Abstract
Background
Asymptomatic severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infections are well documented. Healthcare workers (HCW) are at increased risk of infection due to occupational exposure to infected patients. We aim to determine the prevalence of SARS-CoV-2 antibodies among HCW who did not come to medical attention.
Methods
We prospectively recruited 400 HCW from the National Public Health Laboratory and two COVID-19 designated public hospitals in Klang Valley, Malaysia between 13/4/2020 and 12/5/2020. Quota sampling was used to ensure representativeness of HCW involved in direct and indirect patient care. All participants answered a self-administered questionnaire and blood samples were taken to test for SARS-CoV-2 antibodies by surrogate virus neutralization test.
Findings
The study population comprised 154 (38.5%) nurses, 103 (25.8%) medical doctors, 47 (11.8%) laboratory technologists and others (23.9%). A majority (68.9%) reported exposure to SARS-CoV-2 in the past month within their respective workplaces. Adherence to personal protection equipment (PPE) guidelines and hand hygiene were good, ranging from 91-100% compliance. None (95% CI: 0, 0.0095) of the participants had SARS-CoV-2 antibodies detected, despite 182 (45.5%) reporting some symptoms one month prior to study recruitment. One hundred and fifteen (29%) of participants claimed to have had contact with known COVID-19 persons outside of their workplace.
Interpretation
Zero seroprevalence among HCW suggests a low incidence of undiagnosed COVID-19 infection in our healthcare setting during the first local wave of SARS-CoV-2 infection. The occupational risk of SARS-CoV-2 transmission within healthcare facilities can be prevented by adherence to infection control measures and appropriate use of PPE.
Funding
Own institutional budget and the Fundamental Research Grant Scheme.
Research in Context.
1.1 Evidence before this study
Healthcare workers (HCW) are at higher risk for COVID-19 infection. A recent meta-analysis of 49 studies estimated an overall seroprevalence rate of 8.7% SARS-CoV-2 antibodies among HCW. The seroprevalence was higher in North American (12.7%) and European countries (8.5%) than in Asia (4%). Factors such as sex, ethnicity, profession, location of clinical work, contact with COVID-19 patients, and personal protective equipment (PPE) usage are associated with seroprevalence of SARS-CoV-2 antibodies among HCW.
1.2 Added value of this study
We reported zero seroprevalence of anti-SARS-CoV-2 among HCW working at facilities designated for the management of COVID-19 patients and specimens for COVID-19 testing. Doctors, nurses and laboratory technologists had higher risk of SARS-CoV-2 exposure at workplace but showed no seroconversion. Adherence to infection prevention and control measures (IPC) and PPE usage among the HCW were good.
1.3 Implications of all the available evidence
The risk of SARS-CoV-2 transmission within healthcare facilities can be mitigated with good adherence towards IPC and PPE usage.
Alt-text: Unlabelled box
Introduction
On 25th January 2020, Malaysia had its first confirmed case of coronavirus disease 2019 (COVID-19) [1], a disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [2]. Initially, confirmed cases were mainly imported and daily reported cases were low. Nevertheless, local transmission emerged, following a mass religious event that was held in Kuala Lumpur between late February and early March 2020. This event was attended by an estimated 16,000 people from all over Asia, of whom 14,500 were Malaysians [3]. The event led to massive spikes in local cases and exportation of cases to other countries. As of 14th October 2020, over 17,000 COVID-19 cases and 167 deaths were reported, with an incidence of 55 per 100,000 Malaysian population. Klang Valley, which comprises Selangor state, the Federal Territory (and capital) of Kuala Lumpur and the Federal Territory of Putrajaya, contributed about one third of the national COVID-19 burden [4].
As HCW manage suspected and confirmed COVID-19 patients, they are presumed to have higher risk for COVID-19 infection and, if infected, can possibly transmit the virus to vulnerable patients and other co-workers. According to local management guidelines, any HCW who presented with acute respiratory infection (sudden onset of respiratory infection with at least shortness of breath, cough or sore throat) with or without fever; and had history of travel to or residence in a foreign country within 14 days prior to the onset of illness, close contact with a confirmed case of COVID-19 within 14 days before illness onset, or had attended any event associated with a known COVID-19 outbreak is considered as “patient under investigation (PUI)”, who must undergo Occupational Safety and Health team assessment in their respective healthcare facilities for further management.
The spectrum of COVID-19 severity ranges from mild to critical. According to the Chinese Center for Disease Control and Prevention, about 80% of confirmed COVID-19 patients suffered mild disease [5]. Asymptomatic COVID-19 infections are well documented, but its proportion within total COVID-19 cases remains unclear [6,7]. Nevertheless, current evidence suggests that asymptomatic and pre-symptomatic COVID-19 individuals can transmit the infection to others [8]. Seroprevalence studies are useful to provide information on the proportion of people with past symptomatic or asymptomatic infection. We conducted a serology surveillance of SARS-CoV-2 antibodies among HCW at designated COVID-19 healthcare facilities for evidence of undetected infection in HCW who did not come to medical attention as confirmed cases.
Methods
3.1 Study setting
A cross-sectional study of HCW from Kuala Lumpur Hospital, Sungai Buloh Hospital and the National Public Health Laboratory (NPHL) was conducted from April 13th to May 12th, 2020, corresponding to the post-peak period of first local wave of SARS-CoV-2 infection in Malaysia. The study sites represent two of the three public hospitals which were designated to manage confirmed COVID-19 patients in Klang Valley, Malaysia, with NPHL being a referral laboratory for diagnostic reverse transcription polymerase chain reaction (RT-PCR) testing for suspected COVID-19 cases. The total workforce at these facilities was about 10,000.
3.2 Study participants
Participation in the study was voluntary. Healthcare workers were invited by advertisement and/or internal announcement to participate in the study. Those interested in the study were asked to contact the study team for an appointment. All participants must have at least 30 workdays prior to study enrollment, and were asymptomatic at the point of recruitment. HCW who were previously confirmed with COVID-19 infection or listed as PUI for COVID-19 at the time of study recruitment were excluded from this study. This group of HCW would have been seen by Occupational Safety and Health (OSH) team for further management.
Quota sampling was applied to ensure the recruited study samples were representative of the HCW involved in provision of care for patients directly (e.g. doctors, nurses, assistant medical officers, dentists, and dental surgery assistant), and indirectly (e.g. laboratory technologists, pharmacists, drivers, clerks and other non-clinical staff). The ratio of HCW providing direct and indirect care was about 3:1. Therefore, the quota for HCW involved in direct care was set at 75% of the total sample size.
3.3 Data collection procedure
Each HCW who consented to participate in this study was given a self-administered questionnaire (Supplementary File 1) to capture sociodemographic characteristics, adherence with recommended infection prevention and control (IPC) measures, history of exposure to SARS-CoV-2 and clinical signs and symptoms in the past one month prior to study entry. The questionnaire was modified from the protocol “Assessment of potential risk factors for 2019-novel coronavirus (2019-nCoV) infection among HCW in a healthcare setting”, published by World Health Organization (WHO) [9]. For each participant, 5 mL of peripheral venous blood was collected for SARS-CoV-2 antibody serology testing.
3.4 Definitions and personal protection equipment (PPE) guidelines
The history of close contact and prolonged face-to-face exposure with COVID-19 patients were captured in the questionnaire. Close contact was defined as contact between HCW and patient within one meter distance, with or without PPE. Prolonged face-to-face exposure was defined as face-to-face exposure within one meter distance which lasted at least 15 minutes.
According to local guidelines published in March 2020, use of PPE should be guided by risk assessment concerning anticipated contact during routine patient care. For in-patient facilities, HCW are required to wear full PPE which includes an N95 mask, an isolation gown, gloves, eye protection and a head cover when providing care to PUI or confirmed COVID-19 patients who are intubated, or those who are not intubated but unable to wear a surgical mask. For patients who can wear a surgical mask, HCW may opt for a surgical mask instead of an N95 mask. Full PPE should be worn when collecting oropharyngeal or nasopharyngeal swabs. More comprehensive local PPE guidance in different settings can be found in Supplementary File 2. The HCW are also required to wear surgical masks when they are unable to maintain a physical distance of one meter from other individuals, or in prolonged contact with other co-workers (e.g. meetings, workshop, prayer room, etc), or present in clinical area although not treating PUI or confirmed COVID-19 patients. [10]
3.5 SARS-CoV-2 antibodies serological test
Total circulating neutralizing antibodies against SARS-CoV-2 were tested by using the cPass SARS-CoV-2 Surrogate Virus Neutralization Test (sVNT) kit, according to the manufacturer's instructions (GenScript Biotech, USA) [11]. This test is based on antibody-mediated blockage of virus-host interaction between the receptor binding domain of the viral spike glycoprotein and the angiotensin converting enzyme-2 receptor protein. It has shown sensitivity of 91.3–100% and specificity of 100% in samples collected more than 14 days after onset of illness [12,13], performed well in comparison with other established assays [14], and is capable of distinguishing antibody responses to other known human coronaviruses [13]. To confirm assay performance in our laboratory, the test was first performed on 35 serum samples collected more than 16 days after onset of illness from recovered patients with PCR-confirmed SARS-CoV-2 infection, and 27 archived serum samples from patients with suspected dengue in July 2019, well before the pandemic emerged [15]. These samples were collected in University Malaya Medical Centre, Kuala Lumpur.
3.6 Sample size and statistical analysis
In order to assess the seroprevalence of SARS-CoV-2 antibody among HCW, with a precision of 5% and a 95% confidence interval, and assuming that the prevalence was 50%, with a finite population, we estimated we would need 383 HCW. With the uncertainty about seroprevalence of antibodies against SARS-CoV-2 among the HCW, we used 50% as the prevalence to provide the most conservative sample size.
The analysis was carried out using Statistical Package for the Social Sciences (SPSS) version 26.0 (IBM, USA). Categorical variables were expressed in frequency and percentage. As for continuous variables, mean and standard deviation were used. Seroprevalence of SARS-CoV-2 antibodies was calculated as a proportion, and confidence intervals were calculated using Wilson score interval.
3.7 Ethics approval and consent to participate
This study was registered under National Medical Research Register (NMRR-20-575-54382) and approved by the Medical Research Ethics Committee, Ministry of Health Malaysia. Written consent was taken from all study participants. Retrospective testing of anonymized samples in University Malaya Medical Centre was approved by the hospital's medical ethics committee (no. 2017116-5794), and does not require informed consent.
3.8 Consent for publication
During the process of consent taking, all participants were informed regarding the need of publishing the results. All participants’ personal information were anonymized during the write up, and none of them will be identified when the findings of this study are published.
Role of funding source
The study was mainly funded by own institutional budget. Laboratory testing was part-funded by the Fundamental Research Grant Scheme (FRGS) (grant no. FRGS/1/2020/SKKM0/UM/02/5) from the Ministry of Education, Malaysia, awarded to Yoke Fun Chan and I-Ching Sam. The funders did not have any role in the study design, data collection, data analysis, interpretation and report writing.
Results
4.1 Baseline characteristics
A total of 400 HCW from the National Public Health Laboratory (N=38), Kuala Lumpur Hospital (N=201) and Sungai Buloh Hospital (N=161) were recruited for this study. The three departments with the most participants were the medical (32.3%), emergency (17.5%) and pathology departments (13.5%) (Supplementary File 3, Table 1). This cohort consisted of 154 (38.5%) nurses, 103 (25.8%) medical doctors, 47 (11.8%) laboratory technologists and other professions (Table 1). The mean age was 34.9 ± 7.8 years old, with female predominance (74.3%).
Table 1.
Sociodemographics of Study Participants.
| Sociodemographic characteristics | Result (N = 400) |
|---|---|
| Age in years, mean (±SD) | 34.9 (7.8) |
| Sex, n (%) | |
| Female | 297 (74.3) |
| Male | 103 (25.8) |
| Ethnicity, n (%) | |
| Malay | 291 (72.8) |
| Indian | 62 (15.5) |
| Chinese | 29 (7.3) |
| Others | 18 (4.5) |
| Professional category, n (%) | |
| Nurse | 154 (38.5) |
| Doctor | 103 (25.8) |
| Laboratory technologist | 47 (11.8) |
| Assistant medical officer | 39 (9.8) |
| Health attendant | 32 (8.0) |
| Othersa | 25 (6.3) |
Includes 7 drivers, 3 clerks, 3 assistant food technologists, 2 dentists, 2 physiotherapists, 2 speech therapists, 2 dental surgeon assistants, 1 entomologist, 1 research officer, 1 science officer and 1 pharmacist.
Although all HCW were asymptomatic upon study recruitment, nearly half of the HCW (n=182, 45.5%) reported some symptoms within the month prior to the study period. Fifteen (3.75%) of them reported fever (≥38 ◦C). Among 135 (33.8%) HCW who reported respiratory symptoms, only 12 (8.9%) were associated with fever. The most commonly reported respiratory symptoms were sore throat (70.4%), followed by runny nose (43%), cough (40.7%) and breathlessness (8.1%). Other reported symptoms in the past month included headache (16.5%), fatigue (13.3%), myalgia (8.5%), diarrhea (5.5%), arthralgia (5.3%), nausea/vomiting/anorexia (4.3%), rashes (2%) and conjunctivitis (1.3%). Overall, 66 (16.5%) HCW had symptoms fulfilling the WHO clinical criteria for suspected COVID-19 [16].
4.2 Exposure history to SARS-CoV-2
Overall, 115 of HCW claimed contact with known COVID-19 cases outside of their workplace in the month prior to study enrollment. Among them, 81 (70.4%) reported the contact as more than 14 days prior to study entry, while five (4.3%) did not specify the period.
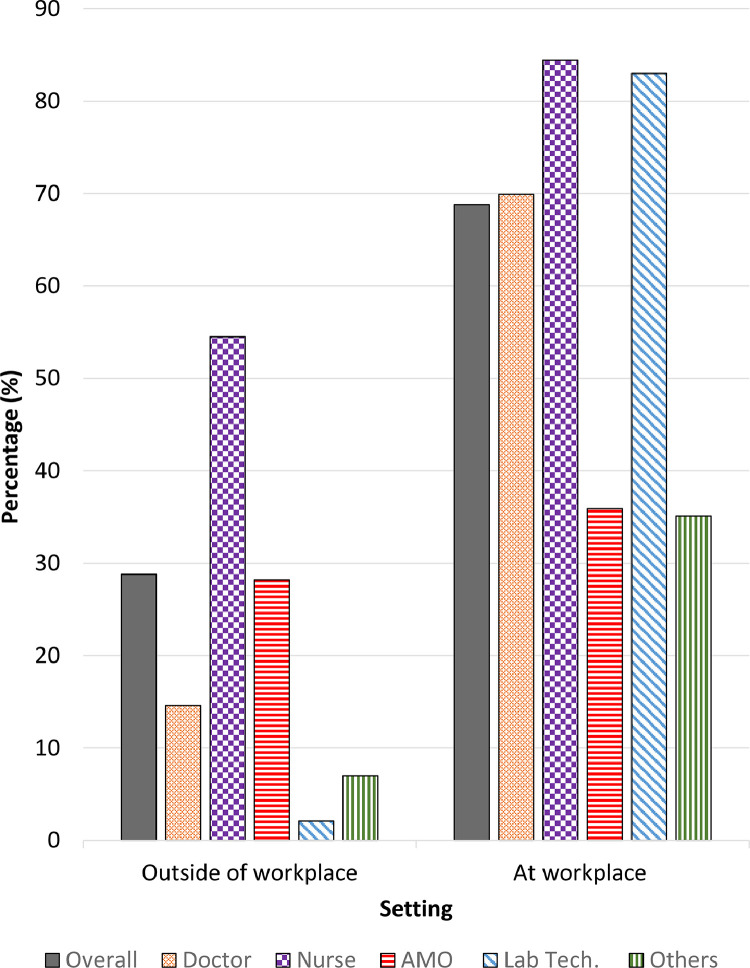
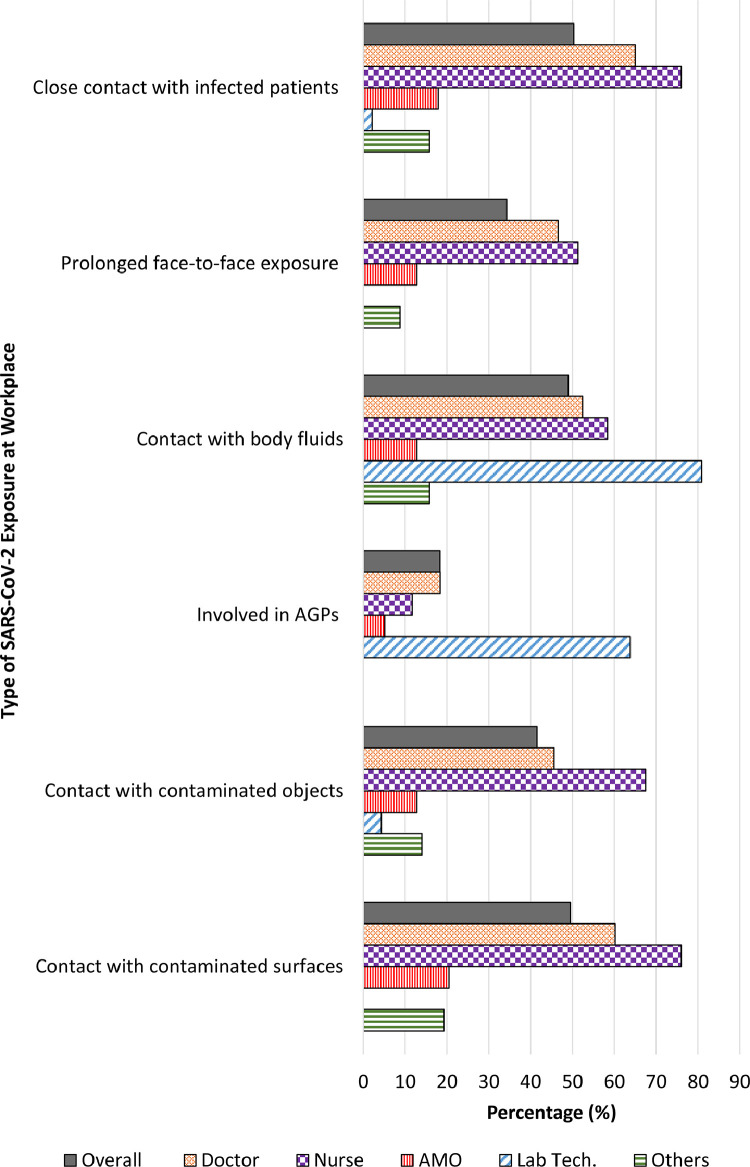
The majority (68.9%) of participants had potential exposure to SARS-CoV-2 at their workplace, within the month prior to study entry (Fig. 1). The source of exposure at the workplace included contact with COVID-19 patients and their bodily fluids, as well as contaminated objects and surfaces. Doctors and nurses were found to have the highest exposure risk when compared to other professionals, ranging from 45–65% and 51–76%, respectively. Laboratory technologists who were not involved in provision of care directly, had contact with patients’ body fluids most of the time. Less than one-fifth (18.3%) of the HCW reported to be involved in aerosol generating procedures (Fig. 2 and Supplementary File 3, Table 2).
Fig. 1.
Setting of Exposure to SARS-CoV-2. AMO: Assistant medical officer; Lab Tech: Laboratory technologist.
Fig. 2.
Type of SARS-CoV-2 Exposure at Workplace. AGPs: Aerosol generating procedures; AMO: Assistant medical officer; Lab Tech: Laboratory technologist.
Table 2.
Adherence of healthcare workers to personal protection equipment usage.
| Type of Exposure at Workplace | Total | Adherence to PPEa, n (%) |
|---|---|---|
| Prolonged face-to-face exposure with infected patients | 137 | 134 (97.8) |
| Handled / contact with body fluids of infected patients | 196 | 196 (100.0) |
| Involved in aerosol generating procedures | 73 | 72 (98.6) |
| Contact with contaminated objects | 166 | 163 (98.2) |
| Contact with contaminated surfaces | 198 | 192 (97.0) |
PPE = Personal protection equipment
4.3 Adherence to infection prevention and control measures
Generally, adherence of the study participants to infection prevention and control (IPC) measures was satisfactory. High compliance with PPE usage (≥97%) were reported by HCW who had prolonged face-to-face exposure with COVID-19 infected patients, involvement in aerosol generating procedures, and contact with infected patients’ body fluids, contaminated objects and surfaces (Table 2). Among them, the level of compliance towards hand hygiene was satisfactory (Table 3).
Table 3.
Level of compliance with hand hygiene among healthcare workers.
| Hand hygiene | Total | Level of Compliance, n (%) |
|||
|---|---|---|---|---|---|
| Always | Most of the time | Occasionally | Rarely | ||
| After close contact with infected patients | 201 | 187 (93.0) | 12 (6.0) | 1 (0.5) | 1 (0.5) |
| After handling / contact with body fluids of infected patients | 196 | 188 (95.9) | 6 (3.1) | 1 (0.5) | 1 (0.5) |
| After contact with contaminated objectsa | 166 | 157 (95.2) | 8 (4.8) | 0 (0.0) | (0.0) |
| After contact with contaminated surfacesb | 198 | 180 (90.9) | 12 (6.1) | 3 (1.5) | 1 (0.5) |
1 respondent did not answer this question
2 respondents did not answer this question
When the analysis was stratified by professional categories, nurses reported good compliance to hand washing across all components, ranging from 94% to 98% (Supplementary File 3, Table 3). Comparatively, doctors and assistant medical officers had poorer hand hygiene compliance (<90%) after direct contact with contaminated objects and surfaces.
4.4 Seroprevalence of total antibodies against SARS-CoV-2
The sVNT was found to detect antibodies in all 35 samples from convalescent COVID-19 cases, and did not detect antibodies in any of the 27 pre-pandemic samples. None of the study participants had SARS-CoV-2 antibodies detected (95% confidence interval (CI): 0, 0.0095).
Discussion
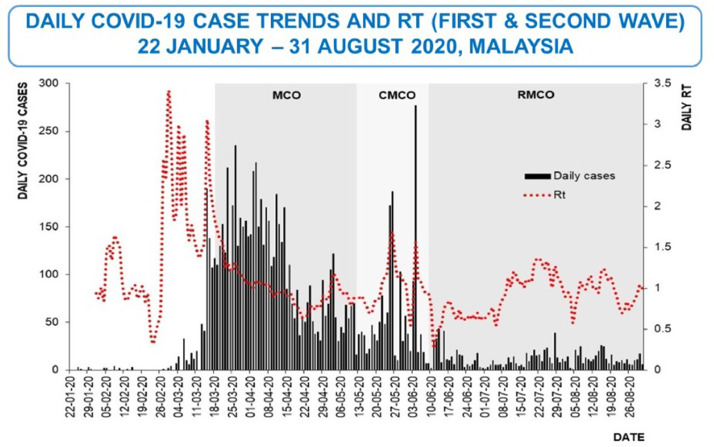
In Malaysia, the first local wave of SARS-CoV-2 transmission started at the end of February and peaked in mid-March to mid-April 2020 (Fig. 3) [17]. During this period, both medical resources and healthcare workers around the country were redirected to combat the disease. Healthcare staff from various hospitals of different departments were deployed to manage suspected and confirmed COVID-19 patients in designated healthcare facilities. This study was conducted during the post-peak period, focusing on HCW with no known prior SARS-CoV-2 infection and who were asymptomatic at the point of study recruitment. The testing strategies during the study period for HCW in Malaysia healthcare facilities targeted those who were symptomatic. According to Ng, O.T. et al, symptom-based polymerase chain reaction testing strategy missed 62% of positive SARS-CoV-2 cases [18]. We were interested to know if HCW could have been infected by SARS-CoV-2 but did not come to medical attention. If there was a high proportion, a change in the policy of HCW testing and also local infection control guidelines would become necessary.
Fig. 3.
Number of New Cases in Malaysia. Adopted from Daily COVID-19 case trends and Rt (first and second wave) 22 January – 31 August 2020, Malaysia, by Ministry of Health Malaysia, 2020. [19].
Prior to this serology surveillance, there were 29 confirmed COVID-19 cases and 1436 PUI cases among HCW in the healthcare facilities included in this study. Our finding of zero seroprevalence suggests that there is a low rate of undetected COVID-19 infection among our HCW. Another Malaysian study of 310 HCW at a non-COVID designated healthcare facility in Sarawak showed a seroprevalence rate of 4.5%, using a rapid antibody test kit. However, the authors reported that two study subjects with faint IgM positive results were false positives [20]. The sVNT used in our study is likely to have a higher specificity than a rapid antibody test kit, thus reducing the risk of false positives [21]. Besides, we hypothesized HCW from designated COVID-19 hospitals would have a better perception of the potential risk of infection involved due to higher occupational exposure, which would lead to better adherence to IPC measures and reduce the transmission risk within the facility itself.
A similar study in China reported zero serological response among 420 HCW who were deployed to Wuhan during this pandemic [22]. The study coupled with our finding suggest that appropriate PPE usage protects HCW from contracting the infection. In contrast, a study of 316 employees in a German tertiary hospital showed that 1.6% were seropositive for antibody against SARS-CoV-2 [23]. Alserehi, H.A. et al conducted a serosurvey of SARS-CoV-2 antibody among 12,621 HCW across 85 hospitals in the Kingdom of Saudi Arabia and found the seroprevalence to be 2.4% [24]. Likewise, a cohort study in Denmark identified 1163/27,292 (4.04%) seropositive HCW [25]. In Kyoto, seroprevalence of SARS-CoV-2 antibody among HCW were reported to be as high as 5.4% [26]. A similar seroprevalence rate was reported by Self, W.H. et al., whereby 194 out of 3,248 (6%) HCW in 13 academic medical centers in the United States were reported to have antibody evidence of previous COVID-19 infection [27]. In a tertiary hospital in Barcelona, 45 (9.3%) out of 578 HCW were seropositive for antibody against SARS-CoV-2 [28]. In the greater New York City, all Northwell HCW were offered free SARS-CoV-2 antibody testing. The results showed 5523 of 40,329 (13.7%) were seropositive [29]. An even higher seropositivity rate was observed in a large acute care hospital in Sweden, where 410/2149 (19.1%) HCW tested seropositive for IgG antibodies [30]. This is in line with a meta-analysis of 49 studies which concluded the seroprevalence of SARS-CoV-2 antibodies among HCW is higher in North America (12.7%), Europe (8.5%), and Africa (8.2%) than in Asia (4%) [31]. We postulate the discrepancy between our study and others could be multifactorial. Firstly, Malaysia had a relatively low COVID-19 incidence rate when compared to these countries during the study period. As of 15th June 2020, the cumulative incidence of COVID-19 in these countries were about 8 to 25 times higher than Malaysia, except Japan which had a lower incidence [32]. HCW seroprevalence may correlate with wider community circulation [33,34]. Secondly, the transmission dynamics of COVID-19 infection could be associated with the disease severity [35], [36], [37]. According to a Malaysian observational study of 5889 confirmed COVID-19 cases diagnosed between February and May 2020, nearly half of the cases were asymptomatic upon admission. Only 3.3% of the patients were ever admitted to intensive care units throughout the course of hospitalization [38]. Thirdly, the variation of participants’ eligibility criteria between the studies may also contribute to the discrepancy. Studies from United States and Spain included HCW who were infected with SARS-CoV-2 previously [27,28]. Besides, the testing method was not standardized across all studies. Any differences between the performances of each testing method can also affect the seroprevalence result. Additionally, there was a delay in response towards this pandemic in some countries. Unpreparedness in handling the surge of patients may result in severe shortage of the PPE, which may contribute to a higher SARS-CoV-2 infection rate among the HCW [39]. Moreover, the variation in PPE guidelines may also explain the difference of HCW seroprevalence between these countries. High seroprevalence of SARS-CoV-2 antibodies among HCW had been reported across various healthcare facilities in the United Kingdom, ranging between 6% and 45% [40], [41], [42], [43], [44], [45]. Some organizations and literature suggested PPE guidelines recommended by the Public Health England (PHE) are insufficient to protect the HCW from SARS-CoV-2 infection [44,[46], [47], [48], [49], [50]]. According to the PHE's PPE guidelines, respirator masks and disposable surgical gowns are not necessary when dealing with suspected or confirmed COVID-19 patients, except when performing selected aerosol-generating procedures or working in ‘high risk acute care areas’ such as intensive care unit, wards with non-invasive ventilation, gastrointestinal endoscopy, etc. [51]. This guidance is different from Malaysia's standards, whereby respirator/surgical mask and isolation gown (fluid-repellent long-sleeved gown) are recommended for HCW who manage suspected and confirmed COVID-19 patients [10].
Numerous studies have stressed the critical importance of strict adherence to IPC measures to prevent patient-to-HCW infections [23,27,[52], [53], [54], [55], [56]], but few have investigated HCW compliance with IPC measures. Interestingly, Barrett et al. did not find any significant association between usage of PPE and COVID-19 infection rate among the HCW in United States [57]. However, our findings of high adherence to infection control guidelines and zero seroprevalence of SARS-CoV-2 antibody in HCWs suggested strict infection control and PPE usage may reduce the risk of SARS-CoV-2 transmission within healthcare facilities. This is further supported by several studies that concluded PPE shortages and reuse of PPE are the main contributors of high COVID-19 transmission risk among the HCW [27,58].
In terms of the history of SARS-CoV-2 exposure, more than a quarter of participants claimed to have contact with a known COVID-19 case outside of their workplace. The participants may assume any contact with infected co-workers within the hospital compound (i.e. pantry, praying area, etc.), but outside of ward as a form of contact beyond workplace. On the other hand, we observed a connection between professional categories and history of SARS-CoV-2 exposure at the workplace. This study found that doctors and nurses have more exposure to SARS-CoV-2 through all types of contact, from patients to respiratory droplets, objects and surfaces. This was in line with the nature of their work, which involves direct provision of care to patients. Laboratory technologists whose main job scope is to process patient specimens were found to have contact with patients’ body fluids most of the time.
As of early May 2020, 359 HCW from Malaysian MOH had been diagnosed with COVID-19. Of these, 73% had acquired COVID-19 from the community, including from coworkers who were infected in the community, 19% had acquired the virus from patients, and the remaining 8% were still under investigation [59]. Public fear of hospitals and HCW remains high. Many countries observed that patients have avoided seeking medical care for other health problems during this pandemic, thus resulting in late presentation with adverse outcomes [60], [61], [62], [63]. Stigmatization and abuse of HCW have also been reported [64], [65], [66], [67]. A study from Spain in March 2020 observed no difference in the COVID-19 infection rate of HCW by professional categories and risk of exposure in respective work departments.[34] Other studies in Spain [28], Italy [54] and Netherlands [68] also suggested the risk to a HCW of contracting COVID-19 infection in a healthcare facility is minimal. Although our study was unable to fully rule out additional risks of COVID-19 infection in a healthcare facility, zero seroprevalence of SARS-CoV-2 antibody among our study subjects should reassure the public that the possibility of any infected HCW who did not come to medical attention is very low.
SARS-CoV-2-specific IgM and IgG antibodies start to appear during the first week of illness, and peak between two to three weeks [69]. After taking into consideration the timeline of seroconversion, serostatus of the HCW in this study most likely reflects COVID-19 transmission in Malaysia between middle of March and April 2020. This period corresponds to the second wave of COVID-19 outbreak in Malaysia following the mass religious event held in Kuala Lumpur [70], with a doubling time of confirmed COVID-19 cases ranging from two to 110 days [71]. Folgueira et al. found a close link between the driving forces of transmission in the community and HCW infection, which suggested that the rates of SARS-CoV-2 infection among HCW could be an indicator of transmission dynamics in the community [34]. If we adopt a similar principle, this study shows that the proportion of the general public in Malaysia infected as of mid-March to April could be very low, and PPE for HCW could have helped in preventing nosocomial transmission. This also signifies Malaysia's successful public health efforts at that time in combating this pandemic via extensive contact tracing and early admission of confirmed COVID-19 cases for isolation and close monitoring.
This study has several limitations. Firstly, the study was limited to selected healthcare facilities, so its findings may not be generalizable to HCW in other workplaces, especially non-COVID designated healthcare facilities. Secondly, we acknowledge the existence of selection bias. Participation in the study was voluntary and all participants were sampled by a non-probabilistic sampling method. HCW who refused to participate or were not sampled might be seropositive for antibody against SARS-CoV-2. Besides, exclusion of the symptomatic cases at the point of study recruitment may underestimate the seroprevalence among HCW. Thirdly, we did not capture the dates of onset for HCW who reported symptoms in the month prior to study enrolment. Therefore, testing for SARS-CoV-2 antibody in a single sample may potentially miss any SARS-CoV-2 infected HCW yet to seroconvert. Nevertheless, all symptomatic HCW were required to report their symptoms to the OSH team for exposure risk assessment and further management. At that period of time, all symptomatic HCW with high-risk exposure (i.e. HCW's eyes, nose, or mouth were exposed while performing or present during aerosol-generating procedures or during which respiratory secretions are likely to be poorly controlled) were swabbed and excluded from work for at least seven days. As for those with medium-risk (i.e. HCW's mucous membranes or hands were exposed during prolonged close contact with a COVID-19 patient) and low-risk exposures (i.e. inconsistencies in adherence to PPE while in close contact with a COVID-19 patient), they were swabbed and excluded from work for at least three days. They were not allowed to return to work until the resolution of fever with improvement in respiratory symptoms and/or tested negative for at least two consecutive swab specimens collected 48 hours apart. Since all symptomatic participants had recovered during study recruitment, they were at least 3–14 days after symptom onset. Fourthly, as with other serological assays, the sVNT used in this study has only been validated in convalescent COVID-19 patients and healthy controls, and not yet in asymptomatic individuals. It has shown sensitivity of 91.3–100% and specificity of 100% in samples collected more than 14 days after onset of illness [12,13], and performed well in comparison with other established assays [14]. SARS-CoV-2 neutralizing antibody levels are positively correlated with disease severity [72,73]. As none of our HCW reported severe symptoms, the likelihood of false negative results cannot be excluded from this study. Fifth, not all infected individuals mount a detectable antibody responses against SARS-CoV-2. Furthermore, only one assay was used for the serological test in this study, although this measured responses to the receptor-binding domain of the spike protein, which is an important immunogenic target. Testing with other assays could have different results. Additionally, as the information of possible COVID-19 exposure and adherence to IPC measures were obtained via a self-administered questionnaire, we were unable to check if the subjects’ responses were true (e.g. whether actual reported case contact had COVID-19 is unknown). Lastly, we also recognize that the small sample size of this study may underestimate the magnitude of undiagnosed COVID-19 infection among the HCW.
Conclusion
This study suggests a low incidence of undetected COVID-19 infection among HCW who did not come to medical attention as confirmed cases during the first local wave of SARS-CoV-2 infection, even at facilities designated to handle COVID-19 patients and specimens for suspected cases. The local testing strategies during the study period were adequate to capture the SARS-CoV-2 infection burden among the HCW at that time. Although the low incidence of undiagnosed COVID-19 infection among the HCW could be attributable to the low transmission dynamics in the community during the study period, this study suggests the role of adherence to IPC measures and appropriate PPE usage in preventing SARS-CoV-2 transmission within healthcare facilities.
Author Contributors
Yuan Liang Woon, Yee Leng Lee, I-Ching Sam, Hani Mat Hussin, Chee Loon Leong, Suresh Kumar Chidambaram, Kalaiarasu M. Peariasamy and Pik Pin Goh conceptualized the study. All authors participated in the design of the study. Yee Leng Lee, Nor Aliya Ayub, Swarna Lata Krishnabahawan, June Fei Wen Lau, Ramani Subramaniam Kalianan, Raj Kumar Sevalingam, Azura Ramli and Chuan Huan Chuah involved in blood taking and data collection. Yoong Min Chong, Yoke Fun Chan and I-Ching Sam performed laboratory testing. Nor Aliya Ayub, Swarna Lata Krishnabahawan, June Fei Wen Lau, Ramani Subramaniam Kalianan, Raj Kumar Sevalingam, Azura Ramli, Chuan Huan Chuah, Yoong Min Chong and Yoke Fun Chan involved in data management. Yuan Liang Woon and Yee Leng Lee performed statistical analysis. Yuan Liang Woon, Yee Leng Lee, Nor Aliya Ayub, Swarna Lata Krishnabahawan, June Fei Wen Lau, Ramani Subramaniam Kalianan, Raj Kumar Sevalingam, Azura Ramli and Chuan Huan Chuah involved in interpretation of data. Yuan Liang Woon, Yee Leng Lee and Yoong Min Chong wrote the original draft. Yoke Fun Chan, I-Ching Sam, Hani Mat Hussin, Chee Loon Leong, Suresh Kumar Chidambaram, Kalaiarasu M. Peariasamy and Pik Pin Goh reviewed and edited the manuscript. All authors read and approved the final manuscript.
Data sharing statement
The dataset analyzed during the current study are available in the figshare repository at https://doi.org/10.6084/m9.figshare.12622703 [74]. The local PPE guidelines and questionnaire used in this study were submitted as supplementary files.
Declaration of Interests
None of the authors have any conflict of interest
Acknowledgments
Funding
Own institutional budget and the Fundamental Research Grant Scheme
Acknowledgments
We would like to thank the Director-General of Health, MOH Malaysia for his permission to publish this study. We would also like to acknowledge the Director of Sungai Buloh Hospital, Kuala Lumpur Hospital and National Public Health Laboratory for their support throughout the study conduct. We are also grateful to Professor Lin-Fa Wang, Duke-NUS Medical School, Singapore, and GenScript Biotech for providing the sVNT testing kits. The authors also wish to thank Mr. Muhammad Al Hafiz bin Hj Adnan and Dr. Tharmini A/P Ravi for their expert assistance in this study.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.lanwpc.2021.100105.
Appendix. Supplementary materials
References
- 1.Abdullah NH. Ministry of Health Malaysia; 2020. KPK press statement January 25, 2020 - detection of new cases infected by 2019 Coronavirus Novel (2019-nCoV) in Malaysia.https://kpkesihatan.com/2020/01/25/kenyataan-akhbar-kpk-25-januari-2020-pengesanan-kes-baharu-yang-disahkan-dijangkiti-2019-novel-coronavirus-2019-ncov-di-malaysia/ [cited 2020 June 3rd]. Available from: [Google Scholar]
- 2.WHO . World Health Organization; 2020. Naming the coronavirus disease (COVID-19) and the virus that causes it.https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it [cited 2020 Mac 30th]. Available from: [Google Scholar]
- 3.Reuters . New Straits Times; 2020. How Sri Petaling Tabligh became Southeast Asia's Covid-19 hotspot.https://www.nst.com.my/news/nation/2020/03/575560/how-sri-petaling-tabligh-became-southeast-asias-covid-19-hotspot [cited 2020 June 3rd]. Available from: [Google Scholar]
- 4.Abdullah NH. Ministry of Health Malaysia; 2020. KPK press statement Oct 14, 2020 - current situation of coronavirus infection 2019 (COVID-19) in Malaysia.https://kpkesihatan.com/2020/10/14/kenyataan-akhbar-kpk-14-oktober-2020-situasi-semasa-jangkitan-penyakit-coronavirus-2019-covid-19-di-malaysia/ [cited 2020 Oct 15th]. Available from: [Google Scholar]
- 5.Wu Z, McGoogan JM., Characteristics of and Important Lessons From the Coronavirus Disease (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention. JAMA. 2019;2020 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 6.Carl H, Jon B, Tom J. Center for Evidence-Based Medicine, University of Oxford; 2020. COVID-19: what proportion are asymptomatic?https://www.cebm.net/covid-19/covid-19-what-proportion-are-asymptomatic/ [cited 2020 June 3rd]. Available from: [Google Scholar]
- 7.Buitrago-Garcia D, Egli-Gany D, Counotte MJ, Hossmann S, Imeri H, Ipekci AM. Occurrence and transmission potential of asymptomatic and presymptomatic SARS-CoV-2 infections: a living systematic review and meta-analysis. PLoS Med. 2020;17(9) doi: 10.1371/journal.pmed.1003346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Furukawa NW, Brooks JT, Sobel J. Centers for Disease Control and Prevention; 2020. Evidence supporting transmission of severe acute respiratory syndrome coronavirus 2 while presymptomatic or asymptomatic.https://wwwnc.cdc.gov/eid/article/26/7/20-1595_article [cited 2020 June 3rd]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.WHO; 2020. Protocol for assessment of potential risk factors for 2019-novel coronavirus (COVID-19) infection among health care workers in a health care setting.https://www.who.int/publications-detail/protocol-for-assessment-of-potential-risk-factors-for-2019-novel-coronavirus-(2019-ncov)-infection-among-health-care-workers-in-a-health-care-setting [cited 2020 March 1st]. Available from: [Google Scholar]
- 10.MOH Malaysia; 2020. Guidelines COVID-19 management in Malaysia No.5/2020.http://covid-19.moh.gov.my/garis-panduan/garis-panduan-kkm (updated on 22 May 2020)[cited 2020 June 3rd]. Available from: [Google Scholar]
- 11.GenScript; 2020. SARS-CoV-2 surrogate virus neutralizationt test kit.https://www.genscript.com/cpass-sars-cov-2-neutralization-antibody-detection-Kit.html [cited 2020 June 2nd]. Available from: [Google Scholar]
- 12.Bond K, Nicholson S, Lim SM, Karapanagiotidis T, Williams E, Johnson D. Evaluation of serological tests for SARS-CoV-2: implications for serology testing in a low-prevalence setting. J Infect Dis. 2020;222(8):1280–1288. doi: 10.1093/infdis/jiaa467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan CW, Chia WN, Qin X, Liu P, Chen MI, Tiu C. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2-spike protein-protein interaction. Nat Biotechnol. 2020;38(9):1073–1078. doi: 10.1038/s41587-020-0631-z. [DOI] [PubMed] [Google Scholar]
- 14.Tan SS, Saw S, Chew KL, Huak CY, Khoo C, Pajarillaga A. Head-to-head evaluation on diagnostic accuracies of six SARS-CoV-2 serological assays. Pathology. 2020;52(7):770–777. doi: 10.1016/j.pathol.2020.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sam IC, Chong YM, Tan CW, Chan YF. Low postpandemic wave SARS-CoV-2 seroprevalence in Kuala Lumpur and Selangor, Malaysia. J Med Virol. 2020 doi: 10.1002/jmv.26426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.WHO COVID-19 case definition Geneva: WHO; 2020 [cited 2020 Dec 10th]. Available from: https://www.who.int/publications/i/item/WHO-2019-nCoV-Surveillance_Case_Definition-2020.1.
- 17.Pfordten D, Ahmad R. The Star; 2020. COVID-19: Cases up by 1,229, bringing total to 83,475 (updated daily) Malaysia.https://www.thestar.com.my/news/nation/2020/03/23/covid-19-current-situation-in-malaysia-updated-daily [cited 2020 Dec 13th]. Available from: [Google Scholar]
- 18.Ng OT, Marimuthu K, Koh V, Pang JX, Linn KZ, Sun J. SARS-CoV-2 seroprevalence and transmission risk factors among high-risk close contacts: a retrospective cohort study. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30833-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.KKMalaysia. Daily COVID-19 case trends and Rt (first and second wave), 22 January - 31 August 2020, Malaysia. Twitter: MOH 2020.
- 20.Ling HS, Pang IX, Fong AYY, Ong TK, Khiew NZ, Cham YL. COVID-19 antibody surveillance among healthcare workers in a non-COVID designated cardiology centre. Authorea. 2020 May 15. [Google Scholar]
- 21.Jacofsky D, Jacofsky EM, Jacofsky M. Understanding antibody testing for COVID-19. J Arthroplasty. 2020 doi: 10.1016/j.arth.2020.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu M, Cheng SZ, Xu KW, Yang Y, Zhu QT, Zhang H. Use of personal protective equipment against coronavirus disease 2019 by healthcare professionals in Wuhan, China: cross sectional study. BMJ. 2020;369:m2195. doi: 10.1136/bmj.m2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korth J, Wilde B, Dolff S, Anastasiou OE, Krawczyk A, Jahn M. SARS-CoV-2-specific antibody detection in healthcare workers in Germany with direct contact to COVID-19 patients. J Clin Virol : Off Publ Pan Am Soc Clin Virol. 2020;128 doi: 10.1016/j.jcv.2020.104437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alserehi HA, Alqunaibet AM, Al-Tawfiq JA, Alharbi NK, Alshukairi AN, Alanazi KH. Seroprevalence of SARS-CoV-2 (COVID-19) among healthcare workers in Saudi Arabia: comparing case and control hospitals. Diagn Microbiol Infect Dis. 2020;99(3) doi: 10.1016/j.diagmicrobio.2020.115273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iversen K, Bundgaard H, Hasselbalch RB, Kristensen JH, Nielsen PB, Pries-Heje M. Risk of COVID-19 in health-care workers in Denmark: an observational cohort study. Lancet Infect Dis. 2020;20(12):1401–1408. doi: 10.1016/S1473-3099(20)30589-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fujita K, Kada S, Kanai O, Hata H, Odagaki T, Satoh-Asahara N. Quantitative SARS-CoV-2 antibody screening of healthcare workers in the southern part of Kyoto city during the COVID-19 peri-pandemic period. Front Public Health. 2020 doi: 10.3389/fpubh.2020.595348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Self WH, Tenforde MW, Stubblefield WB, Feldstein LR, Steingrub JS, Shapiro NI. Seroprevalence of SARS-CoV-2 among frontline health care personnel in a multistate hospital network - 13 academic medical centers. MMWR. 2020;69(35):1121–1226. doi: 10.15585/mmwr.mm6935e2. April-June 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garcia-Basteiro AL, Moncunill G, Tortajada M, Vidal M, Guinovart C, Jimenez A. Seroprevalence of antibodies against SARS-CoV-2 among health care workers in a large Spanish reference hospital. Nat Commun. 2020;11 doi: 10.1038/s41467-020-17318-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moscola J, Sembajwe G, Jarrett M, Farber B, Chang T, McGinn T. Prevalence of SARS-CoV-2 antibodies in health care personnel in the New York city area. JAMA. 2020;324(9):893–895. doi: 10.1001/jama.2020.14765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rudberg AS, Havervall S, Manberg A, Jernbom Falk A, Aguilera K, Ng H. SARS-CoV-2 exposure, symptoms and seroprevalence in healthcare workers in Sweden. Nat Commun. 2020;11(1):5064. doi: 10.1038/s41467-020-18848-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galanis P, Vraka I, Fragkou D, Bilali A, Kaitelidou D. Seroprevalence of SARS-CoV-2 antibodies and associated factors in health care workers: a systematic review and meta-analysis. J Hosp Infect. 2020 doi: 10.1016/j.jhin.2020.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Worldometer. COVID-19 coronavirus pandemic 2020 [cited 2020 June 18th]. Available from: https://www.worldometers.info/coronavirus/#countries.
- 33.Hunter E, Price DA, Murphy E, van der Loeff IS, Baker KF, Lendrem D. First experience of COVID-19 screening of health-care workers in England. Lancet. 2020;395(10234):e77–ee8. doi: 10.1016/S0140-6736(20)30970-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Folgueira MD, Munoz-Ruiperez C, Alonso-Lopez MA, Delgado R. SARS-CoV-2 infection in Health Care Workers in a large public hospital in Madrid, Spain, during March 2020. medRxiv. 2020:2020.04.07.20055723.
- 35.Chaw L, Koh WC, Jamaludin SA, Naing L, Alikhan MF, Wong J. Analysis of SARS-CoV-2 transmission in different settings, Brunei. Emerg Infect Dis. 2020;26(11):2598–2606. doi: 10.3201/eid2611.202263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Y, He Y, Tong J, Qin Y, Xie T, Li J. Characterization of an asymptomatic cohort of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infected individuals outside of Wuhan, China. Clin Infect Dis : Off Publ Infect Dis Soc Am. 2020;71(16):2132–2138. doi: 10.1093/cid/ciaa629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koh WC, Naing L, Chaw L, Rosledzana MA, Alikhan MF, Jamaludin SA. What do we know about SARS-CoV-2 transmission? A systematic review and meta-analysis of the secondary attack rate and associated risk factors. PloS One. 2020;15(10) doi: 10.1371/journal.pone.0240205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lim BHS, Chidambara SK, Wong XC, Pathmanathan MD, Peariasamy KM, Hor CP. Clinical characteristics and risk factors for severe COVID-19 infections in Malaysia: a nationwide observational study. Lancet Reg Health West Pacif. 2020;4(100055) doi: 10.1016/j.lanwpc.2020.100055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pearce N, Lawlor DA, Brickley EB. Comparisons between countries are essential for the control of COVID-19. Int J Epidemiol. 2020;49(4):1059–1062. doi: 10.1093/ije/dyaa108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grant JJ, Wilmore SMS, McCann NS, Donnelly O, Lai RWL, Kinsella MJ. Seroprevalence of SARS-CoV-2 antibodies in healthcare workers at a London NHS Trust. Inf Control Hosp Epidemiol. 2020:1–3. doi: 10.1017/ice.2020.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Houlihan CF, Vora N, Byrne T, Lewer D, Kelly G, Heaney J. Pandemic peak SARS-CoV-2 infection and seroconversion rates in London frontline health-care workers. Lancet. 2020;396(10246):e6–e7. doi: 10.1016/S0140-6736(20)31484-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khalil A, Hill R, Wright A, Ladhani S, O'Brien P. SARS-CoV-2-specific antibody detection in healthcare workers in a UK maternity Hospital: correlation with SARS-CoV-2 RT-PCR results. Clin Infect Dis : Off Publ Infect Dis Soc Am. 2020 doi: 10.1093/cid/ciaa893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pallett SJC, Rayment M, Patel A, Fitzgerald-Smith SAM, Denny SJ, Charani E. Point-of-care serological assays for delayed SARS-CoV-2 case identification among health-care workers in the UK: a prospective multicentre cohort study. Lancet Resp Med. 2020;8(9):885–894. doi: 10.1016/S2213-2600(20)30315-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Poulikakos D, Sinha S, Kalra PA. SARS-CoV-2 antibody screening in healthcare workers in a tertiary centre in North West England. J Clin Virol : Off Publ Pan Am Soc Clin Virol. 2020;129 doi: 10.1016/j.jcv.2020.104545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shields A, Faustini SE, Perez-Toledo M, Jossi S, Aldera E, Allen JD. SARS-CoV-2 seroprevalence and asymptomatic viral carriage in healthcare workers: a cross-sectional study. Thorax. 2020;75(12):1089–1094. doi: 10.1136/thoraxjnl-2020-215414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thomas JP, Srinivasan A, Wickramarachchi CS, Dhesi PK, Hung YM, Kamath AV. Evaluating the national PPE guidance for NHS healthcare workers during the COVID-19 pandemic. Clin Med. 2020 doi: 10.7861/clinmed.2020-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gray S, Clough T, McGee Y, Murphy T, Poulikakos D. Increased risk of COVID-19 in haemodialysis healthcare workers in a tertiary centre in the North West of England. J Hosp Infect. 2020;106(2):390–391. doi: 10.1016/j.jhin.2020.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mortensen N. Royal College of Surgeons of England; 2020. RCS statement on latest Public Health England PPE guidance UK.https://www.rcseng.ac.uk/news-and-events/media-centre/press-releases/rcs-statement-on-latest-public-health-england-ppe-guidance/ [cited 2020 Dec 4th]. Available from: [Google Scholar]
- 49.Resuscitation Council UK; 2020. RCUK statement on PHE PPE guidance UK.https://www.resus.org.uk/about-us/news-and-events/rcuk-statement-phe-ppe-guidance [cited 2020 Dec 4th]. Available from: [Google Scholar]
- 50.Russel P. Medscape; 2020. Updated PPE guidance issued for UK health and care staff UK.https://medscape.com/viewarticle/928020 [cited 2020 Dec 4th]. Available from: [Google Scholar]
- 51.COVID-19: Infection prevention and control (IPC) United Kingdom Public Health England 2020 [cited 2020 Dec 4th]. Available from: https://www.gov.uk/government/publications/wuhan-novel-coronavirus-infection-prevention-and-control.
- 52.Ng K, Poon BH, Puar THK, Quah JLS, Wong YJ, Tan TY. 2020. COVID-19 and the risk to health care workers: a case report annals of internal medicine. March 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wong SC, Kwong RT, Wu TC, Chan JWM, Chu MY, Lee SY. Risk of nosocomial transmission of coronavirus disease 2019: an experience in a general ward setting in Hong Kong. J Hosp Infect. 2020 doi: 10.1016/j.jhin.2020.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alberto P, Milena F, Giulia B, Alberto S, Alberto G, Davie M. SARS-CoV-2 infection in healthcare workers: cross-sectional analysis of an otolaryngology unit. OTO J. 2020 [Google Scholar]
- 55.Canova V, Lederer Schlapfer H, Piso RJ, Droll A, Fenner L, Hoffmann T. Transmission risk of SARS-CoV-2 to healthcare workers -observational results of a primary care hospital contact tracing. Swiss Med Wkly. 2020;150:w20257. doi: 10.4414/smw.2020.20257. [DOI] [PubMed] [Google Scholar]
- 56.Jeremias A, Nguyen J, Levine J, Pollack S, Engellenner W, Thakore A. Prevalence of SARS-CoV-2 infection among health care workers in a tertiary community hospital. JAMA Internal Med. 2020;180(12):1707–1708. doi: 10.1001/jamainternmed.2020.4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barrett ES, Horton DB, Roy J, Gennaro ML, Brooks A, Tischfield J. Prevalence of SARS-CoV-2 infection in previously undiagnosed health care workers in New Jersey, at the onset of the U.S. COVID-19 pandemic. BMC Infect Dis. 2020;20(1):853. doi: 10.1186/s12879-020-05587-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nguyen LH, Drew DA, Graham MS, Joshi AD, Guo CG, Ma W. Risk of COVID-19 among front-line health-care workers and the general community: a prospective cohort study. Lancet Public health. 2020;5(9):e475–ee83. doi: 10.1016/S2468-2667(20)30164-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yon R. Moving forward - Service continuity plan during and post MCP for MOH hospitals, COVID-19 in Malaysia Update. 2020.
- 60.Malik AA, McFadden SM, Elharake J, Omer SB. Determinants of COVID-19 vaccine acceptance in the US. EClinicalMedicine. 2020;26 doi: 10.1016/j.eclinm.2020.100495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.De Filippo O, D'Ascenzo F, Angelini F, Bocchino PP, Conrotto F, Saglietto A. Reduced rate of hospital admissions for ACS during Covid-19 outbreak in northern Italy. N Engl J Med. 2020 doi: 10.1056/NEJMc2009166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ken T. Medscape; 2020. Hospital volumes slashed by more than half during pandemic.https://www.medscape.com/viewarticle/930345 [cited 2020 May 12th]. Available from: [Google Scholar]
- 63.Metzler B, Siostrzonek P, Binder RK, Bauer A, Reinstadler SJ. Decline of acute coronary syndrome admissions in Austria since the outbreak of COVID-19: the pandemic response causes cardiac collateral damage. Eur Heart J. 2020 doi: 10.1093/eurheartj/ehaa314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bongcac DC. CDN Digital; 2020. Cebu groups call to end discrimination, stigma against COVID-19 survivors, frontliners.https://cebudailynews.inquirer.net/305573/cebu-groups-call-to-end-discrimination-stigma-against-covid-19-survivors-frontliners [cited 2020 June 2nd]. Available from: [Google Scholar]
- 65.Kyodo . The Japan Times; 2020. Virus stigma driving Tokyo's front-line hospitals to edge of collapse. [cited 2020 June 2nd]. Available from: https://www.japantimes.co.jp/news/2020/04/25/national/tokyo-front-line-hospital/#.XtWkX2gzY2w. [Google Scholar]
- 66.Papasin HS. Respect frontliners, end stigma vs them Digital News Exchange; 2020 [cited 2020 June 2nd]. Available from: https://www.dnx.news/health/respect-frontliners-end-stigma-vs-them-usls-usg/.
- 67.Reuters . New Straits Times; 2020. Covid-19: Philippines health care workers suffer abuse, stigma.https://www.nst.com.my/world/world/2020/03/579698/covid-19-philippines-health-care-workers-suffer-abuse-stigma [cited 2020 June 2nd]. Available from: [Google Scholar]
- 68.Kluytmans-van den Bergh MFQ, Buiting AGM, Pas SD, Bentvelsen RG, van den Bijllaardt W, van Oudheusden AJG. Prevalence and clinical presentation of health care workers with symptoms of coronavirus disease 2019 in 2 Dutch hospitals during an early phase of the pandemic. JAMA Netw Open. 2020;3(5) doi: 10.1001/jamanetworkopen.2020.9673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee CY, Lin RTP, Renia L, Ng LFP. Serological approaches for COVID-19: epidemiologic perspective on surveillance and control. Front Immunol. 2020;11:879. doi: 10.3389/fimmu.2020.00879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Barker A. NEWS; March, 2020. Coronavirus COVID-19 cases spiked across Asia after a mass gathering in Malaysia. This is how it caught the countries by surprise.https://www.abc.net.au/news/2020-03-19/coronavirus-spread-from-malaysian-event-to-multiple-countries/12066092 [cited 2020 June 2nd]. Available from: [Google Scholar]
- 71.Our World in Data; 2020. Doubling time of total confirmed COVID-19 cases (3-day period)https://ourworldindata.org/grapher/doubling-time-of-covid-cases?country=~MYS [cited 2020 June 2nd]. Available from: [Google Scholar]
- 72.Chen W, Zhang J, Qin X, Wang WX, Xu MM, Wang LF. SARS-CoV-2 neutralizing antibody levels are correlated with severity of COVID-19 pneumonia. Biomed Pharmacother. 2020:130. doi: 10.1016/j.biopha.2020.110629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Meyer B, Reimerink J, Torriani G, Brouwer F, Godeke GJ, Yerly S. Validation and clinical evaluation of a SARS-CoV-2 surrogate virus neutralisation test (sVNT) Emerg Microbes Infect. 2020;9(1):2394–2403. doi: 10.1080/22221751.2020.1835448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Woon Yuan Liang, Lee Yee Leng, Yoong Min Chong, Ayub Nor Aliya, Krishnabahawan Swarna Lata, Lau June Fei Wen. Sociodemographic data and results of the surrogate virus neutralization test to detect anti-SARS-CoV-2 antibodies in asymptomatic healthcare workers. figshare. 2021 doi: 10.6084/m9.figshare.12622703. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.