ABSTRACT
In recent decades, Galleria mellonella (Lepidoptera: Pyralidae) have emerged as a model system to explore experimental aspects of fungal pathogenesis. The benefits of the G. mellonella model include being faster, cheaper, higher throughput and easier compared with vertebrate models. Additionally, as invertebrates, their use is subject to fewer ethical and regulatory issues. However, for G. mellonella models to provide meaningful insight into fungal pathogenesis, the G. mellonella–fungal interactions must be comparable to mammalian–fungal interactions. Indeed, as discussed in the review, studies suggest that G. mellonella and mammalian immune systems share many similarities, and fungal virulence factors show conserved functions in both hosts. While the moth model has opened novel research areas, many comparisons are superficial and leave large gaps of knowledge that need to be addressed concerning specific mechanisms underlying G. mellonella–fungal interactions. Closing these gaps in understanding will strengthen G. mellonella as a model for fungal virulence in the upcoming years. In this review, we provide comprehensive comparisons between fungal pathogenesis in mammals and G. mellonella from immunological and virulence perspectives. When information on an antifungal immune component is unknown in G. mellonella, we include findings from other well-studied Lepidoptera. We hope that by outlining this information available in related species, we highlight areas of needed research and provide a framework for understanding G. mellonella immunity and fungal interactions.
Keywords: Galleria mellonella, lepidoptera, insect immune system, fungal virulence
Galleria mellonella is a useful model for studying fungal infections with Galleria sharing many similarities with mammals' antifungal immune system and interactions with fungal virulence factors.
INTRODUCTION
Galleria mellonella, commonly known as the greater wax moth, is an agricultural pest that destroys beehives due to rapid reproduction and consumption of wax, pollen and honey (Kwadha et al. 2017). This pest can precipitate economic and ecological ruins in certain environments, yet the same qualities make it a good model organism in the laboratory: large sample sizes, fast experiments and easy rearing. Additionally, while G. mellonella are detrimental to honeybees, they are harmless to humans, pose no direct threat to human health and are safe to work with in a laboratory environment. The popularity of G. mellonella as a research tool in medical mycology is growing rapidly, since being affordable, fast in producing results and amenable to high-throughput experiments, G. mellonella can be used for preliminary studies to narrow down a long list of strains, mutants and drugs of interest to later validate in vertebrates (Mylonakis et al. 2005; Kavanagh and Fallon 2010). While mammalian models such as mice or rabbits are still the closest to recapitulating human immunity, they have large drawbacks, including higher cost, lower throughput, specialized housing facilities, larger size, long reproduction times, higher technical skills required and complex regulatory processes. Galleria mellonella models overcome some of these drawbacks. Among the insect models, G. mellonella are especially appealing because they are relatively large (2 cm long), and easy to manage since the larval stages typically used in research can remain within a Petri dish, and require very little special care, training, conditions or expensive food, whereas the common model organisms, Drosophila melanogaster (fruit fly), are smaller, can fly and may require anesthetization prior to manipulation.
Research using G. mellonella as a model for fungal virulence and pathogenesis has increased tremendously, with an ∼5–10-fold increase in yearly papers discussing G. mellonella research with fungi in the past decade according to PubMed database. As in years prior, many of the papers and reviews within the past five have primarily focused on validating fungal virulence and factors associated with virulence in medically important fungi in the G. mellonella model, while there are also large portions of recent literature investigating the antifungal immune functions of the larvae as well as using G. mellonella to discover and evaluate new antifungal pharmaceuticals.
The strength and relevancy of the G. mellonella model for human fungal diseases are only as strong as the relevant comparisons of the G. mellonella (and other lepidopteran) and mammalian immune systems and host–fungal interactions. While there are shortcomings and pitfalls in comparing G. mellonella and mammalian antifungal immunity, there are also significant similarities and orthologous immune pathways, which we will discuss at length below. The details and genetic underpinnings of G. mellonella immunity are not as well established as in humans or mice, and the first whole-genome sequence of G. mellonella was published only in recent years (Lange et al. 2018). Similarly, the mechanisms of many known fungal virulence factors are not fully characterized in G. mellonella, but generalized findings suggest that many have comparable functions. A set of comparisons between G. mellonella and mammalian fungal host–pathogen interactions is summarized in Table 1.
Table 1.
Comparison of the effectors of mammalian and lepidopteran immunity and their responses to fungi. The mammalian and lepidopteran, particularly the gallerian, antifungal immune responses have overlapping properties, functions and outcomes. This includes similar roles of pathogen recognition receptors, inflammatory immune responses and cell-based processes. However, there are many understudied gaps in understanding the details of antifungal immunity in the Galleria model. In these gaps, we include findings in non-Galleria Lepidoptera species to serve as a template or placeholder for the immunological process that could exist in Galleria. The underlined immune factors and processes in the lepidopteran column indicate that these are found in Galleria. Asterisk (*) indicates the factor or process is found in non-Galleria Lepidoptera, such as Bombyx mori and Manduca sexta. Abbreviations: mannose-binding lectin (MBL), mannose receptor (MR), surfactant protein A/D (SP-A, SP-D), mannan-binding lectin associated serine protease (MASP), Toll-like receptor (TLR), interleukin (IL), tumor necrosis factor (TNF), cyclooxygenase (COX), C-type lectins (CLRs), reactive oxygen species (ROS), neutrophil extracellular trap-osis (NETosis), multibinding protein (MBP), thioester-containing protein (TEP), phenoloxidase (PO), phenoloxidase activating protease (PAP), serine protease homologs (SPH), plasmatocyte spreading peptide (PSP), paralytic peptide (PP).
| Immune system function | Mammalian | Lepidopteran | Role in host antifungal immunity | References |
|---|---|---|---|---|
| Pathogen recognition | ||||
| C-type lectins | Dectin-1, Dectin-2, MBL, MR, SP-D, SP-A, MelLec | Unnamed lectins, immulectin-3*, MBP* | Bind to the cell wall components of fungi including: Aspergillus,Candida and Cryptococcus | (Mathacek˜ha et al. 1990; Grubhoffer and Matha 1991; Erwin and Davidson 2002; Turner 2003; Mylonakis et al. 2005; Kiyoura and Tamai 2015; Margalit and Kavanagh 2015; Verma et al. 2015; Campuzano and Wormley 2018; Goyal et al. 2018; Lange et al. 2018) |
| Toll-like receptors | TLR 2/6, TLR 4 | Toll, BmToll9*, Spatzel | Bind to the cell wall components of fungi including: Aspergillus,Candida and Cryptococcus | (Adams 1976; Mathacek˜ha et al. 1990; Goldman et al. 1996; Mukhopadhyay et al. 2012; Satyavathi, Minz and Nagaraju 2014; Liu et al. 2015) |
| Complement system | Classical (antibody initiated, C1q, C1r, C2, C4), lectin (mannose binding lectin-mediated, MASP, C2, C4) and alternative pathways (C3b deposition) leading to C3b opsonization, C3 convertase, C5 convertase, and the membrane attack complex | TEP*, PAPs, SPHs, Phenoloxidases | Recognition of non-self cells, including fungi. Results in the opsonization of fungal cells to mark them for phagocytosis. It also results in cell lysis from the creation of a membrane attack complex (MAC) | (Hamilton, Zhao and Sims 1993; Jiang, Wang and Kanost 1998; Jiang et al. 2003; Komarov et al. 2005; Zou, Wang and Jiang 2005; Kan et al. 2008; Tanaka et al. 2008; Zhao et al. 2011; Wang, Lu and Jiang 2014; Zhang et al. 2015; Phillips and Clark 2017; Shokal and Eleftherianos 2017) |
| Inflammatory response | ||||
| NF-κB signaling | NF-κB1, NF-κB2, RelA, RelB, c-Rel. κB response element | NF-κB family genes, Rel*, Relish, Caudal, Dorsal*, CIF*, κB response element | A conserved pathway with a transcription factor that is able to activate many pro-inflammatory genes at once to help fight off fungal infections. | (Mathacek˜ha et al. 1990; Sun and Faye 1992; Yamano et al. 1998; Gantner et al. 2003; Netea et al. 2004; Kawai and Akira 2007; Cheng et al. 2008; Wu et al. 2010; Liu et al. 2015; Tang et al. 2018; Sarvari, Mikani and Mehrabadi 2020) |
| Cytokine release | IL-1, IL-6, IL-12, TNF | PSP*, PP* | Activate additional immune cells to help clear the infection, including chemotaxis, and stimulating other cells to secrete more cytokines and antimicrobial peptides | (Gemsa, Davis and Wedgwood 1966; Fernández et al. 2005; Woods et al. 2011; Park and Kim 2012; Ishii et al. 2013; Blaser et al. 2016; Tang et al. 2018) |
| Oxidative bursts | NADPH-oxidase (NOX2), superoxide dismutase (SOD), inducible nitric oxide synthase (iNOS) | Phenoloxidases, NADPH-oxidase | The production of reactive oxygen and nitrogen species results in free radicals that can react with fungal membranes, proteins, and genomes. These oxidations can be cytotoxic and destroy the fungus. | (Jiang, Wang and Kanost 1998; Jiang et al. 2003; Zou, Wang and Jiang 2005; Tanaka et al. 2008; Yassine, Kamareddine and Osta 2012; Povelones et al. 2013; Wang, Lu and Jiang 2014; Shokal and Eleftherianos 2017; Moussawi et al. 2019) |
| Antimicrobial peptides | Defensins, protegrins, histatin-5, lysozyme | Galleromycin, galliomycin, cecropin, lysozyme | Antimicrobial peptides bind the surface of pathogens and induce pore formation and membrane disruption, or enter the cell and interfere with cellular processes and organelle integrity (i.e. vacuoles and mitochondria) | (Steinberg et al. 1997; Helmerhorst, Troxler and Oppenheim 2001; Schuhmann et al. 2003; Lee et al. 2004; Feng et al. 2005; Tanaka et al. 2005, 2007; Cheng et al. 2006; Cytryńska et al. 2007; Lu and de Leeuw 2013; Tomalka et al. 2015; Hua et al. 2016; Dekkerová-Chupáčová et al. 2018) |
| Fever | Prostaglandin (COX) | Prostaglandin (COX), Behavioral changes* | Induce temperature-related stress on the fungus and make it less fit for survival/establishing infection. | (Skinner et al. 1991; Mandato et al. 1997; Blanford, Thomas and Langewald 1998; Bundey et al. 2003; Shrestha and Kim 2009; De Roode and Lefèvre 2012; González-Santoyo and Córdoba-Aguilar 2012; Sowa-Jasiłek et al. 2014; Sangbaramou et al. 2018) |
| Opsonization | CLRs, immunoglobulins, complement | CLRs, hemolin, complement-like system* | Factors bind to the pathogen, which allows the host immune cells to recognize and phagocytose the fungus rapidly | (Pendland, Heath and Boucias 1988; Tojo et al. 2000; Yu et al. 2003; Gupta, Wang and Jiang 2005; Ribeiro and Brehélin 2006; Lu and Jiang 2008; Goodridge et al. 2011; Pereira et al. 2018; Clark 2020) |
| Phagocytosis | Macrophages, monocytes, neutrophils, dendritic cells mostly | Granular hemocytes (granulocytes) and plasmatocytes | Allows the removal of the fungus from the extracellular environment | (Herre et al. 2004; Luther et al. 2007; Brown 2011) |
| Phagolysosomal degradation | Lysosome fusion, vATP-ase, acid hydrolases, ROS | Lysosome fusion, vATP-ase, acid hydrolases, ROS | Destruction of the phagocytosed fungus via acid, enzymatic, and oxidative degradation via fusion of the phagosome with lysosomes | (Ratcliffe and Rowley 1984; Ling and Yu 2006; Jung, Sajjadian and Kim 2019) |
| Immune cell aggregates | Platelet aggregation, NETosis, infectious granulomas (macrophages, neutrophils, giant cells, and other phagocytes surrounding dormant fungal cells, which is surrounded by adaptive immune cells like T-cells; Structure contains fibrotic tissue) | NETosis, Nodulation/Encapsulation (Plasmatocytes and Oenocytes surrounding pathogens, rapid degranulation and fibrous polymers, confined phenoloxidase activity) | Preventing movement and continued dissemination of the fungal pathogen, and keeping the immune response concentrated and contained around the site of infection to prevent additional damage | (Gemsa, Davis and Wedgwood 1966; Facchetti et al. 1999; Levashina et al. 2001; Fernández et al. 2005; Yu et al. 2005; Watanabe et al. 2006; Rosales 2011; Vogel et al. 2011; Arai et al. 2013; Tokura et al. 2014; Nazario-Toole and Wu 2017; Inglesfield et al. 2018; Dragotakes et al. 2020) |
The goal of this review is to describe the similarities and differences between the mammalian and G. mellonella antifungal immune systems, as well as fungal virulence strategies in both models. Our understanding of the G. mellonella–fungal immune interactions is sparse in some respects. Consequently, the G. mellonella model is used by some researchers like a ‘black box’ to study fungal virulence—where there is an input (fungus) and output (survival), but the connections between process and mechanism in between these steps are lacking in clarity. In this review, we identify these gaps in knowledge and include findings on antifungal immunity in other lepidopteran species that have overlapping immune-related genes with G. mellonella. Such gaps, as detailed in the following review, exist in the understanding of the G. mellonella complement-like system, specificities of its pattern recognition receptors (PRRs), inflammatory molecules released in response to infection and the specific evasion mechanisms of fungi during infections within the larvae. We hope that presenting the gaps in knowledge in G. mellonella and pairing them with findings in related species will provide a starting point for future research to enhance understanding of the G. mellonella immune system, and strengthen G. mellonella as a murine model alternative. Despite some unknowns, G. mellonella are successfully used in understanding roles of virulence genes, virulent potentials of different fungal species and strains, and efficacy of antifungal therapeutics.
ANTIFUNGAL RESPONSES OF G. MELLONELLA AND MAMMALIAN IMMUNE SYSTEMS
For G. mellonella to be useful in studying human fungal pathogens, there must be considerable similarities between antifungal immunity in mammals and G. mellonella. Such comparisons are challenging given that the ancestors of mammals and insects separated ∼555 million years ago (Erwin and Davidson 2002). An additional challenge is that G. mellonella lack adaptive immunity, which mammals use to produce highly specific immune responses that include immunological memory. The adaptive responses from cytotoxic T-cells (CD8), helper T-cells (Th1, Th2 and Th17) and B-cell-mediated antibody production, which are all involved in antifungal immunity in mammals, are lacking in G. mellonella (Verma et al. 2015).
Despite evolutionary divergence, G. mellonella's immune system is broadly similar to mammalian innate immunity, which makes it useful in studying fungal–host interactions. Broadly, the G. mellonella's immune system consists of PRRs, extracellular responses (Fig. 1), a complement-like system (Fig. 2) and cellular phagocytic cells that facilitate these processes (Fig. 3). Perhaps the greatest difference between mammalian and G. mellonella innate immune systems is the reliance of melanization as an antimicrobial strategy in the latter animals.
Figure 1.

Antifungal recognition and response in G. mellonella and mammals. Fungi are recognized by the mammalian and insect complement/complement-like systems, through the deposition of C3 and TEPs on the fungal surface, respectively, leading to opsonization, phagocytosis and further activation of the complement system, including the MAC (membrane attack complex) in mammals and PO/MC (phenoloxidase and melanization complex) in insects. PRRs like Toll-like receptors (TLRs) and C-type lectins (CLRs) on the surface of immune cells (macrophages and neutrophils in mammals and granulocytes and plasmatocytes in G. mellonella) and soluble circulating CLRs recognize fungus-associated molecular patterns. PRR recognition signals NF-κB and Relish homolog transcription factors and expression of immune genes, including NADPH-oxidase (NOX2), inducible nitric oxide synthase (iNOS) and phenoloxidase (PO), which produce microbicidal reactive oxygen species (ROS). Other immune genes expressed include antimicrobial peptides like lysozyme, defensins and galleromycin, and inflammatory cytokines like tumor necrosis factor (TNF), interleukins 1 and 12 (IL1,12), plasmatocyte spreading peptide (PSP) and paralytic peptide (PP). Pro-inflammatory cytokines signal other cells and cause tissue damage, eicosanoid production and fever. Classes of molecules associated with fungal recognition, immune activation and extracellular responses are in black. Components associated with mammalian (red) and G. mellonella (blue) antifungal immune responses are found below. Shared components or processes are in purple. Asterisks (*) signify immune components inferred from other lepidopteran species (e.g. B. mori and M. sexta) that have comparable immune systems to G. mellonella. Additional abbreviations: CIF (Cecropia immunoresponsive factor), SP-D (surfactant protein D), MBP (multibinding protein), C3 (complement 3), C5 (complement 5), TEP (thioester-containing proteins).
Figure 2.
A comparison of the mammalian complement system and the lepidopteran complement-like system.(A) The mammalian complement system consists of three pathways that have overlapping components and functions. The classical pathway begins with antibody recognition of an antigen on the foreign organism's surface, and the subsequent binding of the C1 complex to the antibodies. In the lectin pathway, mannose binding lectin (MBL) along with mannan-binding lectin serine protease 2 (MASP2) binds to a carbohydrate on the foreign organism. Both the C1 and MBL/MASP complexes hydrolyze C2 and C4. C4b and C2a fragments form a C3 convertase that hydrolyzes C3 into C3b fragments, which binds to foreign surfaces and acts as an opsonin—a compound that promotes phagocytosis. In the alternative pathway, C3 spontaneously hydrolyzes into C3b in plasma, which binds Factor B to form C3bB and is further hydrolyzed by Factor D to form C3bBb, a C3 convertase. Both C3 convertases bind to C3b to form a C5 convertase that hydrolyzes C5. C5b binds C6–9 and forms a membrane attack complex (MAC) that forms a pore in the microbe's cell membrane leading to lysis. (B) Although the G. mellonella complement-like system is unstudied, the complement-like system of other Lepidoptera is partially known. In B. mori and M. sexta, thioester-containing proteins (TEPs) are hypothesized to play C3-like roles. In mosquitoes (inset, yellow), TEP1 is well studied and plays a C3-like role. TEP1 is likely hydrolyzed in hemolymph, and brought to the surface of a pathogen by the leucine-rich repeat proteins (LRRs) LRIM-1 and APL-1, while it is unknown whether LRRs play similar roles in Lepidoptera. TEP proteins may opsonize similar to C3b in mammals. A TEP-convertase that hydrolyzes more TEPs is not known in Lepidoptera; however, a TEP1-convertase in mosquitoes consists of hydrolyzed TEP1 and a serine protease homolog (SPH) SPCLIP1, which activates other serine proteases (CLIPA8). In Lepidoptera, protease activation may occur downstream of TEP deposition, leading to phenoloxidase-activating proteases (PAPs) activation, which then activate phenoloxidases (PO) and SPH. PO and SPH form complexes known as melanization complexes (MCs) that lead to melanization, lysis and oxidative stress at the surface of foreign organisms.
Figure 3.

The cell-based immune response of G. mellonella and mammals. Neutrophils, macrophages and dendritic cells phagocytose fungi in mammals, a process mediated by opsonization and recognition. During opsonization, complement (C3a), antibodies (IgG/M) or soluble C-type lectins (such as SP-D) bind to the fungus. Complement receptor (CR3), FcR receptor and mannose receptors (MR) bind these opsonins, respectively, which triggers phagocytosis. In insects, TEPs, multibinding protein (MBP) and hemolin act as opsonins, but their receptors on G. mellonella phagocytes are not established. C-type lectins (CLRs) such as Dectin-1 in mammals and immulectin-3 (IML-3) in Lepidoptera, and Toll-like receptors (TLRs) bind directly to mannoses, glucans and carbohydrates in the fungal cell wall and trigger phagocytosis. Phagocytosed fungi reside within the phagosome, which merges with lysosomes to form phagolysosomes, where the fungus is killed via acid degradation, reactive oxygen species (ROS), and acid hydrolases in mammals and G. mellonella. Mammalian and G. mellonella also neutralize fungi by forming large structures known as granulomas and nodules, respectively. These contain phagocytic immune cells, fibrotic networks and other immune factors. Galleria mellonella nodules are associated with melanization, and often have dark structures within them. Cell-based antifungal responses are associated with mammals (red) and Lepidoptera (blue). Shared components or processes are in purple, while fungal components are in black. Asterisks (*) signify immune components inferred from other lepidopteran species (e.g. B. mori and M. sexta) that have comparable immune systems to G. mellonella.
Pattern recognition receptors and signaling
PRRs are crucial for recognition and clearance of fungi in G. mellonella and mammals. PRRs are used by immune systems to initially sense foreign organism-specific molecules known as pathogen-associated molecular patterns (PAMPs). In antifungal immunity, the most common PAMPs recognized are carbohydrates.
C-type lectin receptors (CLRs) are soluble and membrane-bound PRRs that bind carbohydrates. In mammals, CLRs play crucial roles in antifungal defense by binding fungal surface carbohydrates like mannans and glucans, as thoroughly reviewed by (Goyal et al. 2018). Once CLRs bind to fungal surfaces, they typically activate inflammatory immune responses, release cytotoxic molecules and induce phagocytosis. CLRs like the Dectin-family, mannose binding lectin (MBL), and surfactant proteins (SP-D/SP-A) are associated with mammalian antifungal defense (Kiyoura and Tamai 2015; Margalit and Kavanagh 2015; Campuzano and Wormley 2018).
Galleria mellonella CLRs have been identified that also bind carbohydrates and promote fungal clearance. In G. mellonella, CLRs specific to fungal carbohydrates β-(1,3)glucan, l-fucose and N-acetyl-d-galactosamine were identified and characterized (Mathacek˜ha et al. 1990; Grubhoffer and Matha 1991). These are comparable carbohydrate-binding antigens to those of mannose binding lectin (MBL), a medically important receptor involved in human antifungal immunity (Turner 2003). In non-Galleria Lepidoptera, additional CLRs are identified such as soluble immulectin-3 that binds β-(1,3)-glucan in Manduca sexta (Yu, Gan and Kanost 1999; Yu et al. 2005). Immulectin-3 coated beads were phagocytosed by hemocytes, indicating that immulectin-3 binding is likely to aid fungal clearance via opsonization-induced phagocytosis. In B. mori silkworms, with which G. mellonella shares 86% of its identifiable genes (Vogel et al. 2011), CLRs such as multibinding protein (BmMBP), which binds fungal mannan are identified (Watanabe et al. 2006). Binding triggers hemocyte aggregation surrounding the fungus in vivo in a process known as nodulation. Nodules are structures of aggregated hemocytes and pathogens, in which microbicidal immune reactions are localized (Satyavathi, Minz and Nagaraju 2014; Tokura et al. 2014). These are reminiscent of human granulomas containing immune cell aggregates during fungal infection (Adams 1976; Goldman et al. 1996; Mukhopadhyay et al. 2012).
Toll receptors are conserved components of both G. mellonella and mammalian immune systems. Toll in insects and Toll-like receptors (TLRs) in mammals are PRRs that bind a diverse array of PAMPs and generally signal immune activation. In humans, there are 10 TLR-family genes, of which fungal PAMPs are primarily recognized by TLR-2 and TLR-4 (Netea et al. 2004). In G. mellonella, three Toll-family genes have been identified based on sequence homology, but are not yet functionally characterized during fungal infections (Vogel et al. 2011). In B. mori,BmToll-3,-4,-6,-7,-8 and -9 Toll genes were upregulated during Beauveria bassiana fungal infection (Cheng et al. 2008; Wu et al. 2010). Similarly, BmSpz-1 (spatzel-1), a key gene and traditional activator of Toll, was highly upregulated following B. bassiana infection in B. mori (Liu et al. 2015).
Typically, downstream of CLR and TLR activation is the NF-κB signaling pathway. NF-κB consists of a family of Rel-homology domain proteins that form dimeric transcription factors (Kawai and Akira 2007; Tang et al. 2018). NF-κB is a crucial mediator of mammalian innate antifungal immunity. Binding of both Dectin-1 and TLR-2 to fungi leads to synergistic NF-κB activation (Gantner et al. 2003). Activated NF-κB localizes to the nucleus and binds to κB sequence enhancer elements to induce immune-related gene expression. NF-κB activation often leads to antimicrobial peptide and pro-inflammatory cytokine responses, although anti-inflammatory responses may be induced. There are hundreds of NF-κB gene targets, emphasizing its important role in immune response and activation, and the nuanced regulation between NF-κB dimer types and functions.
NF-κB-family genes were identified in G. mellonella using bioinformatic approaches and have been functionally implicated in inducing immune activation during bacterial infections (Vogel et al. 2011; Sarvari, Mikani and Mehrabadi 2020). In the Lepidoptera Hyalophora cecropia, a NF-κB-like protein Cecropia immunoresponsive factor (CIF) is activated upon infection or PAMP activation. Interestingly, CIF activation correlates to expression of H. cecropia immune genes containing κB-like binding sequences (Sun and Faye 1992)—indicating the CIF and its target genes have similar immune roles as NF-κB and its target genes. Similar proteins in B. mori are activated in response to PAMPs, and bind to κB-like sequences upstream of immune genes, such as genes encoding many of B. mori antimicrobial peptide genes with κB-like consensus sequences upstream (Yamano et al. 1998; Cheng et al. 2006; Hua et al. 2016). Bombyx mori has two homologs of Rel/NF-κB relish-family genes in Drosophila melanogaster, named BmRelish1 and BmRelish2, that are involved in antimicrobial peptide production (Tanaka et al. 2007). Interestingly, these have antagonistic roles where BmRelish1 activates antimicrobial peptide expression, whereas BmRelish2 represses expression when binding to κB-like sequences (Tanaka et al. 2007). Similarly, B. mori'sBmRel is a homolog of D. melanogaster Rel/NF-κB dorsal/dif-family genes (Tanaka et al. 2005). BmRel has two transcript isoforms,BmRelA and BmRelB, which have different, and sometimes antagonistic, roles in regulating antimicrobial peptides (Tanaka et al. 2005). This shows that Lepidoptera have complex and nuanced regulation of NF-κB-mediated cell responses.
Extracellular immune response
Following NF-κB activation, the mammalian immune system produces short peptides with microbicidal properties, termed antimicrobial peptides. Some of the prominent antifungal peptides include β-defensins, histatin-5, lactoferrin and protegrins (Steinberg et al. 1997; Helmerhorst, Troxler and Oppenheim 2001; Feng et al. 2005). β-Defensins produced primarily by neutrophils have high antifungal activities, induce inflammation and damage tissue (Feng et al. 2005; Lu and de Leeuw 2013; Tomalka et al. 2015). In G. mellonella, fungal infection induces NF-κB–dependent antimicrobial peptide expression. Galleria mellonella produces defensin-like peptides named Galleria defensin, cecropins, gallerimycin and galliomycin, which kill fungi and promote G. mellonella survival (Schuhmann et al. 2003; Lee et al. 2004; Cytryńska et al. 2007; Mak, Zdybicka-Barabas and Cytryńska 2010; Dekkerová-Chupáčová et al. 2018). Galleria mellonella produces a lysozyme, which binds and degrades fungal cell walls (Cytryńska et al. 2007; Sowa-Jasiłek et al. 2014). Lysozyme is found in healthy larval hemolymph, and has similar fungicidal functions as lysozymes found in mammalian sera and fluids (Gemsa, Davis and Wedgwood 1966; Woods et al. 2011; Sowa-Jasiłek et al. 2014).
NF-κB often stimulates pro-inflammatory cytokine expression following fungal infection, such as TNF, IL-6 and IL-12, while simultaneously inducing NOX2(NADPH-oxidase) and iNOS expression to induce oxidative bursts. Inflammatory cytokines and oxidative stressors have important cytotoxic, fungicidal and tissue-damaging effects. Inflammatory cytokines drive more NF-κB activation, which induces more inflammatory cytokines and oxidative bursts (Tang et al. 2018). In mammals, inflammatory cytokines can elicit fever through Cyclooxygenase-2 (COX-2) expression in the brain, resulting in fever-inducing prostaglandin production. Alternatively, fungal mannan and CLR-induced NF-κB activation can directly lead to COX-2 expression and prostaglandin production (Fernández et al. 2005). Febrile responses are common during systemic human fungal infections.
Lepidopteran fungal infection stimulates cytokine production like paralytic peptide (PP) and plasmatocyte-spreading peptide (PSP) mediated by Toll signaling (Park and Kim 2012; Ishii et al. 2013). Hemocytes release PSP and trigger plasmatocyte spreading and the production of eicosanoids like prostaglandins. Galleria mellonella plasmatocytes spread following infection (Mandato et al. 1997); however, there have been no studies showing that this is due to PSP production. Eicosanoids further induce antimicrobial peptides and lysozyme expression (Shrestha and Kim 2009). Eicosanoid release lyses other hemocytes, namely oenocytoids, which release stored immune factors that induce phenoloxidase-mediated oxidative stress (Mandato et al. 1997; Shrestha and Kim 2009; Park and Kim 2012). Galleria mellonella phenoloxidases oxidize catecholamines resulting in black melanin pigment deposition on the pathogen. The oxidative stress from the melanization process kills the foreign organism (González-Santoyo and Córdoba-Aguilar 2012). Additional fungicidal oxidative stress is produced by nitric oxide following PP expression in B. mori (Ishii et al. 2013). PP has not been identified in G. mellonella directly, however, the larvae do exhibit a rigid paralysis in response to injections of synthetic PP (Skinner et al. 1991).
Galleria mellonella are ectothermic and cannot intrinsically regulate their temperatures or directly induce fever, although some insects can trigger behavioral fevers (De Roode and Lefèvre 2012). These occur when an insect increases body temperatures by changing their surroundings to absorb environmental heat. Behavioral fevers fight infection in grasshoppers and locusts due to effects on pathogen viability (Blanford, Thomas and Langewald 1998; Bundey et al. 2003; Sangbaramou et al. 2018). Heat also activates immune responses including antimicrobial peptides and lysozyme expression (Wojda, Kowalski and Jakubowicz 2009; Catalán et al. 2012). Behavioral fevers in fungal-infected Schistocerca gregaria locusts are linked to COX activity and prostaglandin production (Bundey et al. 2003). This demonstrates prostaglandins as conserved fever-inducing molecules in insects and mammals. Although G. mellonella behavioral fever studies have not been published, the larvae exhibit temperature-linked immune responses (Wojda and Jakubowicz 2007; Wojda, Kowalski and Jakubowicz 2009). Further, Thelaira americana caterpillars have behavioral fever upon infection with non-lethal parasites (Karban 1998).
Apolipophorin III (ApoLp-III) is a lipid transport protein with emerging functions in G. mellonella immunity. ApoLp-III is highly expressed during infection and binds to fungal cell walls. Importantly, ApoLp-III leads to increased lysozyme expression, plasmatocyte migration, nodule formation and hemocyte-mediated phagocytosis in G. mellonella (Wiesner et al. 1997; Whitten et al. 2004). ApoLp-III treatment improves survival of fungus-infected G. mellonella (Dettloff et al. 2001; Whitten et al. 2004). ApoE—a mammalian homolog of ApoLp-III—is associated with antifungal immunity, as knockout mice had increased Candida albicans infection susceptibility (Vonk et al. 2004). ApoE4 variant expression is associated with NF-κB-mediated oxidative stress and inflammatory responses, while the ApoE3 variant has anti-inflammatory effects (Brown et al. 2002; Siest, Zaiou and Visvikis 2002; Ophir et al. 2005; Jofre-Monseny et al. 2007; Vitek, Brown and Colton 2009). Another similar mammalian apolipoprotein, ApoA-II, shows antimicrobial effects on Saccharomyces cerevisiae and bacteria due to lipid-binding properties (Motizuki et al. 1998; Motizuki et al. 1999; Siest, Zaiou and Visvikis 2002). Interestingly, ApoA-I decreases pro-inflammatory and tissue damaging neutrophil activation, degranulation and oxidative bursts. Both ApoA-I and ApoA-II interfere with complement system functioning (Blackburn et al. 1991; Hamilton, Zhao and Sims 1993). Importantly, lipid particles containing apolipoproteins bind to cell wall and increased the phagocytic uptake of these components (Carvalho et al. 2000).
Complement and complement-like system
In addition to aforementioned extracellular immune mediators, mammals use a family of soluble proteins to identify and neutralize non-self cells that comprise the complement system. Essentially, complement factors are deposited on a non-self cellular surfaces that trigger an enzymatic cascade resulting in cell lysis and phagocytic immune cell recruitment. Complement-mediated killing and complement-evasion strategies are important to host–fungal interactions (Kozel 1998; Speth et al. 2008). Comparisons of mammalian and lepidopteran complement-like systems are shown in Fig. 2.
In mammals, there are three complement pathways: the classical, lectin and alternative (Sarma and Ward 2011). In the classical pathway, antibodies bind to an antigen on the pathogen's surface. The C1 complex binds to these antibodies and cleaves C2 and C4 into C2a, C2b, C4a, C4b. In the lectin pathway, the mannose-binding lectin and MASP2 complex binds directly to the pathogen's surface carbohydrates and performs these cleavages. The cleaved fragments form C4bC2a, a C3 convertase, which cleaves C3 into C3a and C3b. C3b is deposited on pathogen surface and opsonizes it for phagocyte-mediated clearance. C4bC2a binds C3b to form C5 convertase. In the Alternative pathway, C5 convertase is produced by spontaneously hydrolyzed C3 in the serum. Spontaneous C3b binds Factor B to form C3bB, cleaved by Factor D into C3bBb, then inserts in the pathogen membrane to form C5 convertase. C5 convertases cleave C5 into C5a and C5b. C5b binds C6, C7 and C8, which embed in the membrane. C9 oligomerizes around C5bC6C7C8 and forms the Membrane Attack Complex (MAC) leading to microbial lysis.
Some insects have a complement-like system, which has primarily been studied in mosquitoes and other Diptera, but remains virtually uninvestigated in G. mellonella (Blandin et al. 2004). While we believe the G mellonella model offers advantages over mosquito models, the following findings in mosquitoes can provide crucial insight and a framework for future investigations in G. mellonella and other Lepidoptera, with the caveat that the validity of these comparisons must await experimental validation. In A. gambiae mosquitoes, two leucine-rich repeat proteins, LRIM-1 and APL-1, interact with, and cleave, TEP1 into a ‘cut’ form (Povelones et al. 2009). TEP1, a thioester containing compound, is similar in function and structure to mammalian C3 (Shokal and Eleftherianos 2017). The cut TEP1 localizes to the pathogen surface, where it binds SPCLIP1 and forms TEP1 convertase to cleave soluble TEP1. These deposit on the pathogen and opsonize it, similar to C3b's role in mammals. TEP1 convertase activates serine proteases, which cleave enzymatically inactive serine protease homologs (SPH) like CLIPA8 and activate phenoloxidases (PO) (Yassine, Kamareddine and Osta 2012; Povelones et al. 2013; Moussawi et al. 2019). Active POs and SPHs form large melanization complexes, and PO catalyzes melanization (Kan et al. 2008; Phillips and Clark 2017). Melanization releases toxic and oxidative products that cause lysis and pathogen death (Komarov et al. 2005; Zhao et al. 2011). PO and the lysis-inducing melanization complex in the final stages of mosquito complement could be seen as akin to C5 and the MAC in mammals.
Complement-like systems in Lepidoptera are not well studied and appear to be virtually unstudied in G. mellonella. There are numerous LRR-encoding genes identified in Lepidoptera but none appear orthologous to mosquito LRIM-1 and APL-1 (Zhang et al. 2015). In M. sexta and B. mori three TEP-family genes are reported, but only one, BmTEP2/MsTEP2, has the appropriate thioester group to function similar to TEP1 in mosquitoes. BmTEP1 has a receptor binding domain, indicating it may bind with hemocytes and induce phagocytosis (Tanaka et al. 2008). In M. sexta and B. mori, PO-activating proteases (PAPs) are activated following immune stimulation (Jiang, Wang and Kanost 1998; Jiang et al. 2003; Zou, Wang and Jiang 2005). PAPs activate SPHs through cleavage of pro-SPH (Lu and Jiang 2008; Wang, Lu and Jiang 2014). PAP requires the cleaved SPH as a cofactor to properly activate PO (Yu et al. 2003; Gupta, Wang and Jiang 2005; Wang, Lu and Jiang 2014). The SPH and active PO form large immune and melanization complexes where PO catalyzes catecholamine-based melanization surrounding the pathogen (Phillips and Clark 2017; Clark 2020). The large immune complexes contain other immune components, including CLRs, serine protease regulators, lipid-associated proteins and coagulation factors (Phillips and Clark 2017). Future studies determining the composition and role of the complement-like system in G. mellonella could use these findings in other Lepidoptera as a basis for the investigation.
Immune cell responses and subtypes
Phagocytosis is a fundamental host defense process by which microbes such as pathogenic fungi are handled by the immune system. In mammals, the primary phagocytic immune cells are macrophages, monocytes, neutrophils and dendritic cells. Phagocytosis can be induced by the direct binding of a PRR to a pathogen, such as CLRs and TLRs, resulting in a ‘phagocytic synapse’ (Herre et al. 2004; Luther et al. 2007; Brown 2011; Goodridge et al. 2011). Alternatively, phagocytosis can be induced by opsonins—molecules that enhance the degree to which immune cells recognize, bind and engulf a pathogen mediated by immune phagocyte receptors. Common mammalian opsonins include complement (e.g. C3b, C1 complex), soluble CLRs (e.g. MBL, SP-D) and immunoglobulin (IgG, IgM). In G. mellonella, phagocytosis usually occurs in granular hemocytes and potentially plasmatocytes (Tojo et al. 2000; Ribeiro and Brehélin 2006) in response to infections with a variety of common fungi (Pereira et al. 2018). As in mammals, CLRs and the immunoglobulin-like protein hemolin opsonize fungi for phagocytosis (Ratcliffe and Rowley 1984; Pendland, Heath and Boucias 1988; Ling and Yu 2006; Jung, Sajjadian and Kim 2019). While the lepidopteran complement-like pathway is not functionally characterized, it is hypothesized they play opsonic roles, similar to TEP1 in A. gambiae—a homolog of mammalian C3 (Levashina et al. 2001).
Following phagocytosis, the phagosomal compartment with the engulfed microbe is acidified via fusion with lysosomes. The acidification of mammalian phagolysosomes exhibits ‘bet-hedging’ behavior in which there is a wide range of phagolysosome pH values to give the phagocyte its best chances of destroying the pathogen (Dragotakes et al. 2020). The phagolysosomes acidify via vATP-ases, which activates pathogen-degrading hydrolases. Further, oxidative bursts of ROS are produced via recruitment of NOX. The combination of acidic conditions, hydrolase activity and ROS mediates potent antimicrobial activity that can destroy the fungal pathogen. This process is generally conserved in insects (Rosales 2011; Nazario-Toole and Wu 2017).
In mammals, fungal granulomas consist of a core of fungal organisms surrounded primarily by macrophages, monocytes, giant cells, other phagocytic immune cells, adaptive immune cells and fibrotic tissue. Granulomas can persist for long periods while fungi remain alive within these inflammatory structures. Granuloma restrict fungal growth and movement, deprive nutrients, and provide concentrated centers for inflammatory and cytotoxic immune responses such as ROS (Adams 1976; Goldman et al. 1996; Facchetti et al. 1999; Inglesfield et al. 2018). Similarly, Lepidoptera have granuloma-type responses to fungi. During infection with a wide assortment of pathogens, hemocytes are recruited and encircle pathogens to form a ‘nodule’ structure. Nodules are formed by phagocytic granulocytes and lipid-metabolizing oenocytes. These hemocytes degranulate their contents and release fibrous hemocytin polymers that form aggregate structures (Arai et al. 2013). The cells release pro-inflammatory signals and eicosanoid-mediated immune activation cascade (Shrestha and Kim 2009; Park and Kim 2012). Nodulation activates the melanization pathway, leading to the production of cytotoxic intermediates and high oxidative stress localized within the structure (Tokura et al. 2014; Shu et al. 2016). This ideally kills the fungus and protects the host. The nodulation and granuloma immune responses in G. mellonella and mammals may have overlapping roles in clearing certain fungal pathogens—one example of a fungal pathogen inducing a G. mellonella nodulation response and a mammalian granuloma response is Cryptococcus neoformans, a fungus that notoriously persists within granulomas of mammalian lungs and is seen within nodules when looking at G. mellonella histology (Goldman et al. 1996; Mukhopadhyay et al. 2012; Trevijano-Contador et al. 2015).
One mechanism employed by mammalian neutrophils to defend against pathogens that they cannot phagocytose is to release their nuclear contents extracellularly, creating sticky fibrous traps from their chromatin called NETs (neutrophil extracellular traps) in a process called NETosis. The NETs also contain antimicrobial enzymes, peptides and granular contents that kill the entrapped pathogen, including human pathogens like Candida spp. and Aspergillus spp. (Branzk and Papayannopoulos 2013). NETosis-like processes are found in G. mellonella hemocytes in the context of bacterial challenge (Altincicek et al. 2008).
FUNGAL VIRULENCE FACTORS AND HOST–PATHOGEN INTERACTIONS
The discovery and understanding of how certain factors contribute to a fungus’ virulence has often been elucidated using mammalian models, since mice and rats have comparable immune interactions with fungi as humans. However, mammalian studies are relatively slow, time consuming, expensive and low throughput, which reduces the amount of virulence-associated genes and antifungal treatments that can be evaluated within a certain time frame. For these reasons, G. mellonella offer an attractive alternative model for studying fungal virulence, and the role of certain genes and compounds in establishing an infection.
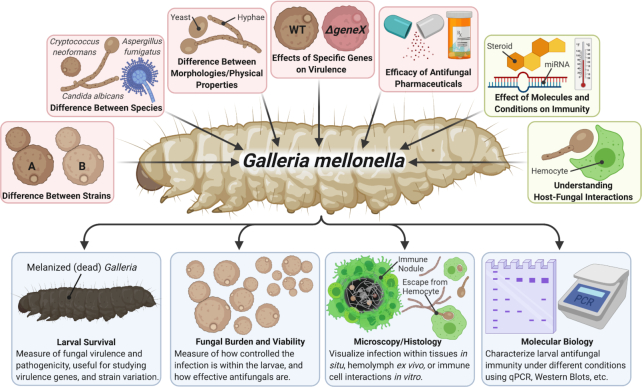
For G. mellonella to be a useful model for extrapolating virulence factor functions during mammalian infection, the virulence factors must be comparably relevant to evading mammalian and G. mellonella immune systems and/or causing tissue damage. In principle, fungal virulence factors have similar functions in and mechanisms of evasion in both G. mellonella and mammalian hosts due to similarities in their antifungal immune systems. In the big picture there appears to be conservation of function between fungal virulence factors in G. mellonella and mammals, but detailed mechanistic studies are limited. Galleria mellonella have been successfully used as a model for infections with human fungal pathogens including, but not limited to: C. neoformans,Cryptococcus gattii, Candida spp., Aspergillus fumigatus,Histoplasma capsulatum and Paracoccidioides brasiliensis. Galleria mellonella have also been used in the study of entomopathogenic fungi, namely Beauveria bassiana, which could help shed additional light on the nature of G. mellonella–fungal interactions. Table 2 compares the roles of fungal virulence factors during infections of mammals and G. mellonella, which highlights gaps in our understanding of fungal virulence in the G. mellonella model.
Table 2.
Fungal virulence factors and known evasion targets within the mammalian and gallerian immune system. Key: # = findings in Galleria with non-human fungal pathogens (i.e. entomopathogenic fungi); * = findings in non-Galleria lepidopteran species; ROS = reactive oxygen species. 'Not specified' indicates that the factor plays a role in virulence within Galleria; however, the mechanism of action or target within the immune system is not known. Overall, this paints a picture that fungal virulence factors have many overlapping properties during infections of Galleria, although there is a lack of many in-depth mechanistic studies.
| Virulence factor | Evasion from component of the mammalian immune system? | Evasion from component of the gallerian immune system? | References |
|---|---|---|---|
| Rodlet layer | C-type lectins, opsonization, phagocytosis, NETosis | Yes (#), not specified | (Altincicek et al. 2008; Aimanianda et al. 2009; Dagenais et al. 2010; Zhang et al. 2011; Branzk and Papayannopoulos 2013; Trevijano-Contador et al. 2015; Shu et al. 2016) |
| Mycotoxins (gliotoxin and fumagillin) | NF-κB signaling (gliotoxin), oxidative bursts, phagocytosis | Oxidative bursts, phagocytosis (fumagillin only) | (Williams et al. 1986; Shah and Larsen 1991; Wiesner and Götz 1993; Pahl et al. 1996; Shah et al. 1998; Yoshida, Abe and Tsunawaki 2000; Tsunawaki et al. 2004; Coméra et al. 2007; Orciuolo et al. 2007; Bruns et al. 2010; Sakamoto et al. 2015; Vij et al. 2020) |
| Capsule/GXM | C-type lectins, Toll-like receptors, complement system, cytokine release, oxidative bursts, opsonization, phagocytosis, phagolysosomal degradation (pH buffering), NETosis | Phagocytosis, oxidative bursts, possibly other interactions | (Bull 1970; Bulmer and Tacker 1975; Small and Mitchell 1989; Vecchiarelli et al. 1995; Kozel et al. 1996; Bland and De Lucca 1998; Vecchiarelli 2000; Shoham et al. 2001; Zaragoza, Taborda and Casadevall 2003; Monari et al. 2005; van Asbeck et al. 2008; Okagaki et al. 2010; Zaragoza et al. 2010; García-Rodas et al. 2011; Crabtree et al. 2012; Okagaki and Nielsen 2012; Rossoni et al. 2013; Lev et al. 2014; Rocha et al. 2015; Huang et al. 2018; Casadevall et al. 2019) |
| Titan cells | Phagocytosis | Phagocytosis | (Small and Mitchell 1989; Kozel et al. 1996; Zaragoza, Taborda and Casadevall 2003; García-Rodas et al. 2011; Okagaki and Nielsen 2012) |
| Superoxide dismutase | Oxidative bursts/ROS | Yes (#), likely oxidative bursts/ROS | (Reeves et al. 2004; Renwick et al. 2007; Fallon, Reeves and Kavanagh 2010; Fallon, Reeves and Kavanagh 2011) |
| Catalase | Oxidative bursts/ROS | Yes*, likely oxidative bursts/ROS | (Wysong et al. 1998; Cox et al. 2003; Nakagawa, Kanbe and Mizuguchi 2003; Cuéllar-Cruz et al. 2008; Xie et al. 2010; Li et al. 2015; Broxton and Culotta 2016; Román et al. 2016) |
| Glutathione | Oxidative bursts/ROS | Yes, likely oxidative bursts/ROS | (Paris et al. 2003; Giles et al. 2006; Wang et al. 2013) |
| Melanin | Oxidative bursts/ROS, antimicrobial peptides, phagocytosis | Maybe, conflicting findings but melanin seems to activate the immune system | (Kuo and Alexander 1967; Bull 1970; Jacobson and Tinnell 1993; Williamson 1994; Wang, Aisen and Casadevall 1995; Salas et al. 1996; Jahn et al. 1997; Doering et al. 1999; Romero-Martinez et al. 2000; Gómez et al. 2001; Rosas and Casadevall 2001; Nosanchuk et al. 2002; Youngchim et al. 2004; Cunha et al. 2005; Morris-Jones et al. 2005; da Silva et al. 2006; Missall et al. 2006; Jackson, Higgins and Lin 2009; Yadav et al. 2011; Chantasingh et al. 2013; Eisenman et al. 2014; Firacative, Duan and Meyer 2014; Tillmann et al. 2015; Liu et al. 2019; Smith and Casadevall 2019; de Sousa et al. 2020) |
| Secreted proteases | Complement system, opsonization, phagocytosis, antimicrobial peptides, nutrient acquisition | Phagocytosis, antimicrobial peptides and non-specified virulence | (Hube et al. 1997; Naglik et al. 2004; Gropp et al. 2009; Meiller et al. 2009; Behnsen et al. 2010; Rambach et al. 2010; Sabiiti et al. 2014; Rapala-Kozik et al. 2015; Stappers et al. 2018; Frazão S de et al. 2020) |
| Acid phosphases | Phagolysosomal degradation (nutrient acquisition) | Phagolysosomal degradation (nutrient acquisition) | (Gago et al. 2014) |
| Urease/ammonia | Phagolysosomal degradation (pH buffering, inhibition of lysosomal fusion) | (Gordon, D'Arcy Hart and Young 1980; Cox et al. 2000; Fernández-Arenas et al. 2009; Vylkova et al. 2011; Bain et al. 2014; Vylkova and Lorenz 2014; Rai et al. 2015) | |
| Efflux pumps | Antimicrobial peptides—Flu1, phagosomal degradation—Afr1 (inhibition of lysosomal fusion) | (Leon-Rodriguez et al. 2018; Ali et al. 2020) | |
| Non-lytic exocytosis and dragotcytosis | Phagosomal degradation | Phagosomal degradation (non-lytic exocytosis only) | (Gordon, D'Arcy Hart and Young 1980; Facchetti et al. 1999; Alvarez and Casadevall 2006; Ma et al. 2006; Mirbod-Donovan et al. 2006; Nicola et al. 2011; Feder et al. 2015; Fu et al. 2018; Seoane and May 2020) |
| Antiphagocytic protein (APP1) | Phagocytosis, complement | Yes, not specified | (Puccia et al. 2011; Bouklas et al. 2017; Orner et al. 2019) |
| Morphological switching and cell wall changes | C-type lectins (α-glucans, β-glucans), phagocytosis (conidia), phagosomal degradation (inhibition of lysosomal fusion) | C-type lectins (α-glucans), phagocytosis (conidia) | (Braun and Calderone 1978; Gopal, Shepherd and Sullivan 1984; Medoff et al. 1986; Latgé 1999; Torosantucci, Chiani and Cassone 2000; Netea et al. 2003; Gantner, Simmons and Underhill 2005; van der Graaf et al. 2005; Marion et al. 2006; Renwick et al. 2006; Sanguinetti et al. 2006; Chai et al. 2011; Edwards, Alore and Rappleye 2011; Mora-Montes et al. 2011; Lewis et al. 2012; Lowman et al. 2014) |
| Filamentation/hyphal growth | Toll-like receptors, phagocytosis, phagosomal degradation (inhibition of lysosomal fusion, escape), physical barriers (epithelium/endothelium) | Yes, not specified | (Lo et al. 1997; Weide and Ernst 1999; Felk et al. 2002; Wasylnka and Moore 2003; Lorenz, Bender and Fink 2004; Sanguinetti et al. 2006; Ghosh et al. 2009; Fuchs et al. 2010; García-Rodas et al. 2011; Seider et al. 2011; Bain et al. 2012; Rai et al. 2015; Westman et al. 2018; Dragotakes, Fu and Casadevall 2019; Seidel et al. 2020) |
| Biofilms | Physical barriers (epithelium/endothelium), oxidative bursts, phagocytosis, antimicrobial peptides | Yes, not specified | (Kuhn et al. 2002; Martinez and Casadevall 2005, 2006a,b, 2007; Mendes et al. 2007; Ben-Ami et al. 2008; Seidler, Salvenmoser and Müller 2008; Xu et al. 2016; Aslanyan et al. 2017; Kong et al. 2017; Sherry et al. 2017; Romera et al. 2019) |
| Age | Oxidative bursts, phagocytosis | Yes, not specified | (Rappleye, Eissenberg and Goldman 2007; Puccia et al. 2011; Thomaz et al. 2013; Bouklas et al. 2017; Orner et al. 2019) |
| Calcineurin | Temperature barriers/resistance, fever | Temperature barriers/resistance | (Morschhäuser et al. 1997; Schaller et al. 1999b; Chen et al. 2012; Casadevall, Kontoyiannis and Robert 2019; Marcos-Zambrano and LJ 2020) |
Rodlet layer
Filamentous fungal conidia (spores) are often hydrophobic, including human pathogen Aspergillus fumigatus. The hydrophobicity comes from the cell wall's rodlet layer composed of extremely hydrophobic proteins (hydrophobins). These effectively cover the cell wall's carbohydrate PAMPs, shielding A. fumigatus conidia from PRRs and subsequent immune activation and pro-inflammatory responses (Aimanianda et al. 2009). Following rodlet layer removal, CLRs bind to the A. fumigatus conidia and induce an immune response. Infection of G. mellonella with B. bassiana mutants lacking hydrophobin genes hyd1 and hyd2 demonstrate similar roles for hydrophobins in entomopathogens (Zhang et al. 2011). The hyd1Δ mutant was less virulent and increased β-glucan PAMP exposure, indicating the rodlet layer plays similar functions in conidial evasion during G. mellonella infection. The hyd2Δ mutant had diminished adherence to the hydrophobic G. mellonella cuticle, which is also important for infection (Zhang et al. 2011).
Aspergillus fumigatus conidia with removed rodlet layers are more easily phagocytosed in mammalian cells, as do the conidia lacking the rodlet layer regulator LaeA in A. fumigatus (Dagenais et al. 2010). Although increased PAMP exposure may increase phagocytosis, there is no difference in carbohydrate presentation in rodlet deficient laeAΔ mutants (Dagenais et al. 2010). This indicates that rodlet layers have physical antiphagocytic properties in line with studies correlating surface hydrophobicity with decreased phagocytosis of Cryptococcus spp. and the bacteria Klebsiella aerogenes (Williams et al. 1986; Vij et al. 2020). Antiphagocytic effects of fungal hydrophobicity have not been directly observed in G. mellonella, although hemocytes have reduced ability to phagocytose hydrophobic beads compared with hydrophilic beads (Wiesner and Götz 1993).
The rodlet layer may help prevent NETosis. Rodlet-deficient A. fumigatus rodAΔ mutant triggered more NETosis compared with rodlet-producing strains (Bruns et al. 2010). Evasion of NETosis is important for virulence, as extracellular neutrophil-mediated death is important for mammalian clearance of A. fumigatus as hyphae are too large to be phagocytosed.
Mycotoxins
Some fungi can produce mycotoxin metabolites, some of which disrupt the host's immune system. Aspergillus spp. hyphae and Candida spp. yeast produce gliotoxin (Shah and Larsen 1991). Gliotoxin induces apoptosis in mammalian monocytes and dendritic cells, but not in neutrophils (Orciuolo et al. 2007). Gliotoxin has additional antiphagocytic properties by disrupting actin polymerization and limiting phagocyte chemotaxis (Shah et al. 1998; Coméra et al. 2007). Gliotoxin strongly inhibits NF-κB-mediated immune activation by interfering with necessary IkB ubiquitination and degradation required for NF-κB translocation and immune activation (Pahl et al. 1996; Sakamoto et al. 2015). Gliotoxin may reduce oxidative bursts and cytotoxic granules normally produced to kill phagocytosed or extracellular fungi (Yoshida, Abe and Tsunawaki 2000; Tsunawaki et al. 2004; Orciuolo et al. 2007). The toxin disrupts assembly of NADPH-oxidase, which produces ROS in phagolysosomes and extracellular environment (Yoshida, Abe and Tsunawaki 2000; Tsunawaki et al. 2004). Aspergillus fumigatus mycotoxin fumagillin has similar immunosuppressive, antiphagocytic, antioxidative burst and antichemotactic effects on mammalian neutrophils (Fallon, Reeves and Kavanagh 2010).
Increased gliotoxin production is linked to virulence of Aspergillus fumigatus clinical isolates in G. mellonella, indicating overlapping functions of gliotoxin in mammalian and G. mellonella immunomodulation (Reeves et al. 2004). In fact, gliotoxin disrupts NADPH-oxidase formation in G. mellonella hemocytes (Renwick et al. 2007), and fumagillin inhibits G. mellonella hemocyte phagocytosis, increases larval susceptibility to A. fumigatus and C. albicans infection, and decreases oxidative bursts, phenoloxidase activity, degranulation and NADPH-oxidase formation (Fallon, Reeves and Kavanagh 2011).
Antioxidant defenses and melanin
Pathogenic fungi have evolved mechanisms to avoid the microbicidal oxidative burst produced by the host. One fungal antioxidant defense is superoxide dismutase (SOD)—a metalloenzyme that converts superoxide created by the host's immune system into oxygen and hydrogen peroxide (Broxton and Culotta 2016). SODs are found in the mitochondria, cytoplasm, and can be secreted extracellularly. SODs contribute to virulence of human fungal pathogens such as C. neoformans,C. albicans and Histoplasma capsulatum because they neutralize superoxide ions during respiratory bursts (Cox et al. 2003). SODs appear to play similar roles in virulence during G. mellonella infection with the fungus B. bassiana (Xie et al. 2010; Li et al. 2015), although the role of C. neoformans or Candida spp. SODs have not been investigated using the G. mellonella model.
Hydrogen peroxide is another mediator of fungal oxidative damage. Fungal catalases resist hydrogen peroxide damage by hydrolyzing it into water and oxygen. CAT1 deletion in C. albicans decreased virulence, survival of neutrophil-mediated immune responses and dissemination within mammalian tissues (Wysong et al. 1998; Nakagawa, Kanbe and Mizuguchi 2003). Conversely, catalase overexpression increased C. albicans' resistance to oxidative damage but not virulence (Román et al. 2016). Candida glabrata catalase mutant Δcta1 showed no difference in murine kidney colonization (Cuéllar-Cruz et al. 2008). In C. neoformans, quadruple catalase mutants did not alter oxidation susceptibility or virulence in mice (Giles et al. 2006). Aspergillus fumigatus catalase mutants were more susceptible to hydrogen peroxide-mediated death and grew smaller pulmonary nodules, but virulence and survival during neutrophil challenge were unchanged (Paris et al. 2003). Tobacco cutworm caterpillars, Spodoptera litura, infected with B. bassiana ΔcatA, ΔcatP and ΔcatD mutants were more likely to survive (Wang et al. 2013). Further, B. bassiana catalase overexpressing strains were more virulent in the armyworm caterpillar Spodoptera exigua (Chantasingh et al. 2013). The conflicting data surrounding catalase's role in virulence indicates possible redundancy in oxidative stress responses. Using G. mellonella to model the roles of Candida,Aspergillus and Cryptococcus catalases in G. mellonella pathogenesis can help clarify their role in mammalian pathogenesis.
Glutathione peroxidase detoxifies hydrogen peroxide by converting it into water, resulting in the oxidation of glutathiones into glutathione disulfide. Glutathione reductases reduce the glutathione disulfide into two glutathiones. Glutathione is a strong antioxidant and neutralizes free radicals during times of oxidative and nitrosative stress. Candida albicans mutants unable to produce glutathione were avirulent in mice, indicating the necessity of glutathione in maintaining antioxidant homeostasis and defense during infection (Yadav et al. 2011). Cryptococcus neoformans grl1Δ mutant cannot reduce glutathione disulfide into glutathione, which disrupts glutathione-based antioxidant defenses. The mutant showed attenuated virulence in mice, decreased resistance to nitrosative stress, and reduced survival of macrophage infection (Missall et al. 2006). In C. albicans infections of G. mellonella and mice, grl1Δ demonstrated reduced virulence while a GRL1 overexpression strain is hypervirulent (Tillmann et al. 2015). Similarities between GRL1’s role in G. mellonella and mouse infection models demonstrate G. mellonella is a relevant model for fungal antioxidant systems during infection.
Melanin is a black-brown pigment that has vital roles in fungal virulence. Many human fungal pathogens, including C. neoformans,A. fumigatus,C. albicans,H. capsulatum,Fonsecaea pedrosoi,Sporothrix schenckii and Paracoccidioides brasiliensis, produce melanin to evade the mammalian immune response (Williamson 1994; Gómez et al. 2001; Nosanchuk et al. 2002; Youngchim et al. 2004; Cunha et al. 2005; Morris-Jones et al. 2005; Smith and Casadevall 2019). These fungi use melanin's strong antioxidant properties to shield host-produced oxidative bursts, during which melanin neutralizes free radicals and protects the fungus (Jacobson and Tinnell 1993). Aspergillus fumigatus,C. neoformans and F. pedrosoi mutants that cannot melanize have decreased virulence and resistance to oxidative in mice (Wang, Aisen and Casadevall 1995; Salas et al. 1996; Jahn et al. 1997; Cunha et al. 2005).
Melanin functions in virulence beyond oxidative damage. Melanins help fungi avoid phagocytosis, and melanized C. neoformans,P. brasiliensis and S. schenckii cells were less likely to be phagocytosed by macrophages following antibody, complement and lectin opsonization, respectively (Wang, Aisen and Casadevall 1995; Romero-Martinez et al. 2000; da Silva et al. 2006). The G. mellonella and mammalian immune systems have arsenals of fungicidal antimicrobial peptides and degradative enzymes, which fungal melanins from C. neoformans and Aspergillus spp. are able to bind, inactivate and/or sequester through electrostatic interactions (Kuo and Alexander 1967; Bull 1970; Doering et al. 1999; Rosas and Casadevall 2001).
While melanin's role in virulence is well defined in mammalian models, its role is less clear in G. mellonella. Cryptococcus neoformans mutants lacking the melanin-producing laccase, Lac1, are less virulent in G. mellonella (Mylonakis et al. 2005). Similarly, there is a correlation between laccase activity, melanization and virulence of Cryptococcus gattii in G. mellonella (Firacative, Duan and Meyer 2014; de Sousa et al. 2020). In contrast, pre-melanized cells are less virulent than non-melanized C. neoformans. Nodulation is greater in pre-melanized infected larvae than contrary to the expected immunoevasive melanin properties (Eisenman et al. 2014). Further, A. fumigatus conidia with melanin defects were more virulent compared with the pigmented wild type, which is unexpected given melanin's anticipated role in virulence (Jackson, Higgins and Lin 2009). Similarly, Fonsecaea monophora non-melanizing mutants are more virulent and result in larger nodule formation in G. mellonella (Liu et al. 2019).
These papers propose possible explanations for the unexpected results. One hypothesis is that melanin is a PAMP in G. mellonella, which would increase recognition, nodulation/inflammation and fungal clearance, similar to MelLec melanin binding in mammalian immunity (Stappers et al. 2018). Additionally, fungal laccases may a have a melanin-independent role in virulence, as is the case in clinical isolates of C. neoformans (Sabiiti et al. 2014; Frazão S de et al. 2020). Another explanation is that A. fumigatus melanin normally blocks PAMPs recognition and overactive inflammatory immune responses, so PAMPs are fully exposed in pigment-less mutants, translating into increased virulence and nodulation. While melanin's fundamental properties remain the same, they overwhelmingly permit fungal proliferation, evasion and disease in mammals, whereas they seem to reduce fungal fitness and elicit stronger immune responses in G. mellonella larvae.
Secreted proteases
Candida albicans releases aspartyl proteases (SAPs) that hydrolyze important proteins involved in host antifungal immunity. Sap9 degrades the peptide Histatin-5, which is found in saliva and provides defense against oral candidiasis (Meiller et al. 2009). Sap-family enzymes degrade the antifungal peptides LL-37 and lactoferrin, as well as cathepsin D protease found in mammalian lysosomes (Naglik et al. 2004; Rapala-Kozik et al. 2015). Mutants lacking SAP1,SAP2,SAP3 or SAP4-6 have reduced virulence in mammals (Hube et al. 1997; Sanglard et al. 1997). Sap1-3 can degrade human complement proteins and render them inactive (Gropp et al. 2009). Similarly, the Alp1 protease in A. fumigatus non-specifically degrades C3, C4 and C5, and IgG, although ALP1 knockouts showed normal virulence in mice (Behnsen et al. 2010). Another A. fumigatus protease degrades CR3 receptor in cerebrospinal fluid, which decreases fungal opsonization and phagocytosis in the brain (Rambach et al. 2010).
For various strains of C. parapsilosis,C. orthopsilosisand C. metapsilosis, increased Sap activity correlates with decreased virulence and phagocytosis in G. mellonella (Gago et al. 2014), although esterases and pseudohyphal formation also correlated with decreased virulence. Similarly, protease and phospholipase activity correlated with virulence in G. mellonella (Rossoni et al. 2013). While these studies do not evaluate individual factors’ contribution to virulence, they are concordant with mammalian models where secreted proteases increase virulence and inactivate host immune factors. Further Aspergillus spp. secrete an protease that cleaves Cecropin A, a common antifungal peptide in G. mellonella hemolymph, which renders it ineffective (Bland and De Lucca 1998).
Secreted hydrolases also help nutrient acquisition under stress. Candida albicans SAPs can degrade bovine serum albumin (BSA) in instances where there are no other nitrogen sources, and SAP-family mutants cannot grow off BSA alone (Sanglard et al. 1997). Cryptococcus neoformans' acid phosphatase Aph1 is used for phosphate acquisition in the phagolysosome. The aph1Δ mutants have reduced virulence in mouse and G. mellonella models, indicating an important role across host species (Lev et al. 2014).
Capsule and titan cells
The large polysaccharide capsule produced around the C. neoformans cell wall is an essential virulence factor (Casadevall, Kontoyiannis and Robert 2019). Acapsular mutants are avirulent in mice and nearly avirulent in G. mellonella (Mylonakis et al. 2005). In part, the capsule masks carbohydrate PAMPs associated with PRR binding and immune activation. Acapsular mutants bind more MBP lectins, and induce stronger immune responses resulting in fungal clearance (van Asbeck et al. 2008). While the capsule masks cell wall PAMPs, the primary capsular polysaccharide—Glucuronoxylomannan (GXM)—is a ligand for TLR2, TLR4 and Dectin-3 (Huang et al. 2018). While Dectin-3 binding to GXM induced pro-inflammatory responses, TLR4 binding to GXM does not (Shoham et al. 2001; Huang et al. 2018). Purified C. neoformans GXM inhibits the pro-inflammatory cytokine secretion (TNF and IL-1β) and increases anti-inflammatory cytokine secretion from mammalian immune cells following LPS stimulation (Vecchiarelli et al. 1995; Vecchiarelli 2000; Monari et al. 2005). Capsular GXM reduces inflammatory cytokine secretion that threaten fungal persistence.
The capsule plays an antiphagocytic role. In addition to blocking opsonization of the cell wall as previously discussed, addition of exogenous capsular polysaccharide inhibits phagocytosis of acapsular mutants (Bulmer and Tacker 1975; Small and Mitchell 1989). Furthermore, capsule size is strongly correlated to decreased phagocytosis and phagocyte adhesion (Kozel et al. 1996). The intact capsule binds complement, however C3 binds sparsely and less efficiently in large capsules unrelated to complement concentration limitations (Kozel et al. 1996; Zaragoza, Taborda and Casadevall 2003). C3 deposits in the capsule's interior in larger capsules, such as those during infection (Zaragoza, Taborda and Casadevall 2003). Inner C3 deposition was associated with low-phagocytosed strains, while strains and cultures with smaller capsules had outer C3 binding and increased phagocytosis. Galleria mellonella hemocytes phagocytose acapsular mutants more than capsular strains in vivo (García-Rodas et al. 2011). Similarly, yeast in nutrient rich media with smaller capsules are more likely to be phagocytosed during G. mellonella infection. These data indicate that capsule plays similar antiphagocytic roles during infections of G. mellonella and mammals.
Cryptococcus neoformans produces large phagocytosis-resistant titan cells that form during pulmonary infections, and enhances fungal virulence (Crabtree et al. 2012; Okagaki and Nielsen 2012). Titan cells are yeast that have cell bodies ranging from 10 to 100 µm in diameter, dense capsules and thicker cell walls (Okagaki et al. 2010; Zaragoza et al. 2010). Titan cells are resistant to phagocytosis due to their large size, but also protect non-Titan cells from phagocytosis during infection through unknown mechanisms (Okagaki and Nielsen 2012). Titan cells and capsular enlargement occur during G. mellonella infections, indicating that the capsule may behave similar in G. mellonella to reduce phagocytosis (García-Rodas et al. 2011).
In mammals, neutrophils undergo NETosis during C. neoformans infection (Rocha et al. 2015). Acapsular mutants increase NETosis when in neutrophils compared with capsulated strains, which is reversed by the addition of exogenous GXM. Isolated GXM inhibits NETosis upon neutrophil stimulation with NETosis-inducing phorbol myristate acetate, whereas the GalXM minor capsular component induces NETosis (Rocha et al. 2015). A NETosis-like process is described in G. mellonella oenocytoids in response to bacterial stimulation (Altincicek et al. 2008). This opens the unexplored possibility that capsular polysaccharides suppress NETosis-like processes in G. mellonella models of C. neoformans infection.
The capsule is also associated with resistance to hydrogen peroxide-induced oxidative damage compared with acapsular mutants or cultures with smaller capsules (Zaragoza et al. 2008). Incubation of C. neoformans and Saccharomyces cerevisiae with shed capsular polysaccharide was protective during hydrogen peroxide treatment. Galleria mellonella hemocytes produced less hydrogen peroxide following infection with a C. neoformans mutant with increased capsular shedding (Ali et al. 2020). The antioxidant role of the capsule may be important during exposure to oxidative stress produced in both mammalian and G. mellonella immune responses.
Upon phagocytosis, pathogens encounter acidic phagolysosomal environments. The capsule buffers phagolysosomal pH to promote survival. Acapsular mutants did not buffer the phagolysosomal pH, but mutants incubated with exogenous capsular polysaccharide formed proto-capsules that partially buffered phagolysosomal pH (Leon-Rodriguez et al. 2018). Due to the conserved role of phagolysosomal acidification this could facilitate persistence within mammalian and G. mellonella phagocytic immune cells.
Persistence within phagolysosome, non-lytic exocytosis and transfer
Fungi resist phagolysosomal acidic and enzymatic degradation using various strategies, including inhibiting phagosome–lysosome fusion and maturation. Aspergillus fumigatus polyketide synthase (Pks1) reduces phagolysosomal fusion and maturation through unknown mechanisms (Jahn et al. 2002). In C. neoformans, overexpression of an efflux pump, AFR1, decreased phagolysosomal maturation in microglia (Orsi et al. 2009). AFR1 overexpression increased virulence and fungal burden in tissues (Sanguinetti et al. 2006). Candida glabrata inhibits phagolysosomal maturation passively and independent of cell survival due to fundamental fungal surface properties (Seider et al. 2011), as well as through a PI3K-mediated process (Rai et al. 2015). Candida glabrata's ability to prevent lysosomal fusion is important for its survival within phagocytes. Candida albicans’ cell wall and filamentation within the phagosome reduced phagolysosomal maturation (Fernández-Arenas et al. 2009; Bain et al. 2014).
Within matured phagolysosome, C. albicans secretes permeases to increase access to amino acids, which are metabolized into ammonia. Secreted ammonia neutralizes the phagolysosome (Vylkova et al. 2011; Vylkova and Lorenz 2014), and allows survival long enough to escape via filamentation. Ammonia also decreases phagosome–lysosome fusion, thus reducing microbial degradation (Gordon, D'Arcy Hart and Young 1980). Many fungi encode ureases that convert urea into ammonia and carbon dioxide. Urease promotes virulence within mouse infection with C. neoformans and Coccidioides posadasii (Cox et al. 2000; Mirbod-Donovan et al. 2006). In abscesses formed by Coccidioides urease mutants, the tissue is more acidic compared with normal (Mirbod-Donovan et al. 2006). Within the murine macrophage phagolysosome, cryptococcal urease increases the pH (Fu et al. 2018). Urease does not impact C. neoformans survival within phagolysosomes, and ure1Δ mutants grow faster. Slower growth in the urease-producing fungus could be linked to long-term persistence and quiescence within phagocytes. Dissimilarly, C. gattii urease is necessary for intracellular replication, as urease mutants do not replicate within macrophages (Feder et al. 2015). Cryptococcus neoformans ure1Δ mutants show decreased non-lytic exocytosis and non-lytic transfer (dragotcytosis) events in murine macrophages, indicating that urease mediates fungal escape strategies (Fu et al. 2018).
Non-lytic exocytosis, or vomocytosis, is a process by which engulfed C. neoformans escape the phagocyte without destroying it (Alvarez and Casadevall 2006; Ma et al. 2006; Seoane and May 2020). This is reported in ex vivo infected murine macrophages, and is potentially linked to pH conditions within phagolysosomes (Nicola et al. 2011). Non-lytic exocytosis may be a mechanism of fungal escape of harsh intracellular conditions. Laccase activity correlates with non-lytic exocytosis, indicating a possible effector that can signal or initiate the process (Frazão S de et al. 2020). Similar non-lytic exocytosis events are seen in Candida spp. (García-Rodas et al. 2011; Bain et al. 2012). Importantly, non-lytic exocytosis occurs in G. mellonella hemocytes infected with C. neoformans (Trevijano-Contador et al. 2015), indicating that fungal ability to escape hostile phagocytes is relevant to G. mellonella models. Galleria mellonella serve as an additional model to study the vomocytosis phenomenon. Another mechanism of escape is through non-lytic transfer known as dragotcytosis, during which Cryptococcus is exocytosed from one murine macrophage and phagocytosed immediately by another, mediated by complement and Fc receptors (Dragotakes, Fu and Casadevall 2019), and is an unstudied process in G. mellonella. Dragotcytosis represents a mechanism of fungal escape from unfavorable intracellular conditions while still avoiding the hostile extracellular environment.
Some fungi undergo morphological switching to escape phagocytes. Under stressors like heat, acidic pH and carbon dioxide, C. albicans undergoes yeast-to-hyphal transitions. Engulfed Candida forms hyphae that place physical stress on phagolysosomal membranes resulting in higher pH (Vylkova et al. 2011; Westman et al. 2018). Filamentation also allows escape from within macrophages (Lorenz, Bender and Fink 2004; Ghosh et al. 2009). Filamentation is necessary for C. albicans pathogenesis and invasion into host tissues, as non-filamentous mutants are avirulent in mice and unable to cause disseminated infection (Lo et al. 1997; Weide and Ernst 1999; Felk et al. 2002). Candida undergoes filamentation in G. mellonella, and non-filamentous mutants were not as virulent as wild type (Fuchs et al. 2010). Aspergillus fumigatus conidia are phagocytosed by macrophages, which germinate and grow hyphae within phagolysosomes. The hyphae stretch the phagolysosome until it fuses with the plasma membrane (Wasylnka and Moore 2003; Seidel et al. 2020). This is non-lytic and allows escape to extracellular space without damage-induced inflammation. There is no evidence for filamentation-based escape from G. mellonella hemocytes in Candida and Aspergillus.
Filamentation, dimorphism and cell wall architecture
Fungi use filamentation and morphological changes to avoid host defenses beyond phagolysosomal escape. Candida hyphae larger than 20 µm slow phagocytosis (Lewis et al. 2012). Similarly, Aspergillus avoids engulfment following germination; conidia are readily phagocytosed by alveolar macrophages, whereas hyphae are too large to be engulfed (Latgé 1999). In G. mellonella hemocytes, germinating Aspergillus conidia are resistant to phagocytosis, and infection with germinated conidia resulted in increased virulence, presumably due to reduced phagocyte-mediate fungal clearance (Renwick et al. 2006).
Fungal morphologies are associated with cell wall changes that affect PRR recognition and subsequent immune responses. Both yeast and hyphal C. albicans effectively mask their β-glucan PAMPs due to cell wall mannan, Dectin-1 binds to C. albicans yeast more than hyphae (Bain et al. 2014), partly due to β-glucan exposure from yeasts’ budding scars that hyphae lack (Gantner, Simmons and Underhill 2005). β-glucan compositions of C. albicans hyphae and yeast differ—hyphae contain more β-(1,6)-glucans and cyclical side chains that trigger Dectin-1-mediated responses (Lowman et al. 2014). In contrast, others report that yeast contain more pro-inflammatory β-(1,6)-glucans (Torosantucci, Chiani and Cassone 2000), while other papers report similar β-glucans between yeast and hyphae (Gopal, Shepherd and Sullivan 1984). Additionally, hyphae contain more chitin compared with yeast (Braun and Calderone 1978). Increased cell wall chitin, and chitin treatment of macrophages block Dectin-1-mediated inflammation (Mora-Montes et al. 2011). Aspergillus fumigatus and C. albicans hyphae bind less TLR-4 resulting in less pro-inflammatory signal induction (Netea et al. 2003; van der Graaf et al. 2005; Chai et al. 2011). In A. fumigatus, TLR-4 signaling loss upon germination is likely due to β-glucans, α-glucans and galactomannan changes (Chai et al. 2011).
Yeast morphologies are associated with virulence in other dimorphic fungi such as H. capsulatum,P. brasiliensis and S. schenckii. Switching to yeast is induced by high temperatures such as the human body temperature of 37°C and these are therefore found as yeast in human infections. The hyphal-to-yeast transition is necessary for H. capsulatum virulence—filamentous H. capsulatum that cannot form yeast cannot infect mice, despite being viable (Medoff et al. 1986). Histoplasma capsulatum and P. brasiliensis produce α-(1,3)-glucan exclusively during yeast phases. The α-(1,3)-glucan layer is important to virulence because its presence on the outer cell wall prevents Dectin-1 recognition of β-glucan (Marion et al. 2006; Rappleye, Eissenberg and Goldman 2007; Edwards, Alore and Rappleye 2011; Puccia et al. 2011). P. lutzii and H. capsulatum are virulent in G. mellonella at both 25°C and 37°C, indicating a reduced importance of yeast morphologies in G. mellonella (Thomaz et al. 2013). However, the H. capsulatum strain G217B—which produces more α-(1,3)-glucan as a yeast—is one exception. During infection with G217B at 37°C, there was greater virulence compared with 25°C-incubated larvae. This difference is not seen during infection with strains producing less α-(1,3)-glucan, indicating overlapping roles of α-glucan in G. mellonella and mammalian virulence.
Fungal cell walls change with increasing generations, with older cells having thicker cell walls in C. glabrata,Candida auris and C. neoformans (Bouklas et al. 2017; Bhattacharya et al. 2019; Orner et al. 2019). Age-thickened cell walls are associated with resistance to oxidative stress, phagocytosis and antifungal drugs, and increased virulence within G. mellonella. Similar to findings in mice, older C. glabrata cells remained viable within G. mellonella whereas younger cells did not (Bouklas et al. 2017). In C. neoformans, older cells have increased LAC1,LAC2 and APP1 expression. The protective effects of fungal age are lost with lac1Δ,lac2Δ and app1Δ mutants during G. mellonella infection, thus implicating these genes as the main effectors of age-linked virulence (Orner et al. 2019). APP1 encodes antiphagocytic protein 1, which competes with C3 to bind complement receptors (CR3), thus inhibiting complement-mediated phagocytosis (Luberto et al. 2003; Stano et al. 2009). Interestingly, these studies support LAC1’s role in virulence G. mellonella. Additionally, APP1-associated virulence implies existence of antifungal complement-like systems in G. mellonella.
Adhesion, biofilms and invasion
Fungal adhesion to the host is often essential for persistence of an infection. Adhesion allows fungi stable entry point for tissue invasion. Fungal adhesion is thoroughly reviewed by (Tronchin et al. 2008), and the genes involved in C. albicans adherence and invasion are established by (Wächtler et al. 2011). Candida albicans is common in healthy human mucosa. However, there is a strong link between Candida adherence and pathogenic potential in patients (Bernhardt et al. 2001). Similarly, A. fumigatus conidia adhere to host epithelium, which facilitates tissue invasion (DeHart et al. 1997).
Hydrophobicity is associated with increased epithelium adhesion; increased C. albicans hydrophobicity correlates to adherence to extracellular matrix (ECM) proteins, such as fibronectin, collagen IV and laminin (Silva, Glee and Hazen 1995). Beauveria bassiana hydrophobicity is important for adherence to hydrophobic surfaces like the G. mellonella cuticle, indicating hydrophobicity's orthologous roles in G. mellonella fungal pathogenesis (Holder and Keyhani 2005).
Cell wall carbohydrates may also contribute to adherence—A. nidulans mutants overexpressing α-(1,3)-glucan synthase have increased adherence to plastic (He, Li and Kaminskyj 2018). Cell wall glycoproteins that encode adhesins such as ALS-family genes, ALA1 and HWP1 are crucial to increased ECM and epithelium adhesion, fungal burdens and virulence in mice (Gaur and Klotz 1997; Kamai et al. 2002; Sundstrom 2002; Sundstrom, Cutler and Staab 2002; Loza et al. 2004; Wächtler et al. 2011). Aspergillus fumigatus produce adhesin-like molecules that bind host ECM (Tronchin et al. 1993; Coulot et al. 1994; Upadhyay et al. 2009; Xu et al. 2012; Xu et al. 2016).
Fungal biofilms are durable structures that form following surface adhesion. They produce thick ECM and polysaccharides that ‘glue’ the fungal community together (Dominguez et al. 2019; Loussert et al. 2010; Martinez and Casadevall 2006b, 2007; Silva-Dias et al. 2015). Biofilms can grow on skin, mucosa and plastic. Candidemia is often associated with nosocomial and medical device-related Candida biofilms (Ben-Ami et al. 2008; Sherry et al. 2017). Biofilms are found in C. neoformans central nervous system infections and A. fumigatus lung infections (Loussert et al. 2010; Aslanyan et al. 2017). Biofilms are often resistant to antifungals like amphotericin, echinocandins and azoles in vitro (Kuhn et al. 2002; Martinez and Casadevall 2006b; Seidler, Salvenmoser and Müller 2008; Kong et al. 2017; Romera et al. 2019). Biofilms are associated with virulence in mice, and increased GXM, SAPs, urease and melanin production (Martinez and Casadevall 2005, 2006a; Mendes et al. 2007; Hasan et al. 2009; Loussert et al. 2010; Benaducci et al. 2016). Biofilms have been shown to dampen the mammalian immune systems’ oxidative bursts, NETosis, inflammatory cytokine production, antimicrobial peptides and phagocytosis (Kernien et al. 2018). Mice infected with strong biofilm-producing Candida isolates have enhanced disease and tissue invasion compared with low biofilm-producing isolates. Similarly, C. neoformans and C. gattii biofilms are more virulent in G. mellonella compared with planktonic cultures (Benaducci et al. 2016). This displays G. mellonella as a potential model for the study of biofilm virulence and treatment.
Following adherence, pathogenic fungi transverse host epithelium and invade tissue, in part using hydrolytic enzymes like serine proteases, phospholipase Bs (PLBs) and SAPs. PLBs break down phospholipids, and increased C. albicans PLB1 expression increases virulence during mucosal infections (Barrett-Bee et al. 1985; Naglik et al. 2003). PLB1 expression increases during Candida spp. and C. neoformans epithelium invasion and mutants have attenuated virulence (Ghannoum 2000; Cox et al. 2001; Mukherjee et al. 2001; Noverr et al. 2003). PLBs degrade host membranes, trigger lysis, damage tissue, activate inflammation and provide entry points for fungal invasion.
Aspergillus fumigatus secretes serine proteases that degrade host laminins in the epithelial basement membrane (Tronchin et al. 1993). Candida Sap-family proteases are linked to fungal invasion. They bind to epithelium during invasion of oral mucosa (Schaller et al. 1999a,b). Sap4 and Sap6 degrade subendothelial ECM, which is prevented by pepstatin A protease inhibitor (Morschhäuser et al. 1997). Candida albicans SAP4 and SAP6 mutants have reduced invasive and tissue-damaging properties during internal organ infection (Felk et al. 2002). SAP1,4 and 6 expression correlates with virulence in G. mellonella, although their roles are not elucidated (Rossoni et al. 2013; Marcos-Zambrano and LJ 2020).
Temperature resistance
To survive within mammalian hosts, fungi must survive at higher temperatures. Evolution of fungal thermotolerance may contribute to emergence of fungal pathogens like C. auris (Casadevall, Kontoyiannis and Robert 2019). The primary mechanism underlying thermotolerance in many pathogenic fungi involves calcineurin, as mutants have reduced thermotolerance (Chen et al. 2012, 2013; Magnani Dinamarco et al. 2012). Aspergillus fumigatus,C. glabrata,C. gattii and C. neoformans mutants lacking calcineurin subunit A (CnaA and CNA1) were less virulent and displayed reduced fungal invasion and proliferation (Odom et al. 1997; Steinbach et al. 2006; Chen et al. 2012, 2013).
Galleria mellonella incubated at 37°C show comparable results to mice when the larvae are during infection with A. fumigatus ΔcnaA mutants (Steinbach et al. 2006). Since G. mellonella are ectothermic, larval temperature can be manipulated to evaluate thermotolerance-associated virulence. Employment of this tool is seen in the study of SwoH, a nucleotide diphosphate kinase gene that interacts with calcineurin in A. fumigatus (Magnani Dinamarco et al. 2012). Galleria mellonella infected with ΔswoH were incubated at 30°C and 37°C. The mutant has typical virulence at lower temperatures, but attenuated virulence at higher temperatures. Similarly designed studies evaluated endoplasmic reticulum Ca+2 ATPase (Eca1)’s role in C. neoformans thermoresistance, in which eca1Δ had attenuated virulence at higher temperatures (Fan et al. 2007).
CONCLUSION
Galleria mellonella is an invertebrate infection model that is an attractive experimental system to study fungal pathogenesis due to the easy, low cost, high-throughput experiments, along with the limited ethical dilemmas such as those associated with higher order vertebrate (e.g. mice, rabbit, non-human primate, etc.) research. The use of G. mellonella is thus in line with initiatives to minimize and optimize vertebrate animal research, while maximizing vertebrate animal welfare (Clark 2018). While there are drawbacks to using G. mellonella including their lack of adaptive immunity, there are strong indicators that murine and G. mellonella antifungal innate immune systems are comparable. Existing literature largely indicates that fungal virulence factors have comparable and overlapping roles in mammalian and G. mellonella hosts, suggesting that G. mellonella studies can be translatable to mammals.
Galleria mellonella are useful tools to elucidate virulence factors, fungal signal/regulatory pathways, and test antifungal pharmaceuticals, and future studies using this model will continue to provide invaluable insights (summarized in Fig. 4). With that being said, G. mellonella are an underused model organism. Consequently, there is a dearth of studies translating the vast knowledge of fungal pathogenesis in mammals to G. mellonella models. Importantly, neither the G. mellonella's complement-like system nor the mechanisms of common fungal virulence factors, such as urease, superoxide dismutase, polysaccharide capsules and adhesion, have been established in G. mellonella models. This makes it difficult to fully understand the strength of the G. mellonella model with certainty. These gaps should serve as a call for investigators to study these and bolster the strength of this alternative model.
Figure 4.
Applications of G. mellonella model in studying fungal pathogenesis and popular methods used. Galleria mellonella can be used to study the differences in virulence between two strains of the same fungal species, between two fungal species, and between two morphological variants or physical characteristics of the same fungal species (i.e. hyphal growth vs yeast growth, pigmented vs non-pigmented, normal vs titan cell, smooth vs wrinkled colony). Galleria mellonella can be used to elucidate virulence genes and study virulence factor function, by comparing infection of wild type and mutant strain lacking the virulence gene of interest. Infected G. mellonella could be treated with pharmaceuticals to test antifungal efficacy, or with compounds (i.e. steroids) or conditions (i.e. temperature) that may directly modulate their immune system. Galleria mellonella are useful models in understanding host–fungal interactions, such as fungal escape from phagocytosis within hemocytes. The uses of G. mellonella often involve measuring larval survival after infection/treatment. Fungal burden and viability could also be assessed to ascertain how well the fungus is replicating within the G. mellonella host. Microscopy and histology are helpful tools in understanding fungal pathogenesis in G. mellonella. Histology could show how a fungus infects and spreads throughout tissue and thus elucidating mechanisms of fungal adhesion, invasion and pathogenesis. Microscopy could be used in similar ways, particularly in visualizing infections in the hemolymph, where melanized nodules and phagocytic immune cells are found, which could describe the severity of the infection and degree to which it is under control. In vitro imaging of hemocytes and fungus allows researchers to understand the dynamics of phagocytosis, phagolysosomal maturation, escape and transfer events. Lastly, molecular biology can investigate the roles virulence factors and immune genes play during the course of fungal infections. Techniques like qPCR (quantitative Polymerase Chain Reaction) and Western blots can reveal the nuanced differences in the immune responses following different infection conditions.
ACKNOWLEDGMENTS
We would like to thank Madhura Kulkarni and Ella Jacobs for additional edits and feedback on the manuscript. All figures were created on BioRender.com.
FUNDING
DFQS and AC are funded by a Johns Hopkins Malaria Research Institute (JHMRI) Pilot Grant Casadevall_123 and National Institute of Allergy and Infectious Diseases R01AI052733. DFQS has been funded by the National Institutes of Health grants 5T32GM008752-18 and 1T32AI138953-01A1.
Contributor Information
Daniel F. Q. Smith, W. Harry Feinstone Department of Molecular Microbiology and Immunology, The Johns Hopkins Bloomberg School of Public Health, Baltimore, MD 21205, USA.
Arturo Casadevall, W. Harry Feinstone Department of Molecular Microbiology and Immunology, The Johns Hopkins Bloomberg School of Public Health, Baltimore, MD 21205, USA.
Conflict of Interest
None declared.
REFERENCES
- Adams DO. The granulomatous inflammatory response. A review. Am J Pathol. 1976;84:164–91. [PMC free article] [PubMed] [Google Scholar]
- Aimanianda V, et al. Surface hydrophobin prevents immune recognition of airborne fungal spores. Nature. 2009;460:1117–21. [DOI] [PubMed] [Google Scholar]
- Ali MF, Tansie SM, Shahan JRet al. Serial passage of Cryptococcus neoformans in Galleria mellonella results in increased capsule and intracellular replication in hemocytes, but not increased resistance to hydrogen peroxide. Pathogens. 2020;9:732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altincicek B, Stötzel S, Wygrecka Met al. Host-derived extracellular nucleic acids enhance innate immune responses, induce coagulation, and prolong survival upon infection in insects. J Immunol. 2008;181:2705–12. [DOI] [PubMed] [Google Scholar]
- Alvarez M, Casadevall A.. Phagosome extrusion and host-cell survival after Cryptococcus neoformans phagocytosis by macrophages. Curr Biol. 2006;16:2161–5. [DOI] [PubMed] [Google Scholar]
- Arai I, Ohta M, Suzuki Aet al. Immunohistochemical analysis of the role of hemocytin in nodule formation in the larvae of the silkworm, Bombyx mori. J Insect Sci. 2013;13:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslanyan L, Sanchez DA, Valdebenito Set al. The crucial role of biofilms in Cryptococcus neoformans survival within macrophages and colonization of the central nervous system. J Fungi. 2017;3:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain JM, Lewis LE, Okai Bet al. Non-lytic expulsion/exocytosis of Candida albicans from macrophages. Fungal Genet Biol. 2012;49:677–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain JM, et al. Candida albicans hypha formation and mannan masking of β-glucan inhibit macrophage phagosome maturation. mBio. 2014;5:e01874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett-Bee K, Hayes Y, Wilson RGet al. A comparison of phospholipase activity, cellular adherence and pathogenicity of yeasts. Microbiology. 1985;131:1217–21. [DOI] [PubMed] [Google Scholar]
- Behnsen J, et al. Secreted Aspergillus fumigatus protease Alp1 degrades human complement proteins C3, C4, and C5. Infect Immun. 2010;78:3585–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ami R, et al. Time to blood culture positivity as a marker for catheter-related candidemia. J Clin Microbiol. 2008;46:2222–6.18480222 [Google Scholar]
- Benaducci T, et al. Virulence of Cryptococcus sp. biofilms in vitro and in vivo using Galleria mellonella as an alternative model. Front Microbiol. 2016;7, 290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt J, Herman D, Sheridan Met al. Adherence and invasion studies of Candida albicans strains, using in vitro models of Esophageal Candidiasis. J Infect Dis. 2001;184:1170–5. [DOI] [PubMed] [Google Scholar]
- Bhattacharya S, Holowka T, Orner EPet al. Gene duplication associated with increased fluconazole tolerance in Candida auris cells of advanced generational age. Sci Rep. 2019;9:5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn WD, et al. Apolipoprotein A-I decreases neutrophil degranulation and superoxide production. J Lipid Res. 1991;32:1911–8. [PubMed] [Google Scholar]
- Blandin S, et al. Complement-like protein TEP1 is a determinant of vectorial capacity in the malaria vector Anopheles gambiae. Cell. 2004;116:661–70. [DOI] [PubMed] [Google Scholar]
- Bland JM, De Lucca AJ.. Identification of cecropin a proteolytic cleavage sites resulting from Aspergillus flavus extracellular protease(s). J Agric Food Chem. 1998;46:5324–7. [Google Scholar]
- Blanford S, Thomas MB, Langewald J. Behavioural fever in the Senegalese grasshopper, Oedaleus senegalensis, and its implications for biological control using pathogens. Ecol Entomol. 1998;23:9–14. [Google Scholar]
- Blaser H, Dostert C, Mak TWet al. TNF and ROS crosstalk in inflammation. Trends Cell Biol. 2016;26:249–61. [DOI] [PubMed] [Google Scholar]
- Bouklas T, et al. Generational distribution of a Candida glabrata population: resilient old cells prevail, while younger cells dominate in the vulnerable host. PLoS Pathog. 2017;13:e1006355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branzk N, Papayannopoulos V.. Molecular mechanisms regulating NETosis in infection and disease. Semin Immunopathol. 2013;35:513–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun PC, Calderone RA.. Chitin synthesis in Candida albicans: comparison of yeast and hyphal forms. J Bacteriol. 1978;133:1472–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CM, Wright E, Colton CAet al. Apolipoprotein E isoform mediated regulation of nitric oxide release. Free Radic Biol Med. 2002;32:1071–5. [DOI] [PubMed] [Google Scholar]
- Brown GD. Innate antifungal immunity: the key role of phagocytes. Annu Rev Immunol. 2011;29:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broxton CN, Culotta VC.. SOD enzymes and microbial pathogens: surviving the oxidative storm of infection. PLoS Pathog. 2016;12:e1005295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns S, et al. Production of extracellular traps against Aspergillus fumigatus in vitro and in infected lung tissue is dependent on invading neutrophils and influenced by hydrophobin RodA. PLoS Pathog. 2010;6:e1000873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull AT. Inhibition of polysaccharases by melanin: enzyme inhibition in relation to mycolysis. Arch Biochem Biophys. 1970;137:345–56. [DOI] [PubMed] [Google Scholar]
- Bulmer GS, Tacker JR.. Phagocytosis of Cryptococcus neoformans by alveolar macrophages. Infect Immun. 1975;11:73–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundey S, Raymond S, Dean Pet al. Eicosanoid involvement in the regulation of behavioral fever in the desert locust, Schistocerca gregaria. Arch Insect Biochem Physiol. 2003;52:183–92. [DOI] [PubMed] [Google Scholar]
- Campuzano A, Wormley FL. Innate immunity against Cryptococcus, from recognition to elimination. J Fungi. 2018;4:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho MDT, Tobias VE, Vendrame CMVet al. Lipoproteins modify the macrophage uptake of triacylglycerol emulsion and of zymosan particles by similar mechanisms. Lipids. 2000;35:55–9. [DOI] [PubMed] [Google Scholar]
- Casadevall A, Kontoyiannis DP, Robert V. On the emergence of Candida auris: climate change, azoles, swamps, and birds. mBio. 2019;10:e01397–19. 10.1128/mBio.01397-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadevall A, et al. The capsule of Cryptococcus neoformans. Virulence. 2019;10:822–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalán TP, Niemeyer HM, Kalergis AMet al. Interplay between behavioural thermoregulation and immune response in mealworms. J Insect Physiol. 2012;58:1450–5. [DOI] [PubMed] [Google Scholar]
- Chai LYA, et al. Aspergillus fumigatus cell wall components differentially modulate host TLR2 and TLR4 responses. Microbes Infect. 2011;13:151–9. [DOI] [PubMed] [Google Scholar]
- Chantasingh D, et al. Identification of catalase as an early up-regulated gene in Beauveria bassiana and its role in entomopathogenic fungal virulence. Biol Control. 2013;67:85–93. [Google Scholar]
- Cheng T-C, et al. Identification and analysis of Toll-related genes in the domesticated silkworm, Bombyx mori. Dev Comp Immunol. 2008;32:464–75. [DOI] [PubMed] [Google Scholar]
- Cheng T, et al. Structures, regulatory regions, and inductive expression patterns of antimicrobial peptide genes in the silkworm Bombyx mori. Genomics. 2006;87:356–65. [DOI] [PubMed] [Google Scholar]
- Chen Y-L, Lehman VN, Lewit Yet al. Calcineurin governs thermotolerance and virulence of Cryptococcus gattii. G3. 2013;3:527–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y-L, et al. Convergent evolution of calcineurin pathway roles in thermotolerance and virulence in Candida glabrata. G3. 2012;2:675–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JM. The 3Rs in research: a contemporary approach to replacement, reduction and refinement. Br J Nutr. 2018;120:S1–S7. [DOI] [PubMed] [Google Scholar]
- Clark KD. Insect hemolymph immune complexes. In: Hoeger U, Harris JR (eds). Vertebrate and Invertebrate Respiratory Proteins, Lipoproteins and other Body Fluid Proteins. Cham: Springer International Publishing, 2020, 123–61. [Google Scholar]
- Coméra C, André K, Laffitte Jet al. Gliotoxin from Aspergillus fumigatus affects phagocytosis and the organization of the actin cytoskeleton by distinct signalling pathways in human neutrophils. Microbes Infect. 2007;9:47–54. [DOI] [PubMed] [Google Scholar]
- Coulot P, et al. Specific interaction of Aspergillus fumigatus with fibrinogen and its role in cell adhesion. Infect Immun. 1994;62:2169–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox GM, Mukherjee J, Cole GTet al. Urease as a virulence factor in experimental cryptococcosis. Infect Immun. 2000;68:443–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox GM, et al. Extracellular phospholipase activity is a virulence factor for Cryptococcus neoformans. Mol Microbiol. 2001;39:166–75. [DOI] [PubMed] [Google Scholar]
- Cox GM, et al. Superoxide dismutase influences the virulence of Cryptococcus neoformans by Affecting growth within macrophages. Infect Immun. 2003;71:173–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree JN, Okagaki LH, Wiesner DLet al. Titan cell production enhances the virulence of Cryptococcus neoformans. Infect Immun. 2012;80:3776–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha MML, et al. Inhibition of melanin synthesis pathway by tricyclazole increases susceptibility of Fonsecaea pedrosoi against mouse macrophages. Microsc Res Tech. 2005;68:377–84. [DOI] [PubMed] [Google Scholar]
- Cuéllar-Cruz M, et al. High resistance to oxidative stress in the fungal pathogen Candida glabrata is mediated by a single catalase, Cta1p, and is controlled by the transcription factors Yap1p, Skn7p, Msn2p, and Msn4p. Eukaryot Cell. 2008;7:814–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cytryńska M, Mak P, Zdybicka-Barabas Aet al. Purification and characterization of eight peptides from Galleria mellonella immune hemolymph. Peptides. 2007;28:533–46. [DOI] [PubMed] [Google Scholar]
- Dagenais TRT, Giles SS, Aimanianda Vet al. Aspergillus fumigatus LaeA-mediated phagocytosis is associated with a decreased hydrophobin layer. Infect Immun. 2010;78:823–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva MB, Marques AF, Nosanchuk JDet al. Melanin in the dimorphic fungal pathogen Paracoccidioides brasiliensis: effects on phagocytosis, intracellular resistance and drug susceptibility. Microbes Infect. 2006;8:197–205. [DOI] [PubMed] [Google Scholar]
- DeHart DJ, Agwu DE, Julian NCet al. Binding and germination of Aspergillus fumigatus conidia on cultured A549 pneumocytes. J Infect Dis. 1997;175:146–50. [DOI] [PubMed] [Google Scholar]
- Dekkerová-Chupáčová J, Borghi E, Morace Get al. Up-Regulation of antimicrobial peptides gallerimycin and galiomicin in Galleria mellonella infected with Candida yeasts displaying different virulence traits. Mycopathologia. 2018;183:935–40. [DOI] [PubMed] [Google Scholar]
- De Roode JC, Lefèvre T. Behavioral immunity in insects. Insects. 2012;3:789–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Sousa HR, et al. Faster Cryptococcus melanization increases virulence in experimental and human cryptococcosis. bioRxiv. 2020; DOI: 10.1101/2020.07.29.222794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettloff M, Wittwer D, Weise Cet al. Lipophorin of lower density is formed during immune responses in the lepidopteran insect Galleria mellonella. Cell Tissue Res. 2001;306:449–58. [DOI] [PubMed] [Google Scholar]
- Doering TL, Nosanchuk JD, Roberts WKet al. Melanin as a potential cryptococcal defence against microbicidal proteins. Med Mycol. 1999;37:175–81. [PubMed] [Google Scholar]
- Dominguez EG, Zarnowski R, Choy HLet al. Conserved role for biofilm matrix polysaccharides in Candida auris drug resistance. mSphere. 2019;4, DOI: 10.1128/mSphereDirect.00680-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragotakes Q, Fu MS, Casadevall A.. Dragotcytosis: elucidation of the mechanism for Cryptococcus neoformans macrophage-to-macrophage transfer. J Immunol. 2019;202:2661–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragotakes Q, et al. Macrophages use a bet-hedging strategy for antimicrobial activity in phagolysosomal acidification. J Clin Invest. 2020;130:3805–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards JA, Alore EA, Rappleye CA.. The yeast-phase virulence requirement for α-glucan synthase differs among Histoplasma capsulatum chemotypes. Eukaryot Cell. 2011;10:87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenman HC, Duong R, Chan Het al. Reduced virulence of melanized Cryptococcus neoformans in Galleria mellonella. Virulence. 2014;5:611–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erwin DH, Davidson EH. The last common bilaterian ancestor. Development. 2002;129:3021–32. [DOI] [PubMed] [Google Scholar]
- Facchetti F, et al. Expression of inducible nitric oxide synthase in human granulomas and histiocytic reactions. Am J Pathol. 1999;154:145–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon JP, Reeves EP, Kavanagh K.. Inhibition of neutrophil function following exposure to the Aspergillus fumigatus toxin fumagillin. J Med Microbiol. 2010;59:625–33. [DOI] [PubMed] [Google Scholar]
- Fallon JP, Reeves EP, Kavanagh K.. The Aspergillus fumigatus toxin fumagillin suppresses the immune response of Galleria mellonella larvae by inhibiting the action of haemocytes. Microbiology. 2011;157:1481–8. [DOI] [PubMed] [Google Scholar]
- Fan W, Idnurm A, Breger Jet al. Eca1, a sarcoplasmic/endoplasmic reticulum Ca2+-ATPase, is involved in stress tolerance and virulence in Cryptococcus neoformans. Infect Immun. 2007;75:3394–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder V, et al. Cryptococcus gattii urease as a virulence factor and the relevance of enzymatic activity in cryptococcosis pathogenesis. FEBS J. 2015;282:1406–18. [DOI] [PubMed] [Google Scholar]
- Felk A, et al. Candida albicans hyphal formation and the expression of the Efg1-regulated proteinases Sap4 to Sap6 are required for the invasion of parenchymal organs. Infect Immun. 2002;70:3689–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Z, Jiang B, Chandra Jet al. Human beta-defensins: differential activity against candidal species and regulation by Candida albicans. J Dent Res. 2005;84:445–50. [DOI] [PubMed] [Google Scholar]
- Fernández-Arenas E, Bleck CKE, Nombela Cet al. Candida albicans actively modulates intracellular membrane trafficking in mouse macrophage phagosomes. Cell Microbiol. 2009;11:560–89. [DOI] [PubMed] [Google Scholar]
- Fernández N, et al. Mannose-containing molecular patterns are strong inducers of Cyclooxygenase-2 expression and prostaglandin E2 production in human macrophages. J Immunol. 2005;174:8154–62. [DOI] [PubMed] [Google Scholar]
- Firacative C, Duan S, Meyer W.. Galleria mellonella model identifies highly virulent strains among all major molecular types of Cryptococcus gattii. PLoS One. 2014;9:e105076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazão S de O, et al. Laccase affects the rate of Cryptococcus neoformans nonlytic exocytosis from macrophages. mBio. 2020;11,DOI: 10.1128/mBio.02085-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs BB, Eby J, Nobile CJet al. Role of filamentation in Galleria mellonella killing by Candida albicans. Microbes Infect. 2010;12:488–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu MS, et al. Cryptococcus neoformans urease affects the outcome of intracellular pathogenesis by modulating phagolysosomal pH. PLoS Pathog. 2018;14:e1007144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gago S, García-Rodas R, Cuesta Iet al. Candida parapsilosis, Candida orthopsilosis, and Candida metapsilosis virulence in the non-conventional host Galleria mellonella. Virulence. 2014;5:278–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantner BN, Simmons RM, Canavera SJet al. Collaborative induction of inflammatory responses by Dectin-1 and Toll-like receptor 2. J Exp Med. 2003;197:1107–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantner BN, Simmons RM, Underhill DM.. Dectin-1 mediates macrophage recognition of Candida albicans yeast but not filaments. EMBO J. 2005;24:1277–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Rodas R, Casadevall A, Rodríguez-Tudela JLet al. Cryptococcus neoformans capsular enlargement and cellular gigantism during Galleria mellonella infection. PLoS One. 2011;6:e24485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Rodas R, González-Camacho F, Rodríguez-Tudela JLet al. The interaction between Candida krusei and murine macrophages results in multiple outcomes, including intracellular survival and escape from killing. Infect Immun. 2011;79:2136–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaur NK, Klotz SA.. Expression, cloning, and characterization of a Candida albicans gene, ALA1, that confers adherence properties upon Saccharomyces cerevisiae for extracellular matrix proteins. Infect Immun. 1997;65:5289–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemsa D, Davis SD, Wedgwood RJ. Lysozyme and serum bactericidal action. Nature. 1966;210:950–1. [DOI] [PubMed] [Google Scholar]
- Ghannoum MA. Potential role of phospholipases in virulence and fungal pathogenesis. Clin Microbiol Rev. 2000;13:122–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, et al. Arginine-induced germ tube formation in Candida albicans is essential for escape from murine macrophage line RAW 264.7. Infect Immun. 2009;77:1596–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles SS, et al. The Cryptococcus neoformans catalase gene family and its role in antioxidant defense. Eukaryot Cell. 2006;5:1447–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D, Cho Y, Zhao Met al. Expression of inducible nitric oxide synthase in rat pulmonary Cryptococcus neoformans granulomas. Am J Pathol. 1996;148:1275–82. [PMC free article] [PubMed] [Google Scholar]
- González-Santoyo I, Córdoba-Aguilar A. Phenoloxidase: a key component of the insect immune system. Entomol Exp Appl. 2012;142:1–16. [Google Scholar]
- Goodridge HS, et al. Activation of the innate immune receptor Dectin-1 upon formation of a 'phagocytic synapse'. Nature. 2011;472:471–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopal PK, Shepherd MG, Sullivan PA.. Analysis of wall glucans from yeast, hyphal and germ-tube forming cells of Candida albicans. Microbiology. 1984;130:3295–301. [DOI] [PubMed] [Google Scholar]
- Gordon AH, D'Arcy Hart P, Young MR. Ammonia inhibits phagosome–lysosome fusion in macrophages. Nature. 1980;286:79–80. [DOI] [PubMed] [Google Scholar]
- Goyal S, Castrillón-Betancur JC, Klaile Eet al. The interaction of human pathogenic fungi with C-type lectin receptors. Front Immunol. 2018;9:1261. 10.3389/fimmu.2018.01261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gropp K, Schild L, Schindler Set al. The yeast Candida albicans evades human complement attack by secretion of aspartic proteases. Mol Immunol. 2009;47:465–75. [DOI] [PubMed] [Google Scholar]
- Grubhoffer L, Matha V. Characteristics of a new L-fucose and N-acetyl-D-galactosamine specific lectin from the integument of Galleria mellonella larvae. 1991. https://agris.fao.org/agris-search/search.do?recordID=US201301741157(27 June 2020, date last accessed). [Google Scholar]
- Gupta S, Wang Y, Jiang H.. Manduca sexta prophenoloxidase (proPO) activation requires proPO-activating proteinase (PAP) and serine proteinase homologs (SPHs) simultaneously. Insect Biochem Mol Biol. 2005;35:241–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez BL, et al. Detection of melanin-like pigments in the dimorphic fungal pathogen Paracoccidioides brasiliensis in vitro and during infection. Infect Immun. 2001;69:5760–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton KK, Zhao J, Sims PJ. Interaction between apolipoproteins A-I and A-II and the membrane attack complex of complement. Affinity of the apoproteins for polymeric C9. J Biol Chem. 1993;268:3632–8. [PubMed] [Google Scholar]
- Hasan F, Xess I, Wang Xet al. Biofilm formation in clinical Candida isolates and its association with virulence. Microbes Infect. 2009;11:753–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmerhorst EJ, Troxler RF, Oppenheim FG. The human salivary peptide histatin 5 exerts its antifungal activity through the formation of reactive oxygen species. Proc Natl Acad Sci. 2001;98:14637–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herre J, et al. Dectin-1 uses novel mechanisms for yeast phagocytosis in macrophages. Blood. 2004;104:4038–45. [DOI] [PubMed] [Google Scholar]
- He X, Li S, Kaminskyj SGW.. Overexpression of Aspergillus nidulans α-1,3-glucan synthase increases cellular adhesion and causes cell wall defects. Med Mycol. 2018;56:645–8. [DOI] [PubMed] [Google Scholar]
- Holder DJ, Keyhani NO.. Adhesion of the entomopathogenic fungus Beauveria (Cordyceps) bassiana to substrata. Appl Environ Microbiol. 2005;71:5260–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H-R, et al. Dectin-3 recognizes glucuronoxylomannan of Cryptococcus neoformans Serotype AD and Cryptococcus gattii Serotype B to initiate host defense against cryptococcosis. Front Immunol. 2018;9, DOI: 10.3389/fimmu.2018.01781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua X-T, Ma X-J, Xue R-Jet al. Characterization of the Bombyx mori Cecropin A1 promoter regulated by IMD pathway. Insect Sci. 2016;23:297–304. [DOI] [PubMed] [Google Scholar]
- Hube B, et al. Disruption of each of the secreted aspartyl proteinase genes SAP1, SAP2, and SAP3 of Candida albicans attenuates virulence. Infect Immun. 1997;65:3529–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglesfield S, et al. Robust phagocyte recruitment controls the opportunistic fungal pathogen mucor circinelloides in innate granulomas in vivo. mBio. 2018;9, DOI: 10.1128/mBio.02010-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii K, et al. Insect cytokine paralytic peptide activates innate immunity via nitric oxide production in the silkworm Bombyx mori. Dev Comp Immunol. 2013;39:147–53. [DOI] [PubMed] [Google Scholar]
- Jackson JC, Higgins LA, Lin X.. Conidiation color mutants of Aspergillus fumigatus are highly pathogenic to the heterologous insect host Galleria mellonella. PLoS One. 2009;4:e4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson ES, Tinnell SB. Antioxidant function of fungal melanin. J Bacteriol. 1993;175:7102–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn B, Langfelder K, Schneider Uet al. PKSP-dependent reduction of phagolysosome fusion and intracellular kill of Aspergillus fumigatus conidia by human monocyte-derived macrophages. Cell Microbiol. 2002;4:793–803. [DOI] [PubMed] [Google Scholar]
- Jahn B, et al. Isolation and characterization of a pigmentless-conidium mutant of Aspergillus fumigatus with altered conidial surface and reduced virulence. Infect Immun. 1997;65:5110–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Wang Y, Kanost MR.. Pro-phenol oxidase activating proteinase from an insect, Manduca sexta: a bacteria-inducible protein similar to Drosophila easter. Proc Natl Acad Sci. 1998;95:12220–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Wang Y, Yu X-Qet al. Prophenoloxidase-activating proteinase-3 (PAP-3) from Manduca sexta hemolymph: a clip-domain serine proteinase regulated by serpin-1J and serine proteinase homologs. Insect Biochem Mol Biol. 2003;33:1049–60. [DOI] [PubMed] [Google Scholar]
- Jofre-Monseny L, et al. Effects of apoE genotype on macrophage inflammation and heme oxygenase-1 expression. Biochem Biophys Res Commun. 2007;357:319–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J, Sajjadian SM, Kim Y.. Hemolin, an immunoglobulin-like peptide, opsonizes nonself targets for phagocytosis and encapsulation in Spodoptera exigua, a lepidopteran insect. J Asia Pac Entomol. 2019;22:947–56. [Google Scholar]
- Kamai Y, Kubota M, Kamai Yet al. Contribution of Candida albicans ALS1 to the pathogenesis of experimental oropharyngeal candidiasis. Infect Immun. 2002;70:5256–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan H, et al. Molecular control of phenoloxidase-induced melanin synthesis in an insect. J Biol Chem. 2008;283:25316–23. [DOI] [PubMed] [Google Scholar]
- Karban R. Caterpillar basking behavior and nonlethal parasitism by tachinid flies. J Insect Behav. 1998;11:713–23. [Google Scholar]
- Kavanagh K, Fallon JP. Galleria mellonella larvae as models for studying fungal virulence. Fungal Biology Reviews, 2010;24:79–83. [Google Scholar]
- Kawai T, Akira S. Signaling to NF-κB by toll-like receptors. Trends Mol Med. 2007;13:460–9. [DOI] [PubMed] [Google Scholar]
- Kernien JF, Snarr BD, Sheppard DCet al. The interface between fungal biofilms and innate immunity. Front Immunol. 2018;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyoura Y, Tamai R. Innate immunity to Candida albicans. Jpn Dent Sci Rev. 2015;51:59–64. [Google Scholar]
- Komarov DA, Slepneva IA, Glupov VVet al. Superoxide and hydrogen peroxide formation during enzymatic oxidation of DOPA by phenoloxidase. Free Radic Res. 2005;39:853–8. [DOI] [PubMed] [Google Scholar]
- Kong Q, et al. In vitro effects of ambroxol on Cryptococcus adherence, planktonic cells, and biofilms. APMIS. 2017;125:634–40. [DOI] [PubMed] [Google Scholar]
- Kozel TR, Tabuni A, Young BJet al. Influence of opsonization conditions on C3 deposition and phagocyte binding of large- and small-capsule Cryptococcus neoformans cells. Infect Immun. 1996;64:2336–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel TR. Complement activation by pathogenic fungi. Res Immunol. 1998;149:309–20. [DOI] [PubMed] [Google Scholar]
- Kuhn DM, George T, Chandra Jet al. Antifungal susceptibility of Candida Biofilms: unique efficacy of amphotericin B lipid formulations and echinocandins. Antimicrob Agents Chemother. 2002;46:1773–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo M-J, Alexander M. Inhibition of the lysis of fungi by melanins. J Bacteriol. 1967;94:624–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwadha CA, Ong'amo GO, Ndegwa PNet al. The biology and control of the greater wax moth, Galleria mellonella. Insects. 2017;8, DOI: 10.3390/insects8020061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange A, Beier S, Huson DHet al. Genome sequence of Galleria mellonella (greater wax moth). Genome Announc. 2018;6, DOI: 10.1128/genomeA.01220-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latgé J-P. Aspergillus fumigatus and Aspergillosis. Clin Microbiol Rev. 1999;12:310–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, et al. Purification, cDNA cloning and expression of an insect defensin from the great wax moth, Galleria mellonella. Insect Mol Biol. 2004;13:65–72. [DOI] [PubMed] [Google Scholar]
- Leon-Rodriguez CMD, Fu MS, Çorbali MOet al. The capsule of Cryptococcus neoformans modulates phagosomal pH through its acid-base properties. mSphere. 2018;3, DOI: 10.1128/mSphere.00437-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levashina EA, Moita LF, Blandin Set al. Conserved role of a complement-like protein in phagocytosis revealed by dsRNA knockout in cultured cells of the mosquito, Anopheles gambiae. Cell. 2001;104:709–18. [DOI] [PubMed] [Google Scholar]
- Lev S, et al. Identification of Aph1, a phosphate-regulated, secreted, and vacuolar acid phosphatase in Cryptococcus neoformans. mBio. 2014;5, DOI: 10.1128/mBio.01649-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis LE, et al. Stage specific assessment of Candida albicans phagocytosis by macrophages identifies cell wall composition and morphogenesis as key determinants. PLoS Pathog. 2012;8:e1002578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Shi H-Q, Ying S-Het al. Distinct contributions of one Fe- and two Cu/Zn-cofactored superoxide dismutases to antioxidation, UV tolerance and virulence of Beauveria bassiana. Fungal Genet Biol. 2015;81:160–71. [DOI] [PubMed] [Google Scholar]
- Ling E, Yu X-Q.. Hemocytes from the tobacco hornworm Manduca sexta have distinct functions in phagocytosis of foreign particles and self dead cells. Dev Comp Immunol. 2006;30:301–9. [DOI] [PubMed] [Google Scholar]
- Liu W, et al. Immune signaling pathways activated in response to different pathogenic micro-organisms in Bombyx mori. Mol Immunol. 2015;65:391–7. [DOI] [PubMed] [Google Scholar]
- Liu Y, Huang X, Liu Het al. Increased virulence of albino mutant of Fonsecaea monophora in Galleria mellonella. Med Mycol. 2019;57:1018–23. [DOI] [PubMed] [Google Scholar]
- Lo H-J, Köhler JR, DiDomenico Bet al. Nonfilamentous C. albicans mutants are avirulent. Cell. 1997;90:939–49. [DOI] [PubMed] [Google Scholar]
- Lorenz MC, Bender JA, Fink GR. Transcriptional response of Candida albicans upon internalization by macrophages. Eukaryot Cell. 2004;3:1076–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loussert C, Schmitt C, Prevost M-Cet al. In vivo biofilm composition of Aspergillus fumigatus. Cell Microbiol. 2010;12:405–10. [DOI] [PubMed] [Google Scholar]
- Lowman DW, et al. Novel structural features in Candida albicans hyphal glucan provide a basis for differential innate immune recognition of hyphae versus yeast. J Biol Chem. 2014;289:3432–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loza L, Fu Y, Ibrahim ASet al. Functional analysis of the Candida albicans ALS1 gene product. Yeast. 2004;21:473–82. [DOI] [PubMed] [Google Scholar]
- Luberto C, et al. Identification of App1 as a regulator of phagocytosis and virulence of Cryptococcus neoformans. J Clin Invest. 2003;112:1080–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luther K, Torosantucci A, Brakhage AAet al. Phagocytosis of Aspergillus fumigatus conidia by murine macrophages involves recognition by the dectin-1 beta-glucan receptor and Toll-like receptor 2. Cell Microbiol. 2007;9:368–81. [DOI] [PubMed] [Google Scholar]
- Lu W, de Leeuw E. Pro-inflammatory and pro-apoptotic properties of human defensin 5. Biochem Biophys Res Commun. 2013;436:557–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z, Jiang H.. Expression of Manduca sexta serine proteinase homolog precursors in insect cells and their proteolytic activation. Insect Biochem Mol Biol. 2008;38:89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnani Dinamarco T, et al. Aspergillus fumigatus calcineurin interacts with a nucleoside diphosphate kinase. Microbes Infect. 2012;14:922–9. [DOI] [PubMed] [Google Scholar]
- Ma H, Croudace JE, Lammas DAet al. Expulsion of live pathogenic yeast by macrophages. Curr Biol. 2006;16:2156–60. [DOI] [PubMed] [Google Scholar]
- Mak P, Zdybicka-Barabas A, Cytryńska M. A different repertoire of Galleria mellonella antimicrobial peptides in larvae challenged with bacteria and fungi. Dev Comp Immunol. 2010;34:1129–36. [DOI] [PubMed] [Google Scholar]
- Mandato CA, Diehl-Jones WL, Moore SJet al. The effects of eicosanoid biosynthesis inhibitors on prophenoloxidase activation, phagocytosis and cell spreading in Galleria mellonella. J Insect Physiol. 1997;43:1–8. [DOI] [PubMed] [Google Scholar]
- Marcos-Zambrano LJ, et al. Candida isolates causing candidemia show different degrees of virulence in Galleria mellonella. Med Mycol. 2020;58:83–92. [DOI] [PubMed] [Google Scholar]
- Margalit A, Kavanagh K. The innate immune response to Aspergillus fumigatus at the alveolar surface. FEMS Microbiol Rev. 2015;39:670–87. [DOI] [PubMed] [Google Scholar]
- Marion CL, Rappleye CA, Engle JTet al. An α-(1,4)-amylase is essential for α-(1,3)-glucan production and virulence in Histoplasma capsulatum. Mol Microbiol. 2006;62:970–83. [DOI] [PubMed] [Google Scholar]
- Martinez LR, Casadevall A.. Cryptococcus neoformans Biofilm formation depends on surface support and carbon source and reduces fungal cell susceptibility to heat, cold, and UV light. Appl Environ Microbiol. 2007;73:4592–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez LR, Casadevall A.. Cryptococcus neoformans cells in biofilms are less susceptible than planktonic cells to antimicrobial molecules produced by the innate immune system. Infect Immun. 2006a;74:6118–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez LR, Casadevall A.. Specific antibody can prevent fungal biofilm formation and this effect correlates with protective efficacy. Infect Immun. 2005;73:6350–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez LR, Casadevall A.. Susceptibility of Cryptococcus neoformans biofilms to antifungal agents in vitro. Antimicrob Agents Chemother. 2006b;50:1021–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathacek˜ha V, Grubhoffer L, Weyda Fet al. Detection of β-1,3-glucan-specific lectin on the surface of plasmatocytes, immunocompetent cells of great wax moth, Galleria mellonella L. Cytobios. 1990;64:35–42. [Google Scholar]
- Medoff G, et al. Irreversible block of the mycelial-to-yeast phase transition of Histoplasma capsulatum. Science. 1986;231:476–9. [DOI] [PubMed] [Google Scholar]
- Meiller TF, et al. A novel immune evasion strategy of Candida albicans: proteolytic cleavage of a salivary antimicrobial peptide. PLoS One. 2009;4:e5039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes A, Mores AU, Carvalho APet al. Candida albicans biofilms produce more secreted aspartyl protease than the planktonic cells. Biol Pharm Bull. 2007;30:1813–5. [DOI] [PubMed] [Google Scholar]
- Mirbod-Donovan F, Schaller R, Hung C-Yet al. Urease produced by Coccidioides posadasii contributes to the virulence of this respiratory pathogen. Infect Immun. 2006;74:504–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missall TA, Pusateri ME, Donlin MJet al. Posttranslational, translational, and transcriptional responses to nitric oxide stress in Cryptococcus neoformans: implications for virulence. Eukaryot Cell. 2006;5:518–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monari C, et al. Glucuronoxylomannan, a Microbial compound, regulates expression of costimulatory molecules and production of cytokines in macrophages. J Infect Dis. 2005;191:127–37. [DOI] [PubMed] [Google Scholar]
- Mora-Montes HM, et al. Recognition and blocking of innate immunity cells by Candida albicans chitin. Infect Immun. 2011;79:1961–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris-Jones R, et al. Synthesis of melanin pigment by Candida albicans in vitro and during infection. Infect Immun. 2005;73:6147–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morschhäuser J, Virkola R, Korhonen TKet al. Degradation of human subendothelial extracellular matrix by proteinase-secreting Candida albicans. FEMS Microbiol Lett. 1997;153:349–55. [DOI] [PubMed] [Google Scholar]
- Motizuki M, Itoh T, Yamada Met al. Purification, primary structure, and antimicrobial activities of bovine apolipoprotein A-II. J Biochem. 1998;123:675–9. [DOI] [PubMed] [Google Scholar]
- Motizuki M, et al. Lipid-binding and antimicrobial properties of synthetic peptides of bovine apolipoprotein A-II. Biochem J. 1999;342:215–21. [PMC free article] [PubMed] [Google Scholar]
- Moussawi LE, Nakhleh J, Kamareddine Let al. The mosquito melanization response requires hierarchical activation of non-catalytic clip domain serine protease homologs. PLoS Pathog. 2019;15:e1008194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee PK, Seshan KR, Leidich SDet al. Reintroduction of the PLB1 gene into Candida albicans restores virulence in vivo. Microbiology. 2001;147:2585–97. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay S, et al. Causes of pulmonary granulomas: a retrospective study of 500 cases from seven countries. J Clin Pathol. 2012;65:51–7. [DOI] [PubMed] [Google Scholar]
- Mylonakis E, et al. Galleria mellonella as a model system to study Cryptococcus neoformans pathogenesis. Infect Immun. 2005;73:3842–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naglik J, Albrecht A, Bader Oet al. Candida albicans proteinases and host/pathogen interactions. Cell Microbiol. 2004;6:915–26. [DOI] [PubMed] [Google Scholar]
- Naglik JR, et al. Differential expression of Candida albicans secreted aspartyl proteinase and phospholipase B genes in humans correlates with active oral and vaginal infections. J Infect Dis. 2003;188:469–79. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y, Kanbe T, Mizuguchi I.. Disruption of the human pathogenic yeast Candida albicans catalase gene decreases survival in mouse-model infection and elevates susceptibility to higher temperature and to detergents. Microbiol Immunol. 2003;47:395–403. [DOI] [PubMed] [Google Scholar]
- Nazario-Toole AE, Wu LP.. Phagocytosis in insect immunity. In: Ligoxygakis P (ed.). Advances in Insect Physiology. Vol. 52, Academic Press, 2017, 35–82. [Google Scholar]
- Netea MG, Van der Graaf C, Van der Meer JWMet al. Recognition of fungal pathogens by Toll-like receptors. Eur J Clin Microbiol Infect Dis. 2004;23:672–6. [DOI] [PubMed] [Google Scholar]
- Netea MG, et al. Aspergillus fumigatus evades immune recognition during germination through loss of Toll-like receptor-4-mediated signal transduction. J Infect Dis. 2003;188:320–26. [DOI] [PubMed] [Google Scholar]
- Nicola AM, Robertson EJ, Albuquerque Pet al. Nonlytic exocytosis of Cryptococcus neoformans from Macrophages occurs in vivo and is influenced by phagosomal pH. mBio. 2011;2, DOI: 10.1128/mBio.00167-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosanchuk JD, et al. Histoplasma capsulatum synthesizes melanin-like pigments in vitro and during mammalian infection. Infect Immun. 2002;70:5124–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noverr MC, Cox GM, Perfect JRet al. Role of PLB1 in pulmonary inflammation and cryptococcal eicosanoid production. Infect Immun. 2003;71:1538–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odom A, Muir S, Lim Eet al. Calcineurin is required for virulence of Cryptococcus neoformans. EMBO J. 1997;16:2576–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okagaki LH, Nielsen K.. Titan cells confer protection from phagocytosis in Cryptococcus neoformans infections. Eukaryot Cell. 2012;11:820–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okagaki LH, et al. Cryptococcal cell morphology affects host cell interactions and pathogenicity. PLoS Pathog. 2010;6:e1000953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ophir G, Amariglio N, Jacob-Hirsch Jet al. Apolipoprotein E4 enhances brain inflammation by modulation of the NF-κB signaling cascade. Neurobiol Dis. 2005;20:709–18. [DOI] [PubMed] [Google Scholar]
- Orciuolo E, et al. Effects of Aspergillus fumigatus gliotoxin and methylprednisolone on human neutrophils: implications for the pathogenesis of invasive aspergillosis. J Leukoc Biol. 2007;82:839–48. [DOI] [PubMed] [Google Scholar]
- Orner EP, Bhattacharya S, Kalenja Ket al. Cell wall-associated virulence factors contribute to increased resilience of old Cryptococcus neoformans cells. Front Microbiol. 2019;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsi CF, et al. The ABC transporter-encoding gene AFR1 affects the resistance of Cryptococcus neoformans to microglia-mediated antifungal activity by delaying phagosomal maturation. FEMS Yeast Res. 2009;9:301–10. [DOI] [PubMed] [Google Scholar]
- Pahl HL, et al. The immunosuppressive fungal metabolite gliotoxin specifically inhibits transcription factor NF-κB. J Exp Med. 1996;183:1829–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris S, et al. Catalases of Aspergillus fumigatus. Infect Immun. 2003;71:3551–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J-A, Kim Y. Toll recognition signal activates oenocytoid cell lysis via a crosstalk between plasmatocyte-spreading peptide and eicosanoids in response to a fungal infection. Cell Immunol. 2012;279:117–23. [DOI] [PubMed] [Google Scholar]
- Pendland JC, Heath MA, Boucias DG.. Function of a galactose-binding lectin from Spodoptera exigua larval haemolymph: opsonization of blastospores from entomogenous hyphomycetes. J Insect Physiol. 1988;34:533–40. [Google Scholar]
- Pereira TC, et al. Recent advances in the use of Galleria mellonella model to study immune responses against human pathogens. J Fungi (Basel). 2018;4, DOI: 10.3390/jof4040128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips DR, Clark KD.. Bombyx mori and Aedes aegypti form multi-functional immune complexes that integrate pattern recognition, melanization, coagulants, and hemocyte recruitment. PLoS One. 2017;12:e0171447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povelones M, Waterhouse RM, Kafatos FCet al. Leucine-rich repeat protein complex activates mosquito complement in defense against plasmodium parasites. Science. 2009;324:258–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povelones M, et al. The CLIP-domain serine protease homolog SPCLIP1 regulates complement recruitment to microbial surfaces in the malaria mosquito Anopheles gambiae. PLoS Pathog. 2013;9:e1003623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puccia R, Vallejo MC, Matsuo ALet al. The paracoccidioides cell wall: past and present layers toward understanding interaction with the host. Front Microbiol. 2011;2, DOI: 10.3389/fmicb.2011.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai MN, Sharma V, Balusu Set al. An essential role for phosphatidylinositol 3-kinase in the inhibition of phagosomal maturation, intracellular survival and virulence in Candida glabrata. Cell Microbiol. 2015;17:269–87. [DOI] [PubMed] [Google Scholar]
- Rambach G, et al. Secretion of a fungal protease represents a complement evasion mechanism in cerebral aspergillosis. Mol Immunol. 2010;47:1438–49. [DOI] [PubMed] [Google Scholar]
- Rapala-Kozik M, et al. Inactivation of the antifungal and immunomodulatory properties of human cathelicidin LL-37 by aspartic proteases produced by the pathogenic yeast Candida albicans. Infect Immun. 2015;83:2518–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappleye CA, Eissenberg LG, Goldman WE.. Histoplasma capsulatum α-(1,3)-glucan blocks innate immune recognition by the β-glucan receptor. Proc Natl Acad Sci. 2007;104:1366–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliffe NA, Rowley AF.. Opsonic activity of insect hemolymph. In: Cheng TC (ed.). Invertebrate Blood: Cells and Serum Factors. Boston, MA: Springer US, 1984, 187–204. [Google Scholar]
- Reeves EP, Messina CGM, Doyle Set al. Correlation between gliotoxin production and virulence of Aspergillus fumigatus in Galleria mellonella. Mycopathologia. 2004;158:73–9. [DOI] [PubMed] [Google Scholar]
- Renwick J, Daly P, Reeves Eet al. Susceptibility of larvae of Galleria mellonella to infection by Aspergillus fumigatus is dependent upon stage of conidial germination. Mycopathologia. 2006;161:377–84. [DOI] [PubMed] [Google Scholar]
- Renwick J, Reeves EP, Wientjes FBet al. Translocation of proteins homologous to human neutrophil p47phox and p67phox to the cell membrane in activated hemocytes of Galleria mellonella. Dev Comp Immunol. 2007;31:347–59. [DOI] [PubMed] [Google Scholar]
- Ribeiro C, Brehélin M.. Insect haemocytes: what type of cell is that?. J Insect Physiol. 2006;52:417–29. [DOI] [PubMed] [Google Scholar]
- Rocha JDB, et al. Capsular polysaccharides from Cryptococcus neoformans modulate production of neutrophil extracellular traps (NETs) by human neutrophils. Sci Rep. 2015;5, Art. no. 1, DOI: 10.1038/srep08008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romera D, Aguilera-Correa JJ, Gadea Iet al. Candida auris: a comparison between planktonic and biofilm susceptibility to antifungal drugs. J Med Microbiol. 2019;68:1353–8. [DOI] [PubMed] [Google Scholar]
- Romero-Martinez R, Wheeler M, Guerrero-Plata Aet al. Biosynthesis and functions of melanin in Sporothrix schenckii. Infect Immun. 2000;68:3696–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Román E, et al. Role of catalase overproduction in drug resistance and virulence in Candida albicans. Future Microbiol. 2016;11:1279–97. [DOI] [PubMed] [Google Scholar]
- Rosales C. Phagocytosis, a cellular immune response in insects. Invertebrate Surviv J. 2011;8, Art. no. 1. [Google Scholar]
- Rosas ÁL, Casadevall A.. Melanization decreases the susceptibility of Cryptococcus neoformans to enzymatic degradation. Mycopathologia. 2001;151:53–6. [DOI] [PubMed] [Google Scholar]
- Rossoni RD, Barbosa JO, Vilela SFGet al. Correlation of phospholipase and proteinase production of Candida with in vivo pathogenicity in Galleria mellonella. Braz J Oral Sci. 2013;12:199–204. [Google Scholar]
- Sabiiti W, et al. Efficient phagocytosis and laccase activity affect the outcome of HIV-associated cryptococcosis. J Clin Invest. 2014;124:2000–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto H, et al. Gliotoxin suppresses NF-κB activation by selectively inhibiting linear ubiquitin chain assembly complex (LUBAC). ACS Chem Biol. 2015;10:675–81. [DOI] [PubMed] [Google Scholar]
- Salas SD, Bennett JE, Kwon-Chung KJet al. Effect of the laccase gene CNLAC1, on virulence of Cryptococcus neoformans. J Exp Med. 1996;184:377–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangbaramou R, Camara I, Huang Xet al. Behavioral thermoregulation in Locusta migratoria manilensis (Orthoptera: Acrididae) in response to the entomopathogenic fungus, Beauveria bassiana. PLoS One. 2018;13:e0206816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanglard D, Hube B, Monod Met al. A triple deletion of the secreted aspartyl proteinase genes SAP4, SAP5, and SAP6 of Candida albicans causes attenuated virulence. Infect Immun. 1997;65:3539–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanguinetti M, et al. Role of AFR1, an ABC transporter-encoding gene, in the in vivo response to fluconazole and virulence of Cryptococcus neoformans. Infect Immun. 2006;74:1352–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma JV, Ward PA. The complement system. Cell Tissue Res. 2011;343:227–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarvari M, Mikani A, Mehrabadi M. The innate immune gene Relish and Caudal jointly contribute to the gut immune homeostasis by regulating antimicrobial peptides in Galleria mellonella. Dev Comp Immunol. 2020;110:103732. [DOI] [PubMed] [Google Scholar]
- Satyavathi VV, Minz A, Nagaraju J. Nodulation: an unexplored cellular defense mechanism in insects. Cell Signal. 2014;26:1753–63. [DOI] [PubMed] [Google Scholar]
- Schaller M, Korting HC, Schäfer Wet al. Secreted aspartic proteinase (Sap) activity contributes to tissue damage in a model of human oral candidosis. Mol Microbiol. 1999b;34:169–80. [DOI] [PubMed] [Google Scholar]
- Schaller M, et al. In vivo expression and localization of Candida albicans secreted aspartyl proteinases during oral candidiasis in HIV-infected patients. J Invest Dermatol. 1999a;112:383–6. [DOI] [PubMed] [Google Scholar]
- Schuhmann B, Seitz V, Vilcinskas Aet al. Cloning and expression of gallerimycin, an antifungal peptide expressed in immune response of greater wax moth larvae, Galleria mellonella. Arch Insect Biochem Physiol. 2003;53:125–33. [DOI] [PubMed] [Google Scholar]
- Seidel C, Moreno-Velásquez SD, Ben-Ghazzi Net al. Phagolysosomal survival enables non-lytic hyphal escape and ramification through lung epithelium during Aspergillus fumigatus infection. Front Microbiol. 2020;11, DOI: 10.3389/fmicb.2020.01955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seider K, et al. The facultative intracellular pathogen Candida glabrata subverts macrophage cytokine production and phagolysosome maturation. J Immunol. 2011;187:3072–86. [DOI] [PubMed] [Google Scholar]
- Seidler MJ, Salvenmoser S, Müller F-MC. Aspergillus fumigatus forms biofilms with reduced antifungal drug susceptibility on bronchial epithelial cells. Antimicrob Agents Chemother. 2008;52:4130–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seoane PI, May RC. Vomocytosis: what we know so far. Cell Microbiol. 2020;22:e13145. [DOI] [PubMed] [Google Scholar]
- Shah DT, Jackman S, Engle Jet al. Effect of gliotoxin on human polymorphonuclear neutrophils. Infect Dis Obstet Gynecol. 1998;6:168–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah DT, Larsen B.. Clinical isolates of yeast produce a gliotoxin-like substance. Mycopathologia. 1991;116:203–8. [DOI] [PubMed] [Google Scholar]
- Sherry L, et al. Biofilm-forming capability of highly virulent, multidrug-resistant Candida auris. Emerg Infect Dis. 2017;23:328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoham S, Huang C, Chen J-Met al. Toll-like receptor 4 mediates intracellular signaling without TNF-α Release in response to Cryptococcus neoformans polysaccharide capsule. J Immunol. 2001;166:4620–6. [DOI] [PubMed] [Google Scholar]
- Shokal U, Eleftherianos I. Evolution and function of thioester-containing proteins and the complement system in the innate immune response. Front Immunol. 2017;8, DOI: 10.3389/fimmu.2017.00759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha S, Kim Y. Various eicosanoids modulate the cellular and humoral immune responses of the Beet Armyworm, Spodoptera exigua. Biosci Biotechnol Biochem. 2009;73:2077–84. [DOI] [PubMed] [Google Scholar]
- Shu M, et al. Mechanisms of nodule-specific melanization in the hemocoel of the silkworm, Bombyx mori. Insect Biochem Mol Biol. 2016;70:10–23. [DOI] [PubMed] [Google Scholar]
- Siest G, Zaiou M, Visvikis S. Human apolipoprotein E concentration in response to diseases and therapeutic treatments. Drug Dev Res. 2002;56:95–110. [Google Scholar]
- Silva-Dias A, Miranda IM, Branco Jet al. Adhesion, biofilm formation, cell surface hydrophobicity, and antifungal planktonic susceptibility: relationship among Candida spp. Front Microbiol. 2015;6, DOI: 10.3389/fmicb.2015.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva TMJ, Glee PM, Hazen KC.. Influence of cell surface hydrophobicity on attachment of Candida albicans to extracellular matrix proteins. J Med Vet Mycol. 1995;33:117–22. [PubMed] [Google Scholar]
- Skinner WS, Dennis PA, Li JPet al. Isolation and identification of paralytic peptides from hemolymph of the lepidopteran insects Manduca sexta, Spodoptera exigua, and Heliothis virescens. J Biol Chem. 1991;266:12873–7. [PubMed] [Google Scholar]
- Small JM, Mitchell TG.. Strain variation in antiphagocytic activity of capsular polysaccharides from Cryptococcus neoformans serotype A. Infect Immun. 1989;57:3751–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DFQ, Casadevall A. The role of melanin in fungal pathogenesis for animal hosts. In: Rodrigues ML (ed.). Fungal Physiology and Immunopathogenesis. Cham: Springer International Publishing, 2019, 1–30. [DOI] [PubMed] [Google Scholar]
- Sowa-Jasiłek A, et al. Studies on the role of insect hemolymph polypeptides: Galleria mellonella anionic peptide 2 and lysozyme. Peptides. 2014;53:194–201. [DOI] [PubMed] [Google Scholar]
- Speth C, Rambach G, Würzner Ret al. Complement and fungal pathogens: an update. Mycoses. 2008;51:477–96. [DOI] [PubMed] [Google Scholar]
- Stano P, et al. App1: an antiphagocytic protein that binds to complement receptors 3 and 2. J Immunol. 2009;182:84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stappers MHT, et al. Recognition of DHN-melanin by MelLec, is required for protective immunity to Aspergillus. Nature. 2018;555:382–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbach WJ, et al. Calcineurin controls growth, morphology, and pathogenicity in Aspergillus fumigatus. Eukaryot Cell. 2006;5:1091–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg DA, et al. Protegrin-1: a broad-spectrum, rapidly microbicidal peptide with in vivo activity. Antimicrob Agents Chemother. 1997;41:1738–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundstrom P, Cutler JE, Staab JF.. Reevaluation of the role of HWP1 in systemic candidiasis by use of Candida albicans strains with selectable marker URA3 targeted to the ENO1 locus. Infect Immun. 2002;70:3281–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundstrom P. Adhesion in Candida spp. Cell Microbiol. 2002;4:461–9. [DOI] [PubMed] [Google Scholar]
- Sun S-C, Faye I. Cecropia immunoresponsive factor, an insect immunoresponsive factor with DNA-binding properties similar to nuclear-factor KB. Eur J Biochem. 1992;204:885–92. [DOI] [PubMed] [Google Scholar]
- Tanaka H, et al. A genome-wide analysis of genes and gene families involved in innate immunity of Bombyx mori. Insect Biochem Mol Biol. 2008;38:1087–110. [DOI] [PubMed] [Google Scholar]
- Tanaka H, et al. A novel Rel protein and shortened isoform that differentially regulate antibacterial peptide genes in the silkworm Bombyx mori. Biochimica Biophysica Acta. 2005;1730:10–21. [DOI] [PubMed] [Google Scholar]
- Tanaka H, et al. Identification and functional analysis of Relish homologs in the silkworm, Bombyx mori. Biochimica Biophysica Acta. 2007;1769:559–68. [DOI] [PubMed] [Google Scholar]
- Tang J, Lin G, Langdon WYet al. Regulation of C-type lectin receptor-mediated antifungal immunity. Front Immunol. 2018;9, DOI: 10.3389/fimmu.2018.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomaz L, García-Rodas R, Guimarães AJet al. Galleria mellonella as a model host to study Paracoccidioides lutzii and Histoplasma capsulatum. Virulence. 2013;4:139–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillmann AT, et al. Contribution of Fdh3 and Glr1 to glutathione redox state, stress adaptation and virulence in Candida albicans. PLoS One. 2015;10:e0126940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tojo S, Naganuma F, Arakawa Ket al. Involvement of both granular cells and plasmatocytes in phagocytic reactions in the greater wax moth, Galleria mellonella. J Insect Physiol. 2000;46:1129–35. [DOI] [PubMed] [Google Scholar]
- Tokura A, et al. Factors functioning in nodule melanization of insects and their mechanisms of accumulation in nodules. J Insect Physiol. 2014;60:40–9. [DOI] [PubMed] [Google Scholar]
- Tomalka J, et al. β-Defensin 1 plays a role in acute mucosal defense against Candida albicans. J Immunol. 2015;194:1788–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torosantucci A, Chiani P, Cassone A.. Differential chemokine response of human monocytes to yeast and hyphal forms of Candida albicans and its relation to the β-1,6 glucan of the fungal cell wall. J Leukoc Biol. 2000;68:923–32. [PubMed] [Google Scholar]
- Trevijano-Contador N, et al. Cryptococcus neoformans induces antimicrobial responses and behaves as a facultative intracellular pathogen in the non mammalian model Galleria mellonella. Virulence. 2015;6:66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronchin G, Bouchara J-P, Larcher Get al. Interaction between Aspergillus fumigatus and basement membrane laminin: binding and substrate degradation. Biol Cell. 1993;77:201–8. [DOI] [PubMed] [Google Scholar]
- Tronchin G, Pihet M, Lopes-Bezerra LMet al. Adherence mechanisms in human pathogenic fungi. Med Mycol. 2008;46:749–72. [DOI] [PubMed] [Google Scholar]
- Tsunawaki S, Yoshida LS, Nishida Set al. Fungal metabolite gliotoxin inhibits assembly of the human respiratory burst NADPH oxidase. Infect Immun. 2004;72:3373–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner MW. The role of mannose-binding lectin in health and disease. Mol Immunol. 2003;40:423–9. [DOI] [PubMed] [Google Scholar]
- Upadhyay SK, Mahajan L, Ramjee Set al. Identification and characterization of a laminin-binding protein of Aspergillus fumigatus: extracellular thaumatin domain protein (AfCalAp). J Med Microbiol. 2009;58:714–22. [DOI] [PubMed] [Google Scholar]
- van Asbeck EC, Hoepelman AI, Scharringa Jet al. Mannose binding lectin plays a crucial role in innate immunity against yeast by enhanced complement activation and enhanced uptake of polymorphonuclear cells. BMC Microbiol. 2008;8:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Graaf CAA, Netea MG, Verschueren Iet al. Differential cytokine production and Toll-Like receptor signaling pathways by Candida albicans Blastoconidia and hyphae. Infect Immun. 2005;73:7458–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecchiarelli A. Immunoregulation by capsular components of Cryptococcus neoformans. Med Mycol. 2000;38:407–17. [DOI] [PubMed] [Google Scholar]
- Vecchiarelli A, et al. Downregulation by cryptococcal polysaccharide of tumor necrosis factor alpha and interleukin-1 beta secretion from human monocytes. Infect Immun. 1995;63:2919–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma A, Wüthrich M, Deepe Get al. Adaptive immunity to fungi. Cold Spring Harb Perspect Med. 2015;5:a019612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vij R, Danchik C, Crawford Cet al. Variation in cell surface hydrophobicity among Cryptococcus neoformans strains influences interactions with amoebas. mSphere. 2020;5, DOI: 10.1128/mSphere.00310-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitek MP, Brown CM, Colton CA. APOE genotype-specific differences in the innate immune response. Neurobiol Aging. 2009;30:1350–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel H, Altincicek B, Glöckner Get al. A comprehensive transcriptome and immune-gene repertoire of the lepidopteran model host Galleria mellonella. BMC Genomics. 2011;12:308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonk AG, et al. Apolipoprotein-E-deficient mice exhibit an increased susceptibility to disseminated candidiasis. Med Mycol. 2004;42:341–8. [DOI] [PubMed] [Google Scholar]
- Vylkova S, Carman AJ, Danhof HAet al. The fungal pathogen Candida albicans autoinduces hyphal morphogenesis by raising extracellular pH. mBio. 2011;2, DOI: 10.1128/mBio.00055-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vylkova S, Lorenz MC. Modulation of phagosomal pH by Candida albicans promotes hyphal morphogenesis and requires Stp2p, a regulator of amino acid transport. PLoS Pathog. 2014;10:e1003995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Aisen P, Casadevall A.. Cryptococcus neoformans melanin and virulence: mechanism of action. Infect Immun. 1995;63:3131–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Lu Z, Jiang H.. Manduca sexta proprophenoloxidase activating proteinase-3 (PAP3) stimulates melanization by activating proPAP3, proSPHs, and proPOs. Insect Biochem Mol Biol. 2014;50:82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z-L, Zhang L-B, Ying S-Het al. Catalases play differentiated roles in the adaptation of a fungal entomopathogen to environmental stresses. Environ Microbiol. 2013;15:409–18. [DOI] [PubMed] [Google Scholar]
- Wasylnka JA, Moore MM.. Aspergillus fumigatus conidia survive and germinate in acidic organelles of A549 epithelial cells. J Cell Sci. 2003;116:1579–87. [DOI] [PubMed] [Google Scholar]
- Watanabe A, Miyazawa S, Kitami Met al. Characterization of a novel C-type lectin, Bombyx mori multibinding protein, from the B. mori Hemolymph: mechanism of wide-range microorganism recognition and role in immunity. J Immunol. 2006;177:4594–604. [DOI] [PubMed] [Google Scholar]
- Weide MR, Ernst JF.. Caco-2 monolayer as a model for transepithelial migration of the fungal pathogen Candida albicans. Mycoses. 1999;42:61–7. [PubMed] [Google Scholar]
- Westman J, Moran G, Mogavero Set al. Candida albicans hyphal expansion causes phagosomal membrane damage and luminal alkalinization. mBio. 2018;9, DOI: 10.1128/mBio.01226-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitten MMA, Tew IF, Lee BLet al. A novel role for an insect apolipoprotein (apolipophorin III) in β-1,3-glucan pattern recognition and cellular encapsulation reactions. J Immunol. 2004;172:2177–85. [DOI] [PubMed] [Google Scholar]
- Wiesner A, Götz P.. Silica beads induce cellular and humoral immune responses in Galleria mellonella larvae and in isolated plasmatocytes, obtained by a newly adapted nylon wool separation method. J Insect Physiol. 1993;39:865–76. [Google Scholar]
- Wiesner A, Losen S, Kopáček Pet al. Isolated apolipophorin III from Galleria mellonella stimulates the immune reactions of this insect. J Insect Physiol. 1997;43:383–91. [DOI] [PubMed] [Google Scholar]
- Williamson PR. Biochemical and molecular characterization of the diphenol oxidase of Cryptococcus neoformans: identification as a laccase. J Bacteriol. 1994;176:656–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P, Lambert PA, Haigh CGet al. The influence of the O and K antigens of Klebsiella aerogenes on surface hydrophobicity and susceptibility to phagocytosis and antimicrobial agents. J Med Microbiol. 1986;21:125–32. [DOI] [PubMed] [Google Scholar]
- Wojda I, Jakubowicz T. Humoral immune response upon mild heat-shock conditions in Galleria mellonella larvae. J Insect Physiol. 2007;53:1134–44. [DOI] [PubMed] [Google Scholar]
- Wojda I, Kowalski P, Jakubowicz T. Humoral immune response of Galleria mellonella larvae after infection by Beauveria bassiana under optimal and heat-shock conditions. J Insect Physiol. 2009;55:525–31. [DOI] [PubMed] [Google Scholar]
- Woods CM, Hooper DN, Ooi EHet al. Human lysozyme has fungicidal activity against nasal fungi. Am J Rhinol Allergy. 2011;25:236–40. [DOI] [PubMed] [Google Scholar]
- Wu S, Zhang X, Chen Xet al. BmToll9, an arthropod conservative Toll, is likely involved in the local gut immune response in the silkworm, Bombyx mori. Dev Comp Immunol. 2010;34:93–6. [DOI] [PubMed] [Google Scholar]
- Wysong DR, Christin L, Sugar AMet al. Cloning and sequencing of a Candida albicans Catalase gene and effects of disruption of this gene. Infect Immun. 1998;66:1953–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wächtler B, Wilson D, Haedicke Ket al. From attachment to damage: defined genes of Candida albicans mediate adhesion, invasion and damage during interaction with oral epithelial cells. PLoS One. 2011;6:e17046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X-Q, Wang J, Huang B-Fet al. A new manganese superoxide dismutase identified from Beauveria bassiana enhances virulence and stress tolerance when overexpressed in the fungal pathogen. Appl Microbiol Biotechnol. 2010;86:1543–53. [DOI] [PubMed] [Google Scholar]
- Xu X-Y, Chen F, Sun Het al. Important factors mediates the adhesion of Aspergillus fumigatus to alveolar epithelial cells with E-cadherin. Am J Transl Res. 2016;8:2419–25. [PMC free article] [PubMed] [Google Scholar]
- Xu X, et al. E-cadherin mediates adhesion and endocytosis of Aspergillus fumigatus blastospores in human epithelial cells. Chin Med J. 2012;125:617–21. [PubMed] [Google Scholar]
- Yadav AK, Desai PR, Rai MNet al. Glutathione biosynthesis in the yeast pathogens Candida glabrata and Candida albicans: essential in C. glabrata, and essential for virulence in C. albicans. Microbiology. 2011;157:484–95. [DOI] [PubMed] [Google Scholar]
- Yamano Y, Matsumoto M, Sasahara Ket al. Structure of genes for cecropin A and an inducible nuclear protein that binds to the promoter region of the genes from the silkworm, Bombyx mori. Biosci Biotechnol Biochem. 1998;62:237–41. [DOI] [PubMed] [Google Scholar]
- Yassine H, Kamareddine L, Osta MA.. The mosquito melanization response is implicated in defense against the entomopathogenic fungus Beauveria bassiana. PLoS Pathog. 2012;8:e1003029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida LS, Abe S, Tsunawaki S.. Fungal gliotoxin targets the onset of superoxide-generating NADPH oxidase of human neutrophils. Biochem Biophys Res Commun. 2000;268:716–23. [DOI] [PubMed] [Google Scholar]
- Youngchim S, Morris-Jones R, Hay RJet al. Production of melanin by Aspergillus fumigatus. J Med Microbiol. 2004;53:175–81. [DOI] [PubMed] [Google Scholar]
- Yu X-Q, Gan H, Kanost MR. Immulectin, an inducible C-type lectin from an insect, Manduca sexta, stimulates activation of plasma prophenol oxidase. Insect Biochem Mol Biol. 1999;29:585–97. [DOI] [PubMed] [Google Scholar]
- Yu X-Q, Jiang H, Wang Yet al. Nonproteolytic serine proteinase homologs are involved in prophenoloxidase activation in the tobacco hornworm, Manduca sexta. Insect Biochem Mol Biol. 2003;33:197–208. [DOI] [PubMed] [Google Scholar]
- Yu X-Q, Tracy ME, Ling Eet al. A novel C-type immulectin-3 from Manduca sexta is translocated from hemolymph into the cytoplasm of hemocytes. Insect Biochem Mol Biol. 2005;35:285–95. [DOI] [PubMed] [Google Scholar]
- Zaragoza O, García-Rodas R, Nosanchuk JDet al. Fungal cell gigantism during mammalian infection. PLoS Pathog. 2010;6:e1000945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaragoza O, Taborda CP, Casadevall A.. The efficacy of complement-mediated phagocytosis of Cryptococcus neoformans is dependent on the location of C3 in the polysaccharide capsule and involves both direct and indirect C3-mediated interactions. Eur J Immunol. 2003;33:1957–67. [DOI] [PubMed] [Google Scholar]
- Zaragoza O, et al. Capsule enlargement in Cryptococcus neoformans confers resistance to oxidative stress suggesting a mechanism for intracellular survival. Cell Microbiol. 2008;10:2043–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Xia YX, Kim Bet al. Two hydrophobins are involved in fungal spore coat rodlet layer assembly and each play distinct roles in surface interactions, development and pathogenesis in the entomopathogenic fungus, Beauveria bassiana. Mol Microbiol. 2011;80:811–26. [DOI] [PubMed] [Google Scholar]
- Zhang X, et al. Phylogenetic analysis and expression profiling of the pattern recognition receptors: insights into molecular recognition of invading pathogens in Manduca sexta. Insect Biochem Mol Biol. 2015;62:38–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P, Lu Z, Strand MRet al. Antiviral, anti-parasitic, and cytotoxic effects of 5,6-dihydroxyindole (DHI), a reactive compound generated by phenoloxidase during insect immune response. Insect Biochem Mol Biol. 2011;41:645–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Z, Wang Y, Jiang H.. Manduca sexta prophenoloxidase activating proteinase-1 (PAP-1) gene: organization, expression, and regulation by immune and hormonal signals. Insect Biochem Mol Biol. 2005;35:627–36. [DOI] [PMC free article] [PubMed] [Google Scholar]