohol abuse within the past 6 months or use of illegal drugs; pathological EEG and those containing more that 15% artefactual segments; more than 2 missing items per questionnaire. In total, 102 patients were included into the study. Part of the dataset has previously been published [46].
Questionnaires
To evaluate fatigue, depressive symptoms, sleepiness and sleep characteristics, the following self-report instruments were filled by the patients immediately before the EEG recording:
Multidimensional Fatigue Inventory (MFI-20; [47]) is a 20-item questionnaire covering five fatigue dimensions: general fatigue, physical fatigue, mental fatigue, reduced activity and reduced motivation. Dimensional scores range between 4 and 20, with higher scores indicating higher degree of fatigue. As the MFI-20 has not yet been standardized specifically for MDD patients, we used norm values obtained from a sample representative of the German population [48]. The influence of age and sex on fatigue scores was taken into account, since the norm values are stated for both sexes and three age groups. Only patients with scores exceeding the 75th percentile on the dimension of general fatigue were included into the study.
Beck Depression Inventory version II (BDI-II; [49]) is a 21-item questionnaire, assessing the severity of depression; except for items 16 and 18, each item has four possible responses, ranging in intensity from 0 to 3; the sum score ranges from 0 to 63.
Epworth Sleepiness Scale (ESS; [50]) is an 8-item questionnaire, assessing trait daytime sleepiness. On a 4-point scale (0–3), respondents are asked to rate their usual probability of dozing off while engaged in eight different activities (e.g., “sitting and reading”, “as a passenger in a car for an hour without a break”, “sitting quietly after a lunch without alcohol”). The sum score ranges between 0 and 24 with scores over 10 indicating mild excessive daytime sleepiness [51].
Stanford Sleepiness Scale (SSS; [52]) is a questionnaire assessing state sleepiness, with a seven-point rating scale: 1 (feeling active, vital, alert); 2 (functioning at high levels, but not at peak; able to concentrate); 3 (awake, but relaxed; responsive but not fully alert); 4 (somewhat foggy, let down); 5 (foggy; losing interest in remaining awake; slowed down); 6 (sleepy, woozy, fighting sleep; preferring to lie down); 7 (no longer fighting sleep, sleep onset soon).
Multiple items of German sleep inventory (SF-A/R; [53]) were used to estimate sleep quality and total time in bed the night before the EEG recording. The sleep quality score, ranging from 1 (impaired) to 5 (excellent), consists of items assessing sleep onset latency, quantity and duration of the time awake, early awakening and overall sleep characteristics (e.g., steady, deep, undisturbed). Total time in bed according to SF-A/R is calculated from items assessing time of going to sleep in the evening and time of getting up in the morning.
EEG recording and preprocessing
The recordings took place between 8 am and 3 pm in a dimly lit booth with sound attenuation; participants were instructed to close the eyes, relax and not fight against sleep pressure. Fifteen minutes of resting eyes-closed EEG was recorded using a 40-channel QuickAmp amplifier (Brain Products GmbH, Gilching, Germany) from 31 monopolar referenced electrodes according to an extended international 10–20 system with impedances kept below 10 kΩ and at a sampling rate of 1 kHz. A total of four bipolar referenced electro-oculogram (EOG) electrodes were placed above and below the right eye and at the canthus of each eye. Data were recorded and preprocessed using the BrainVision Analyzer 2.0 software (Brain Products GmbH, Gilching, Germany). Preprocessing included: filtering (70 Hz low-pass, 0.5 Hz high-pass, 50 Hz notch filter with ± 2 Hz range); rejection of periods with open eyes based on visual EOG screening; rejection of eye movement and muscle artifacts using independent component analysis (ICA). Remaining muscle/sweat/movement artifacts were excluded from the analysis after visual inspection; thereafter, data were re-referenced to a common average.
Brain arousal regulation was assessed using the freely available VIGALL 2.1 algorithm (available at https://research.uni-leipzig.de/vigall/), which attributes one of seven EEG vigilance stages to each 1-s EEG segment. EEG vigilance stages range from active wakefulness to sleep onset (see Table 2 for scoring criteria). Consequently, using scoring criteria presented in Table 3, we calculated an arousal stability score for each subject, indicating the degree of arousal decline. Referring to the replicated finding of a hyperstable arousal regulation in depressed patients, who typically do not reach states of drowsiness and sleep in a 15-min resting EEG, we divided the patients into two groups: patients reaching EEG vigilance stages indicating arousal states of increased drowsiness and sleep (“hypoaroused fatigue”; arousal stability score ≤ 6) and patients not reaching these states (“non-hypoaroused fatigue”; arousal stability score ≥ 7).
Table 2.
Scoring criteria of EEG vigilance stages (see the VIGALL 2.1 manual [33] for detailed description); higher scores correspond to higher arousal levels
| EEG characteristic | VIGALL stage classification | Stage score |
|---|---|---|
| Low amplitude, predominantly beta EEG (12–25 Hz) without horizontal SEM | 0 | 7 |
| Alpha frequency (8–12 Hz), dominant in occipital regions | A1 | 6 |
| Alpha frequency (8–12 Hz), dominant in parietal regions | A2 | 5 |
| Alpha frequency (8–12 Hz), dominant in frontal regions | A3 | 4 |
| Low amplitude, predominantly beta EEG (12–25 Hz) with horizontal SEM | B1 | 3 |
| Predominantly delta (2–4 Hz) or theta (4–7 Hz) EEG | B2/3 | 2 |
| K-complexes or sleep spindles | C | 1 |
VIGALL 2.1 Vigilance Algorithm Leipzig 2.1, EEG electroencephalogram, SEM slow eye movements
Table 3.
Scoring criteria of arousal stability score
| Scoring criteria | Arousal stability score | |
|---|---|---|
| ≥ 2/3 of all segments classified as 0 or A1 | 11 | Non-hypoaroused fatigue |
| ≥ 2/3 of all segments classified as 0, A1, A2 or A3 | 10 | |
| ≥ 1/3 of segments from minutes 11–15 classified as B1 | 9 | |
| ≥ 1/3 of segments from minutes 6–10 classified as B1 | 8 | |
| ≥ 1/3 of segments from minutes 1–5 classified as B1 | 7 | |
| ≥ 1/3 of segments from minutes 11–15 classified as B2/3 | 6 | Hypoaroused fatigue |
| ≥ 1/3 of segments from minutes 6–10 classified as B2/3 | 5 | |
| ≥ 1/3 of segments from minutes 1–5 classified as B2/3 | 4 | |
| ≥ 1 C-stage occurred in minutes 11–15 | 3 | |
| ≥ 1 C-stage occurred in minutes 10–6 | 2 | |
| ≥ 1 C-stage occurred in minutes 5–1 | 1 |
Statistical analyses
Statistical analyses were conducted using SPSS Statistics 23 (IBM corp.; Armonk, NY, USA). Differences between groups were analyzed using nonparametric tests (Chi2, Mann–Whitney U). The Spearman correlation coefficient was used as measure of association, since tested variables were non-normally distributed. The two-tailed significance level was set to p = 0.05. The effect size ɳ2 was calculated with R version 4.0.2 [63] according to [64]; the bootstrapped 90% confidence intervals are based on 10,000 replications.
Results
Descriptive analyses
A total of 102 patients with a current MDD diagnosis according to the ICD-10 and fatigue of clinically significant severity constituted the study sample (see Table 4 for description and scores). Thereof, 57% were unmedicated (i.e., no AD medication for the past 7 days or first dose < 24 h before the recording), 28% were treated with SSRIs and 15% with SSRI combined with other AD medication (see Table 4 for details). No differences between medicated and unmedicated subjects were found concerning the arousal stability score (M ± SD: 8.2 ± 1.9 vs 7.6 ± 2.7), BDI-II score (M ± SD: 28.7 ± 8.4 vs 30.1 ± 8.8), MFI-20 dimensions, trait (M ± SD: 9.2 ± 4.8 vs 8.5 ± 3.3) or state sleepiness (M ± SD: 3.5 ± 1.1 vs 3.7 ± 1.3).
Table 4.
Characteristics of the total sample and the arousal groups; comparison between the hypoaroused fatigue group (arousal stability score ≤ 6) and the non-hypoaroused fatigue group (arousal stability score ≥ 7)
| Total sample (N = 102) | Non-hypoaroused fatigue (n = 78) | Hypoaroused fatigue (n = 24) | Test value | p value | |
|---|---|---|---|---|---|
| Demographic variables | |||||
| Age | 37.7 ± 12.5 [18–75] | 38.4 ± 12.2 | 35.3 ± 13.5 | Z = − 1.28 | 0.201 |
| Gender (F/M ratio) | 61/41 | 45/33 | 16/8 | X2 = 0.62 | 0.433 |
| MFI-20 dimensions | |||||
| General fatigue | 15.0 ± 2.4 [10–20] | 14.9 ± 2.4 | 15.4 ± 2.5 | Z = − 0.60 | 0.547 |
| Mental fatigue | 14.8 ± 3.2 [4–20] | 14.4 ± 3.3 | 15.9 ± 2.3 | Z = − 2.09 | 0.037 |
| Physical fatigue | 14.0 ± 3.2 [4–20] | 13.7 ± 3.3 | 14.8 ± 2.7 | Z = − 1.34 | 0.180 |
| Reduced activity | 15.5 ± 2.9 [8–20] | 15.3 ± 2.8 | 16.2 ± 3.1 | Z = − 1.67 | 0.095 |
| Reduced motivation | 13.5 ± 3.1 [4–20] | 13.5 ± 3.1 | 13.7 ± 3.5 | Z = − 0.49 | 0.625 |
| AD medication (yes/no) | 44/58 | 37/41 | 7/17 | X2 = 2.49 | 0.114 |
| (Es-) citalopram | 25 | 4 | |||
| (Es-) citalopram/mirtazapine | 4 | 1 | |||
| (Es-) citalopram/opipramol | 1 | 1 | |||
| (Es-) citalopram/trimipramine | 2 | – | |||
| Venlafaxine | – | 1 | |||
| Sertraline | 5 | – | |||
| Arousal stability score | 7.8 ± 2.4 | 8.9 ± 1.6 [7–11] | 4.5 ± 1.3 [2–6] | ||
| Arousal related variables | |||||
| Time of EEG recording (h:min) | 11:02 ± 1:50 | 10:53 ± 1:45 | 11:31 ± 2:04 | Z = − 1.14 | 0.254 |
| Coffee prior to EEG (yes/no) | 61/41 | 48/30 | 13/11 | Χ2 = 0.42 | 0.519 |
| Time of coffee consumption (h:min) | 8:05 ± 1:12 | 8:07 ± 1:15 | 8:00 ± 1:03 | Z = − 0.24 | 0.814 |
| Clinical data | |||||
| History of suicide attempts (yes/no) | 11/87, n = 98 | 7/67, n = 74 | 4/20, n = 24 | Χ2 = 0.94 | 0.331 |
| ICD-10 diagnosis (F32/F33 ratio) | 61/41 | 44/34 | 17/7 | Χ2 = 1.58 | 0.208 |
| Familya history of affective disorders (yes/no) | 16/53 | 11/39 | 5/14 | Χ2 = 0.14 | 0.708 |
Bold values denote statistical significance at the p < 0.05 level
Mean ± SD [range]
MFI-20 Multiple Fatigue Inventory, AD antidepressant, F32 depressive episode, F33 recurrent depressive disorder
aFirst degree relatives
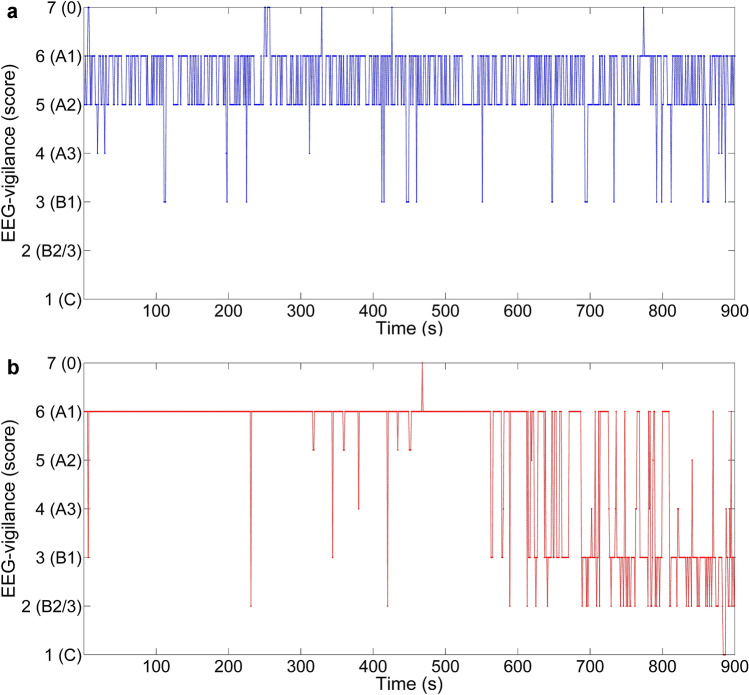
In this sample of fatigued MDD patients, 76.5% stayed in higher arousal states (n = 78; non-hypoaroused fatigue group), whereas 23.5% reached arousal states of increased drowsiness or sleep (n = 24; hypoaroused fatigue group) during the 15-min EEG recording. No differences concerning arousal biasing variables (see “arousal related variables” in Table 4) were found, as well as no effect of medication on arousal stability score (M ± SD; hypoaroused medicated vs hypoaroused unmedicated: 5.1 ± 0.9 vs 4.2 ± 1.4, Z = − 1.60, p = 0.11; non-hypoaroused medicated vs non-hypoaroused unmedicated: 8.8 ± 1.5 vs 9.0 ± 1.7, Z = − 0.50, p = 0.62). Representative examples of the time courses of scored EEG vigilance over the entire recording period for either group are presented in Fig. 1.
Fig. 1.
Time course of scored EEG vigilance over 900 consecutive 1-s segments. a Subject assigned to the non-hypoaroused fatigue group (arousal stability score = 10) and (b) subject assigned to the hypoaroused fatigue group (arousal stability score = 3). Each 1-s EEG segment was classified according to the scoring criteria presented in Table 2
Between-group comparisons: BDI-II, ESS, SSS and SF-A/R item comparisons
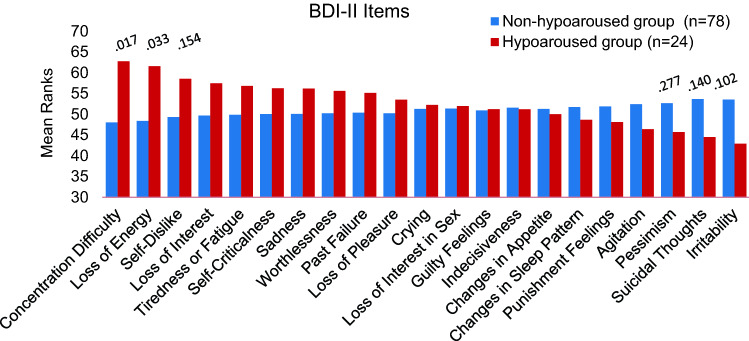
BDI-II item comparisons revealed that patients assigned to the hypoaroused group reported significantly higher scores on item “loss of energy” (Z = − 2.13, p = 0.033; ɳ2 = 0.044, 90% CI 0.003–0.128) and “concentration difficulty” (Z = − 2.40, p = 0.017; ɳ2 = 0.056, 90% CI 0.009–0.139) than patients in the non-hypoaroused group (see Fig. 2).
Fig. 2.
Beck Depression Inventory-II items comparison between the hypoaroused fatigue group and the non-hypoaroused fatigue group. Items are sorted according to mean ranks differences; six smallest p values are presented above the bars
Although the Mann–Whitney U test revealed no significant difference concerning the item “suicidal thoughts” (Z = − 1.47, p = 0.14), 64% of the non-hypoaroused group vs 42% of the hypoaroused group reported having suicidal ideations (Chi2 = 3.81, p = 0.051; ɳ2 = 0.037, 90% CI 0.0008–0.126; “I don’t have any thoughts of killing myself”—0, “I have thoughts of killing myself, but I would not carry them out”—1; “I would like to kill myself”—2; “I would kill myself if I had the chance”—3). Given the clinical relevance of suicidal ideations we further performed an exploratory correlation analysis, wherein the item “suicidal thoughts” and arousal stability score correlated significantly when age, sex and depression severity (total BDI-II score) were included in a partial correlation analysis as control variables (rho = 0.27, p = 0.018).
Patients in the hypoaroused group reported significantly higher scores on daytime trait sleepiness and state sleepiness, and experienced significantly higher mental fatigue than patients in the non-hypoaroused group. No group differences were found concerning sleep quality and total time in bed as measured by SF-A/R items (see Table 5).
Table 5.
Outcome variables for the total sample and comparison between hypoaroused fatigue group (arousal stability score ≤ 6) and non-hypoaroused fatigue group (arousal stability score ≥ 7)
| Total sample (N = 102) | Non-hypoaroused fatigue (n = 78) | Hypoaroused fatigue (n = 24) | Test value | p value | Effect size ɳ2 (90% CI) | |
|---|---|---|---|---|---|---|
| Daytime sleepiness | ||||||
| Trait (ESS) | 8.8 ± 4.0 [0–19] | 8.3 ± 4 | 10.4 ± 4 | Z = − 2.20 | 0.028 | 0.047 (0.004–0.134) |
| State (SSS) |
3.6 ± 1.2 [1–6] n = 99 |
3.4 ± 1.2 n = 75 |
4.1 ± 1.2 | Z = − 2.31 | 0.021 | 0.054 (0.004–0.138) |
| BDI-II score | 29.5 ± 8.6 [16–53] | 29.2 ± 8.8 | 30.3 ± 7.9 | Z = − 0.73 | 0.468 | 0.005 (.00–0.05) |
| SF-A/R | ||||||
| Sleep quality |
2.7 ± 1.0 n = 95 |
2.7 ± 1.03 n = 73 |
2.6 ± 0.7 n = 22 |
Z = − 0.23 | 0.819 | 0.0006 (.00–0.03) |
| Total time in bed (h:min) |
7:13 ± 1:38 [1:00–11:30] n = 100 |
7:09 ± 1:44 [1:00–11:30] n = 76 |
7:24 ± 1:19 [5:15–10:30] n = 24 |
Z = − 0.32 | 0.749 | 0.001 (.00–0.037) |
Bold values denote statistical significance at the p< 0.05 level
Mean ± SD [range]; ESS Epworth Sleepiness cale, SSS Stanford Sleepiness Scale, BDI-II Beck Depression nventory version 2, SF-A/R sleep inventory (refers to sleep during the night before EEG recording)
Explorative sensitivity analysis
To examine possible effects of the medication on the aforementioned findings, separate analyses for the unmedicated and medicated groups were conducted (see supplementary). Comparably, the hypoaroused group reported higher scores than the non-hypoaroused group on the BDI-II items “concentration difficulty” and “lack of energy” in both medicated and unmedicated patients. However, the difference reached the level of significance only in the unmedicated group. The item “suicidal thoughts” and arousal stability score correlated significantly when age, sex and depression severity (total BDI-II score) were included as control variables in a partial correlation analysis in the unmedicated (rho = 0.29, p = 0.03) but not in the medicated (rho = 0.06, p = 0.69) group. With or without medication, the hypoaroused group reported higher state and trait sleepiness, and higher mental fatigue, although only the difference in state sleepiness within the unmedicated group reached statistical significance.
Discussion
In the present study, we aimed to explore whether stratifying MDD patients with fatigue by brain arousal, as assessed with an EEG-based resting-state measure, would reveal two subgroups with distinct clinical features. We found that the subgroup with downregulated brain arousal (hypoaroused group) scored significantly higher on BDI-II items “concentration difficulty” and “lack of energy”, on state sleepiness (accessed by SSS) and trait daytime sleepiness (accessed by ESS), and on the MFI-20 dimension “mental fatigue”. In contrast, suicidal thoughts, as measured by one of the BDI-II items, were more frequent in the non-hypoaroused subgroup.
Regarding the BDI-II measure of depressive symptoms, significantly more pronounced concentration difficulties in the hypoaroused group may have resulted from the instability of brain arousal which interferes with the ability to concentrate, and hence contributes to greater mental fatigue, as measured by the MFI-20. Notably, “concentration difficulties” was the most predictive for antidepressant response among 31 clinical and demographic features, as reported recently in a study using a machine learning approach [54]. Moreover, patients in the hypoaroused group complained about a greater loss of energy, despite similar levels of physical and general fatigue. This may reflect the fact that within typical (hyperaroused) depression, patients do not suffer from lack of energy or drive but from inhibition of drive with exhaustion and high inner tension—a completely different psychopathological syndrome [5, 6].
The between-group comparison of the BDI-II item “suicidal thoughts” was marginally significant (p = 0.051), with suicidal ideations more often reported by the non-hypoaroused group. Subsequent exploratory correlation analyses revealed a positive correlation (rho = 0.27, p = 0.018) of brain arousal with the propensity towards suicidal ideation after adjusting for age, sex, and severity of depressive symptoms, which are known parameters associated with suicidal tendencies [55]. This finding supports the view of hyperarousal as a potential pathophysiological mechanism associated with suicidal ideation [56], and is in line with a recent quantitative EEG sleep study by Dolsen et al. [57]. The authors provided evidence, after adjusting for age, sex, depressive and insomnia symptoms, that hyperarousal during sleep and suicidal ideations may be associated in MDD patients. Therefore, our results support the view of an upregulated brain arousal as a worthwhile target in clinical research which may help to better understand the mechanisms underlying suicidal ideation [58].
Regarding subjective trait daytime sleepiness and state sleepiness at the time of the EEG, increased sleepiness was associated with downregulated arousal, as it has previously been reported for healthy individuals [28]. Both findings support the validity of the VIGALL classification. It is conceivable that patients with downregulated brain arousal were drowsy or fell asleep due to poor sleep quality the night before the recording, since nighttime sleep can affect daytime vigilance, however, no between-group differences of sleep parameters were found. This indicates that group differences concerning arousal and state/trait sleepiness are independent of the sleep characteristics in the night preceding the EEG recording.
Despite the profound fatigue all patients experienced, less than a quarter of the sample (23.5%) reached arousal stages of increased drowsiness or fell asleep within the 15-min eyes-closed EEG during quiet rest. This proportion is markedly low compared to those found in (1) a comparable healthy sample without fatigue (48.3%; [37]) and (2) a 10-years-older sample with cancer-related fatigue of comparable severity (59%; [43]). The fact that only a minority of fatigued patients in our sample shows a pronounced decline of brain arousal is in line with earlier studies reporting an upregulated brain arousal in patients with MDD as assessed with VIGALL [37, 41, 42].
There was no effect of medication on arousal neither within the total sample, nor within the arousal groups. The effect of hypoarousal in medicated and unmedicated patients showed the same direction as found in the total sample; nonetheless, the level of significance was only reached within the unmedicated group.
We suggest stratifying patients along the dimension of brain arousal. Differentiating fatigue into a hypo- and hyperaroused subtype in depressed patients may be of relevance for personalized treatment. There is some evidence that treatment with psychostimulants may be beneficial in patients with hypo- but not hyperaroused fatigue (reviewed in [6, 45]). Furthermore, a better response to antidepressants such as selective serotonin reuptake inhibitors has been observed in MDD patients with upregulated brain arousal [46].
Several limitations of the current study should be mentioned: first, the assessment of sleep behavior of the night preceding the EEG recording was based on subjective ratings. Second, the MFI questionnaire that had not yet been standardized for the depressed populations required a cut-off definition for scores representing fatigue of clinically significant severity. Previous studies support our choice of the cut-off [21, 59], e.g., [60] reported that 93.8% of their depressed sample (cf. 93.6% in our sample) complained about clinically significant fatigue as assessed with the Fatigue Questionnaire [61]. Third, inclusion of medicated patients can be seen as a limitation, since fatigue has been described as an adverse reaction to antidepressant treatment (for review [62]). However, in our sample, no differences concerning fatigue scores were found between medicated and unmedicated patients.
Stratifying fatigued MDD patients by arousal may lead to subgroups, which are more homogeneous concerning pathophysiology and symptomatology. Brain arousal may be a worthwhile target in clinical researchofor better understanding the mechanisms underlying suicidal tendencies and to improve treatment response.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank our colleagues Dr. Philippe Jawinski and Dr. Roland Mergl for their valuable advices on statistical methods.
Author contributions
GS, CU, UH, CS and TH contributed to the study conception and design. Material preparation, data collection and analysis were performed by GS, CU and FMS. The first draft of the manuscript was written by GS and CU and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was in part supported within the framework of the cooperation between the German Depression Foundation and the Deutsche Bahn Stiftung gGmbH.
Compliance with ethical standards
Conflict of interest
UH was an advisory board member for Lilly, Lundbeck, Servier, Takeda and Otsuka; a consultant for Bayer and Nycomed; and a speaker for Bristol-Myers Squibb, Medice Arzneimittel, Novartis, and Roche. CU, GS, FMS, CS, TH declare no conflict of interest.
Ethical approval
This retrospective study was performed in line with the principles of the 1964 Declaration of Helsinki. The study protocol was approved by the Ethics Committee of Leipzig University (#236-2007).
Consent to participate
Informed consent was obtained from all participants included in the study.
Footnotes
Galina Surova and Christine Ulke contributed equally and are co-first authors.
References
- 1.World Health Organisation, WHO (2018) Fact sheet No 369: Depression. Available at: http://www.who.int/mediacentre/factsheets/fs369/en/. Accessed 1 May 2020
- 2.Hegerl U. Largely unnoticed flaws in the fundamentals of depression diagnosis: The semantics of core symptoms. Aust N Z J Psychiatry. 2014;48(12):1166–1166. doi: 10.1177/0004867414559550. [DOI] [PubMed] [Google Scholar]
- 3.Ghanean H, Ceniti AK, Kennedy SH. Fatigue in patients with major depressive disorder: prevalence, burden and pharmacological approaches to management. CNS Drugs. 2018;32(1):65–74. doi: 10.1007/s40263-018-0490-z. [DOI] [PubMed] [Google Scholar]
- 4.Morris G, Berk M, Galecki P, et al. The neuro-immune pathophysiology of central and peripheral fatigue in systemic immune-inflammatory and neuro-immune diseases. Mol Neurobiol. 2016;53:1195–1219. doi: 10.1007/s12035-015-9090-9. [DOI] [PubMed] [Google Scholar]
- 5.Hegerl U, Lam RW, Malhi GS, et al. Conceptualising the neurobiology of fatigue. Aust N Z J Psychiatry. 2013;47:312–316. doi: 10.1177/0004867413481505. [DOI] [PubMed] [Google Scholar]
- 6.Hegerl U, Ulke C. Fatigue with up-vs downregulated brain arousal should not be confused. Prog Brain Res. 2016;229:239–254. doi: 10.1016/bs.pbr.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Pariante CM, Lightman S. The HPA axis in major depression: classical theories and new developments. Trends Neurosci. 2008;31:464–468. doi: 10.1016/j.tins.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Silverman MN, Heim CM, Nater UM, Marques AH, Sternberg EM. Neuroendocrine and immune contributors to fatigue. PM&R. 2010;2(5):338–346. doi: 10.1016/j.pmrj.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kayumov L, Rotenberg V, Buttoo K, et al. Interrelationships between nocturnal sleep, daytime alertness, and sleepiness. J Neuropsychiatry Clin Neurosci. 2000;12(1):86–90. doi: 10.1176/jnp.12.1.86. [DOI] [PubMed] [Google Scholar]
- 10.Ulke C, Sander C, Jawinski P, et al. Sleep disturbances and upregulation of brain arousal during daytime in depressed versus non-depressed elderly subjects. World J Biol Psychiatry. 2017;8:633–640. doi: 10.1080/15622975.2016.1224924. [DOI] [PubMed] [Google Scholar]
- 11.Carney RM, Freedland KE, Veith RC. Depression, the autonomic nervous system, and coronary heart disease. Psychosom Med. 2005;67:29–33. doi: 10.1097/01.psy.0000162254.61556.d5. [DOI] [PubMed] [Google Scholar]
- 12.Bower JE, Ganz PA, Aziz N. Altered cortisol response to psychologic stress in breast cancer survivors with persistent fatigue. Psychosom Med. 2005;67:277–280. doi: 10.1097/01.psy.0000155666.55034.c6. [DOI] [PubMed] [Google Scholar]
- 13.Chaudhuri A, Behan PO. Fatigue in neurological disorders. Lancet. 2004;363(9413):978–988. doi: 10.1016/S0140-6736(04)15794-2. [DOI] [PubMed] [Google Scholar]
- 14.Forsythe LP, Helzlsouer KJ, MacDonald R, et al. Daytime sleepiness and sleep duration in long-term cancer survivors and non-cancer controls: results from a registry-based survey study. Support Care Cancer. 2012;20:2425–2432. doi: 10.1007/s00520-011-1358-7. [DOI] [PubMed] [Google Scholar]
- 15.Sater R, Gudesblatt M, Kresa-Reahl K, et al. The relationship between objective parameters of sleep and measures of fatigue, depression, and cognition in multiple sclerosis. Mult Scler J Exp Transl Clin. 2015;1:1–8. doi: 10.1177/2055217315577828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ulke C, Surova G, Sander C, et al. Fatigue in cancer and neuroinflammatory and autoimmune disease: CNS arousal matters. Brain Sci. 2020;10(9):569. doi: 10.3390/brainsci10090569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barsevick A, Frost M, Zwinderman A, Hall P, Halyard M, GENEQOL Consortium I’m so tired: biological and genetic mechanisms of cancer-related fatigue. Qual Life Res. 2010;19(10):1419–1427. doi: 10.1007/s11136-010-9757-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stone EA, Lin Y, Sarfraz Y, Quartermain D. The role of the central noradrenergic system in behavioral inhibition. Brain Res Rev. 2011;67:193–208. doi: 10.1016/j.brainresrev.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ding Q, Whittemore R, Redeker N. Excessive daytime sleepiness in stroke survivors: an integrative review. Biol Res Nurs. 2016;18:420–431. doi: 10.1177/1099800415625285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stoppe M, Meyer K, Schlingmann M, Olbrich S, Then Bergh F. Hyperstable arousal regulation in multiple sclerosis. Psychoneuroendocrinology. 2019;110:104417. doi: 10.1016/j.psyneuen.2019.104417. [DOI] [PubMed] [Google Scholar]
- 21.Anderson KO, Getto CJ, Mendoza TR, et al. Fatigue and sleep disturbance in patients with cancer, patients with clinical depression, and community-dwelling adults. J Pain Symptom Manage. 2003;25(4):307–318. doi: 10.1016/s0885-3924(02)00682-6. [DOI] [PubMed] [Google Scholar]
- 22.Rojí R, Centeno C. The use of methylphenidate to relieve fatigue. Curr Opin Support Palliat Care. 2017;11(4):299–305. doi: 10.1097/SPC.0000000000000296. [DOI] [PubMed] [Google Scholar]
- 23.Papakostas GI, Nutt DJ, Hallett LA, Tucker VL, Krishen A, Fava M. Resolution of sleepiness and fatigue in major depressive disorder: a comparison of bupropion and the selective serotonin reuptake inhibitors. Biol Psychiatry. 2006;60(12):1350–1355. doi: 10.1016/j.biopsych.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 24.Cooper JA, Tucker VL, Papakostas GI. Resolution of sleepiness and fatigue: a comparison of bupropion and selective serotonin reuptake inhibitors in subjects with major depressive disorder achieving remission at doses approved in the European Union. J Psychopharmacol (Oxford) 2014;28(2):118–124. doi: 10.1177/0269881113514878. [DOI] [PubMed] [Google Scholar]
- 25.Mustian KM, Alfano CM, Heckler C, et al. Comparison of pharmaceutical, psychological, and exercise treatments for cancer-related fatigue: a meta-analysis. JAMA Oncol. 2017;3(7):961–968. doi: 10.1001/jamaoncol.2016.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sander C, Hensch T, Wittekind DA, Böttger D, Hegerl U. Assessment of wakefulness and brain arousal regulation in psychiatric research. Neuropsychobiology. 2015;72:195–205. doi: 10.1159/000439384. [DOI] [PubMed] [Google Scholar]
- 27.Huang J, Sander C, Jawinski P, et al. Test-retest reliability of brain arousal regulation as assessed with VIGALL 2.0. Neuropsychiatr Electrophysiol. 2015;1(1):13. [Google Scholar]
- 28.Jawinski P, Kittel J, Sander C, et al. Recorded and reported sleepiness: the association between brain arousal in resting state and subjective daytime sleepiness. Sleep. 2017;40(7):10. doi: 10.1093/sleep/zsx099. [DOI] [PubMed] [Google Scholar]
- 29.Pfaff D, Ribeiro A, Matthews J, Kow LM. Concepts and mechanisms of generalized central nervous system arousal. Ann N Y Acad Sci. 2008;1129:11–25. doi: 10.1196/annals.1417.019. [DOI] [PubMed] [Google Scholar]
- 30.Jawinski P, Kirsten H, Sander C, et al. Human brain arousal in the resting state: a genome-wide association study. Mol Psychiatry. 2019;24(11):1599–1609. doi: 10.1038/s41380-018-0052-2. [DOI] [PubMed] [Google Scholar]
- 31.Hegerl U, Sander C, Hensch T. Arousal regulation in affective disorders. In: Frodl T, editor. Systems neuroscience in depression. Amsterdam: Academic Press; 2016. pp. 341–370. [Google Scholar]
- 32.Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med. 2013;11:126. doi: 10.1186/1741-7015-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hegerl et al (2017) Vigilance Algorithm Leipzig (VIGALL) Version 2.1—Manual. Available from: http://research.uni-leipzig.de/vigall/. Accessed 1 June 2020
- 34.Hegerl U, Hensch T. The vigilance regulation model of affective disorders and ADHD. Neurosci Biobehav Rev. 2014;44:45–57. doi: 10.1016/j.neubiorev.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 35.Ulke C, Huang J, Schwabedal JTC, Surova G, Mergl R, Hensch T. Coupling and dynamics of cortical and autonomic signals are linked to central inhibition during the wake–sleep transition. Sci Rep. 2017;7:11804. doi: 10.1038/s41598-017-09513-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang J, Ulke C, Sander C, et al. Impact of brain arousal and time-on-task on autonomic nervous system activity in the wake–sleep transition. BMC Neurosci. 2018;19(1):18. doi: 10.1186/s12868-018-0419-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hegerl U, Wilk K, Olbrich S, Schoenknecht P, Sander C. Hyperstable regulation of vigilance in patients with major depressive disorder. World J Biol Psychiatry. 2012;13:436–446. doi: 10.3109/15622975.2011.579164. [DOI] [PubMed] [Google Scholar]
- 38.Strauss M, Ulke C, Paucke M, et al. Brain arousal regulation in adults with attention-deficit/hyperactivity disorder (ADHD) Psychiatry Res. 2018;261:102–108. doi: 10.1016/j.psychres.2017.12.043. [DOI] [PubMed] [Google Scholar]
- 39.Ulke C, Mauche N, Makiol C, et al. Successful treatment in a case of ultra-rapid cycling bipolar disorder is reflected in brain arousal regulation. Bipolar Disord. 2018;20:77–80. doi: 10.1111/bdi.12610. [DOI] [PubMed] [Google Scholar]
- 40.Wittekind DA, Spada J, Gross A, et al. Early report on brain arousal regulation in manic vs depressive episodes in bipolar disorder. Bipolar Disord. 2016;18:502–510. doi: 10.1111/bdi.12440. [DOI] [PubMed] [Google Scholar]
- 41.Schmidt FM, Pschiebl A, Sander C, et al. Impact of serum cytokine levels on EEG-measured arousal regulation in patients with major depressive disorder and healthy controls. Neuropsychobiology. 2016;73:1–9. doi: 10.1159/000441190. [DOI] [PubMed] [Google Scholar]
- 42.Ulke C, Tenke CE, Kayser J, Sander C, et al. Resting EEG measures of brain arousal in a multisite study of major depression. Clin EEG Neurosci. 2018;50(1):3–12. doi: 10.1177/1550059418795578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Olbrich S, Sander C, Jahn I, et al. Unstable EEG-vigilance in patients with cancer-related fatigue (CRF) in comparison to healthy controls. World J Biol Psychiatry. 2012;13:146–152. doi: 10.3109/15622975.2010.545434. [DOI] [PubMed] [Google Scholar]
- 44.Shen J, Hossain N, Streiner DL, et al. Excessive daytime sleepiness and fatigue in depressed patients and therapeutic response of a sedating antidepressant. J Affect Disord. 2011;134(1–3):421–426. doi: 10.1016/j.jad.2011.04.047. [DOI] [PubMed] [Google Scholar]
- 45.Hegerl U, Hensch T. Why do stimulants not work in typical depression? Aust N Z J Psychiatry. 2017;51:20–22. doi: 10.1177/0004867416676369. [DOI] [PubMed] [Google Scholar]
- 46.Schmidt FM, Sander C, Dietz M-E, et al. Brain arousal regulation as response predictor for antidepressant therapy in major depression. Sci Rep. 2017;7:45187. doi: 10.1038/srep45187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smets EM, Garssen B, Bonke B, De Haes JC. The multidimensional fatigue inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res. 1995;39:315–325. doi: 10.1016/0022-3999(94)00125-o. [DOI] [PubMed] [Google Scholar]
- 48.Schwarz R, Krauss O, Hinz A. Fatigue in the general population. Oncol Res Treat. 2003;26:140–144. doi: 10.1159/000069834. [DOI] [PubMed] [Google Scholar]
- 49.Beck AT, Guth D, Steer RA, Ball R. Screening for major depression disorders in medical inpatients with the beck depression inventory for primary care. Behav Res Ther. 1997;35:785–791. doi: 10.1016/s0005-7967(97)00025-9. [DOI] [PubMed] [Google Scholar]
- 50.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 51.Sander C, Hegerl U, Wirkner K, et al. Normative values of the Epworth sleepiness scale (ESS), derived from a large German sample. Sleep Breath. 2016;20:1337–1345. doi: 10.1007/s11325-016-1363-7. [DOI] [PubMed] [Google Scholar]
- 52.Hoddes E, Zarcone V, Dement W. The development and use of stanford sleepiness scale (SSS) Psychophysiology. 1972;9:150. [Google Scholar]
- 53.Jaworska N, de la Salle S, Ibrahim MH, Blier P, Knott V. Leveraging machine learning approaches for predicting antidepressant treatment response using electroencephalography (EEG) and clinical data. Front Psychiatry. 2019;9:768. doi: 10.3389/fpsyt.2018.00768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Görtelmeyer R. Schlaffragebogen A und B-Revidierte Fassung (SF-A/R und SF-B/R) Göttingen: Hogrefe; 2011. [Google Scholar]
- 55.Nock M, Borges G, Bromet E, et al. Cross-national prevalence and risk factors for suicidal ideation, plans and attempts. Br J Psychiatry. 2008;192(2):98–105. doi: 10.1192/bjp.bp.107.040113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kang GE, Patriquin MA, Nguyen H, et al. Objective measurement of sleep, heart rate, heart rate variability, and physical activity in suicidality: a systematic review. J Affect Disord. 2020;273:318–327. doi: 10.1016/j.jad.2020.03.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dolsen MR, Cheng P, Arnedt JT, et al. Neurophysiological correlates of suicidal ideation in major depressive disorder: hyperarousal during sleep. J Affect Disord. 2017;212:160–166. doi: 10.1016/j.jad.2017.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Calati R, Nemeroff CB, Lopez-Castroman J, Cohen LJ, Galynker I. Candidate biomarkers of suicide crisis syndrome: what to test next? A concept paper. Int J Neuropsychopharmacol. 2020;23(3):192–205. doi: 10.1093/ijnp/pyz063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Singer S, Kuhnt S, Zwerenz R, et al. Age-and sex-standardised prevalence rates of fatigue in a large hospital-based sample of cancer patients. Br J Cancer. 2011;105(3):445. doi: 10.1038/bjc.2011.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ferentinos P, Kontaxakis V, Havaki-Kontaxaki B, Dikeos D, Papadimitriou G. The fatigue questionnaire: standardization in patients with major depression. Psychiatry Res. 2010;177(1):114–119. doi: 10.1016/j.psychres.2009.01.029. [DOI] [PubMed] [Google Scholar]
- 61.Chalder T, Berelowitz G, Pawlikowska T, et al. Development of a fatigue scale. J Psychosom Res. 1993;37(2):147–153. doi: 10.1016/0022-3999(93)90081-p. [DOI] [PubMed] [Google Scholar]
- 62.Kelly K, Posternak M, Alpert JE. Toward achieving optimal response: understanding and managing antidepressant side effects. Dialogues Clin Neurosci. 2008;10(4):409–418. doi: 10.31887/DCNS.2008.10.4/kkelly. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.R Core Team (2020). R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/. Accessed 20 Sept 2020
- 64.Rosenthal R, DiMatteo MR. Meta-analysis: recent developments in quantitative methods for literature reviews. Annu Rev Psychol. 2001;52:59–82. doi: 10.1146/annurev.psych.52.1.59. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.