Abstract
Background:
Stevens-Johnson syndrome and toxic epidermal necrolysis are severe, life-threatening mucocutaneous drug reactions with a high morbidity and mortality that require immediate medical care. Several immunomodulatory drugs are used for the treatment but evidence of their efficacy is limited. Cyclosporine has recently been found to have a promising role in SJS/TEN owing to its potent antiapoptotic activity.
Aims:
This open label prospective study was conducted to determine the efficacy, safety, and tolerability of cyclosporine in patients with SJS/TEN.
Methods:
This study was conducted at a tertiary care teaching hospital of South Rajasthan during a period of 4 years (August 2015 to July 2019). Data regarding clinical profile, causative drug(s), disease severity, associated comorbidities, treatment received, and outcome were recorded in a predesigned proforma. SCORTEN prognostic score was calculated for each patient at the time of admission. Cyclosporine was administered in a dose of 5 mg/kg body weight in two divided dosage until reepithelization.
Results:
Out of 16 patients 10 were males and 6 were females. Mean age of patients was 30.62 ± 16.98 years (range: 7–63). Most of the patients, i.e., 8 out of 16 had TEN, 5 patients had SJS, and 3 patients had SJS/TEN overlap. Mean ± SD delay between onset and admission was 3.812 ± 1.377 days (range: 2–7). Among the suspected drugs, antiepileptics (43.7%) formed the major group. Mean duration of reepithelization was 10.5 ± 3.46 days (range: 7–15). Based on the SCORTEN, the expected mortality was 2.55 with mean predicted mortality rate of 16.43% with SD of 19.3.
Limitations:
1) Sample size was small. 2) Placebo control trial could not be done due to the severity of the disease.
Conclusion:
We recommend cyclosporine (5 mg/kg/day) as the first line-specific immunomodulatory agent in SJS/TEN on account of its efficacy, safety, rapid reepithelization, decrease hospital stay, and reduced morbidity and mortality.
Keywords: Cyclosporin, granulysin, Stevens-Johnson syndrome, toxic epidermal necrolysis, SCORTEN
Introduction
Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are rare, potentially life threatening, severe mucocutaneous adverse reactions characterized by extensive epidermal detachment, erosion of mucosae, and severe constitutional symptoms.[1] The incidence rate is 0.5-1.4 million per year, and the average mortality is estimated to be 25-35%.[2] SJS and TEN are considered a disease continuum and involve skin detachment of <10% and >30% of body surface area (BSA), respectively. SJS/TEN overlap describes patients with skin detachment of 10-30% of BSA.[3]
Most cases of SJS and TEN are druginduced. Although any drug can cause SJS/TEN, the majority of reactions can be attributed to a group of highrisk drugs such as carbamazepine, phenytoin, allopurinol, lamotrigine, oxicam, and other nonsteroidal antiinflammatory drugs, sulfonamide antibiotics, and nevirapine.[4]
As SJS-TEN is a fatal condition, prompt withdrawal of culprit drug, supportive care, and early institution of immunomodulating drugs are the mainstay of treatment. Though several treatment protocols exist, none has been universally accepted. Several studies have demonstrated variable success with corticosteroids,[5,6,7] intravenous immunoglobulin,[8,9] plasmapheresis,[10] cyclophosphamide,[11] and tumor necrosis factor-α inhibitors.[12]
Cyclosporine has recently been found to have a promising role in SJS/TEN owing to its potent antiapoptotic activity.[13] Only a few Indian studies[14,15] are available on cyclosporine treatment in SJS/TEN. We report our experience of treating successfully 16 cases of SJS/TEN with cyclosporine.
Methods
This open label prospective study was conducted at a tertiary care teaching hospital of South Rajasthan during a period of 4 years (August 2015-July 2019). Ethical clearance was taken from the institutional review board. All of the consecutive patients with clinical diagnosis of SJS, SJS-TEN overlap, and TEN were enrolled in the study. All patients were hospitalized in the isolation ward of dermatology department. All suspected drugs were withdrawn. Data regarding clinical profile, causative drug(s), disease severity, associated comorbidities, treatment received, and outcome were recorded in a predesigned proforma. Informed consent was taken from the patients. SCORTEN prognostic score was calculated for each patient at the time of admission. Cyclosporine was administered in a dose of 5 mg/kg body weight in two divided dosage from the day of admission until complete reepithelization.
Supportive care was provided including barrier nursing, maintaining ambient temperature of 30°C, fluid and electrolyte balance, and high calorie containing diets. Appropriate antibiotics were administered in patients with evidence of sepsis. Referral to other specialties was done whenever needed.
Efficacy of cyclosporine was assessed by the average number of days for stabilization of the disease progression, rate of reepithelization of skin, duration of hospitalization, tolerance to treatment, and rate of mortality at complete recovery and compared with the predicted death estimated by the SCORTEN at the time of admission. The actual death rates were compared to the predicted rates by standardized mortality ratio analysis {(sum of observed deaths/sum of expected deaths) × 100}. The SCORTEN calculation was as per study of Bastuji-Garin et al.[16] Stabilization of disease was defined when new lesions ceased to appear. Progression of disease was evaluated by any increase in erosions, blistering, and positive Nikolsky's sign. Reepithelization was defined as complete healing of the skin without any erosion.
The safety and tolerability parameters were assessed by adverse events and routine investigations performed on a weekly basis (complete hemogram, fasting blood sugar, liver function tests, serum urea, creatinine, and serum electrolytes). Blood pressure monitoring was done on daily basis.
Results
A total of 16 consecutive patients were enrolled in the study. Out of 16 patients, 10 were males and 6 were females. Mean age of patients was 30.62 ± 16.98 years (range: 7- 63). Most of the patients, i.e., 8 out of 16 had TEN, 5 patients had SJS, and 3 patients had SJS/TEN overlap. Mean ± SD delay between onset and admission was 3.812 ± 1.377 days (range: 2-7). Among the suspected drugs, antiepileptics (43.7%) formed the major group causing SJS/TEN followed by antiretroviral drugs (12.5%), paracetamol and itraconazole (6.3% each), and unidentified in 31.2%. Two or more mucosae were involved in every patient of which oral and conjunctiva were the most commonly affected. Fever was the most common constitutional symptoms (13 out of 16 patients). Cyclosporine was tolerated well by all the patients.
Mean duration from initiation of cyclosporine and disease stabilization was 3.94 ± 1.12 days. Mean duration of reepithelization was 10.5 ± 3.46 days (range: 7-15). Mean duration of hospital stay was 13.75 ± 3.67 days (range: 9-19). Clinical profile, SCORTEN, and clinical outcome parameters are shown in Table 1. Response to cyclosporine in a few representative patients is shown in Figures 1a, b, 2a, b and 3a, b.
Table 1.
Clinical profile, SCORTEN, and clinical outcome parameters
| Age | Sex | Clinical diagnosis | Causal drug | Co-morbidity | SCORTEN at day 0 | Delay in admission (days) | Stabilization duration (days) | Reepithelization duration (days) | Hospital stay (days) |
|---|---|---|---|---|---|---|---|---|---|
| 35 | F | TEN | Phenytoin | MS | 2 | 3 | 5 | 14 | 17 |
| 30 | F | SJS/TEN | Nevirapine | HIV | 2 | 3 | 6 | 15 | 17 |
| 55 | M | TEN | Carbamazepine | HTN | 4 | 2 | 3 | 7 | 9 |
| 25 | F | SJS/TEN | Unknown | - | 2 | 5 | 5 | 9 | 15 |
| 40 | M | SJS | Carbamazepine | - | 1 | 4 | 3 | 7 | 9 |
| 35 | M | TEN | Unknown | - | 2 | 4 | 3 | 15 | 19 |
| 9 | M | SJS/TEN | Unknown | - | 1 | 3 | 4 | 9 | 13 |
| 46 | F | SJS | Efavirenz | HIV | 1 | 5 | 2 | 6 | 9 |
| 28 | M | TEN | Phenytoin | - | 2 | 4 | 5 | 15 | 18 |
| 22 | M | SJS | Unknown | - | 2 | 3 | 4 | 13 | 17 |
| 5 | M | SJS | Phenytoin | - | 1 | 3 | 3 | 7 | 9 |
| 44 | F | TEN | Carbamazepine | Diabetes | 3 | 3 | 5 | 11 | 13 |
| 11 | M | SJS | Paracetamol | - | 0 | 2 | 3 | 7 | 10 |
| 7 | M | TEN | Unknown | - | 1 | 4 | 3 | 8 | 10 |
| 63 | M | TEN | Carbamazepine | HTN | 4 | 7 | 5 | 15 | 18 |
| 25 | F | TEN | Itraconazole | - | 2 | 6 | 4 | 10 | 15 |
Figure 1.
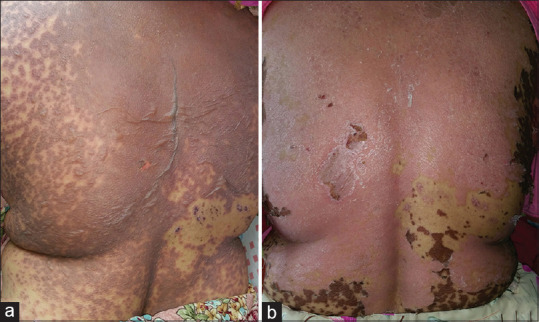
(a) Extensive blistering and detachment of the skin over back in a female with TEN due to carbamazepine. (b) Reepithelization of the skin at day 10 of cyclosporine use
Figure 2.
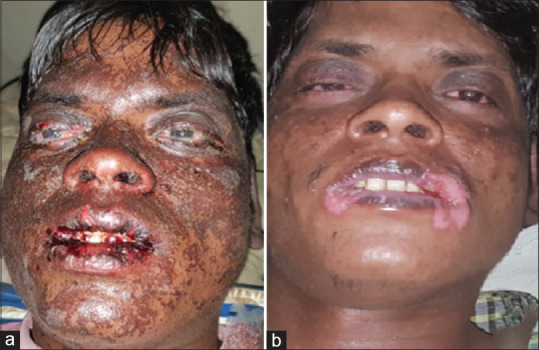
(a) Extensive mucosal involvement with epidermal necrolysis in a male patient with TEN due to Phenytoin. (b) Reepithelization of the skin and improvement in mucosal lesions on day 15 of cyclosporine use
Figure 3.
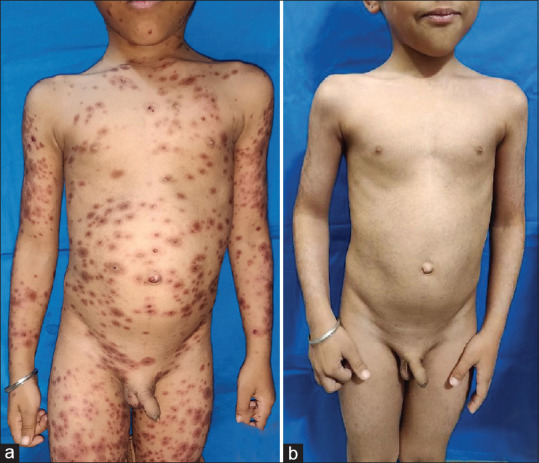
(a) Dusky erythematous macules and atypical target lesions all over the body in a child of Stevens-Johnson syndrome due to paracetamol. (b) Complete resolution of lesions at day 7 of cyclosporine treatment
Based on the SCORTEN, the expected mortality was 2.55 with the mean predicted mortality rate of 16.43% and a standard deviation of 19.3, in patients treated with cyclosporine [Table 2]. Standard mortality rate could not be calculated because there was no death with cyclosporine treatment.
Table 2.
Data of mortality of patients of SJS/TEN treated with cyclosporine
| SCORTEN | Expected mortality (%) | No. of patients | No. of death | |
|---|---|---|---|---|
| Predicted | Actual | |||
| 0-1 | 3.2 | 6 | 0.19 | 0 |
| 2 | 12.1 | 7 | 0.85 | 0 |
| 3 | 35.3 | 1 | 0.35 | 0 |
| 4 | 58.3 | 2 | 1.16 | 0 |
| 5-7 | 90 | 0 | 0 | 0 |
| Total | 16 | 2.55 | 0 | |
Discussion
SJS/TEN are severe life-threatening mucocutaneous adverse drug reactions with high morbidity and mortality. The management essentials include early recognition of the condition, prompt withdrawal of the culprit drug, meticulous supportive care, referral if required, initiation of specific therapy, management of complications, and prevention of future episodes.[1] Traditionally, systemic corticosteroids have remained the mainstay of therapy of SJS and TEN in most centers. The rationale is that both these conditions are immune-mediated processes, and corticosteroids suppress the intensity of the reaction, prevent/decrease the necrolysis of the skin, reduce fever, and prevent damage to internal organs when given at an early stage and in sufficiently high dosage. Although corticosteroids successfully control disease activity in SJS/TEN, they may be associated with increase in the rate of infective complications, delayed healing, and prolonged hospital stay.[17]
The present understanding of mechanism of SJS/TEN involves activation of cytotoxic T-cells by a culprit drug with the consequent release of granulysin and activation of caspase cascade resulting in keratinocyte apoptosis.[18] Cyclosporine inhibits the activation of CD4+ and CD8+ (cytotoxic) T-cells in the epidermis by suppressing interlekuin-2 production from activated T-helper cells. Cyclosporine has also been shown to inhibit TNF-α production. TNF-α is another key cytokine involved in the amplification of apoptotic pathways implicated in SJS/TEN.[19] Many case reports, case series, open trials, and retrospective studies have documented the efficacy of cyclosporine in SJS/TEN [Table 3].[20,21,22,23] Some of these reports and meta analyses, in fact, suggest the superiority of cyclosporine over other therapies including intravenous immunoglobulin, corticosteroids, cyclophosphamide, and supportive care alone.[24,25,26,27,28,29,30,31] One cohort study, however, has questioned beneficial effect of cyclosporine in epidermal necrolysis.[32]
Table 3.
Studies of cyclosporine in treatment of Stevens-Johnson syndrome and toxic epidermal necrolysis
| Study | Study design | Treatment regimen | No. of patients treated with Cyclosporine | Hospitalization duration (Days) | SCORTEN predicted mortality | Observed mortality | Conclusion |
|---|---|---|---|---|---|---|---|
| Valeyrie-Allanore et al.,[24] 2010 | Prospective open trial (2005-2010) | 3 mg/kg/d × 10d, 2 mg/kg/d × 10d, 1 mg/kg/d × 10d | 29 | 16.2±9 | 2.75 | 0 | Both the death rates and the progression of detachment seemed lower than expected, suggesting a possible usefulness of cyclosporine in SJS and TEN. |
| Reese et al.,[20] 2011 | Case series | 5 mg/kg/d for 5 d to a month | 4 | - | - | 0 | Cyclosporine is efficacious with rapid response and reepithelization. Short-term use of cyclosporine did not have adverse reactions or increased infections. |
| Singh et al.,[14] 2013 | Prospective open trial (2011-2012) | 3 mg/kg/d × 7d, 2 mg/kg/d × 7d | 11 | 18±5 | 1.1 | 0 | Cyclosporine has encouraging role in the management of uncomplicated cases of SJS, SJS-TEN overlap, or TEN. |
| Kirchhof et al.,[25] 2014 | Retrospective study (2001-2011) | 3-5 mg/kg/d for 7d | 15 | 16±8 | 2.4 | 1 | Relative mortality benefit of Cyclosporine over IVIg in patients with SJS/ TEN. |
| Lee et al,[27] 2016 | Retrospective study (2011-2014) | 3 mg/kg/d × 10 d, 2 mg/kg/d × 10 d, 1 mg/kg/d × 10 d | 24 | 20±15 | 7.2 | 3 | Relative mortality benefit of Cyclosporine over supportive care in patients with SJS/TEN. |
| Saoji et al.,[21] 2016 | Case series | 3-5 mg/kg/d for 10 d | 5 | 12.4 | - | 0 | Cyclosporine even without systemic corticosteroids is safe and effective for the treatment of TEN. |
| Gonzalez-Herrada et al,[26] 2017 | Retrospective (2001-2010) and prospective study (2011-2015) | 3 mg/kg/d until reepithelialization subsequently decreasing by 10 mg/day every 48 h | 49 | - | 11.8 | 5 | Cyclosporine reduces mortality in epidermal necrolysis patients. |
| Mohanty et al.,[15] 2017 | Retrospective study (2014-2015) | 5 mg/kg/d for 10 d | 19 | 20.39±5.40 | 3.11 | 1 | Cyclosporine (5 mg/ kg/day) for 10 days from onset of SJS/TEN may decrease the risk of dying, may provide faster healing of lesions, and might lead to early discharge from hospital. |
| Conner et al.,[22] 2018 | Case series | 3 mg/kg/d until reepithelialization | 4 | - | - | 1 | Rapid stabilization, rapid reepithelialization, low mortality rate, and shortened hospital length of stay with cyclosporine therapy |
| Vinay et al.,[23] | Case series | 3 mg/kg in divided dose | 5 | - | - | 0 | Predictable bio-availability and rapid reepithelialisation with intravenous form has potential of reducing hospital stay and incidence of secondary nosocomial infections in SJS/TEN. |
| Present study | Prospective open trial (2015-2019) | 5 mg/kg/d until reepithelialization | 16 | 13.75±3.67 | 2.55 | 0 | Cyclosporine (5 mg/ kg/day) can be used as the first line-specific immunomodulatory agent in SJS/TEN on account of its efficacy, safety, rapid reepithelization, decrease hospital stay, and reduced morbidity and mortality. |
Optimal dosing of cyclosporine for SJS/TEN is unclear. Various studies have utilized different dosing approaches. Generally, a dose of 3-5 mg/kg body weight, as oral capsules or solution, in two divided doses with an average treatment duration of 10-14 days has been used. We treated our patients with 5 mg/kg/day cyclosporine divided in twice daily dose with an average duration of 7-15 days.
Most common side effects associated with cyclosporin treatment are hypertension and renal toxicity, but they are not seen in treatment with short duration as used in SJS/TEN.[1] None of our patient experienced these side effects, and the treatment was well tolerated despite being administered to acutely ill patients. Cyclosporine is an immunosuppressant and may place patients at increased risk for developing lymphomas and other malignancies, particularly those related to the skin. But due to shorter duration of treatment in SJS/TEN, the risk of malignancy or infection incurred from cyclosporine treatment is likely to be negligible.[30]
As indicated in one case report, initiation of cyclosporine therapy is not contraindicated for the treatment of SJS/TEN in HIV-infected patients. However, the short course of cyclosporine therapy needs to be preferred.[21] Our two HIV positive patients were also treated successfully with cyclosporine without any complications.
Limitation
-
1)
Sample size was small
-
2)
Placebo control trial could not be done due to the severity of the disease.
Conclusion
On account of paucity of randomized control studies on ideal therapeutic agents in SJS/TEN, an experience and evidence-based approach is needed in the management of SJS/TEN. We recommend cyclosporine (5 mg/kg/day) as the first line-specific immunomodulatory agent in SJS/TEN on account of its (i) efficacy, (ii) safety, (iii) rapid reepithelization, (iv) decrease hospital stay, and (v) reduced morbidity and mortality. A randomized control trial and comparison with other immunomodulatory agents would further lend support to our view and provide valuable data to treat this dreaded drug-induced condition.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
Dr. Mohammed Zahid, M.D. Scholar Dermatology, RNT Medical College, Udaipur (Rajasthan).
References
- 1.Gupta LK, Martin AM, Agarwal N, D'Souza P, Das S, Kumar R, et al. Guidelines for the management of Stevens-Johnson syndrome/toxic epidermal necrolysis: An Indian perspective. Indian J Dermatol Venereol Leprol. 2016;82:603–25. doi: 10.4103/0378-6323.191134. [DOI] [PubMed] [Google Scholar]
- 2.Harr T, French LE. Toxic epidermal necrolysis and Stevens-Johnson syndrome. Orphanet J Rare Dis. 2010;5:39. doi: 10.1186/1750-1172-5-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bastuji-Garin S, Rzany B, Stern RS, Shear NH, Naldi L, Roujeau JC. Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol. 1993;129:92–6. [PubMed] [Google Scholar]
- 4.Mockenhaupt M, Viboud C, Dunant A, Naldi L, Halevy S, Bouwes Bavinck JN, et al. Stevens-Johnson syndrome and toxic epidermal necrolysis: Assessment of medication risks with emphasis on recently marketed drugs. The EuroSCAR-study. J Invest Dermatol. 2008;128:35–44. doi: 10.1038/sj.jid.5701033. [DOI] [PubMed] [Google Scholar]
- 5.Tripathi A, Ditto AM, Grammer LC, Greenberger PA, McGrath KG, Zeiss CR, et al. Corticosteroid therapy in an additional 13 cases of Stevens-Johnson syndrome: A total series of 67 cases. Allergy Asthma Proc. 2000;21:101–5. doi: 10.2500/108854100778250914. [DOI] [PubMed] [Google Scholar]
- 6.Kim KJ, Lee DP, Suh HS, Lee MW, Choi JH, Moon KC, et al. Toxic epidermal necrolysis: Analysis of clinical course and SCORTEN-based comparison of mortality rate and treatment modalities in Korean patients. Acta Derm Venereol. 2005;85:497–502. doi: 10.1080/00015550510038232. [DOI] [PubMed] [Google Scholar]
- 7.Das S, Roy AK, Biswas I. A six-month prospective study to find out the treatment outcome, prognosis and offending drugs in toxic epidermal necrolysis from an urban institution in Kolkata. Indian J Dermatol. 2013;58:191–3. doi: 10.4103/0019-5154.110826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trent JT, Kirsner RS, Romanelli P, Kerdel FA. Analysis of intravenous immunoglobulin for the treatment of toxic epidermal necrolysis using SCORTEN: The University of Miami Experience. Arch Dermatol. 2003;139:39–43. doi: 10.1001/archderm.139.1.39. [DOI] [PubMed] [Google Scholar]
- 9.Al-Mutairi N, Arun J, Osama NE, Amr Z, Mazen AS, Ibtesam el-A, et al. Prospective, noncomparative open study from Kuwait of the role of intravenous immunoglobulin in the treatment of toxic epidermal necrolysis. Int J Dermatol. 2004;43:847–51. doi: 10.1111/j.1365-4632.2004.02048.x. [DOI] [PubMed] [Google Scholar]
- 10.Narita YM, Hirahara K, Mizukawa Y, Kano Y, Shiohara T. Efficacy of plasmapheresis for the treatment of severe toxic epidermal necrolysis: Is cytokine expression analysis useful in predicting its therapeutic efficacy? J Dermatol. 2011;38:236–45. doi: 10.1111/j.1346-8138.2010.01154.x. [DOI] [PubMed] [Google Scholar]
- 11.Heng MC, Allen SG. Efficacy of cyclophosphamide in toxic epidermal necrolysis. Clinical and pathophysiologic aspects. J Am Acad Dermatol. 1991;25:778–86. doi: 10.1016/s0190-9622(08)80969-3. [DOI] [PubMed] [Google Scholar]
- 12.Fischer M, Fiedler E, Marsch WC, Wohlrab J. Antitumour necrosis factor-alpha antibodies (infliximab) in the treatment of a patient with toxic epidermal necrolysis. Br J Dermatol. 2002;146:707–9. doi: 10.1046/j.1365-2133.2002.46833.x. [DOI] [PubMed] [Google Scholar]
- 13.Stern RS, Divito SJ. Stevens-Johnson syndrome and toxic epidermal necrolysis: Associations, outcomes, and pathobiology-Thirty years of progress but still much to be done. J Invest Dermatol. 2017;137:1004–8. doi: 10.1016/j.jid.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh GK, Chatterjee M, Verma R. Cyclosporine in Stevens-Johnson syndrome and toxic epidermal necrolysis and retrospective comparison with systemic corticosteroid. Indian J Dermatol Venereol Leprol. 2013;79:686–92. doi: 10.4103/0378-6323.116738. [DOI] [PubMed] [Google Scholar]
- 15.Mohanty S, Das A, Ghosh A, Sil A, Gharami RC, Bandyopadhyay D, et al. Effectiveness, safety and tolerability of cyclosporine versus supportive treatment in Stevens-Johnson syndrome/toxic epidermal necrolysis: A record-based study. Indian J Dermatol Venereol Leprol. 2017;83:312–6. doi: 10.4103/ijdvl.IJDVL_201_16. [DOI] [PubMed] [Google Scholar]
- 16.Bastuji-Garin S, Fouchard N, Bertocchi M, Roujeau JC, Revuz J, Wolkenstein P. SCORTEN: A severity-of-illness score for toxic epidermal necrolysis. J Invest Dermatol. 2000;115:149–53. doi: 10.1046/j.1523-1747.2000.00061.x. [DOI] [PubMed] [Google Scholar]
- 17.Chave TA, Mortimer NJ, Sladden MJ, Hall AP, Hutchinson PE. Toxic epidermal necrolysis: Current evidence, practical management and future directions. Br J Dermatol. 2005;153:241–53. doi: 10.1111/j.1365-2133.2005.06721.x. [DOI] [PubMed] [Google Scholar]
- 18.Chung WH, Hung SI, Yang JY, Su SC, Huang SP, Wei CY, et al. Granulysin is a key mediator for disseminated keratinocyte death in Stevens Johnson syndrome and toxic epidermal necrolysis. Nat Med. 2008;14:1343–50. doi: 10.1038/nm.1884. [DOI] [PubMed] [Google Scholar]
- 19.Remick DG, Nguyen DT, Eskandari MK, Strieter RM, Kunkel SL. Cyclosporine A inhibits TNF production without decreasing TNF mRNA levels. Biochem Biophys Res Commun. 1989;16:551–5. doi: 10.1016/0006-291x(89)92634-x. [DOI] [PubMed] [Google Scholar]
- 20.Reese D, Henning JS, Rockers K, Ladd D, Gilson R. Cyclosporine for SJS/TEN: A case series and review of the literature. Cutis. 2011;87:24–9. [PubMed] [Google Scholar]
- 21.Saoji V, Hazare S, Choudhary S. Successful use of cyclosporine in the treatment of toxic epidermal necrolysis: A case series. Indian J Drugs Dermatol. 2016;2:24–7. [Google Scholar]
- 22.Conner CD, McKenzie E, Owen CE, Callen JP. The use of cyclosporine for Stevens-Johnson syndrome-toxic epidermal necrolysis spectrum at the University of Louisville: A case series and literature review. Dermatol Online J. 2018;24:4. [PubMed] [Google Scholar]
- 23.Vinay K, Kaushik A, Kumaran MS, Parsad D. Intravenous cyclosporine in treatment of Stevens-Johnson syndrome/toxic epidermal necrolysis: A case series. Dermatol Ther. 2019;32:e12957. doi: 10.1111/dth.12957. [DOI] [PubMed] [Google Scholar]
- 24.Valeyrie-Allanore L, Wolkenstein P, Brochard L, Ortonne N, Maitre B, Revuz J, et al. Open trial of ciclosporin treatment for Stevens-Johnson syndrome and toxic epidermal necrolysis. Br J Dermatol. 2010;163:847–53. doi: 10.1111/j.1365-2133.2010.09863.x. [DOI] [PubMed] [Google Scholar]
- 25.Kirchhof MG, Miliszewski MA, Sikora S, Papp A, Dutz JP. Retrospective review of Stevens-Johnson syndrome/toxic epidermal necrolysis treatment comparing intravenous immunoglobulin with cyclosporine. J Am Acad Dermatol. 2014;71:941–7. doi: 10.1016/j.jaad.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 26.González-Herrada C, Rodríguez-Martín S, Cachafeiro L, Lerma V, González O, Lorente JA, et al. Cyclosporin use in epidermal necrolysis is associated with an important mortality reduction: Evidence from three different approaches. J Invest Dermatol. 2017;137:2092–100. doi: 10.1016/j.jid.2017.05.022. [DOI] [PubMed] [Google Scholar]
- 27.Lee HY, Fook-Chong S, Koh HY, Thirumoorthy T, Pang SM. Cyclosporine treatment for Stevens-Johnson syndrome/toxic epidermal necrolysis: Retrospective analysis of a cohort treated in a specialized referral center. J Am Acad Dermatol. 2017;76:106–13. doi: 10.1016/j.jaad.2016.07.048. [DOI] [PubMed] [Google Scholar]
- 28.Ng QX, De Deyn MLZQ, Venkatanarayanan N, Ho CYX, Yeo WS. A meta-analysis of cyclosporine treatment for Stevens-Johnson syndrome/toxic epidermal necrolysis. J Inflamm Res. 2018;11:135–42. doi: 10.2147/JIR.S160964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Papp A, Sikora S, Evans M, Song D, Kirchhof M, Miliszewski M, et al. Treatment of toxic epidermal necrolysis by a multidisciplinary team. A review of literature and treatment results. Burns. 2018;44:807–15. doi: 10.1016/j.burns.2017.10.022. [DOI] [PubMed] [Google Scholar]
- 30.Gilbert M, Scherrer LA. Efficacy and safety of cyclosporine in Stevens-Johnson syndrome and toxic epidermal necrolysis. Dermatol Ther. 2019;32:e12758. doi: 10.1111/dth.12758. [DOI] [PubMed] [Google Scholar]
- 31.Morgado-Carrasco D, Fustà-Novell X, Iranzo P. FR-Ciclosporin as a first line treatment in epidermal necrolysis. Actas Dermosifiliogr. 2019;110:601–3. doi: 10.1016/j.ad.2018.01.014. [DOI] [PubMed] [Google Scholar]
- 32.Poizeau F, Gaudin O, Le Cleach L, Duong TA, Hua C, Hotz C, et al. Cyclosporine for epidermal necrolysis: Absence of beneficial effect in a retrospective cohort of 174 patients-exposed/unexposed and propensity score-matched analyses. J Invest Dermatol. 2018;138:1293–300. doi: 10.1016/j.jid.2017.12.034. [DOI] [PubMed] [Google Scholar]


