Abstract
A 66‐year‐old man with hypopharyngeal carcinoma with a single bone metastasis was treated with definitive chemo/radiotherapy omitting local treatment of the single bone lesion. He remained relapse‐free for 6 years. We have concluded that radiotherapy‐dependent abscopal effect might have allowed to avoid ablative treatment of metastatic site.
Keywords: abscopal effect, chemotherapy, head and neck cancer, oligometastatic, radiotherapy
A 66‐year‐old man with hypopharyngeal carcinoma with a single bone metastasis was treated with definitive chemo/radiotherapy omitting local treatment of the single bone lesion. He remained relapse‐free for 6 years. We have concluded that radiotherapy‐dependent abscopal effect might have allowed to avoid ablative treatment of metastatic site.
Key Clinical Message.
Treatment of primary disease and metastatic sites improves survival of oligometastatic head and neck cancer patients. Selected cases could benefit from radiotherapy‐dependent abscopal effect avoiding local treatment of metastases.
1. INTRODUCTION
Distant metastasis at presentation is an uncommon event for head and neck squamous cell carcinoma (HNSCC), approximately 10% of stage IV diseases. 1 This condition is classified as stage IV of disease according to American Joint Committee on Cancer (AJCC) TNM Cancer Staging Manual, 8th Edition. 2
The disease presenting with metastases limited in number and location can be defined oligometastatic disease. 3
Oligometastatic HNSCC patients appear to have a better prognosis than those with widespread metastatic involvement. 4
According to national comprehensive cancer network guidelines, the therapeutic options for patients with metastatic disease include locoregional treatment based on primary site and/or combined systemic therapy. 5
Chemotherapy based on cisplatin and fluorouracil (5FU) in combination with cetuximab, a monoclonal antibody directed against the epidermal growth factor receptor (EGFR), has been the standard care for metastatic HNSCC 6 since the advent of immune checkpoint inhibitors (ICIs) which have demonstrated favorable results. 7 Currently, pembrolizumab alone or in combination with platinum and 5‐fluorouracil has indication as first‐line treatment in selected programmed death‐ligand 1 (PD‐L1) positive recurrent or metastatic HNSCC. 8
There are no specific treatment guidelines for oligometastatic HNSCC patients, but retrospective data suggest that an aggressive treatment of the primary disease and definitive local treatment of metastatic sites improves survival outcome. 4 Moreover, an American review of cases suggests that the application of full course of irradiation for HNSCC with synchronous metastases can allow longer survival that abbreviated course. 9
2. CASE REPORT
In February 2014, a 66‐year‐old man referring long‐lasting sore throat and pain when swallowing was evaluated by endoscopic examination with evidence of ulcerative lesion sited in the lateral pharyngeal wall and extended to pharyngoepiglottic fold. The biopsy described high‐grade squamous cell carcinoma, human papillomavirus (HPV)‐16, and Epstein‐Bar virus (EBV) status negative, p53 hyper‐expressed.
Computed tomography (CT) scan of soft tissue of the neck, chest, and abdomen showed a voluminous mass on the left side of the hypopharynx and multiple pathological left cervical lymph nodes (Figure 1A).
FIGURE 1.
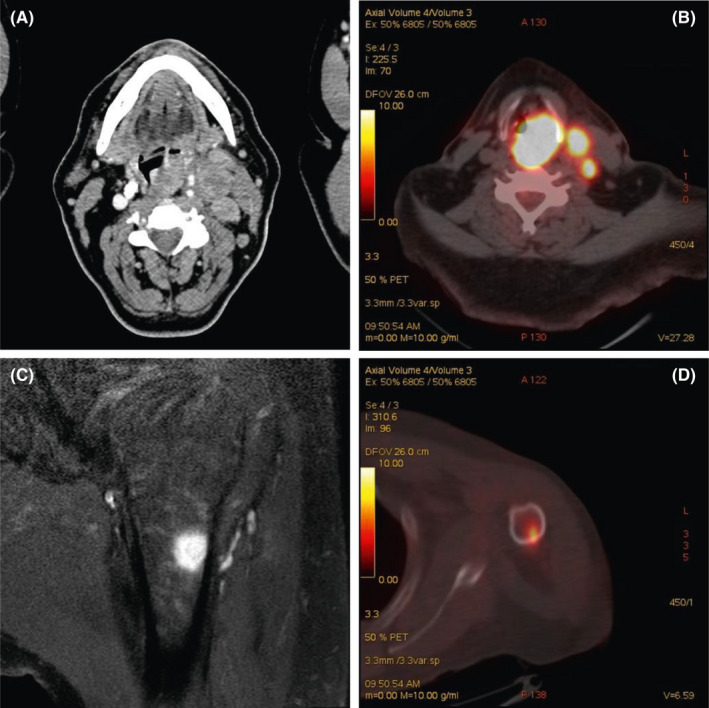
A: Computed tomography (CT) scan (laryngeal windows) of the patient after administration of intravenous contrast media. Axial CT image showing solid lesion with homogeneous contrast enhancement at level of hypopharynx, on the left side, associated with metastatic lymph nodes (level III A). B: PET‐CT demonstrate intense uptake of the lesion and lymph nodes. C: Magnetic resonance showing a round‐shape lesion in the humerus with hypointense signal on T1‐weighted sequences. D: PET‐CT showing intense uptake of the lesion in the humerus
Cancer staging 18‐Fluorodeoxyglucose (FDG)‐positron emission tomography (PET) scan confirmed locally advanced hypopharyngeal lesion (Figure 1B), with even an increased FDG intake in the proximal diaphysis of the left humerus (Figure 1D). A subsequent left arm resonance imaging (MRI) showed a round‐shape lesion in the proximal diaphysis of humerus (Figure 1C).
In order to confirm metastatic nature of the arm lesion excluding its primary origin or its benign nature, we suggested to the patient to undergo a CT‐guided biopsy, but the patient refused.
We finally classified the disease as cT4b cN2b M1, according to American Joint Committee on Cancer (AJCC) TNM Cancer Staging Manual, 7th Edition, 10 and we opted for a standard treatment for stage IVB hypopharynx cancer as a preservative strategy, considering it an oligometastatic disease: induction chemotherapy (IC) followed by radiotherapy (IC + RT). 11
From March 2014 to May 2014 the patient received three courses of chemotherapy based on docetaxel 75 mg/mq day (D) 1, cisplatin 75 mg /mq (D1), and 5‐fluorouracil 750 mg/mq in 96 hours continuous infusion starting from D1 (TPF regimen).
CT scan evaluation after induction therapy showed a partial response of the hypopharyngeal lesion (not shown) and MRI of the humerus a reduction of the lesion size (not shown). From a clinical point of view, patient was completely pain free.
Considering the clinical benefit and radiological partial response both on the primary and on the metastatic site, we decided to employ locoregional radiotherapy alone.
Total amount of radiotherapy was 70 Gray (Gy) on the oropharynx, rhinopharynx, hypopharynx, larynx, bilateral jugular‐digastric lymph nodes; 40 Gy on the cervical and V level lymph nodes.
CT and PET scans performed after 2 months from the end of radiotherapy showed, respectively, an ulterior response at primary site (Figure 2A) and absence of pathological uptake both on primary (Figure 2B) and single metastatic site (Figure 2D), left arm MRI confirmed complete resolution of the single bone lesion (Figure 2C).
FIGURE 2.
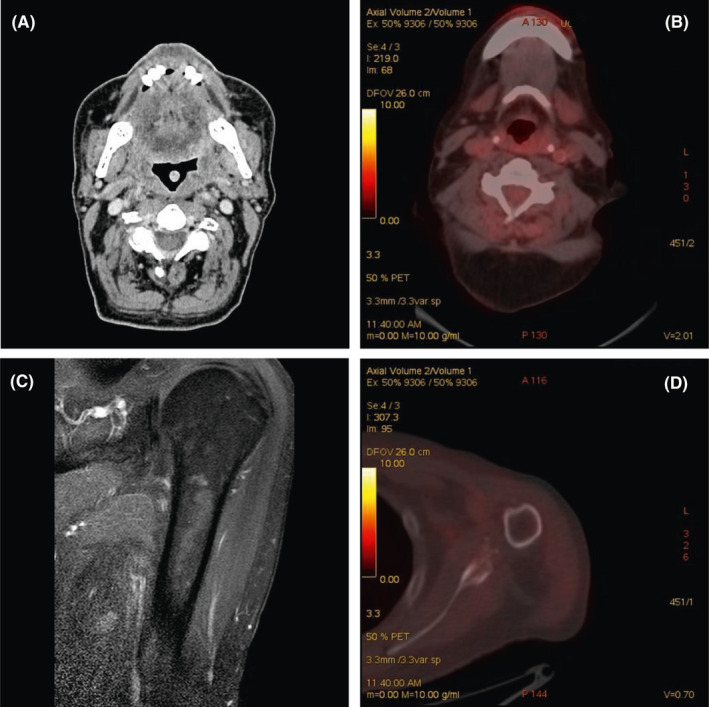
A: Computed tomography (CT) scan (laryngeal windows) of the patient after administration of intravenous contrast media. Axial CT image showing an almost complete resolution of the solid lesion and complete lymph nodal response. B: PET‐CT showing complete response after therapy in head and neck district. C: Magnetic resonance after therapy showing complete resolution of the lesion. D: PET‐CT showing complete response after therapy in the humerus
On the basis of the radiological response, in absence of symptoms, we decided to not employ an ablative metastatic‐directed treatment, reserving it in case of pain or radiological progression. Thus, we planned regular radiological and clinical follow up.
The patient remained relapse‐free for 6 years with no grade 2‐3 adverse events reported; then, he had a local relapse and died for complications.
3. DISCUSSION
Metastatic disease ranges from disseminated to oligometastatic disease and this presentation can occur since the onset of the disease (synchronous metastases) or at the time of recurrence (metachronous metastases). Oligometastatic disease implies notions of relative low kinetics of progression and organotropism, and there are evidence that oligometastatic state can be treated “more curatively” with aggressive treatment of primary disease than with systemic treatment. 12
This assumption has relevance in HNSCC because it frequently affects frail patients with poor performance status, concomitant diseases, and malnutrition in whom the possibility to avoid toxicities of long‐lasting polychemotherapy results is particularly important.
Referring to our clinical case, we considered the single bone lesion as a secondary localization of HNSCC because both locally advanced hypopharyngeal lesion and humerus showed similar high FDG intake suggesting the same nature and MRI showed an area of abnormal signal strongly suggestive of metastatic localization.
We decided to use TPF induction chemotherapy 13 followed by radiation‐based locoregional treatment (ie, IC + RT), 11 the standard treatment for stage IVB hypopharynx cancer. To note, the role of neoadjuvant therapy in the management of locally advanced head and neck cancer is still matter of debate: A clear advantage in survival has not been shown yet except for the nasopharyngeal carcinoma. 14 In addition, this option could give us the possibility of changing strategy at time of reassessment depending on response of disease.
Following international guidelines, after obtaining partial response of the disease, we recommended locoregional radiotherapy alone.
The radiological evaluation after treatment completion showed a dramatic tumor response at distance from the irradiated volume, a rare event known as “abscopal effect.”
The first in vivo evidence of “abscopal effect” occurred in 1908, 15 in 1953 Mole used for the first time the wards “abscopal effect”, 16 and over years, sporadic abscopal effects were described in clinical cases, most of them regarding renal cell carcinoma, melanoma and lymphoma, traditional considered immunogenic. 17
The immune system stimulation has been proposed to be the driver of the abscopal effect: preclinical data show that radiotherapy induces in tumor cells DNA accumulation in the cytosol and it actives a cascade of intracellular events bringing to interferon secretion from cancer cells and consequent dendritic cell recruitment and activation, essential for priming T cells 18 , 19 In conclusion, radiotherapy acts as an in situ vaccine converting tumor cells into a site for priming of tumor‐specific T cells. 19
HNSCC cells produce immunosuppressive mediators evading the host immune system and promoting metastasis, 20 so it is unclear whether the immunogenicity of HNSCC makes this cancer really suitable to engage the abscopal effect.
In our patient, the radiotherapy‐dependent abscopal effect has allowed to obtain an unusual long‐lasting response even in the absence of metastasis‐directed treatment.
There is in vivo evidence that p53‐dependent signals might be responsible for the abscopal effect in model system, via a pro‐apoptotic pathway. 21
In our clinical case, we treated a patient with p53 hyper‐expressed HNSCC, that is the immunohistochemical expression of mutations of TP53 gene. 22
We can conclude that other biological features of the disease rather than p53 wild type, the site of origin of the neoplasia, the number and localization of metastases, and patient's characteristics (including his efficient immune system), might have been involved in the achievement of radiotherapy‐dependent abscopal effect.
4. CONCLUSION
Oligometastatic head and neck tumors display a well‐known better prognosis than those with widespread metastases.
There is evidence that aggressive treatment of the primitive lesion associated with treatment of metastatic sites improves survival without effects of long‐lasting polychemotherapy.
Our clinical case suggests the possibility, in selected cases of HNSCC, to use radiotherapy‐dependent abscopal effect of aggressive treatment of primitive site to avoid ablative treatment of metastases. Patients selection criteria for this therapeutic approach could be represented by number of metastases and organs involved and biological features of the disease.
Because “abscopal effect” is a rare condition, methods to increase its prevalence are being studied. One of these is combining radiotherapy with immunotherapy in order to obtain a synergic effect.
Recently, a maxillary sinus cancer patient treated with immunotherapy and radiotherapy achieved a dramatic response of disease, underlying the possibility of obtaining “abscopal effect” in head and neck tumor. 23
At the present time, prospective randomized studies are necessary to define the better approach to oligometastatic disease. It is essential to select patients for systemic treatment alone, combination of systemic and ablative metastases‐directed treatments, aggressive treatment of primary combined to ablative metastases‐directed treatments or aggressive treatment of primary site alone (including evaluation of combination of radio/immunotherapy).
CONFLICT OF INTEREST
No author has any conflict of interest.
AUTHOR CONTRIBUTIONS
All authors have contributed substantially to the work. In particular:
Giulia Mazzaschi, MD1, wrote the paper.
Chiara Tommasi, MD1, wrote the paper.
Elisabetta Pietri MD1, wrote the paper.
Luigi Corcione, MD2, performed the pathology analysis.
Annamaria De Giorgi MD3, performed a critical revision.
Paola Bini, MD4, performed the literature review.
Simona Bui, MD1, conceived the paper.
All authors have approved the submitted version.
ACKNOWLEDGMENTS
We don't have to list contributions from anyone who does not meet the criteria for authorship
Mazzaschi G, Tommasi C, Pietri E, et al. Abscopal effect as part of treatment of oligometastatic head and neck cancer: A case report. Clin Case Rep. 2021;9:1334–1338. 10.1002/ccr3.3758
DATA AVAILABILITY STATEMENT
Data are available on request from the authors.
REFERENCES
- 1. Marur S, Forastiere AA. Head and neck cancer: Changing epidemiology, diagnosis, and treatment. Mayo Clin. Proc. 2008;83:489‐501. [DOI] [PubMed] [Google Scholar]
- 2. Lydiatt WM, Patel SG, O'Sullivan B, et al. Head and neck cancers‐major changes in the American Joint Committee on cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67(2):122‐137. 10.3322/caac.21389 [DOI] [PubMed] [Google Scholar]
- 3. Guckenberger M, Lievens Y, Bouma AB, et al. Characterisation and classification of oligometastatic disease: a European Society for Radiotherapy and Oncology and European Organisation for Research and Treatment of Cancer consensus recommendation. Lancet Oncol. 2020;21:e18‐e28. [DOI] [PubMed] [Google Scholar]
- 4. Vengaloor Thomas T, Packianathan S, Bhanat E, et al. Oligometastatic head and neck cancer: Comprehensive review. Head Neck. 2020;42:2194‐2201. [DOI] [PubMed] [Google Scholar]
- 5. Pfister DG, Spencer S, Adelstein D, et al. Head and neck cancers, version 2.2020, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Canc. Netw. 2020;18(2):873‐878. 10.6004/jnccn.2020.0031 [DOI] [PubMed] [Google Scholar]
- 6. Vermorken JB, Mesia R, Rivera F, et al. Platinum‐Based Chemotherapy plus Cetuximab in Head and Neck Cancer A bs tr ac t. N Engl J Med, vol. 359 www.nejm.org (2008) [DOI] [PubMed] [Google Scholar]
- 7. Cohen EEW, Bryan Bell R, Bifulco CB, et al. The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of squamous cell carcinoma of the head and neck (HNSCC). J. Immunother. Cancer. 2019;7:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Burtness B, Harrington KJ, Greil R, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE‐048): a randomised, open‐label, phase 3 study. Lancet. 2019;394:1915‐1928. [DOI] [PubMed] [Google Scholar]
- 9. Ampil FL, Kim DD, Ghali GE, Baluna RG. How intensive should radiotherapy for head and neck cancer with synchronous distant metastases be? Review of cases. J. Oral Maxillofac. Surg. 2012;70:730‐733. [DOI] [PubMed] [Google Scholar]
- 10. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th Edition of the AJCC Cancer Staging Manual and the Future of TNM. Ann Surg Oncol. 2010;17(6):1471‐1474. 10.1245/s10434-010-0985-4 [DOI] [PubMed] [Google Scholar]
- 11. Dietz A, Wichmann G, Kuhnt T, et al. Induction chemotherapy (IC) followed by radiotherapy (RT) versus cetuximab plus IC and RT in advanced laryngeal/hypopharyngeal cancer resectable only by total laryngectomy—final results of the larynx organ preservation trial DeLOS‐II. Ann. Oncol. 2018;29:2105‐2114. [DOI] [PubMed] [Google Scholar]
- 12. Florescu C, Thariat J. Local ablative treatments of oligometastases from head and neck carcinomas. Critical Reviews in Oncology/Hematology. 2014;91:47‐63. [DOI] [PubMed] [Google Scholar]
- 13. Vermorken JB, Remenar E, Herpen C, et al. Cisplatin, Fluorouracil, and Docetaxel in Unresectable Head and Neck Cancer A bs t r ac t. www.nejm.org (2007). [DOI] [PubMed] [Google Scholar]
- 14. Zhang Y, Chen L, Hu G‐Q, et al. Gemcitabine and Cisplatin Induction Chemotherapy in Nasopharyngeal Carcinoma. N. Engl. J. Med. 2019;381:1124‐1135. [DOI] [PubMed] [Google Scholar]
- 15. Mc Culloch HD. On the analogy between spontaneous recoveries from cancer and the specific immunity induced by X ray irradiations of the lymphatic glands involved. Br. Med. J. 1908;2:1146‐1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mole, RH Whole body irradiation; radiobiology or medicine? Br. J. Radiol. 1953;26:234‐241. [DOI] [PubMed] [Google Scholar]
- 17. Abuodeh Y, Venkat P, Kim S. Systematic review of case reports on the abscopal effect. Curr. Probl. Cancer. 2016;40:25‐37. [DOI] [PubMed] [Google Scholar]
- 18. Lhuillier C, Rudqvist NP, Elemento O, Formenti SC, Demaria S. Radiation therapy and anti‐tumor immunity: Exposing immunogenic mutations to the immune system. Genome Med. 2019;11:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tugce Yilmaz M, Elmali A, Yazici G, Yilmaz MT. Abscopal Effect, From Myth to Reality: From Radiation Oncologists’ Perspective. Cureus. 2019; 10.7759/cureus.3860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ferris RL. Immunology and Immunotherapy of Head and Neck Cancer. J Clin Oncol. 2015;33:3293‐3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Strigari L, Mancuso M, Ubertini V, et al. Abscopal effect of radiation therapy: Interplay between radiation dose and p53 status. Int. J. Radiat. Biol. 2014;90:248‐255. [DOI] [PubMed] [Google Scholar]
- 22. Hashmi AA, Hussain ZF, Hashmi SK, et al. Immunohistochemical over expression of p53 in head and neck Squamous cell carcinoma: clinical and prognostic significance. BMC Res. Notes. 2018;11:433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yazici G, Gullu I, Cengiz M, et al. The Synergistic Effect of Immune Checkpoint Blockade and Radiotherapy in Recurrent/Metastatic Sinonasal Cancer. Cureus. 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on request from the authors.


