Key Clinical Message
Abnormal acid‐base status (metabolic acidosis or alkalosis), inappropriate urine electrolytes excretion (high or low Na+ and Cl−), and higher required dose of potassium supplement (4‐5 mmol/kg) are suggestive of non‐TPP causes of hypokalemia.
Keywords: acid‐base, electrolytes, hyperthyroidism, hypokalemia, paralysis, urine
Hypokalemic paralysis in hyperthyroidism does not always make it thyrotoxic periodic paralysis. We describe two young patients with known hyperthyroidism‐manifested sudden onset of muscle paralysis and profound hypokalemia. A rapid assessment of blood acid‐base status and timely spot urine electrolytes excretion uncovered the underlying causes of hypokalemia.

1. INTRODUCTION
Muscle weakness to paralysis caused by hypokalemia is called hypokalemic paralysis, a life‐threatening but reversible emergency. It can be divided into two groups: one is hypokalemic periodic paralysis (HypoPP) due to an acute shift of potassium (K+) into cells and the other non‐HypoPP resulting from a large body deficit of K+ related to gastrointestinal, adrenal, and renal tubular disorders. 1 The most common etiologies in HypoPP are familial periodic paralysis in Western countries and nonfamilial thyrotoxic periodic paralysis (TPP) characterized by a triad of hyperthyroidism, muscle paralysis, and acute hypokalemia in Asia. 2 Due to globalization, TPP has been increasingly reported in Western countries. Activation of Na+‐K+‐ATPase together with reduced K+ efflux in the skeletal muscle may be a mechanism for TPP. 3 , 4 , 5 , 6 , 7 Unlike high dose of K+ supplement required in non‐HypoPP, K+ dose should be minimal in HypoPP to avoid rebound hyperkalemia on recovery. 8
The presence of hyperthyroidism and severe hypokalemia is often said to be the signature of TPP. However, patients with hyperthyroidism may have chronic hypokalemia of divert causes such as use of diuretics, laxatives, or concurrent renal tubular disorders. 9 , 10 , 11 These nonthyroid causes of hypokalemia are often misdiagnosed as TTP with improper management especially when severe hypokalemia with paralysis is the primary presentation. In this report, we describe two young patients with hyperthyroidism‐manifested profound hypokalemia and paralysis due to surreptitious use of loop diuretics and Gitelman's syndrome rather than TPP to illustrate the importance of the assessment of blood acid‐base and spot urine electrolytes.
2. CASE REPORTS
2.1. Case 1
A 24‐year‐old Taiwanese male presented with sudden onset of bilateral legs paralysis. He had primary hyperthyroidism due to Graves’ disease on carbimazole 10 mg thrice daily. He reported having carbohydrate‐rich meal ingestion prior to this presentation. His blood pressure was 124/58 mmHg, pulse rate 98 beats/min regularly, respiratory rate 19 breaths/min, and temperature 36.1°C. The thyroid gland was enlarged. A neurologic examination revealed symmetrical areflexia and total paralysis of the lower extremities. The major laboratory findings were marked hypokalemia and hypochloremic metabolic alkalosis (Table 1). Electrocardiography revealed first‐degree atrioventricular block and prominent U waves. He was presumptively diagnosed with TPP, and potassium chloride (KCl) at a rate of 10 mmol/h was administered. However, spot urine biochemistry showed low urinary K+, sodium (Na+), chloride (Cl−), and divalent cation excretions in favor of K+ deficit rather than shift into cells (Figure 1). After a total of KCl supplementation 250 mmol (rate 20‐30 mmol/h), muscle strength recovered at plasma K+ concentration 2.6 mmol/L. Upon questioning the drug history, he confessed the covert use of furosemide 120 mg daily to keep his body image. Despite the controlled hyperthyroidism, he developed hypokalemic paralysis again due to diuretics three months later.
TABLE 1.
Biochemical studies
| Normal range | Case 1 | Case 2 | |
|---|---|---|---|
| Plasma | |||
| Sodium | (136‐145 mmol/L) | 139 | 136 |
| Potassium | (3.5‐5.1 mmol/L) | 1.7 a | 1.8 a |
| Chloride | (98‐107 mmol/L) | 96 a | 94 a |
| Total calcium | (2.2‐2.6 mmol/L) | 2.4 | 2.5 |
| Phosphate | (0.8‐1.4 mmol/L) | 1.3 | 1.6 |
| Magnesium | (0.6‐1.0 mmol/L) | 0.8 | 0.5 a |
| pH | (7.350‐7.450) | 7.466 a | 7.461 a |
| PCO2 | (35.0‐45.0 mmHg) | 40.1 | 41.8 |
| Bicarbonate | (23‐25 mmol/L) | 28.3 a | 28.7 a |
| BUN | (2.9‐8.9 mmol/L) | 4.6 | 3.6 |
| Creatinine | (44‐80 μmol/L) | 62 | 44 |
| TSH | (0.25‐5.0 μIU/mL) | <0.03 a | 0.05 a |
| Free T4 | (0.8‐2.0 ng/dL) | 4.63 a | 3.27 a |
| Urine | |||
| Sodium | (mmol/L) | 34 | 161 |
| Chloride | (mmol/L) | 52 | 159 |
| Creatinine | (mmol/L) | 8.4 | 12.8 |
| Potassium/creatinine | (<2 mmol/mmol) | 1.02 | 10.1 a |
| TTKG | (<3‐4) | 2.8 | 30.1 a |
| Calcium/creatinine | (0.1‐0.3 mmol/mmol) | 0.18 | 0.09 a |
| FEphosphate | (%) | 2.7 | 12.2 a |
| FEmagnesium | (%) | 0.9 | 14.1 a |
Abbreviations: BUN, blood urea nitrogen; FEmagnesium, fractional excretion of magnesium; FEphosphate, fractional excretion of phosphate; TSH, thyroid‐stimulating hormone; TTKG, transtubular potassium gradient.
Denotes an abnormal value.
FIGURE 1.
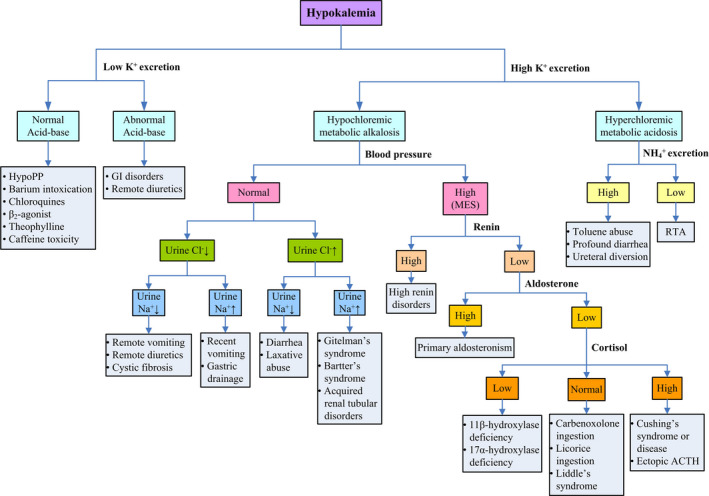
Algorithm for the investigation of hypokalemic patients. (Abbreviation: ACTH, Adrenocorticotropic hormone; Cl−, chloride; GI, gastrointestinal; HypoPP, hypokalemic periodic paralysis; K+, potassium; Na+, sodium; NH4 +, ammonium; MES, mineralocorticoid excess state; RTA, renal tubular acidosis)
2.2. Case 2
A 22‐year‐old Taiwanese female presented with generalized muscle paralysis and inability to ambulate in the morning. She had the history of primary hyperthyroidism due to Graves’ disease treated with propylthiouracil 50 mg thrice daily and experienced intermittent muscle weakness and paresthesia of both hands since senior high school. Her blood pressure was 115/53 mmHg and pulse rate 109 beats/min. The thyroid gland was enlarged. Muscle strength was markedly reduced, and deep tendon reflex was absent. The laboratory findings were notable for severe hypokalemia and hypochloremic metabolic alkalosis (Table 1). An electrocardiogram revealed sinus tachycardia and U waves. A presumptive diagnosis of TPP was made, and she was treated with KCl supplement at a rate of 10 mmol/h However, spot urine biochemistry showed high urinary K+, Na+, and Cl− excretions in favor of K+ deficit (Figure 1). With KCl supplementation 320 mmol (rate 20‐30 mmol/h), recovery of muscle strength was achieved at plasma K+ concentration 2.5 mmol/L. She was also found to have hypomagnesemia (0.8 mmol/L) with renal magnesium wasting and hypocalciuria, consistent with Gitelman's syndrome. Molecular analysis confirmed compound heterozygous mutation in SLC12A3 (R83Q and c.1670‐191C→T) (Figure 2). Aside from the control of her hyperthyroidism, she was put on higher dose of oral KCl (1‐1.5 mmol/kg/d) and magnesium oxide (1 gm/day) without recurrent muscle paralysis.
FIGURE 2.

Molecular analysis confirmed compound heterozygous mutation in SLC12A3 (R83Q and c.1670‐191C→T)
3. DISCUSSION
These two young patients with hyperthyroidism‐manifested sudden onset of muscle paralysis associated with profound hypokalemia and were initially diagnosed to have TPP treated with low dose of KCl. However, both of them had obvious metabolic alkalosis in the blood rather than relatively normal acid‐base status observed in TPP. The timely spot urine clearly showed low urinary K+, Na+, and Cl− excretions in one and high urinary K+, Na+, and Cl− excretions the other, contrary to the findings of low urinary K+ but normal Na+ and Cl− in TPP (Table 2). A much higher dose of KCl (4‐5 mmol/kg) was needed to achieve muscle recovery in them. 12 Their causes of hypokalemia were secondary to the surreptitious use of diuretics and Gitelman's syndrome, respectively.
TABLE 2.
Differentiation between TPP and non‐TPP in hyperthyroidism
| TPP (potassium shift) | Non‐TPP (potassium deficit) | |
|---|---|---|
| Age | 20‐30 years old | Variable |
| Gender | Male predominance | Variable |
| Family history | No periodic paralysis | Variable |
| Muscle weakness | Recurrent & transient | Newly develop |
| Renal potassium excretion | ↓ | ↑ |
| Acid‐base | Relatively normal | Metabolic acidosis/alkalosis |
| Plasma phosphate | ↓ | ↓ or ↑ |
| Urine sodium | Normal | ↓ or ↑ |
| Urine phosphate | ↓ | ↓ or ↑ |
| Urine calcium | ↑ | ↓ or ↑ |
| Dose of potassium required | Low | High |
| Rebound hyperkalemia | Often | Very rare |
Abbreviation: TPP, thyrotoxic periodic paralysis.
In hyperthyroidism, the clinical features of TPP and non‐TPP causes are often indistinguishable. They may not have overt symptoms of hyperthyroidism and may share similar precipitating factors as well as electrocardiographic findings of hyperthyroidism (sinus tachycardia, atrioventricular block, and high QRS voltage) and hypokalemia (flat T waves, ST depression, QT prolongation, and prominent U waves). 13 Aside from the meticulous history and careful physical examination, there are still several important clues to separate TPP (K+ shift) from non‐TPP (K+ deficit) in hyperthyroidism as shown in Table 2. For example, patients with TPP usually have normal acid‐base status because the quantity of extracellular K+ influx in exchange of intracellular Na+ and hydrogen is small and the extracellular fluid content of bicarbonate is large. K+ loss via renal or nonrenal route as in non‐TPP causes is usually accompanied by Cl− or bicarbonate loss, leading to metabolic acidosis or metabolic alkalosis. Additionally, patients with renal K+ loss usually have high urinary Na+ and Cl− excretions whereas those with remote or extrarenal K+ loss have aberrant or inappropriate Na+ vs Cl− excretions, as typically shown in both cases. 14 Blood and urine divalents are also helpful to aid in diagnosis. Hypophosphatemia with hypophosphaturia and hypercalciuria are other biochemical features of TPP. 15 , 16 , 17 However, the decreased excretions of divalent cations are characteristic of remote or extrarenal K+ loss, as shown in case 1 with surreptitious use of loop diuretics. Hypomagnesemia with renal magnesium wasting but hypocalciuria is typical findings to discriminate the disorders of distal convoluted tubule from that of loop of Henle as shown in case 2 with Gitelman's syndrome.
Several different causes of hypokalemia in the presence of hyperthyroidism have been increasingly reported. 9 , 10 , 11 Of note, the coexistence of Gitelman's syndrome and hyperthyroidism has been reported, mostly in young female of eastern Asian population. 11 In addition to diuretics, thyrotoxic patients may take laxatives for various purposes including weight reduction. 9 Hypokalemic paralysis can be caused by renal tubular acidosis in patients with hyperthyroidism with or without concomitant Sjogren's syndrome. 18 , 19 Mineralocorticoid excess states, such as primary aldosteronism and pheochromocytoma, presenting severe hypokalemia and hypertension have been reported in patients with hyperthyroidism. 10 , 20
With respect to therapy of severe hypokalemia, the dose of KCl (1 mmol/kg) should be minimized to prevent rebound hyperkalemia on recovery in TPP but a much higher dose of KCl is needed in the disorders of K+ deficit. In general, there is a deficit approximately 750 mmol when plasma K+ concentration close to 2.0 mmol/L. 21 In our patients, they required 250 and 320 mmol of KCl to restore muscle strength, respectively. Nevertheless, the treatment of the underlying disorders of hypokalemia is still the key in addition to the control of hyperthyroidism.
In conclusion, the presence of profound hypokalemia with paralysis in the setting of hyperthyroidism does not always make the diagnosis of TPP. The rapid assessment of blood acid‐base status and timely urine electrolytes (Na+, K+, and Cl−) excretion can help distinguish between TPP and non‐TPP. Prompt recognition of the underlying etiologies with appropriate management avoids the potential complications.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
Dr Wen‐Fang Chiang: drafted the paper and conducted the literature review. Dr Jenq‐Shyong Chan and Kun‐Lin Wu: assisted with critical revisions of the writing. Dr Shih‐Hua Lin: took responsibility for the work as a whole and approved the final submitted version of the manuscript.
Chiang W‐F, Chan J‐S, Wu K‐L, Lin S‐H. Hypokalemic paralysis in hyperthyroidism: Not all that glitter are gold. Clin Case Rep. 2021;9:1283–1287. 10.1002/ccr3.3754
Funding information
This study was supported in part by a grant from the Armed Forces Taoyuan General Hospital (TYAFGH‐D‐110030).
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Lin SH, Davids MR, Halperin ML. Hypokalaemia and paralysis. QJM. 2003;96:161‐169. [DOI] [PubMed] [Google Scholar]
- 2. Lin SH. Thyrotoxic periodic paralysis. Mayo Clin Proc. 2005;80:99‐105. [DOI] [PubMed] [Google Scholar]
- 3. Maciel RM, Lindsey SC, Dias da Silva MR. Novel etiopathophysiological aspects of thyrotoxic periodic paralysis. Nat Rev Endocrinol. 2011;7:657‐667. [DOI] [PubMed] [Google Scholar]
- 4. Brown MJ, Brown DC, Murphy MB. Hypokalemia from beta2‐receptor stimulation by circulating epinephrine. N Engl J Med. 1983;309:1414‐1419. [DOI] [PubMed] [Google Scholar]
- 5. Ryan DP, da Silva MR, Soong TW, et al. Mutations in potassium channel Kir2.6 cause susceptibility to thyrotoxic hypokalemic periodic paralysis. Cell. 2010;140:88‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cheng CJ, Lin SH, Lo YF, et al. Identification and functional characterization of Kir2.6 mutations associated with non‐familial hypokalemic periodic paralysis. J Biol Chem. 2011;286:27425‐27435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lin SH, Huang CL. Mechanism of thyrotoxic periodic paralysis. J Am Soc Nephrol. 2012;23:985‐988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lin SH, Lin YF. Propranolol rapidly reverses paralysis, hypokalemia, and hypophosphatemia in thyrotoxic periodic paralysis. Am J Kidney Dis. 2001;37:620‐623. [PubMed] [Google Scholar]
- 9. Chiang WF, Hsu YJ, Chang CC, Lin SH. Hypokalemic paralysis in a young obese female. Clin Chim Acta. 2012;413:1295‐1297. [DOI] [PubMed] [Google Scholar]
- 10. Gunatilake SSC, Bulugahapitiya U. Coexistence of primary hyperaldosteronism and graves' disease, a rare combination of endocrine disorders: is it beyond a coincidence‐a case report and review of the literature. Case Rep Endocrinol. 2017;2017:1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhou H, Liang X, Qing Y, et al. Complicated Gitelman syndrome and autoimmune thyroid disease: a case report with a new homozygous mutation in the SLC12A3 gene and literature review. BMC Endocr Disord. 2018;18:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sung CC, Cheng CJ, Chiang WF, et al. Etiologic and therapeutic analysis in patients with hypokalemic nonperiodic paralysis. Am J Med. 2015;128:289‐296.e281. [DOI] [PubMed] [Google Scholar]
- 13. Hsu YJ, Lin YF, Chau T, Liou JT, Kuo SW, Lin SH. Electrocardiographic manifestations in patients with thyrotoxic periodic paralysis. Am J Med Sci. 2003;326:128‐132. [DOI] [PubMed] [Google Scholar]
- 14. Wu KL, Cheng CJ, Sung CC, et al. Identification of the causes for chronic hypokalemia: importance of urinary sodium and chloride excretion. Am J Med. 2017;130:846‐855. [DOI] [PubMed] [Google Scholar]
- 15. Norris KC, Levine B, Ganesan K. Thyrotoxic periodic paralysis associated with hypokalemia and hypophosphatemia. Am J Kidney Dis. 1996;28:270‐273. [DOI] [PubMed] [Google Scholar]
- 16. Lin SH, Chu P, Cheng CJ, Chu SJ, Hung YJ, Lin YF. Early diagnosis of thyrotoxic periodic paralysis: spot urine calcium to phosphate ratio. Crit Care Med. 2006;34:2984‐2989. [DOI] [PubMed] [Google Scholar]
- 17. Katz AI, Emmanouel DS, Lindheimer MD. Thyroid hormone and the kidney. Nephron. 1975;15:223‐249. [DOI] [PubMed] [Google Scholar]
- 18. Mason AM, Golding PL. Renal tubular acidosis and autoimmune thyroid disease. Lancet. 1970;2:1104‐1107. [DOI] [PubMed] [Google Scholar]
- 19. Baldini C, Ferro F, Mosca M, Fallahi P, Antonelli A. The association of Sjogren syndrome and autoimmune thyroid disorders. Front Endocrinol (Lausanne). 2018;9:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Housni B, Elharroudi T, Soufi M, Bouziane M, Azzouzi A. Graves' disease allied with multiple pheochromocytoma. Indian J Endocrinol Metab. 2013;17:323‐325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sterns RH, Cox M, Feig PU, Singer I. Internal potassium balance and the control of the plasma potassium concentration. Medicine (Baltimore). 1981;60:339‐354. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


