Abstract
Dermatoscopy is a non-invasive, handy tool, which is increasingly being used in diagnosis and prognostication of pigmentary dermatoses. Dermatoscopic changes in pigmentary pattern, scaling, and vasculature help us to differentiate among the myriad of hypo and hyper pigmentary diseases. This review gives a brief overview of the dermatoscopic features of pigmentary diseases, which are commonly encountered in clinical practice. We also provide a diagnostic approach based on salient dermatoscopic features.
Keywords: Dermatoscopy, dermoscopy, exogenous ochronosis, lichen planus pigmentosus, lichen sclerosus et atrophicus, melasma, pigmentary diseases, vitiligo
Introduction
Dermatoscopy or epiluminescence microscopy is a rapidly evolving imaging modality. Akin to a stethoscope to an internist, a dermatoscope has now become an integral part of dermatology practice. Its application has evolved from early diagnosis of melanoma and non-melanoma skin cancers to inflammatory diseases, pigmentary diseases, infections, and infestations.[1] However, the knowledge of dermatoscopy in ethnic skin (Fitzpatrick skin phototype IV–VI) is limited.[2] This section of the symposium deals with dermatoscopic features of pigmentary diseases, with special emphasis on ethnic skin.
Dermatoscopy of normal skin
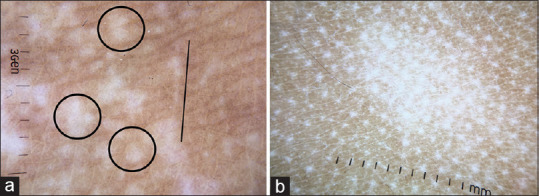
Before discussing the dermatoscopic features of various dermatoses, it is essential to understand the dermatoscopic features of normal skin. Under a dermatoscope, ethnic glabrous non-facial skin shows thin lines arranged in a reticular pattern [Figure 1a].[2] This represents the pigmented basal layer as seen through a horizontal section. Interspersed between these reticular lines are pinpoint white dots representing the eccrine duct openings. Parallel brown lines representing skin folds can also be seen. Dermatoscopy of facial skin shows pseudo network pattern, which is caused due to the interruption of the pigmented epidermis by appendageal openings [Figure 1b].[2]
Figure 1.
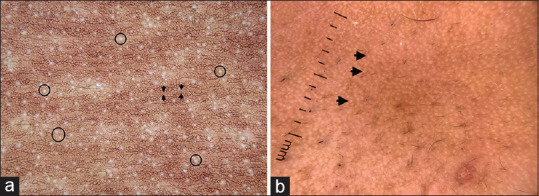
Dermatoscopic images of normal skin captured by dermlite DL4 dermatoscope in polarized mode, 10× (a) Glabrous non-facial skin showing brownish thin lines arranged in a reticular pattern. The intervening white dots represent eccrine duct openings (circles). Parallel brown lines (arrow) represent skin folds. (b) Facial skin showing pseudo-reticular pattern, due to interruption of the pigment network by appendageal openings (arrows)
Dermatoscopy of hypopigmentary diseases
Vitiligo
Vitiligo is the most common acquired depigmentary disease with significant psychological morbidity.[3] Vitiligo of ethnic skin differs considerably from that in the lighter phototypes because of the contrast provided by darker skin tone and the associated stigma. Early vitiligo, especially in children is difficult to differentiate from pityriasis alba, indeterminate leprosy, and nevus depigmentosus (ND). Dermatoscopy is a non-invasive imaging modality, which aids in establishing diagnosis in such situation. The salient dermatoscopic features of vitiligo include diffuse white glow, distorted pigment network (absent, broken, or reverse pigment network; residual pigmentation), perifollicular pigmentary changes (perifollicular depigmentation, perifollicular re-pigmentation), leukotrichia, micro-Koebnerisation, starburst appearance, and tapioca sago appearance.[4,5]
Diffuse white glow
Diffuse white glow is the hallmark dermatoscopic feature of vitiligo wherein the lesion resembles glow from a full moon [Figure 2a-d]. This is due to the complete reflection of the incident light from the upper dermal collagen fibers, which otherwise would have been absorbed by the melanin in the basal layer.[6,7] Diffuse white glow can also be seen in idiopathic guttate hypomelanosis (IGH) and piebaldism.
Figure 2.
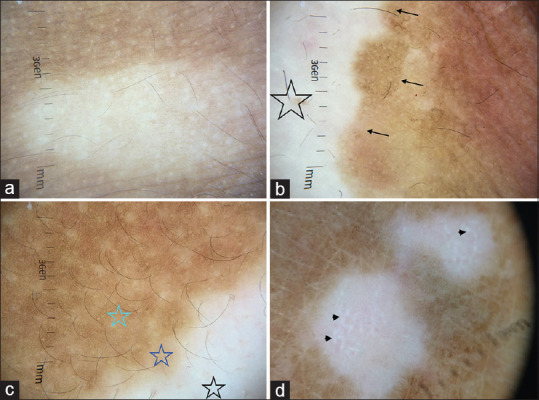
Dermatoscopy images of vitiligo captured by dermlite DL4 dermatoscope in polarized mode, 10× (a) Diffuse white glow due to complete loss of pigment network at places (b) Stable vitiligo patch showing complete loss of pigment network (star) and well defined hyperpigmented borders (arrows) (c) Progressive vitiligo showing areas of broken pigment network at the edge of the lesion (purple star). This can be contrasted with areas of absent pigment network (black star) and normal reticular pigment network (green star), the three different zones represent the dermatoscopic trichrome sign (d) Reverse pigmentary network (photo courtesy Dr Manoj Nayak, Department of Dermatology AIIMS Rishikesh)
Distorted pigment network
Due to the loss of melanocytes from the basal layer, the normal reticular pigmentary network seen in glabrous non-facial skin is absent in vitiligo [Figure 2b]. The margins of evolving vitiligo show areas of broken pigmentary network with ill-defined margins [Figure 2c]. Reverse pigmentary network is a peculiar phenomenon wherein reticular white lines, with central relatively more pigmented holes are seen [Figure 2d]. Reverse pigmentary network has also been reported in malignant melanoma.
Perifollicular pigmentary changes
Perifollicular pigmentary changes are common in vitiligo. It is pertinent to understand that the use of terms perifollicular depigmentation or perifollicular re-pigmentation should take into account the background pigmentation. Perifollicular depigmentation refers to loss of perifollicular pigment when interfollicular pigment network is preserved [Figure 3a]. It is one of the markers of disease activity.[8] Perifollicular re-pigmentation refers to the re-occurrence of pigmentary network around the hair follicle in a lesion, which previously had a complete loss of pigment network. It is one of the signs of re-pigmenting vitiligo [Figure 3b]. Perifollicular repigmentation should be differentiated from perifollicular residual pigmentation, which is retention of pigment globules around the follicle with loss of pigment network in the interfollicular epidermis. Perifollicular repigmentation, which occurs spontaneously or upon treatment, also looks similar to residual pigmentation.[9] Both are indistinguishable on dermoscopy and can be assessed only by longitudinal evaluation. In this situation, history, clinical morphology and treatment history should be taken into consideration. Perifollicular pigmentary changes can also be associated with leukotrichia.[4,9]
Figure 3.
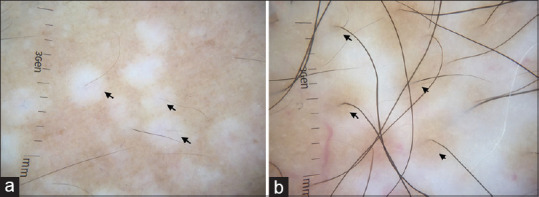
Perifollicular pigmentary changes in vitiligo (a) Perifollicular depigmentation (arrows) in a case of progressive vitiligo. The interfollicular pigment network is preserved. (b) Repigmenting vitiligo (arrows) showing perifollicular re-pigmentation. Dermlite DL4 dermatoscope in polarized mode, 10×
Dermatoscopic markers of disease activity in vitiligo
Certain dermatoscopic features are linked with disease activity and suggest progressive vitiligo.[4,5] These include Ill-defined margins with reduced pigment network (trichrome lesion), micro-Koebnerisation, peripheral white globules (Polka-dot, tapioca sago) and star-burst/feathery pattern.[4] Micro-Koebnerisation (also known as comet-tail appearance) is among the well studied dermatoscopic features of vitiligo and appears as linearly arranged areas of depigmentation. They are generally not appreciated through naked eye examination [Figure 4a]. Multiple white globules surrounding a primary depigmented macule is another marker of active vitiligo. It has been variously described metaphorically as polka-dot/tapioca sago appearance [Figure 4a]. Starburst pattern is a metaphorical description for depigmented lines extending radially from a central primary lesion [Figure 4b].
Figure 4.
Dermatoscopic markers of disease activity (a) multiple hypopigmented dots surrounding a primary lesion (polka-dot/tapioca-sago appearance, circle) and micro-Koebnerization (line), Dermlite DL4 dermatoscope in polarized mode, 10× (b) Star burst pattern, multiple radiating white lines from a primary lesion, Dermlite photo pro dermatoscope in polarized mode, 10×
Dermatoscopic markers of stable disease in vitiligo
Dermatoscopic features of stable vitiligo include complete absence of pigmentary network with or without island of pigment retention, sharp borders, perilesional hyperpigmentation [Figure 2b] and leukotrichia. Repigmenting vitiligo shows reappearance of perifollicular pigment network [Figure 3b].[9]
Evidence for dermatoscopic markers of disease activity in vitiligo
The evidence for dermatoscopic markers of disease activity in vitiligo is limited to case series and cross-sectional observational studies. No longitudinal studies till date have assessed the dermatoscopic markers of disease activity. Chuh and Zawar reported a pattern of depigmentation in which residual reservoirs of perifollicular pigment signified focally active or repigmenting vitiligo.[10] Meng et al.[5] studied 176 patients with hypopigmented disorders out of which 97 were diagnosed with vitiligo. Residual perifollicular pigmentation was observed in 91.94% (57/62) of patients with progressing vitiligo and 62.86% (22/35) of those with stable vitiligo. However, residual perifollicular pigmentation was absent in the 79 non-vitiligo depigmented cases.
In a study by Jha et al. follicular changes constituted the most consistent dermatoscopic features in vitiligo lesions.[5] While perifollicular pigmentation was predominantly observed in lesions of active/unstable vitiligo, perifollicular depigmentation was typically encountered in stable vitiligo. In this same study, there was altered pigment network (which included absent pigment network, reduced pigment network, and reversed pigment network) in 32 cases (91.4%) of unstable vitiligo. Apart from these certain specific morphological appearances on dermatoscopy have been reported to be suggestive of unstable vitiligo, such as the starburst appearance, comet tail, trichrome, and salt and pepper patterns.
The readers are advised caution in attributing perifollicular changes to disease activity since these changes are dynamic and the above-mentioned studies have assessed the changes in a cross-sectional study design. Longitudinal studies are required for a more confirmative answer.
Idiopathic guttate hypomelanosis
IGH is an acquired depigmentary disease predominantly affecting the photo-damaged skin of the elderly. When noticed, it can be confused with vitiligo and can cause considerable distress to the patients. Similar to vitiligo, dermatoscopy of IGH shows loss of pigment network and diffuse white glow. However, the dermatoscopic appearance of margins are diagnostic with four characteristic morphological variants described; amoeboid, petaloid, feathery and nebuloid [Figure 5a-d].[11,12] The amoeboid pattern shows pseudopod like extensions at the periphery, petaloid shows leaf like extensions, feather like striations are seen in feathery, whereas the margins are indistinct in nebuloid form.
Figure 5.
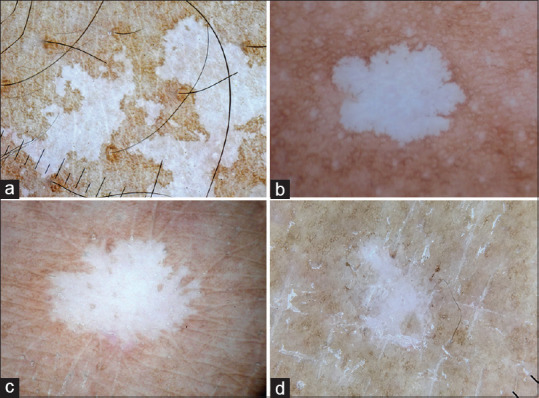
Dermatoscopy images of idiopathic guttate hypomelanosis showing well-defined margins and complete loss of pigment network (a) Amoeboid pattern with pseudopod like extensions at the margins (b) petaloid pattern with leaf-like extension at the margins (c) Feathery pattern with feather-like striations (d) Nebuloid pattern with indistinct margins. Dermlite photo pro dermatoscope in polarized mode, 10x
Lichen sclerosus et atrophicus
Extra genital lichen sclerosus et atrophicus (LSA) can be a diagnostic challenge wherein, it can be misdiagnosed as guttate vitiligo, IGH, and morphea. The dermatoscopic features are however typical in LSA and often helps in alleviating patient anxiety by differentiating it from vitiligo. The early inflammatory phase shows background erythema, follicular keratinous plugs, and linear telangiectatic vessels [Figure 6a].[13,14] The histological correlates of these findings are dermal inflammation, follicular plugging, and dilated upper dermal vessels. The late sclerotic phase shows loss of pigment network, linear fibrotic strands representing upper dermal fibrosis with or without follicular plugging [Figure 6b].[13,14] LSA of ethnic skin in addition shows peppering of brown and blue-gray pigment dots and globules [Figure 6a]. This is due to basal cell vacuolization and subsequent melanin incontinence.
Figure 6.
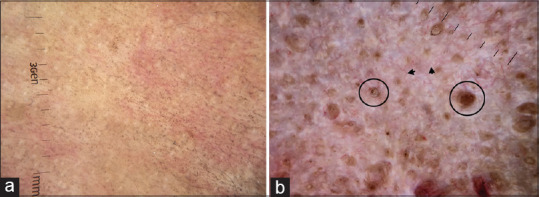
Dermatoscopic images of lichen sclerosus et atrophicus (a) Early inflammatory phase characterized by background erythema, ill-focused vessels, and peppering of pigment dots (b) Late sclerotic stage characterized by structureless linear white strands representing upper dermal fibrotic bands and follicular plugs (circle). Dermlite DL4 dermatoscope in polarized mode, 10×
Nevus depigmentosus
Nevus pigmentosus is a nevoid disorder caused due to defective transfer of melanosomes to epidermal keratinocytes. It is often first noticed in infancy and early childhood and can be confused with vitiligo, causing considerable apprehension. Dermatoscopy of nevus depigmentosus shows whitish area containing faint albeit uniform pigment network and feathery margins [Figure 7a].[15] Leukotrichia is not seen. Unlike vitiligo, diffuse white glow is absent since the pigmentary network is disturbed, but not completely lost and melanocytes absorb some of the incident light.
Figure 7.
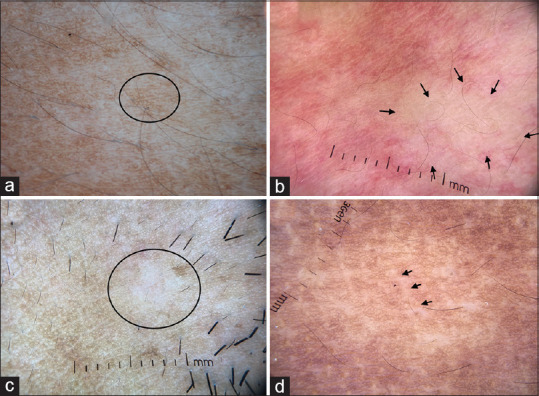
(a) Dermatoscopic image of nevus depigmentosus showing feathery margins and broken pigmentary network (circle). The diffuse white glow of vitiligo is characteristically missing. (b) Dermatoscopy of nevus anemicus showing focal areas of reduced/absent vasculature (arrows). (c) Dermatoscopic image of progressive macular hypomelanosis showing focal loss of pigment network (circle) and prominent skin markings. (d) Dermatoscopic images of borderline lepromatous leprosy showing focal areas of broken pigmentary network and white chrysalis like structures (arrow). Lesional paucity of appendageal openings and hair follicles can be appreciated. Dermlite DL4 dermatoscope in polarized mode, 10×
Nevus anemicus
Nevus anemicus is a rare congenital vascular malformation caused due to localized sensitivity of cutaneous vasculature to catecholamines. It presents as hypopigmented patches and can be confused with segmental nevus depigmentosus and segmental vitiligo. The apparent hypopigmentation is not due to pigment loss, but paleness caused due to localized vasoconstriction. The dermatoscopy shows a preserved pigment network but an obvious paucity of vessels in the lesional skin [Figure 7b]. At times there might be compensatory flare of cutaneous vasculature in the surrounding skin.
Progressive macular hypomelanosis
Progressive macular hypomelanosis (PMH) is an acquired hypopigmentary disease occurring over the seborrheic areas in middle-aged women and men. The clinical differentials of PMH include pityriasis alba, early vitiligo, hypopigmented mycosis fungoides, and borderline lepromatous leprosy. Dermatoscopy of PMH shows focal white areas with broken pigmentary network, widened skin lines and minimal, focal white scaling [Figure 7c].[1] The diffuse white glow of vitiligo is absent; appendages are preserved and no abnormal vascular structures are seen.
Leprosy
The lepromatous spectrum of leprosy often shows pigmentary disturbances, which can be striking in ethnic skin. Indeterminate leprosy over the face can be misdiagnosed as pityriasis alba and early vitiligo and borderline lepromatous leprosy can be confused with PMH and mycosis fungoides. Dermatoscopy of well-established leprosy shows yellowish-orange areas, white chrysalis structures with broken pigmentary network, and loss/diminution of hair follicle and appendages [Figure 7d].[16]
Pityriasis alba
Pityriasis alba is a type of sub-acute eczema affecting the head and neck area of children. Its clinical differentials include indeterminate leprosy and early vitiligo. Dermoscopy of pityriasis alba shows focal white areas with broken pigmentary network and diffuse fine scaling [Figure 8a].[17]
Figure 8.
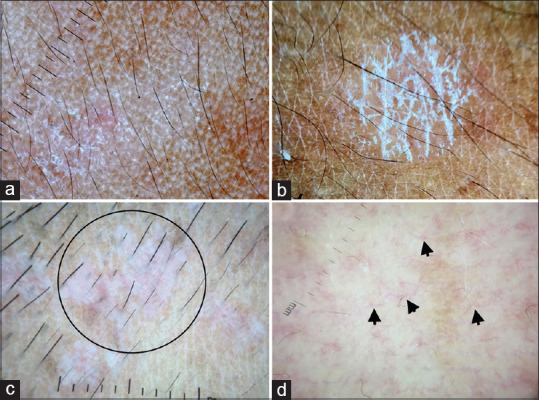
(a) Pityriasis alba showing focal areas of broken pigment network and diffuse fine scaling, Dermlite photo pro dermatoscope in polarized mode, 10×. (b) Pityriasis versicolor showing accentuated double-edged scales at skin creases, Dermlite photo pro dermatoscope in polarized mode, 10×. (c) Epidermodysplasia verruciformis showing broken pigmentary network, background erythema and ill-focused dotted vessels (circle), Dermlite DL4 dermatoscope in polarized mode, 10× (d) Hypopigmented mycosis fungoides showing areas of broken pigmentary network and spermatozoa like vessels (arrow), Dermlite DL4 dermatoscope in polarized mode, 10×
Pityriasis versicolor
The dermatoscopic features of pityriasis versicolor are characteristic and shows faint pigment network with white scales. The scales are prominent in physiological skin lines and typically appear as double-edged when lesion is stretched [Figure 8b]. Scales can also be diffuse and perifollicular.[18]
Epidermodysplasis verruciformis
Epidermodysplasia verruciformis (EDV) is a genodermatosis predisposing to cutaneous infection by certain human papilloma virus serotypes. Pityriasis versicolor like macules is one of the cutaneous features of this disease. Dermatoscopy of pityriasis versicolor like macules in EDV shows focal areas of broken pigmentary network, diffuse scaling, background erythema, and ill-focused dotted vessels.[19] There is no accentuation of scales in skin creases [Figure 8c].
Hypopigmented mycosis fungoides
Hypopigmented mycosis fungoides commonly affects adolescents and young adults of Asian descent, with head and neck being the most common area of involvement. Dermatoscopy of hypopigmented mycosis fungoides shows broken pigmentary network and spermatozoa like blood vessels [Figure 8d].[20]
Post kala azar dermal leishmaniasis
Post kala azar dermal leishmaniasis (PKDL) presents as hypopigmented macules, papules and plaques. Face, neck, proximal extremities, and trunk are commonly affected and the lesions spread in a descending fashion. The muzzle area of the face is typically involved. Dermatoscopy features vary depending on the type of lesion being evaluated. Patch lesions show focal areas of loss of pigment network and yellowish structureless area representing dermal granulomas [Figure 9a]. Whereas plaque lesions show yellowish orange areas and unfocussed vessels akin to other granulomatous dermatoses [Figure 9b and 9c].
Figure 9.
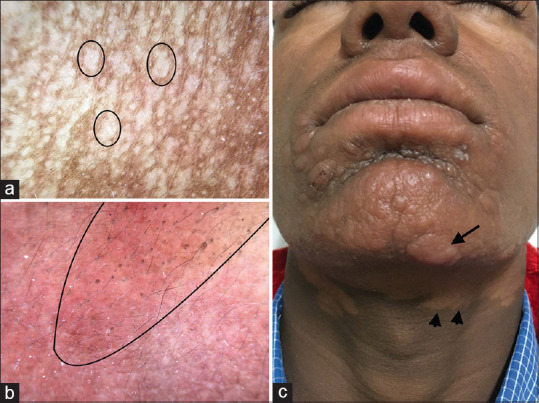
(a) Dermatoscopic evaluation of patch lesions of post kala-azar dermal leishmaniasis (PKDL) showing loss of pigmentary network and yellowish structureless area representing dermal granulomas (circle). Dermlite DL4 dermatoscope in polarized mode, 10× (b) Dermatoscopy of plaque lesions show yellowish-orange structureless areas and unfocussed vessels representing dermal granuloma, Dermlite DL4 dermatoscope in polarized mode, 10× (c) PKDL involving the muzzle area of the face and neck showing reddish-brown plaques (arrow) and hypopigmented patches (arrowhead)
Dermatoscopy of Hyperpigmentary Diseases
Melasma
Melasma is a common hyperpigmentary disease seen in clinical practice. The diagnosis is often apparent on clinical examination; however, it can sometimes be mistaken for maturity-onset pigmentation and lichen planus pigmentosus. The most common dermatoscopy pattern of melasma is exaggerated pseudo network [Figure 10a]. Other features include brownish dots and globules [Figure 10b], annular and arcuate structures, and telangiectasia.[2] Typically in melasma, the pigmentation spares the follicular, and appendageal opening, which are obliterated in exogenous ochronosis
Figure 10.
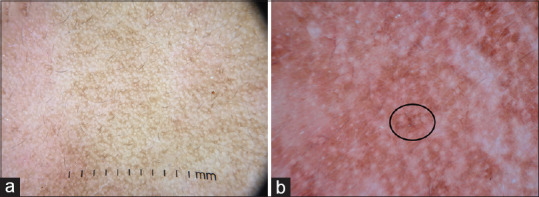
(a) Dermatoscopy of melasma showing exaggerated pseudoreticular network in comparison to the normal pseudoreticular network in the left half of the image (b) Brownish dots and globules (circle). The appendageal openings are spared. Dermlite DL4 dermatoscope in polarized mode, 10×
Exogenous ochronosis
Exogenous ochronosis is an iatrogenic dermatoses caused due to unsupervised topical use of hydroquinone. The histopathology of exogenous ochronosis classically shows banana bodies, which are exogenous yellow to brown material in the papillary and middle dermis, pigment incontinence, and collagen degeneration. The dermatoscopic features include bluish-gray pigment globules in arciform or annular patterns producing a curvilinear appearance. The appendageal openings are obliterated [Figure 11a and 11b].[21] Dermatoscopic features of background melasma can be apparent.
Figure 11.
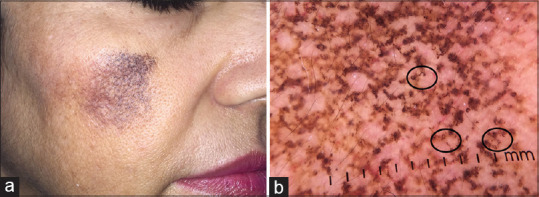
(a) A case of exogenous ochronosis (b) Dermatoscopic image showing arcuate and semicircular structures (circle). The appendageal openings are obliterated. Dermlite DL4 dermatoscope in polarized mode, 10×
Acquired dermal macular hyperpigmentation
It is a hypernym, which includes diseases of dermal pigmentation lichen planus pigmentosus, pigmented cosmetic dermatitis, and erythema dyschromicum perstans.[22]
Dermatoscopy of ADMH shows pigment dots, globules, and blotches.[22,23] Depending on the disease severity, the pigment structures show various arrangements. They can be arranged in a dotted pattern, arcuate/Chinese letter pattern, hem-like pattern, reticular pattern, and diffuse [Figure 12a-d]. Dotted pattern [Figure 12a] is commonly seen and better appreciated in lesions over neck and trunk. Arcuate/Chinese letter pattern is more apparent in facial lesions [Figure 12b] and hem-like pattern are seen in folded skin; with time, untreated patients progress to complete reticular pattern [Figure 12c]. In diffuse pattern, there is diffuse involvement with pigment dots and globules, sparing only the appendageal openings [Figure 12d]. Akin to melasma, the follicular and appendageal openings and skin creases are spared in ADMH. The other dermatoscopic features include exaggerated pseudo reticular pattern and brown dots [Figure 13a and 13b].
Figure 12.
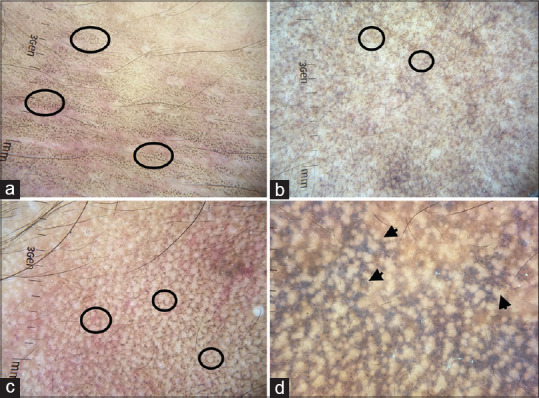
Dermatoscopic images of acquired dermal macular hyperpigmentation showing pigment dots and globules (a) Dotted pattern (circle), discrete black pigment dots sparing the skin creases over the abdomen (b) Arcuate pattern over face showing brownish broken lines (circle) (c) Complete reticular pattern showing brownish reticular rings (circle) (d) Diffuse involvement with sparing of the appendageal openings (arrow). Dermlite DL4 dermatoscope in polarized mode, 10×
Figure 13.
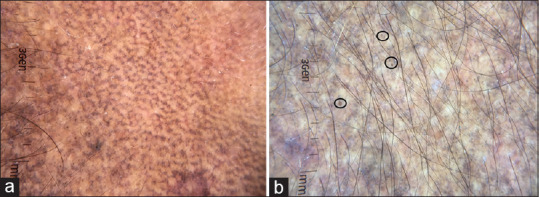
Dermatoscopic images of acquired dermal macular hyperpigmentation showing (a) Exaggerated pseudo network (b) Brown dots. Dermlite DL4 dermatoscope in polarized mode, 10×
Nevus of Ota
Nevus of Ota, nevus of Ito, and Hori's nevus are dermal melanocytosis characterized by bluish-grey pigmentation. The dermatoscopic feature of nevus of Ota has been described in one case series, which includes a bluish structureless area and scattered brown-grey dots [Figure 14a and b].[24]
Figure 14.
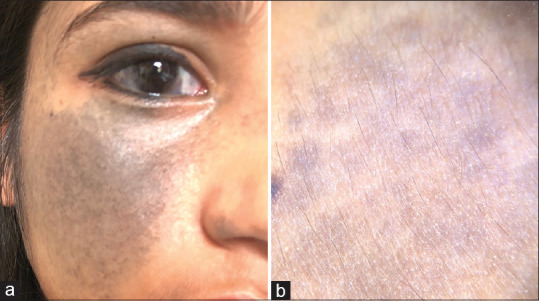
(a) Nevus of Ota (b) Dermatoscopic image showing bluish-grey structureless area (Photo courtesy, Dr Payal Chauhan Assistant Professor, Himalayan Institute of Medical Sciences)
Maturational hyperpigmentation
Maturational hyperpigmentation is an evolving entity predominantly affecting south Asians and Africans. It is said to be a cutaneous marker of metabolic syndrome. Dermatoscopy of maturational hyperpigmentation shows exaggerated pseudo network and prominent perifollicular accentuation of pigmentation appearing as a brownish ring [Figure 15a].[25]
Figure 15.
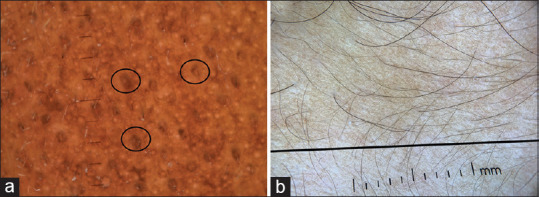
(a) Maturational pigmentation of dermatoscopy showing exaggerated pseudo network and prominent perifollicular accentuation of pigmentation appearing as brownish ring (circles) (Photo courtesy, Dr. Shekhar Neema, MD, FEBDV Armed Forces Medical College, Pune) (b) Exaggerated pseudo network in a case of pigmentary demarcation lines over upperlimb. The line demarcates the pigmentary demarcation line. Dermlite photo pro dermatoscope in polarized mode, 10×
Pigmentary demarcation lines
Pigmentary demarcation lines (PDL) are sites of transition from hyperpigmentation to hypopigmented or normal skin, which is more prominent in darker skin phototypes. Eight groups of PDL have been described. Dermatoscopy of PDL shows exaggerated pseudo network [Figure 15b].[26]
Primary cutaneous amyloidosis
Macular and biphasic amyloidosis are common causes of pigmentation over upper back and extremities in ethnic skin. Dermatoscopy of macular amyloidosis shows a central hub surrounded by brownish pigmentation.[27] The brownish pigmentation can be arranged radially or in a leaf-like pattern giving a “hub and spoke” appearance [Figure 16a].[27] In lichen/biphasic amyloidosis the central hub is replaced by a white scar-like area.
Figure 16.
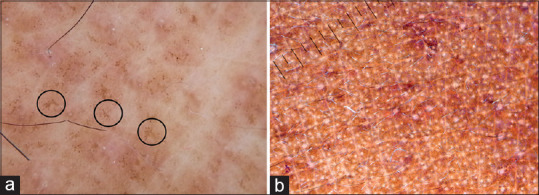
(a) Dermatoscopy of macular amyloidosis showing a central hub and radially arranged spokes (hub and spoke pattern, circles), Dermlite DL4 dermatoscope in polarized mode, 10× (b) Frictional melanosis showing dispersed pigment dots, Dermlite photo pro dermatoscope in polarized mode, 10×
Frictional melanosis
Frictional melanosis is not uncommon among Indians. The use of bathing stone and loofah has been incriminated in its causation. When it occurs over the upper back, it can be confused with macular amyloidosis. However, dermatoscopy can differentiate both the conditions. Frictional melanosis shows fine brownish stippling in a rippled reticular pattern [Figure 16b].[1,27] The central hub seen in macular amyloidosis is typically lacking. On the facial lesions, dermatoscopy shows a patchy arrangement of bluish-gray pigment globules with dilated follicular openings.[28]
Terra firma-forme dermatosis
Terra firma-forme dermatosis is caused due to increased retention and delayed exfoliation of keratinocytes. Large brown polygonal scales arranged in a cobblestone pattern are seen on dermatoscopy [Figure 17a].[29]
Figure 17.
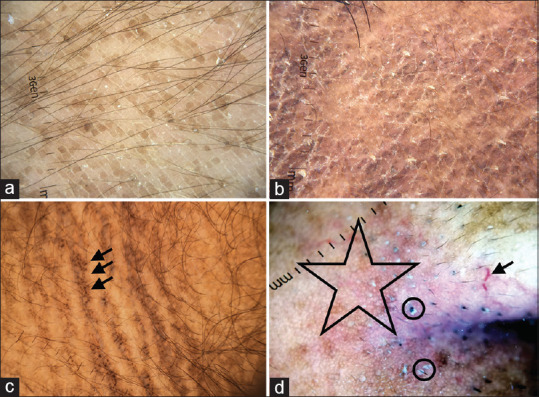
(a) Terra firma-forme dermatosis showing large brown polygonal scales arranged in cobblestone pattern on dermatoscopy, Dermlite DL4 dermatoscope in polarized mode, 10×. (b) Confluent and reticulate papillomatosis showing brownish homogenous polygonal scales separated by whitish/pale striae arranged in a cobblestone pattern. White fine scales over the surface can be appreciated, Dermlite DL4 dermatoscope in polarized mode, 10×. (c) Acanthosis nigricans shows multiple cristae and sulci. Pigment dots over cristae are characteristic of acanthosis nigricans (arrow), Dermlite photo pro dermatoscope in polarized mode, 10×. (d) Dermoscopy of seborrheic melanosis over nasal ala showing prominent pseudo network (star), ill-focussed vessels (arrow), prominent follicle openings and whitish-yellow excrescences of sebum coating the vellus hair shafts (circle), Dermlite photo pro dermatoscope in polarized mode, 10×
Confluent and reticulated papillomatosis
Confluent and reticulated papillomatosis (CARP) is a Malassezia associated hyperproliferative disorder affecting the seborrheic distribution of young adults. Its dermatoscopic features include brownish homogenous polygonal scales separated by whitish/pale striae arranged in a cobblestone pattern, covered by white fine scales [Figure 17b].[30]
Acanthosis nigricans
Acanthosis nigricans is a cutaneous marker of insulin resistance and metabolic syndrome. When it occurs over the face, it can be misdiagnosed as maturational pigmentation, lichen planus pigmentosus, and melasma. Dermatoscopy of acanthosis nigricans shows multiple cristae and sulci, which are better appreciated in non-polarized mode [Figure 17c]. Pigmented dots and globules are specific clues for AN and are better seen in polarized mode.[31]
Seborrheic melanosis
Seborrheic melanosis or sebomelanosis is an ill-defined entity reported among pigmented races.[32,33] It is considered to be post-inflammatory sequelae of seborrheic dermatosis, unique to pigmented races. The described dermatoscopic features of sebomelanosis include prominent pseudonetwork, brown granular structures, ill-focussed vessels, prominent follicle openings, and whitish-yellow excrescences of sebum coating the vellus hair shafts [Figure 17d].[32]
Fixed drug eruption
The late phase of fixed drug eruption (FDE) can present with isolated pigmentation, which can clinically mimic erythema dyschromicum perstans and post-inflammatory hyperpigmentation. Dermatoscopy in FDE shows pigment dots and globules with various shades ranging from brown to grayish [Figure 18]. This depends on the location of melanin in the dermis due to pigment incontinence.
Figure 18.
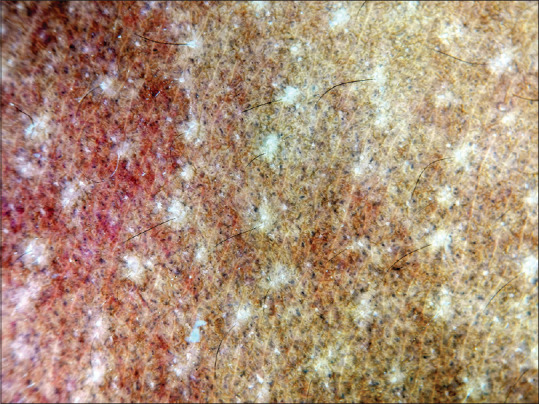
Dermatoscopy of fixed drug eruption showing greyish pigment dots. Dermlite photo pro dermatoscope in polarized mode, 10×
Conclusion
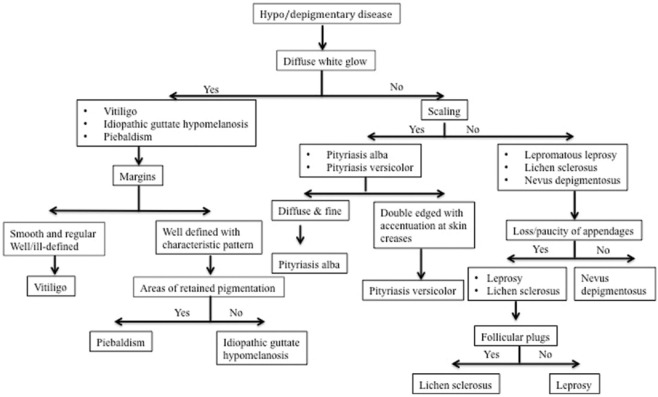
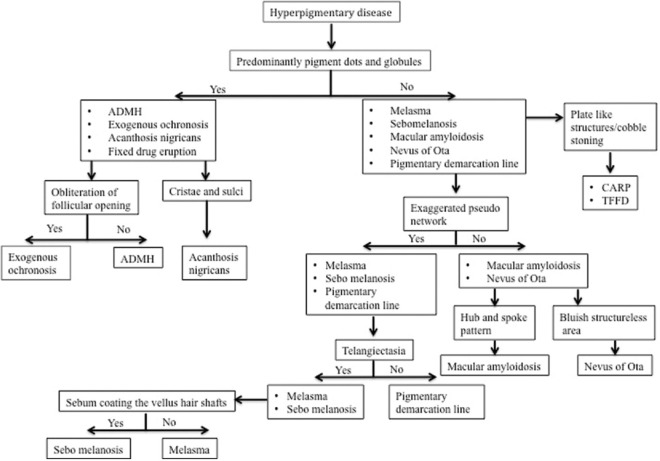
Dermatoscopic knowledge of hypopigmentary and hyperpigmentary dermatosis helps in clinical diagnosis and differentiation from another dermatosis. Dermatoscopic approach to diagnosis of hypopigmentary and hyperpigmentary dermatoses is summarized in the flowcharts Figure 19 and 20 respectively.
Figure 19.
Dermatoscopic approach to hypopigmentary diseases
Figure 20.
Dermatoscopic approach to hyperpigmentary diseases. CARP, Confluent, and reticulated papillomatosis; TFFD, Terra firma-forme dermatosis
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Errichetti E, Stinco G. Dermoscopy in general dermatology: A practical overview. Dermatol Ther (Heidelb) 2016;6:471–507. doi: 10.1007/s13555-016-0141-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chatterjee M, Neema S. Dermoscopy of pigmentary disorders in brown skin. Dermatol Clin. 2018;36:473–85. doi: 10.1016/j.det.2018.05.014. [DOI] [PubMed] [Google Scholar]
- 3.Parsad D, Dogra S, Kanwar AJ. Quality of life in patients with vitiligo. Health Qual Life Outcomes. 2003;1:58. doi: 10.1186/1477-7525-1-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jha AK, Sonthalia S, Lallas A. Dermoscopy as an evolving tool to assess vitiligo activity. J Am Acad Dermatol. 2018;78:1017–9. doi: 10.1016/j.jaad.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 5.Kumar Jha A, Sonthalia S, Lallas A, Chaudhary RKP. Dermoscopy in vitiligo: Diagnosis and beyond. Int J Dermatol. 2018;57:50–4. doi: 10.1111/ijd.13795. [DOI] [PubMed] [Google Scholar]
- 6.Lister T, Wright PA, Chappell PH. Optical properties of human skin. J Biomed Opt. 2012;17:0909011. doi: 10.1117/1.JBO.17.9.090901. [DOI] [PubMed] [Google Scholar]
- 7.Masuda Y, Ogura Y, Inagaki Y, Yasui T, Aizu Y. Analysis of the influence of collagen fibres in the dermis on skin optical reflectance by Monte Carlo simulation in a nine-layered skin model. Ski Res Technol. 2018;24:248–55. doi: 10.1111/srt.12421. [DOI] [PubMed] [Google Scholar]
- 8.Sonthalia S, Arora R, Sarkar R. Novel dermoscopic findings of perifollicular depigmentation and evolving leukotrichia in areas of clinically unaltered pigmentation: An early predictive sign of impending vitiligo! Pigment Int. 2014;1:28. [Google Scholar]
- 9.Nirmal B, Antonisamy B, Peter CVD, George L, George AA, Dinesh GM. Cross-sectional study of dermatoscopic findings in relation to activity in vitiligo: BPLeFoSK criteria for stability. J Cutan Aesthet Surg. 12:36–41. doi: 10.4103/JCAS.JCAS_75_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chuh AAT, Zawar V. Demonstration of residual perifollicular pigmentation in localized vitiligo--a reverse and novel application of digital epiluminescence dermoscopy. Comput Med Imaging Graph. 2004;28:213–7. doi: 10.1016/j.compmedimag.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Errichetti E, Stinco G. Dermoscopy of idiopathic guttate hypomelanosis. J Dermatol. 2015;42:1118–9. doi: 10.1111/1346-8138.13035. [DOI] [PubMed] [Google Scholar]
- 12.Ankad B, Beergouder S. Dermoscopic evaluation of idiopathic guttate hypomelanosis: A preliminary observation. Indian Dermatol Online J. 2015;6:164–7. doi: 10.4103/2229-5178.156383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larre Borges A, Tiodorovic-Zivkovic D, Lallas A, Moscarella E, Gurgitano S, Capurro M, et al. Clinical, dermoscopic and histopathologic features of genital and extragenital lichen sclerosus. J Eur Acad Dermatology Venereol. 2013;27:1433–9. doi: 10.1111/j.1468-3083.2012.04595.x. [DOI] [PubMed] [Google Scholar]
- 14.Errichetti E, Lallas A, Apalla Z, Di Stefani A, Stinco G. Dermoscopy of morphea and cutaneous lichen sclerosus: Clinicopathological correlation study and comparative analysis. Dermatology. 2017;233:462–70. doi: 10.1159/000484947. [DOI] [PubMed] [Google Scholar]
- 15.Oiso N, Kawada A. The diagnostic usefulness of dermoscopy for nevus depigmentosus. Eur J Dermatology. 2011;21:639–40. doi: 10.1684/ejd.2011.1414. [DOI] [PubMed] [Google Scholar]
- 16.Vinay K, Kamat D, Chatterjee D, Narang T, Dogra S. Dermatoscopy in leprosy and its correlation with clinical spectrum and histopathology: A prospective observational study. J Eur Acad Dermatology Venereol. 2019;33:1947–51. doi: 10.1111/jdv.15635. [DOI] [PubMed] [Google Scholar]
- 17.Al-Refu K. Dermoscopy is a new diagnostic tool in diagnosis of common hypopigmented macular disease: A descriptive study. Dermatology Reports. 2018;11:7916. doi: 10.4081/dr.2018.7916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaur I, Jakhar D, Singal A. Dermoscopy in the evaluation of pityriasis versicolor: A cross sectional study. Indian Dermatol Online J. 2019;10:682–5. doi: 10.4103/idoj.IDOJ_502_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Afra TP, Vinay K, Razmi TM, Khader A, Hafi NAB. Novel dermoscopic features of pityriasis versicolor-like macules in epidermodysplasia verruciformis. Pediatr Dermatol. 2020;37:230–2. doi: 10.1111/pde.14031. [DOI] [PubMed] [Google Scholar]
- 20.Lallas A, Apalla Z, Lefaki I, Tzellos T, Karatolias A, Sotiriou E, et al. Dermoscopy of early stage mycosis fungoides. J Eur Acad Dermatology Venereol. 2013;27:617–21. doi: 10.1111/j.1468-3083.2012.04499.x. [DOI] [PubMed] [Google Scholar]
- 21.Gil I, Segura S, Martínez-Escala E, Lloreta J, Puig S, Vélez M, et al. Dermoscopic and reflectance confocal microscopic features of exogenous ochronosis. Arch Dermatol. 2010;146:1021–5. doi: 10.1001/archdermatol.2010.205. [DOI] [PubMed] [Google Scholar]
- 22.Vinay K, Bishnoi A, Parsad D, Saikia UN, Sendhil Kumaran M. Dermatoscopic evaluation and histopathological correlation of acquired dermal macular hyperpigmentation. Int J Dermatol. 2017;56:1395–9. doi: 10.1111/ijd.13782. [DOI] [PubMed] [Google Scholar]
- 23.Sharma V, Gupta V, Pahadiya P, Vedi K, Arava S, Ramam M. Dermoscopy and patch testing in patients with lichen planus pigmentosus on face: A cross-sectional observational study in fifty Indian patients. Indian J Dermatology, Venereol Leprol. 2017;83:656–62. doi: 10.4103/ijdvl.IJDVL_469_16. [DOI] [PubMed] [Google Scholar]
- 24.Elmas ÖF, Kilitçi A. Dermoscopic findings of nevus of Ota. Balkan Med J. 2020;37:116–8. doi: 10.4274/balkanmedj.galenos.2019.2019.11.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sonthalia S, Agrawal M, Sharma P, Pandey A. Maturational hyperpigmentation: Cutaneous marker of metabolic syndrome. Dermatol Pract Concept. 2020:e2020046. doi: 10.5826/dpc.1002a46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Russo F, Flori ML, Taddeucci P, Rubegni P, Cinotti E. Pigmentary demarcation lines of Voigt-Futcher: Dermoscopic and reflectance confocal microscopy features. Ski Res Technol. 2020;26:440–2. doi: 10.1111/srt.12795. [DOI] [PubMed] [Google Scholar]
- 27.Chuang YY, Lee DD, Lin CS, Chang YJ, Tanaka M, Chang YT, et al. Characteristic dermoscopic features of primary cutaneous amyloidosis: A study of 35 cases. Br J Dermatol. 2012;167:548–54. doi: 10.1111/j.1365-2133.2012.11066.x. [DOI] [PubMed] [Google Scholar]
- 28.Mutalik SD, Pethe SV, Nikam BP RY. Facial frictional melanosis in Indian patients: Defining the entity. Clin Dermatol Rev. 2019;3:78–83. [Google Scholar]
- 29.Errichetti E, Stinco G. Dermoscopy in terra firma-forme dermatosis and dermatosis neglecta. Int J Dermatol. 2017;56:1481–3. doi: 10.1111/ijd.13686. [DOI] [PubMed] [Google Scholar]
- 30.Errichetti E, Maione V, Stinco G. Dermatoscopy of confluent and reticulated papillomatosis (Gougerot-Carteaud syndrome) J Dtsch Dermatol Ges. 2017;15:836–8. doi: 10.1111/ddg.13259. [DOI] [PubMed] [Google Scholar]
- 31.Nirmal B. Dermatoscopy image characteristics and differences among commonly used standard dermatoscopes. Indian Dermatol Online J. 2017;8:233–4. doi: 10.4103/idoj.IDOJ_319_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verma S, Vasani R, Chandrashekar L, Thomas M. Seborrheic melanosis: An entity worthy of mention in dermatological literature. Indian J Dermatology, Venereol Leprol. 2017;83:285–9. doi: 10.4103/0378-6323.203248. [DOI] [PubMed] [Google Scholar]
- 33.Arshdeep , Sonthalia S, Kaliyadan F, Errichetti E, Jha A, Lallas A. Seborrheic melanosis and dermoscopy: Lumping better than splitting. Indian J Dermatol Venereol Leprol. 2018;84:585–7. doi: 10.4103/ijdvl.IJDVL_175_18. [DOI] [PubMed] [Google Scholar]