Abstract
Background
The side effects of busulfan on male reproduction are serious, so fertility preservation in children undergoing busulfan treatment is a major worldwide concern. Human placental mesenchymal stem cells (hPMSCs) have advantages such as stable proliferation and lower immunogenicity that make them an ideal material for stimulating tissue repair, especially restoring spermatogenesis. The protective effects of hPMSCs in busulfan-induced Sertoli cells and in busulfan-treated mouse testes have not been determined. Our study aimed to elaborate the protective effect and potential mechanisms of hPMSCs in busulfan-treated testes and Sertoli cells.
Methods
First, we developed a mouse model of busulfan-induced testicular toxicity in vivo and a mouse Sertoli cell line treated with busulfan in vitro to assess the protective effect and mechanisms of hPMSC treatment on spermatogenesis. Then, the length, width, and weight of the testes were monitored using Vernier calipers. Furthermore, at 1 week and 4 weeks after the transplantation of hPMSCs, histological sections of testes were stained with hematoxylin-eosin, and the seminiferous tubules with fluid-filled cavities were counted. Through ELISA analysis, testosterone levels and MDA, SOD, LDH, and CAT activities, which are associated with ROS, were detected. Markers of ROS, proliferation (Ki67), and apoptosis (Annexin V) were evaluated by FACS. Next, the fluorescence intensity of proliferation markers (BrdU and SCP3), an antioxidant marker (SIRT1), a spermatogenesis marker (PLZF), and autophagy-related genes (P62 and LC3AB) were detected by fluorescence microscopy. The mRNA expression of γ-H2AX, BRCA1, PARP1, PCNA, Ki67, P62, and LC3 was determined by qRT-PCR.
Results
hPMSCs restored disrupted spermatogenesis, promoted improved semen parameters, and increased testosterone levels, testis size, and autophagy in the testis toxicity mouse model induced by busulfan. hPMSCs suppressed the apoptosis of Sertoli cells and enhanced their rate of proliferation in vitro. Additionally, hPMSCs protected against oxidative stress and decreased oxidative damage in the testis toxicity mouse model induced by busulfan. Furthermore, hPMSCs increased the expression of proliferation genes (PCNA and KI67) and decreased the mRNA levels of apoptotic genes such as γ-H2AX, BRCA1, and PARP1.
Conclusions
This research showed that hPMSC injection ameliorated busulfan-induced damage in the testis by reducing apoptosis/oxidative stress and promoting autophagy. The present study offers an idea for a new method for clinical treatment of chemotherapy-induced spermatogenesis.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13287-021-02275-z.
Keywords: Busulfan, Spermatogenesis, Human placental mesenchymal stem cells, Autophagy, Reactive oxygen species, Apoptosis
Background
Spermatogenesis of mammals is a profoundly organized and dynamic cell differentiation process with three stages: mitosis, meiosis, and spermatogenesis [1]. In the process of spermatogenesis, Sertoli cells play crucial roles that include regulating multidirectional differentiation and spontaneous recovery of spermatogonia stem cells (SSCs), providing nourishment as well as fundamental support for developing germ cells and so on [2]. Deviation in these processes could lead to abnormal characteristics in the morphology, function, and motility of the sperm [3]. For instance, the spermatogenesis process is susceptible to toxicity, contamination, anticancer medicine, and other factors [4–6]. In particular, spermatogonial progenitor cells (SPCs) are sensitive to the toxicity of busulfan, but the molecular mechanism remains largely unknown [7].
Busulfan is a common chemotherapeutic drug that prevents cellular division, and consequently, germ cells, which have high rates of division, are vulnerable to busulfan [8]. Several studies have focused on the mechanism of busulfan toxicity, such as ROS-induced apoptosis [9]. Nevertheless, the potential mechanism induced by busulfan is very intricate since it probably involves multiple biological reactions, e.g., reactive oxygen species, proliferation, apoptosis, and autophagy [10]. Thus, the side effects of busulfan on male reproduction are so severe that fertility preservation in children undergoing busulfan treatment is a major worldwide concern.
Mesenchymal stem cells (MSCs) hold great promise for rehabilitating the microenvironment of spermatogenesis. For instance, mesenchymal stem cells derived from the human placenta are in clinical trials for a variety of restorative applications [11]. Compared with MSCs from other sources, human placental mesenchymal stem cells (hPMSCs) have the advantages of stable proliferation and low immunogenicity. These characteristics of hPMSCs make them an ideal material for stimulating tissue repair. Our group confirmed that hPMSCs ameliorate premature ovarian insufficiency via NRF2/HO-1 activation [12]. However, the mechanism by which hPMSCs restore spermatogenesis in busulfan-induced mouse testes is not clear.
Autophagy is a highly conserved membrane-trafficking process for degrading long-lived proteins and organelles by lysosomes. Autophagy starts with the development of a vesicle with a double membrane, which is named an autophagosome. The autophagosome moves along cytoskeletal structures and merges with lysosomes, creating an autolysosome [13]. To date, more than forty autophagy-related (ATG) proteins have been identified [14, 15]. Upon induction of autophagy in mammals, LC3 is activated by ATG3 and ATG7 and attaches to the lipid-containing membrane. The lipid-containing membrane serves as a scaffold to establish phagophores that become enclosed autophagosomes. Autophagy participates in many non-pathological processes, such as preimplantation development [16], ectoplasmic specialization assembly in Sertoli cells [17], and spermatid differentiation [18]. In addition, oxidative stress above normal physiological levels can damage sperm DNA, leading to male infertility [19]. A previous study also showed that apoptosis is a key physiological process in the development of testes [20]. However, the function of autophagy, apoptosis, and oxidative stress in busulfan-induced spermatogenesis remains largely unknown.
To date, the protective effects of hPMSCs in mouse testes treated with busulfan and in mouse Sertoli cells induced by busulfan have not been elaborated. Therefore, our research aimed to identify whether hPMSCs could ameliorate chemotherapy-induced damage in the testes of mice by reducing apoptosis/oxidative stress and promoting autophagy.
Methods
Preparation of hPMSCs
After being dissected and immediately placed in solution, samples of the human placenta were thoroughly rinsed in PBS containing antibiotic-antimycotic (100 U/ml penicillin G and 100 mg/ml streptomycin; Thermo Fisher Scientific) for 60 min on ice. The samples were split into quadrants, and then, the villous chorion and chorionic plate were diced to small pieces no more than 1 mm in length after the removal of the amniotic membrane layer. To release the cells, the minced tissues were enzymatically digested by trypsin (10 g) in culture medium for 60 min at 37 °C, and dispase (4 mg/ml, Thermo Fisher Scientific) plus Collagenase Type IV (3 mg/ml, Thermo Fisher Scientific) was added. The samples were centrifuged for 5 min at room temperature in culture medium containing 10% fetal bovine serum (FBS) before the reaction was ended. Approximately 3 × 107 cells were transplanted into each 10-cm cell culture dish. The cells started growing adherently after 3 days, and within 1 week, cell clusters formed. All experiments were performed using 3rd–4th-generation hPMSCs.
RNA extraction and reverse transcription-polymerase chain reaction (RT-PCR)
After hPMSC treatment, a Qiagen RNeasy Mini Kit (Qiagen, USA) was used to extract total RNA from the testes of the four groups of mice. A Prime-Script RT Reagent Kit (Takara, Japan) was used to reverse transcribe RNA into cDNA, SYBR Premix Ex Taq (Takara, Japan) was used to perform quantitative real-time polymerase chain reaction (PCR) utilizing a Thermal Cycler Dice Real-Time System (Takara, Japan), and the 2-ΔΔCt calculation method was employed to analyze the data. The primer sequences for the genes γ-H2AX, BRCA I, PARP1, PCNA, KI67, P62, and LC3 are shown in Supplementary Table.
Sertoli cell preparation and treatment
The TM4 mouse Sertoli cell line (Procell, China) was acquired and cultured at 1 × 105 cells per well utilizing specific complete medium for TM4 (Procell, China) in an incubator. The cell medium was changed every 2 days, and the cells were trypsinized and passaged at 80–90% confluence. Busulfan was added to Sertoli cells based on a previously described approach with slight alterations [21]. Briefly, Sertoli cells were treated with 10− 4 μM busulfan and cultured in an incubator for 48 h. After that, hPMSCs were cocultured with the busulfan-induced Sertoli cells for 48 h using a Transwell system. The Sertoli cells were then divided into three groups: the control group (untreated), BU group (busulfan-treated), and BU/hPMSC group (treated with hPMSCs after busulfan treatment).
Experimental mouse model
Nanjing Medical University provided male C57BL/6 mice. They were fed as described in our previous study [22]. A mouse model of testicular toxicity induced by busulfan was established using the previously described approach with some alterations. Briefly, busulfan was dissolved in DMSO and diluted with distilled water to 5 mg/mL. Busulfan (40 mg/kg) was injected into the enterocoelia of mice. Approximately 5 × 106 hPMSCs suspended in normal saline were injected into the testes of mice, which were subsequently sacrificed at 1 or 4 weeks after the hPMSC treatment. Consequently, the mice were divided into three groups: the control group (untreated), BU group (busulfan treated), and BU/hPMSC group (treated with hPMSCs after busulfan treatment).
Testes measurement and histological analysis
The blood and testes were acquired for ELISA detection and HE staining, respectively. Euthanasia was used on all mice, and the blood, testes, and epididymis were collected for follow-up experiments. Furthermore, the length, width, and weight of the testes were measured using Vernier calipers. After the transplantation of hPMSCs, the testes of mice were fixed with paraformaldehyde. The tissues were dehydrated and then clarified by xylene and paraffin-embedded, and the paraffin-embedded blocks were cut into slices of 5-μm thickness. As described in a previous study, slices of 5-μm thickness were stained with hematoxylin-eosin and examined with an optical microscope [23]. Five typical sections containing convoluted tubules with vacuoles were examined from each testis.
ELISA analysis
Serum levels of testosterone, superoxide dismutase (SOD), malondialdehyde (MDA), catalase (CAT), and lactate dehydrogenase (LDH) were measured by ELISA (Cayman Chemical, USA). In short, 50-μl culture medium or serum was added to the plate and incubated at 37 °C for 30 min. Then, the wells were washed 5 times for 10 s each, and the cells were incubated with 50 mol/l HRP-coupling reagent at 37 °C for 60 min. The wells were washed five times for 10 s each and incubated with a mixture of substrate B and A solution (50 mol/L) at 37 °C for 30 min; last, the reaction was ended by adding 50 μl stop solution. Finally, the absorbance was measured with a spectrophotometer (BioTek, USA).
Fluorescence-activated cell sorting (FACS) analysis
To detect ROS, Ki67, and Annexin V, Sertoli cells were collected. The mouse testes were digested with 0.25% trypsin-EDTA to produce a single-cell suspension. Furthermore, the Fixation and Permeabilization Solution Kit (BD, USA) was used to fix and permeabilize the digested cells. The cells were labeled with FITC-conjugated anti-Annexin V (BD, USA), PE-conjugated anti-Ki67 (BD, USA), and PE-conjugated anti-ROS (Abcam, USA) antibodies and their isotype controls at 4 °C for 30 min. After that, flow cytometry (Beckman, USA) was used for analysis according to the manufacturer’s instructions.
BrdU assay and immunofluorescence of SCP3, SIRT1, PPLZF, P62, and LC3AB
First, the slides were dewaxed with xylene, rehydrated with isopropyl alcohol, incubated with a proteinase K solution, fixed with formaldehyde, and eventually incubated in a DNA labeling solution and an antibody solution. Anti-scp3 antibody, anti-SIRT1 antibody, anti-PLZF antibody, anti-P62 antibody, and anti-LC3AB antibody (Abcam, USA) were used for backstaining, and 4% PFA was used for fixing (Sigma, USA); after that, 0.1% Triton X-100 (Sigma, USA) was used for permeating, and 4% bovine serum albumin (BSA, Sigma, USA) was used for blocking. The slides were incubated with the above five antibodies, and BrdU staining was performed overnight at 4 °C. The sections were stained with FITC-conjugated secondary antibodies (Jackson ImmunoResearch, West Grove), stained with Hoechst 33342 (Beyotime Biotechnology, China), and evaluated with a fluorescence microscope (Olympus, Japan).
Statistical analysis
All the experiments in this study were repeated at least three times. Data are expressed as the mean ± standard deviation. SPSS 22.0 software was used for one-way ANOVA to analyze significant differences, which were defined as a P value less than 0.05.
Results
hPMSCs restored disrupted spermatogenesis and raised the levels of testosterone in a mouse model of busulfan-induced testicular toxicity
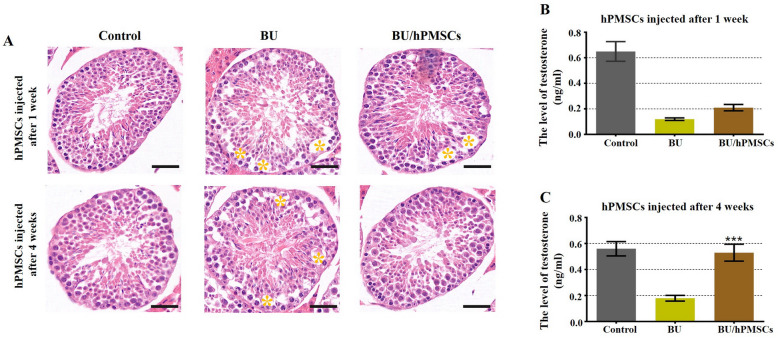
To investigate the therapeutic potential of hPMSCs to restore busulfan-disrupted spermatogenesis, testosterone levels and phenotypic characteristics of the vas deferens in the three groups were determined by ELISA and HE staining, respectively (Fig. 1). The histopathological pictures show that busulfan markedly damaged spermatogenic cells, while complete spermatogenesis occurred in the control group. After the busulfan treatment, obvious vacuolation (marked by a yellow quincunx) was observed in the basal compartment in paraffin sections (Fig. 1a). The results suggested that the testis toxicity mouse model induced by busulfan was successfully established. One week after hPMSC injection, cell vacuolation in the basement membrane was slightly reduced. Surprisingly, cell vacuolation disappeared entirely in the hPMSC-treated group at 4 weeks. Compared with that in the BU group, the testosterone level in the HPMSC group was slightly increased at 1 week (Fig. 1b). Nevertheless, the testosterone level of the hPMSC-treated group was greatly increased at 4 weeks compared with that of the BU group (Fig. 1c).
Fig. 1.
Human placental mesenchymal stem cells (hPMSCs) rescued spermatogenesis and promoted testosterone levels in a busulfan-induced testis toxicity mouse model. a Images of HE-stained mouse testis sections in the three groups (the control group and the 1- and 4-week hPMSC treatment groups). Scale bar = 20 μm. n = 3 for each group. b Testosterone levels were examined 1 week after hPMSC injection in the three groups. c Testosterone levels were detected 4 weeks after hPMSC treatment in the three groups. The error bars indicate the SD. ***p < 0.001
hPMSCs promoted improved semen parameters and increased weight and size of testes in a mouse model of busulfan-induced testicular toxicity
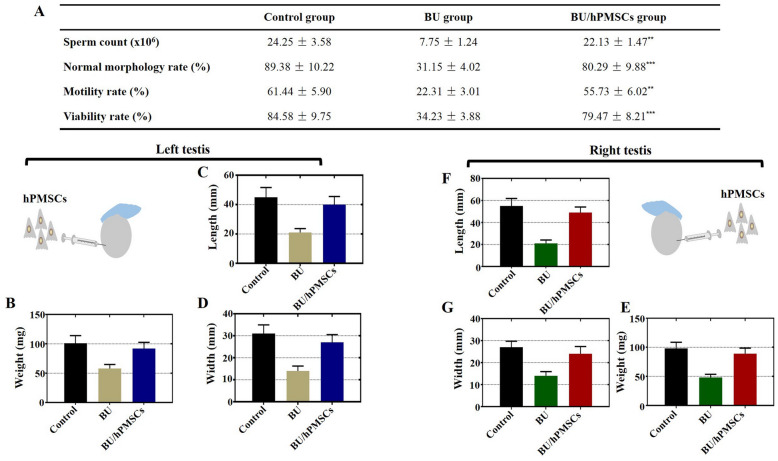
The sperm count of the BU group presented a more than threefold decline compared to that of the control group. The sperm count returned to a level similar to that of the control group at 4 weeks after hPMSC therapy (Fig. 2a). Next, the ratio of sperm cells with normal morphology to those with abnormal morphology decreased greatly in the BU group but rapidly recovered in the BU/hPMSC group compared with that in the control group (Fig. 2a). Additionally, we observed a nearly threefold decrease in the proportion of motile cells in the BU group, and this proportion was restored at 4 weeks after hPMSC treatment (Fig. 2a). Similarly, the proportion of viable cells declined dramatically in the BU group but returned to the level of the control group at 4 weeks after hPMSC therapy (Fig. 2a). Finally, to determine whether hPMSCs restore testicular function, we measured and compared the weight, length, and width of the mouse left and right testes among the three groups. In the BU group, the width, length, and weight of both the right and left testes were reduced by half. Moreover, the three parameters above markedly increased to normal levels at 4 weeks after hPMSC treatment (Fig. 2b–g).
Fig. 2.
hPMSCs promoted increased testicular weight and size and improved semen parameters in a busulfan-induced testis toxicity mouse model. a The sperm count, normal morphology, motility, and proportion of viable cells were determined in the three groups after hPMSC therapy. b–d Variation in the weight, length, and width of the mouse left testis in the three groups after hPMSC treatment. e–g Variation in the weight, length, and width of the mouse right testis in the three groups after hPMSC treatment. The error bars indicate the SD. **p < 0.01,***p < 0.001
hPMSCs suppressed the apoptosis of Sertoli cells and enhanced the rate of proliferation
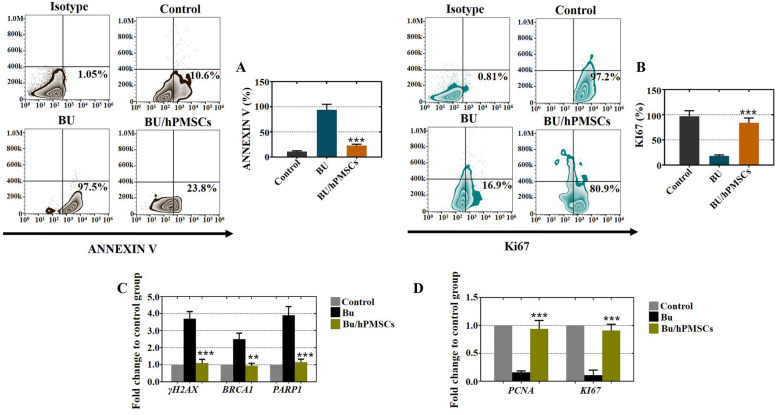
To investigate the role of hPMSCs in enhancing the proliferation and inhibiting the apoptosis of busulfan-induced Sertoli cells, FACS analysis was employed to quantitatively assess cell viability by the proliferation marker of Ki67 and the apoptosis marker of ANNEXIN V. As shown in Fig. 3a, in the BU/hPMSC group, the rate of apoptosis significantly decreased by 23.8%, which was similar to that of the control group but lower than that of the BU group. Additionally, the proliferation rate of the BU/hPMSC group increased by 80.9%, which was higher than that of the BU group (Fig. 3b). Moreover, we used qRT-PCR to detect the expression levels of apoptotic genes (γ-H2AX, BRCA1, and PARP1) and proliferative genes (PCNA and Ki67). As shown in Fig. 3c, after hPMSC treatment, the expression levels of γ-H2AX, BRCA1, and PARP1 in the BU/hPMSC group were similar to those in the control group. The expression patterns of PCNA and Ki67 in the BU/hPMSC group were similar to those in the control group but were greatly decreased in the BU group (Fig. 3d). Briefly, hPMSCs suppressed the apoptosis of Sertoli cells and enhanced the rate of proliferation.
Fig. 3.
In cultured Sertoli cells treated with busulfan, hPMSCs inhibited cell apoptosis and enhanced cell proliferation. a The FACS results indicated that hPMSC treatment inhibited the rate of apoptosis (Annexin V) in Sertoli cells. b The FACS results showed that hPMSC treatment improved the proliferation rate (Ki67) of Sertoli cells. c, d The qRT-PCR results showed that hPMSC treatment suppressed the expression of apoptotic genes (γ-H2AX, BRCA1, and PARP1) and increased the expression of proliferative genes (PCNA and Ki67). All experiments were carried out three times, and the error bars indicate the SD. **p < 0.01, ***p < 0.001
hPMSCs enhanced cell proliferation and alleviated apoptosis in vitro
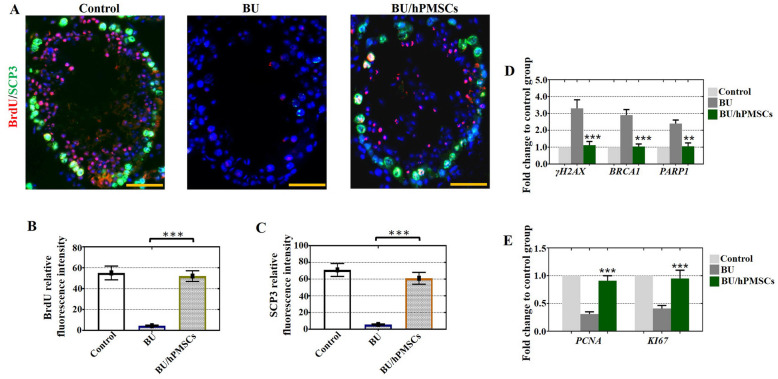
To explore whether hPMSCs boosted cell proliferation and alleviated apoptosis in vitro, a mouse model of busulfan-induced testicular toxicity was introduced to assess cell apoptosis and proliferation features after hPMSC injection. As expected, hPMSC treatment successfully increased the relative fluorescence intensities of BrdU and SCP3 in the BU/hPMSC group compared with the BU group (Fig. 4a–c). In agreement with the fluorescence microscopy results, we observed a rising trend in the mRNA levels of PCNA and Ki67 in the BU/hPMSC group compared with the BU group (Fig. 4e). Moreover, the mRNA levels of apoptotic genes such as γ-H2AX, BRCA1, and PARP1 revealed a decreasing trend in the BU/hPMSC group compared with the BU group (Fig. 4d).
Fig. 4.
hPMSCs improved cell proliferation and alleviated apoptosis of testes in the busulfan-induced testis toxicity mouse model. a–c The fluorescence intensities of BrdU (red) and SCP3 (green) in the three groups were detected by fluorescence microscopy. d, e The mRNA expression of γ-H2AX, BRCA1, PARP1, PCNA, and Ki67 was determined by qRT-PCR. The error bars indicate the SD. **p < 0.01, ***p < 0.001
hPMSCs increased oxidative protection and decreased oxidative damage in a mouse model of busulfan-induced testicular toxicity
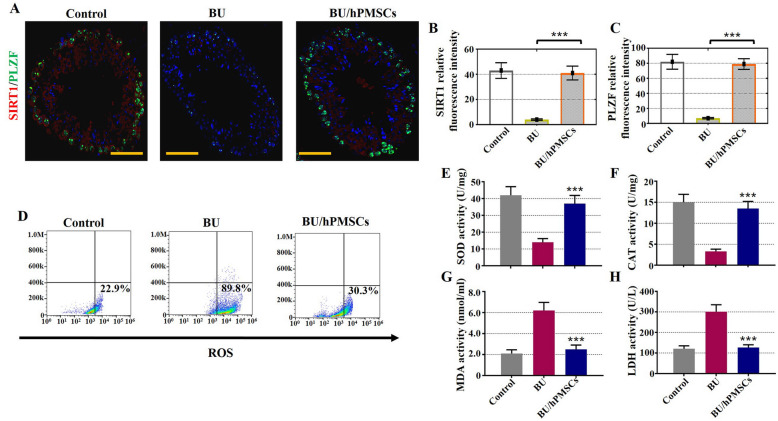
We next sought to verify whether hPMSCs protect against oxidative stress and decrease oxidative damage in the busulfan-induced testis toxicity mouse model. The results of fluorescence microscopy, FACS, and ELISA indicated that hPMSCs rescued spermatogenic function by suppressing oxidative stress. As observed by fluorescence microscopy, hPMSCs successfully enhanced the relative fluorescence intensities of SIRT1 and PLZF in the BU/hPMSC group compared with the BU group (Fig. 5a–c). FACS analysis showed that after hPMSC treatment, the proportion of ROS+ Sertoli cells was significantly lower in the BU/hPMSC group (30.3%) than in the BU group (89.8%) (Fig. 5d). Finally, the levels of antioxidant enzymes such as SOD and CAT recovered and maintained an increasing trend in the BU/hPMSC group, while MDA and LDH showed a declining trend.
Fig. 5.
hPMSCs alleviated oxidative damage in a busulfan-induced testis toxicity mouse model. a–c Fluorescence microscopy was used to detect the relative fluorescence intensity of SIRT1 (red) and PLZF (green) in the three groups. d FACS analysis was applied to measure the percentage of ROS+ Sertoli cells in the three groups. e–h ELISA analysis was employed to examine the expression of SOD, CAT, MDA, and LDH. The error bars indicate the SD. ***p < 0.001
hPMSCs promoted autophagy in a mouse model of busulfan-induced testicular toxicity
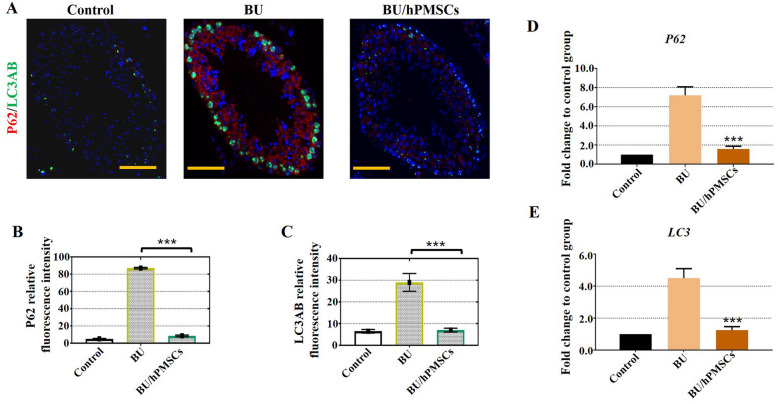
To further confirm the relationship between autophagy and spermatogenesis, fluorescence microscopy was used to investigate the colocalization of autophagy-associated proteins, including p62 and LC3. The results showed that the immunofluorescence signals of the autophagy markers p62 and LC3 increased in the BU group (Fig. 6a, b), indicating that autophagy was inhibited under busulfan induction. However, LC3 fluorescence vanished at 4 weeks after hPMSC treatment (Fig. 6a, b). A hallmark of autophagy, LC3 acts as a scaffold to recognize p62 and regulate protein turnover in autophagy. Moreover, the fluorescence of p62, a specific substrate of autophagy and an LC3-binding protein, disappeared at 4 weeks after hPMSC treatment. Additionally, in agreement with the immunofluorescence results, the mRNA levels of p62 and LC3 showed trends similar to those of their protein levels (Fig. 6d–e).
Fig. 6.
hPMSCs promoted autophagy in a busulfan-induced testis toxicity mouse model. a–c Fluorescence microscopy was employed to monitor the relative fluorescence intensities of p62 (red) and LC3AB (green) in the three groups. d–e qRT-PCR assays revealed the mRNA levels of the autophagy-related genes P62 and LC3. The error bars indicate the SD. ***p < 0.001
Discussion
Chemotherapy with busulfan is an effective treatment for leukemia, especially in children. Nevertheless, the male reproductive system could be damaged by busulfan and develop oligospermia or azoospermia and finally permanent male sterility [24, 25]. In addition, previous studies have reported that male sterility in mice induced by busulfan is similar to that in humans [8, 26]. In the current research, we found that hPMSC treatment restored spermatogenesis in a mouse model of busulfan-induced testicular toxicity and boosted the proliferation of busulfan-induced mouse Sertoli cells by reducing apoptosis/oxidative stress and promoting autophagy. Consequently, we sought to determine the underlying mechanisms by which hPMSCs improve spermatogenesis by using different analyses.
The previous studies have shown that transplantation of testicular endothelial cells alone could restore spermatogenesis in mice after chemotherapy-induced depletion of spermatogonial stem cells (SSC) [27], autologous spermatogonial stem cell (SSC) transplantation could rescue some forms of male infertility caused by Cldn11 deficiency [28] or alginate oligosaccharides improved testis and blood metabolomes to support the recovery of spermatogenesis [29]. Our research also confirmed that hPMSCs are ideal for restoring spermatogenesis in the testes of mice induced by busulfan [22]. However, the interaction between transplantation of hPMSCs and testis toxicity induced by busulfan has not been explored. By using histological analysis, we confirmed that the number of seminiferous tubules with fluid-filled cavities decreased obviously after hPMSC transplantation (Fig. 1). Additionally, the testosterone level of the hPMSC-treated group returned to normal at 4 weeks after hPMSC treatment, which agreed with the results of our preceding study [22] (Fig. 1). The weight, size, and semen parameters of the testis recovered to normal values after hPMSC injection (Fig. 2). In accordance with our outcomes, busulfan-induced testes of mice transplanted with TECs were similar in size to those of control-injected mice [27]. Our results revealed that the overall number of sperm cells as well as the ratio of sperm cells with normal morphology to those with abnormal morphology were obviously elevated after hPMSC injection (Fig. 2).
Busulfan treatment was shown to lead to ROS-mediated apoptosis [30]; apoptosis is a crucial process in testis development because it manages the proportion of germ cells and Sertoli cells to maintain efficient spermatogenesis [31]. Our study showed that hPMSCs have the ability to suppress the apoptosis of Sertoli cells and enhance their rate of proliferation in vivo and in vitro (Figs. 3 and 4). Moreover, our previous research also confirmed this conclusion since it demonstrated that hPMSCs alleviated cell apoptosis and enhanced cell proliferation to improve premature ovarian insufficiency [12]. ROS are inhibited in stem cell self-renewal and believed to be destructive for spermatogenesis [7]. Our results demonstrated that treatment with hPMSCs suppressed the levels of ROS (Fig. 5). Similar to our findings, hPMSCs were shown to protect against oxidative damage in CD4+ T cells by activating Akt-regulated Nrf2 antioxidant signaling [32].
Early studies discovered that the lysosomes of the seminiferous epithelium displayed a cyclical pattern and that autophagy was active in Sertoli cells [33, 34]. In the current study, we found that the autophagy markers p62 and LC3 aggregated in Sertoli cells and mouse testes after busulfan induction not only at the protein level but also at the mRNA level (Fig. 6). As two primary markers of autophagy, p62 and LC3 accumulate when autophagy is inhibited and are reduced when autophagy is induced [35]. Additionally, autophagy is inhibited by mTOR after busulfan treatment in mouse spermatogonial progenitor cells [36]. Collectively, the results from these studies showed that autophagy protected spermatogenesis and Sertoli cells from busulfan-induced stress, which may provide evidence for an important role for hPMSCs in the clinic.
Conclusions
In this study, we investigated the ability of hPMSCs to protect spermatogenic cells against busulfan-induced injury. By upregulating proliferation markers (Ki67, PCNA, BrdU, and SCP3), antioxidant markers (SIRT1, PLZF, SOD, and CAT), and autophagy markers (p62 and LC3AB) and downregulating apoptosis markers (Annexin V, γ-H2AX, BRCA1, and PARP1) and oxidative markers (MDA and LDH), hPMSCs have substantial potential for repairing busulfan-disrupted spermatogenesis through repressing oxidative stress and apoptosis and enhancing cell proliferation and autophagy. Therefore, these results implied that hPMSCs played a vital role in enhancing cell proliferation and autophagy as well as inhibiting apoptosis and oxidative stress to restore male fertility impaired by busulfan.
Supplementary Information
Additional file 1: Supplemental Table 1. Designations, sequences, and the sizes of real-time PCR amplicons.
Abbreviations
- hPMSCs
Human placental mesenchymal stem cells
- ROS
Reactive oxygen species
- MSCs
Mesenchymal stem cells
- SSC
Spermatogonial stem cell
- SPCs
Spermatogonial progenitor cells
- ELISA
Enzyme-linked immunosorbent assay
- FACS
Fluorescence-activated cell sorting
- RT-PCR
reverse transcription-polymerase chain reaction
- qPCR
Quantitative polymerase chain reaction
- HE
Hematoxylin and eosin
- MDA
Malondialdehyde
- SOD
Superoxide dismutase
- CAT
Catalase
- LDH
Lactate dehydrogenase
Authors’ contributions
J.L. and Z.L. performed qPCR, HE staining, ELISA, and immunofluorescence experiments in vivo and in vitro. M.S. and J.L. took part in the statistical analysis and drawing figures. Western blot and FACS assays were carried out by J.L., L.T., and L.Z. W.X., L.T., and L. Z participated in the mice feeding. B.H. and H.L. designed all experiments and composed the manuscript. The authors read and approved the final manuscript.
Funding
This study is supported by the National Natural Science Foundation of China (81801515, 81801478), Suzhou talent training program (GSWS2019005), Suzhou introduce expert team of clinical medicine (SZYJTD201708), and Open project of State Key Laboratory of Reproductive Medicine (SKLRM-K202008).
Availability of data and materials
All the data generated or analyzed during this study are included in this published rticle.
Declarations
Ethics approval and consent to participate
The use of human placental mesenchymal stem cells was in accordance with the relevant guidelines, and regulations and the experimental protocols were approved by the Medical Ethics Committee of the Suzhou Hospital Affiliated to Nanjing Medical University.
Our investigation using experimental animals and ethical approval were conducted on the basis of the Nanjing Medical University Animal Center’s specific guidelines and standards.
We obtained informed consents from the donors.
Consent for publication
Not applicable
Competing interests
The authors declare no conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jiafeng Lu, Zhenxing Liu and Mingkai Shu contributed equally to this work.
Contributor Information
Boxian Huang, Email: huangboxiannj@163.com.
Hong Li, Email: hongliszivf@163.com.
References
- 1.Nishimura H, L’Hernault SW. Spermatogenesis. Curr Biol. 2017;27(18):R988–R994. doi: 10.1016/j.cub.2017.07.067. [DOI] [PubMed] [Google Scholar]
- 2.Hai Y, Hou J, Liu Y, Liu Y, Yang H, Li Z, He Z. The roles and regulation of Sertoli cells in fate determinations of spermatogonial stem cells and spermatogenesis. Semin Cell Dev Biol. 2014;29:66–75. doi: 10.1016/j.semcdb.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 3.Matzuk MM, Lamb DJ. The biology of infertility: research advances and clinical challenges. Nat Med. 2008;14(11):1197–1213. doi: 10.1038/nm.f.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou Q, Wang M, Yuan Y, Wang X, Fu R, Wan H, Xie M, Liu M, Guo X, Zheng Y, Feng G, Shi Q, Zhao XY, Sha J, Zhou Q. Complete meiosis from embryonic stem cell-derived germ cells in vitro. Cell Stem Cell. 2016;18(3):330–340. doi: 10.1016/j.stem.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 5.Levine H, Jørgensen N, Martino-Andrade A, Mendiola J, Weksler-Derri D, Mindlis I, Pinotti R, Swan SH. Temporal trends in sperm count: a systematic review and meta-regression analysis. Hum Reprod Update. 2017;23(6):646–659. doi: 10.1093/humupd/dmx022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu FJ, Dong WY, Zhao H, Shi XH, Zhang YL. Effect of molybdenum on reproductive function of male mice treated with busulfan. Theriogenology. 2019;126:49–54. doi: 10.1016/j.theriogenology.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Morimoto H, Iwata K, Ogonuki N, Inoue K, Atsuo O, Kanatsu-Shinohara M, Morimoto T, Yabe-Nishimura C, Shinohara T. ROS are required for mouse spermatogonial stem cell self-renewal. Cell Stem Cell. 2013;12(6):774–786. doi: 10.1016/j.stem.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Panahi M, et al. Busulfan induced azoospermia: stereological evaluation of testes in rat. Vet Res Forum. 2015;6(4):273–278. [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang X, Xia Q, Wei R, Song H, Mi J, Lin Z, Yang Y, Sun Z, Zou K. Melatonin protects spermatogonia from the stress of chemotherapy and oxidation via eliminating reactive oxidative species. Free Radic Biol Med. 2019;137:74–86. doi: 10.1016/j.freeradbiomed.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 10.Xu LL, Liu ML, Wang JL, Yu M, Chen JX. Saligenin cyclic-o-tolyl phosphate (SCOTP) induces autophagy of rat spermatogonial stem cells. Reprod Toxicol. 2016;60:62–68. doi: 10.1016/j.reprotox.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 11.Trounson A, McDonald C. Stem cell therapies in clinical trials: progress and challenges. Cell Stem Cell. 2015;17(1):11–22. doi: 10.1016/j.stem.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 12.Ding C, Zou Q, Wu Y, Lu J, Qian C, Li H, Huang B. EGF released from human placental mesenchymal stem cells improves premature ovarian insufficiency via NRF2/HO-1 activation. Aging (Albany NY) 2020;12(3):2992–3009. doi: 10.18632/aging.102794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147(4):728–741. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 14.Mizushima N, Yoshimori T, Ohsumi Y. The role of Atg proteins in autophagosome formation. Annu Rev Cell Dev Biol. 2011;27(1):107–132. doi: 10.1146/annurev-cellbio-092910-154005. [DOI] [PubMed] [Google Scholar]
- 15.Yang Z, Klionsky DJ. An overview of the molecular mechanism of autophagy. Curr Top Microbiol Immunol. 2009;335:1–32. doi: 10.1007/978-3-642-00302-8_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsukamoto S, Kuma A, Murakami M, Kishi C, Yamamoto A, Mizushima N. Autophagy is essential for preimplantation development of mouse embryos. Science. 2008;321(5885):117–120. doi: 10.1126/science.1154822. [DOI] [PubMed] [Google Scholar]
- 17.Liu C, Wang H, Shang Y, Liu W, Song Z, Zhao H, Wang L, Jia P, Gao F, Xu Z, Yang L, Gao F, Li W. Autophagy is required for ectoplasmic specialization assembly in sertoli cells. Autophagy. 2016;12(5):814–832. doi: 10.1080/15548627.2016.1159377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shang Y, Wang H, Jia P, Zhao H, Liu C, Liu W, Song Z, Xu Z, Yang L, Wang Y, Li W. Autophagy regulates spermatid differentiation via degradation of PDLIM1. Autophagy. 2016;12(9):1575–1592. doi: 10.1080/15548627.2016.1192750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rehman R, Amjad S, Tariq H, Zahid N, Akhter M, Ashraf M. Oxidative stress and male infertility: a cross sectional study. J Pak Med Assoc. 2020;70(3):461–466. doi: 10.5455/JPMA.12992. [DOI] [PubMed] [Google Scholar]
- 20.Forand A, Bernardino-Sgherri J. A critical role of PUMA in maintenance of genomic integrity of murine spermatogonial stem cell precursors after genotoxic stress. Cell Res. 2009;19(8):1018–1030. doi: 10.1038/cr.2009.50. [DOI] [PubMed] [Google Scholar]
- 21.Li B, He X, Zhuang M, Niu B, Wu C, Mu H, Tang F, Cui Y, Liu W, Zhao B, Peng S, Li G, Hua J. Melatonin ameliorates busulfan-induced spermatogonial stem cell oxidative apoptosis in mouse testes. Antioxid Redox Signal. 2018;28(5):385–400. doi: 10.1089/ars.2016.6792. [DOI] [PubMed] [Google Scholar]
- 22.Qian C, Meng Q, Lu J, Zhang L, Li H, Huang B. Human amnion mesenchymal stem cells restore spermatogenesis in mice with busulfan-induced testis toxicity by inhibiting apoptosis and oxidative stress. Stem Cell Res Ther. 2020;11(1):290. doi: 10.1186/s13287-020-01803-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ding C, Zou Q, Wang F, Wu H, Chen R, Lv J, Ling M, Sun J, Wang W, Li H, Huang B. Human amniotic mesenchymal stem cells improve ovarian function in natural aging through secreting hepatocyte growth factor and epidermal growth factor. Stem Cell Res Ther. 2018;9(1):55. doi: 10.1186/s13287-018-0781-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ganjalikhan Hakemi S, et al. The effects of olive leaf extract on the testis, sperm quality and testicular germ cell apoptosis in male rats exposed to busulfan. Int J Fertil Steril. 2019;13(1):57–65. doi: 10.22074/ijfs.2019.5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pacey AA, Eiser C. The importance of fertility preservation in cancer patients. Expert Rev Anticancer Ther. 2014;14(5):487–489. doi: 10.1586/14737140.2014.883283. [DOI] [PubMed] [Google Scholar]
- 26.Panahi S, Abdollahifar MA, Aliaghaei A, Nazarian H, Paktinat S, Abdi S, Farahani RM. Application of stereological methods for unbiased estimation of sperm morphology in the mice induced by busulfan. Anat Cell Biol. 2017;50(4):301–305. doi: 10.5115/acb.2017.50.4.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhang DH, Kim BJ, Kim BG, Schadler K, Baek KH, Kim YH, Hsiao W, Ding BS, Rafii S, Weiss MJ, Chou ST, Kolon TF, Ginsberg JP, Ryu BY, Ryeom S. Testicular endothelial cells are a critical population in the germline stem cell niche. Nat Commun. 2018;9(1):4379. doi: 10.1038/s41467-018-06881-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanatsu-Shinohara M, Ogonuki N, Matoba S, Ogura A, Shinohara T. Autologous transplantation of spermatogonial stem cells restores fertility in congenitally infertile mice. Proc Natl Acad Sci U S A. 2020;117(14):7837–7844. doi: 10.1073/pnas.1914963117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao Y, Zhang P, Ge W, Feng Y, Li L, Sun Z, Zhang H, Shen W. Alginate oligosaccharides improve germ cell development and testicular microenvironment to rescue busulfan disrupted spermatogenesis. Theranostics. 2020;10(7):3308–3324. doi: 10.7150/thno.43189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iskandar K, Rezlan M, Yadav SK, Foo CHJ, Sethi G, Qiang Y, Bellot GL, Pervaiz S. Synthetic lethality of a novel small molecule against mutant KRAS-expressing cancer cells involves AKT-dependent ROS production. Antioxid Redox Signal. 2016;24(14):781–794. doi: 10.1089/ars.2015.6362. [DOI] [PubMed] [Google Scholar]
- 31.Rodriguez I, Ody C, Araki K, Garcia I, Vassalli P. An early and massive wave of germinal cell apoptosis is required for the development of functional spermatogenesis. EMBO J. 1997;16(9):2262–2270. doi: 10.1093/emboj/16.9.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiong Y, Wang Y, Zhang J, Zhao N, Zhang H, Zhang A, Zhao D, Yu Z, Yin Y, Song L, Xiong Y, Luan X. hPMSCs protects against d-galactose-induced oxidative damage of CD4(+) T cells through activating Akt-mediated Nrf2 antioxidant signaling. Stem Cell Res Ther. 2020;11(1):468. doi: 10.1186/s13287-020-01993-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yefimova MG, Messaddeq N, Harnois T, Meunier AC, Clarhaut J, Noblanc A, Weickert JL, Cantereau A, Philippe M, Bourmeyster N, Benzakour O. A chimerical phagocytosis model reveals the recruitment by Sertoli cells of autophagy for the degradation of ingested illegitimate substrates. Autophagy. 2013;9(5):653–666. doi: 10.4161/auto.23839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Y, Zhou Y, Wang X, Qian W, Han X. Microcystin-LR induces autophagy and apoptosis in rat Sertoli cells in vitro. Toxicon. 2013;76:84–93. doi: 10.1016/j.toxicon.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 35.Borgeson E, et al. Lipoxin A4 attenuates obesity-induced adipose inflammation and associated liver and kidney disease. Cell Metab. 2015;22(1):125–137. doi: 10.1016/j.cmet.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wei R, Zhang X, Cai Y, Liu H, Wang B, Zhao X, Zou K. Busulfan suppresses autophagy in mouse spermatogonial progenitor cells via mTOR of AKT and p53 signaling pathways. Stem Cell Rev Rep. 2020;16(6):1242–1255. doi: 10.1007/s12015-020-10027-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplemental Table 1. Designations, sequences, and the sizes of real-time PCR amplicons.
Data Availability Statement
All the data generated or analyzed during this study are included in this published rticle.