Abstract
BACKGROUND:
While childbearing protects against risk of epithelial ovarian cancer (EOC), few studies have explored the impact on maternal EOC risk of sex of offspring, which may affect the maternal environment during pregnancy.
METHODS:
We performed a pooled analysis among parous participants from 12 case-controls studies comprising 6,872 EOC patients and 9,101 controls. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated using multivariable logistic regression for case-control associations and polytomous logistic regression for histotype-specific associations, all adjusted for potential confounders.
RESULTS:
In general, no associations were found between offspring sex and EOC risk. However, compared to bearing only female offspring, bearing one or more male offspring was associated with increased risk of mucinous EOC (OR=1.45; 95%CI=1.01–2.07), which appeared to be limited to women reporting menarche before age 13 compared to later menarche (OR=1.71 vs 0.99; P-interaction=0.02). Bearing increasing numbers of male offspring was associated with greater risks of mucinous tumors (OR=1.31, 1.84, 2.31, for 1, 2 and 3 or more male offspring, respectively; trend-p = 0.005). Stratifying by hormonally-associated conditions suggested that compared to bearing all female offspring, bearing a male offspring was associated with lower risk of endometrioid cancer among women with a history of adult acne, hirsutism, or polycystic ovary syndrome (OR=0.49, 95%CI=0.28–0.83) but with higher risk among women without any of those conditions (OR=1.64 95%CI=1.14–2.34; P-interaction=0.003).
CONCLUSION:
Offspring sex influences the childbearing-EOC risk relationship for specific histotypes and conditions. These findings support the differing etiologic origins of EOC histotypes and highlight the importance of EOC histotype-specific epidemiologic studies. These findings also suggest the need to better understand how pregnancy affects EOC risk
Keywords: epithelial ovarian cancer, offspring sex, mucinous ovarian cancer, endometrioid ovarian cancer, case-control study, pooled analysis
INTRODUCTION
Ovarian cancer is the fifth most common cancer among women in developed countries and the most fatal gynecological malignancy(1). In 2018, more than 295,000 women were newly diagnosed with the disease and over 185,000 women died from it worldwide(1). More than 70% of cases are diagnosed at late stages when 5-year survival is less than 30%(2). This high fatality coupled with the lack of a screening test for early detection(3) makes it critical to understand risk factors in order to help inform prevention strategies(4).
Ever bearing children is associated with about a 30% decrease in risk of epithelial ovarian cancer (EOC) in general (5) and increasing parity increases protection (6), although the magnitudes of the relationship vary by histotype (7, 8). The exact mechanism underlying the protective effect of pregnancy remains unknown, although it is frequently attributed to ovulation suppression that accompanies pregnancy(9). However, an ovulation alone cannot explain the magnitude of the protective effect(10), suggesting that other pregnancy-associated factors may impact EOC risk. Alterations in the maternal hormonal and immune milieus may be such factors(11–13). Fetal sex potentially affects these environments during pregnancy(14–21), can impact maternal physiology(22, 23), and is associated with conditions that have long-term maternal health consequences(24, 25). Together these data support the possibility that offspring sex may impact maternal EOC risk.
Few epidemiologic studies have explored the relationship between offspring sex and EOC, and results have been inconsistent(26–30). Methodological limitations including small sample sizes overall and for specific histotypes may account for these disparate findings. EOC is a heterogeneous disease consisting of distinct histotypes exhibiting varied risk factor profiles(8) and likely having distinct etiologic pathways(31). The main aim of this study was to evaluate the associations between offspring sex and EOC in an international collaborative investigation using pooled data from 12 case-control studies participating in the Ovarian Cancer Association Consortium (OCAC). Secondarily, we wished to evaluate associations by histotype. The large sample size of the pooled analysis enabled more robust estimates of the associations between offspring sex and EOC overall and by histotype than previously reported. In addition, the pooled analysis enabled exploration of potential interactions with hormonally-associated exposures.
METHODS
Study population
OCAC was established in 2005 to promote collaborative research on epidemiologic and genetic factors associated with EOC(32). The present analysis included participant-level data for parous women from 12 OCAC case-control studies conducted in Australia, Canada, Germany, the United Kingdom, and the United States with available information on offspring sex(33–45). Characteristics of the studies are shown in Table 1. Because offspring sex was inconsistently reported for non-singleton births across studies and because non-singleton births may differentially impact EOC risk relative to singleton births, we excluded subjects with any non-singleton births (n=528) from current analyses, resulting in 16,343 parous women with all singleton births. We then excluded women missing covariate data (n=35) and women missing offspring sex information (n=335), resulting in a total sample of 15,973 participants for data analysis (6,872 EOC patients and 9,101 controls). All participants provided informed consent and all participating institutions obtained approval from relevant ethics committees.
Table 1.
Characteristics of the 12 Ovarian Cancer Case-Control Studies from the Ovarian Cancer Association Consortium, Conducted in Australia, Europe, and North America Between 1989 and 2010
| Response Rate % | Controls, n(%) | All cancer, n(%) | Mucinous, n(%) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Region | Study Name | Study Period | Study Type | Method of Data Collection | Matching Variables | Cases | Controls | Total number of parous women2 | Age (years), mean (SD) | Never gave birth to a boy | Ever gave birth to a boy | Never gave birth to a boy | Ever gave birth to a boy | Adjusted OR (95% CI) model3 | Never gave birth to a boy | Ever gave birth to a boy | Adjusted OR (95% CI) model3 |
| AUS | Australia | Australian Ovarian Cancer Study | 2002–2005 | Population-based | Self-administered questionnaire | Age (5-year categories) Age (3 age groups: | 84 | 47 | 2337 | 57.9 (11.1) | 233 (19.3) | 972 (80.7) | 218 (19.3) | 914 (80.7) | 1.11 (0.89, 1.40) | 16 (12.9) | 108 (87.1) | 1.90 (1.07, 3.39) |
| CON | USA | Connecticut Ovarian Cancer Study | 1998–2003 | Population-based | In-person interview | 35–49 years, 50–64 years, and 65–79 years) | 69 | 61 | 826 | 55.70 (10.96) | 105 (23.2) | 348 (76.8) | 90 (24.1) | 283 (75.9) | 1.09 (0.76, 1.56) | 6 (16.2) | 31 (83.8) | 1.70 (0.66, 4.41) |
| GER | Germany | Germany Ovarian Cancer Study | 1993–1996 | Population-based | Self-administered questionnaire | Age and study region | 58 | 51 | 642 | 56.3 (10.6) | 125 (29.0) | 306 (71.0) | 75 (35.6) | 136 (64.5) | 0.70 (0.47, 1.05) | 9 (33.3) | 18 (66.7) | 0.77 (0.30, 1.99) |
| HAW | USA | Hawaii Ovarian Cancer Case- Control Study | 1993–2008 | Population-based | In-person interview | Age (5-year categories, race/ethnicity) | 78 | 80 | 1507 | 56.5 (13.9) | 181 (20.2) | 715 (79.8) | 128 (21.0) | 483 (79.0) | 1.06 (0.80, 1.41) | 25 (22.5) | 86 (77.5) | 1.14 (0.67, 1.94) |
| HOP | USA | Hormones and Ovarian Cancer Prediction Study | 2003–2008 | Population-based | In-person interview | Age (5-year categories), Race, Telephone prefix | 71 | 68 | 2176 | 59.4 (12.4) | 315 (20.7) | 1207 (79.3) | 146 (22.3) | 508 (77.7) | 0.94 (0.73, 1.20) | 10 (25.0) | 30 (75.0) | 0.97 (0.43, 2.17) |
| NCO | USA | North Carolina Ovarian Cancer Study | 1999–2008 | Population-based | In-person interview | Age (5-year categories, race/ethnicity) | 67 | 60 | 1819 | 56.5 (11.0) | 207 (22.8) | 700 (77.2) | 206 (22.6) | 706 (77.4) | 1.07. (0.84, 1.37) | 14 (17.1) | 68 (82.9) | 2.27 (1.16, 4.45) |
| NJO | USA | New Jersey Ovarian Cancer Study | 2002–2008 | Population-based | In-person interview | No matching Age (3 age groups: | 47 | 40 | 524 | 62.4 (11.1) | 92 (25.1) | 275 (74.9) | 41 (26.1) | 116 (73.9) | 1.12 (0.69, 1.82) | 2 (40.0) | 3. (60.0) | - |
| SON | Canada | Southern Ontario Ovarian Cancer Study | 1989–1993 | Population-based | In-person interview | 35–49 years, 50–64 years, and 65–79 years) | 71 | 65 | 792 | 56.7 (11.7) | 88 (18.8) | 379 (81.2) | 82 (25.2) | 243 (74.8) | 0.76 (0.53, 1.10) | 12 (20.0) | 48 (80.0) | 0.81 (0.40, 1.67) |
| TBO | USA | Tampa Bay Ovarian Cancer Study | 2000-present | Population-based | In-person interview | Age (5-year categories, race) | 68 | 60 | 163 | 61.3 (10.2) | 23 (31.1) | 51 (68.9) | 18 (20.2) | 71 (79.8) | 1.53 (0.60, 3.89) | 0 (0.0) | 1 (100.0) | - |
| TOR | Canada | Familial Ovarian Tumour Study (FOTS) AND Health Watch (HW) | FOTS: 1995-1999 and 2000-2003; HW: 1995-2000 | Population-based | In-person interview | Age (5-year categories) | 50 | 80 | 1135 | 57.3 (12.2) | 48 (17.1) | 233 (82.9) | 164 (19.2) | 690 (80.8) | 1.04 (0.71, 1.52) | 23 (18.3) | 103 (81.7) | 1.34 (0.73, 2.46) |
| UKO | UK | United Kingdom Ovarian cancer Population Study | 2006–2010 | Hospital- based | In-person interview | No matching | 86 | 97 | 1268 | 63.5 (8.0) | 180 (21.7) | 648 (78.3) | 98 (22.3) | 342 (77.7) | 1.05 (0.76, 1.45) | 11 (28.2) | 28 (71.8) | 1.17 (0.51, 2.69) |
| USC | USA | Los Angeles County Case- Control Studies of Ovarian Cancer | 1993–2009 | Population-based | In-person interview | Age (5-year categories, race/ethnicity) | 73 | 73 | 2784 | 56.9 (11.2) | 362 (21.7) | 1308 (78.3) | 212 (19.0) | 902 (81.0) | 1.17 (0.95, 1.44) | 19 (23.2) | 63 (76.8) | 0.92 (0.52, 1.64) |
| Pooled | - | - | - | 15973 | 58.0 (11.6) | 1959 (21.5) | 7142 (78.5) | 1478 (21.5) | 5394 (78.5) | 1.04 (0.95, 1.13) | 147 (20.0) | 587 (80.0) | 1.25 (1.00, 1.56) | |||||
Although TOR controls were limited to relatives and in-laws, it should not affect the exposure of interest, offspring sex Thus, cases and controls from TOR can all be included in the currect analysis
Excludes women with non-singleton births (n=528), missing core data (n=35), and missing offspring sex data (n=335)
Adjusted for age at diagnosis/reference date (continous), race (Black, White, Asian, Other), duration of oral contraceptive use (never, less than 1 years, 1–4 years, 5–9 years, and more than 10 years) and number of full-term pregnancies (1, 2, 3, 4, 5+)
Study variables
Information on offspring sex for each pregnancy lasting six months or longer (full-term) was self-reported. Based on our previous work, we classified women according to the number of male offspring(26). Ever having given birth to a boy was defined as reporting at least one male offspring among all singleton full-term births. Giving birth to all boys was defined as reporting a male offspring for each full-term, singleton pregnancy. The number of boys was calculated by summing the total number of pregnancies resulting in male offspring. The number of girls was calculated by subtracting the number of boys from the total number of full-term pregnancies. The fraction of births that were boys was defined as the total number of male offspring divided by the number of full-term pregnancies.
Information on other relevant variables and potential confounders was obtained from the OCAC core dataset and included age at diagnosis (cases) or interview (controls), race, education, body mass index (BMI) at 18 years of age, recent BMI (defined as previously reported as BMI 1 year prior or 5 year prior to diagnosis/interview or at diagnosis/interview(46)), total duration of oral contraceptive (OC) use, number of full-term pregnancies (parity), family history of ovarian or breast cancer, smoking status, and history of endometriosis, adult acne, hirsutism, polycystic ovary syndrome (PCOS), and irregular periods.
Statistical analysis
We used unconditional logistic regression to estimate odds ratios (ORs) and their 95% confidence intervals (95%CIs) for associations between bearing male offspring and EOC risk among parous women. The main multivariate model was adjusted for study site, age at reference (continuous), duration of OC use (never, less than 1 year, 1–4 years, 5–9 years, 10+ years), parity (1, 2, 3, 4, 5+ offspring) and race (white, black, Asian, other). We also considered adjustment for additional ovarian cancer risk factors including education (less than high school, high school, post-high school, college graduate, post graduate), family history of ovarian or breast cancer (yes/no), history of breastfeeding (yes/no), BMI at 18 (<18.5 / 18.5–24.9 / 25–30 / >=30 kg/m2), recent BMI (<18.5 / 18.5–24.9 / 25–30 / >=30 kg/m2), history of endometriosis (yes/no), history of irregular periods (yes/no), history of polycystic ovary syndrome (PCOS), adult acne, or hirsutism (yes/no), smoking history (never, ever), and age at menarche (<13 years/ >=13 years). These factors did not change the association between bearing a male offspring and EOC risk in general by more than 10% and were therefore not included in final models. Where they did alter associations by more than 10%, we present both the parsimonious model and the more adjusted model.
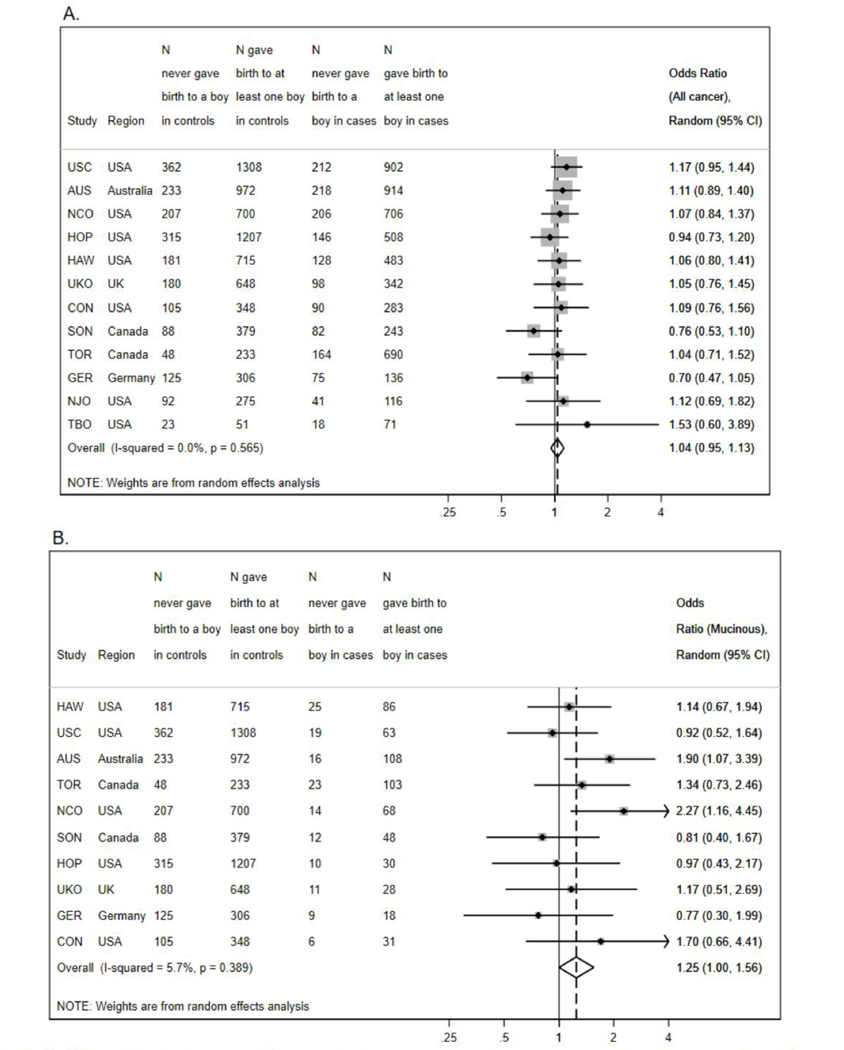
Random effects meta-analyses across study sites of all cancer histotypes showed no evidence of heterogeneity (I2=0.0%; p-het=0.57 Figure 1). Consequently, all analyses were performed using the pooled dataset adjusted for study site. We performed polytomous logistic regression to evaluate associations between bearing male offspring and EOC risk by the main histotypes (high-grade serous, mucinous, endometrioid, clear cell). We further stratified analyses by number of full-term births to separate associations with offspring sex from those with parity. We also explored models containing terms for total number of male and total number of female offspring and models containing terms for total number of full-term pregnancies and fraction of boys.
Figure 1:
Association Between Offspring Sex and Epithelial Ovarian Cancer (EOC) among Participants in 12 Population-Based, Case-Control Studies in Australia, Europe, and North America from 1989–2010.
Footnote: Results presented according to study site and overall and are adjusted for age at diagnosis/reference date (continous), race (Black, White. Asian, Other), duration of oral contraceptive use (never, less than 1 years. 1–4 years, 5–9 years, and more than 10 years) and number of full-term pregnancies (1, 2, 3, 4, 5+).
Association for (a) EOC in general and for (b) the mucinous histotype.
To identify potential interactions between offspring sex and hormonally-associated exposures for EOC in general and by specific histotypes, we performed stratified analyses by history of endometriosis (associated with excess estrogens(47) or reduced progesterone(48)), history of acne or hirsutism or PCOS (associated with excess androgens(49–51)), age at menarche less than 13 (which is associated with excess estrogens and increased ovulations(52–54)), recent BMI greater than or equal to 30 kg/m2 (which is associated with hormonal imbalances(55, 56)), history of irregular periods (associated with hormonal dysregulation(57)), history of ever using oral contraceptives (associated with altered hormonal milieu(58–60)), and history of ever smoking cigarettes (associated with anti-estrogenic effects(61)). Interactions and linear trends were assessed with Wald statistics. Stata/SE version 15.1 (StataCorp, College Station, TX) was used to conduct all analyses. All tests were two-sided with significance level of 5%.
RESULTS
Among parous controls, the study-specific frequency of never bearing a male offspring ranged from 17% to 31%, whereas among parous cases it ranged from 19% to 36% (Table 1). Compared to controls, women with EOC were less likely to have used OCs, had more than one child, attained a college education, reported a history of acne, hirsutism, or PCOS, and reported a history of irregular periods. Case women were more likely to have higher recent BMI, reported histories of endometriosis, and family histories of breast or ovarian cancer (Table 2).
Table 2.
Characteristics of Participants in the Ovarian Cancer Association Consortium (Australia, Europe, and North America), 1989–20101
| Controls (N=9101) n (%) | Cases (N=6872) n (%) | P-Value | |
|---|---|---|---|
| Age, years, mean (SD) | 57.5 (11.8) | 58.6 (11.3) | <0.0001 |
| Race | 0.42 | ||
| White | 7544 (83.0) | 5633 (82.2) | |
| Black | 331 (3.6) | 269 (3.9) | |
| Asian | 331 (3.6) | 276 (4.0) | |
| Other | 880 (9.7) | 677 (9.9) | |
| Education | <0.001 | ||
| Less than High School | 1233 (15.5) | 1336 (22.4) | |
| High School | 2530 (31.9) | 1958 (32.9) | |
| Post High School Training | 1964 (24.8) | 1419 (23.8) | |
| College Graduate | 1194 (15.1) | 710 (11.9) | |
| Post graduate | 1011 (12.7) | 535 (9.0) | |
| Body Mass Index (BMI) at 18, kg/m^2 | 0.064 | ||
| <18.5 | 1246 (16.3) | 792 (15.4) | |
| 18.5–24.9 | 5689 (74.3) | 3788 (73.8) | |
| 25–29.9 | 551 (7.2) | 429 (8.4) | |
| ≥30 | 168 (2.2) | 121 (2.4) | |
| Recent Body Mass Index (BMI), kg/m^2 | |||
| <18.5 | 108 (1.67) | 68 (1.50) | 0.006 |
| 18.5–24.9 | 2874 (44.43) | 1906 (42.07) | |
| 25–29.9 | 1370 (30.24 | ||
| 1975 (30.53) | ) | ||
| ≥30 | 1512 (23.37) | 1187 (26.2) | |
| Duration of Oral Contraceptive Use, years | <0.001 | ||
| 0 | 3031 (33.7) | 2917 (43.0) | |
| <1 | 1203 (13.4) | 1070 (15.8) | |
| 1–4 | 1986 (22.1) | 1277 (18.8) | |
| 5–9 | 1466 (16.3) | 894 (13.2) | |
| 10+ | 1316 (14.6) | 619 (9.1) | |
| Number of Full Term Pregnancies | <0.001 | ||
| 1 | 1493 (16.4) | 1356 (19.7) | |
| 2 | 3659 (40.2) | 2632 (38.3) | |
| 3 | 2282 (25.1) | 1664 (24.2) | |
| 4 | 1010 (11.1) | 726 (10.5) | |
| 5+ | 657 (7.2) | 494 (7.2) | |
| Endometriosis | <0.001 | ||
| No | 8381 (94.5) | 6180 (92.5) | |
| Yes | 485 (5.5) | 501 (7.5) | |
| Smoking Status | 0.33 | ||
| Never Smoker | 4426 (54.7) | 3206 (53.4) | |
| Former Smoker | 1171 (14.5) | 902 (15.0) | |
| Current Smoker | 2501 (30.9) | 1894 (31.6) | |
| Acne or Hirsutism or Polycystic ovary syndrome (PCOS) | 0.004 | ||
| No | 3906 (77.1) | 2831 (79.7) | |
| Yes | 1157 (22.9) | 720 (20.3) | |
| Irregular periods | 0.001 | ||
| No | 5692 (81.3) | 4079 (83.6) | |
| Yes | 1308 (18.7) | 798 (16.4) | |
| Age at Menarche | |||
| <13 years | 4068 (44.96) | 2972 (43.51) | 0.069 |
| ≥13 years | 4981 (55.04) | 3859 (56.49) | |
| Family History of Breast or Ovarian Cancer in first-relative | <0.001 | ||
| No | 7516 (85.4) | 4846 (80.7) | |
| Yes | 1285 (14.6) | 1156 (19.3) | |
Missing data are as follows: race 15 controls, 17 cases; education 1169 controls, 914 cases; BMI at 18 1447 controls, 1742 cases; recent BMI 2632 controls, 2341 cases; duration of oral contraceptive use 99 controls, 95 cases; endometriosis 235 controls, 191 cases; smoking 1003 controls, 870 cases; acne or hirsutism or PCOS 4038 controls, 3321 cases; irregular period 2101 controls, 1995 cases; age at menarche 52 controls, 41 cases; family history of breast or ovarian cancer first-relative 300 controls, 870 cases.
Compared to bearing all females, ever having borne a male was not associated with EOC overall (OR=1.05; 95%CI=0.96–1.14; Table 3); however, bearing a male offspring was associated with increased risk of mucinous histotype (OR=1.25; 95%CI=1.02–1.54). This association strengthened when we further adjusted for hormonally-associated conditions (endometriosis, irregular periods, acne or PCOS or hirsutism, smoking, history of early menarche and recent BMI; OR=1.45; 95%CI=1.01–2.07). Similarly, giving birth only to boys was not associated with EOC risk overall, whereas compared to giving birth to at least one girl, bearing all male offspring was associated with increased risk of mucinous tumors (OR=1.29; 95%CI=1.07–1.55). The association was slightly strengthened when further adjusted for hormonally-associated conditions (OR=1.35; 95%CI=0.99–1.84). Increasing number of male offspring was associated with increasing risk of mucinous ovarian cancer in both the most parsimonious model (OR=1.16, 1.56, 1.55, for 1, 2 and 3 or more male offspring compared to all female offspring, respectively; trend-p = 0.006) and in a model additionally controlling for hormonally-associated conditions (OR=1.31, 1.84, 2.31, for 1, 2 and 3 or more male offspring, respectively; trend-p = 0.005). There were no associations between increasing number of male offspring and EOC risk overall or for any other histotypes.
Table 3:
Adjusted Pooled Odds Ratios for the Association Between Offspring Sex and Epithelial Ovarian Cancer Among Parous Women with Only Singleton Births in the Ovarian Cancer Association Consortium (Australia, Europe, and North America), 1989–2010
| Controls | All Cancers | HGSOC | Mucinous | Clear cell | Endometriod | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N(%) | Cases, n (%) | Adjusted OR1 (95% CI) | Adjusted OR2 (95% CI) | Cases, n (%) | Adjusted OR1 (95% CI) | Adjusted OR2 (95% CI) | Cases, n (%) | Adjusted OR1 (95% CI) | Adjusted OR2 (95% CI) | Cases, n (%) | Adjusted OR1 (95% CI) | Adjusted OR2 (95% CI) | Cases, n (%) | Adjusted OR1 (95% CI) | Adjusted OR2 (95% CI) | |
| Gave birth to a boy | ||||||||||||||||
| Never | 1959 (21.5) | 1478 (21.5) | ref | ref | 548 (20.4) | ref | ref | 147 (20.0) | ref | ref | 88 (23.7) | ref | ref | 181 (23.4) | ref | ref |
| Ever | 7142 (78.5) | 5394 (78.5) | 1.05 (0.96, 1.14) | 1.06 (0.93, 1.21) | 2135 (79.6) | 1.06 (0.94, 1.20) | 1.03 (0.87, 1.22) | 587 (80.0) | 1.25 (1.02, 1.54) | 1.45 (1.01, 2.07) | 283 (76.3) | 1.14 (0.87, 1.49) | 1.06 (0.70, 1.61) | 593 (76.6) | 1.03. (0.85, 1.25) | 1.06 (0.78, 1.44) |
| Gave birth to all boys | ||||||||||||||||
| No | 7077 (77.8) | 5257 (76.5) | ref | ref | 2133 (79.5) | ref | ref | 521 (71.0) | ref | ref | 269 (72.5) | ref | ref | 589 (76.1) | ref | ref |
| Yes | 2024 (22.2) | 1615 (23.5) | 0.99 (0.91, 1.08) | 1.02 (0.90, 1.16) | 550 (20.5) | 0.91 (0.81, 1.02) | 0.96 (0.82, 1.13) | 213 (29.0) | 1.29 (1.07, 1.55) | 1.35 (0.99, 1.84) | 102 (27.5) | 1.02 (0.79, 1.31) | 0.90 (0.60, 1.35) | 185 (23.9) | 0.93 (0.77, 1.13) | 0.80 (0.58, 1.10) |
| Number of boys | ||||||||||||||||
| No boy | 1959 (21.5) | 1478 (21.5) | ref | ref | 548 (20.4) | ref | ref | 147 (20.0) | ref | ref | 88 (23.7) | ref | ref | 181 (23.4) | ref | ref |
| 1 boy | 3826 (42.0) | 2910 (42.3) | 1.04 (0.95, 1.13) | 1.05 (0.92, 1.20) | 1130 (42.1) | 1.08 (0.95, 1.22) | 1.04 (0.87, 1.24) | 309 (42.1) | 1.16 (0.93, 1.44) | 1.31 (0.90, 1.91) | 186 (50.1) | 1.20 (0.91, 1.57) | 1.19 (0.78, 1.82) | 339 (43.8) | 1.03. (0.84, 1.26) | 1.07 (0.78, 1.48) |
| 2 boys | 2244 (24.7) | 1723 (25.1) | 1.09 (0.98, 1.21) | 1.12 (0.96, 1.31) | 683 (25.5) | 1.05 (0.90, 1.22) | 1.05 (0.86, 1.29) | 193 (26.3) | 1.56 (1.20, 2.02) | 1.84 (1.18, 2.87) | 74 (20.0) | 1.00 (0.70, 1.42) | 0.70 (0.39, 1.24) | 195 (25.2) | 1.10 (0.86, 1.41) | 1.11 (0.75, 1.64) |
| 3 or more boys | 1072 (11.8) | 761 (11.1) | 0.99 (0.86, 1.15) | 0.93 (0.75, 1.16) | 322 (12.0) | 0.95 (0.77, 1.16) | 0.86 (0.65, 1.13) | 85 (11.6) | 1.55 (1.08, 2.23) | 2.31 (1.24, 4.29) | 23 (6.2) | 0.75 (0.43, 1.31) | 0.75 (0.34, 1.67) | 59 (7.6) | 0.68 (0.47, 1.00) | 0.54 (0.29, 1.02) |
| P for Trend | 0.90 | 0.65 | 0.56 | 0.32 | 0.006 | 0.005 | 0.24 | 0.28 | 0.08 | 0.07 | ||||||
| Continuous3 | ||||||||||||||||
| number of boys | 9101 (100.0) | 6872 (100.0) | 0.93 (0.90, 0.96) | 0.91 0.87, 0.96) | 2683 (100.0) | 0.95 (0.91, 0.99) | 0.93 (0.87, 0.99) | 734 (100.0) | 1.03 (0.95, 1.11) | 1.02 (0.88, 1.17) | 371 (100.0) | 0.70 (0.62, 0.80) | 0.73 (0.60, 0.88) | 774 (100.0) | 0.81. (0.74, 0.88) | 0.80 (0.70, 0.91) |
| number of girls | 9101 (100.0) | 6872 (100.0) | 0.92 (0.89, 0.95) | 0.91 (0.87, 0.96) | 2683 (100.0) | 0.96 (0.92, 1.00) | 0.95 (0.90, 1.02) | 734 (100.0) | 0.88 (0.81, 0.96) | 0.80 (0.69, 0.94) | 371 (100.0) | 0.73 (0.64, 0.82) | 0.81 (0.67, 0.98) | 774 (100.0) | 0.85. (0.78, 0.92) | 0.88 (0.77, 1.00) |
| Fraction of births that were boys,4 per 25% increase | 9101 (100.0) | 6872 (100.0) | 1.01 (0.99, 1.04) | 1.01 (0.98, 1.05) | 2683 (100.0) | 1.00 (0.96, 1.03) | 1.00 (0.95, 1.04) | 734 (100.0) | 1.09 (1.03, 1.16) | 1.13 (1.03, 1.24) | 371 (100.0) | 1.01 (0.94, 1.09) | 0.98 (0.87, 1.09) | 774 (100.0) | 1.00 (0.95, 1.06) | 0.97 (0.89, 1.06) |
| Stratified by number of birth episodes5 | ||||||||||||||||
| Among with exactly 1 birth | ||||||||||||||||
| Girl | 742 (49.7) | 651 (48.0) | ref | ref | 230 (52.0) | ref | ref | 71 (42.8) | ref | ref | 45 (43.7) | ref | ref | 75 (43.4) | ref | ref |
| Boy | 751 (50.3) | 705 (52.0) | 1.02 (0.88, 1.20) | 1.11 (0.87, 1.41) | 212 (48.0) | 0.96 (0.76, 1.20) | 1.06 (0.77, 1.47) | 95 (57.2) | 1.22 (0.86, 1.72) | 1.31 (0.73, 2.34) | 58 (56.3) | 1.22 (0.80, 1.86) | 1.58 (0.79, 3.15) | 98 (56.6) | 1.27 (0.91, 1.78) | 0.98 (0.56, 1.69) |
| Among women with exacly 2 births | ||||||||||||||||
| No boy | 873 (23.9) | 558 (21.2) | ref | ref | 217 (21.4) | ref | ref | 56 (19.4) | ref | ref | 29 (19.5) | ref | ref | 76 (24.4) | ref | ref |
| 1 boy | 1924 (52.6) | 1423 (54.1) | 1.14 (1.00, 1.30) | 1.02 (0.84, 1.25) | 564 (55.6) | 1.20 (1.00, 1.44) | 1.01 (0.78, 1.30) | 146 (50.7) | 1.16 (0.83, 1.61) | 1.27 (0.71, 2.25) | 87 (58.4) | 1.30 (0.84, 2.01) | 1.00 (0.52, 1.90) | 166 (53.4) | 0.99. (0.74, 1.33) | 1.12 (0.69, 1.81) |
| 2 boys | 862 (23.6) | 651 (24.7) | 1.15 (0.99, 1.35) | 1.12 (0.89, 1.40) | 233 (23.0) | 1.07 (0.86, 1.33) | 1.01 (0.75, 1.36) | 86 (29.9) | 1.58 (1.10, 2.28) | 1.89 (1.02, 3.52) | 33 (22.1) | 1.15 (0.69, 1.93) | 0.61 (0.27, 1.42) | 69 (22.2) | 0.92 (0.65, 1.32) | 0.92 (0.51, 1.64) |
| P for trend | 0.07 | 0.35 | 0.56 | 0.95 | 0.01 | 0.04 | 0.59 | 0.26 | 0.66 | 0.77 | ||||||
| Among women with exactly 3 births | ||||||||||||||||
| No boy | 262 (11.5) | 209 (12.6) | ref | ref | 79 (11.4) | ref | ref | 15 (9.3) | ref | ref | 12 (15.6) | ref | ref | 24 (13.7) | ref | ref |
| 1 boy | 822 (36.0) | 562 (33.8) | 0.86 (0.69, 1.08) | 0.91 (0.66, 1.27) | 250 (36.1) | 1.02 (0.75, 1.37) | 1.06 (0.69, 1.62) | 53 (32.9) | 1.21 (0.65, 2.26) | 2.08 (0.59, 7.35) | 28 (36.4) | 0.71 (0.35, 1.42) | 0.82 (0.27, 2.51) | 54 (30.9) | 0.72 (0.42, 1.22) | 0.76 (0.32, 1.73) |
| 2 boys | 874 (38.3) | 682 (41.0) | 0.97 (0.78, 1.20) | 0.97 (0.70, 1.34) | 277 (40.0) | 1.03 (0.77, 1.39) | 1.10 (0.72, 1.66) | 68 (42.2) | 1.52 (0.83, 2.81) | 2.33 (0.67, 8.09) | 27 (35.1) | 0.66 (0.33, 1.34) | 0.54 (0.17, 1.71) | 82 (46.9) | 1.00 (0.60, 1.65) | 0.97 (0.44, 2.15) |
| 3 boys | 324 (14.2) | 211 (12.7) | 0.82 (0.63, 1.06) 0.23 | 0.79 (0.54, 1.18) 0.31 | 87 (12.6) | 0.89 (0.62, 1.29) 0.57 | 0.92 (0.55, 1.52) 0.78 | 25 (15.5) | 1.44 (0.72, 2.89) 0.22 | 2.59 (0.66, 10.10) 0.16 | 10 (13.0) | 0.63 (0.26, 1.50) 0.28 | 0.55 (0.13, 2.22) 0.31 | 15 (8.6) | 0.43 (0.21, 0.88) 0.05 | 0.33 (0.10, 1.13) 0.11 |
| P for trend | 0.23 | 0.31 | 0.57 | 0.78 | 0.22 | 0.16 | 0.28 | 0.31 | 0.05 | 0.11 | ||||||
Adjusted for study sites, age at diagnosis/reference date (continous), race (Black, White, Asian, Other), duration of oral contraceptive use (never, less than 1 years, 1–4 years, 5–9 years, and more than 10 years) and number of full-term pregnancies (1, 2, 3, 4, 5+); Two hundrad twenty three women with missing data in race or oral contraceptive use were excluded from the analysis.
Further adjsuted for endometriosis (yes, no), smoking (ever, never), acne or hirsutism or PCOS (yes, no), irrgular periods (yes, no), recent BMI (<18.5, 18.5–24.9, 24.9–30, ≥30), and age at menarche (<13 years, ≥13 years).
Models did not adjust for total number of full term pregnancies
Models adjust for total number of full term pregnancies as a continous variable
Adjusted for study sites, age at diagnosis/reference date (continous), race (Black, White, Asian, Other) and duration of oral contraceptive use (never, less than 1 years, 1–4 years, 5–9 years, and more than 10 years)
In models including separate quantitative terms for total number of male offspring and total number of female offspring, each additional offspring was associated with about an 8% decrease in EOC risk overall regardless of whether the offspring was male (OR=0.93; 95%CI=0.90–0.96) or female (OR=0.92; 95%CI=0.89–0.95) (Table 3). While the point estimates for high-grade serous, clear cell, and endometrioid subtypes were similar for both male and female offspring, for the mucinous histotype, each additional female offspring was associated with a 12% decrease in risk (OR=0.88; 95%CI=0.810.96) whereas each male offspring was not associated with risk (OR=1.03; 95%CI=0.95–1.11). The results from models controlling for total number of full-term births also showed that a 25% increase in the fraction of births that were boys was associated with a 9% increase in risk of mucinous EOC (OR=1.09; 95%CI=1.03–1.16). Fraction of male births was not associated with risk of the other subtypes.
Stratifying by number of offspring (Table 3) yielded similar patterns of risk associated with increasing male offspring for the mucinous histotype. Among women with exactly one full-term birth, bearing a male offspring was associated with a 22% increased risk of mucinous cancer compared to bearing a female offspring. Among women with exactly two births, compared to bearing all female offspring, bearing exactly one male offspring was associated with a 16% increased risk of mucinous tumors, whereas bearing two male offspring was associated with a 58% increased risk (P-trend=0.01).
For mucinous histotype, we further observed interactions with age at menarche (Table 4). Compared to never giving birth to a boy, ever bearing a male offspring was associated with an increased risk of mucinous cancer among women with menarche before age 13 (OR=1.71, 95%CI=1.23–2.38) but no increased risk associated with menarche at a later age (OR=0.99, 95%CI=0.76–1.30; P-interaction=0.02). Results were similar when we examined interactions between menarche and giving birth to all boys (OR=1.55 for early menarche versus OR=1.08 for later menarche; P-interaction=0.08). Among women with menarche prior to age 13, increasing number of male offspring was associated with increasing risk of mucinous tumor (ORs for bearing 1, 2, 3+ male offspring: 1.54, 2.34, 2.24 compared to no male offspring; P-trend =0.002). Among women with later menarche no trend was observed (ORs for bearing 1, 2, 3+ male offspring: 0.94, 1.16, 1.20; P-trend=0.32; P-interaction=0.10). Consistent with this observation, each 25% increase in fraction of male offspring was associated with a significant 18% increase in mucinous cancer among women with earlier menarche but no increase in women with later menarche (P-interaction=0.01). We also observed an interaction between age at menarche and bearing female offspring, with each female offspring associated with a significant 21% reduced risk of mucinous tumors among women with earlier menarche but little or no association among women with later menarche (OR=0.79 versus 0.94 for each female offspring in women with and without early menarche, respectively; P-interaction=0.02). There was no interaction between age at menarche and bearing male offspring (OR=1.04 versus 1.01 for each male offspring in women with and without early menarche, respectively; P-interaction=0.51).
Table 4:
Adjusted Pooled Odds Ratios for the Association Between Offspring Sex and Mucinous Epithelial Ovarian Cancer Stratified by History of Estrogenic Conditions Among Parous Women with Only Singleton Births in the Ovarian Cancer Association Consortium (Australia, Europe, and North America), 1989–20101
| Age at Menarche <13 years | Age at Menarche >13 years | |||||
|---|---|---|---|---|---|---|
| Controls N(%) | Cases N(%) | Adjusted OR (95% Cl) | Controls N(%) | Cases N(%) | Adjusted OR (95% Cl) | |
| Gave birth to a boy | ||||||
| Never | 916(22.52) | 56(18.24) | ref | 1032 (20.72) | 91 (21.46) | ref |
| Ever | 3152 (77.48) | 251 (81.76) | 1.71 (1.23, 2.38) | 3949 (79.28) | 333 (78.54) | 0.99 (0.76, 1.30) |
| P for interaction | 0.02 | |||||
| Gave birth to all boys | ||||||
| Not all boys | 3124 (76.79) | 205 (66.78) | ref | 3913 (78.56) | 314 (74.06) | ref |
| All boys | 944 (23.21) | 102 (33.22) | 1.55 (1.18, 2.04) | 1068 (21.44) | 110 (25.94) | 1.08 (0.83, 1.40) |
| P for interaction | 0.08 | |||||
| No. of boys | ||||||
| No boy | 916(22.52) | 56(18.24) | ref | 1032 (20.72) | 91 (21.46) | ref |
| 1 boy | 1663 (40.88) | 132 (43.00) | 1.54(1.09, 2.18) | 2137 (42.90) | 176 (41.51) | 0.94 (0.71, 1.24) |
| 2 boys | 1010 (24.83) | 86 (28.01) | 2.34(1.55, 3.53) | 1222 (24.53) | 105 (24.76) | 1.16 (0.82, 1.63) |
| 3 or more boys | 479 (11.77) | 33 (10.75) | 2.24 (1.27, 3.98) | 590(11.85) | 52 (12.26) | 1.20 (0.75, 1.93) |
| P for trend | 0.002 | 0.32 | ||||
| P for interaction | 0.10 | |||||
| Number of boys2 | 1919 (100.00) | 132 (100.00) | 1.04 (0.92, 1.18) | 4981 (100.00) | 424 (100.00) | 1.01 (0.90, 1.12) |
| P for interaction | 0.51 | |||||
| Number of girls2 | 1919 (100.00) | 132 (100.00) | 0.79 (0.69, 0.91) | 4981 (100.00) | 424 (100.00) | 0.94 (0.84, 1.05) |
| P for interaction | 0.02 | |||||
| Fraction of births that were boys, 25% increase3 | 1919 (100.00) | 132 (100.00) | 1.18(1.09, 1.28) | 4981 (100.00) | 424 (100.00) | 1.03 (0.95, 1.11) |
| P for interaction | 0.01 | |||||
Adjusted for study sites, age at diagnosis/reference date (continous), race (Black, White, Asian, Other), duration of oral contraceptive use (never, less than 1 years, 1–4 years, 5–9 years, and more than 10 years) and number of full-term pregnancies (1, 2, 3, 4, 5+)
Adjsuted for each other
Models adjust for total number of full term pregnancies as a continous variable
No other interactions between hormonal-associated exposures and EOC were observed, except for self-reported history of acne or hirsutism or PCOS and risk of endometrioid cancer (Table 5). Compared to bearing all female offspring, bearing at least one male offspring was associated with reduced risk of endometrioid cancer among women with a history of any of those conditions (OR=0.49, 95%CI=0.28–0.83), but an increased risk among women with no history of any of those conditions (OR=1.64 95%CI=1.14–2.34; P-interaction=0.003). Results were similar when we examined the interaction between reported history of acne/hirsutism/PCOS and number of male offspring (ORs for bearing 1, 2 or 3+ male offspring: 0.47, 0.52, 0.47 versus 1.69, 1.59. 0.78, for women with and without this history, respectively, P-interaction=0.007). An interaction was also observed between reported history of those androgenic conditions and bearing female offspring, with each female offspring associated with reduced endometrioid cancer risk in women with no reported history compared to those with such a history (OR=0.80 vs 1.02 for each female offspring in women without and with a history, respectively; P-interaction 0.03). There appeared to be no interaction between a history of those androgenic conditions and bearing male offspring (OR=0.82 vs 0.87 for each male offspring in women without and with a history, respectively; P-interaction=0.44).
Table 5:
Adjusted Pooled Odds Ratios for the Association Between Offspring Sex and Endometrioid Epithelial Ovarian Cancer Stratified by History of Androgenic Conditions Among Parous Women with Only Singleton Births in the Ovarian Cancer Association Consortium (Australia, Europe, and North America), 1989–20101
| History of Acne or Hirsutism or PCOS | No history of Acne or Hirsutism or PCOS | |||||
|---|---|---|---|---|---|---|
| Controls N(%) | Cases N(%) | Adjusted OR (95% Cl) | Controls N(%) | Cases N(%) | Adjusted OR (95% Cl) | |
| Gave birth to a boy | ||||||
| Never | 253 (21.9) | 28 (34.1) | ref | 832 (21.3) | 48(18.8) | ref |
| Ever | 904 (78.1) | 54 (65.9) | 0.49 (0.28, 0.83) | 3074 (78.7) | 207 (81.2) | 1.64(1.14, 2.34) |
| P for interaction | 0.003 | |||||
| Gave birth to all boys | ||||||
| Not all boys | 894 (77.3) | 65 (79.3) | ref | 3075 (78.7) | 197 (77.3) | ref |
| All boys | 263 (22.7) | 17 (20.7) | 0.88 (0.49, 1.59) | 831 (21.3) | 58 (22.7) | 0.93 (0.67, 1.28) |
| P for interaction | 0.56 | |||||
| No. of boys | ||||||
| No boy | 253 (21.9) | 28 (34.1) | ref | 832 (21.3) | 48(18.8) | ref |
| 1 boy | 492 (42.5) | 27 (32.9) | 0.47 (0.27, 0.85) | 1606 (41.1) | 127 (49.8) | 1.69 (1.18, 2.44) |
| 2 boys | 275 (23.8) | 18(22.0) | 0.52 (0.26, 1.06) | 1004 (25.7) | 64 (25.1) | 1.59 (1.02, 2.49) |
| 3 or more boys | 137 (11.8) | 9 (11.0) | 0.47 (0.16, 1.67) | 464(11.9) | 16(6.3) | 0.78 (0.39, 1.56) |
| P for trend | 0.21 | 0.47 | ||||
| P for interaction | 0.007 | |||||
| Number of boys2 | 1157 (100.0) | 82 (100.0) | 0.77 (0.60, 0.99) | 3906 (100.0) | 255 (100.0) | 0.82 (0.71, 0.95) |
| P for interaction | 0.44 | |||||
| Number of girls2 | 1157 (100.0) | 82 (100.0) | 1.02 (0.81, 1.30) | 3906 (100.0) | 255 (100.0) | 0.80 (0.69, 0.93) |
| P for interaction | 0.03 | |||||
| Fraction of births that were boys, 25% increase3 | 1157 (100.0) | 82 (100.0) | 0.85 (0.72, 1.00) | 3906 (100.0) | 255 (100.0) | 1.06 (0.96, 1.15) |
| P for interaction | 0.03 | |||||
Adjusted for study sites, age at diagnosis/reference date (continous), race (Black, White, Asian, Other), duration of oral contraceptive use (never, less than 1 years, 1–4 years, 5–9 years, and more than 10 years) and number of full-term pregnancies (1, 2, 3, 4, 5+)
Adjsuted for each other
Models adjust for total number of full term pregnancies as a continous variable
DISCUSSION
In this pooled analysis of data from 6,872 parous women with EOC and 9,101 parous controls, sex of offspring was not associated with maternal EOC risk overall. However, bearing male offspring was associated with less protection against mucinous cancers. When examining the per-pregnancy association, offspring sex was not associated with EOC risk overall or for high-grade serous, clear cell, and endometrioid histotypes, but was associated with risk of mucinous tumors. In particular, bearing female offspring was associated with decreased risk of mucinous tumors among parous women, whereas bearing male offspring appeared to have no relation to that histotype. We observed no interactions between offspring sex and hormonally-associated exposures, except among women with mucinous tumors and menarche prior to age 13 and among women with endometrioid tumors and a history of acne, hirsutism, or PCOS. Among women with menarche before age 13, bearing male children was associated with higher risk of mucinous cancer than in women with later menarche. Among women with a history of acne, hirsutism, or PCOS, bearing male children was associated with lower risk of endometrioid cancer than in women without those conditions.
Five studies have reported the association between offspring sex and ovarian cancer risk(26–30), including two studies included in this pooled analysis (HOPE and AUS). In the HOPE Study, conducted in western Pennsylvania, USA from 2003–2008, compared to bearing all female offspring, bearing any male offspring was associated with lower risk of EOC (OR=0.92) and bearing all male offspring was associated with even lower risk (OR=0.86)(30). A earlier population-based study of 511 cases and 1136 controls conducted in eastern Pennsylvania, USA from 1994–1998 by the same group reported similar findings – relative to all female offspring, bearing all male offspring was associated with decreased EOC risk (OR=0.80)(26). These findings were supported by a nested case-control study within the population-based Swedish Fertility Register that included 7,407 women diagnosed with EOC between 1961 and 2001 and 37,658 controls(27): compared to bearing all female offspring, bearing a male child was associated with reduced EOC risk in a dose-response fashion (ORs: 0.92, 0.87, 0.82, for 1, 2 or 3+ boys, compared to all girls)(27). In contrast, the Australia-wide population-based study (AUS) conducted between 2002 and 2005 and included in this pooled analysis reported no association between offspring sex and EOC for parous women in general but a 2-fold increased risk of the mucinous histotype associated with bearing only male offspring(29). Notably, excluding AUS data from the current analysis did not appreciably affect the observed association with mucinous tumors. A population-based cohort study of 5,092 EOC cases in the Norwegian national registry also reported no EOC-offspring sex association in general(28). However, that study reported an increased risk of endometrioid tumors among women who gave birth only to girls compared to those who gave birth only to boys (incidence ratio 1.35 based on 475 cases).
Although there are histotype differences in the magnitude of the protective effect, greater parity has consistently been associated with reduced EOC risk(7, 8), especially among non-mucinous disease; however, the mechanism underlying this association remains unknown. Two theories have dominated the literature: suppressed ovulation(9) and lowered gonadotropin levels(62). Pregnancy, regardless of fetal sex, should equally affect ovulation and gonadotropin secretion; thus, our results suggest the possibility of additional mechanisms. Reducing inflammation(12) and altering circulating steroid hormones(11) have been postulated. During pregnancy, both maternal hormonal and immune milieus differ by fetal sex. Carriage of a male fetus is associated with lower maternal levels of estradiol and hCG(14, 15, 18) and higher maternal levels of progesterone(16) and testosterone(19). While the role of hCG in EOC etiology is unclear, progesterone is believed to protect against EOC while estrogens and androgens may increase risk(11) in a histotype-specific way(20, 21). Whether the observed maternal hormonal differences by fetal sex are large enough to matter in the context of the high hormonal levels of pregnancy is unknown. Women carrying male fetuses also exhibit more proinflammatory/proangiogenic immune milieus than women carrying female fetuses(17). Pregnancy outcomes also vary by offspring sex, with preterm birth, higher birth weight, and gestational diabetes associated with males(63–65), and increased risk of maternal hypertensive disorders and asthma flares associated with females(66, 67). Genetic and metabolic profiles of the placenta also vary by fetal sex(68), and both hormones and cells derived from the fetoplacental unit persist in maternal circulation for years after pregnancy ends(69). Moreover, male-origin microchimerism, which arises predominantly but not exclusively from fetal cells acquired during pregnancy(70) and persists for decades after pregnancy(71), has recently been associated with reduced rates of ovarian cancer(72). Fetal sex also influences maternal physiology(22, 23), and pregnancy conditions that differ by fetal sex, such as preeclampsia and gestational diabetes, may impact future maternal health outcomes(24, 25). Together, these observations suggest that fetal sex-based differences can have long-term health consequences and support a potential link between offspring sex and EOC risk.
Despite this apparent biologic plausibility, the results of this study did not show any overall relationship between offspring sex and EOC risk. However, we did observe relationships with offspring sex for the mucinous histotype in general and specifically for women with menarche prior to age 13. We further observed an association for endometrioid tumors in relation to maternal androgenic conditions.
It is now accepted that while pregnancy protects against EOC in general, the protection varies by histotype. In the Ovarian Cancer Cohort Consortium (OCCC), ever bearing offspring provided a 31% decrease in risk in general, with a greatest protection seen for the clear cell histotype (RR=0.35, 95%CI:0.27–0.47) and the least protection observed in the serous histotype (RR=0.81, 95%CI=0.73–0.90) (8). The Million Women Study also reported a differing protective effect against EOC associated with every bearing offspring based on histotype, with the greatest effect seen among clear cell cases and the least seen among serous cases (7). Both studies also report histotype differences based on the number of offspring. Given these differences in protective effect of pregnancy by hisotype, it is possible that the relationship between offspring sex and EOC could also vary by histotype.
Thus, while our histotype-specific observations are plausible, the underlying biologic reasons for these observations are unclear. Mucinous EOC is a relatively infrequent histotype, representing some 5–20% of cases(73); however, epidemiologic evidence supports a substantially different risk-factor profile than that of the other histotypes(74). Notably, apart from pregnancy, the relationships between hormonal exposures and mucinous tumors are less pronounced or perhaps nonexistent compared to other histotypes(74), suggesting that alteration in the hormonal milieu may not account for our mucinous-disease findings in general and among women with menarche prior to age 13. In addition to higher endogenous estrogen exposure, earlier age at menarche is associated with earlier and more prolonged ovulation(52, 53). That observation, however, cannot explain the mucinous-specific association because increasing lifetime ovulations are associated with increased ovarian cancer risk overall(75–77). Moreover, histotype-specific results show no relationship between lifetime ovulations and the mucinous subtype(77). Similarly, it is unclear why the relationship between offspring sex and endometrioid tumors should vary based on history of androgenic conditions, as endometrioid tumors are more closely associated with estrogenic exposures(78–80) and possibly higher circulating androgen levels in the post-menopause(20).
Regardless of the underlying biology, our findings underscore the need to further understand the mechanisms whereby pregnancy impacts EOC risk. Moreover, they reflect the heterogeneous etiologic nature of ovarian cancer(81), which is no longer believed to be a single disease but a group of diseases with separate etiologic origins. EOC histotypes exhibit differing clinical behavior and are believed to have different or differentially evolved cells of origin leading to distinct carcinogenic pathways(82). Epidemiologic studies further support the multifactorial origin of EOC, with most well-established risk factors exhibiting substantial heterogeneity by histotype(8, 74). Our results lend further population-based support to the distinct etiology of EOC histotypes, and in particular for that of mucinous tumors compared to the others(8, 74).
A strength of the present study is the use of participant-level data from 12 population-based case-control studies spanning three continents. The large sample size resulted in increased statistical power to examine histotype-specific associations, which individual studies could not adequately do. In addition, pooling data from population-based case-control studies with detailed lifestyle, reproductive, and medical history data enabled us to control for potential confounders and to stratify by hormonally-associated exposures, which the population-based registry studies were unable to do. The included studies were all population-based, and the majority of studies used in-person interviews to obtain data on offspring sex and other exposures, increasing the generalizability of findings. Study-specific data were carefully cleaned, harmonized, and entered into a single dataset, further increasing confidence in the quality of the data and allowing us to adjust for a single set of standard confounders. Finally, all available OCAC studies with information on offspring sex were included, thus mitigating the possibility of publication bias.
Despite these strengths, some limitations should be considered. First, data were self-reported; thus, potential confounding variables could be influenced by case/control status, which could distort our findings. Moreover, due to missing data, we were not able to assess relationships between offspring sex and some factors that may influence ovarian cancer risk, such as age at first pregnancy. We also can not eliminate the possibility of unknown confounders influencing results. Selection bias is also a concern as controls participating in these studies may differ from cases by factors related to offspring sex or EOC risk, including unknown factors that could not be accounted for in the analyses. Validation in prospective cohorts is needed to address these concerns. Because our study population was predominately white, we could not evaluate the impact of offspring sex in non-white women and how it may differ across race. Finally, we cannot eliminate the possibility that our findings are due to chance.
In conclusion, offspring sex appears to affect differentially EOC risk based on histotype and, possibly, in combination with other host factors. Our findings support the distinct etiologic pathways among EOC histotypes and suggest that current etiologic models of EOC may be incomplete. Our findings also suggest the need to better understand how pregnancy affects EOC risk. Confirmation of these findings in prospective cohorts is needed to improve our understanding of EOC etiology, thereby paving the way for new avenues of prevention research for this highly fatal disease.
Acknowledgements
We are grateful to the family and friends of Kathryn Sladek Smith for their generous support of the Ovarian Cancer Association Consortium through their donations to the Ovarian Cancer Research Fund. We thank the study participants, doctors, nurses, research staff, clinical and scientific collaborators, health care providers, and health information sources who have contributed to the many studies contributing to this manuscript.
Acknowledgements for individual studies: AUS: The AOCS also acknowledges the cooperation of the participating institutions in Australia, and the contribution of the study nurses, research assistants and all clinical and scientific collaborators. The complete AOCS Study Group can be found at www.aocstudy.org. We would like to thank all of the women who participated in this research program; CON: The cooperation of the 32 Connecticut hospitals, including Stamford Hospital, in allowing patient access, is gratefully acknowledged. This study was approved by the State of Connecticut Department of Public Health Human Investigation Committee. Certain data used in this study were obtained from the Connecticut Tumor Registry in the Connecticut Department of Public Health. The authors assume full responsibility for analyses and interpretation of these data; GER: The German Ovarian Cancer Study (GER) thank Sabine Behrens for competent technical assistance; UKO: We particularly thank I. Jacobs, M. Widschwendter, E. Wozniak, A. Ryan, J. Ford and N. Balogun for their contribution to the study.
Funding:
The Ovarian Cancer Association Consortium was supported by a grant from the Ovarian Cancer Research Fund thanks to donations by the family and friends of Kathryn Sladek Smith (PPD/RPCI.07).
Funding for individual studies: AUS: The Australian Ovarian Cancer Study (AOCS) was supported by the U.S. Army Medical Research and Materiel Command (DAMD17–01-1–0729), National Health & Medical Research Council of Australia (199600, 400413 and 400281), Cancer Councils of New South Wales, Victoria, Queensland, South Australia and Tasmania and Cancer Foundation of Western Australia (Multi-State Applications 191, 211 and 182). AOCS gratefully acknowledges additional support from Ovarian Cancer Australia and the Peter MacCallum Foundation; CON: National Institutes of Health (R01-CA074850; R01-CA080742); DKE: Ovarian Cancer Research Fund; GER: German Federal Ministry of Education and Research, Programme of Clinical Biomedical Research (01 GB 9401) and the German Cancer Research Center (DKFZ); HAW: U.S. National Institutes of Health (R01-CA58598, N01-CN-55424 and N01-PC-67001); HOP: University of Pittsburgh School of Medicine Dean’s Faculty Advancement Award (F. Modugno), Department of Defense (DAMD17–02-1–0669) and NCI (K07-CA080668, R01-CA95023, MO1-RR000056 R01-CA126841); NCO: National Institutes of Health (R01-CA76016) and the Department of Defense (DAMD17–02-1–0666); NJO: National Cancer Institute (NIH-K07 CA095666, R01-CA83918, NIH-K22-CA138563, and P30-CA072720) and Rutgers Cancer Institute of New Jersey; SON: National Health Research and Development Program, Health Canada, grant 6613–1415-53; TBO: National Institutes of Health (R01-CA106414-A2), American Cancer Society (CRTG-00–196-01-CCE), Department of Defense (DAMD17–98-1–8659), Celma Mastery Ovarian Cancer Foundation; TOR: NIH grants R01 CA063678 and R01 CA063682; UKO: The UKOPS study was funded by The Eve Appeal (The Oak Foundation) with investigators supported by the National Institute for Health Research University College London Hospitals Biomedical Research Centre and MRC core funding (MR_UU_12023); USC: P01CA17054, P30CA14089, R01CA61132, N01PC67010, R03CA113148, R03CA115195, N01CN025403, and California Cancer Research Program (00–01389V-20170, 2II0200);
Footnotes
Conflict of Interest: Usha Menon has stock ownership awarded to her by UCL in Abcodia. No other authors report conflicts of interest.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES:
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Kosary CL, et al. SEER Cancer Statistics Review, 1975–2014, National Cancer Institute. Bethesda, MD, https://seercancergov/csr/1975_2014/, based on November 2016 SEER data submission, posted to the SEER web site, April 2017. [Google Scholar]
- 3.Moyer VA. Screening for ovarian cancer: U.S. Preventive Services Task Force reaffirmation recommendation statement. Ann Intern Med. 2012;157(12):900–4. [DOI] [PubMed] [Google Scholar]
- 4.Modugno F, Edwards RP. Ovarian cancer: prevention, detection, and treatment of the disease and its recurrence. Molecular mechanisms and personalized medicine meeting report. Int J Gynecol Cancer. 2012;22(8):S45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cho KR, Shih Ie M. Ovarian cancer. Annu Rev Pathol. 2009;4:287–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sung HK, Ma SH, Choi JY, Hwang Y, Ahn C, Kim BG, et al. The Effect of Breastfeeding Duration and Parity on the Risk of Epithelial Ovarian Cancer: A Systematic Review and Meta-analysis. J Prev Med Public Health. 2016;49(6):349–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaitskell K, Green J, Pirie K, Barnes I, Hermon C, Reeves GK, et al. Histological subtypes of ovarian cancer associated with parity and breastfeeding in the prospective Million Women Study. International journal of cancer. 2018;142(2):281–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wentzensen N, Poole EM, Trabert B, White E, Arslan AA, Patel AV, et al. Ovarian Cancer Risk Factors by Histologic Subtype: An Analysis From the Ovarian Cancer Cohort Consortium. J Clin Oncol. 2016;34(24):2888–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fathalla MF. Incessant ovulation--a factor in ovarian neoplasia? Lancet. 1971;2(7716):163. [DOI] [PubMed] [Google Scholar]
- 10.Risch HA, Weiss NS, Lyon JL, Daling JR, Liff JM. Events of reproductive life and the incidence of epithelial ovarian cancer. Am J Epidemiol. 1983;117(2):128–39. [DOI] [PubMed] [Google Scholar]
- 11.Risch HA. Hormonal etiology of epithelial ovarian cancer, with a hypothesis concerning the role of androgens and progesterone. J Natl Cancer Inst. 1998;90(23):1774–86. [DOI] [PubMed] [Google Scholar]
- 12.Ness RB, Cottreau C. Possible role of ovarian epithelial inflammation in ovarian cancer. J Natl Cancer Inst. 1999;91(17):1459–67. [DOI] [PubMed] [Google Scholar]
- 13.Toriola AT, Grankvist K, Agborsangaya CB, Lukanova A, Lehtinen M, Surcel HM. Changes in pre-diagnostic serum C-reactive protein concentrations and ovarian cancer risk: a longitudinal study. Ann Oncol. 2011;22(8):1916–21. [DOI] [PubMed] [Google Scholar]
- 14.Obiekwe BC, Chard T. Human chorionic gonadotropin levels in maternal blood in late pregnancy: relation to birthweight, sex and condition of the infant at birth. Br J Obstet Gynaecol. 1982;89(7):543–6. [DOI] [PubMed] [Google Scholar]
- 15.Adamcova K, Kolatorova L, Skodova T, Simkova M, Parizek A, Starka L, et al. Steroid hormone levels in the peripartum period - differences caused by fetal sex and delivery type. Physiol Res. 2018;67(Supplementum 3):S489–s97. [DOI] [PubMed] [Google Scholar]
- 16.Boroditsky RS, Reyes FI, Winter JS, Faiman C. Serum human chorionic gonadotropin and progesterone patterns in the last trimester of pregnancy: relationship to fetal sex. Am J Obstet Gynecol. 1975;121(2):238–41. [DOI] [PubMed] [Google Scholar]
- 17.Enninga EA, Nevala WK, Creedon DJ, Markovic SN, Holtan SG. Fetal sex-based differences in maternal hormones, angiogenic factors, and immune mediators during pregnancy and the postpartum period. Am J Reprod Immunol. 2015;73(3):251–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toriola AT, Vaarasmaki M, Lehtinen M, Zeleniuch-Jacquotte A, Lundin E, Rodgers KG, et al. Determinants of maternal sex steroids during the first half of pregnancy. Obstet Gynecol. 2011;118(5):1029–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steier JA, Ulstein M, Myking OL. Human chorionic gonadotropin and testosterone in normal and preeclamptic pregnancies in relation to fetal sex. Obstet Gynecol. 2002;100(3):552–6. [DOI] [PubMed] [Google Scholar]
- 20.Ose J, Poole EM, Schock H, Lehtinen M, Arslan AA, Zeleniuch-Jacquotte A, et al. Androgens Are Differentially Associated with Ovarian Cancer Subtypes in the Ovarian Cancer Cohort Consortium. Cancer Res. 2017;77(14):3951–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trabert B, Michels KA, Anderson GL, Brinton LA, Falk RT, Geczik AM, et al. Circulating androgens and postmenopausal ovarian cancer risk in the Women’s Health Initiative Observational Study. Int J Cancer. 2019;145(8):2051–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clifton VL, Murphy VE. Maternal asthma as a model for examining fetal sex-specific effects on maternal physiology and placental mechanisms that regulate human fetal growth. Placenta. 2004;25 Suppl A:S45–52. [DOI] [PubMed] [Google Scholar]
- 23.Stark MJ, Dierkx L, Clifton VL, Wright IM. Alterations in the maternal peripheral microvascular response in pregnancies complicated by preeclampsia and the impact of fetal sex. J Soc Gynecol Investig. 2006;13(8):573–8. [DOI] [PubMed] [Google Scholar]
- 24.Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care. 2002;25(10):1862–8. [DOI] [PubMed] [Google Scholar]
- 25.Ying W, Catov JM, Ouyang P. Hypertensive Disorders of Pregnancy and Future Maternal Cardiovascular Risk. J Am Heart Assoc. 2018;7(17):e009382.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gierach GL, Modugno F, Ness RB. Gender of offspring and maternal ovarian cancer risk. Gynecol Oncol. 2006;101(3):476–80. [DOI] [PubMed] [Google Scholar]
- 27.Baik I, Lambe M, Liu Q, Cnattingius S, Mucci LA, Riman T, et al. Gender of offspring and maternal risk of invasive epithelial ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2007;16(11):2314–20. [DOI] [PubMed] [Google Scholar]
- 28.Albrektsen G, Heuch I, Thoresen S, Kvale G. Twin births, sex of children and maternal risk of ovarian cancer: a cohort study in Norway. Br J Cancer. 2007;96(9):1433–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jordan SJ, Green AC, Nagle CM, Olsen CM, Whiteman DC, Webb PM, et al. Beyond parity: association of ovarian cancer with length of gestation and offspring characteristics. Am J Epidemiol. 2009;170(5):607–14. [DOI] [PubMed] [Google Scholar]
- 30.Fu Z, Moysich K, Ness RB, Modugno F. Gender of offspring and risk of ovarian cancer: The HOPE study. Cancer Epidemiol. 2019;64:101646.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Committee on the State of the Science in Ovarian Cancer R, Board on Health Care S, Institute of M, National Academies of Sciences E, Medicine. Ovarian Cancers: Evolving Paradigms in Research and Care. Washington (DC): National Academies Press (US) Copyright 2016 by the National Academy of Sciences. All rights reserved.; 2016. [Google Scholar]
- 32.Ramus SJ, Vierkant RA, Johnatty SE, Pike MC, Van Den Berg DJ, Wu AH, et al. Consortium analysis of 7 candidate SNPs for ovarian cancer. Int J Cancer. 2008;123(2):380–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Merritt MA, Green AC, Nagle CM, Webb PM. Talcum powder, chronic pelvic inflammation and NSAIDs in relation to risk of epithelial ovarian cancer. Int J Cancer. 2008;122(1):170–6. [DOI] [PubMed] [Google Scholar]
- 34.Risch HA, Bale AE, Beck PA, Zheng W. PGR +331 A/G and increased risk of epithelial ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2006;15(9):1738–41. [DOI] [PubMed] [Google Scholar]
- 35.Royar J, Becher H, Chang-Claude J. Low-dose oral contraceptives: protective effect on ovarian cancer risk. Int J Cancer. 2001;95(6):370–4. [DOI] [PubMed] [Google Scholar]
- 36.Goodman MT, Lurie G, Thompson PJ, McDuffie KE, Carney ME. Association of two common single-nucleotide polymorphisms in the CYP19A1 locus and ovarian cancer risk. Endocr Relat Cancer. 2008;15(4):1055–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lo-Ciganic WH, Zgibor JC, Bunker CH, Moysich KB, Edwards RP, Ness RB. Aspirin, nonaspirin nonsteroidal anti-inflammatory drugs, or acetaminophen and risk of ovarian cancer. Epidemiology. 2012;23(2):311–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lurie G, Wilkens LR, Thompson PJ, McDuffie KE, Carney ME, Terada KY, et al. Combined oral contraceptive use and epithelial ovarian cancer risk: time-related effects. Epidemiology. 2008;19(2):237–43. [DOI] [PubMed] [Google Scholar]
- 39.Schildkraut JM, Iversen ES, Wilson MA, Clyde MA, Moorman PG, Palmieri RT, et al. Association between DNA damage response and repair genes and risk of invasive serous ovarian cancer. PLoS One. 2010;5(4):e10061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bandera EV, King M, Chandran U, Paddock LE, Rodriguez-Rodriguez L, Olson SH. Phytoestrogen consumption from foods and supplements and epithelial ovarian cancer risk: a population-based case control study. BMC Womens Health. 2011;11:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Risch HA, Marrett LD, Howe GR. Parity, contraception, infertility, and the risk of epithelial ovarian cancer. Am J Epidemiol. 1994;140(7):585–97. [DOI] [PubMed] [Google Scholar]
- 42.Pal T, Permuth-Wey J, Betts JA, Krischer JP, Fiorica J, Arango H, et al. BRCA1 and BRCA2 mutations account for a large proportion of ovarian carcinoma cases. Cancer. 2005;104(12):2807–16. [DOI] [PubMed] [Google Scholar]
- 43.Balogun N, Gentry-Maharaj A, Wozniak EL, Lim A, Ryan A, Ramus SJ, et al. Recruitment of newly diagnosed ovarian cancer patients proved challenging in a multicentre biobanking study. J Clin Epidemiol. 2011;64(5):525–30. [DOI] [PubMed] [Google Scholar]
- 44.Ness RB, Cramer DW, Goodman MT, Kjaer SK, Mallin K, Mosgaard BJ, et al. Infertility, fertility drugs, and ovarian cancer: a pooled analysis of case-control studies. Am J Epidemiol. 2002;155(3):217–24. [DOI] [PubMed] [Google Scholar]
- 45.Wu AH, Pearce CL, Tseng CC, Templeman C, Pike MC. Markers of inflammation and risk of ovarian cancer in Los Angeles County. Int J Cancer. 2009;124(6):1409–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Olsen CM, Nagle CM, Whiteman DC, Ness R, Pearce CL, Pike MC, et al. Obesity and risk of ovarian cancer subtypes: evidence from the Ovarian Cancer Association Consortium. Endocr Relat Cancer. 2013;20(2):251–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen P, Wang DB, Liang YM. Evaluation of estrogen in endometriosis patients: Regulation of GATA-3 in endometrial cells and effects on Th2 cytokines. J Obstet Gynaecol Res. 2016;42(6):669–77. [DOI] [PubMed] [Google Scholar]
- 48.Al-Sabbagh M, Lam EW, Brosens JJ. Mechanisms of endometrial progesterone resistance. Mol Cell Endocrinol. 2012;358(2):208–15. [DOI] [PubMed] [Google Scholar]
- 49.Nisenblat V, Norman RJ. Androgens and polycystic ovary syndrome. Curr Opin Endocrinol Diabetes Obes. 2009;16(3):224–31. [DOI] [PubMed] [Google Scholar]
- 50.Diamanti-Kandarakis E Polycystic ovarian syndrome: pathophysiology, molecular aspects and clinical implications. Expert Rev Mol Med. 2008;10:e3. [DOI] [PubMed] [Google Scholar]
- 51.Goodman NF, Cobin RH, Futterweit W, Glueck JS, Legro RS, Carmina E. American association of clinical endocrinologists, Amercian college of endocrinology, and androgen excess and PCOS society disease state clinical review: guide to the best practices in the evaluation and treament of polycystic syndrome -- part 1. Endocr Pract. 2015;21(11):1291–300. [DOI] [PubMed] [Google Scholar]
- 52.Apter D, Vihko R. Early menarche, a risk factor for breast cancer, indicates early onset of ovulatory cycles. J Clin Endocrinol Metab. 1983;57(1):82–6. [DOI] [PubMed] [Google Scholar]
- 53.Vihko R, Apter D. Endocrine characteristics of adolescent menstrual cycles: impact of early menarche. J Steroid Biochem. 1984;20(1):231–6. [DOI] [PubMed] [Google Scholar]
- 54.Bernstein L, Pike MC, Ross RK, Henderson BE. Age at menarche and estrogen concentrations of adult women. Cancer Causes Control. 1991;2(4):221–5. [DOI] [PubMed] [Google Scholar]
- 55.Kopelman PG. Hormones and obesity. Baillieres Clin Endocrinol Metab. 1994;8(3):549–75. [DOI] [PubMed] [Google Scholar]
- 56.Pasquali R Obesity and androgens: facts and perspectives. Fertil Steril. 2006;85(5):1319–40. [DOI] [PubMed] [Google Scholar]
- 57.Rosenfield RL. Clinical review: Adolescent anovulation: maturational mechanisms and implications. J Clin Endocrinol Metab. 2013;98(9):3572–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gaspard UJ, Romus MA, Gillain D, Duvivier J, Demey-Ponsart E, Franchimont P. Plasma hormone levels in women receiving new oral contraceptives containing ethinyl estradiol plus levonorgestrel or desogestrel. Contraception. 1983;27(6):577–90. [DOI] [PubMed] [Google Scholar]
- 59.Brenner PF, Mishell DR Jr., Stanczyk FZ, Goebelsmann U. Serum levels of d-norgestrel, luteinizing hormone, follicle-stimulating hormone, estradiol, and progesterone in women during and following ingestion of combination oral contraceptives containing dl-norgestrel. Am J Obstet Gynecol. 1977;129(2):133–40. [DOI] [PubMed] [Google Scholar]
- 60.Mishell DR Jr., Thorneycroft IH, Nakamura RM, Nagata Y, Stone SC. Serum estradiol in women ingesting combination oral contraceptive steroids. Am J Obstet Gynecol. 1972;114(7):923–8. [DOI] [PubMed] [Google Scholar]
- 61.Baron JA, La Vecchia C, Levi F. The antiestrogenic effect of cigarette smoking in women. Am J Obstet Gynecol. 1990;162(2):502–14. [DOI] [PubMed] [Google Scholar]
- 62.Cramer DW, Welch WR. Determinants of ovarian cancer risk. II. Inferences regarding pathogenesis. J Natl Cancer Inst. 1983;71(4):717–21. [PubMed] [Google Scholar]
- 63.Zeitlin J, Saurel-Cubizolles MJ, De Mouzon J, Rivera L, Ancel PY, Blondel B, et al. Fetal sex and preterm birth: are males at greater risk? Hum Reprod. 2002;17(10):2762–8. [DOI] [PubMed] [Google Scholar]
- 64.Di Renzo GC, Rosati A, Sarti RD, Cruciani L, Cutuli AM. Does fetal sex affect pregnancy outcome? Gend Med. 2007;4(1):19–30. [DOI] [PubMed] [Google Scholar]
- 65.Retnakaran R, Kramer CK, Ye C, Kew S, Hanley AJ, Connelly PW, et al. Fetal sex and maternal risk of gestational diabetes mellitus: the impact of having a boy. Diabetes Care. 2015;38(5):844–51. [DOI] [PubMed] [Google Scholar]
- 66.Shiozaki A, Matsuda Y, Satoh S, Saito S. Impact of fetal sex in pregnancy-induced hypertension and preeclampsia in Japan. J Reprod Immunol. 2011;89(2):133–9. [DOI] [PubMed] [Google Scholar]
- 67.Clifton V Maternal asthma during pregnancy and fetal outcomes: potential mechanisms and possible solutions. Curr Opin Allergy Clin Immunol. 2006;6(5):307–11. [DOI] [PubMed] [Google Scholar]
- 68.Gong S, Sovio U, Aye IL, Gaccioli F, Dopierala J, Johnson MD, et al. Placental polyamine metabolism differs by fetal sex, fetal growth restriction, and preeclampsia. JCI Insight. 2018;3(13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nelson JL. Microchimerism and human autoimmune diseases. Lupus. 2002;11(10):651–4. [DOI] [PubMed] [Google Scholar]
- 70.Gammill HS, Nelson JL. Naturally acquired microchimerism. Int J Dev Biol. 2010;54(2–3):531–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bianchi DW, Zickwolf GK, Weil GJ, Sylvester S, DeMaria MA. Male fetal progenitor cells persist in maternal blood for as long as 27 years postpartum. Proc Natl Acad Sci U S A. 1996;93(2):705–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hallum S, Jakobsen MA, Gerds TA, Pinborg A, Tjønneland A, Kamper-Jørgensen M. Male origin microchimerism and ovarian cancer. International Journal of Epidemiology. 2020. [DOI] [PubMed] [Google Scholar]
- 73.Seidman JD, Kurman RJ, Ronnett BM. Primary and metastatic mucinous adenocarcinomas in the ovaries: incidence in routine practice with a new approach to improve intraoperative diagnosis. Am J Surg Pathol. 2003;27(7):985–93. [DOI] [PubMed] [Google Scholar]
- 74.Risch HA, Marrett LD, Jain M, Howe GR. Differences in risk factors for epithelial ovarian cancer by histologic type. Results of a case-control study. Am J Epidemiol. 1996;144(4):363–72. [DOI] [PubMed] [Google Scholar]
- 75.Yang HP, Murphy KR, Pfeiffer RM, George N, Garcia-Closas M, Lissowska J, et al. Lifetime Number of Ovulatory Cycles and Risks of Ovarian and Endometrial Cancer Among Postmenopausal Women. Am J Epidemiol. 2016;183(9):800–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Webb PM, Green A, Cummings MC, Purdie DM, Walsh MD, Chenevix-Trench G. Relationship between number of ovulatory cycles and accumulation of mutant p53 in epithelial ovarian cancer. J Natl Cancer Inst. 1998;90(22):1729–34. [DOI] [PubMed] [Google Scholar]
- 77.Trabert B, Tworoger SS, O’Brien KM, Townsend MK, Fortner RT, Iversen ES, et al. The Risk of Ovarian Cancer Increases with an Increase in the Lifetime Number of Ovulatory Cycles: An Analysis from the Ovarian Cancer Cohort Consortium (OC3). Cancer Res. 2020;80(5):1210–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee AW, Ness RB, Roman LD, Terry KL, Schildkraut JM, Chang-Claude J, et al. Association Between Menopausal Estrogen-Only Therapy and Ovarian Carcinoma Risk. Obstet Gynecol. 2016;127(5):828–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Beral V, Gaitskell K, Hermon C, Moser K, Reeves G, Peto R. Menopausal hormone use and ovarian cancer risk: individual participant meta-analysis of 52 epidemiological studies. Lancet. 2015;385(9980):1835–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pearce CL, Templeman C, Rossing MA, Lee A, Near AM, Webb PM, et al. Association between endometriosis and risk of histological subtypes of ovarian cancer: a pooled analysis of case-control studies. Lancet Oncol. 2012;13(4):385–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kurman RJ, Shih Ie M. Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer--shifting the paradigm. Hum Pathol. 2011;42(7):918–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jarboe EA, Folkins AK, Drapkin R, Ince TA, Agoston ES, Crum CP. Tubal and ovarian pathways to pelvic epithelial cancer: a pathological perspective. Histopathology. 2009;55(5):619. [DOI] [PubMed] [Google Scholar]