Abstract
Our previous study showed that flowers of Agastache rugosa had higher phenolic levels and higher antibacterial and antioxidant capacity compared to those of the leaves and stems. The aim of this study was to provide information on the variation in primary and secondary metabolites during flower development in A. rugosa by using high performance liquid chromatography (HPLC) and assays of total anthocyanin (TAC), flavonoid (TFC), and phenolic content (TPC), as well as gas chromatography time-of-flight mass spectrometry (GC-TOFMS) analysis. Assays of TPC, TAC, and TFC showed that the floral bud (stage I) contained higher TPC than did the partially open flower (stage II) and fully open flower (stage III). However, the TFC was the highest at stage II, and the highest TAC was observed at stage III. Furthermore, HPLC analysis revealed that the level of total phenylpropanoids, including rosmarinic acid, tilianin, acacetin, 4-hydroxybenzoic acid, caffeic acid, chlorogenic acid, trans-cinnamic acid, rutin, (-)-epicatechin, quercetin, and kaempferol, was higher in stages I and II, but the concentrations of rutin and rosmarinic acid were highest in stage III. A total of 43 compounds, including amino acids, organic acids, phenolic compounds, sugars, photorespiration-related compounds, and intermediates of the tricarboxylic acid cycle, were identified through GC-TOFMS analysis. Of these compounds, most amino acids decreased during flower development. In contrast, the increase in concentrations of glucose and sucrose were observed from stages I to III. In this study, health-beneficial compounds were identified and quantified in flowers of A. rugosa. Accordingly, our results suggests that A. rugosa flowers can potentially be used as biomaterials for pharmaceuticals, cosmetics, food, and related industries.
Supplementary information
The online version contains supplementary material available at (10.1007/s12298-021-00945-z).
Keywords: Agastache rugosa, Phenylpropanoid, Metabolic profiling, Rosmarinic acid biosynthesis
Introduction
Agastache rugosa is a perennial herb and it has various medicinal properties such as anti-atherogenic, antibacterial, antifungal, anti-inflammation, anti-melanogenesis, antipyretic, antitumor, antiviral, and carminative activities (An et al. 2018; Park et al. 2020b). It is widely distributed in the country such as China, Japan, and Korea. In Korea it has been used as a traditional medicine for the curing of anorexia, anxiety, bacterial infections, cholera, diarrhea, miasma, nausea, and vomiting (Tuan et al. 2012; Lee et al. 2016; An et al. 2018; Park et al. 2020b). Both leaves and flowers of A. rugosa are edible. The products of this plants were commercially used in meat marinades, salad dressings, salad, and tea. The wide range of health benefits of these products might be due to the presence of various secondary metabolites in A. rugosa. There are several phytochemical studies proved that the A. rugosa consist of essential oils, flavonoids, lignans, non-volatile terpenoids phenolic acids, and sterols (Janicsak et al. 1999; Lee et al. 2002; Estrada-Reyes et al. 2004; Lim et al. 2013; Zielińska and Matkowski 2014). Among these, compounds such as acacetin, tilianin, and some flavonoids have various medicinal properties (An et al. 2018; Park et al. 2020b).
Rosmarinic acid, tilianin, and acacetin are typical compounds present in A. rugosa. Biosynthesis pathways of these compounds consist of the phenylalanine-derived branch (phenylpropanoid) pathway and the tyrosine-derived branch pathway. First, L-phenylalanine was catalyzed to trans-cinnamate by phenylalanine ammonia-lyase (ArPAL). The trans-Cinnamate was catalyzed to 4-coumarate by 4-hydroxylase (ArC4H) and 4-coumarate was converted to 4-coumaroyl-CoA by 4-coumarate:CoA ligase (Ar4CL). In contrast, L-tyrosine was transformed to 4-hydroxyphenylpyruvate using tyrosine aminotransferase (ArTAT) and 4-hydroxyphenylpyruvate was catalyzed to 4-hydroxyphenyllacetate by hydroxyphenylpyruvate reductase (ArHPPR). Then, 4-coumaroyl-CoA and 4-hydroxyphenyllactate were simultaneously catalyzed to rosmarinic acid by hydroxycinnamoyl-CoA:hydroxyphenyllacetate hydroxycinnamoyl transferase (ArRAS), 3-hydroxycinnamoyl (Ar3-H), and 3′-hydroxycinnamoyl (Ar3′-H). Meanwhile, 4-coumaroyl-CoA was transformed to naringenin chalcone by chalcone synthase (ArCHS), and naringenin chalcone was catalyzed to acacetin by chalcone isomerase (ArCHI), flavone synthase (ArFS), and apigenin 4′-O-methyltransferase (ArA4OMT). Finally, acacetin was transformed into tilianin by glucosyltransferase (ArGT) (Tuan et al. 2012). Previous studies reported that phenolic biosynthesis is stimulated due to the activation of key enzymes which leads to the up-regulation of key genes (PAL and CHS) in the phenylpropanoid branch. In order to understand the gene expression profile in different flowering stages of Korean A. rugosa, we had analyzed the phenylpropanoid pathway gene expression and phenylpropanoid content (Sharma et al. 2016, 2019).
Plant secondary metabolites are molecules that are essential for plant growth and development, reproduction, and protection. Especially, phenolic compounds, which are widely present in plant species and have various biological properties linked with plant defense against biotic and abiotic stresses (Douglas 1996; Higdon et al. 2007; Park et al. 2017). In addition, phenolics are beneficial for human health because of their biological effects, such as radical scavenging, antioxidant (Bily et al. 2004), antiviral, antispasmodic, anti-inflammatory, and antibacterial activities (Diaz et al. 2004).
Metabolic profiling could be utilized to determine diverse cell- or organism-specific responses to biological or environmental conditions through detection and quantitation of low-molecular-weight intermediates produced from many catabolic reactions and the biosynthetic pathways in plants. Among these techniques, gas chromatography time-of-flight mass spectrometry (GC–TOFMS) is regarded as an effective tool for qualitative and quantitative detection of various metabolites. In particular, GC–TOFMS could be used for the detection of low-molecular-weight metabolites because of enhanced resolution, scan times, and mass accuracy (Clarke and Haselden 2008; Park et al. 2017; Vaidyanathan and Goodacre 2003; Yeo et al. 2018).
To date, there are very few studies regarding the phytochemical compositions and components in A. rugosa. In addition, in A. rugosa flowering stage the characterization of primary and secondary metabolic compounds has not yet been fully characterized. Moreover, a quantitative method has yet to expose the composition changes during the different flowering stages in A. rugosa, and the importance of the period during the different flowering stages of Korean A. rugosa harvest. Thus, this study aimed to examine the changes in the biosynthesis of phenolic compounds during flower development using high-performance liquid chromatography (HPLC) and quantitative real-time polymerase chain reaction (qRT-PCR), and comprehensively describe variation in primary and secondary metabolites during flower development in A. rugosa by using HPLC, TAC, TFC, and TPC assays, and GC-TOFMS.
Materials and methods
Plant materials
The flowers of Korean mint (also Agastache rugosa) were harvested from the greenhouse at the Chungnam National University, Daejeon, Korea. These were separated into three stages. The flowers of stages I (floral bud), II (partially open flower), and III (fully open flower) were harvested at 6, 8, and 10 weeks of age, respectively (Fig. 1).
Fig. 1.

Three stages of Agastache rugosa flower development. (a) Floral bud (stage I); (b) partially open flower (stage II); (c) fully open flower (stage III) were harvested on the 5th, 15th, and 25th day after blooming, respectively (color figure online)
Total RNA extraction and cDNA synthesis
Total RNA of flowers at the three different developmental stages was extracted using a Plant RNA Mini Kit (Geneaid, Sijhih, Taiwan), referring to the manufacturer’s instruction. The isolated total RNA (1 μg) was subjected to reverse transcription using the Superscript II First Strand Synthesis Kit (Invitrogen; Carlsbad, CA, USA) and oligo (dT)20 primer according to the manufacturer’s protocols.
Quantitative real-time PCR for gene expression analysis
The sets of gene-specific primers for phenylpropanoid and rosmarinic acid biosynthesis were used for the assay of quantitative real-time PCR (qRT-PCR) (Table S1). The cDNA product, which was diluted 20-fold, was subjected to PCR amplification in triplicate on a CFX96TM Real-Time System combined with a C1000TM Thermal Cycler (Bio-Rad, USA) using the BioFACT™ 2X Real-time PCR Kit with SFCgreen® I (BIOFACT, Daejeon, Korea). The qRT-PCR reaction volume, cyclic conditions, and quantifying gene expression were done according to the protocol described in a previous study by Park et al. (2021b).
Phenylpropanoid extraction and HPLC analysis
Sample extraction and HPLC analysis of phenylpropanoids were performed according to Park et al. (2020a). The freeze-dried sample powders (100 mg) of A. rugosa flowers at the different flower developmental stages were added to a sterile 15 mL tube, followed by the addition of 1.5 mL of aqueous methanol (80%, v/v). After sonication at 60 °C for 1 h, the crude extract was centrifuged at 15,000 rpm for 20 min. Then, the supernatant was transferred into a fresh 50 mL tube, and the entire procedure was repeated twice. The collected aliquots were desiccated using nitrogen gas and re-dissolved in 2 mL of aqueous methanol (80%, v/v). The resulting extract was filtered into a 1.5 mL vial via 0.45 μm PTFE syringe filters. The HPLC analysis system, gradient program, and analysis conditions were similar to those described by Park et al. (2020a). Peak identification was achieved by performing spike tests and comparing them with the retention time of individual chemical standards, and the quantification was performed using the corresponding calibration curves (Fig. S1).
Total phenolic, flavonoid, anthocyanin content
Approximately 100 mg of dried powders of each flowering stage (Stages I, II, and III) were extracted with 2 mL of aqueous methanol (80%, v/v). The extract was vortexed for 30 s, sonicated for 1 h, and centrifuged at 12,000 rpm for 20 min. After centrifugation, the supernatant was transferred into a clear 1.8 mL Eppendorf tube and filtered through a PTFE hydrophilic syringe filter (0.45 µm) into a brown vial. The extracts were used for further experiments such as estimation of total phenolic, flavonoid, and anthocyanin content (TPC, TFC, and TAC). The quantification of TPC, TFC, and TAC were done according to the protocol described in a previous study by Park et al. (2016a), Kim et al. 2003, and Sutharut and Sudarat (2012), respectively.
Metabolic profiles using GC-TOFMS analysis
Hydrophilic metabolites were extracted and analyzed according to a formerly reported method (Park et al. 2021a). A 1 mL sample of methanol/water/chloroform (2.5:1:1, v/v/v) and 60 µL of ribitol (0.2 mg/mL), as an internal standard, were added into a 10 mL tube containing freeze-dried sample powders (10 mg) of A. rugosa flowers, followed by incubation in a compact thermomixer at 1200 rpm and 37 °C for 30 min and centrifugation at 9000 rpm for 10 min. Next, the supernatant (800 μL) was transferred to a fresh 10 mL tube containing 400 μL of deionized water, followed by centrifugation at 11,000 rpm for 15 min. The methanol/water phase was evaporated for 3 h and the remainder was freeze-dried for 18 h. For derivatization, each lyophilized sample was mixed with 80 μL of methoxyamine hydrochloride/pyridine (20 mg/mL, in pyridine) and then shaken at 30 °C for 90 min. After centrifugation, 80 μL of N-methyl-N-(trimethylsilyl) trifluoroacetamide was incubated at 37 °C for 30 min. The GC-TOF–MS analysis system and conditions were taken from a previous study (Park et al. 2021a). ChromaTOF was utilized to identify peaks using an in-house library and comparison with reference compounds. The quantitative calculation was based on the corrected peak area ratios relative to the peak area of the internal standard.
In vitro antioxidant assays
2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging activity of the methanol extracts from the three stages of A. rugosa flowers was evaluated according to our previous method (Park et al. 2019). 200 μL of 0.05-mM DPPH solution was added to each reaction mixture containing 3.8 mL of the extract (31.25–1000 ppm) and then incubated at 37 °C for 30 min in the dark. The absorbance was measured at 517 nm. Furthermore, SOD-like activity extracts from the three stages of A. rugosa flowers was determined according to our previous method (Park et al. 2019). 120 μL of 50 mM Tris–HCl buffer (pH 8.5) and 20 μL of 7.2 mM pyrogallol solution were added to mixture containing 100 μL of the extract (31.25–1000 ppm) and then incubated at 25 °C for 10 min, followed by the addition of 40 μL of 1 M hydroxylamine hydrochloride (NH2OH. HCl) to stop the reaction. The absorbance was measured at 420 nm. As a positive control ascorbic acid was used in these assays.
Statistical analysis
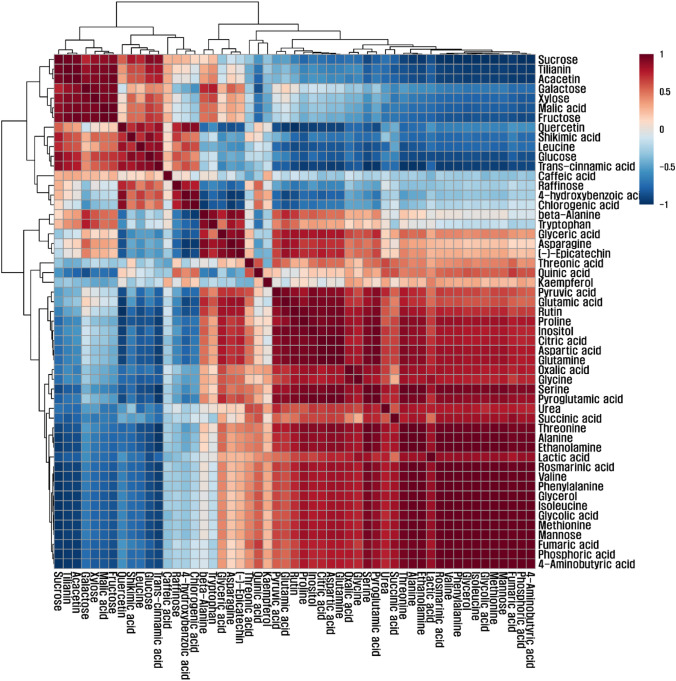
Data from HPLC and qRT-PCR were subjected to comparison using Duncan’s multiple range test (DMRT) at p < 0.05 and statistically evaluated by analysis of variance (ANOVA) using SAS software 9.3 (SAS Institute Inc., Cary, NC, USA). The data from GC-TOFMS were subjected to principal component analysis (PCA) and hierarchical clustering analysis (HCA) with Pearson’s correlation analysis for the detected metabolites was performed based on MetaboAnalyst 3.0. The resulting correlation coefficients were visualized via using Multi Experiment Viewer 4.4.0 software (Multi Experiment Viewer version 4.4.0, Dana-Farber Cancer Institute, Boston, MA, USA, http://www.tm4.org/mev/). Z-value in Fig. 3 was calculated by Yu et al. (2016).
Fig. 3.
Overview of metabolic changes in different developmental stages of A. rugosa flowers. Flower bud (stage I), partially open flower (stage II), and fully open flower (stage III) were harvested on the 5th, 15th, and 25th day after blooming, respectively. Each colored boxes (Left to right) under each compound represent stage 1, stage 2, and stage 3. The scale bar indicates the z-score transformed average values of metabolites, and the colored squares box (blue-to-red) represent relative metabolite abundance in the different flower developmental stages. * Z-score = (X—) / σ; X, each value in the data set; , average of all values in the data set; σ, the standard deviation of a sample (color figure online)
Results
Expression level of phenylpropanoid-related genes in different flowering stages of A. rugosa
Six genes (ArPAL, ArC4H, ArCHS, ArTAT, ArHPPR, and ArRAS) related to phenylpropanoid and rosmarinic acid biosynthesis pathway in A. rugosa were investigated through qRT-PCR analysis. In stage II, the expression levels of ArPAL and ArC4H were 15.78 and 2.39 times higher than that in stage I. The expression levels of ArTAT, ArHPPR, and ArRAS substantially increased in stage III. On the other hand, the expression levels of ArCHS decreased as the flowering stages progressed (Fig. 2).
Fig. 2.
Expression of phenylpropanoid biosynthesis related genes in three stages of Agastache rugosa flower development (stage I, stage II, and stage III) by using RT-PCR. ArPAL: phenylalanine ammonia-lyase; Ar4CL: 4-coumaroyl-CoA by 4-coumarate:CoA ligase; ArCHS: chalcone synthase; ArTAT: tyrosine aminotransferase; ArHPPR: hydroxyphenylpyruvate reductase; ArRAS; hydroxycinnamoyl-CoA:hydroxyphenyllactate hydroxycinnamoyl transferase. Mean values with a different letter (a, b, and c, respectively) were significantly different (p < 0.05, ANOVA, DMRT)
HPLC analysis of different flowering stages of A. rugosa
Eleven phenolic compounds, namely, rosmarinic acid, tilianin, acacetin, 4-hydroxybenzoic acid, caffeic acid, chlorogenic acid, trans-cinnamic acid, rutin, (-)-epicatechin, quercetin, and kaempferol, were identified and quantified through HPLC analysis. The highest levels (mg/g dw) of rosmarinic acid (5.23 ± 0.22) and rutin (0.49 ± 0.00) were observed at stage III, but the levels of 4-hydroxybenzoic acid (0.48 ± 0.024), chlorogenic acid (1.78 ± 0.03), trans-cinnamic acid (0.11 ± 0.01), and quercetin (4.53 ± 0.15) were higher at stage I. Furthermore, the levels (mg/g dw) of caffeic acid (0.12 ± 0.00), (-)-epicatechin (0.72 ± 0.02), tilianin (12.52 ± 0.46), and acacetin (3.02 ± 0.08) were higher in stage II. The levels of kaempferol were not significantly different (Table 1).
Table 1.
HPLC analysis of phenylpropanoids in the different flowering stages of Agastache rugosa
| Compounds | Stage I | Stage II | Stage III |
|---|---|---|---|
| 4-Hydroxybenzoic acid | 0.48 ± 0.02a | 0.26 ± 0.02c | 0.32 ± 0.04b |
| Chlorogenic acid | 1.78 ± 0.03a | 1.16 ± 0.02c | 1.41 ± 0.04b |
| Caffeic acid | 0.09 ± 0.00b | 0.12 ± 0.00a | 0.07 ± 0.00c |
| trans-Cinnamic acid | 0.11 ± 0.01a | 0.07 ± 0.00b | 0.01 ± 0.00c |
| Quercetin | 4.53 ± 0.15a | 1.70 ± 0.05b | 1.51 ± 0.09b |
| (-)-Epicatechin | 0.22 ± 0.02c | 0.72 ± 0.02a | 0.67 ± 0.03b |
| Rutin | 0.00 ± 0.00c | 0.40 ± 0.01b | 0.49 ± 0.00a |
| Kaempferol | 0.14 ± 0.01a | 0.12 ± 0.01a | 0.14 ± 0.01a |
| Tilianin | 11.47 ± 0.68b | 12.52 ± 0.46a | 7.13 ± 0.20c |
| Acacetin | 2.45 ± 0.01b | 3.02 ± 0.08a | 0.79 ± 0.05c |
| Rosmarinic acid | 2.17 ± 0.11c | 2.67 ± 0.08b | 5.24 ± 0.22a |
| Total | 23.45 ± 0.90a | 22.78 ± 0.42a | 17.70 ± 0.36b |
Mean values with a different letter (a, b, and c, respectively) were significantly different (p < 0.05, ANOVA, DMRT)
TPC, TFC, and TAC of different flowering stages of A. rugosa
TPC, TFC, and TAC were measured using a spectrophotometer (Table 2). Flowers of A. rugosa at the different stages of development were extracted using aqueous methanol. The TFC varied in the flowers at the different stages and ranged from 0.64 to 0.68 mg RE/g dw. The highest TFC (mg RE/g dw) was detected in stage II (0.68 ± 0.01), followed by stage I (0.66 ± 0.004) and stage III (0.64 ± 0.003). Furthermore, the highest TPC (mg RE/g dw) was detected in stage I (3.96 ± 0.06), followed by stage II (3.61 ± 0.05) and stage III (3.05 ± 0.15). The highest TAC was detected in stage III (15.96 ± 0.30).
Table 2.
Total phenolic content (TPC), total flavonoid content (TFC), and total anthocyanin content (TAC) in different stages of the flowers of Agastache rugosa
| Methanol Extract | Stage I | Stage II | Stage III |
|---|---|---|---|
| TPC (mg GAE/g) | 3.96 ± 0.06a | 3.61 ± 0.05b | 3.05 ± 0.15c |
| TFC (mg RE/g) | 0.66 ± 0.004b | 0.68 ± 0.01a | 0.64 ± 0.003c |
| TAC (mg CGE/g) | 7.13 ± 0.12c | 10.67 ± 1.0b | 15.96 ± 0.3a |
Mean values with a different letter (a, b, and c, respectively) were significantly different (p < 0.05, ANOVA, DMRT)
Metabolic profiling of different stages of flowers of A. rugosa
We identified 39 metabolites, including amino acids, organic acids, phenolic compounds, sugars, photorespiration-related compounds, and intermediates of the tricarboxylic acid cycle (Table S2). A total of 18 amino acids were found, and a decrease in concentrations of serine, isoleucine, proline, threonine, glutamic acid, phenylalanine, aspartic acid, methionine, pyroglutamic acid, glycine, 4-aminobutyric acid, alanine, and glutamine was observed during flower development. In addition, TCA cycle intermediates, including succinic, citric, and fumaric acids, decreased from stages I to III. In contrast, the concentrations of glucose and sucrose increased during flower development (Fig. 3). A score plot of the PCA showed an overview of the differences among flowers at the three different stages of development, and a loading plot was created to calculate the correlations among the 54 metabolites (Figs. 4, 5). The flowers were apparently differentiated with the two principal components (PCs), which explained 87.02% of the total variance (PC1, 64.19%; PC2, 22.3%), via separation of the metabolites of stages I, II, and III. The metabolite profiles of stages I and III were separated by PC1. In addition, PC2 resolved the separation of stage II from stages I and III (Fig. 4). HCA was conducted using Pearson’s correlation results to determine the relationships between primary and secondary metabolites of flowers of A. rugosa at the different developmental stages. The results showed the degree of correlation among 54 primary and secondary metabolites. Specifically, kaempferol, (-)-epicatechin, rutin, and rosmarinic acid were positively correlated with sucrose and glucose, which were implicated in phenylpropanoid biosynthesis. Moreover, citric and fumaric acids, involved in flavonoid biosynthesis, had strong positive correlations with secondary metabolites (Fig. 5).
Fig. 4.
Score plot (a) and loading plot (b) of principal components 1 and 2 of the principal component analysis (PCA) results acquired from polar metabolite data on the three stages of Agastache rugosa flower samples. Stage I, flower bud; stage II, partially open flower; stage III, fully open flower (color figure online)
Fig. 5.
Cluster analysis and correlation matrix of 53 metabolites from the three stages of Agastache rugosa flowers. Each square indicates the Pearson correlation coefficient of a pair of compounds. In addition, the value of correlation coefficient is represented as the intensity of the red or blue colors (color figure online)
In vitro antioxidant assays of different flowering stages of A. rugosa
SOD-like and DPPH scavenging activities were determined using extracts from the three stages of A. rugosa flowers (Fig. 6). SOD-like scavenging activity was slightly higher in stage III compared to stage I and stage II at most concentrations from 31.25 to 1000 ppm (Fig. 6a). Similarly, stage III showed the higher DPPH scavenging activity than that of stage I and stage II at most concentrations (Fig. 6b).
Fig. 6.
Antioxidant activity of the methanol extracts of the three stages of Agastache rugosa flowers. (a) DPPH radical scavenging assay, absorbance in vertical axis means that each sample was measured at 700 nm wavelength using spectrophotometer (b) Superoxide radical scavenging assay, inhibition of vertical axis was the efficiency of superoxide radical removal of the sample extract
Discussion
The floral bud (stage I) had the highest TPC, but the partially open flower (stage II) contained the highest TFC. The highest TAC was observed at the fully open flower stage (stage III). Furthermore, HPLC analysis revealed that the levels of 4-hydroxybenzoic acid, chlorogenic acid, trans-cinnamic acid, and quercetin were higher in stage I but the levels of caffeic acid, (-)-epicatechin, tilianin, and acacetin were higher in stage II. However, the levels of rutin and rosmarinic acid were higher in stage III. These results were in accordance with those of a previous study that showed that the highest TPC occurred in the bud formation stage, followed by the flower formation and full flowering stage, and the highest TFC was observed at the flower formation stage, followed by the bud formation and full flowering stage of Carthamus tinctorius red flowers. Similarly, Schmitzer et al. (2010) studied the TAC of four stages (floral bud, partially open flower, fully open flower, and senescent flower) of flower development in rose cultivars (Rosa × hybrida), such as KORverlandus and The Fairy (pink flowering cultivars), Gärtnerfreude, MORedfar, and POUIeas (red flowering cultivars), and Sea Foam, NOAschnee, and KOReb (white flowering cultivars). Most cultivars showed increasing patterns of TAC during flower developmental stages. In particular, the highest TAC was observed at the fully open flower stage of POUIeas, NOAschnee, and Gärtnerfreude (Schmitzer et al. 2010). On the other hand, the TAC decreased during flower development of Rosa × hybrida L. ‘KORcrisett’ (Schmitzer et al. 2009). These results showed that stage III A. rugosa flower accumulate highest amount of TAC which might be useful for the nutraceutical and traditional medicine, as colorant and as food.
The qRT-PCR analysis showed higher expression of ArPAL and ArC4H at stage II and higher expression of ArTAT, ArHPPR, and ArRAS at stage III. This was consistent with the results of HPLC analysis and assays of TPC, TFC, and TAC. In particular, the higher gene expression of ArTAT, ArHPPR, and ArRAS supported the higher accumulation of rosmarinic acid in stage III. Park et al. (2016c) reported increased expression levels of ArTAT, ArHPPR, and ArRAS with silver nitrate and yeast extract treatment, which enabled enhanced production of rosmarinic acid in the cell culture of A. rugose (Park et al. 2016c).
The in vitro antioxidant assays revealed that antioxidant activities were higher in the fully open flower stage (stage III) compared to the floral bud (stage I) and the partially open flower (stage II). These properties might be due to the higher TAC level in stage III since anthocyanins are strong antioxidant (Kahkonen and Heinonen 2003). Furthermore, phenolic compounds (4-hydroxybenzoic acid, chlorogenic acid, caffeic acid, trans-cinnamic acid, quercetin, (-)-epicatechin, rutin, kaempferol, tilianin, acacetin, and rosmarinic acid) identified in this study have been reported to possess effective antioxidant properties. Phenolic acids (4-hydroxybenzoic acid, caffeic acid, chlorogenic acid, and trans-cinnamic acid) are considered to be potential antioxidants (Kang et al. 1996; Natella et al. 1999) as well as flavonoids (quercetin, (-)-epicatechin rutin, kaempferol, rosmarinic acid, tilianin, and acacetin) have been reported to possess effective antioxidant activities (Duenas et al. 2010; Morel et al. 1993; Torel et al. 1986; Tuan et al. 2012). From this result, it has been shown that stage III A. rugosa flower accumulates highest values for the secondary metabolic compounds, which has various potential health benefits due to their highest antioxidant properties.
The amino acids as building blocks were used in the synthesis of secondary metabolites containing N precursors of signaling molecules, structural proteins, and enzymes (Borghi and Fernie 2017). In this study, a decreasing pattern of most amino acids was observed according to the flowering developmental stages, reflecting intermediate or precursor supply to support high contents of secondary metabolites during earlier flowering. This was consistent with the results of a previous study showing that the higher TAC in purple kohlrabi (Brassica oleracea var. gongylodes) was associated with lower levels of amino acids (Park et al. 2017). Moreover, the Lingonberry (Vaccinium vitisidaea L.) producing a high amount of anthocyanins showed lower levels of free amino acids (Lee and Finn 2012).
In this study, the TAC and carbohydrates (i.e., glucose and sucrose) increased from stages I to III. Previous studies have shown that glucose and sucrose are involved in phenylpropanoid and flavonoid biosynthesis. Payyavula et al. (2013) showed that exogenous sucrose supply could substantially enhance the expression of phenylpropanoid and flavonoid biosynthesis-related and sugar metabolism-related genes and improved the production of phenolics in plantlets of Solanum tuberosum L. (S. tuberosum L.). In addition, the purple or red flesh of S. tuberosum L. had a higher content of glucose and sucrose compared to those with white or yellow flesh (Payyavula et al. 2013). Similarly, Park et al. (2018) reported that violet flowers of Magnolia Liliiflora Desr. had a higher content of anthocyanins and phenolic compounds, as well as a higher content of carbohydrates, including glucose and sucrose, compared to white flowers of Magnolia Denudata Desr. (Park et al. 2018). The Arabidopsis thaliana mutant pho3 contained a higher endogenous abundance of glucose, sucrose, and other carbohydrates, causing improved production of anthocyanins (Lloyd and Zakhleniuk 2004). Furthermore, increasing sucrose concentrations enhanced phenylpropanoid- and flavonoid-biosynthetic pathway genes and production of phenolic compounds in Vitis vinifera cell cultures (Ferri et al. 2011). Similarly, the exogenous sucrose supply improved the production of polyphenolics (i.e., wogonin, baicalin, and baicalein) in hairy root cultures of Scutellaria baicalensis (Park et al. 2016b). From our results, it has been inferred that the carbohydrates (glucose and sucrose) significantly triggered the anthocyanin biosynthetic pathway which leads to the highest accumulation of TAC in the stage III A. rugosa flower. Thus the stage III flower showed high antioxidant capability when compared to stage I and II flowers.
Conclusions
Primary and secondary metabolites were quantified at different flower developmental stages of A. rugosa using GC-TOFMS and HPLC and the TPC, TFC, and TAC were assessed. The methanolic extracts of flowers had significant antioxidant activities. Particularly, stage III contained the highest total anthocyanin level, explains why they had the highest antioxidant effects than stage I and stage II flowers. This present study describes that there are synergistic antioxidant abilities that are derived from phenolic compounds in different flower developmental stages of A. rugosa.
Supplementary information
Acknowledgements
This study was supported by a grant from Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (20110010231).
Author contributions
S.Y.L. and S.U.P. designed and carried out the experimental part of the manuscript. H.J.Y., C.H.P., Y.E.P., H.H., and J.K.K. performed the experiments and wrote the paper. H.J.Y., C.H.P., and Y.E.P. were involved in analyzing and monitoring the data and experiment. H.J.Y. and C.H.P. wrote the manuscript. All of the authors read and approved the final manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare no competing interests, including financial and non-financial interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hyeon Ji Yeo, Chang Ha Park are contributed equally to this work.
Contributor Information
Sook Young Lee, Email: seedbank@chosun.ac.kr.
Sang Un Park, Email: supark@cnu.ac.kr.
References
- An JH, Yuk HJ, Kim DY, Nho CW, Lee D, Ryu HW, Oh SR. Evaluation of phytochemicals in Agastache rugosa (Fisch. & C.A.Mey.) Kuntze at different growth stages by UPLC-QTof-MS. Ind Crops Prod. 2018;112:608–616. [Google Scholar]
- Bily AC, Burt AJ, Ramputh AL, Livesey J, Regnault-Roger C, Philogene BR, Arnason JT. HPLC-PAD-APCI/MS assay of phenylpropanoids in cereals. Phytochem Anal. 2004;15(1):9–15. doi: 10.1002/pca.735. [DOI] [PubMed] [Google Scholar]
- Borghi M, Fernie AR. Floral metabolism of sugars and amino acids: Implications for pollinators' preferences and seed and fruit set. Plant Physiol. 2017;175(4):1510–1524. doi: 10.1104/pp.17.01164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke CJ, Haselden JN. Metabolic profiling as a tool for understanding mechanisms of toxicity. Toxicol Pathol. 2008;36(1):140–147. doi: 10.1177/0192623307310947. [DOI] [PubMed] [Google Scholar]
- Diaz AM, Abad MJ, Fernandez L, Silvan AM, De Santos J, Bermejo P. Phenylpropanoid glycosides from Scrophularia scorodonia: In vitro anti-inflammatory activity. Life Sci. 2004;74(20):2515–2526. doi: 10.1016/j.lfs.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Douglas CJ. Phenylpropanoid metabolism and lignin biosynthesis: from weeds to trees. Trends Plant Sci. 1996;1(6):171–178. [Google Scholar]
- Duenas M, Gonzalez-Manzano S, Gonzalez-Paramas A, Santos-Buelga C. Antioxidant evaluation of O-methylated metabolites of catechin, epicatechin and quercetin. J Pharmaceut Biomed. 2010;51(2):443–449. doi: 10.1016/j.jpba.2009.04.007. [DOI] [PubMed] [Google Scholar]
- Estrada-Reyes R, Hernández EA, García-Argáez A, Hernández MS, Linares E, Bye R, Heinze G, Martínez-Vázquez M. Comparative chemical composition of Agastache mexicana subsp: mexicana and A. Mexicana subsp xolocotziana Biochem Syst Ecol. 2004;32:685–694. [Google Scholar]
- Ferri M, Righetti L, Tassoni A. Increasing sucrose concentrations promote phenylpropanoid biosynthesis in grapevine cell cultures. J Plant Physiol. 2011;168(3):189–195. doi: 10.1016/j.jplph.2010.06.027. [DOI] [PubMed] [Google Scholar]
- Higdon JV, Delage B, Williams DE, Dashwood RH. Cruciferous vegetables and human cancer risk: epidemiologic evidence and mechanistic basis. Pharmacol Res. 2007;55(3):224–236. doi: 10.1016/j.phrs.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janicsak G, Mathe I, Mikklosy-Vari V, Blunden G. Comparative studies of the rosmarinic and caffeic acid contents of Lamiaceae species. Biochem Syst Ecol. 1999;27:733–738. [Google Scholar]
- Kahkonen MP, Heinonen M. Antioxidant activity of anthocyanins and their aglycons. J Agr Food Chem. 2003;51(3):628–633. doi: 10.1021/jf025551i. [DOI] [PubMed] [Google Scholar]
- Kang Y-H, Park Y-K, Lee G-D. The nitrite scavenging and electron donating ability of phenolic compounds. Korean J Food Sci Technol. 1996;28:232–239. [Google Scholar]
- Kim DO, Jeong SW, Lee CY. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem. 2003;81(3):321–326. [Google Scholar]
- Lee J, Finn CE. Lingonberry (Vaccinium vitis-idaea L.) grown in the Pacific Northwest of North America: Anthocyanin and free amino acid composition. J Funct Foods. 2012;4(1):213–218. [Google Scholar]
- Lee CH, Kim HN, Kho YE. Agastinol and agastenol: novel lignans from Agastache rugosa and their evaluation in an apoptosis inhibition assay. J Nat Prod. 2002;65:414–416. doi: 10.1021/np010425e. [DOI] [PubMed] [Google Scholar]
- Lee TH, Park SJ, Yoo GJ, Jang CY, Kim MH, Kim SH, Kim SY. Demethyleugenol β-glucopyranoside isolated from Agastache rugose decreases melanin synthesis via down-regulation of MITF and SOX9. J Agric Food Chem. 2016;64:7733–7742. doi: 10.1021/acs.jafc.6b03256. [DOI] [PubMed] [Google Scholar]
- Lim SS, Jang JM, Park WT, Park SU. Chemical composition of essential oils from flower and leaf of Korean mint, Agastache rugosa. Asian J Chem. 2013;25:4361–4363. [Google Scholar]
- Lloyd JC, Zakhleniuk OV. Responses of primary and secondary metabolism to sugar accumulation revealed by microarray expression analysis of the Arabidopsis mutant, pho3. J Exp Bot. 2004;55(400):1221–1230. doi: 10.1093/jxb/erh143. [DOI] [PubMed] [Google Scholar]
- Morel I, Lescoat G, Cogrel P, Sergent O, Pasdeloup N, Brissot P, Cillard P, Cillard J. Antioxidant and iron-chelating activities of the flavonoids catechin, quercetin and diosmetin on iron-loaded rat hepatocyte cultures. Biochem Pharmacol. 1993;45(1):13–19. doi: 10.1016/0006-2952(93)90371-3. [DOI] [PubMed] [Google Scholar]
- Natella F, Nardini M, Di Felice M, Scaccini C. Benzoic and cinnamic acid derivatives as antioxidants: Structure-activity relation. J Agr Food Chem. 1999;47(4):1453–1459. doi: 10.1021/jf980737w. [DOI] [PubMed] [Google Scholar]
- Park CH, Baskar TB, Park SY, Kim SJ, Arasu MV, Al-Dhabi NA, Kim JK, Park SU. Metabolic profiling and antioxidant assay of metabolites from three radish cultivars (Raphanus sativus) Molecules. 2016;21(2):157. doi: 10.3390/molecules21020157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CH, Kim YS, Li X, Kim HH, Arasu MV, Al-Dhabi NA, Lee SY, Park SU. Influence of different carbohydrates on flavonoid accumulation in hairy root cultures of Scutellaria baicalensis. Nat Prod Commun. 2016;11(6):799–802. [PubMed] [Google Scholar]
- Park WT, Arasu MV, Al-Dhabi NA, Yeo SK, Jeon J, Park JS, Lee SY, Park SU. Yeast extract and silver nitrate induce the expression of phenylpropanoid biosynthetic genes and induce the accumulation of rosmarinic acid in Agastache rugosa cell culture. Molecules. 2016;21(4):426. doi: 10.3390/molecules21040426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CH, Yeo HJ, Kim NS, Eun PY, Kim SJ, Arasu MV, Al-Dhabi NA, Park SY, Kim JK, Park SU. Metabolic profiling of pale green and purple kohlrabi (Brassica oleracea var. gongylodes) Appl Biol Chem. 2017;60(3):249–257. [Google Scholar]
- Park CH, Park SY, Lee SY, Kim JK, Park SU. Analysis of metabolites in white flowers of Magnolia denudata Desr. and violet flowers of Magnolia liliiflora Desr. Molecules. 2018;23(7):1558. doi: 10.3390/molecules23071558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CH, Yeo HJ, Baskar TB, Park YE, Park JS, Lee SY, Park SU. In vitro antioxidant and antimicrobial properties of flower, leaf, and stem extracts of Korean mint. Antioxidants-Basel. 2019;8(3):75. doi: 10.3390/antiox8030075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CH, Park SY, Park YJ, Kim JK, Park SU. Metabolite Profiling and Comparative Analysis of Secondary Metabolites in Chinese Cabbage, Radish, and Hybrid xBrassicoraphanus. J Agric Food Chem. 2020;68(47):13711–13719. doi: 10.1021/acs.jafc.0c04897. [DOI] [PubMed] [Google Scholar]
- Park WT, Yeo SK, Sathasivam R, Park JS, Kim JK, Park SU. Influence of light-emitting diodes on phenylpropanoid biosynthetic gene expression and phenylpropanoid accumulation in Agastache rugosa. Appl Biol Chem. 2020;63:25. [Google Scholar]
- Park CH, Park YE, Yeo HJ, Yoon JS, Park SY, Kim JK, Park SU. Comparative Analysis of Secondary Metabolites and Metabolic Profiling between Diploid and Tetraploid Morus alba L. J Agric Food Chem. 2021;69(4):1300–1307. doi: 10.1021/acs.jafc.0c06863. [DOI] [PubMed] [Google Scholar]
- Park CH, Xu H, Yeo HJ, Park YE, Hwang GS, Park NI, Park SU. Enhancement of the flavone contents of Scutellaria baicalensis hairy roots via metabolic engineering using maize Lc and Arabidopsis PAP1 transcription factors. Metab Eng. 2021;64:64–73. doi: 10.1016/j.ymben.2021.01.003. [DOI] [PubMed] [Google Scholar]
- Payyavula RS, Singh RK, Navarre DA. Transcription factors, sucrose, and sucrose metabolic genes interact to regulate potato phenylpropanoid metabolism. J Exp Bot. 2013;64(16):5115–5131. doi: 10.1093/jxb/ert303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitzer V, Veberic R, Osterc G, Stampar F. Changes in the phenolic concentration during flower development of rose 'KORcrisett'. J Am Soc Hortic Sci. 2009;134(5):491–496. [Google Scholar]
- Schmitzer V, Veberic R, Osterc G, Stampar F. Color and phenolic content changes during flower development in groundcover rose. J Am Soc Hortic Sci. 2010;135(3):195–202. [Google Scholar]
- Sharma A, Thakur S, Kumar V, Kanwar MK, Kesavan AK, Thukral AK, Bhardwaj R, Alam P. Ahmad P (2016) Pre-sowing seed treatment with 24-Epibrassinolide ameliorates pesticide stress in Brassica juncea L. through the modulation of stress markers. Front. Plant Sci. 2016;7:1569. doi: 10.3389/fpls.2016.01569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Yuan H, Kumar V, Ramakrishnan M, Kohli SK, Kaur R, Thukral AK, Bhardwaj R, Zheng B. Castasterone attenuates insecticide induced phytotoxicity in mustard. Ecotoxicol Environ Saf. 2019;179:50–61. doi: 10.1016/j.ecoenv.2019.03.120. [DOI] [PubMed] [Google Scholar]
- Sutharut J, Sudarat J. Total anthocyanin content and antioxidant activity of germinated colored rice. Int Food Res J. 2012;19:215–221. [Google Scholar]
- Torel J, Cillard J, Cillard P. Antioxidant activity of flavonoids and reactivity with peroxy radical. Phytochemistry. 1986;25:383–385. [Google Scholar]
- Tuan PA, Park WT, Xu H, Park NI, Park SU. Accumulation of tilianin and rosmarinic acid and expression of phenylpropanoid biosynthetic genes in Agastache rugosa. J Agr Food Chem. 2012;60(23):5945–5951. doi: 10.1021/jf300833m. [DOI] [PubMed] [Google Scholar]
- Vaidyanathan S, Goodacre R. Metabolome and proteome profiling for microbial characterization. In: Harrigan GG, Goodacre R, editors. Metabolic profiling: its role in biomarker discovery and gene function analysis. Boston, US, MA: Springer; 2003. pp. 27–28. [Google Scholar]
- Yeo HJ, Park CH, Lee KB, Kim JK, Park JS, Lee JW, Park SU. Metabolic analysis of Vigna unguiculata sprouts exposed to different light-emitting diodes. Nat Prod Commun. 2018;13(10):1349–1354. [Google Scholar]
- Yu N, Wei S, Li M, Yang J, Li K, Jin L, Xie Y, Giesy JP, Zhang X, Yu H. Effects of perfluorooctanoic acid on metabolic profiles in brain and liver of mouse revealed by a high-throughput targeted metabolomics approach. Sci Rep. 2016;6:1–10. doi: 10.1038/srep23963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielińska S, Matkowski A. Phytochemistry and bioactivity of aromatic and medicinal plants from the genus Agastache (Lamiaceae) Phytochem Rev. 2014;13:391–416. doi: 10.1007/s11101-014-9349-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.