Abstract
The processes of hypertrophic scar formation are extremely complex, and current animal models have limitations in terms of the complete characterization of lesions. An ideal animal model is indispensable for exploring the complex progression of scar formation to elucidate its pathophysiology and to perform therapeutic testing. This study aimed to establish a long‐term, consistent and easily testable animal model by injecting anhydrous alcohol into the dorsal trunk dermis of rabbits. The rabbits were injected with different amounts of anhydrous alcohol. Anhydrous alcohol was infiltrated into the subcutaneous and superficial fascia. The optimal amount of anhydrous alcohol was determined by measuring the area and thickness of the scar. The typical model was established by determining the optimum dosage, and then we analysed the histological characteristics and fibrosis‐associated protein expression. The dermal scar was generated by treating with 2 ml/kg anhydrous alcohol and displayed histopathologic features that characterize human hypertrophic scarring, including a parallel collagen fibre orientation, dermal and epidermal thickening, broad collagen deposition and the loss of dermal adnexal structures. The expression of fibrotic pan‐markers was also enhanced. Moreover, the scar features and duration were compared between the anhydrous alcohol model and the rabbit ear model. Our results show that injecting anhydrous alcohol in the rabbit model thickened the dermal tissue, stimulated dermal fibroproliferation and resulted in hypertrophic scars with protein and histologic features similar to those seen in humans. Taken together, the findings from this study show that our model could be a feasible and useful tool for further research on the pathogenesis of hypertrophic scars.
Keywords: anhydrous alcohol, animal model, fibrosis, hypertrophic scar
1. INTRODUCTION
Hypertrophic scarring is a fibroproliferative dermal disorder that can occur during the wound healing process after skin trauma or severe burn injury and is characterized by an excessive deposition of collagen and other extracellular matrix (ECM) proteins. 1
The processes occurring during wound healing in the skin after dermal injury are complex and include prolonged re‐epithelialization, increased mechanical stress, protracted haemostasis and exaggerated inflammation. 2 Due to the complexity and the involvement of multiple stages in these processes, an ideal animal model is indispensable for simulating the complex progression, elucidating the pathophysiology and performing therapeutic testing. Various animal models, including pigs, 3 rabbits, 4 and mice, 5 have been developed to replicate skin injury, but the methods are very cumbersome. Therefore, we attempted to establish a reproducible, long‐term, consistent and easily testable animal model of hypertrophic scar.
Anhydrous alcohol is widely used as sclerotherapy in the treatment of vascular malformation, but this treatment could produce serious complications due to muscle fibrosis. 6 Moreover, chronic alcohol abuse could induce a fibrotic response in various organs, such as skeletal muscle, heart and liver. 7 , 8 In view of the aforementioned findings, we established an animal model of congenital muscular torticollis (CMT) by injection of anhydrous alcohol in the sternocleidomastoid muscle (SCM) of rabbits in our previous study. 9 Furthermore, bottulinum toxin type A reduces SCM fibrosis in CMT. During the experiment, we found that when the injection area was located in the subcutaneous and superficial fascia, fibrosis will occur in the rabbit skin simultaneously. Moreover, inflammation, re‐epithelialization, ECM deposition, contraction and scar formation were observed, which are part of the skin repair process in wound healing. 10 This phenomenon has attracted our attention.
The common methods of establishing animal models of scar formation have included hypertrophic scarring or keloid implantation in immunodeficient animals (athymic mice and rats); hypertrophic scarring or keloid implantation in an immune‐privileged site (hamster cheek pouch); hypertrophic scarring or keloid induction via chemically mediated injury (guinea pigs); hypertrophic scarring or keloid induction in specific anatomic sites (rabbit ear); and hypertrophic scarring or keloid induction in deep dermal wounds in a porcine model. 11 However, the above‐mentioned methods rarely used sclerotic‐reagent injection. Taken together, these findings prompted us to clarify whether anhydrous alcohol injection is an effective way to establish a scar animal model and what the optimal dose is. Here, we investigated the gross appearance, thickness and histological changes of the skin of rabbits induced by injection of different doses of anhydrous alcohol. We further evaluated the fibrotic marker Col I, Col III and the myofibroblast marker α‐SMA and evaluated the severity by Observer Scar Assessment value analysis.
2. MATERIALS AND METHODS
2.1. Injection procedure of rabbit anhydrous alcohol model versus surgical procedure of the rabbit ear model
Sixty male New Zealand white rabbits weighing between 2.0 ~ 2.5 kg and aged between 90 ~ 120 days were used in this study. The rabbits were obtained from the Bengbu Medical College animal laboratory. The rabbits were assigned to 12 groups (5 rabbits in each group) according to the dose and concentration of anhydrous alcohol injected. Anhydrous alcohol was diluted to three different concentrations with distilled water: 50%, 75%, and 100% [volume of alcohol / [volume of H2O + (volume of alcohol)]. The injected dosage at each concentration was divided into three levels: 1; 2; and 3 ml/kg. Group numbers were generated by permutation and combination. The dorsal trunk was prepared for injection by shaving and disinfecting it with 75% ethanol scrubs after the rabbits were intravenously anaesthetized with 20% pentobarbital (20 ml) via the ear margin vein. Each rabbit had two test areas (one per side) with infiltration of the subcutaneous and superficial fascia. Scars were evaluated with digital photography (α‐6000, Sony, Japan), which were taken under a natural bright light at 1 metre distance, at 15, 30, 60 and 90 days to compare with normal rabbit skin and human hypertrophic scar. Fifteen days after injection, the injected site tissue was harvested from anaesthetized rabbits for the thickness measurement with Vernier calipers. The optimum group was chosen for further experiments.
One 1.0 cm2 square defect was created on each ear using a no. 11 scalpel blade. The defects involved the epidermis, dermis and perichondrium. A dissecting microscope was used to ensure removal of the perichondrium. Haemostasis was achieved by applying manual pressure, and each ear was covered with Vaseline and sterile gauze.
2.2. Observer scar assessment scale
All scars in the rabbit anhydrous alcohol model and ear model at different time points were evaluated using the observer scar assessment scale, 12 , 13 which needs to be filled out by the observer and was performed independently by two board‐certified plastic surgeons. The observer determines scores for the items vascularization, pigmentation, pliability, thickness and relief. Each item is scored on a 10‐point scale, whereby a score of 10 reflects the worst grade scar. The total score of the observer scale is determined by adding the scores for each item.
2.3. Histology
Samples from the anhydrous alcohol model were harvested on days 15, 30, 60 and 90 under anaesthesia. Half of the samples were analysed by Western blotting (the samples for Western blotting assay were stored in liquid nitrogen until all samples had been collected), while the other half were fixed in 10% neutral‐buffered formalin and embedded in paraffin. The histological sections (3 μm) were stained with haematoxylin and eosin (H and E), Masson's trichrome stain and immunohistochemical stains. Immunohistochemical staining was performed by the EnVision method using a Col I antibody (1:150 in PBS, Abcam, ab34710, USA), α‐SMA antibody (1:200 in PBS, Abcam, ab5694, USA) and Col III antibody (1:1000 in PBS, Abcam, ab7778, USA). During the deparaffinization and rehydration processes, the slides were placed in an incubator (60°C) for 40 min and then placed in two xylol containers for 5 min and three containers containing different concentrations of ethanol solution (100%, 80% and 75%). The sections were then washed in distilled water. During the antigen retrieval stage, the slides were placed in a container with citric acid (0.01M, pH 6.0, G1202, Servicebio, Wuhan, China) and heated on medium power for 8 minutes until boiling, then turned off the microwave oven, kept warm for 8 minutes and then transferred to medium‐low power for heating 7 minutes. During this process, care should be taken to avoid excessive evaporation of buffer and sections drying. To cool to room temperature before proceeding, the sections were placed in PBS (PH7.4) and washed 3 times. Then, the slides were incubated with primary antibody overnight at 4 °C and placed in a wet box containing a small amount of water. After that, the tissue sections were covered with secondary antibody labelled with HRP (1:200 in PBS, G1215, Servicebio, Wuhan, China) and incubated at room temperature for 50 minutes. In the developing stage, freshly prepared DAB chromogenic reagent was added to the sections. The reaction was monitored by microscopy until the nuclei turned brown‐yellow. The reaction was stopped by washing the tissue in running tap water. Sections were then incubated with haematoxylin for 3 minutes to counterstain the cells nuclei. Finally, the samples were dehydrated successively in a gradient of ethanol solutions (75%, 85% and 2 changes of pure ethanol) for 6 minutes each. The tissue was then cleared in xylene for 5 minutes and mounted with neutral gum (China National Pharmaceutical Group Chemical Reagent Co., LTD).
Images were acquired at 200× and 400× magnification using an Olympus microscope (Olympus, BX53). Ten randomly selected photomicrographs (200×) of each dermal sample were digitized using Image‐Pro Plus software. For immunohistochemical staining, α‐SMA, Col I and Col III were labelled in samples to evaluate the presence of myofibroblast and the degree of fibrosis. The positive staining percentages of Col I, α‐SMA and Col III were determined semi‐quantitatively using Image‐Pro Plus software.
2.4. Western blotting
Total protein in the samples and normal skin tissues was extracted using RIPA lysis buffer. The protein concentration was determined with the Bradford method; 50 μg of protein was mixed with loading buffer, separated by 10% SDS‐PAGE and transferred onto nitrocellulose membranes. After blocking for 2 hours at room temperature with 10% BSA, the membranes were incubated with the appropriate primary antibody overnight at 4°C, and the membrane was washed and then incubated with HRP‐conjugated secondary antibodies for 1 hour at room temperature, after which it was exposed by using an ECL reagent kit. The following antibodies were used: Col I antibody (1:10000 in PBS, Abcam, ab34710,USA), α‐SMA antibody (1:200 in PBS, Abcam, ab5694, USA) and Col III antibody (1:10000 in PBS, Abcam, ab7778, USA).
2.5. Statistical analysis
All the samples were analysed three times independently, and all data are expressed as the mean ± SD. The thickness differences between the nine groups were assessed by one‐way ANOVA. The immunofluorescence staining results and the differences between two groups were analysed using unpaired t tests. P < .05 was considered statistically significant.
3. RESULTS
3.1. Anhydrous alcohol‐induced fibrotic‐like trauma in rabbits
Fifteen days after injection, the skin injection area was altered between the groups. The skin was almost completely healed, and no scar was seen in group 1 and group 2, which were injected at dosages of 1 and 2 ml/kg respectively. Only a few atrophic scars were observed in group 2, in which the injection dosage was increased to 3 ml/kg; however, the lesion area was too small to be observed and detected. As the concentration increased to 75%, the skin texture in the injection area became slightly hard, and the colour turned slightly red. However, this reaction soon disappeared and left behind a slight scar. When we adjusted the dose to 100%, a serious inflammatory reaction was present. The colour of the skin turned red, the texture became hard, and the dermis became thicker with subcutaneous adhesion. When the injection dosage was increased to 3 ml/kg of 100% anhydrous alcohol, these characteristics became more obvious, and the area of the injury became large enough to be detected in downstream experiments (Figure 1A). However, this dosage caused deeper and larger areas of skin necrosis. The subsequent exfoliation resulted in extensive skin defects (23.2 ± 4.8 cm2) with exposure of underlying oedematous muscles. Such a large skin defect could be difficult to heal, increasing the risk of inflammation, nutrient depletion and infection, which might have been the cause of death in one rabbit from this group. It indicated that 3 ml/kg of 100% anhydrous alcohol is not the ideal dose.
FIGURE 1.
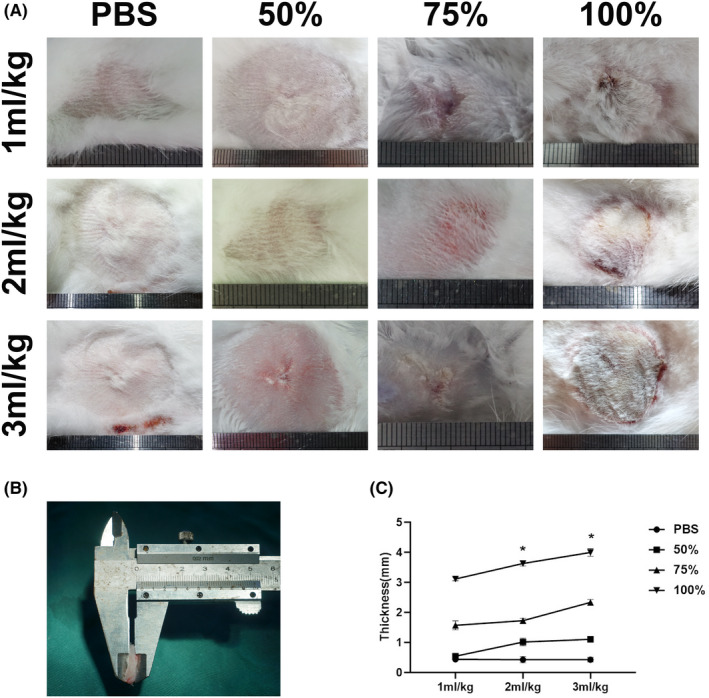
The fibrotic scar formation in rabbit with different dose of anhydrous alcohol injection at 15 days. (A) The images showed the size of lesion in rabbit with injection of PBS, 50% anhydrous alcohol, 75% anhydrous alcohol and 100% anhydrous alcohol respectively. The injection volume was according to the body weight with 1, 2 and 3 ml/kg respectively. (B) The measurement of fibrotic scar thickness of each group with Vernier Calipers and corresponding statistic values (C). Data are presented as means ± SD; Error bars = SD
The dermal scar was also quantified by measuring the thickness with Vernier calipers (Figure 1B). The thickness was enhanced in dermal tissue treated with anhydrous alcohol injection when it was compared among the groups. The dermis thickened significantly in a dose‐dependent manner (Figure 1C). According to one‐way ANOVA, the data showed that there was a significant difference between the groups. In the 100% anhydrous alcohol groups, the 2 ml/kg model lesions displayed marked dermal thickening, which was significantly increased compared with that in the 1 ml/kg model (3.625 ± 0.081 mm vs. 3.118 ± 0.059 mm, P < .01). The 3 ml/kg model lesions had significantly increased dermal thickness compared with the 1 ml/kg model samples or the 2 ml/kg model samples (4.000 ± 0.1273 mm vs. 3.118 ± 0.059 mm, P < .001, and 4.000 ± 0.1273 mm vs. 3.625 ± 0.081 mm, P < .01).
Overall, considering the tissue proliferation levels and the model survival rate, 100% 2 ml/kg anhydrous alcohol was the ideal injection dosage. Therefore, we chose this treatment for further experiments thereafter.
3.2. Comparison between the anhydrous alcohol model and the rabbit ear model
To further and comprehensively evaluate the characteristics of the lesion model, we compared the characteristics between the long‐term superficial fascia anhydrous alcohol injection model and the rabbit ear model 14 (Table 1). Each group had 15 samples, and rabbits in the two groups did not differ in age (P > .05). The operation duration in the anhydrous alcohol model group was much shorter than that in the rabbit ear model group, and the operation was easier to repeat. The duration of fibrotic hyperplasia was much longer in the anhydrous alcohol model group; on average, it lasted for nearly 30 days. At 30 days after the operation, the scar in the rabbit ear began to become flattened, and the colour began to fade (Figure S1). The defects had recovered almost completely at 60 days, while the scar was still evident in the anhydrous alcohol model group (Figure 2A). Moreover, the Observer Scar Assessment provided a comprehensive analysis of anhydrous alcohol‐induced trauma. Compared with those for the ear model, the pliability, thickness, relief and summary scores were significantly and gradually enhanced for the injection model group (Table 1).
Table 1.
Characteristics of anhydrous alcohol model versus rabbit ear model
| Variable | Anhydrous alcohol model | Rabbit ear model | P value |
|---|---|---|---|
| Age (days) | 102 ± 9 (range 91 ~ 120) | 103 ± 10 (range 90 ~ 120) | .969 |
| Operation duration (min) | 1.15 ± 1.16 | 8.73 ± 4.30 | .000 |
| Fibrotic hyperplasia duration(day) | 74.27 ± 15.86 | 41.13 ± 15.28 | .001 |
| Observer scar assessment | |||
| Vascularization | 4.47 ± 2.39 | 5.20 ± 2.31 | .339 |
| Pigmentation | 3.3 ± 1.95 | 3.5 ± 1.92 | .852 |
| Pliability | 7.73 ± 1.16 | 3.27 ± 1.92 | .000 |
| Thickness | 7.46 ± 7.47 | 3.20 ± 1.32 | .000 |
| Relief | 7.13 ± 1.13 | 3.60 ± 1.35 | .000 |
| Summary scores | 30.80 ± 3.41 | 18.73 ± 4.64 | .000 |
FIGURE 2.
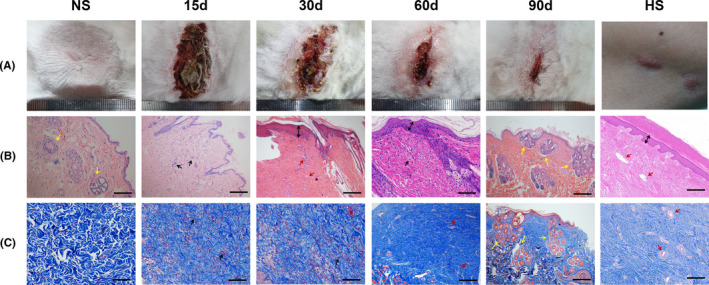
The anhydrous alcohol injection induced the dermal fibrotic model. (A) Visual inspection of the normal rabbit skin (NS) and dermal scar characteristic 15, 30, 60, 90 days after injection. The last one is human hypertrophic scar (HS) on jaw. Panel B and C, respectively, corresponds to H&E and Masson's trichrome stain in panel A. (Scale bar = 100 μm. Yellow arrows represent adnexal structures, black arrows represent immune cells, double‐line arrows represent the thickened epidermis, red arrows represent blood vessels)
3.3. Abnormal fibrotic tissues were significantly associated with anhydrous alcohol‐induced trauma
Unlike hypertrophic scar, the normal rabbit skin presents a well‐developed dermis (Figure 2A NS). Appendages and hair follicles are evenly distributed in the deep dermis, surrounded by loose and well‐arranged collagen fibres (Figure 2B,C NS). Fifteen days after injection, cutaneous necrosis and subcutaneous tissue hyperplasia were caused by anhydrous alcohol dehydration (Figure 2A 15D). A large number of fibroblasts had proliferated and inflammatory cells had infiltrated the dermal tissue. The number of dermal adnexal structures decreased compared with the normal rabbit skin group. Collagen fibres in the dermis began to accumulate in small amounts (Figure 2B,C 15D). The wound healing was accompanied by a large amount of collagen deposited, the thick and broad deposition of collagen in the affected dermal tissue presented at 30 and 60 days, the scar in the injection area bulged, and the tissue became hard. Over time, these fibrotic histological characteristics continued to develop. The excessive deposition of collagen increased, and the thickness of the epidermis also increased; meanwhile, a large number of blood vessels regenerated in the dermis (Figure 2A‐C 30, 60 days). Both of these features were morphologically similar to human hypertrophic scar (Figure 2A‐C HS). At 90 days, scar proliferation began to decrease, and the tissue became flattened and softened. The deposition of collagen was significantly decreased compared with that observed at 60 days. The adnexal structures were more regenerated than those in normal skin (Figure 2A‐C 90 days).
To evaluate the effect of anhydrous alcohol on fibroblast‐to‐myofibroblast differentiation and fibroproliferative induction, expression of α‐SMA and the fibrosis marker Col I and Col III at 15, 30, 60, 90 days and normal skin was detected by immunohistochemical and Western blotting analysis.
The levels of α‐SMA, Col I and Col III protein in the dermal scar were increased 30 days after injection of anhydrous alcohol (100%, 2 ml/kg). At day 60, the dermal scar showed large amounts of brown‐stained collagen (Figure 3A,C), an increase in the number of inflammatory cells and vascular penetration deep within the reticular dermis. The deep vessels presented α‐SMA uptake, indicating the presence of many myofibroblasts. By day 90, the presence of inflammatory cells and brown‐stained collagen were diminished compared to those at day 60, and myofibroblasts seemed to be present in the regenerated adnexal structures (Figure 3B). The ratio of the expression of Col I and Col III protein constituents showed a significant difference among the five groups (P < .0001) (Figure 3A,C). The number of myofibroblasts in dermal scars, which were identified as α‐SMA‐positive cells, was increased significantly in both the 15 or 60‐day models compared with that in the skin of the placebo group rabbits. Ninety days after injection, the number of α‐SMA‐positive area was significantly reduced compared to that in the 60‐day model (Figure 3D).
FIGURE 3.
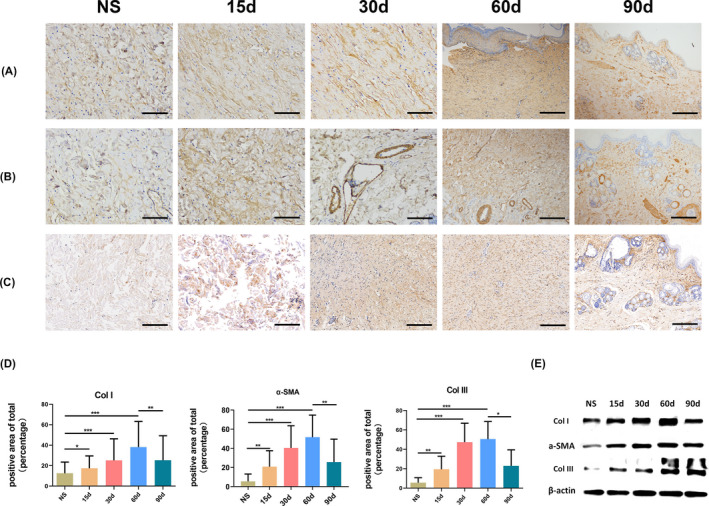
The anhydrous alcohol‐induced scar tissues express fibrotic pan‐markers. The immunohistochemistry results of Col I(A), α‐SMA(B) and Col III(C) of normal rabbit skin (NS) and 15, 30, 60, 90 d after injection. (Scale bar = 100 μm) (D) Image analysis was performed to quantify the positive area of total using Image‐Pro Plus 6.0. ***P < .001; **P < .01; *P < .05; Data are presented as means ± SD; Error bars = SD. (E) Western blot was performed to analyse the expression of Col I, α‐SMA and Col III between normal dermal tissue and dermal scar at different times
The protein expression of Col I, α‐SMA and Col III was significantly increased in response to anhydrous alcohol (100%, 2 ml/kg) injection. The expression levels of both proteins showed injection time‐dependent effects. However, their expression was decreased in the 90‐day group compared with that in the 60‐day group (Figure 3E). The protein expression levels of Col I, α‐SMA and Col III were consistent with the immunohistochemical results.
These indicators showed that anhydrous alcohol‐induced trauma could serve as a novel, successful and reproducible long‐term animal model for hypertrophic scar studies.
4. DISCUSSION
A number of animal models have been developed to reproduce the various aspects of injury to elucidate the pathophysiology and to explore potential treatment interventions. Understanding the advantages and limitations of these animal models is essential for the design and development of treatments that are clinically relevant to humans. 15 Approximately 30 years ago, Shetlar reported a scar model involving human keloid tissue implantation in athymic mice, 16 and the histological evaluation of this model revealed that the tissue preserved the characteristics of keloids. Some progress has been with the advent of the subcutaneous heterologous graft, as shown by the implantation of full‐thickness human skin grafts on the back of nude mice 17 and the regeneration of human skin on the back of nude mice by transplantation of a cultured bioengineered skin equivalent. 18 However, subcutaneous heterologous grafting does not allow the study of epidermis‐dermis interactions and impedes the evaluation of topical agent efficacy. 11 Moreover, these models exhibit partial suppression of the animal immune system, leading to some conclusions that would be difficult to extrapolate to human disease. 19 The porcine model 3 , 20 is recognized as an excellent model because hypertrophic wound healing progression in this model resembles that observed in humans, and this species has cones 21 , 22 (skin appendages and fat domus) in the skin. However, the experiments are restricted by the large size, high price and difficult feeding and operation of the porcine model.
Anhydrous alcohol can have a direct cytotoxic effect on tissue due to the dehydration and denaturation of proteins, and it will induce a strong inflammatory reaction locally. 23 We hypothesized that injection of anhydrous alcohol would cause skin fibrosis. The rabbit was selected as the model animal of choice because of its low cost, sufficient experimental skin area, and strong anti‐infection characteristic. To determine the optimal injection dose and concentration, we used several doses and performed observations for 90 days. The results showed that 2 ml/kg of 100% anhydrous alcohol were the optimal concentrations.
The most commonly used rabbit scar model in previous studies was the rabbit ear model, which involved the punching or excision of wounds approximately 5‐6 mm in diameter on the ventral surface of rabbit ears. The lesions involved the epidermis, dermis and perichondrium. 14 We compared the characteristics of the anhydrous alcohol model and the rabbit ear model, and the results suggested that it is easier to implement the anhydrous alcohol model. Fibrotic hyperplasia extended over nearly one month, and the scar was remarkable. The long‐term model was beneficial for exploring the mechanism of scar formation.
Hypertrophic scarring is characterized by excessive collagen deposition, which results in a stiff, elevated surface. The fibres are oriented parallel to the epidermal surface, with nodules consisting of myofibroblasts. The histopathologic features of this model reflect the features of human hypertrophic scarring, including parallel collagen fibre orientation, dermal and epidermal thickening, widespread collagen deposition and the loss of dermal adnexal structures.
The accumulation of collagen fibrogenesis and myofibroblasts are key features of the development of excess extracellular matrix and fibrosis. This reflects the importance of the cytoplasmic microfilamentous apparatus, as indicated by the typical pattern of α‐SMA staining. 24 The 15 to 60‐day anhydrous alcohol model revealed a significant increase in the expression of Col I, α‐SMA and Col III, compared with that in rabbit skin of the placebo group.
However, the limitation of this model is that it could not permanently mimic the features of human hypertrophic scarring. Tissue regeneration is more frequently observed in other animals than in humans in nature. Some animals have the ability to regenerate entire organs, including the skin. 10 , 25 Clearly, human skin lacks this ability in general. It instead replaces the tissue defect with scar tissue after trauma. Hair follicles and skin appendages are generally missing in the scar. In our study, the histological results of the 90‐day model showed that when there was a decline in scar formation, the regeneration of appendages could be found. Then, the hair will regenerate completely, the skin will be softened, and the scar will disappear. This phenomenon rarely occurs in hypertrophic scars in humans. On the other hand, this may also provide new clues for scar‐free healing research.
In conclusion, an animal model of hypertrophic scarring can be established by injection of anhydrous alcohol. This model exhibited both the hypertrophic and regression stages, which exhibited the protein and histological features of human hypertrophic scars. This model provides a feasible tool for further research on the pathogenesis of hypertrophic scarring.
CONFLICT OF INTEREST
All authors have no conflict of interest to disclose.
ETHICAL APPROVAL
They were handled according to procedures approved by the Bengbu Medical College Animal Care and Use Committee.
Supporting information
Fig S1
Zu W, Jiang B, Liu H. Establishment of a long‐term hypertrophic scar model by injection of anhydrous alcohol: A rabbit model. Int J Exp Path. 2021;102:105–112. 10.1111/iep.12389
Wenxuan Zu and Banghong Jiang contributed equally to this work
Funding information
This work was supported by Education Department Project of Anhui Province (KJ2019A0345) and National Natural Science Foundation, China (81272100, 81372065).
REFERENCES
- 1. Zhu Z, Ding J, Shankowsky HA, et al. The molecular mechanism of hypertrophic scar. J Cell Commun Sign. 2013;7(4):239‐252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li G, Zhou R, Zhang Q, et al. Fibroproliferative effect of microRNA‐21 in hypertrophic scar derived fibroblasts. Exp Cell Res. 2016;345(1):93‐99. [DOI] [PubMed] [Google Scholar]
- 3. Debruler DM, Blackstone BN, Mcfarland KL, et al. Effect of skin graft thickness on scar development in a porcine burn model. Burns. 2018;44(4):917‐930. [DOI] [PubMed] [Google Scholar]
- 4. Kloeters O, Tandara A, Mustoe TA. Hypertrophic scar model in the rabbit ear: a reproducible model for studying scar tissue behavior with new observations on silicone gel sheeting for scar reduction. Wound Repair Regen. 2008;16(4):582. [DOI] [PubMed] [Google Scholar]
- 5. Wang X, Zhang Y, Jiang BH, et al. Study on the role of Hsa‐miR‐31‐5p in hypertrophic scar formation and the mechanism. Exp Cell Res. 2017;361(2):201‐209. [DOI] [PubMed] [Google Scholar]
- 6. Lee BB, Do YS, Byun HS, et al. Advanced management of venous malformation with ethanol sclerotherapy: mid‐term results. J Vasc Surg. 2003;37(3):533‐538. [DOI] [PubMed] [Google Scholar]
- 7. Bhopale KK, Amer SM, Kaphalia L, et al. Proteomic profiling of liver and plasma in chronic ethanol feeding model of hepatic alcohol dehydrogenase‐deficient deer mice. Alcohol Clin Exp Res. 2017;41(10):1675‐1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Steiner JL, Pruznak AM, Navaratnarajah M, et al. Alcohol differentially alters extracellular matrix and adhesion molecule expression in skeletal muscle and heart. Alcohol Clin Exp Res. 2015;39(8):1330‐1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jiang B, Zu W, Xu J, et al. Botulinum toxin type A relieves sternocleidomastoid muscle fibrosis in congenital muscular torticollis. Int J Biol Macromol. 2018;112:1014‐1020. [DOI] [PubMed] [Google Scholar]
- 10. Seifert AW, Maden M. New insights into vertebrate skin regeneration. Int Rev Cell Mol Biol. 2014;310:129‐169. [DOI] [PubMed] [Google Scholar]
- 11. Ramos ML, Gragnani A, Ferreira LM. Is there an ideal animal model to study hypertrophic scarring? J Burn Care Res. 2008;29(2):363‐368. [DOI] [PubMed] [Google Scholar]
- 12. Draaijers LJ, Tempelman FR, Botman YA, et al. The patient and observer scar assessment scale: a reliable and feasible tool for scar evaluation. Plast Reconstr Surg. 2004;113(7):1960‐1967. [DOI] [PubMed] [Google Scholar]
- 13. Hoogewerf CJ, van Baar ME, Middelkoop E, et al. Patient reported facial scar assessment: directions for the professional. Burns. 2014;40(2):347‐353. [DOI] [PubMed] [Google Scholar]
- 14. Uzun H, Bitik O, Hekimoglu R, et al. Angiotensin‐converting enzyme inhibitor enalapril reduces formation of hypertrophic scars in a rabbit ear wounding model. Plast Reconstr Surg. 2013;132(3):e361‐e371. [DOI] [PubMed] [Google Scholar]
- 15. Abdullahi A, Amini‐Nik S, Jeschke MG. Animal models in burn research. Cell Mol Life Sci. 2014;71(17):3241‐3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shetlar MR, Shetlar CL, Hendricks L, et al. The use of athymic nude mice for the study of human keloids. Proc Soc Exp Biol Med. 1985;179(4):549‐552. [DOI] [PubMed] [Google Scholar]
- 17. Yang DY, Li SR, Wu JL, et al. Establishment of a hypertrophic scar model by transplanting full‐thickness human skin grafts onto the backs of nude mice. Plast Reconstr Surg. 2007;119(1):104‐111. [DOI] [PubMed] [Google Scholar]
- 18. Escámez MJ, García M, Larcher F, et al. An in vivo model of wound healing in genetically modified skin‐humanized mice. J Invest Dermatol. 2004;123(6):1182‐1191. [DOI] [PubMed] [Google Scholar]
- 19. Cameron AM, Adams DH, Greenwood JE, et al. A novel murine model of hypertrophic scarring using subcutaneous infusion of bleomycin. Plast Reconstr Surg. 2014;133(1):69‐78. [DOI] [PubMed] [Google Scholar]
- 20. Rapp SJ, Rumberg A, Visscher M, et al. Establishing a reproducible hypertrophic scar following thermal injury: a porcine model. Plast Reconstr Surg Glob Open. 2015;3(2):e309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Matsumura H, Engrav LH, Gibran NS, et al. Cones of skin occur where hypertrophic scar occurs. Wound Repair Regen. 2001;9(4):269‐277. [DOI] [PubMed] [Google Scholar]
- 22. Zhu KQ, Engrav LH, Gibran NS, et al. The female, red Duroc pig as an animal model of hypertrophic scarring and the potential role of the cones of skin. Burns. 2003;29(7):649‐664. [DOI] [PubMed] [Google Scholar]
- 23. Su L, Fan X, Zheng L, et al. Absolute ethanol sclerotherapy for venous malformations in the face and neck. J Oral Maxillofac Surg. 2010;68(7):1622‐1627. [DOI] [PubMed] [Google Scholar]
- 24. Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol. 2003;200(4):500‐503. [DOI] [PubMed] [Google Scholar]
- 25. Seifert AW, Kiama SG, Seifert MG, et al. Skin shedding and tissue regeneration in African spiny mice (Acomys). Nature. 2012;489(7417):561‐565. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1


