Fig. 5.
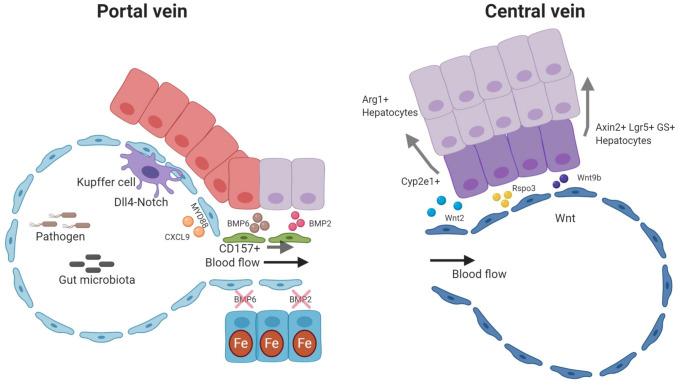
Angiocrine factors control liver function. The hepatic endothelium is not just a passive conduit for blood. Instead, it acts as instructive gatekeeper regulating the hepatic microenvironment through EC-derived angiocrine factors. Pericentral LSEC are a prime example of angiocrine signaling. They modulate hepatocytic function, by secreting angiocrine Wnt2, Wnt9b, and Rspo3 to establish the spatial division of labor of hepatocytes (“metabolic liver zonation”). Concurrently, angiocrine Wnt-signaling fuels Axin2+, Lgr5+, and glutamine synthase (GS)+ pericentral hepatocytes to maintain the pericentral niche during tissue homeostasis. Moreover, angiocrine Wnt-signaling plays a pivotal role in regulating xenobiotic functions by modulating the hepatic cytochrome activity such as CYP2E1. In the portal zone, hepatocytes are actively involved in gluconeogenesis and urea cycle with increased expression of arginase (Arg1). Portal vein EC and periportal LSEC have high Notch activity with upregulated Dll4 expression. Angiocrine DLL4 may orchestrate monocyte recruitment to establish a niche for KC. Likewise, the hepatic immune zonation is further maintained by LSEC driven CXCL9 and through MYD88 pathway. Periportal LSEC also serve as a niche for resident LSEC progenitors expressing CD157. Midlobular LSEC control iron homeostasis by secretion of the angiocrine factors BMP2 and BMP6