Abstract
Brown adipose tissue (BAT) and stimulating adaptive thermogenesis have been implicated as anti-obese and anti-diabetic tissues due to their ability to dissipate energy as heat by the expression of UCP1. We have recently demonstrated that TRB3 impairs differentiation of brown preadipocytes via inhibiting insulin signaling. However, the roles of the protein in BAT function and thermogenesis in vivo have not yet been established. For this study we tested the hypothesis that TRB3 mediates obesity- and diabetes-induced impairments in BAT differentiation and function, and that inhibition of TRB3 improves BAT function. TRB3 expression was increased in BAT from high-fat fed mice and ob/ob mice, which was associated with decreased UCP1 expression. Incubation of brown adipocytes with palmitate increased TRB3 expression and decreased UCP1. Knockout of TRB3 in mice displayed higher UCP1 expression in BAT and cold resistance. Incubation of brown adipocytes with ER stressors increased TRB3 but decreased UCP1 and ER stress markers were elevated in BAT from high-fat fed mice and ob/ob mice. Finally, high-fat feeding in TRB3KO mice were protected from obesity-induced glucose intolerance and displayed cold resistance and higher expression of BAT-specific markers. These data demonstrate that high-fat feeding and obesity increase TRB3 in BAT, resulting in impaired tissue function.
Keywords: TRB3, Brown Adipose Tissue, Obesity, Type 2 Diabetes
INTRODUCTION
Brown adipose tissue plays an important role in both basal and inducible energy expenditure in the form of thermogenesis through the expression of the tissue-specific uncoupling protein (UCP1)[1] and adaptive thermogenesis have been thought to prevent or treat obesity and diabetes [2], the idea of which has been well established in rodent studies. Activation of UCP1 decreases in ATP production resulting in regulation of fuel metabolism and fatty acid oxidation is necessary for UCP1 function and BAT [3], suggesting the BAT as an anti-obese and anti-diabetic tissue.
TRB3 is a member of the Tribbles family that lacks catalytically active kinase domains [4]. TRB3 is highly conserved through the evolutionary process and has numerous functions, including cell cycle regulation, apoptosis, differentiation, and metabolism [5–13]. TRB3 interacts with its target proteins, regulating their activities or functions [5,7,14,15]. In liver, TRB3 inhibits Akt through direct binding and therefore results in impaired insulin signaling, decreased glycogen content, and increased hepatic glucose output [9,10]. Our laboratory and other groups have recently shown the expression of TRB3 in skeletal muscle [7,16–20]. Our previous studies found that TRB3 in skeletal muscle regulates metabolism and tissue mass via interaction with Akt [12,21,22]. The role of TRB3 in BAT has less been understood and remained to be elucidated. Our recent study demonstrated that TRB3 inhibits BAT differentiation and function by suppressing Akt and insulin signaling [23]. However, the roles of the protein in BAT function and thermogenesis in vivo have not yet been established.
In the current study we tested the hypothesis that that TRB3 mediates obesity- and diabetes-induced impairments in BAT differentiation and function, and that inhibition of TRB3 improves BAT function. We found that TRB3 expression is increased in BAT from high-fat fed mice and ob/ob mice. TRB3 knockout mice display higher UCP1 expression in BAT, cold resistance, and increased BAT differentiation markers but decreased lipid synthesis markers. ER stress, which was increased in BAT from ob/ob and HFD fed mice, induces TRB3 and decreases UCP1. TRB3KO mice are protected from obesity-induced impairments in BAT differentiation and function. These data demonstrate that TRB3 mediates obesity- and diabetes-induced impairments in BAT differentiation and metabolism.
MATERIALS AND METHODS
Animals
C57BL/6 wild type mice from Charles River, or whole body TRB3 knockout mice [24] were maintained in a pathogen-free animal facility under standard 12-hour light/12-hour dark cycle, and unless indicated, were maintained on a chow diet (16% of calorie from fat; Bio-Serv F4031). For the high-fat diet study, mice were fed a high-fat diet (60% of calorie from fat; Bio-Serv F3282) beginning at the age of 6 weeks for 16 weeks. Mice were studied at 8–12 weeks of age, as specified in figure legends. All procedures were performed in accordance with NIH guidelines and approved by the Institutional Animal Care and Use Committee of the University of South Carolina.
Cell culture
Immotalized brown preadipocytes were maintained and differentiated as described [25–27].
RNA isolation and Real-time PCR analysis
Total RNA was extracted from brown preadipocytes or mouse brown adipose tissue using TRIzol (Life Technologies). First strand cDNA was synthesized using High Capacity cDNA kit (Life Technologies). The following primers were used for the Real-time PCR: TBP, F 5′-ACCCTTCACCAATGACTCCTATG-3′, R 5′-TGACTGCAGCAAATCGCTTGG-3′; TRB3, F 5′-TCTCCTCCGCAAGGAACCT-3′, R 5′-TCTCAACCAGGGATGCAAGAG-3′; PRDM16, F 5′-GACATTCCAATCCCACCAGA-3′, R 5′-CACCTCTGTATCCGTCAGCA-3′; UCP1, F 5′-ACTGCCACACCTCCAGTCATT-3′, R 5′-CTTTGCCTCACTCAGGATTGG-3′; PPARγ, F 5′-TCAGCTCTGTGGACCTCTCC-3′, R 5′-ACCCTTGCATCCTTCACAAG-3′; CHOP, F 5′-CCACCACACCTGAAAGCAGAA-3′, R 5′-GGTGCCCCCAATTTCATCT-3′; GRP78, F 5′-CTGGACTGAATGTCATGAGGATCA- 3′, R 5′-CTCTTATCCAGGCCATATGCAATAG-3′; Dio2, F 5′-CAGTGTGGTGCACGTCTCCAATC-3′, R 5′-TGAACCAAAGTTGACCACCAG-3′; XBP1s, F 5′-AAGAACACGCTTGGGAATGG-3′, R 5′-CTGCACCTGCTGCGGAC-3′; PGC1α, F 5′-CCCTGCCATTGTTAAGACC-3′, R 5′-TGCTGCTGTTCCTGTTTTC-3′. Relative mRNA levels were calculated with the PCR product for each primer set normalized to TBP RNA.
Western blot analysis and antibodies
Tissues and cells were rapidly processed in lysis buffer [12,28]. Western blot analyses were used to assess protein and phosphorylation levels of various molecules. Primary antibodies purchased from commercial sources included α-tubulin, UCP1 (Santa Cruz). Secondary antibodies used were horseradish peroxidase (HRP)-conjugated anti-mouse (EMD Millipore) and HRP-conjugated anti-goat (Promega). Antibody to TRB3 was a gift from Dr. Montminy [9]. All the indicated antibodies were used at the dilutions suggested by the manufacturer. Blots were developed using ECL reagents (Thermo Fisher Scientific), and bands were visualized and quantified using ImageJ [29].
Statistical analysis
Data are means ± S.E.M. All data were compared using Student’s t-test or two-way ANOVA.
RESULTS
High-fat feeding and obesity increase TRB3 expression in brown adipose tissue and decrease UCP1 expression.
To determine if high-fat feeding and obesity affect TRB3 expression in mouse brown adipose tissue (BAT), mice were fed a high-fat diet for six weeks. High-fat feeding increased body weight, blood glucose, and serum insulin concentrations [12], and significantly increased TRB3 mRNA expression in BAT by 73% (Fig. 1a). The high-fat diet significantly decreased a BAT-specific uncoupling protein UCP1 mRNA expression by 56% (Fig. 1a). Similarly, TRB3 mRNA expression was up-regulated in BAT from 12-week-old obese (ob/ob) mice compared to control mice by 110% (Fig. 1b), whereas UCP1 expression was down-regulated by 58% (Fig. 1b). The expression of genes involved in BAT differentiation, including PRDM16 (PR domain containing 16), Dio2 (Iodothyronine 2), PGC1alpha (Peroxisome proliferator-activated receptor gamma coactivator 1 alpha) was also decreased in ob/ob mice (Fig. 1b). To determine if fatty acids regulates the TRB3 and UCP1 expression in BAT, brown preadipocytes were studied. Brown preadipocytes were differentiated for 6 days and incubated in the presence or absence of palmitate for 16 hrs. Palmitate incubation at 0.25 mM and 0.75 mM, physiological dosages [30], increased TRB3 mRNA expression by 70% and 74%, respectively (Fig. 1c). Incubation of differentiated brown adipocytes with palmitate significantly decreased UCP1 expression by 30% and 52% (Fig. 1d). Taken together, all of these studies demonstrate that high fat diet and obesity regulate the expression of TRB3 and UCP1 in mouse BAT.
Fig. 1. Regulation of TRB3 expression in mouse brown adipose tissue.
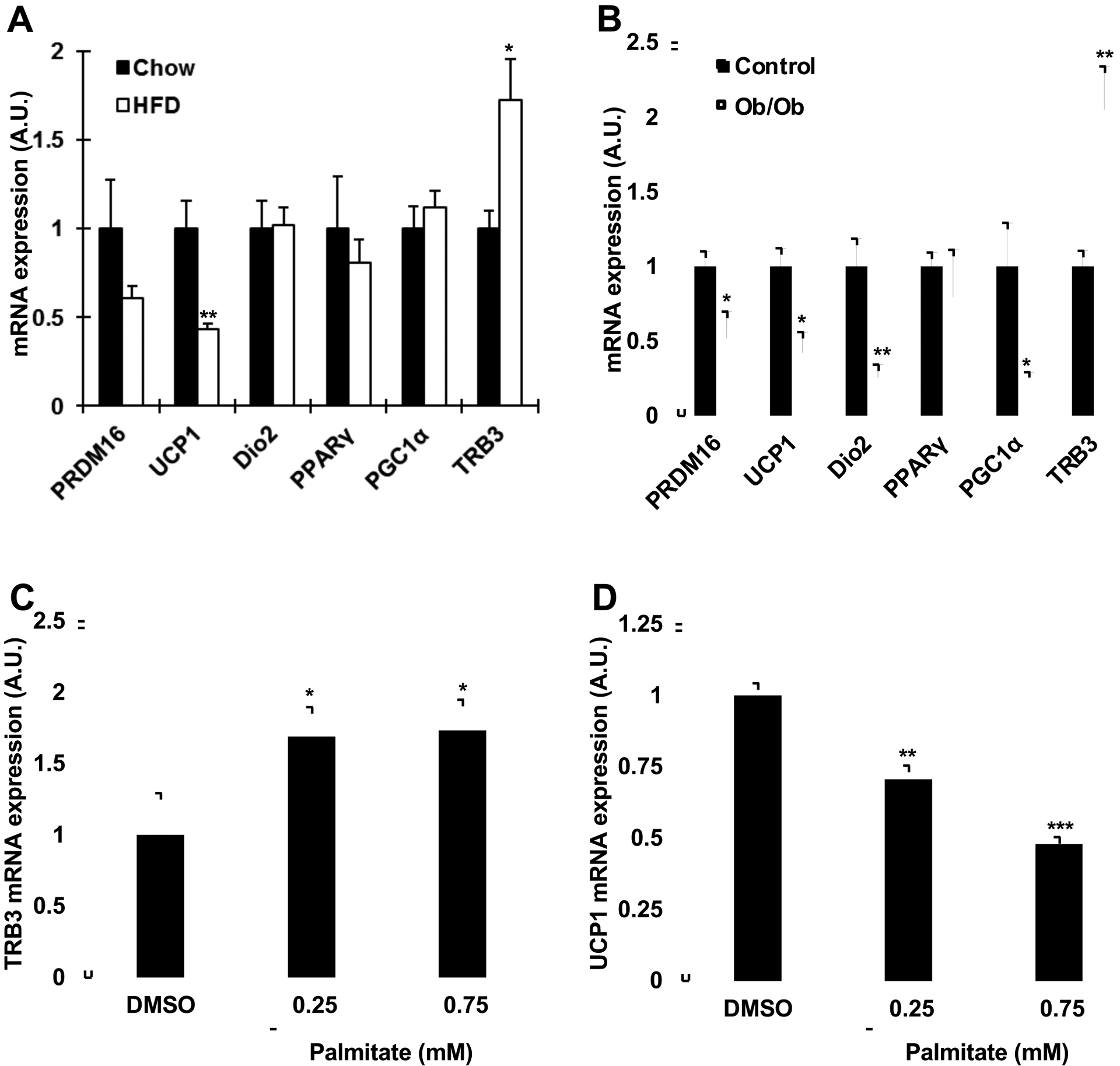
(a) Male C57BL/6 mice (8 weeks old) were fed a high fat diet for 6 weeks. Brown adipose tissue was dissected to determine mRNA expression of TRB3 and other genes involved in brown adipose tissue differentiation (n=5–6). (b) Male ob/ob mice and their controls (12 weeks old) were euthanized and brown adipose tissue was collected to determine mRNA expression of TRB3 and genes involved in brown adipose tissue differentiation (n=4). (c and d) Brown preadipocytes were differentiated 6 days and incubated with 0, 0.25, or 0.75 mM of palmitate with 2% BSA for 16h to determine TRB3 (c) and UCP1 (d) expression (n=4). * indicates p<0.05, ** indicates p<0.01, and *** indicates p<0.001 vs. control.
TRB3 knockout mice are protected from cold exposure
Based on our current data showing that TRB3 was increased with altered metabolic states and our previous work demonstrating that overexpression of TRB3 in brown preadipocyte cells impaired brown adipocyte differentiation and UCP1 expression [23], we next determined if deletion of TRB3 in BAT would improve BAT activity and thermogenesis. To test this hypothesis, we studied TRB3 knockout (TRB3KO) mice that were previously generated [24]. Wild type and TRB3KO mice were exposed to cold (4°C) temperature for 120 min and rectal temperature was determined. TRB3KO and wild type mice had similar body temperature at room temperature (Fig. 2a and b). One hundred twenty minutes of cold exposure significantly decreased body temperature in wild type mice but the decrease was not seen in TRB3KO mice (Fig. 2a and b), suggesting that TRB3 is an important regulator of cold-induced thermogenic responses in BAT. Consistently, UCP1 expression was higher in BAT from TRB3KO mice compared to BAT from wild type mice (Fig. 2c and d). mRNA expression of genes involved in BAT differentiation and function, CIDEA, Dio2, and UCP1 was higher in TRB3KO mice and the expression of PRDM16, and mitochondrial genes, mTFAM, and NRF1 was unchanged (Fig. 2e). There was no significant difference in BAT weight (data not shown).
Fig. 2. The functional assessment of brown adipose tissue in TRB3KO mice.
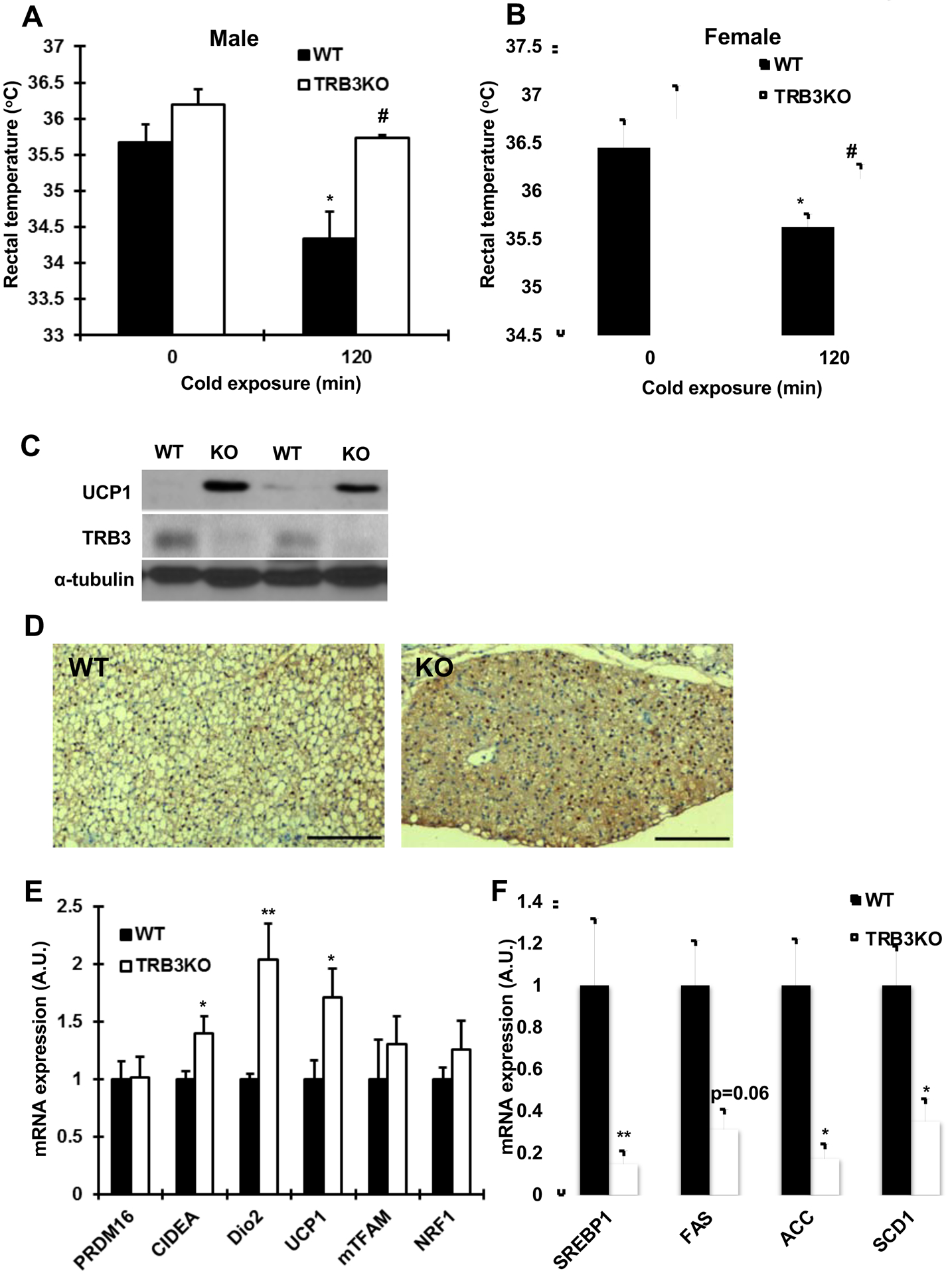
(a,b) Core body temperature was measured in TRB3KO males (a) and females (b) at 4°C (n=5–6). (c) Protein lysates from brown adipose tissue were subjected to Western blot analysis to determine UCP1 expression. (d) Representative histology and UCP1 immunohistochemistry of the brown adipose tissue from wild type (WT) and TRB3KO (KO) mice. Bars indicate 250 μm. We examined 4 independent animals for the analysis. (e,f) Real-time PCR analysis was performed to determine mRNA expression of genes involved in brown adipose tissue differentiation and metabolism (e) and genes involved in lipid metabolism (f) (n=3–8). Data are the means ± S.E.M. * indicates p<0.05 and ** indicates p<0.01 vs. basal or control. # indicates p<0.05 vs. corresponding control.
Since TRB3KO mice also revealed less lipid accumulation in BAT (Fig. 2d), we determined gene expression involved in lipid synthesis and found that mRNA expression of lipid synthesis genes was significantly lower in TRB3KO mice, including SREBP1, FAS, ACC and SCD1 (Fig. 2f). Thus, deletion of TRB3 in BAT improved BAT differentiation and UCP1 expression. Furthermore, TRB3 regulates lipid metabolism in BAT.
ER stress induces TRB3 and inhibits UCP1
Our previous study demonstrated that ER stress induces TRB3 expression in skeletal muscle during high fat diet [12] and in multiple cell lines [31]. We hypothesized that ER stress induces TRB3 expression in BAT, which in turn decreases UCP1 expression. Brown preadipocytes were differentiated for 6 days and incubated in the presence or absence of an ER stressor, Tunicamycin, for 4 hrs. Incubation of the cells with Tunicamycin significantly increased TRB3 mRNA expression by 21.8-fold (Fig. 3a), which was associated with decreased UCP1 expression by 36% (Fig. 3b). Given that mRNA expression of TRB3 was increased and UCP1 was decreased in BAT from HFD and Ob/Ob mice, we next examined if the increased ER stress is causative for the impaired BAT function in the mice. The expression of ER stress markers, including GRP78 (PR domain containing 16), CHOP (CCAAT/enhancer-binding protein homologous protein), XBP1s (X-box binding protein 1 spliced form) was increased in BAT from HFD and ob/ob mice mice (Fig. 1b). These data suggest that obesity and diabetes increase ER stress in BAT and this may increase TRB3 and decrease UCP1 expression.
Fig. 3. Effect of ER stress and TRB3 on UCP1 expression.
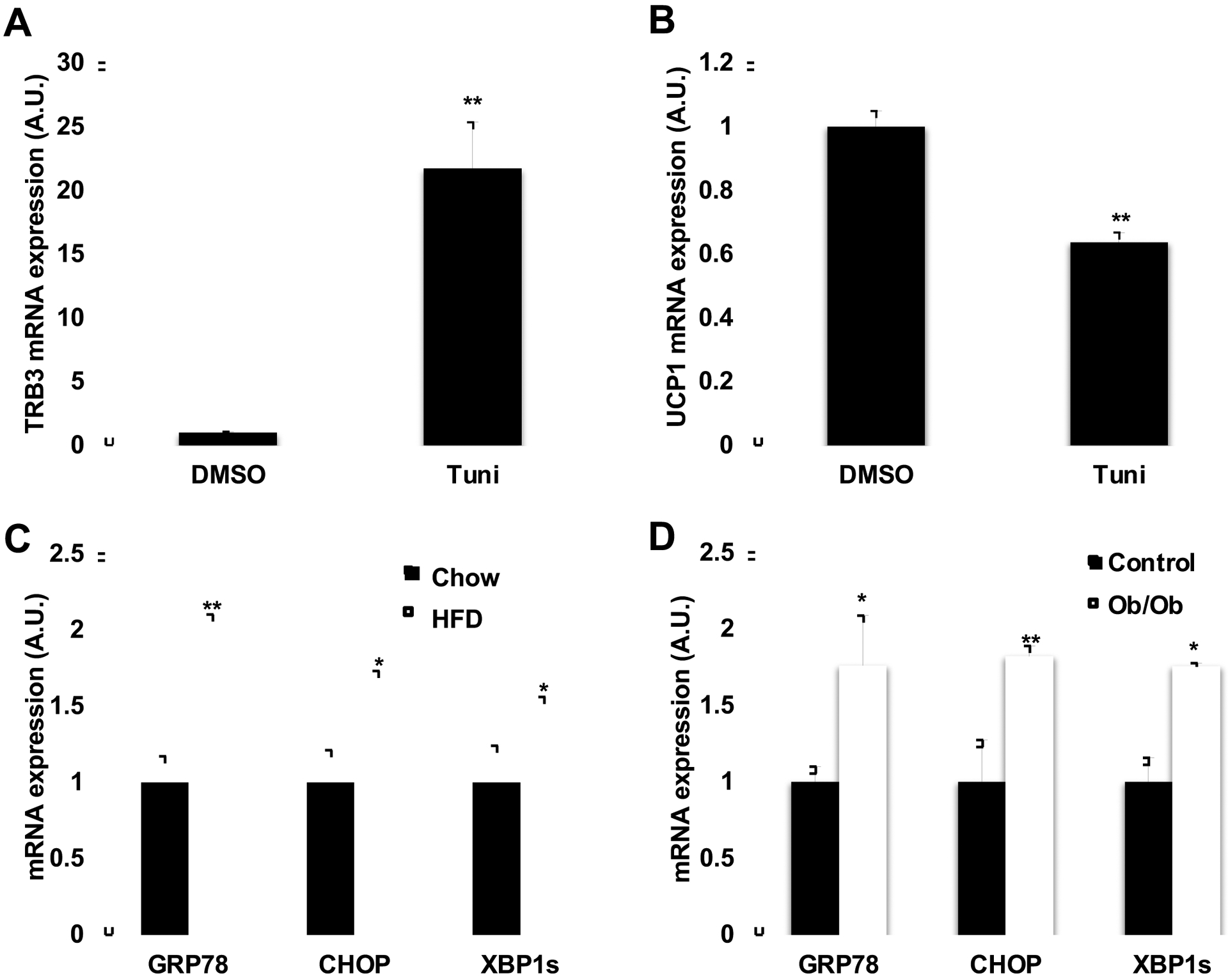
(a,b) Brown preadipocytes were differentiated to brown adipose cells for 8 days. Cells were incubated with an ER stressor, tunicamycin (1 μg ml−1) for 4h and were collected to determine TRB3 (a) and UCP1 (b) mRNA expression using Real-time PCR analysis (n=5). (c,d) Real-time PCR analysis was performed to determine mRNA expression of genes involved in ER stress in BAT from high fat fed mice (c) or ob/ob mice (d) (n=4–6). Data are the means ± S.E.M. * indicates p<0.05, and ** indicates p<0.01 vs. basal or controls.
TRB3 knockout mice are protected from HFD-induced impairment in BAT function.
Our recent studies demonstrated that overexpression of TRB3 in brown preadipocytes inhibits differentiation of the cells and UCP1 expression [23], and that TRB3KO mice fed a HFD for 8 weeks display lower body weight, improved glucose metabolism, and insulin signaling [12]. Given that TRB3 expression was increased with HFD (Fig. 1a) and that TRB3 inhibits UCP1 expression in BAT [23], we next determined if TRB3 mediates the impairment in BAT function in related to HFD in mice. Wild type and TRB3KO male mice were fed a HFD for 16 weeks as previously described [12]. TRB3KO showed the improved glucose tolerance with lower area under the curve by 18% (Fig. 4 a and b). Wild type and TRB3KO mice were kept at cold (4°C) temperature for 6 hrs and rectal temperature was determined. TRB3KO mice had higher body temperature at room temperature compared to wild type mice (Fig. 4c). Six hours of cold exposure decreased body temperature in both groups but the decrease was significantly less in TRB3KO mice (Fig. 4c), indicating that HFD-induced impairment in BAT function is ameliorated in TRB3KO mice. mRNA expression of UCP1 expression was higher in BAT from TRB3KO mice compared to BAT from wild type mice by 6.3-folds (Fig. 4d). Other genes involved in BAT differentiation and function, Dio2, CIDEA, PPARγ, and PGC1α were higher in TRB3KO mice and the expression of PRDM16, and ER stress markers, GRP78, CHOP, and XBP1s was unchanged (Fig. 4d). These data demonstrate that TRB3 plays an important role in HFD-induced impairments in BAT function and differentiation in mice.
Fig. 4. The effects of knockout of TRB3 on high-fat diet-induced BAT impairment.
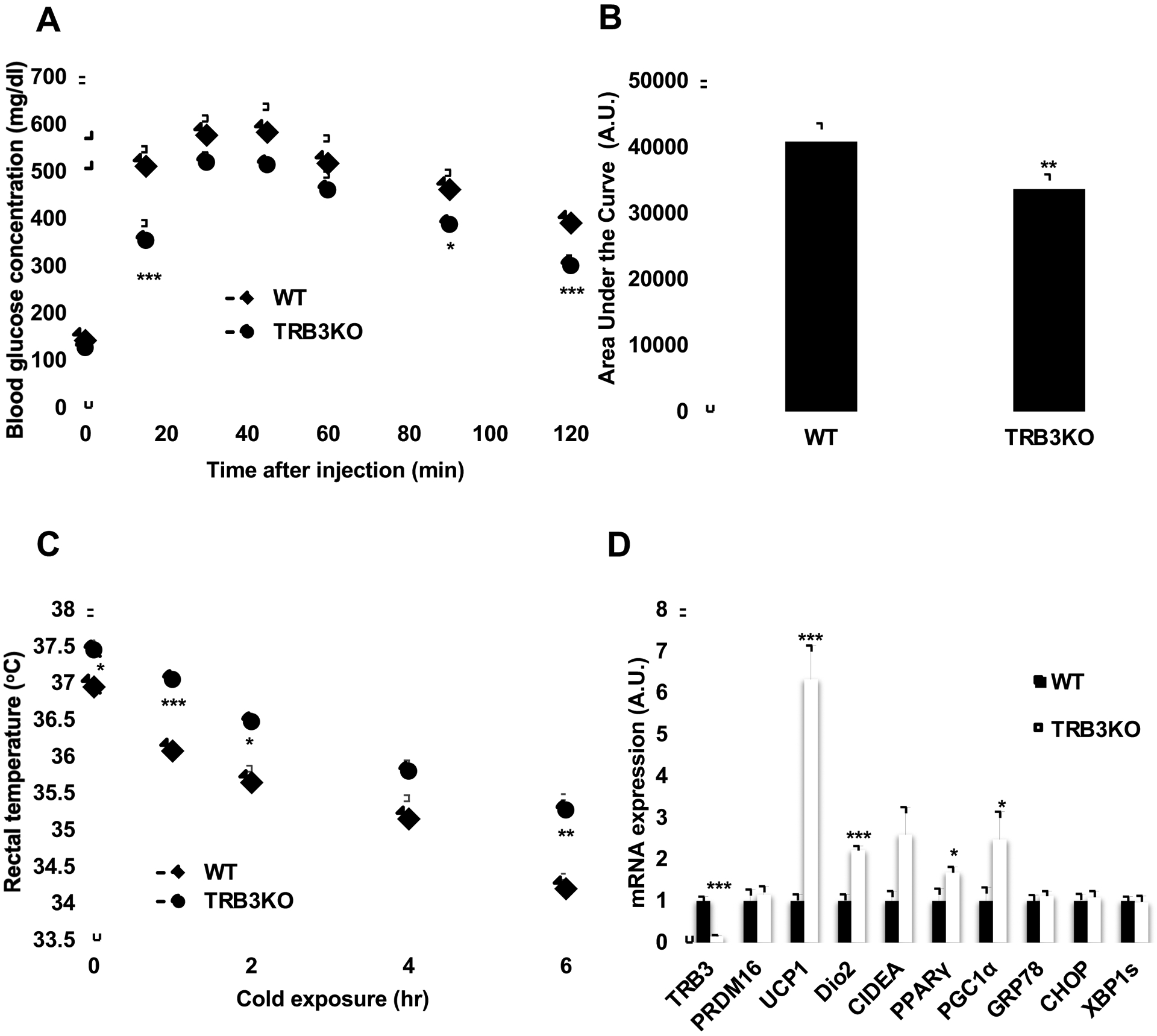
(a-d) Wild type (WT) and TRB3KO mice at 6 weeks of age were placed on a high-fat diet for 16 weeks (n=5). (a,b) At 12 weeks of high-fat diet, mice were fasted for 14 h to perform a glucose tolerance test. TRB3KO mice had improved glucose tolerance (a) and lower area under the curve during the glucose tolerance test (b). (c) At 16 weeks of high-fat diet, mice were placed at 4°C for 6 h. Core temperature was measured at indicated times. (d) mRNA from brown adipose tissue was collected and used to determine mRNA expression for multiple genes. Data are the means ± S.E.M. * indicates p<0.05, ** indicates p<0.01, and *** indicates p<0.001 vs. WT.
DISCUSSION
Activation of thermogenesis has been known to prevent obesity and decrease the incidence of diabetes in humans and mice by increasing energy expenditure and decreasing fat storage [32,33], whereas impairment of thermogenesis results in obesity and diabetes [34,35], suggesting the role of BAT as an anti-obese and anti-diabetic tissues. These fidnings suggest that thermogenic tissue may be important in humans and could serve as a target for the treatment of obesity and diabetes. However, the signaling molecules that regulate BAT differentiation and activity in response to high fat diet have not yet been elucidated. In the current study we tested the hypothesis that TRB3 mediates HFD-induced impairment in BAT differentiation and function. We found that expression of BAT TRB3 was increased in high-fat fed mice and ob/ob mice and that TRB3KO mice were cold resistant and displayed higher UCP1 expression in BAT. Finally, knockout of TRB3 in mice significantly improved BAT function during HFD. These data strongly support the role of TRB3 on BAT differentiation and function during HFD.
We recently reported that TRB3KO mice fed a HFD displayed lower weight gain, lower insulin concentration, improved insulin signaling, and improved glucose homeostasis. Consistent with these results, our current study performed a separate experiment feeding TRB3KO mice on high fat diet for 16 weeks and found that TRB3KO showed improved glucose metabolism. Based on these results, effects of TRB3 on whole body glucose metabolism could be more pronounced under stress conditions, such as high fat feeding, as Okamoto et al. reported no change in body weight and glucose metabolism in TRB3KO mice on chow diet [24].
We found that a high-fat diet in mice and ob/ob mice increase TRB3 expression in BAT, which in turn inhibits UCP1 expression and BAT activity. The mechanism by which these conditions increase TRB3 expression is not known but our results indicate that ER stress mediates the induction of TRB3 in BAT. ER stress has emerged as a key player for the development of insulin resistance in multiple tissues [36–39] and TRB3 expression has been shown to be induced by ER stress in multiple cell lines via ATF4/CHOP pathway [31] and mouse skeletal muscle [12]. In support of this hypothesis we found that incubation of brown preadipocytes with an ER stressor, tunicamycin (50 ng/ml), during 8 days of differentiation results in increased TRB3 mRNA expression by 21-fold but decreased UCP1 mRNA expression by 36% [23]. Furthermore, a previous study demonstrated that heat production in BAT is significantly decreased by thapsigargin, an additional ER stressor, by 23% [40]. Our current results also demonstrated that incubation of differentiated brown adipocytes with tunicamycin induced TRB3 and suppressed UCP1. Furthermore, BAT from high fat fed mice and ob/ob mice showed increased ER stress markers. Taken together, these data support the hypothesis that elevated ER stress in BAT could mediate the effects of high fat feeding, obesity, and type 2 diabetes to increase TRB3 expression in mouse BAT.
In summary, a high-fat diet induces expression of TRB3 in BAT, which results in a decrease in UCP1 expression. Knockdown of TRB3 in mice significantly improves UCP1 expression and BAT function to utilize energy. ER stress mediates HFD-induced TRB3 expression in BAT. TRB3KO mice were protected from HFD-induced impairment in BAT function. We conclude TRB3 plays an important role in obesity- and/or type 2 diabetes-induced impairments in metabolism and function in BAT. Our data also suggest that TRB3 may be a new therapeutic target for effectively managing BAT function.
Highlights.
TRB3 was increased and UCP1 was decreased in BAT from high-fat fed and ob/ob mice.
Knockout of TRB3 improved UCP1 expression and BAT function.
ER stress increased TRB3 and decreased UCP1 in brown adipocytes.
Knockout of TRB3 prevented mice from obesity-induced impairments in thermogenesis.
ACKNOWLEDGEMENTS
The authors thank Dr. M. Montminy (Salk Institute) for providing TRB3 antibody and Regeneron for TRB3KO mice. This work was supported by NIH grants to H.J.K. (R03AR066825 and P20GM109091).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Lowell BB, Flier JS, Brown adipose tissue, beta 3-adrenergic receptors, and obesity, Annu Rev Med 48 (1997) 307–316. 10.1146/annurev.med.48.1.307. [DOI] [PubMed] [Google Scholar]
- [2].Lowell BB, Spiegelman BM, Towards a molecular understanding of adaptive thermogenesis, Nature. 404 (2000) 652–660. [DOI] [PubMed] [Google Scholar]
- [3].Gonzalez-Hurtado E, Lee J, Choi J, Wolfgang MJ, Fatty acid oxidation is required for active and quiescent brown adipose tissue maintenance and thermogenic programing, Mol Metab 7 (2018) 45–56. 10.1016/j.molmet.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wu M, Xu LG, Zhai Z, Shu HB, SINK is a p65-interacting negative regulator of NF-kappaB-dependent transcription, J.Biol.Chem 278 (2003) 27072–27079. [DOI] [PubMed] [Google Scholar]
- [5].Mata J, Curado S, Ephrussi A, Rorth P, Tribbles coordinates mitosis and morphogenesis in Drosophila by regulating string/CDC25 proteolysis, Cell. 101 (2000) 511–522. [DOI] [PubMed] [Google Scholar]
- [6].Cunha DA, Igoillo-Esteve M, Gurzov EN, Germano CM, Naamane N, Marhfour I, Fukaya M, Vanderwinden JM, Gysemans C, Mathieu C, Marselli L, Marchetti P, Harding HP, Ron D, Eizirik DL, Cnop M, Death protein 5 and p53-upregulated modulator of apoptosis mediate the endoplasmic reticulum stress-mitochondrial dialog triggering lipotoxic rodent and human beta-cell apoptosis, Diabetes. 61 (2012) 2763–2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kato S, Du K, TRB3 modulates C2C12 differentiation by interfering with Akt activation, Biochem Biophys.Res Commun 353 (2007) 933–938. [DOI] [PubMed] [Google Scholar]
- [8].Bezy O, Vernochet C, Gesta S, Farmer SR, Kahn CR, TRB3 blocks adipocyte differentiation through the inhibition of C/EBPbeta transcriptional activity, Mol Cell Biol. 27 (2007) 6818–6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Du K, Herzig S, Kulkarni RN, Montminy M, TRB3: a tribbles homolog that inhibits Akt/PKB activation by insulin in liver, Science. 300 (2003) 1574–1577. [DOI] [PubMed] [Google Scholar]
- [10].Koo SH, Satoh H, Herzig S, Lee CH, Hedrick S, Kulkarni R, Evans RM, Olefsky J, Montminy M, PGC-1 promotes insulin resistance in liver through PPAR-alpha-dependent induction of TRB-3, Nat.Med 10 (2004) 530–534. [DOI] [PubMed] [Google Scholar]
- [11].Qi L, Heredia JE, Altarejos JY, Screaton R, Goebel N, Niessen S, Macleod IX, Liew CW, Kulkarni RN, Bain J, Newgard C, Nelson M, Evans RM, Yates J, Montminy M, TRB3 links the E3 ubiquitin ligase COP1 to lipid metabolism, Science. 312 (2006) 1763–1766. [DOI] [PubMed] [Google Scholar]
- [12].Koh HJ, Toyoda T, Didesch MM, Lee MY, Sleeman MW, Kulkarni RN, Musi N, Hirshman MF, Goodyear LJ, Tribbles 3 mediates endoplasmic reticulum stress-induced insulin resistance in skeletal muscle, Nat Commun 4 (2013) 1871. 10.1038/ncomms2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Takahashi Y, Ohoka N, Hayashi H, Sato R, TRB3 suppresses adipocyte differentiation by negatively regulating PPARgamma transcriptional activity, J Lipid Res. 49 (2008) 880–892. [DOI] [PubMed] [Google Scholar]
- [14].Rorth P, Szabo K, Texido G, The level of C/EBP protein is critical for cell migration during Drosophila oogenesis and is tightly controlled by regulated degradation, Mol Cell. 6 (2000) 23–30. [DOI] [PubMed] [Google Scholar]
- [15].Seher TC, Leptin M, Tribbles, a cell-cycle brake that coordinates proliferation and morphogenesis during Drosophila gastrulation, Curr Biol. 10 (2000) 623–629. [DOI] [PubMed] [Google Scholar]
- [16].Koh HJ, Arnolds DE, Fujii N, Tran TT, Rogers MJ, Jessen N, Li Y, Liew CW, Ho RC, Hirshman MF, Kulkarni RN, Kahn CR, Goodyear LJ, Skeletal muscle-selective knockout of LKB1 increases insulin sensitivity, improves glucose homeostasis, and decreases TRB3, Mol Cell Biol. 26 (2006) 8217–8227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Du K, Ding J, Insulin regulates TRB3 and other stress-responsive gene expression through induction of C/EBPbeta, Mol Endocrinol. 23 (2009) 475–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Matos A, Ropelle ER, Pauli JR, Frederico MJ, de Pinho RA, Velloso LA, de Souza CT, Acute exercise reverses TRB3 expression in the skeletal muscle and ameliorates whole body insulin sensitivity in diabetic mice, Acta Physiol (Oxf). 198 (2010) 61–69. [DOI] [PubMed] [Google Scholar]
- [19].Mortensen OH, Frandsen L, Schjerling P, Nishimura E, Grunnet N, PGC-1alpha and PGC-1beta have both similar and distinct effects on myofiber switching toward an oxidative phenotype, Am J Physiol Endocrinol.Metab 291 (2006) E807–E816. [DOI] [PubMed] [Google Scholar]
- [20].Yacoub Wasef SZ, Robinson KA, Berkaw MN, Buse MG, Glucose, dexamethasone, and the unfolded protein response regulate TRB3 mRNA expression in 3T3-L1 adipocytes and L6 myotubes, Am.J.Physiol Endocrinol.Metab 291 (2006) E1274–E1280. [DOI] [PubMed] [Google Scholar]
- [21].Choi RH, McConahay A, Jeong HW, McClellan JL, Hardee JP, Carson JA, Hirshman MF, Goodyear LJ, Koh HJ, Tribbles 3 regulates protein turnover in mouse skeletal muscle, Biochem Biophys Res Commun 493 (2017) 1236–1242. 10.1016/j.bbrc.2017.09.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Choi RH, McConahay A, Silvestre JG, Moriscot AS, Carson JA, Koh HJ, TRB3 regulates skeletal muscle mass in food deprivation-induced atrophy, Faseb j (2019) fj201802145RR. 10.1096/fj.201802145RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Jeong HW, Choi RH, McClellan JL, Piroli GG, Frizzell N, Tseng YH, Goodyear LJ, Koh HJ, Tribbles 3 Inhibits Brown Adipocyte Differentiation and Function by Suppressing Insulin Signaling, Biochem Biophys Res Commun (2016). 10.1016/j.bbrc.2016.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Okamoto H, Latres E, Liu R, Thabet K, Murphy A, Valenzeula D, Yancopoulos GD, Stitt TN, Glass DJ, Sleeman MW, Genetic deletion of Trb3, the mammalian Drosophila tribbles homolog, displays normal hepatic insulin signaling and glucose homeostasis, Diabetes. 56 (2007) 1350–1356. [DOI] [PubMed] [Google Scholar]
- [25].Tseng YH, Kriauciunas KM, Kokkotou E, Kahn CR, Differential roles of insulin receptor substrates in brown adipocyte differentiation, Mol Cell Biol 24 (2004) 1918–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Tseng YH, Butte AJ, Kokkotou E, Yechoor VK, Taniguchi CM, Kriauciunas KM, Cypess AM, Niinobe M, Yoshikawa K, Patti ME, Kahn CR, Prediction of preadipocyte differentiation by gene expression reveals role of insulin receptor substrates and necdin, Nat.Cell Biol 7 (2005) 601–611. [DOI] [PubMed] [Google Scholar]
- [27].Fasshauer M, Klein J, Kriauciunas KM, Ueki K, Benito M, Kahn CR, Essential role of insulin receptor substrate 1 in differentiation of brown adipocytes, Mol Cell Biol 21 (2001) 319–329. 10.1128/mcb.21.1.319-329.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Fujii N, Boppart MD, Dufresne SD, Crowley PF, Jozsi AC, Sakamoto K, Yu H, Aschenbach WG, Kim S, Miyazaki H, Rui L, White MF, Hirshman MF, Goodyear LJ, Overexpression or ablation of JNK in skeletal muscle has no effect on glycogen synthase activity, Am J Physiol Cell Physiol 287 (2004) C200–C208. [DOI] [PubMed] [Google Scholar]
- [29].Nihalani D, Meyer D, Pajni S, Holzman LB, Mixed lineage kinase-dependent JNK activation is governed by interactions of scaffold protein JIP with MAPK module components, EMBO J 20 (2001) 3447–3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Jove M, Planavila A, Laguna JC, Vazquez-Carrera M, Palmitate-induced interleukin 6 production is mediated by protein kinase C and nuclear-factor kappaB activation and leads to glucose transporter 4 down-regulation in skeletal muscle cells, Endocrinology 146 (2005) 3087–3095. [DOI] [PubMed] [Google Scholar]
- [31].Ohoka N, Yoshii S, Hattori T, Onozaki K, Hayashi H, TRB3, a novel ER stress-inducible gene, is induced via ATF4-CHOP pathway and is involved in cell death, EMBO J. 24 (2005) 1243–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Chondronikola M, Volpi E, Borsheim E, Porter C, Annamalai P, Enerback S, Lidell ME, Saraf MK, Labbe SM, Hurren NM, Yfanti C, Chao T, Andersen CR, Cesani F, Hawkins H, Sidossis LS, Brown Adipose Tissue Improves Whole Body Glucose Homeostasis and Insulin Sensitivity in Humans, Diabetes (2014). 10.2337/db14-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ferrer-Lorente R, Cabot C, Fernandez-Lopez JA, Alemany M, Combined effects of oleoyl-estrone and a beta3-adrenergic agonist (CL316,243) on lipid stores of diet-induced overweight male Wistar rats, Life Sci 77 (2005) 2051–2058. 10.1016/j.lfs.2005.04.008. [DOI] [PubMed] [Google Scholar]
- [34].Feldmann HM, Golozoubova V, Cannon B, Nedergaard J, UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality, Cell Metab 9 (2009) 203–209. 10.1016/j.cmet.2008.12.014. [DOI] [PubMed] [Google Scholar]
- [35].Lowell BB, Susulic V, Hamann A, Lawitts JA, Himms-Hagen J, Boyer BB, Kozak LP, Flier JS, Development of obesity in transgenic mice after genetic ablation of brown adipose tissue, Nature. 366 (1993) 740–742. [DOI] [PubMed] [Google Scholar]
- [36].Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, Hotamisligil GS, Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes, Science. 306 (2004) 457–461. [DOI] [PubMed] [Google Scholar]
- [37].Wang S, Kaufman RJ, The impact of the unfolded protein response on human disease, J Cell Biol. 197 (2012) 857–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hotamisligil GS, Endoplasmic reticulum stress and the inflammatory basis of metabolic disease, Cell. %19;140 (2010) 900–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Walter P, Ron D, The unfolded protein response: from stress pathway to homeostatic regulation, Science. 334 (2011) 1081–1086. [DOI] [PubMed] [Google Scholar]
- [40].de Meis L, Oliveira GM, Arruda AP, Santos R, Costa RM, Benchimol M, The thermogenic activity of rat brown adipose tissue and rabbit white muscle Ca2+-ATPase, IUBMB Life 57 (2005) 337–345. 10.1080/15216540500092534. [DOI] [PubMed] [Google Scholar]


