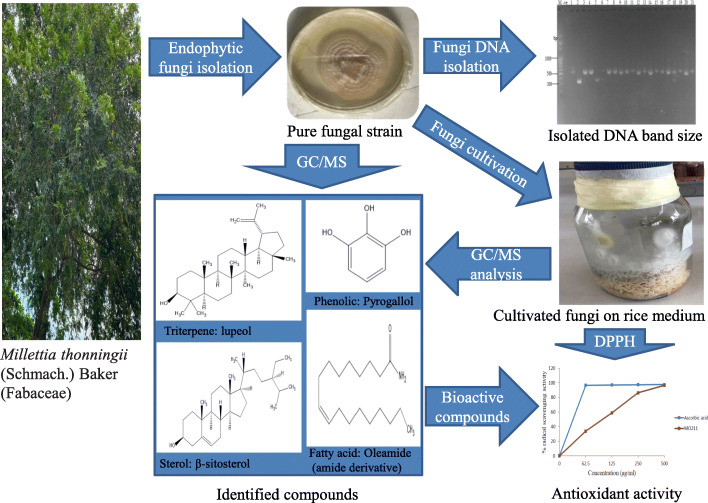
Abstract
Background
Plants with an ethnobotanical history are known to harbor diverse group of endophytic fungi, which constitute major natural sources of bioactive compounds. In the present study, we evaluated the antioxidant activity of endophytic fungi from eight Nigerian ethnomedicinal plants. Endophytic fungi were isolated from the leaves of Acalypha ornata, Albizia zygia, Alchornea cordifolia, Chrysophyllum albidum, Ficus exasperata, Gomphrena celosioides, Millettia thonningii, and Newbouldia laevis.
Methods
Endophytic fungi were isolated from the leaves of selected plants via surface sterilization. Isolated fungi were identified by internal transcribed spacer (ITS-rDNA) sequence analysis. Pure fungal strains were subjected to fermentation process on solid rice medium and metabolites extracted using ethyl-acetate. Fungal crude extracts were screened for antioxidant activity using 2, 2- diphenyl-1-picrylhydrazyl (DPPH) radical scavenging and reduction of ferric ion assays. Gas chromatography/mass spectrometry (GC/MS) analysis was used to identify the major chemical constituents in active fungal extracts.
Results
A total of eighteen fungal endophytes with fungal codes CU (061 and 062); ZA (161, 162, 163, and 164); LO (261); CA (041, 042, and 043); FE (081, 082, and 084); GE (091); MO (211 and 212); and NA (021 and 022) were isolated from the eight ethnomedicinal plants A. ornata, A. zygia, A. cordifolia, C. albidum, F. exasperata, G. celosioides, M. thonningii, and N. laevis respectively. ZA 163 and MO 211 fungal extracts showed significant (p < 0.05) radical scavenging activity with IC50 values of 50.53 ± 0.01 and 86.69 ± 0.02 μg/ml respectively. Fungal extract CA 041 demonstrated significantly (p < 0.01) higher iron chelating activity than standard gallic acid with absorbance values of 0.803 and 1.107 at 250 and 500 μg/ml concentrations respectively. Pyrogallol, phenol, 2,6-dimethoxy-, phytol, dl-alpha-tocopherol, alpha-tocospiro, oleamide, methyl stearate, oleic acid, palmitic acid, campesterol, stigmasterol, β-sitosterol, urs-12-en-24-oic acid, 3-oxo-, methyl ester, lup-20(29)-en-3-one, and lupeol were detected in the selected active extracts.
Conclusion
These results showed that leaves of the selected Nigerian plants harbor diverse group of endophytic fungi, which can be potential antioxidant resource.
Graphical abstract
Keywords: Nigerian plants, Endophytic fungi, Ethnomedicinal plants, ITS-rDNA, Free radical scavenging, Secondary metabolites
Background
Endophytes are microorganisms that reside within plant tissues and do not cause any deleterious effect to the plants. These microorganisms consist of bacterial and fungal communities that colonize and spend the whole or part of their life cycle inside tissues of the host, without causing noticeable symptoms of plant diseases [1, 2]. Endophytic fungi are found in almost plant parts and act as chemical synthesizers inside the host plant, producing a wide range of bioactive secondary metabolites [3, 4].
Endophytic fungi from the genera Colletotrichum, Fusarium, Alternaria and Aspergillus, isolated from medicinally important plants exhibit a variety of biological activities such as anticancer, antimicrobial, antifungal, immunomodulatory, antitubercular, and antioxidant activities with wide application in agrochemical and pharmaceutical industries [5–8]. These biological activities demonstrated by endophytes have been attributed to isolated and identified secondary metabolites such as alkaloids, terpenoids, steroids, quinones, isocoumarin derivatives, flavanoids, peptides, and phenols present in the fungal extracts [9–14]. Therefore, exploring endophytic fungi that reside in medicinal plant species would provide vast opportunities to discover new medicinally important metabolites [15].
Production of reactive oxygen species known as free radicals occurs frequently in all cells as part of normal metabolic process. However, free radicals have been implicated in the pathogenesis of quite a number of chronic diseases such as diabetes, neurological disorders, and cancer [16]. Free radical scavenging property of antioxidant agents inhibits or delays cellular damage. Dietary intake as well as adequate amount of exogenous antioxidants would prevent the pathological conditions induced by free radicals [17]. However, the use of synthesized antioxidants in the prevention of free radical damage may exude toxic side effects thus the search for natural antioxidant agents is required [18, 19]. Endophytic fungi can synthesize secondary metabolites with antioxidant activity that can interrupt the chain reaction of reactive oxygen species [20, 21].
Medicinal plants growing in natural habitats are promising hosts of endophytic fungi that might produce bioactive secondary metabolites of pharmaceutical relevance. Different plant parts, especially the leaves, stems, and roots are considered an enormous repository of these fungal endophytes with reported cytotoxic, antifungal, antiviral, and antimicrobial activities [22–24]. The genetic resources of these plants found in Nigeria are a veritable source of pharmaceuticals and therapeutics with significant information on their phytochemistry. Additionally, these plants are used locally in the treatment of various diseases. Hence, this study was carried out to isolate and screen endophytic fungi with antioxidant activity from eight selected ethnomedicinal plants namely: Acalypha ornata Hochst. ex A. Rich (Euphorbiaceae), Albizia zygia (DC.) J.F. Macbr. (Fabaceae), Alchornea cordifolia (Schum. & Thonn.) Müll-Arg (Euphorbiaceae), Chrysophyllum albidum G. Don (Sapotaceae), Ficus exasperata Vahl (Moraceae), Gomphrena celosioides Mart. (Amaranthaceae), Millettia thonningii (Schmach.) Baker (Fabaceae), and Newbouldia laevis (P. Beauv.) Seem. ex Bureau (Bignoniaceae).
Methods
Collection of plant samples and identification
Fresh and healthy leaf samples of Acalypha ornata Hochst. ex A. Rich, Albizia zygia (DC.) J.F. Macbr., Alchornea cordifolia (Schum. & Thonn.) Müll-Arg, Chrysophyllum albidum G. Don, Ficus exasperata Vahl, Gomphrena celosioides Mart., Millettia thonningii (Schmach.) Baker, and Newbouldia laevis (P. Beauv.) Seem. ex Bureau were collected within the main campus of the University of Lagos, Lagos State, Nigeria in the month of March, 2019. The plant samples were identified and authenticated by Dr. George I. Nodza at the Herbarium of the Department of Botany, University of Lagos where herbarium specimen and voucher specimen number (LUH 8256–62; 8264) for each sample was deposited. Each sample was kept separately in a sealed plastic bag and brought to the laboratory on the same day for the isolation of fungal endophytes.
Isolation of endophytic fungi
The collected plant samples were surface sterilized according to the method previously described [25, 26] with modifications. To remove dust and debris, samples were washed thoroughly under running tap water, followed by washing in distilled water. The leaf samples were cut into small segments measuring 2–4 mm using sterile razor blades. Surface sterilization of leaf segments was carried out by sequential immersion in 70% ethanol for 60 s, 0.5% sodium hypochlorite for 5 min, 70% ethanol for 30 s, followed by a final rinse in sterilized distilled water for 5 min. Sterilized leaf segments were air-dried aseptically and placed on petri dishes containing potato dextrose agar amended with 0.01% gentamycin antibiotic for the direct contact of cut edges with the nutrient media. Three segments of each plant sample were placed on PDA plates, sealed with parafilm and incubated at 27 °C for 5–7 days. Samples were checked daily for visual growth of fungi.
Purification and preservation of endophytic fungi
Following the incubation period, different fungal strains emerged from each sample and individual strains were isolated by transferring hyphal tips onto a fresh PDA medium. This process was repeated several times until a pure endophytic fungal strain with uniform colony was obtained. Pure endophytic fungi isolates were transferred separately to PDA slants and 15% (v/v) sterilized glycerol solution. Growth of endophytic fungi strains in both media was observed for 3–5 days. Glycerol stock solution and PDA slants were maintained at − 20 °C and 4 °C respectively till further use.
Molecular identification
Identification of endophytic fungi was performed using molecular characterization and sequencing of the internal transcribed spacer region (ITS).
Endophytic fungi DNA extraction
Deoxyribonucleic acid (DNA) extraction of fungal strains was done using Zymo fungal / bacteria DNA extraction kit (Zymo Research Corp., South Africa) according to the manufacturer’s instructions. Briefly, pure endophytic fungi strains isolated from the plant samples were grown in potato dextrose broth (PDB) for 6–7 days. Following the formation of endophytes colony, fungal mycelium was drained and homogenized in 200 μL of phosphate buffered saline to aid lysis. About 50 mg of homogenate was harvested into a 1.5 ml microcentrifuge tube and 750 μL lysis solution was added to the tube. The tube was vortex vigorously for 30–60 s and 400 μL of supernatant was transferred into a Zymo-Spin™ IV spin filter in a collection tube and centrifuged at 10,000 rpm for 1 min. After centrifugation, 1200 μL of Fungal/Bacterial DNA binding buffer was added to filtrate in the collection tube. 800 μL of the mixture was then transferred to a Zymo-Spin™ IIC column in a collection tube. The reaction mixture was centrifuged at 10,000 rpm for 1 min. The flow through was discarded and the remaining mixture was centrifuged again at 10,000 rpm for 1 min in the same tube. The column was pre-washed with 200 μL DNA Pre-Wash buffer and centrifuged at 10,000 rpm for 1 min. This was followed by a wash with 500 μL Fungal/Bacterial DNA Wash Buffer and centrifuged at 10,000 rpm for 1 min. The DNA was eluted from the Zymo-Spin™ IIC column to a sterile 1.5 ml microcentrifuge tube using 100 μL of DNA Elution Buffer. The purity and concentration of extracted DNA was evaluated using a NANODROP (ND 1000) spectrophotometer (Thermo Scientific, USA).
Amplification and sequencing
Polymerase chain reaction was carried out to amplify the ITS gene of specific DNA of each fungus using the primer pair ITS-1 (5′-TCCGTAGGTGAACCTGCGG) and ITS-4 (5′-TCCTCCGCTTATTGATATGC) as previously described [27, 28]. PCR was done using the Solis Biodyne 5x Hot FIREPol® Master Mix Ready to Load. PCR reaction was performed in a total volume of 20 μL containing 1 x blend master mix buffer, 1.5 mM MgCl2, 200 μM of each deoxynucleoside triphosphate (dNTP), 25 pmol ITS1 and ITS4, 2 units of hot FIREPol DNA polymerase and 10–200 ng of DNA. Sterile distilled water was used to make up the reaction mixture. PCR amplification was performed in PTC 100 Peltier Thermal Cycler (MJ Research) using the following protocols: denaturation at 95 °C for 15 min; 35 amplification cycles at 95 °C for 30 s; primer-specific annealing at 58 °C for 1 min and elongation at 72 °C for 90 s; a final elongation at 72 °C for 10 min. Amplified DNA was checked by electrophoresis at 80 V for 90 min in a 1.5% agarose gel stained with ethidium bromide. The band to PCR product was visualized with a photodocumentation system (Cleaver Scientific, UK).
The amplified products were purified with exo sap and sequenced by Epoch Life science, USA using Sanger sequencing method. Identification of endophytic fungi was performed on the basis of similarity of amplified sequence with ITS sequence data from strains available in the US National Centre for Biotechnology Information (NCBI) database using Basic Local Alignment Search Tool (BLAST) N sequence match routines.
Fermentation and extraction of isolated fungal metabolites
Cultivation of isolated fungal endophytes was carried out in a conical flask containing sterilized rice medium as previously described [25, 29] with modifications. Approximately 200 g of rice was soaked in 200 ml sterile water for 10 min in a glass bottle. The flask with its content was autoclaved at 121 °C for 20 min and allowed to cool. Three to four pieces of pure mycelia agar plugs were inoculated aseptically into the cooled solid rice medium. A flask of autoclaved solid rice medium without inoculum served as the control. Fungal strain was allowed to grow on rice medium at room temperature (27 ± 2 °C) for 4–6 weeks with a routine check. After the incubation period, about 500 ml of ethyl-acetate was added into the culture flask and left overnight at room temperature. The crude ethyl-acetate fungal mixture was filtered through a Whatman filter paper No. 1 under vacuum using a Buchner funnel. Collected supernatant was evaporated to dryness under vacuum on a rotary evaporator (Buchi, Switzerland) at 40 °C to obtain crude fungal extract.
In vitro antioxidant activity of fungal extracts
DPPH radical scavenging activity
Free radical scavenging activities of fungal extracts were measured using 2, 2-diphenyl-1-picryl-hydrazyl (DPPH) as previously described [30, 31]. Briefly, 2 ml of varying concentrations (62.5–500 μg/ml) of fungal extracts and positive standard (ascorbic acid) prepared in methanol was mixed with 2 ml of 0.135 mM DPPH in methanol. The mixture was shaken vigorously and incubated in the dark at room temperature for 30 min. Mixture of methanol and DPPH without fungal extract served as the control. Absorbance of samples was measured at 517 nm against the blank tube that contains methanol and used to maintain zero of the UV-vis spectrophotometer. The assay was carried out in three replicates. Inhibition of DPPH was calculated as a percentage of radical scavenging activity of each fungal extract using the formula:
Percentage of radical scavenging activity = [(Acontrol - Asample)/Acontrol] × 100.
Where A is the absorbance reading. IC50 value (μg/mL) was calculated from percentage of radical scavenging activity against extract concentrations.
Reduction of Fe3+ ions by ortho-phenanthroline
O-phenanthroline assay was used to determine the reducing capacity of fungal extracts as previously described [32, 33]. A reaction mixture containing 1 ml of 2 mM phenanthroline, 2 ml of 0.2 mM ferric chloride hexahydrate (FeCl3.6H2O), and 2 ml of different concentrations (62.5–500 μg/ml) of fungal extracts was shaken and incubated at ambient temperature for 10 min. Absorbance was measured at 510 nm using a UV-vis spectrophotometer. Gallic acid served as positive standard.
Identification of bioactive constituents by GC/MS
Gas chromatography/mass spectrometry (GC/MS) analysis of bioactive fungal extracts was performed using Agilent 7820A gas chromatograph coupled to an Agilent 5975C inert mass selective detector (MSD) with triple axis detector operated in an electron impact (EI) mode with ionization energy of 70 eV. An HP-5 capillary column coated with 5% phenyl methyl siloxane (30 m × 0.32 mm diameter × 0.25 μm film thickness) was used for the separation. Sample preparation was performed according to the procedure described [34]. The sample (1 μL, diluted 1: 100 in dichloromethane) was injected in splitless mode at an injection temperature of 300 °C. Purge flow to split vent was 15 ml/min at 0.75 min with a total flow of 16.654 ml/min. Helium was used as the carrier gas at the flow rate of 1.487 ml/min with initial nominal pressure of 1.4902 psi and an average velocity of 44.22 cm/sec. The oven temperature was initially programmed at 40 °C for 1 min then ramped at 12 °C / min to 300 °C for 10 min. Run time was 32.667 min with a hold time of 5 °C / min. Identification of the chromatographic peaks was based on comparisons of their relative retention times and mass spectra with those obtained from the NIST14.L library data.
Statistical analysis
All assays were carried out in three replicates and results are expressed as mean ± standard error of mean (SEM). IC50 values were obtained by interpolations from standard curves. Data were analyzed using Graph Pad Prism 8.
Results
Isolation of endophytic fungi
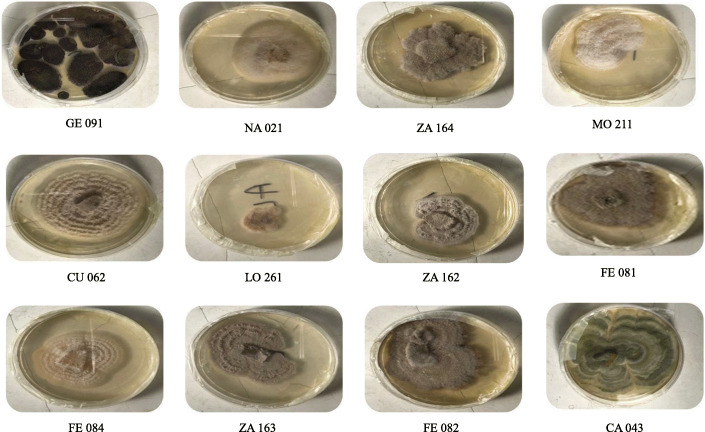
Eighteen pure fungal endophytes were successfully isolated from surface sterilized fresh leaf samples of eight medicinal plants: Acalypha ornata, Albizia zygia, Alchornea cordifolia, Chrysophyllum albidum, Ficus exasperata, Gomphrena celosioides, Millettia thonningii, and Newbouldia laevis (Fig. 1). The leaves of A. zygia were found to host the highest number of endophytic fungi with four isolates: ZA 161, ZA 162, ZA 163, and ZA 164 (Table 1). This was followed by the leaves of C. albidum with three isolates: CA 041, CA 042, and CA 043 and F. exasperata with three isolates: FE 081, FE 082, and FE 083 (Table 1). Two endophytic fungal isolates were obtained from the leaves of A. ornata (CU 061 and CU 062); M. thonningii (MO 211 and MO 212); N. laevis (NA 021 and NA 022). A. cordifolia and G. celosioides leaf samples hosted one fungal isolate LO 261 and GE 091 respectively (Table 1). Pictorial representation of isolated endophytic fungi is as shown in Fig. 2.
Fig. 1.
Pictures of selected plants from University of Lagos, main campus, Nigeria used for fungal isolation
Table 1.
Identification of endophytic fungi isolated from selected ethnomedicinal plants from University of Lagos, Nigeria
| S/N | Plant source | Voucher specimen number | Number of isolated endophytic fungi | Fungal code |
|---|---|---|---|---|
| 1. | Albizia zygia (DC.) J.F. Macbr. (Fabaceae) | LUH 8256 | 4 | ZA 161 |
| ZA 162 | ||||
| ZA 163 | ||||
| ZA 164 | ||||
| 2. | Chrysophyllum albidum G. Don (Sapotaceae) | LUH 8262 | 3 | CA 041 |
| CA 042 | ||||
| CA 043 | ||||
| 3. | Ficus exasperata Vahl (Moraceae) | LUH 8264 | 3 | FE 081 |
| FE 082 | ||||
| FE 084 | ||||
| 4. | Acalypha ornata Hochst. ex A. Rich (Euphorbiaceae) | LUH 8260 | 2 | CU 061 |
| CU 062 | ||||
| 5. | Millettia thonningii (Schmach.) Baker (Fabaceae) | LUH 8261 | 2 | MO 211 |
| MO 212 | ||||
| 6. | Newbouldia laevis (P. Beauv.) Seem. ex Bureau (Bignoniaceae) | LUH 8257 | 2 | NA 021 |
| NA 022 | ||||
| 7. | Alchornea cordifolia (Schum. & Thonn.) Müll-Arg (Euphorbiaceae) | LUH 8258 | 1 | LO 261 |
| 8. | Gomphrena celosioides Mart. (Amaranthaceae) | LUH 8259 | 1 | GE 091 |
Fig. 2.
Pictures of isolated endophytic fungi species on culture plate
Extraction of fungal metabolites
All the fungi isolates were cultivated on solid media and extracted with ethyl-acetate to afford fungal extracts of varying yield, which range from 44 to 2002 mg per 200 g rice medium (Table 2).
Table 2.
Yield of fungal extracts and reduction in ferric ion activity of standard and endophytic fungi
| S/N | Standard and Fungal code | Yield of endophytic fungi extract (mg) in 200 g of rice medium | Absorbance readings at varying concentrations (μg/ml) | |||
|---|---|---|---|---|---|---|
| 62.5 | 125 | 250 | 500 | |||
| 1. | Gallic acid | – | 0.479 ± 0.011 | 0.486 ± 0.004 | 0.499 ± 0.009 | 0.521 ± 0.004 |
| 2. | ZA 161 | 0233 | 0.277 ± 0.001* | 0.302 ± 0.002* | 0.333 ± 0.005* | 0.460 ± 0.009* |
| 3. | ZA 162 | 1390 | 0.212 ± 0.017* | 0.240 ± 0.003* | 0.233 ± 0.021* | 0.453 ± 0.086 |
| 4. | ZA 163 | 0044 | 0.304 ± 0.002* | 0.310 ± 0.005* | 0.424 ± 0.009* | 1.190 ± 0.001* |
| 5. | ZA 164 | 0080 | 0.219 ± 0.005* | 0.240 ± 0.006* | 0.278 ± 0.006* | 0.324 ± 0.006* |
| 6. | CA 041 | 0900 | 0.296 ± 0.005* | 0.412 ± 0.009* | 0.843 ± 0.011* | 1.070 ± 0.038* |
| 7. | CA 042 | 1280 | 0.230 ± 0.003* | 0.238 ± 0.005* | 0.453 ± 0.118 | 0.932 ± 0.002* |
| 8. | CA 043 | 0930 | 0.233 ± 0.004* | 0.256 ± 0.005* | 0.478 ± 0.047 | 0.994 ± 0.002* |
| 9. | FE 081 | 1650 | 0.219 ± 0.007* | 0.249 ± 0.005* | 0.280 ± 0.002* | 0.387 ± 0.011* |
| 10. | FE 082 | 1247 | 0.300 ± 0.012* | 0.320 ± 0.002* | 0.382 ± 0.021* | 0.852 ± 0.008* |
| 11. | FE 084 | 1960 | 0.239 ± 0.010* | 0.245 ± 0.005* | 0.336 ± 0.011* | 0.869 ± 0.064* |
| 12. | CU 061 | 2002 | 0.428 ± 0.046 | 0.357 ± 0.010* | 0.249 ± 0.004* | 0.142 ± 0.003* |
| 13. | CU 062 | 0066 | 0.452 ± 0.018 | 0.472 ± 0.007 | 0.316 ± 0.048 | 0.179 ± 0.019* |
| 14. | MO 211 | 0044 | 0.307 ± 0.007* | 0.341 ± 0.012* | 0.351 ± 0.004* | 0.440 ± 0.007* |
| 15. | MO 212 | 1402 | 0.236 ± 0.004* | 0.272 ± 0.007* | 0.356 ± 0.034 | 0.441 ± 0.021 |
| 16. | NA 021 | 0539 | 0.224 ± 0.007* | 0.251 ± 0.004* | 0.252 ± 0.020* | 0.301 ± 0.005* |
| 17. | NA 022 | 0166 | 0.263 ± 0.001* | 0.330 ± 0.008* | 0.367 ± 0.004* | 0.462 ± 0.003* |
| 18. | LO 261 | 1149 | 0.259 ± 0.005* | 0.332 ± 0.005* | 0.490 ± 0.006 | 0.803 ± 0.011* |
| 19. | GE 091 | 1010 | 0.352 ± 0.002* | 0.400 ± 0.003* | 0.640 ± 0.024* | 0.977 ± 0.009* |
Absorbance readings are expressed as mean ± SEM. * p < 0.01 significant difference between concentrations of same extract
Antioxidant activity of fungal extracts
DPPH radical scavenging activity
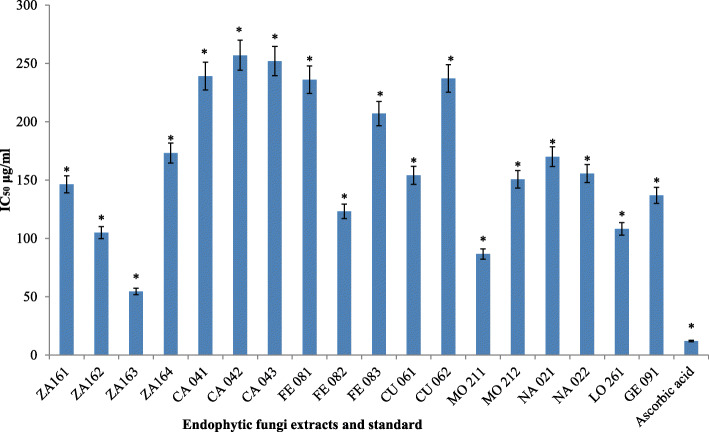
Figure 3 showed the IC50 values of fungal extracts, which was used to indicate antioxidant capacity. Extracts of all endophytic fungal isolates exhibited significant (p < 0.05) radical scavenging activity with an IC50 value range of 50.53 μg/ml to 257.0 μg/ml. The standard ascorbic acid revealed a value of 12.07 μg/ml. The highest antioxidant activity with an IC50 value of 50.53 ± 0.01 μg/ml was displayed by extract of endophytic fungi ZA163 isolated from the leaves of Albizia zygia. This was followed by extract of fungal isolate MO211 obtained from the leaves of Millettia thonningii with an IC50 value of 86.69 ± 0.02 μg/ml. Extracts of fungal isolates CA 041, CA 043, and CA 042 isolated from Chrysophyllum albidum leaves showed the least radical scavenging activity with IC50 values of 239.20 ± 0.04, 252.0 ± 0.11 and 257.0 ± 0.11 μg/ml respectively.
Fig. 3.
IC50 values of standard and extracts of endophytic fungi in DPPH radical scavenging assay.* p < 0.05 significant difference between concentrations of same extract
Reduction of Fe3+ ions by ortho-phenanthroline
Except for extracts of endophytic fungi CU 061 and CU 062 isolated from Acalypha ornata, all fungal extracts showed a dose dependent antioxidant activity with an increase in absorbance value as concentration increases (Table 2). At 250 and 500 μg/ml concentrations, fungal extracts CA 041 from Chrysophyllum albidum and GE 091 from Gomphrena celosioides demonstrated significantly (p < 0.01) higher antioxidant activity (0.843 and 1.070; 0.640 and 0.977 respectively) than standard gallic acid (0.499 and 0.521). Also at 500 μg/ml concentration, the absorbance values of fungal extracts ZA 163 (1.190) from Albizia zygia; LO 261 (0.803) from Alchornea cordifolia; CA 042 (0.932) and CA 043 (0.994) from C. albidum; FE 084 (0.869) and FE 082 (0.852) from Ficus exasperata; demonstrated significant (p < 0.01) higher antioxidant capability than gallic acid (Table 2).
Identification of bioactive constituents by GC/MS
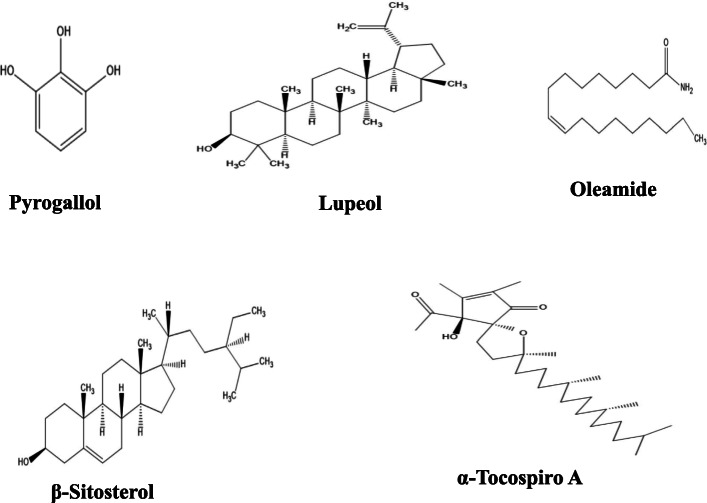
Table 3 shows the retention time, molecular weight, molecular formula, and class of identified chemical constituents present in endophytic fungal extracts ZA163, MO211, LO 261, FE 082, and FE 084 with a substantial percentage compositions and quality match ranging from 86 to 99%. The chromatograms are shown in Figs. 4, 5, 6, 7, 8. The identified compounds include phenol and tocopherol (phenol, 2-methoxy; phenol, 2,6-dimethoxy-; phenol, 2,6-dimethoxy-4-(2-propenyl); pyrogallol; phenol, 2,2′-methylenebis [6-(1,1-dimethylethyl)-4-methyl; alpha tocospiro A; alpha tocospiro B, dl-alpha-tocopherol; and gamma tocopherol); terpenoids (phytol; lup-20(29)-en-3-one; lupeol; and urs-12-en-24-oic acid, 3-oxo-, methyl ester); sterols (campesterol; stigmasterol; and sitosterol); and fatty acids and its amide derivative (methyl stearate; oleic acid; linoleic acid; palmitic acid; and oleamide). Figure 9 shows the chemical structures of pyrogallol (phenolic), lupeol (terpenoid), and β-sitosterol (sterol) as representatives of the major classes of secondary metabolites as well as oleamide (amide derivative of fatty acid) and α-tocospiro A (tocopherol) present in the investigated endophytic fungi extracts.
Table 3.
Identified chemical constituents of bioactive fungal extracts
| S/N | Name of compound/ Molecular weight (g/mol)/ Class of identified compound | Molecular formular | RT (min) | Percent composition | ||||
|---|---|---|---|---|---|---|---|---|
| ZA163 | MO211 | LO261 | FE082 | FE084 | ||||
| 1. | Phenol, 2-methoxy- (124.14) Phenolic | C7H8O2 | 6.73 | – | 0.46 | – | – | – |
| 2. | Phenol, 2,6-dimethoxy- (154.16) Phenolic | C8H10O3 | 9.88 | – | 0.54 | – | – | – |
| 3. | 1,2,3-Benzenetriol / pyrogallol (126.11) Phenolic | C6H6O3 | 10.84 | 10.72 | 19.84 | 1.46 | – | 31.33 |
| 4. | Phenol, 2,6-dimethoxy-4-(2-propenyl)- (194.23) Phenolic | C11H14O3 | 12.48 | 0.67 | – | 0.50 | – | – |
| 5. | Naphthalene, 1,6-dimethyl-4-(1-methylethyl)- (198.30) Phenolic | C15H18 | 13.19 | – | 0.52 | – | – | – |
| 6. | n-Hexadecanoic acid / palmitic acid (256.42) Fatty acid | C16H32O2 | 15.64 | – | 0.71 | – | 2.69 | – |
| 7. | 9-Octadecenoic acid (Z)-, methyl ester (296.49) Fatty acid | C19H36O2 | 16.69 | 1.56 | 1.48 | 1.71 | 2.82 | 0.62 |
| 8. | Phytol (296.54) Terpenoid | C20H40O | 16.79 | – | – | – | 1.09 | – |
| 9. | Methyl stearate (298.50) Fatty acid | C19H38O2 | 16.88 | 0.56 | 0.26 | 0.41 | – | – |
| 10. | Oleic acid (282.46) Fatty acid | C18H34O2 | 17.28 | 7.26 | 9.28 | – | – | 0.93 |
| 11. | 9,12-Octadecadienoic acid (Z, Z)-, linoleic acid/linoelaidic (280.45) Fatty acid | C18H32O2 | 17.72 | 0.40 | 0.37 | 0.05 | 0.07 | – |
| 12. | 9-Octadecenamide, (Z)- / oleamide (281.48) Fatty acid | C18H35NO | 18.69 | 1.83 | – | 11.04 | – | 1.12 |
| 13. | Phenol, 2,2′-methylenebis[6-(1,1-dimethylethyl)-4-methyl (340.50) Phenolic | C23H32O2 | 19.05 | – | – | – | 0.19 | – |
| 14. | Alpha-tocospiro A / B (462.71) Tocopherol | C29H50O4 | 21.86 | 0.62 | – | – | 4.92 | 0.86 |
| 15. | DL-alpha-tocopherol / gamma tocopherol (430.71) Tocopherol | C29H50O2 | 23.37 | 0.15 | 0.06 | – | 1.47 | – |
| 16. | Campesterol (400.68) Sterol | C28H48O | 24.07 | – | 0.09 | – | – | 0.93 |
| 17 | Stigmasterol (412.69) Sterol | C29H48O | 24.64 | 0.45 | 0.13 | – | 0.94 | – |
| 18. | Gamma/ beta. -Sitosterol (414.71) Sterol | C29H50O | 24.77 | 0.42 | 0.14 | – | 1.79 | – |
| 19 | Lup-20 (29)-en-3-one (424.70) Terpenoid | C30H48O | 25.29 | 0.26 | – | – | 4.04 | – |
| 20 | Lupeol (426.72) Terpenoid | C30H50O | 25.50 | – | – | – | 3.41 | – |
| 21. | Urs-12-en-24-oic acid, 3-oxo-, methyl ester, (+)- (468.70) Terpenoid | C31H48O3 | 26.45 | – | 0.15 | – | – | – |
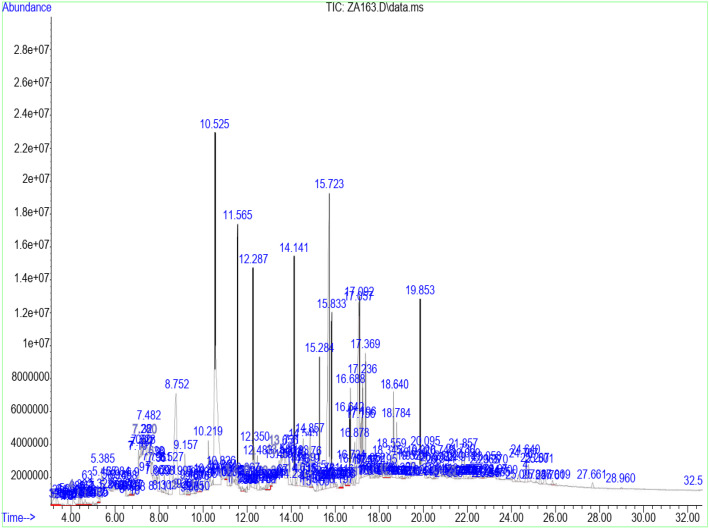
Fig. 4.
GC/MS chromatogram of fungal extract ZA163
Fig. 5.
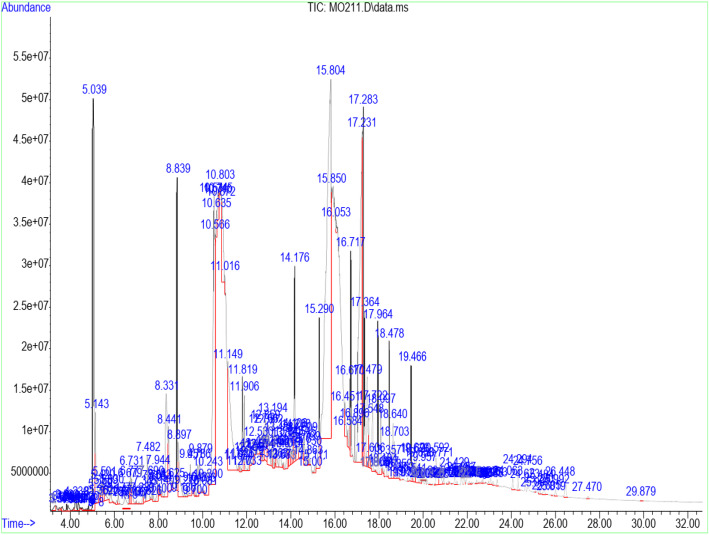
GC/MS chromatogram of fungal extract MO211
Fig. 6.
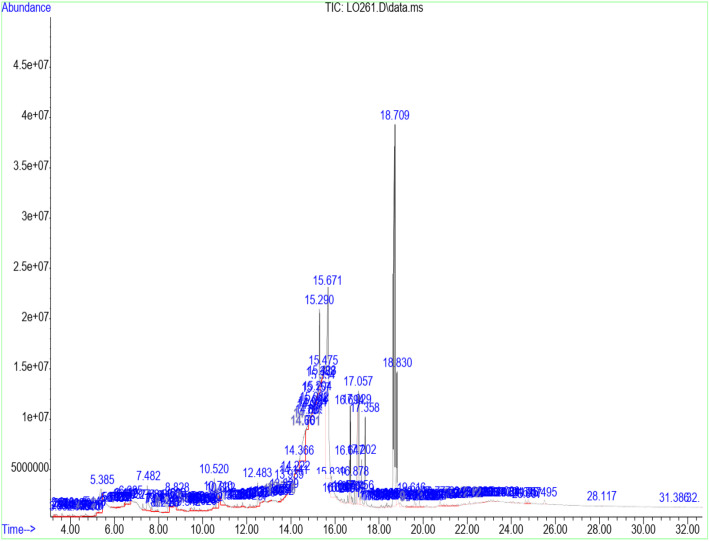
GC/MS chromatogram of fungal extract LO 261
Fig. 7.
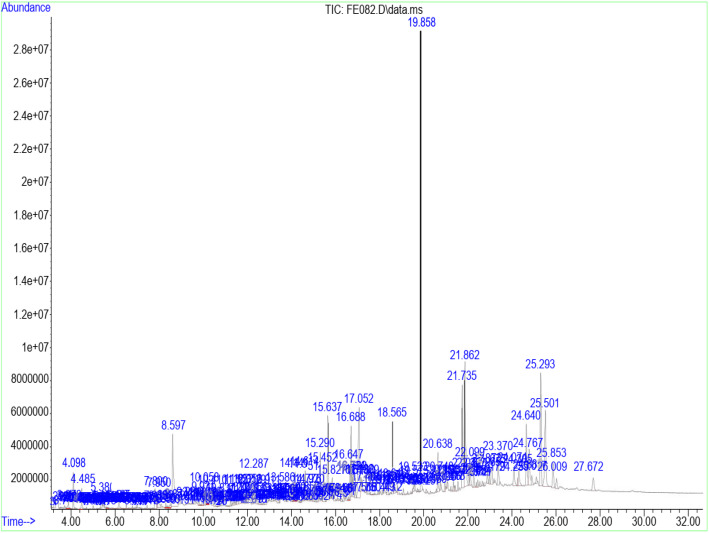
GC/MS chromatogram of fungal extract FE 082
Fig. 8.
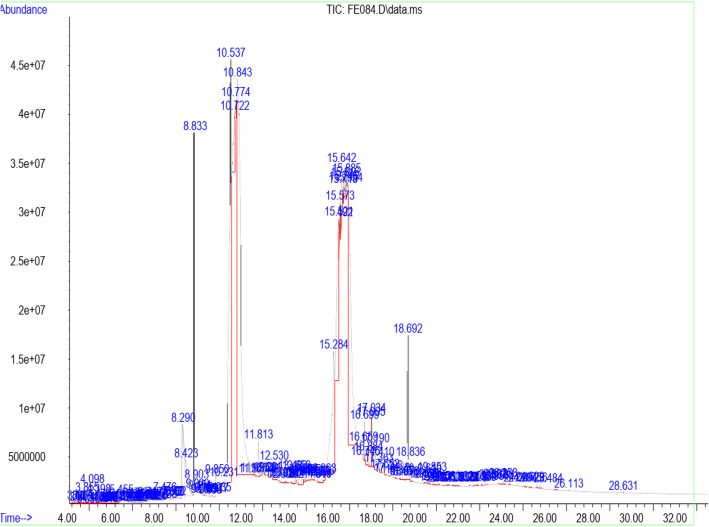
GC/MS chromatogram of fungal extract FE 084
Fig. 9.
Chemical structures of representative of phenolic (pyrogallol), terpenoid (lupeol), fatty acid (oleamide), sterol (β-sitosterol), and tocopherol (α-tocospiro A) compounds present in extracts of the isolated endophytic fungi
Fourteen compounds (0.06 to 19.84% percent composition) were identified in fungal extract MO211. Pyrogallol was recorded as the most abundant compound with percent composition of 19.84% while dl-alpha-tocopherol (0.06%) was the least identified compound. A total of twelve compounds were identified in ZA163 fungal extract, pyrogallol (10.72%) was the most abundant compound while dl-alpha-tocopherol (0.15%) was least identified. This was followed by fungal extract FE 082 with eleven identified compounds. Alpha tocospiro (4.92%) appeared as the most abundant while linoleic acid (0.07%) was the least identified compound. Fungal extracts FE 084 and LO 261 recorded six compounds each with pyrogallol (31.33%) and 9-octadecenamide (11.04%) recorded as most abundant compounds and 9-octadecenoic acid (Z)-, methyl ester (0.62%) and linoleic acid (0.05%) as least identified compounds respectively (Table 3).
Discussion
In this study, eighteen endophytic fungi were successfully isolated from surface sterilized fresh leaf samples of eight medicinal plants. To the best of our knowledge, this is the first report describing the isolation of endophytic fungi residing in the leaf cells of the selected plant samples. After size separation by agarose gel electrophoresis of the ITS PCR products, the PCR products of the plant samples exhibited DNA band size range of 400–700 bp. Detailed taxonomic assignment of fungal isolates remain unresolved due to limitations inherent in fungal ITS sequences. The results obtained from this study thus confirmed that leaves of the medicinal plants: Acalypha ornata, Albizia zygia, Alchornea cordifolia Chrysophyllum albidum, Ficus exasperata, Gomphrena celosioides, Millettia thonningii, and Newbouldia laevis are host to endophytic fungi.
The crude fungal extracts showed vary degrees of antioxidant activity in the DPPH radical scavenging and reduction of ferric ion assays. DPPH (2, 2-diphenyl-1-picryl-hydrazyl) is a stable free radical that produces purple color in methanol. Antioxidant activity of the fungal extracts was measured by discoloration to yellow color following the formation of non-radical (2,2-diphenyl-1-hydrazine) molecule [17]. Fungal extracts ZA163 and MO211 exhibited significant (p < 0.05) DPPH radical scavenging activity.
The metal chelating capacity of a compound may also serve as a significant indicator of its potential antioxidant activity [35]. The iron chelating activity of all fungal extracts was determined by reaction with ortho-phenanthroline. Fe3+ is reduced to Fe2+ by an antioxidant and the formed Fe2+ rapidly react with phenanthroline to form a stable red orange colored complex [33, 36]. Isolated fungal extracts ZA 163, LO 261, CA 041, CA 042, CA 043, FE 082, FE 084, and GE 091 from A. zygia, A. cordifolia, C. albidum, F. exasperata, and G. celosioides medicinal plants demonstrated iron chelating capability. Similar observations of antioxidant activity of endophytic fungi isolates have been reported in some medicinal plants such as Bauhinia racemosa, Distylium chinense, Euphorbia hirta, Guazoma tomentosa, Phoenix dactylifer, Phyllanthus amarus, and Senna spectabilis [4, 37–41]. Our results suggest that endophytic fungi that reside in the leaves of A. ornata, A. zygia, A. cordifolia, C. albidum, F. exasperata, G. celosioides, M. thonningii, and N. laevis showed promising antioxidant activity.
Fungal extracts ZA163, MO211, LO 261, FE 082, and FE 084 were among the fungal extracts that exhibited effective antioxidant activity with a substantial yield of extract, thus were selected for phytochemical analysis via GC/MS analytical technique. The identified compounds represented phenolic, terpenoid, and sterol classes of secondary metabolites as well as tocopherols and fatty acids. Most of these chemical constituents have been reported to demonstrate remarkable antioxidant activity. Phenolic compounds act as natural antioxidants that exert therapeutic effects such as anti-inflammatory, antidiabetic, antimicrobial, antiviral and vasodilatory effects and prevent various forms of diseases [42–44]. Tocopherol plays an important role as a lipid antioxidant in stabilizing subcellular membranes [45]. Fatty acids are able to reduce oxidative stress from free radicals by exerting an antioxidant role [46, 47]. Pyrogallol (phenol), alpha tocospiro (tocopherol), and oleamide (amide derivative of fatty acid) are present as the major components in the investigated endophytic fungi extracts. Previous studies have reported the isolation and characterization of graphislactone A, a phenolic benzopyranone antioxidant compound from the endophytic fungus Cephalosporium sp. inhabiting the medicinal plant Trachelospermum jasminoides [11]. Similarly, isolation and characterization of pestacin and isopestacin, a coumarone antioxidant and antifungal agents from the endophytic fungus Pestalotiopsis microspora that resides in the medicinal plant Terminalia morobensis was reported in literature [12, 13]. These results suggest that these compounds and others identified in fungal extracts ZA163 from Albizia zygia, MO211 from Millettia thonningii, LO 261 from Alchornea cordifolia, FE 082 and FE 084 from Ficus exasperata could be responsible for the antioxidant activity demonstrated by the endophytes.
Conclusion
The results obtained from this study established that endophytic fungi isolated from medicinal plants: Acalypha ornata, Albizia zygia, Alchornea cordifolia, Chrysophyllum albidum, Ficus exasperata, Gomphrena celosioides, Millettia thonningii, and Newbouldia laevis commonly used in Nigeria local herbal remedies may be a potential source of bioactive compounds, which may be used for the development of antioxidant drugs. Phytochemical analysis revealed the presence of phenols, tocopherols, sterols, terpenoids, and fatty acids that may have contributed to the observed effect. These findings indicate that endophytic fungi hold promise for large scale production of free radical scavenging agents, which can be developed as drug molecules used in the intervention of oxidative stress-mediated diseases.
Acknowledgements
The authors are thankful to FOWM Laboratory, for providing necessary facilities required for molecular identification of the isolated endophytic fungi. The assistance of Mrs. Sefiat Elesho during collection of plant samples; Mr. Abdulrahman Usman during isolation of endophytic fungi; Mrs. Faustina Ezeamaramu during molecular analysis is acknowledged.
Abbreviations
- PDA
Potato dextrose agar
- ITS
Internal transcribed spacer region
- DNA
Deoxyribonucleic acid
- PDB
Potato dextrose broth
- PCR
Polymerase chain reaction
- dNTP
Deoxynucleoside triphosphate
- NCBI
National Centre for Biotechnology Information
- BLAST
Basic Local Alignment Search Tool
- DPPH
2, 2-diphenyl-1-picryl-hydrazyl
- UV-vis
Ultraviolet-visible
- GC/MS
Gas chromatography/mass spectrometry
- O-phenanthroline
Ortho-phenanthroline
- IC50
Half maximal inhibitory concentration
- SEM
Standard error of mean
Authors’ contributions
AAA and MI designed the study and coordinated the project. Plant collection, identification and isolation of endophytic fungi from plant samples were carried out by MI, EO, AA, CO, and BA. Molecular analysis of isolated endophytes was carried out by MF, AA, CO, and BA. Antioxidant investigation was conducted by MI, AA, CO, and BA. Analysis of GCMS results was performed by MI, EO, and AAA. The manuscript was written by MI and reviewed by EO, MF, and AAA. All authors read and approved the final manuscript for submission.
Funding
Not applicable.
Availability of data and materials
The datasets generated and/or analyzed during the present study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Petrini O, Fisher P. Occurrence of fungal endophytes in twigs of Salix fragilis and Quercus robur. Mycol Res. 1990;94(8):1077–1080. doi: 10.1016/S0953-7562(09)81336-1. [DOI] [Google Scholar]
- 2.Venieraki A, Dimou M, Katinakis P. Endophytic fungi residing in medicinal plants have the ability to produce the same or similar pharmacologically active secondary metabolites as their hosts. Hell Plant Prot J. 2017;10(2):51–66. doi: 10.1515/hppj-2017-0006. [DOI] [Google Scholar]
- 3.Strobel GA. Endophytes as sources of bioactive products. Microb Infect. 2003;5(6):535–544. doi: 10.1016/S1286-4579(03)00073-x. [DOI] [PubMed] [Google Scholar]
- 4.Mefteh FB, Daoud A, Bouket AC, Thissera B, Kadri Y, Cherif-Silini H, Eshelli M, Alenezi FN, Vallat A, Oszako T, Kadri A, Ros-García JM, Rateb ME, Gharsallah N, Belbahri L. Date palm trees root-derived Endophytes as fungal cell factories for diverse bioactive metabolites. Int J Mol Sci. 2018;19(1986):1–22. doi: 10.3390/ijms19071986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dhayanithy G, Subban K, Chelliah J. Diversity and biological activities ofendophytic fungi associated with Catharanthus roseus. BMC Microbio. 2019;19(22):1–14. doi: 10.1186/s12866-019-1386-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fatima N, Mukhtar U, Ihsan U, Qazi MA, Jadoon M, Ahmed S. Biological Evaluation of Endophytic Fungus Chaetomium sp. NF15 of Justicia adhatoda L.: A Potential Candidate for Drug Discovery. Jundishapur J. Microbiol. 2016;9(6):e29978. doi: 10.5812/jjm.29978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Idris A, Al-tahir I, Idris E. Antibacterial activity of endophytic fungi extracts from the medicinal plant Kigelia Africana. Egypt Acad J Biol Sci. 2013;5(1):1–9. doi: 10.21608/eajbsg.2013.16639. [DOI] [Google Scholar]
- 8.Kumar S, Kaushik N. Endophytic fungi isolated from the Oil-seed crop Jatropha curcas produces oil and exhibit antifungal activity. PLoS One. 2013;8:e56202. doi: 10.1371/journal.pone.0056202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao J, Zhou L, Wang J, Shan T, Zhong L, Liu X, Gao X. Endophytic fungi for producing bioactive compounds originally from their host plants. Curr Res Tech Edu Topics in Appl Microbio Microb Biotech. 2010;1:567–576. [Google Scholar]
- 10.Bano N, Rizvi IF, Sharma N, Siddiqui M, Khan MA, Akhtar S. Production of bioactive secondary metabolites from endophytic fungi. Int Res J Eng Tech. 2016;3(6):1859–1866. [Google Scholar]
- 11.Song YC, Huang WY, Sun C, Wang FW, Tan RX. Characterization of graphislactone a as the antioxidant and free radical-scavenging substance from the culture of Cephalosporium sp. IFB-E001, an endophytic fungus in Trachelospermum jasminoides. Biol Pharm Bull. 2005;28(3):506–509. doi: 10.1248/bpb.28.506. [DOI] [PubMed] [Google Scholar]
- 12.Strobel G, Ford E, Worapong J, Harper JK. Isopestacin, an isobenzofuranone from Pestalotiopsis microspora, possessing antifungal and antioxidant activities. Phytochemistry. 2002;60(2):179–183. doi: 10.1016/S0031-9422(02)00062-6. [DOI] [PubMed] [Google Scholar]
- 13.Harper JK, Arif AM, Ford EJ, Strobel GA. Pestacin (I): a 1,3-Dihydro Isobenzofuran from Pestalotiopsis microspora possessing antioxidant and antimycotic activities. Tetrahedron. 2003;59(14):2471–2476. doi: 10.1016/S0040-4020(03)00255-2. [DOI] [Google Scholar]
- 14.Toghueo RMK, Boyom FF. Endophytic fungi from Terminalia species: a comprehensive review. J Fungi. 2019;5(43):1–20. doi: 10.3390/jof5020043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang WY, Cai YZ, Xing J, Corke H, Sun M. A potential antioxidant resource: endophytic fungi from medicinal plants. Econ Bot. 2007;61(1):14–30. doi: 10.1663/0013-0001(2007)61[14:APAREF]2.0.CO;2. [DOI] [Google Scholar]
- 16.Bharathidasan R, Panneerselvam A. Antioxidant activity of the endophytic fungi isolated from mangrove environment of karankadu, Ramanathapuram district. Int J Pharm Sci Res. 2012;3(8):2866–2869. [Google Scholar]
- 17.Gunasekaran S, Sathiavelu M, Arunachalam S. In vitro antioxidant and antibacterial activity of endophytic fungi isolated from Mussaenda luteola. J Appl Pharm Sci. 2017;7(8):234–238. [Google Scholar]
- 18.Radulovic N, Stankov-Jovanovic V, Stojanovic G, Smelcerovic A, Spiteller M, Asakawa Y. Screening of in-vitro antimicrobial and antioxidant activity of nine Hypericum species from the Balkans. Food Chem. 2007;103(1):15–21. doi: 10.1016/j.foodchem.2006.05.062. [DOI] [Google Scholar]
- 19.Bhattacharya S, Debnath S, Saha AK. Evaluation of antioxidant activity of two endophytic fungi isolated from Ananus comosus L. Eur J Biomed Pharm. 2018;5(1):896–899. [Google Scholar]
- 20.White JF, Torres MS. Is plant endophyte-mediated defensive mutualism the result of oxidative stress protection? Physiol Plant. 2010;138:440–446. doi: 10.1111/j.1399-3054.2009.01332.x. [DOI] [PubMed] [Google Scholar]
- 21.Hamilton CE, Gundel PE, Helander M, Saikkonen K. Endophytic mediation of reactive oxygen species and antioxidant activity in plants: a review. Fungal Divers. 2012;54(1):1–10. doi: 10.1007/s13225-012-0158-9. [DOI] [Google Scholar]
- 22.Strobel G, Daisy B. Bioprospecting for microbial endophytes and their natural products. Microbio Mol Bio Rev. 2003;67(4):491–502. doi: 10.1128/MMBR.67.4.491-502.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tolulope RA, Adeyemi AI, Erute MA, Abiodun TS. Isolation and screening of endophytic fungi from three plants used in traditional medicine in Nigeria for antimicrobial activity. Int J Green Pharm. 2015;9:58–62.
- 24.Ibrahim M, Kaushik N, Sowemimo A, Chhipa H, Koekemoer T, Van de Venter M, Odukoya OA. Antifungal and antiproliferative activities of endophytic fungi isolated from the leaves of Markhamia tomentosa. Pharm Bio. 2016;55(1):590–595. doi: 10.1080/13880209.2016.1263671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kjer J, Debbab A, Aly AH, Proksch P. Methods for isolation of marine derived endophytic fungi and their bioactive secondary products. Nat Protoc. 2009;5:479–490. doi: 10.1038/nprot.2009.233. [DOI] [PubMed] [Google Scholar]
- 26.Petrini O. Taxonomy of endophytic fungi of aerial plant tissues. In: Fokkenna NJ, Van Den HJ, editors. Microbiology of the Phyllosphere. Cambridge University Press: Cambridge; 1986. pp. 175–187. [Google Scholar]
- 27.White TJ, Bruns T, Lee S, Taylor JW. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. San Diego, CA: Academic Press; 1990. [Google Scholar]
- 28.Zarrini M, Ganj F, Faramarzi S. Analysis of the rDNA internal transcribed spacer region of the Fusarium species by polymerase chain reaction- restriction fragment length polymorphism. Biomed Rep. 2016;4(4):471–474. doi: 10.3892/br.2016.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alade GO, Moody JO, Bakare AG, Awotona OR, Adesanya S, Lai D, Debbab A, Proksch P. Metabolites from endophytic fungus; Pestalotiopsis clavispora isolated from Phoenix reclinata leaf. Future J Pharm Sci. 2018;4(2):273–275. doi: 10.1016/j.fjps.2018.10.001. [DOI] [Google Scholar]
- 30.Jeong SM, Kim SY, Kim DR, Namk C, Ahn DU, Lee SC. Effect of seed roasting conditions on the antioxidant activity of defatted sesame meal extracts. J Food Sci. 2004;69:C377–C381. doi: 10.1111/j.1365-2621.2004.tb10701.x. [DOI] [Google Scholar]
- 31.Parvez MK, Alam P, Arbab AH, Al-Dosari MS, Alhowiriny TA, Alqasoumi SI. Analysis of antioxidative and antiviral biomarkers b-amyrin, b-sitosterol, lupeol, ursolic acid in Guiera senegalensis leaves extract by validated HPTLC methods. Saudi Pharm J. 2018;26(5):685–693. doi: 10.1016/j.jsps.2018.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Afroze F, Hossain MT. Proximate analysis, phytochemical screening and antioxidant activity of Psidium guajava leaves growing in coastal area of Bangladesh. World J Pharm Pharm Sci. 2015;4:140–151. [Google Scholar]
- 33.Islam MZ, Hossain MT, Hossen F, Akter MS, Mokammel MA. In-vitro antioxidant and antimicrobial activity of Bougainvillea glabra flower. J Med Plant Res. 2016;10:228–236. doi: 10.3923/rjmp.2016.228.236. [DOI] [Google Scholar]
- 34.Liu ZL, Yang K, Bai PH, Zhou L, Liu SL, Liu QZ. Gas chromatography-mass spectrometric analysis of essential oil of aerial parts of Glycosmis parviflora (Sims) little (Rutaceae) Trop J Pharm Res. 2014;13(2):275–280. doi: 10.4314/tjpr.v13i2.17. [DOI] [Google Scholar]
- 35.Silvia MTA, Lydia R, Orly W, Moussayoudim BH. Green tea catechins as brain-permeable, natural iron chelators-antioxidants for the treatment of neurodegenerative disorders. Mol Nutr Food Res. 2006;50:229–234. doi: 10.1002/mnfr.200500156. [DOI] [PubMed] [Google Scholar]
- 36.Yefrida SH, Alif A, Efdi M, Aziz H. Modification of phenanthroline method to determine antioxidant content in tropical fruits methanolic extract. Res. J. Chem. 2018;22:28–35. [Google Scholar]
- 37.Srinivasan K, Jagadish LK, Shenbhagaraman R, Muthumary J. Antioxidant activity of endophytic fungus Phyllosticta sp. isolated from Guazuma tomentosa. J. Phytol. 2010;2(6):37–41. [Google Scholar]
- 38.Chapla VM, Zeraik ML, Ximenes VF, Zanardi LM, Lopes MN, Cavalheiro AJ, Silva DHS, Young MCM, da Fonseca LM, Bolzani VS, Araujo AR. Bioactive secondary metabolites from Phomopsis., an endophytic fungus from Senna spectabilis. Molecules. 2014;19(5):6597–6608. doi: 10.3390/molecules19056597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kandasamy P, Manogaran S, Dhakshinamoorthy M, Kannan KP. Evaluation of antioxidant and antibacterial activities of endophytic fungi isolated from Bauhinia racemosa Lam and Phyllanthus amarus Schum and Thonn. J Chem Pharm Res. 2015;7(9):366–379. [Google Scholar]
- 40.Akpotu MO, Eze PM, Abba CC, Nwachukwu CU, Okoye FBC, Esimone CO. Metabolites of Endophytic Fungi isolated from Euphorbia hirta growing in southern Nigeria. Chem Sci Rev Lett. 2017;6(21):12–19. [Google Scholar]
- 41.Duan X, Xu F, Qin D, Gao T, Shen W, Zuo S, Yu B, Xu J, Peng Y, Dong J. Diversity and bioactivities of fungal endophytes from Distylium chinense, a rare waterlogging tolerant plant endemic to the three gorges reservoir. BMC Microbio. 2019;19:279. doi: 10.1186/s12866-019-1670-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shahidi F, Ambigaipalan P. Phenolics and polyphenolics in foods, beverages and spices: antioxidant activity and health effects - a review. J Func Food. 2015;18:820–897. doi: 10.1016/j.jff.2015.06.018. [DOI] [Google Scholar]
- 43.Gonçalves S, Moreira E, Grosso C, Andrade PB, Valentão P, Romano A. Phenolic profile, antioxidant activity and enzyme inhibitory activities of extracts from aromatic plants used in Mediterranean diet. J Food Sci Tech. 2017;54(1):219–227. doi: 10.1007/s13197-016-2453-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Comunian TA, Ravanfar R, de Castro I, A Dando R, Favaro-Trindade CS, Abbaspourrad A. Improving oxidative stability of echium oil emulsions fabricated by Microfluidics:effect of ionic gelation and phenolic compounds. Food Chem. 2017;233:125–134. doi: 10.1016/j.foodchem.2017.04.085. [DOI] [PubMed] [Google Scholar]
- 45.Wang X, Quinn PJ. Vitamin E and its function in membranes. Pog Lipid Res. 1999;38(4):309–336. doi: 10.1016/S0163-7827(99)00008-9. [DOI] [PubMed] [Google Scholar]
- 46.Tsaluchidu S, Cocchi M, Tonello L, Puri BK. Fatty acids and oxidative stress in psychiatric disorders. BMC Psych. 2008;8(Suppl 1):S5. doi: 10.1186/147-244X-8-S1-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gülçin I. Antioxidant activity of food constituents: an overview. Arch Tox. 2012;86(3):345–391. doi: 10.1007/s00204-011-0774-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the present study are available from the corresponding author on reasonable request.