Abstract
Background:
The classic triad of fat embolism syndrome consists of pulmonary distress, mental status change, and petechial rash. Typically, symptoms manifest 24–48 hours after a long bone fracture, but case reports have demonstrated fat embolism syndrome without long bone fracture. These cases are initiated by a stress response, mobilizing free fatty acids into the circulation.
Case Description:
Herein, we present the case of a 70-year-old male who presented with the left-sided hemiparesis and was subsequently found to have tandem lesions of the right internal carotid artery (ICA) and right middle cerebral artery (MCA) warranting emergent mechanical thrombectomy (MT). The ensuing pathology report determined the source of ischemic stroke to be caused by fat embolism, a rare and intriguing case of cryptogenic large vessel occlusion (LVO) with unique features distinguishing it from other reports in the literature.
Conclusion:
According to the biochemical theory, a catecholamine surge can precipitate fat globules forming in the circulatory system, leading to tissue hypoxia, injury, and ischemia. While the majority of cerebral fat emboli cause reversible ischemia of small diameter vessels, our case presents with LVO and tandem lesions in both the ICA and MCA resulting in infarct and residual hemiparesis.
Keywords: Endovascular, Fat emboli, Interventional neurosurgery, Neurointervention, Thrombectomy

INTRODUCTION
Fat embolism, described in the literature as early as 1862, is characterized by the release of fat droplets into the systemic circulation, commonly as a complication of long bone fracture or following an orthopedic procedure. Less commonly, severe burns, infection, kidney transplant, liposuction, cardiopulmonary bypass, gastrectomy, and transfusions may result in fat emboli syndrome (FES).[11] More often than not, fat embolism occurs without significant clinical consequence.[4] Allardyce et al. identified fat globules circulating in the vasculature in 95% of asymptomatic patients following a femur fracture,[1] while only 0.15–4% of patients with long bone fractures develop clinical symptoms consistent with FES.[5,13,17,23] The classic triad of fat embolism syndrome consists of pulmonary distress, mental status change, and petechial rash, though a variety of symptoms (or lack thereof) may be present. Herein, we present the case of a 70-year-old male who presented with the left-sided hemiparesis and was subsequently found to have tandem lesions of the right internal carotid artery (ICA) and right middle cerebral artery (MCA) warranting emergent mechanical thrombectomy (MT). The ensuing pathology report determined the source of ischemic stroke to be a fat embolism, a rare and intriguing case of cryptogenic large vessel occlusion (LVO) with unique features distinguishing it from other reports in the literature.
CLINICAL PRESENTATION
A 70-year-old male with hypertension presented to the emergency department after being involved in a motor vehicle collision in the early afternoon. He awoke that morning with the left-sided weakness and impaired coordination. He initially proceeded to carry out his daily routine, until the time of the accident. Emergency medical services at the scene noted left facial droop and left upper extremity weakness, without other apparent injury, and initiated a stroke code. His time last known well (LNW) was 14 h before presentation. Baseline modified Rankin scale (MRS) was 0.
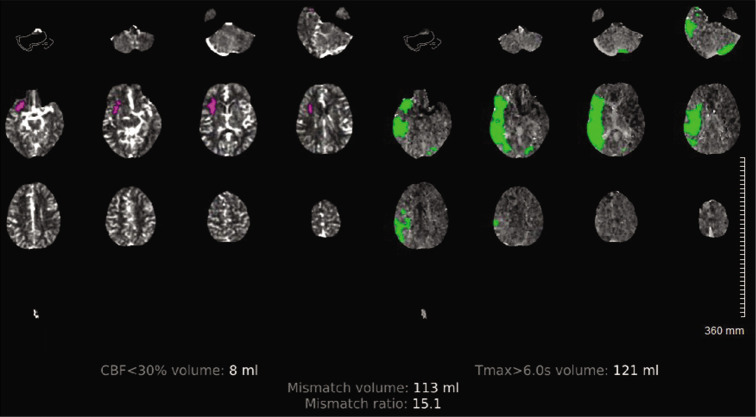
On arrival, blood pressure, heart rate, and oxygen saturation were within normal limits and National Institute of Health Stroke Scale (NIHSS) was calculated to be 4 for partial left facial droop and left upper extremity drift. No other injuries were identified. Computed tomography (CT) head without contrast demonstrated a hyperdense MCA [Figure 1]. CT angiography of the head and neck demonstrated abrupt vessel cutoff at the right ICA origin with reconstitution of the distal MCA territory through collateral arterial flow [Figure 2]. CT perfusion indicated decreased cerebral blood volume in the distal MCA territory with a core infarct of 8 cc and salvageable penumbra of 121 cc, with a mismatch volume of 113 cc and mismatch ratio of 15.5 [Figure 3]. No tPA thrombolysis was given as the patient presented 14 h from the LNW. Given the large volume of salvageable penumbra, the patient was consented and then taken for emergent diagnostic subtraction angiography with MT.
Figure 1:
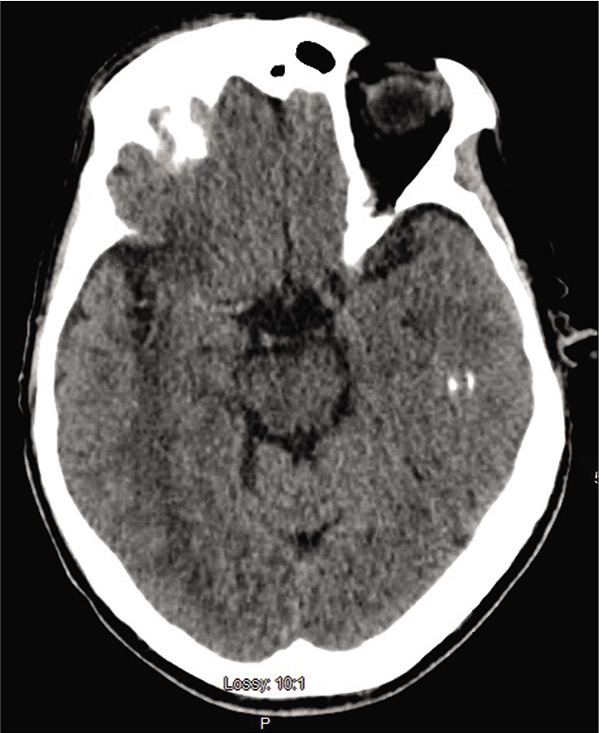
Initial axial noncontrast head computed tomography demonstrating a hyperdense right middle cerebral artery vessel and early ischemic changes in the insular region.
Figure 2:
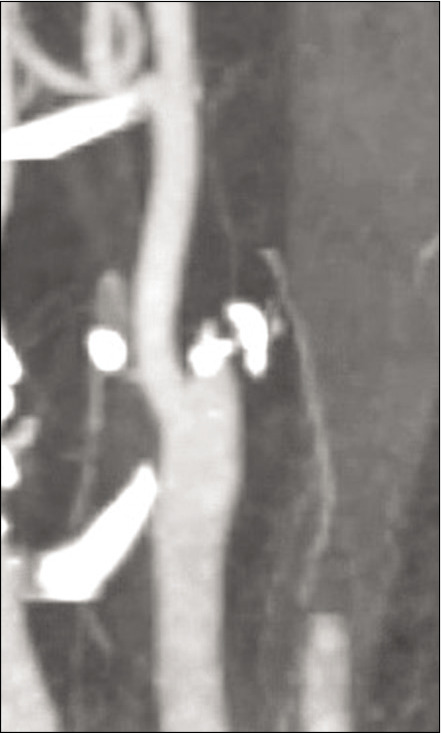
Computed tomography angiography of the neck demonstrating abrupt cutoff of the right internal carotid artery just distal to the bifurcation.
Figure 3:
Computed tomography perfusion demonstrating a small area of core infarct (left) with relatively large penumbra (right).
Intraoperatively, there was complete occlusion at the proximal right ICA [Figure 4]. After passing the carotid bulb with a microcatheter to the level of the carotid ICA, microinjection demonstrated absence of flow, suggesting an additional clot in the right MCA. A combination of balloon angioplasty and MT was used to recanalize the right ICA, but the proximal M1 remained occluded. Multiple passes with direct aspiration were attempted and thrombolysis in cerebral infarction-2B flow restoration was achieved without restoration of the M2 inferior division [Figure 5]. Thrombotic material was collected in the suction canister and sent for pathologic analysis [Figures 6 and 7]. Pathologic analysis demonstrated a degenerating thrombus with features suggestive of fat embolus. No evidence of malignancy was identified.
Figure 4:
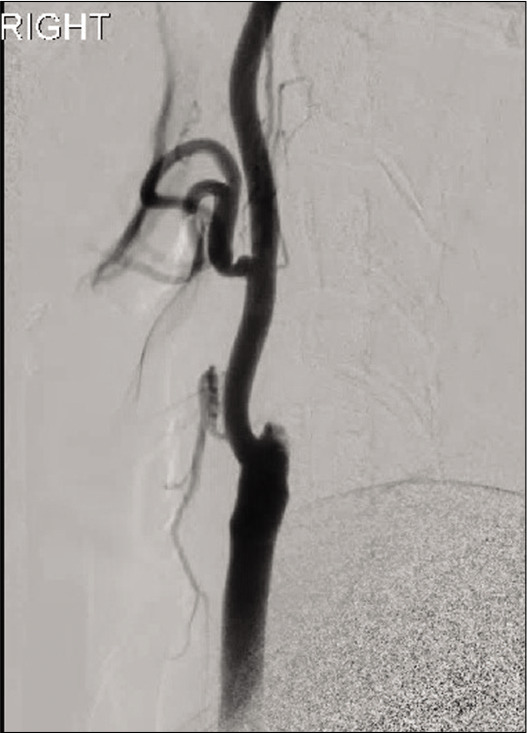
Initial right common carotid angiogram demonstrating abrupt cutoff of the right internal carotid artery just distal to the bifurcation.
Figure 5:
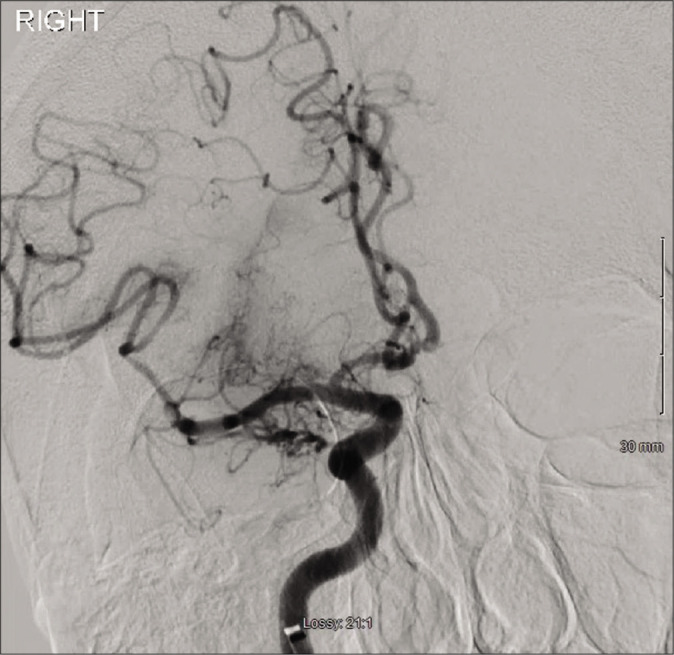
Final anterior-posterior angiogram of the right internal carotid artery. Thrombolysis in cerebral infarction-2B flow was reestablished with branch cutoff of the inferior M2 division noted.
Figure 6:
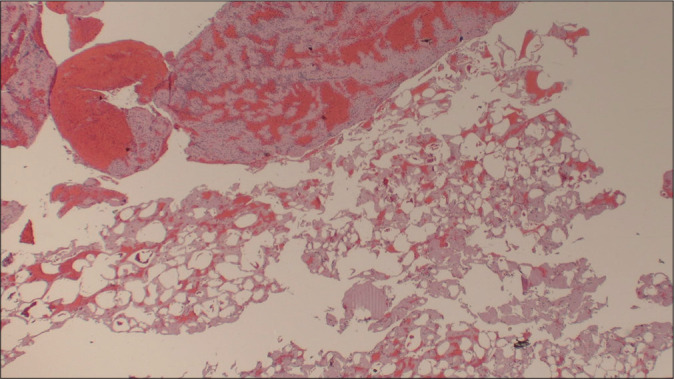
Hematoxylin and eosin stain (×20) shows large vacuolated spaces of microscopic fat globules.
Figure 7:
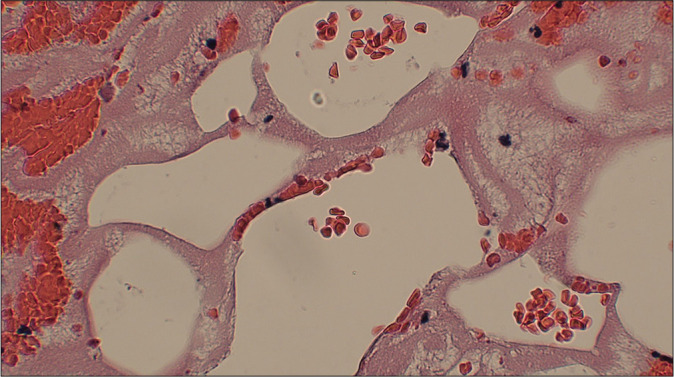
Hematoxylin and eosin stain (×100) with magnification of the fat globules with associated red blood cells with minimal hematopoietic cellularity, indicating that it is unlikely from bone marrow source.
The patient was monitored in the intensive care unit after the procedure. Magnetic resonance imaging (MRI) of the brain on postprocedure day 1 showed right MCA territory infarct [Figure 8]. The patient remained with an NIHSS score of 4 postprocedure with continued mild left-sided hemiparesis. He was subsequently discharged home on postprocedure day 8 with outpatient physical therapy and MRS of 1.
Figure 8:
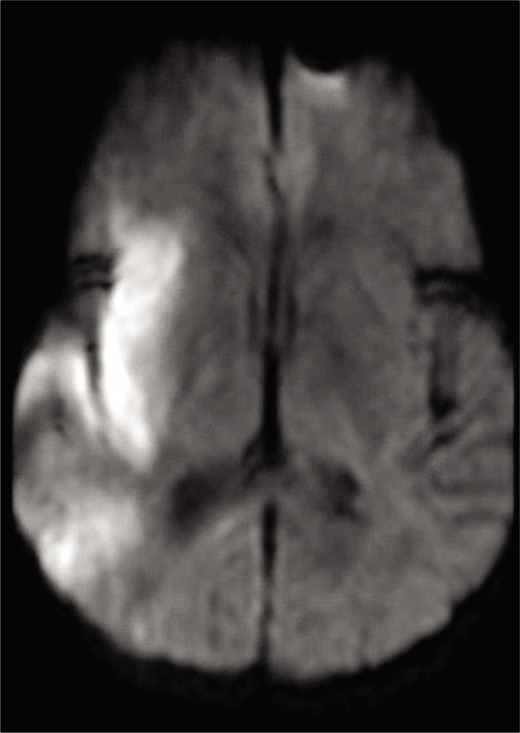
Axial magnetic resonance imaging brain, DWI sequence postprocedure demonstrating hyperintensity in the right middle cerebral artery territory, primarily in the insular region, consistent with restricted diffusion. There is some scattered hyperintensity posteriorly consistent with “starfield” pattern.
DISCUSSION
The case presented has several unique features that distinguish it from prior reports of FES described in the literature. While the classic triad of FES consists of pulmonary distress, mental status change, and petechial rash, a variety of symptoms (or lack thereof) may be present. Gurd and
Wilson described at least two major criteria or one major and four minor criteria to be presented to diagnose FES.[9] Major criteria consist of petechial rash, respiratory insufficiency, or cerebral involvement while minor criteria consist of fever, retinal changes, jaundice, renal signs, thrombocytopenia, anemia, high ESR, and fat macroglobulinemia.[9] Patients less frequently may also display anemia, coagulopathy, increased lipase, retinopathy, tachycardia, and pyrexia.[3] Our patient presents with isolated neurologic manifestations, which has only been reported once in the literature.[3] Neurologic manifestations from cerebral fat emboli can range from headache and confusion to stupor, rigidity, convulsions, and coma. These neurological deficits are usually accompanied by respiratory distress and a corporeal petechial rash. In this case, the patient interestingly presented with isolated left-sided hemiparesis and facial droop without other systemic findings.
Second, fat emboli typically affects small caliber vessels, while FES presenting as a LVO is exceedingly rare.[21] The only case report described in the literature is a case of LVO secondary to fat embolism attributed to dermal fillers harvested from a patient’s own adipose tissue and implanted into the face.[12] Given the rarity, other explanations of the vacuolated histology (presumed to be fat emboli) were explored. No case reports supported displaced adipose tissue or catheter or stent lubricant. One possible alternative explanation included fat from ruptured atheromatous plaque. However, foamy histiocytes (typically present with an atheromatous plaque) were not seen in our specimen.
In the case presented, the patient had no long bone fracture. While the majority FES reported in the literature is in association with long bone fracture, less common etiologies include severe burns, infection, kidney transplant, liposuction, cardiopulmonary bypass, gastrectomy, and transfusions.[11] Traditional understanding has been centered around the mechanical theory of fat embolism by which free fat particles from fractured bone marrow enter venous circulation at the site of the fracture and embolize to the intracranial vasculature either paradoxically through a cardiac defect such as a patent foramen ovale or through pulmonary arteriovenous shunting.[7] In the setting of other etiologies, it is apparent that the mechanical theory cannot explain the whole picture. The biochemical (or sedimentation) theory postulates that fat embolism syndrome is mediated by a stress response.[3] Catecholamines released in stress and trauma activate the adenylyl cyclase pathway which, in turn, activates lipase, hydrolyzing stored triglycerides to free fatty acids and glycerol that then enter systemic circulation.[2] Independent of the hypothesized mechanism, fat emboli generally arise from plasma fat when chylomicrons coalesce and fuse to form larger fat globules within the circulatory system.[13,16,18,19] Endothelial damage subsequently results from toxic free fat and capillary obstruction by fat globules, prompting release of vasoactive substances and platelet aggregation. In turn, occlusive emboli form manifesting in ischemia and infarct distal to the occlusion.[6,8] In our case, given no identifiable traditional preceding risk factor for fat embolus, we can postulate that our patient may have developed this LVO secondary to a stress response, bringing to light a rare case of fat embolism without preceding long bone trauma.
Despite an overall mortality rate of 7.7–36%,[23] the literature illustrates that cerebral dysfunction secondary to FES is often reversible in the majority of cases.[8,10,15,20] The number and size of the hyperintense lesions on MRI demonstrating ischemia or infarct are variable but directly correlate with the degree of neurologic injury, while indirectly correlating with probability of clinical recovery.[14,22] Fat emboli of very small diameter can cause reversible ischemia and usually only transient perivascular edema, typically providing opportunity for gradual recovery from cerebral dysfunction.[21] In our case, the patient experienced multifocal infarcts secondary to fat emboli restricting perfusion in LVO territory rather than traditional small diameter vessels. As such, our patient’s neurological recovery persisted in contrast to the majority of cases in the literature, highlighting the need for quick management of LVO no matter the etiology to prevent irreversible neurological injury.
CONCLUSION
Fat embolism syndrome typically presents with respiratory distress, mental status change, and petechial rash. While cerebral fat emboli in itself are not a clinical rarity, our case presents numerous unique aspects. Namely, our patient demonstrated isolated neurological manifestations in the absence of long bone fracture. According to the biochemical theory, a catecholamine surge can precipitate fat globules forming in the circulatory system, leading to tissue hypoxia, injury, and ischemia. While the majority of cerebral fat emboli cause reversible ischemia of small diameter vessels, our case presents with LVO and tandem lesions in both the ICA and MCA resulting in infarct and residual hemiparesis.
Footnotes
How to cite this article: Fowler JB, Fiani B, Sarhadi K, Cortez V. Cerebral fat embolism in the absence of a long bone fracture: A rare case report. Surg Neurol Int 2021;12:78.
Contributor Information
James B. Fowler, Email: james-fowler@augustana.edu.
Brian Fiani, Email: bfiani@outlook.com.
Kasra Sarhadi, Email: ksarhadi@uw.edu.
Vladimir Cortez, Email: vladimiradriancortez1@gmail.com.
Declaration of patient consent
Patient’s consent was not required as the identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Allardyce DB, Meek RN, Woodruff B, Cassim MM, Ellis D. Increasing our knowledge of the pathogenesis of fat embolism: A prospective study of 43 patients with fractured femoral shafts. J Trauma. 1974;14:955–62. [PubMed] [Google Scholar]
- 2.Baker PL, Pazell JA, Peltier LF. Free fatty acids, catecholamines, and arterial hypoxia in patients with fat embolism. J Trauma. 1971;11:1026–30. doi: 10.1097/00005373-197112000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Bardana D, Rudan J, Cervenko F, Smith R. Fat embolism syndrome in a patient demonstrating only neurologic symptoms. Can J Surg. 1998;41:398–402. [PMC free article] [PubMed] [Google Scholar]
- 4.Chang RN, Kim JH, Lee H, Baik HJ, Chung RK, Kim CH, et al. Cerebral fat embolism after bilateral total knee replacement arthroplasty-a case report. Korean J Anesthesiol. 2010;59:S207–10. doi: 10.4097/kjae.2010.59.S.S207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeFroda SF, Klinge SA. Fat embolism syndrome with cerebral fat embolism associated with long-bone fracture. Am J Orthop (Belle Mead NJ) 2016;45:E515–21. [PubMed] [Google Scholar]
- 6.Erdem E, Namer IJ, Saribas O, Aras T, Tan E, Bekdik C, et al. Cerebral fat embolism studied with MRI and SPECT. Neuroradiology. 1993;35:199–201. doi: 10.1007/BF00588493. [DOI] [PubMed] [Google Scholar]
- 7.Gossling HR, Pellegrini VD., Jr Fat embolism syndrome: A review of the pathophysiology and physiological basis of treatment. Clin Orthop Relat Res. 1982;165:68–82. [PubMed] [Google Scholar]
- 8.Gregorakos L, Sakayianni K, Hroni D, Harizopoulou V, Markou N, Georgiadou F, et al. Prolonged coma due to cerebral fat embolism: Report of two cases. J Accid Emerg Med. 2000;17:144–6. doi: 10.1136/emj.17.2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gurd AR, Wilson RI. The fat embolism syndrome. J Bone Joint Surg Br. 1974;56B:408–16. [PubMed] [Google Scholar]
- 10.Ibañez NC, Collado RN, Gaviña IS, de Medrano MG, Chicharro FM. Fat embolism syndrome without respiratory failure. Clin Intensive Care. 2011;10:101–3. [Google Scholar]
- 11.Ihn YK, Baik JH. Cerebral fat embolism as a rare complication of postgastrectomy: Case report. J Korean Radiol Soc. 2004;51:591–4. [Google Scholar]
- 12.Liu L, Yin M, Liu S, Hu M, Zhang B. Facial filler causes stroke after development of cerebral fat embolism. Lancet. 2020;395:449. doi: 10.1016/S0140-6736(20)30001-5. [DOI] [PubMed] [Google Scholar]
- 13.Muller C, Rahn BA, Pfister U, Meinig RP. The incidence, pathogenesis, diagnosis, and treatment of fat embolism. Orthop Rev. 1994;23:107–17. [PubMed] [Google Scholar]
- 14.Parchani A, Shaikh N, Bhat V, Kattren MA. Fat embolism syndrome: Clinical and imaging considerations: Case report and review of literature. Indian J Crit Care Med. 2008;12:32–6. doi: 10.4103/0972-5229.40948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parizel PM, Demey HE, Veeckmans G, Verstreken F, Cras P, Jorens PG, et al. Early diagnosis of cerebral fat embolism syndrome by diffusion-weighted MRI (starfield pattern) Stroke. 2001;32:2942–4. [PubMed] [Google Scholar]
- 16.Peltier LF. Fat embolism. Clin Orthop Relat Res. 2004;422:148–53. [Google Scholar]
- 17.Pinney SJ, Keating JF, Meek RN. Fat embolism syndrome in isolated femoral fractures: Does timing of nailing influence incidence? Injury. 1998;29:131–3. doi: 10.1016/s0020-1383(97)00154-x. [DOI] [PubMed] [Google Scholar]
- 18.Replogle RL. The nature of blood sludging, and its relationship to the pathophysiological mechanisms of trauma and shock. J Trauma. 1969;9:675–83. doi: 10.1097/00005373-196908000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Robb HJ. Microembolism in the pathophysiology of shock. Angiology. 2016;16:405–11. [Google Scholar]
- 20.Ryu CW, Lee DH, Kim TK, Kim SJ, Kim HS, Lee JH, et al. Cerebral fat embolism: Diffusion-weighted magnetic resonance imaging findings. Acta Radiol. 2005;46:528–33. doi: 10.1080/02841850510021481. [DOI] [PubMed] [Google Scholar]
- 21.Satoh H, Kurisu K, Ohtani M, Arita K, Okabayashi S, Nakahara T, et al. Cerebral fat embolism studied by magnetic resonance imaging, transcranial doppler sonography, and single photon emission computed tomography. J Trauma. 1997;43:345–8. doi: 10.1097/00005373-199708000-00023. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi M, Suzuki R, Osakabe Y, Asai JI, Miyo T, Nagashima G, et al. Magnetic resonance imaging findings in cerebral fat embolism. J Trauma. 1999;46:324–7. doi: 10.1097/00005373-199902000-00021. [DOI] [PubMed] [Google Scholar]
- 23.Tsai IT, Hsu CJ, Chen YH, Fong YC, Hsu HC, Tsai CH. Fat embolism syndrome in long bone fracture-clinical experience in a tertiary referral center in Taiwan. J Chin Med Assoc. 2010;73:407–10. doi: 10.1016/S1726-4901(10)70088-5. [DOI] [PubMed] [Google Scholar]