Abstract
Open heart transcatheter mitral valve implantation using the Edwards‐Sapien 3 for mitral annular calcification is a safe procedure, because surgeons do not have to decalcify. And also, surgeons can resect the anterior mitral leaflet to prevent left ventricular outflow tract obstruction and deploy the valve under direct visualization.
Keywords: left ventricular outflow tract obstruction, mitral annular calcification, mitral valve replacement, sapien 3, transatrial approach, transcatheter mitral valve implantation
Open heart transcatheter mitral valve implantation using the Edwards‐Sapien 3 for mitral annular calcification is a safe procedure, because surgeons do not have to decalcify. And also, surgeons can resect the anterior mitral leaflet to prevent left ventricular outflow tract obstruction and deploy the valve under direct visualization.

1. INTRODUCTION
Transcatheter mitral valve implantation during an open heart mitral valve surgery with mitral annular calcification using a transcatheter aortic valve implantation prosthesis is a new procedure. Surgeons can resect the anterior mitral leaflet to prevent left ventricular outflow tract obstruction and deploy the valve under direct visualization.
Open heart surgical use of a transcatheter aortic valve implantation (TAVI) prosthesis for mitral stenosis (MS) with severe MAC appears to decrease the risk of atrio‐ventricular disruption following decalcification and circumflex artery injury and simplifies the procedure. 1 , 2 And this procedure has no risk of systolic anterior motion of the anterior mitral valve leaflet (AML) compared to percutaneous transcatheter mitral valve implantation (TMVI), because the AML is resected in open surgery. A potential problem with this procedure that should be addressed is a left ventricular outflow tract (LVOT) obstruction by the prosthesis, especially by the felt strip at the prosthesis. 1 This problem may especially occur when preoperative LVOT is reduced by myocardial hypertrophy, which is usually observed when an associated aortic stenosis (AS) is present.
2. CASE
A 74‐year‐old, 65‐kg, 174‐cm man with a history of hypertension, dyslipidemia, nephropathy due to type 2 diabetes, and emphysema presented with New York Heart Association class Ⅱ due to AS and MS.
Echocardiography showed severe MS with a mean pressure gradient (PG) of 12 mmHg and a mitral valve (MV) area of 0.99 cm2 (Figure 1A). The left ventricle (LV) was hypertrophic, and the LV ejection fraction was 70%. AS was also severe, and the aortic valve (AV) area was 0.74 cm2. MAC was severe as shown on computed tomography (CT) (Figure 1B). The preoperative aortomitral angle was 110゜ (Figure 1C). The width of the preoperative LVOT in the direction of placing the TAVI prosthesis was 20.3 mm (Figure 1D). The extra space after performing TMVI becomes neo‐LVOT. We can estimate postprocedural LVOT obstruction taking measurement of neo‐LVOT at the baseline CT scan.
FIGURE 1.
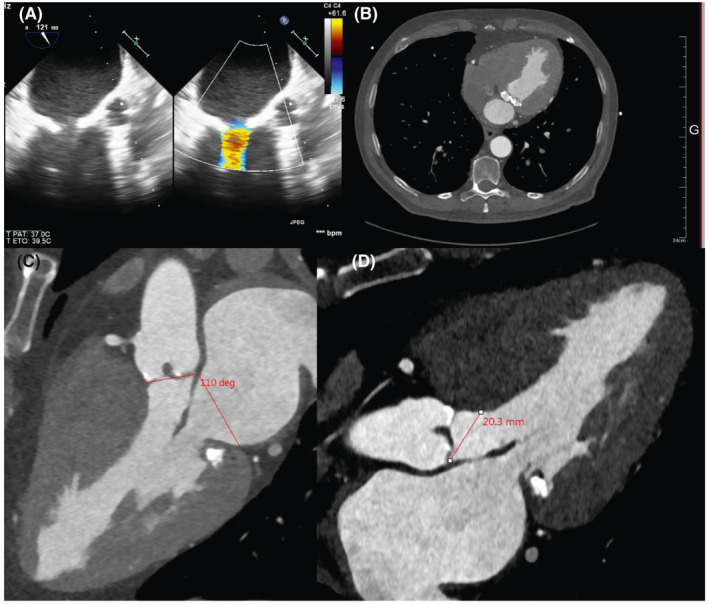
A, Preoperative transesophageal echocardiography showed calcified MV and MAC with turbulent flow at the MV in systole. B, Preoperative CT showed MAC. C, Aortomitral angle was 110゜. D, Preoperative LVOT. The measurement is parallel to the TAVI prosthesis
After a median sternotomy on cardiopulmonary bypass with cardioplegic cardiac arrest, the calcified AV was resected through an aortotomy. The aortic annulus was small, and a 19‐mm AVALUS valve sizer (Medtronic, Minneapolis, MN, USA) was suitable. The left atrium was opened parallel to the interatrial groove. We resected the A2 portion of the valve to prevent anterior MV protrusion in the LVOT and secondary LVOT obstruction. We sewed anchor sutures around the mitral annulus (2‐0 coated braided polyester sutures with pledgets) in places where needles exhibited no resistance around or across calcifications. These sutures were used to fix the final position of the TAVI prosthesis once inflated. We measured the mitral annulus using valve sizers of mitral mechanical valve, and we chose a valve that was exactly the same size as the annulus. As previously described, 2 after washing a 26‐mm Sapien‐Edwards AV (Edwards Lifesciences, Inc), felt strip was sewn to the Sapien to cover the polyethylene terephthalate portion (Figure 2A). The prosthesis was mounted upside down on the short delivery catheter. A Safari XS (Boston Scientific) was passed into the catheter to avoid injury of the LV. The balloon was inflated, and the valve was deployed under direct visualization (Figure 2B). During that step, it was necessary to assess the position of the A1 and A2 parts of the mitral annulus on the felt strip to reduce the amount of covered stent into the LVOT. Usually, the width of the felt strip is approximately 1 cm and a residual 5‐6 mm width in the atrial side is likely to avoid LVOT obstruction. Then, the valve was fixed in its position by passing the previous anchor sutures across the bottom of the Sapien and tied with the Corknot device (LSI Solutions) to prevent valve migration. These sutures, in our opinion, also reduced paravalvular leaks (PVL) (Figure 2C). After we closed the left atrium, we replaced the AV with a 19‐mm AVALUS (Medtronic). Visualization through the aortic annulus confirmed that only a limited part of the felt strip was positioned into the LVOT; thus, obstruction of the LVOT was not likely to occur (Figure 2D). After we closed the aorta, the cross‐clamp was removed after 96 min. While weaning from cardiopulmonary bypass, we temporarily stopped the bypass flow and checked the PG between the LV (109/12 mm Hg) (needle through the ventricular wall) and the ascending aorta (94/56 mm Hg).
FIGURE 2.

A, Felt strip sewn to the Sapien to cover the polyethylene terephthalate portion. B, Valve implantation. C, After the valve implantation, anchor sutures were sewn to the felt strip and tied thereafter. D, Visualization via the aortotomy enabled us to view the Sapien at the mitral position, and there was no obstruction at the LVOT
Transthoracic echocardiography 1 year postoperatively showed smooth opening of the Sapien at mitral position (Figure 3A). There is no PVL at the mitral prosthesis, and transmitral pressure gradient is 3 mm Hg (Figure 3B). There is no PVL at the aortic valve, and mean transaortic pressure gradient is 22 mm Hg. There is no significant LVOT obstruction.
FIGURE 3.

A, Valve opening of the Sapien at mitral position is smooth. B, There is no PVL at the mitral prosthesis, and transmitral pressure gradient is 3 mm Hg
3. DISCUSSION
Mitral valve replacement for mitral valve disease with MAC is sometimes a challenging operation because surgeons must try to decalcify MAC to secure a soft annulus, which they can suture. Alain et al 3 reported en bloc decalcification of MAC and reconstruction of the annulus with either valve repair or replacement. In that report, they performed valve repair in 67 patients and valve replacement in 1 patient, and they experienced no left ventricular rupture; the freedom from all valve‐related complications at 9 years was 85.1%. 3 However, this method seems to require high skill and experience.
Mayra et al 1 reported a multicenter global registry involving the acceptable outcomes of early experience with TMVI in patients with MAC. 1 However, the 30‐day and 1‐year all‐cause mortality rates were 25% and 53.7%, respectively. It was concluded that LVOT obstruction is the most important and independent predictor of 30‐day and 1‐year mortality. 4
Although cross‐clamping and cardioplegia were required, this technique allows precise assessment of positioning along A1 and A2 of the covered part of the TAVI and resection of the A2 portion of the valve. Indeed, these two points are advantages in avoiding LVOT obstruction compared to percutaneous TMVI. John‐Peder et al 5 reported a case of LVOT obstruction after transapical TMVI, and the patient had to undergo additional surgery to resect the A1‐A2‐A3 parts of the MV. Robin et al 6 reported that aortomitral angle <120゜ is a predictor of LVOT obstruction after mitral valve replacement. In this case, aortomitral angle is 110゜, so the risk of LVOT obstruction was high. This open heart TMVI was suitable for this case in that it could prevent LVOT obstruction.
In percutaneous TMVI, a laceration of the AML may be performed to prevent LVOT obstruction (LAMPOON procedure). Vasilis et al 7 reported five successful cases of patients at risk for LVOT obstruction who underwent the LAMPOON procedure and percutaneous TMVI using the Sapien 3; however, this technique does not appear to be appropriate for calcified AML.
The mean pressure gradient measured by echocardiography after AV replacement using AVALUS is between 10 and 15 mm Hg, so this patient's peak to peak PG at aortic valve was acceptable. 8
Patients with AS and mitral disease associated with MAC can be treated with associated classical AV replacement and transatrial open heart TMVI. This procedure for mitral valve disease is not a standard procedure, but if the calcification at mitral anulus is significant and the risk of left ventricular rupture due to decalcification is high, this procedure is a good choice. During balloon inflation, this approach allows a very good assessment of the width of the covered TAVI prosthesis positioning into the LVOT and might play a major role in reducing the risk of LVOT obstruction. However, long‐term durability of this valve in mitral position is not clear, so more experience is needed to confirm this preliminary result.
CONFLICT OF INTEREST
Dr L. Leroux is a proctor for Edwards Lifescience. The other authors declare that there are no conflicts of interest.
AUTHOR CONTRIBUTIONS
Shohei Morita: wrote this paper. Shinya Takahashi: edited and reviewed the article. Mathieu Pernot: treated the patient. Lionel Leroux: treated the patient and collected the data of the patient. Louis Labrousse: treated the patient and collected the data of the patient. He is the supervisor. All authors: read and approved the final manuscript.
CONSENT FOR PUBLICATION
The patient consented to the reporting of this case in a scientific publication.
ACKNOWLEDGMENTS
Published with written consent of the patient.
Morita S, Takahashi S, Pernot M, Leroux L, Labrousse L. Open heart mitral valve replacement using the Edwards‐Sapien 3 for severe mitral annular calcification prevents left ventricular outflow tract obstruction. Clin Case Rep. 2021;9:1424–1427. 10.1002/ccr3.3796
DATA AVAILABILITY STATEMENT
The data of the patient can be found in Haut Leveque Hospital in Bordeaux.
REFERENCES
- 1. Guerrero M, Dvir D, Himbert D, et al. Transcatheter mitral valve replacement in native mitral valve disease with severe mitral annular calcification: Results from the first multicenter global registry. J Am Coll Cardiol Intv. 2016;9:1361‐1371. [DOI] [PubMed] [Google Scholar]
- 2. Lee R, Fukuhara S, George I, Borger MA. Mitral valve replacement with a transcatheter valve in the setting of severe mitral annular calcification. J Thorac Cardiovasc Surg. 2016;151:e47‐e49. [DOI] [PubMed] [Google Scholar]
- 3. Alain FC, Michel P, Jean FF, John YMR. Extensive calcification of the mitral valve annulus: pathology and surgical management. J Thorac Cardiovasc Surg. 1996;111:718‐730. [DOI] [PubMed] [Google Scholar]
- 4. Guerrero M, Urena M, Himbert D, et al. 1‐year outcomes of transcatheter mitral valve replacement in patients with severe mitral annular calcification. J Am Coll Cardiol. 2018;71:1841‐1853. [DOI] [PubMed] [Google Scholar]
- 5. Kvitting JPE, Nielsen NE, Vanhanen I, Baranowski J. Surgical management of outflow tract obstruction after transapical mitral valve implantation. J Card Surg. 2018;33:545‐547. [DOI] [PubMed] [Google Scholar]
- 6. Varghese R, Itagaki S, Anyanwu AC, Trigo P, Fischer G, Adams DH. Predicting systolic anterior motion after mitral valve reconstruction: using intraoperative transoesophageal echocardiography to identify those at greatest risk. Eur J Cardiothorac Surg. 2014;45:132‐138. [DOI] [PubMed] [Google Scholar]
- 7. Babaliaros VC, Greenbaum AB, Khan JM, Rogers T, Wang DD, Eng MH. Intentional percutaneous laceration of the anterior mitral leaflet to prevent outflow obstruction during transcatheter mitral valve replacement. JACC Cardiovasc Interv. 2017;10:798‐809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Klautz RJM, Kappetein AP, Lange R, Dagenais F, Labrousse L, Bapat V. Safety, effectiveness and haemodynamic performance of a new stented aortic valve bioprosthesis. Eur J Cardiothorac Surg. 2017;52:425‐431. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data of the patient can be found in Haut Leveque Hospital in Bordeaux.


