Abstract
In ethnomedicine, plant parts and compounds are used traditionally to treat different diseases. Neem (Azadirachta indica A. Juss) is the most versatile and useful medicinal plant ever found. Its every part is rich in bioactive compounds, which have traditionally been used to treat different ailments including infectious diseases. Bioactive compounds such as nimbolide, azarirachtin, and gedunin of neem are reported to have a tremendous ability to regulate numerous biological processes in vitro and in vivo. The present review article aims to explore the importance of neem extracts and bioactive compounds in the regulation of different biological pathways. We have reviewed research articles up to March 2020 on the role of neem in antioxidant, anti-inflammatory, antiangiogenic, immunomodulatory, and apoptotic activities. Studies on the concerned fields demonstrate that the bioactive compounds and extracts of neem have a regulatory effect on several biological mechanisms. It has been unveiled that extensive research is carried out on limonoids such as nimbolide and azarirachtin. It is evidenced by different studies that neem extracts are the potential to scavenge free radicals and reduce ROS-mediated damage to cells. Neem can be used to normalize lipid peroxidation and minimize ROS-mediated cell death. Besides, neem extracts can significantly reduce the release of proinflammatory cytokines and elevate the count of CD4 + and CD8 + T-cells. This review indicates the pivotal roles of A. indica in the regulation of different biological pathways. However, future investigations on other bioactive compounds of neem may reveal different therapeutic potentials.
Keywords: Neem, Azadirachta indica, Biological pathways, Antioxidant, Anti-inflammatory, Antiapoptotic activity
Introduction
As per the World Health Organization, the majority of the people (80%) from developing countries depend on ethnomedicines for primary health care. Contrarily, half of the World’s population still relies on ethnomedicines obtained from plants’ active ingredients (Oyebode et al. 2016). Azadirachta indica or neem is one of the most important medicinal plants ever found in the history of humankind. The use of A. indica is from prehistory to contemporary. Siddha medicine (10,000 B.C. to 4000 B.C.), practiced in south India, is believed to be the oldest medicinal system. As per the Tamil literature, neem or margosa was the first medicinal plant found a place in the Siddha system (Kumar and Navaratnam 2013). Neem has been used from time immemorial for ailments such as smallpox and infectious diseases. A. indica is mainly found in India and neighboring countries. It has been used as a medicinal plant in the Indian subcontinent for more than 4500 years (Kumar and Navaratnam 2013). Plausibly, its uses were started during the Indian great Harappan culture of Indus Civilization. In 1922, during the time of excavations, scientists found several medicinal products including neem leaves from ancient deeds and ruins of Harappa and Mohenjo Daro. Scientists found evidence on the use of A. indica on a skull having cranial surgery. These discoveries suggest the use of A. indica in both surgical and phytochemical processes in the world’s most ancient and developed civilizations (Kumar and Navaratnam 2013). At the beginning of the twentieth century, A. indica was distributed to the other places of the world by Indian immigrants. Now, neem tree can be found almost in 72 countries in Asia, Africa, and central and South America (Jhariya et al. 2013).
The divine tree A. indica has tremendous medicinal importance in modern medicine, Unani, Ayurveda, and Homoeopathy. Neem tree in Sanskrit is called as “Arishtha”, which means “reliever of sickness”. In 1942, the United States National Academy of Sciences published a report entitled “Neem—a tree for solving global problems” (Biswas et al. 2002). A. indica has several bioactive compounds obtained by modern high-throughput techniques such as HPLC–MS, LC–MS, GC–MS, NMR, and infrared ray spectroscopy (Atawodi and Atawodi 2009). Chemical investigations and characterization of neem compounds were extensively carried out in the middle of the twentieth century (Biswas et al. 2002). Neem compounds can be classified into two major sections isoprenoids and non-isoprenoids (Tiwari et al. 2014). Isoprenoids are classified into diterpenoids and triterpenoids (Seriana et al. 2019). Diterpenoids are further sub-classified into protomeliacin, azadirone, gedunin, amoorstatin with vepinin, vilasinin, and C-Seco meliacins. Azaridone, azadiradione, and epoxyazadiradione fall into azadirone sub-class (Tan and Luo 2011; Haldar et al. 2013; Ponnusamy et al. 2015). In 1942, Siddiqui isolated the first bitter compound nimbin under the sub-class C-Seco meliacins from neem seed (Siddiqui 1942). Nimbin itself is an inactive compound, but it can be changed into salannin due to enzymatic reaction. Further enzymatic modification and oxidation lead to the formation of azadiractin. Azadirachtin is the most active compound of neem, which is toxic for insects. The bitterness of neem is mainly for the accumulation of limonoids. The occurrence of limonoids in meliaceae is called as meliacins. On the other hand, non-isoprenoids contain amino acids, polysaccharides, and polyphenolic compounds, coumarin, sulphurous compound, dihydrochalcone, aliphatic compounds, and tannins (Saxena and Kumar 2009).
Active compounds of A. indica play a major role in disease management by modulating several biochemical, genetic pathways, and other biological processes (Alzohairy 2016). The first polyphenolic flavonoids obtained from fresh leaves are quercetin and ß-sitosterol, which have a tremendous effect as anti-fungal and antibacterial activities (Mahmoud et al. 2011; Jaisinghani 2017). Besides, several biological and pharmacological activities of neem compounds have been reported such as antioxidant, anti-inflammatory, antiarthritic, antipyretic, antiviral, spermicidal, hypoglycemic, anthelminthic, antigastric ulcer, and antitumour activities (Gupta et al. 2019). However, several reviews have been published so far to summarize the biological role and therapeutic significance of A. indica. In this review, an attempt has been made to critically evaluate the emerging role of A. indica in signaling pathways inter-linked with different biological processes. Amongst the biological processes antioxidant, anti-inflammatory, antiangiogenic, immunomodulatory, and apoptotic activities of A. indica have been extensively studied. The present review focuses on these biological activities attributed to different parts of A. indica extracts and bioactive compounds.
Botanical description and taxonomic position of A. indica:
Azadirachta indica A. Juss (neem) under the family meliaceae is an evergreen tree found in the Indian subcontinent. It can grow and survive under a wide range of agro-climatic conditions (Lokanadhan et al. 2012). Its favorable growth requires soil pH ranging from 6.2 to 7.0 (Bhowmik et al. 2010). It can be found an altitude about 1500 m and rainfall ranging from 450 to 1150 mm. Neem trees thrive in extended dried weather conditions even at the poor quality of soil (Saxena and Kumar 2009). It is a fast-growing tree with having an average height of 20–30 m with a diameter of 4–5 feet. Neem leaves are imparipinnate having 5–15 leaflets. Its green drupes fruits become yellow after ripening (Alzohairy 2016). The taxonomic position of A. indica is shown in Table 1.
Table 1.
Taxonomic description of A. indica
| Kingdom | Plantae |
|---|---|
| Division | Magnoliophyta |
| Order | Rutales |
| Suborder | Rutinae |
| Family | Meliaceae |
| Subfamily | Melioideae |
| Tribe | Melieae |
| Genus | Azadirachta |
| Species | Indica |
Biological activities of A. indica and its compounds
Antioxidant activity
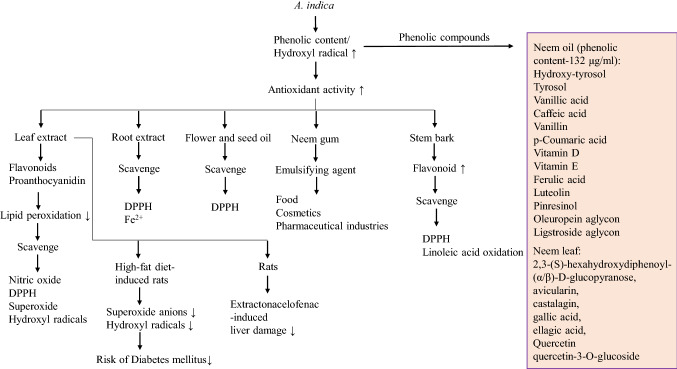
Multiple studies have shown that neem extracts are potential antioxidants in nature (Airaodion et al. 2019; Septiyani and Wibowo 2019; Sharma et al. 2019; Diatta et al. 2019; Asghar et al. 2017). Neem leaf compounds such as azadirachtin and nimbin have tremendous antioxidant activity (Ghimeray et al. 2009). It is reported that aqueous extract of neem leaves is beneficial to extractonacelofenac-induced liver damage in rats (Soumendra et al. 2009). Antioxidant properties of neem extracts are attributed to the presence of polyphenols (Pokhrel et al. 2015; Al-Hashemi and Hossain 2016). Hence, total polyphenolic content is important to retain the antioxidant activity of different neem plant parts (Fig. 1). The phenolic contents of neem can be varied based on geographical location and other abiotic factors (Ghimeray et al. 2009). A study based on HPLC analysis has revealed that neem oil contains phenolic compounds such as hydroxy-tyrosol, tyrosol, vanillic acid, caffeic acid, vanillin, p-Coumaric acid, vitamin D, vitamin E, ferulic acid, luteolin, pinresinol, oleuropein aglycon, and ligstroside aglycon (Gosse et al. 2005). It is reported that drying of neem leaves favors retaining the activities of polyphenolic compounds (Sejali and Anuar 2011). However, total polyphenol content and radical scavenging activity of leaf extract are proportionally linked with antiradical and antimicrobial properties. Study has shown the similarities between A. indica leaves and other synthetic conservatives on lipid oxidation in raw chilled beef patties (Ouerfelli et al. 2019). Methanolic extract of A. indica leaves are potential to scavenge nitric oxide (6.38 mg/ml), 1,1-diphenyl-2-picryl hydrazyl (DPPH) (6.65 mg/ml), superoxide (9.21 mg/ml), and hydroxyl radicals (4.35 mg/ml) (Mali et al. 2019). The results of scavenging activity are represented as equivalent to their IC50 values. The scavenging activity has also been studied with ethanolic extract of neem roots. In this study, ethanolic extract of neem root showed remarkable ability to scavenge DPPH and free ferrous ion against L-ascorbic acid, butylated hydroxy anisole as a positive control (Hossain et al. 2014). It has been observed that the use of ethanolic extract of neem leaves stabilize palm by reducing peroxidation. Bioactive compounds of neem leaves possess significant antioxidant activity, as the IC50 (%) inhibition values are found to be 12.54 ± 0.0173 and 48.8233 ± 0.0251 for DPPH and β-carotene linoleic, respectively (Elaigwu et al. 2019). A. indica flowers and seed oil have also antioxidant potentiality. However, neem seed oil shows more antioxidant activity than flowers due to the presence of more phenolic content (Nahak and Sahu 2011; Varghese B and Naithani 2002). Neem gum is an exudate of the tree. Due to high antioxidant activity, neem gum can be used as an emulsifying agent in food, cosmetics, and pharmaceutical industries (Malviya et al. 2017). In addition to it, the aqueous extract of A. indica stem bark having high phenolic and flavonoids contents has remarkable antioxidant activity (Anokwuru et al. 2011; Sultana et al. 2007). The oil obtained from A. indica has the tremendous potentiality to enhance the efficacy of commercially available antioxidant drugs. It is reported that encapsulation and bio-emulsion with A. indica oil improve the antioxidant activity of ceftriaxone (Ameta et al. 2017). Ethanolic extract of A. indica leaves contains some major polyphenolic compounds such as avicularin, castalagin, gallic acid, 2,3-(S)-hexahydroxydiphenoyl-(α/β)-D-glucopyranose, ellagic acid, quercetin, and quercetin-3-O-glucoside. These compounds have significant antioxidant and cytotoxic activity (Abdelhady et al. 2015). The high free radical scavenging property of neem leaves is maybe due to the presence of hydroxyl group in the chemical structure of the phenolic compounds (Nahak and Sahu 2010a). It is reported that aqueous, methanolic, and ethanolic extract of neem bark and roots are rich in antioxidants. However, this activity is comparatively more in mahaneem (Melia azedarach) (Nahak and Sahu 2010b). In addition to phenolic content, methanolic extract of A. indica leaves contains flavonoids and proanthocyanidin. These bioactive phytochemicals possess antioxidant as well as antimicrobial activities (Pokhrel et al. 2015; Vergallo and Panzarini 2019; Deka et al. 2013). It is reported that subfraction of neem leaves ethanolic extract has a protective role against pBR322 DNA, superoxides, free radicals, and oxidative damage of the red blood cells by hydrogen peroxide (Manikandan et al. 2009). Essential fatty acids are capable to reduce the chance of breast cancer. A study has shown that combination of curcumin from turmeric and neem having strong antioxidant activity moderately reduces the inhibition of essential unsaturated α-lenoleic acid in breast cancer cells (Cheung et al. 2016). Clinically hydroxyapatite (HA) is extensively used in the field of dentistry and orthopedics due to its mechanical strength, biocompatibility, osteoconductivity, etc. It is in this context that biomimetization of HA with A. indica leaves possesses high antioxidant potentiality, which may be more beneficial for dentistry and orthopedics applications (Nagaraj and Samiappan 2019). Oxidative stress due to free radicals causes damage to protein, lipid membranes, and nucleic acids, which ultimately causes cell death in both types of Diabetes mellitus. A study has shown that leaf extract of A. indica potentially normalizes lipid peroxidation, superoxide anions, and hydroxyl radicals in high-fat diet induced rats (Shrivastava et al. 2012). A similar kind of observation was reported earlier, where the antioxidant activity of aqueous seed extract of A. indica was shown to inhibit lipid peroxidation as well as lipoxygenase activity (Rao et al. 1998). Chewing sticks obtained from neem may be beneficial to periodontal diseases. Pathogen like Streptococcus mutans is considered to initiate dental caries and dental decay in periodontal diseases. It is in this context that dried neem stick having antimicrobial activity is efficacious to inhibit S. mutans (Dani et al. 2016; Chava et al. 2012). Leaf extract of neem has been shown to inhibit P. gingivalis, a potential risk factor of periodontal disease. However, the accumulation of polyphenols obtained from leaf extract can synergistically increase the antioxidant activity of mucosal surface in complex with bacterial, lysozyme, or red blood cells (Heyman et al. 2017).
Fig. 1 .
Antioxidant activities of different A. indica extracts and their bioactive compounds. The box is showing total phenolic compounds obtained from A. indica oil and leaves. The signs “↑” and “↓” indicate more and less respectively
Anti-inflammatory activity
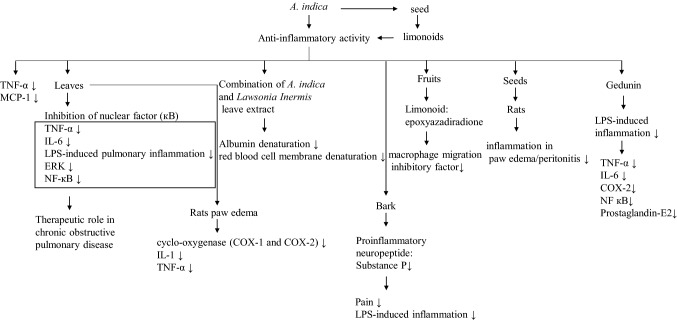
Some bioactive phytochemicals are potential anti-inflammatory in nature (Fig. 2). The anti-inflammatory activity of A. indica has extensively been studied and successfully observed in both acute and chronic inflammation (Dutta et al. 2016; Emran et al. 2015; Jagadeesh et al. 2014). Neem is more effective than commercially available non-steroidal anti-inflammatory drugs such as ibuprofen. Neem can remarkably reduce the release of monocyte chemotactic protein-1 (MCP-1) and tumor necrosis factor (TNF-α) (Kang et al. 2014). Neem root is also anti-inflammatory, but its activity is less than aspirin (Patil et al. 2012). It has been reported that neem seeds extracted in n-hexane contain limonoids, which show anti-inflammatory activity (Akihisa et al. 2011). Methanolic extract of neem leaves has tremendous anti-inflammatory activity (Schumacher et al. 2011). This study has shown inhibition of TNF-α-induced damage and inhibition of nuclear factor (кB). The neem seed oil has anti-inflammatory activity. 2 mL per kilogram body weight 1 study, it has been shown that a combination of ethanolic extract of A. indica and Lawsonia Inermis leaf extract has potent anti-inflammatory activity. This combination having 200 µg/mL of concentration shows a protective function against albumin and red blood cell membrane denaturation (Annavarapu et al. 2016). Aqueous extract of neem leaf also reduces inflammation in paw edema of rats, but the action is less than commercially available dexamethasone (Mosaddek and Rashid 2008). In contrast, methanolic extract of neem leaves shows a reduction in the release of proinflammatory cytokines such as TNF-α, IL-6 in cigarette smoke, and lipopolysaccharide (LPS)-induced pulmonary inflammation. On the other hand, it attenuates the activation of extracellular signal-regulated kinase (ERK) and NF-кB (Lee et al. 2017). These results suggest the therapeutic role of neem leaf extract in chronic obstructive pulmonary disease. Neem fruits contain limonoid epoxyazadiradione. Epoxyazadiradione inhibits macrophage migration inhibitory factor in both human and malaria parasites, and results in the release of different proinflammatory cytokines (Alam et al. 2012). Total polysaccharides and fractioned by ion-exchange chromatography obtained from neem seed show reduction of inflammation in paw edema/peritonitis in rats (de Paulo Pereira et al. 2012). Another study, conducted in the rat paw edema model, has shown the anti-inflammatory effect of neem leaf extracted in methanol, chloroform, petroleum ether, and water. All kinds of extracts show attenuation inflammation by decreasing cyclo-oxygenase (COX-1 and COX-2), IL-1, and TNF-α (Umar et al. 2014). Neem bark extract is capable to reduce pain and inflammation. Besides, study has shown the reduction of substance P, a proinflammatory neuropeptide, in lipopolysaccharide-induced inflammation in neuroblastoma cells using neem bark extract (Reddy et al. 2018). Gedunin modulates innate immune response by inhibiting lipopolysaccharide-induced release of TNF-α and IL-6. It has been shown that gedunin also inhibits the production of prostaglandin E2, expression of cyclo-oxygenase-2, and translocation of nuclear factor kB inside the nucleus macrophages (Borges et al. 2015).
Fig. 2 .
Anti-inflammatory activities of different A. indica extracts and their bioactive compounds in the modulation of different signaling pathways. The signs “↑” and “↓” indicate more and less respectively
Anti-angiogenic activity
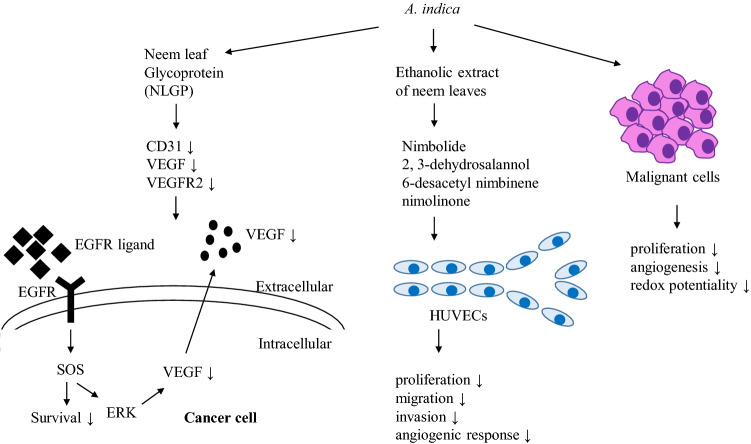
A. indica has potential antiangiogenic properties (Fig. 3). Neem leaf glycoprotein (NLGP) is a good immunomodulator, which is reported to regulate both local and systemic immunity by modulating effector NK cells, NKT, and CD8 + T cells. It has been shown that NLGP downregulates CD31, VEGF, VEGFR2, and normalize vascular tone in melanoma and carcinoma bearing mice model (Banerjee et al. 2014). A study has been conducted on the ethanolic extract of neem leaves (EENL) on human umbilical vein endothelial cells (HUVECs). It is observed that EENL remarkably reduces the HUVEC mediated angiogenesis in both in vivo and in vitro. Compounds such as nimolinone, nimbolide, 6-desacetyl nimbinene, and 2, 3-dehydrosalannol, of EENL significantly attenuate the proliferation, migration, invasion, and angiogenic response of HUVECs (Mahapatra et al. 2012). Bioactive components of neem seeds, leaves, flowers, and fruits have potential anti-cancer effects, which show significant inhibition of malignant cell proliferation, angiogenesis, and redox potentiality. In this context, inhibition of NF-кB pathway may be the partial cause of cancer prevention (Hao et al. 2014). Ethanolic fraction of neem leaves (EFNL) has an anti-cancer effect against mammary carcinogenesis. In vivo study conducted on rats shows down-regulation of vascular endothelial growth factor A (VEGF-A) while treating with EFNL (Arumugam et al. 2014). Silver nanoparticles obtained by green synthesis using neem leaves show the reduction of neovascularization. The application of such nanoparticles shows a remarkable reduction of viable blood vessels at the chorioallantoic membrane in developing embryonated eggs of chicken, which results in the death of the embryo (Khandia et al. 2015).
Fig. 3 .
Anti-angiogenic activities of different A. indica extracts and their bioactive compounds in the regulation of different angiogenic pathways. The signs “↑” and “↓” indicate more and less respectively
Immunomodulatory functions
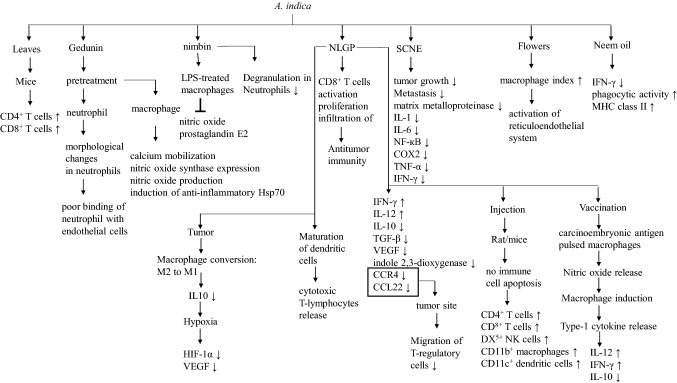
Extracts from different parts of neem are known to have immense immunomodulation functions (Talpur and Ikhwanuddin 2013; Durrani et al. 2008). A study conducted on BALB/c-mice showed increased counts of CD4 + and CD8 + cells upon treatment of aqueous extract of neem leaves. This treatment also showed increased counts of lymphocytes and monocytes (Beuth et al. 2006). In this context, NLGP is involved in the activation, proliferation, and infiltration of CD8 + T cells and responsible for anti-tumor immunity (Mallick et al. 2013a, b). In another study, it has been shown that NLGP promotes monocyte migration and T-cell-mediated killing of tumor cells (Chakraborty et al. 2010). Gedunin can modulate the function of macrophage and neutrophil. Pretreatment of neutrophil with gedunin shows weaker binding of neutrophil with endothelial cells due to morphological changes to neutrophils. Besides, pretreatment of macrophages to gedunin shows alteration in calcium mobilization, nitric oxide synthase expression, nitric oxide production, and induction of anti-inflammatory heat-shock protein (Hsp70) (Fig. 4) (Conte et al. 2015). Similar to gedunin, nimbin also modulate the function of macrophage and neutrophil. It inhibits nitric oxide release and prostaglandin E2 in LPS-treated macrophages. Nimbin minimizes degranulation in neutrophils (Kaur et al. 2004). Highly pure supercritical carbon dioxide neem leaf extract (SCNE) has been shown to downregulate proinflammatory pathways in the case of oral squamous cell carcinoma. Treatment with SCNE significantly reduces tumor growth, metastasis, and activity of matrix metalloproteinase. Additionally, SCNE suppresses pro-cancer inflammatory cytokines such as IL-1, IL-6, NF-кB, COX-2, TNF-α, and IFN-γ (Morris et al. 2019). Treatment with neem oil also reduces the activity of IFN-γ. Intraperitoneal injection of neem oil in mice shows increased phagocytic activity and expression of MHC class II molecules (Upadhyay et al. 1992). A single-blind randomized study has been conducted on elderly immunodeficient patients having age 65.87 ± 5.87 years. In this study, patients are treated with neem bark powder. The result of the study shows increased counts of both CD4 + and CD8 + cells after treatment (Akmal et al. 20,165). Aqueous extract of A. indica flowers possesses both humoral and cell-mediated responses. This extract shows the elevation of the macrophage index, which signifies the activation of reticuloendothelial system (Shah et al. 2009). Downregulation of CD4 + CD25 + FOXp3 + T-regulatory cells is a better immunotherapeutic approach to control cancer. It is reported that NLGP is a valuable player to control tumors in murine model by downregulating CD4 + CD25 + FOXp3 + T-regulatory cells. In tumor microenvironment, NLGP produces anti-tumor niche by upregulating IFN-γ, IL-12, and downregulating IL-10, TGF-β, VEGF, indole 2,3-dioxygenase. NLGP downregulates C–C chemokine receptor 4 (CCR4) and its ligand C–C chemokine ligand 4 (CCL22), which further restricts the migration of regulatory-T cells into the tumor site (Chakraborty et al. 2011). Injection of NLGP into rats and mice shows no immune cell apoptosis; instead, the treatment significantly increases the number of DX5 + NK cells, CD11b + macrophages, CD11c + dendritic cells, CD4 + , and CD8 + T cells (Mallick et al. 2013a, b). As mentioned earlier, NLGP is associated with enhanced CD8 + count and activity. A study conducted in murine B16 melanoma model demonstrates that CD8 + normalizes tumor microenvironment to inhibit melanoma growth (Barik et al. 2015). It has been reported that vaccination of carcinoembryonic antigen-pulsed macrophages with NLGP (CEAMϕNLGP) enhances nitric oxide release, which is involved in macrophage induction and type-1 cytokine release. Activation of macrophage, in turn, is associated with the upregulation of the synthesis of IL-12, IFN-γ, and down-regulation of IL-10 (Sarkar et al. 2009). NLGP is also associated with the maturation of dendritic cells. This activation results in the stimulation of T cells, which in turn generates cytotoxic T-lymphocytes (Roy et al. 2011). NLGP can efficiently convert M2 (CD11b + F4/80high) macrophage to M1 (CD11b + F4/80low) macrophage in tumor microenvironment. This conversion is associated with a low level of IL-10, which generates hypoxia in tumor core and downregulates hypoxia-inducible factor (HIF)-1α and VEGF (Goswami et al. 2016).
Fig. 4 .
Immunomodulatory functions of different A. indica extracts and their bioactive compounds in the regulation of different immune cells and immunomodulatory components. The signs “↑” and “↓” indicate more and less respectively. The sign “┴” represents inhibition of the particular pathway
Effect of A. indica on apoptosis
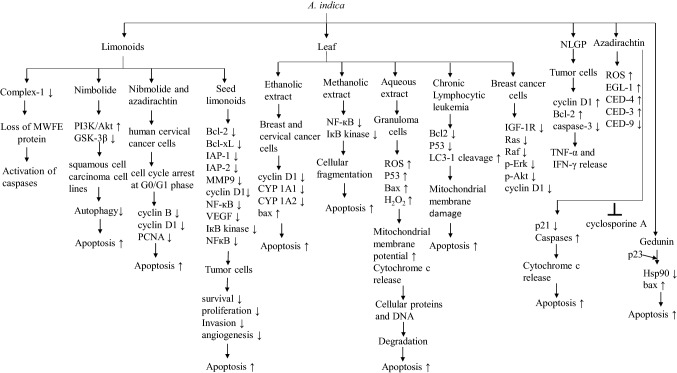
Neem significantly introduces increased reactive oxygen species (ROS) production and mitochondrial fragmentation (Yadav et al. 2016). It results in the decrease of oxidative phosphorylation Complex-1 (mitochondrial NADH-ubiquinone oxidoreductase) and the loss of MWFE protein, which in turn activates caspases (Yadav et al. 2016). Limonoids are known to have apoptotic features (Fig. 5) (Kashif et al. 2019). Nimbolide, an important limonoid obtained from A. indica is known to have tremendous anti-proliferative and apoptotic effects. The study conducted by Sophia et al. on hamster oral oncogenesis model has shown the regulation of proteins in autophagy and apoptosis by nimbolide. Nimbolide downregulates PI3K/Akt pathway and upregulates GSK-3β, which in turn inhibits autophagy and induces apoptosis in cancer (Sophia et al. 2018). Nibmolide and azadirachtin promote apoptosis in human cervical cancer cells. These limonoids are responsible for cell cycle arrest at G0/G1 phase, which in turn downregulates cell cycle-associated proteins proliferating cell nuclear antigen (PCNA), cyclin D1, and cyclin B (Priyadarsini et al. 2010). It is in this context that limonoids obtained from neem seeds also exert apoptosis (Kikuchi et al. 2011). The study demonstrates that limonoid restricts the expression of proteins associated with cancer cell survival, proliferation, invasion, and angiogenesis. Proteins such as VEGF, cyclin D1, IAP-1, IAP-2, MMP9, NF-кB, Bcl-xL, and Bcl-2 are significantly downregulated by limonoid (Gupta et al. 2010). Neem leaf extract potentially accelerates apoptosis in 4T1 breast cancer in the mouse model. It is reported that 250 mg/kg and 500 mg/kg bodyweight of neem leaf extract can induce apoptosis in breast cancer (Othman et al. 2011). Treatment of human breast and cervical cancer cells with ethanolic extract of neem leaves shows the suppression of cancer cells through apoptosis. This suppression is accomplished through the modulation of bax, cyclin D1, and cytochrome P450 monooxygenase (CYP 1A1, and CYP 1A2) expression (Sharma et al. 2014). Methanolic extract of neem leaves shows apoptosis by cellular fragmentation. This mechanism is facilitated by the inhibition of NF-кB and IкB kinase (Schumacher et al. 2011). Aqueous neem leaf extract is associated with ROS-mediated apoptosis in granuloma cells. Increased ROS production enhances the expression of p53 and bax proteins and increases H2O2 level. This promotes the enhancement of mitochondrial membrane potential, cytochrome c release, which results in the degradation of cellular proteins and DNA (Chaube et al. 2014). In this context, the oral dose of neem leaf extract significantly induced mitochondrial membrane damage and promotes apoptosis in chronic lymphocytic leukemia in adult patients. It has been shown that treatment with neem leaf extract significantly inhibits Bcl-2 and p53, and induces LC3-1 cleavage (Chitta et al. 2014). Aqueous extract of neem leaf promotes degeneration and apoptosis in rat oocytes by overexpression of bax protein and DNA fragmentation (Chaube et al. 2006). Neem leaf extract induces single dose-radiation and ionization radiation-mediated apoptotic cell death. It inhibits NAIP, BIRC6, BIRC8, NOL3, and induces BAK1, BAX, BCL10, CASP1, CASP10 CARD8, and CRADD (Veeraraghavan et al. 2011). Neem oil significantly increases the percentage of necrosis and apoptosis in cancer cells. It increases the expression of caspases 3, 8, and 9 (Kashif et al. 2018). In another study, it has been shown that ethanolic neem leaf extract targets insulin-like growth factor (IGF) signaling pathway in breast cancer cells. It remarkably reduces the expression of p-Akt, cyclin D1, Raf, p-Erk, Ras, and IGF-1R (Elumalai et al. 2012). Both ethanolic and aqueous extract of neem leaves enhances apoptosis in leukemia and colon cancer cells through increased ROS production and mitochondrial membrane destabilization (Roma et al. 2015). NLGP can promote apoptosis by releasing cytotoxic cytokines such as TNF-α and IFN-γ. In tumor cells, NLP acts to downregulate cyclin D1, Bcl-2, and upregulate caspase-3 (Bose et al. 2007). Azadirachtin is a potent non-toxic antifilarial agent against Setarai cervi. It significantly increases ROS production, pro-apoptotic factors such as EGL-1, CED-4, and CED-3 and downregulates antiapoptotic CED-9 (Mukherjee et al. 2019). Azadirachtin obtained from neem oil activates caspase cascade, which promotes apoptotic and autophagic cell death. Especially, less p21 enhances the activation of the caspase pathway in the presence of azadirachtin. It results in the release of apoptosis-inducing factor and cytochrome c from mitochondria and this mechanism is p53-independent (Srivastava et al. 2012). As mentioned earlier, mitochondria play an important role in apoptosis. It is reported that azadirachtin inhibits cyclosporine A, which is a potent identifier of mitochondria in apoptosis (Huang et al. 2013). The inhibition of Hsp90 is a therapeutic approach to cancer. Binding of Hsp90 with co-chaperone p23 results in the overexpression of the antiapoptotic protein Hsp70. It is reported that gedunin binds with p23 and inactivate Hsp90 machinery to induce apoptosis (Patwardhan et al. 2013). Gedunin shows antiproliferation, DNA fragmentation, and apoptosis of human embryonal cancer cells. Its treatment shows inhibition of Hsp90, surviving, and upregulation of bax and p53 (Tharmarajah et al. 2017). Similar kinds of results are obtained using gedunin-loaded nanoliposome in non-small-cell lung cancer (Nwokwu et al. 2017).
Fig. 5 .
Apoptotic functions of different A. indica extracts and their bioactive compounds in the regulation of different signaling pathways. The signs “↑” and “↓” indicate more and less, respectively. The sign “┴” represents inhibition of the particular pathway
Conclusions
Azadirachta indica (neem) is one of the most useful medicinal plants having preventive functions against wide range of diseases. Extracts obtained from different parts of the neem tree such as leaf, flower, seed, bark, and root contain numerous bioactive phytochemicals, which are known to have tremendous therapeutic potentials. Extensive studies on the concerned fields demonstrate that these bioactive compounds of neem have different regulatory effects on several biological processes such as inflammation, apoptosis, angiogenesis, and immunomodulation. Amongst the bioactive compounds, limonoids such as nimbolide and azarirachtin are extensively studied. These compounds show potential anti-cancer activity by reducing autophagy and elevating PI3K/Akt signal transduction. Different parts of neem tree show remarkable antioxidant activity. Studies have shown that neem extracts are the potential to scavenge free radicals and reduce ROS-mediated damage to cells. Use of neem in case of diabetes mellitus may have therapeutic importance as it normalizes lipid peroxidation and reduces ROS-mediated cell death. Additionally, neem extracts are anti-inflammatory, which significantly minimizes the release of proinflammatory cytokines such as TNF-α and IL-6. Furthermore, neem extracts have immense immunomodulatory functions, which increase the count of CD4 + and CD8 + T cells. These studies are indicating the pivotal roles of A. indica in the regulation of different biological pathways. Hence, neem may be used as therapeutic in a wide range of diseases. However, there is a need to study more on other bioactive compounds of neem to reveal therapeutic potentials.
Author contributions
SS, RPS, and GB have performed the literature search, data organization, and interpretation. SS, RPS, and GB have reviewed and constructed the manuscript. SS has written the manuscript.
Declarations
Ethical statements
There are no biological samples or subjects included in this study.
References
- Abdelhady MIS, Bader A, Shaheen U, El-Malah Y, Abourehab MAS, Barghash MF. Azadirachta indica as a source for antioxidant and cytotoxic polyphenolic compounds. Niosci Biotech Res Asia. 2015;12:1209–1222. [Google Scholar]
- Airaodion AI, Olatoyinbo PO, Ogbuagu U, Ogbuagu EO, Akinmolayan JD, Adekale OA, Awosanya OO, Agunbiade AP, Oloruntoba AP, Obajimi OO, Adeniji AR, Airaodion EO. Comparative assessment of phytochemical content and antioxidant potential of Azadirachta indica and Parquetina nigrescens leaves. APRJ. 2019;2:1–14. [Google Scholar]
- Akihisa T, Takahashi A, Kikuchi T, Takagi M, Watanabe K, Fukatsu M, Fujita Y, Banno N, Tokuda H, Yasukawa K. The melanogenesis-inhibitory, anti-inflammatory, and chemopreventive effects of limonoids in n-hexane extract of Azadirachta indica A. Juss. (neem) seeds. J Oleo Sci. 2011;60:53–59. doi: 10.5650/jos.60.53. [DOI] [PubMed] [Google Scholar]
- Akmal M, Zarnigar RB. Evaluation of chale neem (Azadirachta Indica) as an immunomodulator in elderly persons: a single blind randomized placebo controlled study. RRJoP. 2016;6:48–52. [Google Scholar]
- Alam A, Haldar S, Thulasiram HV, Kumar R, Goyal M, Iqbal MS, Pal C, Dey S, Bindu S, Sarkar S, Pal U, Maiti NC, Bandyopadhyay U. Novel anti-inflammatory activity of epoxyazadiradione against macrophage migration inhibitory factor: inhibition of tautomerase and proinflammatory activities of macrophage migration inhibitory factor. J Biol Chem. 2012;287:24844–24861. doi: 10.1074/jbc.M112.341321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hashemi ZSS, Hossain MA (2016) Biological activities of different neem leaf crude extracts used locally in Ayurvedic medicine. Pac Sci Rev A: Nat Sci Eng 18:128e131
- Alzohairy MA. Therapeutics role of Azadirachta indica (Neem) and their active constituents in diseases prevention and treatment. Evid Based Complement Alternat Med. 2016 doi: 10.1155/2016/7382506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameta RK, Sangani CB, Pansuriya B, Mali M, Kataria P, Patel M, Kawad M. DTAB catalysed Azadirachta indica oil/water bio-emulsion for enhancing the antioxidant efficacy of ceftriaxone. J In Silico In Vitro Pharmacol. 2017;3:22. [Google Scholar]
- Annavarapu TR, Renuka P, Akhil P, Divya P, Devi Priyanka P. Evaluation of the anti-inflammatory activity of combination of ethanol extracts of Azadirachta indica (neem) and Lawsonia inermis (Henna) Asian J Pharm Clin Res. 2016;9:256–258. [Google Scholar]
- Anokwuru CP, Ajibaye O, Adesuyi A. Comparative antioxidant activity of water extract of Azadiractha indica stem bark and Telfairia occidentalis leaf. Curr Res J Biol Sci. 2011;3:430–434. [Google Scholar]
- Arumugam A, Agullo P, Boopalan T, Nandy S, Lopez R, Gutierrez C, Narayan M, Rajkumar L. Neem leaf extract inhibits mammary carcinogenesis by altering cell proliferation, apoptosis, and angiogenesis. Cancer Biol Ther. 2014;15:26–34. doi: 10.4161/cbt.26604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asghar MS, Khan MS, Qurashi NA, Jabeen F, Wajid M, Farooq Z. Antioxidant potential of Allium sativum, Cinnamomum zeylanicum and Azadirachta indica against free radicals and their antimicrobial activity against isolated microbes from diseased Tilapia. J Entomol Zool Stud. 2017;5:1973–1979. [Google Scholar]
- Atawodi SE, Atawodi JC. Azadirachta indica (neem): a plant of multiple biological and pharmacological activities. Phytochem Rev. 2009;8:601–620. [Google Scholar]
- Banerjee S, Ghosh T, Barik S, Das A, Ghosh S, Bhuniya A, Bose A, Baral R. Neem leaf glycoprotein prophylaxis transduces immune dependent stop signal for tumor angiogenic switch within tumor microenvironment. PLoS ONE. 2014;9:e110040. doi: 10.1371/journal.pone.0110040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barik S, Banerjee S, Sarkar M, Bhuniya A, Roy S, Bose A, Baral R. Neem leaf glycoprotein optimizes effector and regulatory functions within tumor microenvironment to intervene therapeutically the growth of B16 melanoma in C57BL/6 mice. Trials Vaccinol. 2015;4:e80–e87. [Google Scholar]
- Beuth J, Schneider H, Ko HL. Enhancement of immune responses to neem leaf extract (Azadirachta indica) correlates with antineoplastic activity in BALB/c-mice. Vivo. 2006;20:247–251. [PubMed] [Google Scholar]
- Bhowmik D, Chiranjib YJ, Tripathi KK, Sampath Kumar KP. Herbal remedies of Azadirachta indica and its Medicinal application. J Chem Pharm Res. 2010;2:62–72. [Google Scholar]
- Biswas K, Chattopadhyay I, Banerjee RK, Bandyopadhyay U. Biological activities and medicinal properties of neem (Azadirachta indica) Curr Sci. 2002;82:1336–1345. [Google Scholar]
- Borges PV, Moret KH, Maya-Monteiro CM, Souza-Silva F, Alves CR, Batista PR, Caffarena ER, Pacheco P, Henriques Md, Penido C. Gedunin binds to myeloid differentiation protein 2 and impairs lipopolysaccharide-induced toll-like receptor 4 signaling in macrophages. Mol Pharmacol. 2015;88:949–961. doi: 10.1124/mol.115.098970. [DOI] [PubMed] [Google Scholar]
- Bose A, Haque E, Baral R. Neem leaf preparation induces apoptosis of tumor cells by releasing cytotoxic cytokines from human peripheral blood mononuclear cells. Phytother Res. 2007;21:914–920. doi: 10.1002/ptr.2185. [DOI] [PubMed] [Google Scholar]
- Chakraborty K, Bose A, Chakraborty T, Sarkar K, Goswami S, Pal S. Baral R (2010) Restoration of dysregulated CC chemokine signaling for monocyte/macrophage chemotaxis in head and neck squamous cell carcinoma patients by neem leaf glycoprotein maximizes tumor cell cytotoxicity. Cell Mol Immunol. 2010;7:396–408. doi: 10.1038/cmi.2010.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty T, Bose A, Barik S, Goswami KK, Banerjee S, Goswami S, Ghosh D, Roy S, Chakraborty K, Sarkar K, Baral R. Neem leaf glycoprotein inhibits CD4+CD25+Foxp3+ Tregs to restrict murine tumor growth. Immunotherapy. 2011;3:949–969. doi: 10.2217/imt.11.81. [DOI] [PubMed] [Google Scholar]
- Chaube SK, Prasad PV, Khillare B, Shrivastav TG. Extract of Azadirachta indica (neem) leaf induces apoptosis in rat oocytes cultured in vitro. Fertil Steril. 2006;1:1223–1231. doi: 10.1016/j.fertnstert.2005.11.034. [DOI] [PubMed] [Google Scholar]
- Chaube SK, Shrivastav TG, Tiwari M, Prasad S, Tripathi A, Pandey AK (2014) Neem (Azadirachta indica L.) leaf extract deteriorates oocyte quality by inducing ROS-mediated apoptosis in mammals. Springerplus 3:464 [DOI] [PMC free article] [PubMed]
- Chava VR, Manjunath SM, Rajanikanth AV, Sridevi N. The efficacy of neem extract on four microorganisms responsible for causing dental caries viz Streptococcus mutans, Streptococcus salivarius, Streptococcus mitis and Streptococcus sanguis: an in vitro study. J Contemp Dent Pract. 2012;13:769–772. doi: 10.5005/jp-journals-10024-1227. [DOI] [PubMed] [Google Scholar]
- Cheung TKN, Nigam PS, Owusu-Apenten R. Antioxidant activity of curcumin and neem (Azadirachta indica) powders: Combination studies with ALA Using MCF-7 breast cancer cells. JALSI. 2016;4:1–12. [Google Scholar]
- Chitta KS, Khan AN, Ersing N, Swaika A, Masood A, Paulus A, Qadeer A, Advani P, Sher T, Miller KC, Lee K, Chanan-Khan AA. Neem leaf extract induces cell death by apoptosis and autophagy in B-chronic lymphocytic leukemia cells. Leuk Lymphoma. 2014;55:652–661. doi: 10.3109/10428194.2013.807927. [DOI] [PubMed] [Google Scholar]
- Conte FP, Ferraris FK, Costa TE, Pacheco P, Seito LN, Verri WA, Jr, Cunha FQ, Penido C, Henriques MG. Effect of gedunin on acute articular inflammation and hypernociception in mice. Molecules. 2015;20:2636–2657. doi: 10.3390/molecules20022636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani S, Prabhu A, Chaitra KR, Desai NC, Patil SR, Rajeev R. Assessment of Streptococcus mutans in healthy versus gingivitis and chronic periodontitis: A clinico-microbiological study. Contemp Clin Dent. 2016;7:529–534. doi: 10.4103/0976-237X.194114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Paulo PL, da Silva KES, da Silva RO, Assreuy AMS, Pereira MG. Anti-inflammatory polysaccharides of Azadirachta indica seed tegument. Rev Bras Farmacogn. 2012;22:617–622. [Google Scholar]
- Deka H, Das S, Lahan JP, Yadav RNS. In-vitro free radical scavenging, antioxidant and antibacterial activity of Azadirachta Indica A. Juss. of Assam. Advances in Life Sciences. 2013;3:1–4. [Google Scholar]
- Diatta K, Diatta W, Fall AD, Dieng SIM, Mbaye AI, Akpoto-Kougblenou AAJ, Bassene E. Contribution to the study of leaves: Azadirachta indica A. Juss (Meliaceae): Evaluation of the antioxidant activity. AJRIB. 2019;2:1–8. [Google Scholar]
- Durrani FR, Chand DN, Jan M, Sultan A, Durrani Z, Akhtar S. Immunomodulatory and growth promoting effects of neem leaves infusion in broiler chicks. Sarhad J Agric. 2008;24:655–659. [Google Scholar]
- Dutta G, Deori C, Das S. Anti-inflammatory activity of ethanolic extract of Azadirachta indica leaves on experimental animal models. J Evolution Med Dent Sci. 2016;5:6335–6338. [Google Scholar]
- Elaigwu ED, Ogo OA, Efiong EE, Oche OG. Effects of ethanolic leaf extracts of neem (Azadirachta indica) on oxidative stability of palm oil. Res J Phytochem. 2019;13:1–10. [Google Scholar]
- Elumalai P, Gunadharini DN, Senthilkumar K, Banudevi S, Arunkumar R, Benson CS, Sharmila G, Arunakaran J. Ethanolic neem (Azadirachta indica A. Juss) leaf extract induces apoptosis and inhibits the IGF signaling pathway in breast cancer cell lines. Biomed Prev Nutr. 2012;2:59–68. [Google Scholar]
- Emran TB, Uddin MMN, Rahman A, Uddin Z, Islam M (2015) Phytochemical, antimicrobial, cytotoxic, analgesic and anti-Inflammatory properties of Azadirachta Indica: A therapeutic study. Bioanal Biomed S12.
- Ghimeray AK, Jin C, Ghimire BK, Cho DH. Antioxidant activity and quantitative estimation of azadirachtin and nimbin in Azadirachta Indica A. Juss grown in foothills of Nepal. Afr J Biotechnol. 2009;8:3084–3091. [Google Scholar]
- Gosse B, Amissa AA, Adje FA, Niamke FB. Analysis of components of neem (Azadirachta indica) oil by diverse chromatographic techniques. J Liq Chrom & Rel Technol. 2005;28:2225–2233. [Google Scholar]
- Goswami KK, Sarkar M, Ghosh S, Saha A, Ghosh T, Guha I, Barik S, Banerjee S, Roy S, Bose A, Dasgupta P, Baral R. Neem leaf glycoprotein regulates function of tumor associated M2 macrophages in hypoxic tumor core: Critical role of IL-10/STAT3 signaling. Mol Immunol. 2016;80:1–10. doi: 10.1016/j.molimm.2016.10.008. [DOI] [PubMed] [Google Scholar]
- Gupta SC, Prasad S, Reuter S, Kannappan R, Yadav VR, Ravindran J, Hema PS, Chaturvedi MM, Nair M, Aggarwal BB. Modification of cysteine 179 of IкBα kinase by nimbolide leads to down-regulation of NF-кB-regulated cell survival and proliferative proteins and sensitization of tumor cells to chemotherapeutic agents. J Biol Chem. 2010;285:35406–35417. doi: 10.1074/jbc.M110.161984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Ansari S, Gupta S, Narwani M, Gupta M, Singh M. Therapeutics role of neem and its bioactive constituents in disease prevention and treatment. J Pharmacogn Phytochem. 2019;8:680–691. [Google Scholar]
- Haldar S, Kolet SP, Thulasiram HV. Biocatalysis: fungi mediated novel and selective 12- β or 17-β hydroxylation on basic limonoid skeleton. Green Chem. 2013;15:1311–1317. [Google Scholar]
- Hao F, Kumar S, Yadav N, Chandra D. Neem components as potential agents for cancer prevention and treatment. Biochim Biophys Acta. 2014;1846:247–257. doi: 10.1016/j.bbcan.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyman L, Houri-Haddad Y, Heyman SN, Ginsburg I, Gleitman Y, Feuerstein O. Combined antioxidant effects of neem extract, bacteria, red blood cells and lysozyme: possible relation to periodontal disease. BMC Complement Altern Med. 2017;17:399. doi: 10.1186/s12906-017-1900-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain MD, Sarwar MS, Dewan SM, Hossain MS, Shahid-Ud-Daula A, Islam MS. Investigation of total phenolic content and antioxidant activities of Azadirachta indica roots. Avicenna J Phytomed. 2014;4:97–102. [PMC free article] [PubMed] [Google Scholar]
- Huang J, Lv C, Hu M, Zhong G. The mitochondria-mediate apoptosis of Lepidopteran cells induced by azadirachtin. PLoS ONE. 2013;8:e58499. doi: 10.1371/journal.pone.0058499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagadeesh K, Srinivas K, Revankar SP. Anti inflammatory effect of Azadirachta Indica (neem) in albino rats—an experimental study. IOSR J Pharm. 2014;4:34–38. [Google Scholar]
- Jaisinghani RN. Antibacterial properties of quercetin. Microbiol Res. 2017;8:6877. [Google Scholar]
- Jhariya MK, Raj A, Sahu KP, Paikra PR. Neem- A tree for solving global problem. IJAR. 2013;3:1–3. [Google Scholar]
- Kang JJ, Samad MA, Kim KS, Bae S. Comparative anti-inflammatory effects of anti-arthritic herbal medicines and Ibuprofen. Nat Prod Commun. 2014;9:1351–1356. [PubMed] [Google Scholar]
- Kashif M, Kim D, Kim G. In vitro anti-proliferative and apoptosis-inducing effect of a methanolic extract of Azadirachta indica oil on selected cancerous and noncancerous cell lines. Asian Pac J Trop Med. 2018;11:555–561. [Google Scholar]
- Kashif M, Hwang Y, Kim WJ, Kim G. In-vitro morphological assessment of apoptosis induced by nimbolide; A limonoid from Azadirachta Indica (neem tree) Iran J Pharm Res. 2019;18:846–859. doi: 10.22037/ijpr.2019.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur G, Sarwar Alam M, Athar M. Nimbidin suppresses functions of macrophages and neutrophils: relevance to its antiinflammatory mechanisms. Phytother Res. 2004;18:419–424. doi: 10.1002/ptr.1474. [DOI] [PubMed] [Google Scholar]
- Khandia R, Munjal A, Bangrey RS, Mehra R, Dhama K, Sharma NC. Evaluation of silver nanoparticle mediated reduction of neovascularisation (angiogenesis) in chicken model. Adv Anim Vet Sci. 2015;3:372–376. [Google Scholar]
- Kikuchi T, Ishii K, Noto T, Takahashi A, Tabata K, Suzuki T, Akihisa T. Cytotoxic and apoptosis-inducing activities of limonoids from the seeds of Azadirachta indica (neem) J Nat Prod. 2011;74:866–870. doi: 10.1021/np100783k. [DOI] [PubMed] [Google Scholar]
- Kumar VS, Navaratnam V. Neem (Azadirachta indica): prehistory to contemporary medicinal uses to humankind. Asian Pac J Trop Biomed. 2013;3:505–514. doi: 10.1016/S2221-1691(13)60105-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JW, Ryu HW, Park SY, Park HA, Kwon OK, Yuk HJ, Shrestha KK, Park M, Kim JH, Lee S, Oh SR, Ahn KS. Protective effects of neem (Azadirachta indica A. Juss.) leaf extract against cigarette smoke- and lipopolysaccharide-induced pulmonary inflammation. Int J Mol Med. 2017;40:1932–1940. doi: 10.3892/ijmm.2017.3178. [DOI] [PubMed] [Google Scholar]
- Lokanadhan S, Muthukrishnan P, Jeyaraman S. Neem products and their agricultural applications. J Biopest. 2012;5(Suppl):72–76. [Google Scholar]
- Mahapatra S, Young CY, Kohli M, Karnes RJ, Klee EW, Holmes MW, Tindall DJ, Donkena KV. Antiangiogenic effects and therapeutic targets of Azadirachta indica leaf extract in endothelial cells. Evid Based Complement Alternat Med. 2012;2012:303019. doi: 10.1155/2012/303019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud DA, Hassanein NM, Youssef KA, Abou Zeid MA. Antifungal activity of different neem leaf extracts and the nimonol against some important human pathogens. Braz J Microbiol. 2011;42:1007–1016. doi: 10.1590/S1517-838220110003000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mali AS, Thorat MB, Ghadge DM, Nikam KA, Sawant S, Shaikh F, Vhatkar N, Shinde S, Gonghade K, Tamboli MA (2019) Antinociceptive and antioxidant activities of methanolic extract of leaves of Azadirachta Indica (Neem). bioRxiv. http://doi.org/10.1101/694505
- Mallick A, Barik S, Goswami KK, Banerjee S, Ghosh S, Sarkar K, Bose A, Baral R. Neem leaf glycoprotein activates CD8(+) T cells to promote therapeutic anti-tumor immunity inhibiting the growth of mouse sarcoma. PLoS ONE. 2013;8:e47434. doi: 10.1371/journal.pone.0047434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallick A, Ghosh S, Banerjee S, Majumder S, Das A, Mondal B, Barik S, Goswami KK, Pal S, Laskar S, Sarkar K, Bose A, Baral R. Neem leaf glycoprotein is non-toxic to physiological functions of Swiss mice and Sprague Dawley rats: histological, biochemical and immunological perspectives. Int Immunopharmacol. 2013;15:73–83. doi: 10.1016/j.intimp.2012.11.006. [DOI] [PubMed] [Google Scholar]
- Malviya R, Sharma PK, Dubey SK. Antioxidant potential and emulsifying properties of neem (Azadirachita indica, Family Meliaceae) gum polysaccharide. Pharm Anal Acta. 2017;8:9. [Google Scholar]
- Manikandan P, Anandan R, Nagini S. Evaluation of Azadirachta indica leaf fractions for in vitro antioxidant potential and protective effects against H2O2-induced oxidative damage to pBR322 DNA and red blood cells. J Agric Food Chem. 2009;57:6990–6996. doi: 10.1021/jf901351n. [DOI] [PubMed] [Google Scholar]
- Morris J, Gonzales CB, De La Chapa JJ, Cabang AB, Fountzilas C, Patel M, Orozco S, Wargovich MJ. The highly pure neem leaf extract, SCNE, inhibits tumorigenesis in oral squamous cell carcinoma via disruption of pro-tumor inflammatory cytokines and cell signaling. Front Oncol. 2019;9:890. doi: 10.3389/fonc.2019.00890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosaddek ASM, Rashid MMU. A comparative study of the anti-inflammatory effect of aqueous extract of neem leaf and dexamethasone. Bangladesh J Pharmacol. 2008;3:44–47. [Google Scholar]
- Mukherjee N, Joardar N, Sinha Babu SP. Antifilarial activity of azadirachtin fuelled through reactive oxygen species induced apoptosis: a thorough molecular study on Setaria cervi. J Helminthol. 2019;93:519–528. doi: 10.1017/S0022149X18000615. [DOI] [PubMed] [Google Scholar]
- Nagaraj A, Samiappan S. Presentation of antibacterial and therapeutic anti-inflammatory potentials to hydroxyapatite via biomimetic with Azadirachta indica: An in vitro anti-inflammatory assessment in contradiction of LPS-induced stress in RAW 264.7 Cells. Front Microbiol. 2019;10:1757. doi: 10.3389/fmicb.2019.01757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahak G, Sahu RK. In vitro antioxidative acitivity of Azadirachta indica and Melia azedarach leaves by DPPH scavenging assay. J Am Sci. 2010;6:123–128. [Google Scholar]
- Nahak G, Sahu RK. Antioxidant activity in bark and roots of neem (Azadirachta indica) and mahaneem (Melia azedarach) C J Pharm Sci. 2010;4:28–34. [Google Scholar]
- Nahak G, Sahu RK. Evaluation of antioxidant activity of flower and seed oil of Azadirachta indica A. Juss J Appl Nat Sci. 2011;3:78–81. [Google Scholar]
- Neem leaf extract inhibits mammary carcinogenesis by altering cell proliferation, apoptosis, and angiogenesis. Cancer Biol Ther 15:26–34 [DOI] [PMC free article] [PubMed]
- Nwokwu CDU, Samarakoon SR, Karunaratne DN, Katuvawila NP, Pamunuwa GK, Ediriweera MK, Tennekoon KH. Induction of apoptosis in response to improved gedunin by liposomal nano-encapsulation in human non-small-cell lung cancer (NCI-H292) cell line. Trop J Pharm Res. 2017 doi: 10.4314/tjpr.v16i9.6. [DOI] [Google Scholar]
- Othman F, Motalleb G, Lam Tsuey Peng S, Rahmat A, Fakurazi S, Pei Pei C. Extract of Azadirachta indica (neem) leaf induces apoptosis in 4T1 breast cancer BALB/c mice. Cell J. 2011;13:107–116. [PMC free article] [PubMed] [Google Scholar]
- Ouerfelli M, Villasante J, Ben Kaab LB, Almajano M. Effect of neem (Azadirachta indica L.) on lipid oxidation in raw chilled beef patties. Antioxidants (Basel) 2019;8:piiE305. doi: 10.3390/antiox8080305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyebode O, Kandala NB, Chilton PJ, Lilford RJ. Use of traditional medicine in middle-income countries: a WHO-SAGE study. Health Policy Plan. 2016;31:984–991. doi: 10.1093/heapol/czw022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil PR, Patil VM, Verma S, Vijayanath V, Surpur RR. A study of anti-inflammatory activity of Azadirachta indica (Neem) root in Wistar albino rats. Medico-Legal Update. 2012;12:104–106. [Google Scholar]
- Patwardhan CA, Fauq A, Peterson LB, Miller C, Blagg BS, Chadli A. Gedunin inactivates the co-chaperone p23 protein causing cancer cell death by apoptosis. J Biol Chem. 2013;288:7313–7325. doi: 10.1074/jbc.M112.427328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokhrel B, Rijal S, Raut S, Pandeya A. Investigations of antioxidant and antibacterial activity of leaf extracts of Azadirachta indica. Afr J Biotechnol. 2015;14:3159–3163. [Google Scholar]
- Ponnusamy S, Haldar S, Mulani F, Zinjarde S, Thulasiram H, Kumar AR. Gedunin and azadiradione: Human pancreatic alpha-amylase inhibiting limonoids from neem (Azadirachta indica) as anti-diabetic agents. PLoS ONE. 2015;10:e0140113. doi: 10.1371/journal.pone.0140113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priyadarsini RV, Murugan RS, Sripriya P, Karunagaran D, Nagini S. The neem limonoids azadirachtin and nimbolide induce cell cycle arrest and mitochondria-mediated apoptosis in human cervical cancer (HeLa) cells. Free Radic Res. 2010;44:624–634. doi: 10.3109/10715761003692503. [DOI] [PubMed] [Google Scholar]
- Rao AD, Devi KN, Thyagaraju K. Isolation of antioxidant principle from Azadirachta seed kernels: determination of its role on plant lipoxygenases. J Enzyme Inhib. 1998;14:85–96. doi: 10.3109/14756369809036547. [DOI] [PubMed] [Google Scholar]
- Reddy M, Thangavelu L, Roy A. Substance P inhibitory activity of Azadirachta indica bark extract-In vitro analysis. J Adv Pharm Edu Res. 2018;8:29–32. [Google Scholar]
- Roma A, Ovadje P, Steckle M, Nicoletti L, Saleem A. Pandey S (2015) Selective induction of apoptosis by Azadarichta indica leaf extract by targeting oxidative vulnerabilities in human cancer cells. J Pharm Pharm Sci. 2015;18:729–746. doi: 10.18433/j3vg76. [DOI] [PubMed] [Google Scholar]
- Roy S, Goswami S, Bose A, Chakraborty K, Pal S, Haldar A, Basu P, Biswas J, Baral R. Neem leaf glycoprotein partially rectifies suppressed dendritic cell functions and associated T cell efficacy in patients with stage IIIB cervical cancer. Clin Vaccine Immunol. 2011;18:571–579. doi: 10.1128/CVI.00499-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar K, Bose A, Haque E, Chakraborty K, Chakraborty T, Goswami S, Ghosh D, Baral R. Induction of type 1 cytokines during neem leaf glycoprotein assisted carcinoembryonic antigen vaccination is associated with nitric oxide production. Int Immunopharmacol. 2009;9:753–760. doi: 10.1016/j.intimp.2009.02.016. [DOI] [PubMed] [Google Scholar]
- Saxena N, Kumar Y. Chemistry of azadirachtin and other bioactive isopreniods from Neem (Azadirachta indica A. Juss). In: Neem, a Treatise, K. K. Singh, S. Phogat, R. S. Dhillon, A. Tomar (ed). IK International Publishing House Pvt. Ltd., New Delhi, India. 2009, pp 208–230
- Schumacher M, Cerella C, Reuter S, Dicato M, Diederich M. Anti-inflammatory, pro-apoptotic, and anti-proliferative effects of a methanolic neem (Azadirachta indica) leaf extract are mediated via modulation of the nuclear factor-κB pathway. Genes Nutr. 2011;6:149–160. doi: 10.1007/s12263-010-0194-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sejali SNF, Anuar MS. Effect of drying methods on phenolic contents of neem (Azadirachta indica) leaf powder. J Herbs Spic Med Plants. 2011;2:119–131. [Google Scholar]
- Septiyani R, Wibowo C. Identification of active compounds and testing the antioxidant properties of neem leaf extract. AIP Conf Proc. 2019;2094:020034. doi: 10.1063/1.5097503. [DOI] [Google Scholar]
- Seriana I, Akmal M, Darusman Wahyuni S. Neem leaves extract (Azadirachta indica A. Juss) on male reproductive system: a mini-review. IOP Conf Series. 2019;399:012106. [Google Scholar]
- Shah AS, Gunjal MA, Juvekar AR. Immunomostimulatory activity of aqueous extract of Azadirachta indica flowers on specific and non specific immune response. J Nat Remed. 2009;9(1):35–42. [Google Scholar]
- Sharma C, Vas AJ, Goala P, Gheewala TM, Rizvi TA, Hussain A. Ethanolic neem (Azadirachta indica) leaf extract prevents growth of MCF-7 and HeLa cells and potentiates the therapeutic index of cisplatin. J Oncol. 2014;2014:321754. doi: 10.1155/2014/321754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma G, Thakur A, Lal S, Nadda RK. Comparative analysis of phytochemical, antibacterial, and antioxidant activity of different extracts of Azadirachta indica leaves. Asian J Pharm Clin Res. 2019 doi: 10.22159/ajpcr.2019.v12i3.29286. [DOI] [Google Scholar]
- Shrivastava A, Chaturvedi U, Sonkar R, Khanna AK, Saxena JK, Bhatia G. Antioxidant effect of Azadirachta Indica on high fat diet induced diabetic charles foster rats. Appl Biochem Biotechnol. 2012;167:229–236. doi: 10.1007/s12010-012-9681-0. [DOI] [PubMed] [Google Scholar]
- Siddiqui S. A note on the isolation of three new bitter principles from the nim oil. Curr Sci. 1942;11:278–279. [Google Scholar]
- Sophia J, Kowshik J, Dwivedi A, Bhutia SK, Manavathi B, Mishra R, Nagini S. Nimbolide, a neem limonoid inhibits cytoprotective autophagy to activate apoptosis via modulation of the PI3K/Akt/GSK-3β signalling pathway in oral cancer. Cell Death Dis. 2018;9:1087. doi: 10.1038/s41419-018-1126-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soumendra D, Anirbandeep B, Uttam B, Nilendra C, Bikash R , Kumar CT, Anjan D, Kumar PT (2009) Antioxidant and hepatoprotective effect of Azadirachta indica leaf extractonaceclofenac induced hepatotoxicity in rats. JPR. http://doi.org/10.18579/jpcrkc/2009/8/2/79778
- Srivastava P, Yadav N, Lella R, Schneider A, Jones A, Marlowe T, Lovett G, O'Loughlin K, Minderman H, Gogada R, Chandra D. Neem oil limonoids induces p53-independent apoptosis and autophagy. Carcinogenesis. 2012;33:2199–2207. doi: 10.1093/carcin/bgs269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana B, Anwar F, Przybylski R. Antioxidant activity of phenolic components present in barks of Azadirachta indica, Terminalia arjuna, Acacia nilotica, and Eugenia jambolana Lam. trees. Food Chem. 2007;104:1106–1114. [Google Scholar]
- Talpur AD, Ikhwanuddin M. Azadirachta indica (neem) leaf dietary effects on the immunity response and disease resistance of Asian seabass, lates calcarifer challenged with Vibrio harveyi. Fish Shellfish Immunol. 2013;34:254–264. doi: 10.1016/j.fsi.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Tharmarajah L, Ediriweera SSR, MK, Piyathilaka P, Tennekoon KH, Senathilake KS, Rajagopalan U, Galhena PB, Thabrew I, In vitro anti-cancer effect of gedunin on human teratocarcinomal (NTERA-2) cancer stem-like cells. Biomed Res Int. 2017;2017:2413197. doi: 10.1155/2017/2413197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari R, Verma AK, Chakraborty S, Dhama K, Singh SV. Neem (Azadirachta indica) and its potential for safeguarding health of animals and humans: A review. J Biol Sci 14:110–123 Tan QG, Luo XD (2011) Meliaceous limonoids: chemistry and biological activities. Chem Rev. 2014;111:7437–7522. doi: 10.1021/cr9004023. [DOI] [PubMed] [Google Scholar]
- Umar MI, Asmawi MZ, Sadikun A, Abdul Majid AM, Atangwho IJ, Khadeer Ahamed MB, Altaf R, Ahmad A. Multi-constituent synergism is responsible for anti-inflammatory effect of Azadirachta indica leaf extract. Pharm Biol. 2014;52:1411–1422. doi: 10.3109/13880209.2014.895017. [DOI] [PubMed] [Google Scholar]
- Upadhyay SN, Dhawan S, Garg S, Talwar GP. Immunomodulatory effects of neem (Azadirachta indica) oil. Int J Immunopharmacol. 1992;14:1187–1193. doi: 10.1016/0192-0561(92)90054-o. [DOI] [PubMed] [Google Scholar]
- Varghese B, Naithani SC. Desiccation-induced changes in lipid peroxidation, superoxide level and antioxidant enzymes activity in neem (Azadirachta indica A. Juss) seeds. Acta Physiol Plant. 2002;24:79–87. [Google Scholar]
- Veeraraghavan J, Aravindan S, Natarajan M, Awasthi V, Herman TS, Aravindan N. Neem leaf extract induces radiosensitization in human neuroblastoma xenograft through modulation of apoptotic pathway. Anticancer Res. 2011;31:161–170. [PubMed] [Google Scholar]
- Vergallo C, Panzarini E, Din L. High performance liquid chromatographic profiling of antioxidant and anti-diabetic flavonoids purified from Azadirachta indica (neem) leaf ethanolic extract. Pure Appl Chem. 2019;91:1631–1640. [Google Scholar]
- Yadav N, Kumar S, Kumar R, Srivastava P, Sun L, Rapali P, Marlowe T, Schneider A, Inigo JR, O'Malley J, Londonkar R, Gogada R, Chaudhary AK, Yadava N, Chandra D. Mechanism of neem limonoids-induced cell death in cancer: Role of oxidative phosphorylation. Free Radic Biol Med. 2016;90:261–271. doi: 10.1016/j.freeradbiomed.2015.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]