Abstract
Background:
Vasogenic edema in the setting of acute ischemic stroke can be attributed to the opening of transient receptor potential 4 channels, which are expressed in the setting of injury and regulated by sulfonylurea receptor 1 (SUR1) proteins. Glibenclamide, also known as glyburide, RP-1127, Cirara, and BIIB093, is a second-generation sulfonylurea that binds SUR1 at potassium channels and may significantly reduce cerebral edema following stroke, as evidenced by recent clinical trials. This review provides a comprehensive analysis of clinical considerations of glibenclamide use and current patient outcomes when administered in the setting of acute ischemic stroke to reduce severe edema.
Methods:
National databases (MEDLINE, EMBASE, Cochrane, and Google scholar databases) were searched to identify studies that reported on the clinical outcomes of glibenclamide administered immediately following acute ischemic stroke.
Results:
The pharmacological mechanism of glibenclamide was reviewed in depth as well as the known indications and contraindications to receiving treatment. Eight studies were identified as having meaningful clinical outcome data, finding statistically significant differences in glibenclamide treatment groups ranging from matrix metalloproteinase-9 serum levels, midline shift, modified Rankin Scores, National Institute of Health Stroke Score, and mortality endpoints.
Conclusion:
Studies analyzing the GAMES-Pilot and GAMES-PR trials suggest that glibenclamide has a moderate, however, measurable effect on intermediate biomarkers and clinical endpoints. Meaningful conclusions are limited by the small sample size of patients studied.
Keywords: BIIB093, Cerebral edema, Glibenclamide, Glyburide, Pharmacology

INTRODUCTION
Cerebral edema is a complication affecting over 70,000 patients in the United States with acute ischemic stroke each year, resulting in fatality rates approaching eighty percent.[26]
Cerebral edema results in the setting of increased intracranial cellular permeability due to cellular damage caused by stroke, head trauma, infection, intracranial lesions, or medication side effects.[46] Physiologically, brain tissue volume increases as edema induces intracellular swelling which then lowers perfusion to the brain, causing further damage to both intracellular and extracellular brain tissue.[39] Clinical presentation may be asymptomatic, yet if left untreated can lead to autonomic dysfunction, coma, and death from brainstem compression and herniation.[14] This intracranial edema can be attributed to the opening of transient receptor potential 4 channels (TRPM4), which are expressed in the setting of injury and regulated by sulfonylurea receptor 1 (SUR1) proteins.[2] Cell depolarization and resulting sodium influx, intracellular edema, and cell death follows.[39] Thus, blocking the SUR1-TRPM4 channel is advantageous in preventing edema following acute ischemic stroke, as they are transcriptionally upregulated in all neurovascular cells, co-assembling with aquaporin-4 channels causing cellular swelling of astrocytes.[2,33,43]
Glibenclamide, also known as glyburide, RP-1127, Cirara, and BIIB093, is a second-generation sulfonylurea drug that binds the SUR1 protein at potassium adenosine triphosphate channels (KATP). Although traditionally used to treat diabetes mellitus type 2, recent clinical trials have demonstrated efficacy in preventing cerebral edema in the setting of stroke. Studies have shown efficacy in inhibiting the SUR1-TRPM4 complex to prevent sodium and water influx into the cell and resulting edema.[13] Furthermore, glibenclamide has shown benefits in preserving the integrity of the vascular endothelium during cell damage, reducing edema, and inhibiting neuronal cell death from elevated intracranial pressure.[2,14,43] This review provides a comprehensive analysis of individual patient considerations, the pharmacological mechanism of glibenclamide, and outcomes of patients from clinical trials.
Patient selection
When severe or malignant edema is a complication of ischemic stroke, symptoms related to cerebral swelling specifically may be difficult to distinguish from residual deficits of stroke if eloquent regions of the brain have been affected from ischemia. Nevertheless, severe edema would be characterized by rapid decline in clinical picture, uncharacteristic of the stroke presentation. Some of the predictors and early signs of malignant edema may present as signs of increased intracranial pressure such as depressed consciousness, gaze palsy, and vomiting.[15] Other signs that are more specific for stroke-induced cerebral edema may be higher National Institutes of Health Stroke Scale (NIHSS) scores or the necessity for ventilation, which may be indicative of increased midline shift accompanied by herniation and respiratory depression.[45] Glibenclamide may be indicated in this setting and although the side effect profile of glibenclamide is minimal, some considerations are worth noting.
In the clinical pilot studies first investigating the use of glibenclamide for cerebral edema, hypoglycemia was a significant concern given the primary use of this pharmacotherapy for diabetes. However, despite close monitoring of blood glucose throughout the course of study, hypoglycemia was not reported to contribute to poorer outcomes in the setting of acute ischemic stroke.[6,31,32,35] However, complications of hypoglycemia have been described as being more pronounced in geriatric populations.[8] Furthermore, given that glibenclamide is a sulfonylurea, it is critical to screen for patients who may have a history of allergic reactions to sulfa drugs. Furthermore, those who are genetically susceptible to hemolytic anemia when exposed to sulfa drugs, such as patients with glucose-6-phosphate deficiency, should be avoided.[23,38] Conflicting reports of risk factors in patients with preexisting cardiovascular disease exist and although mortality is reportedly higher in cohorts of patients treated with glibenclamide compared to metformin in diabetes patients, clear contraindications are not currently defined.[6,22,28,38]
At present, there are no clear contraindications for use in pregnancy. Glibenclamide has been shown to cross the placenta in pregnancy and exposure to the drug in utero has been reported, however, the drug is digested by placental microsomes and toxicity in utero has not been reported.[19] Furthermore, the safety and efficacy of glibenclamide have not been clinically established in children and adolescents, however, both diabetes mellitus type 2 and ischemic stroke are exceeding rare in this age group. At present, the age requirement of the drug is not limited and patients in clinical trials have been treated from age 18 to 80 years old.[18,31] In the setting of hepatic diseases or renal impairment, glibenclamide is not contraindicated; however, initial and maintenance therapies should be decreased to reduce likelihood of hypoglycemia.[8,38] Given glibenclamide is metabolized by hepatic enzyme cytochrome P2C9, interactions with other ongoing therapies that induce or inhibit hepatic enzymes such as salicylates, sulfonamides, drugs containing fibric acid, warfarin, carbamazepine, phenobarbital, rifampin, St. John’s Wort, and dexamethasone may cause unwanted interaction and alter desired drug levels.[6,8,38] Weight gain is also a notable concern for diabetic patients on long-term therapy, however, not necessarily concerning for use in acute stroke.[6,8]
Recent study of development of glibenclamide for the prevention of cerebral edema following acute ischemic stroke has been primarily through intravenous (IV) administration.[18,30,31,33] However, oral application of glibenclamide has also been recently described in setting of ischemic stroke patient cohorts.[11,16] A significant limitation of oral administration is the time for absorption, estimated to be nine hours, which requires higher dosing and may have delayed effect, notwithstanding the practical limitation of a stroke patient having to swallow a pill.[11] In pilot studies on investigational use of glibenclamide for cerebral edema, the indication to start the IV drug administration, for study purposes was within 10 h of ischemic stroke.[37] Thus, IV administration may be desirable in acute setting due to immediate bioavailability, especially in cases when treatment is delayed several hours after stroke. Another important favorable aspect of glibenclamide is that many of the treatment modalities currently used to treat ischemic stroke do not conflict with administration of glibenclamide. The use of tissue plasminogen activator (tPA) has not been reported to significantly alter the indication of whether or not glibenclamide may be given, although some studies have suggested that matrix metalloproteinase-9 (MMP-9) serums levels may be elevated in the setting of tPA administration.[7,9] Furthermore, glibenclamide may be used in conjunction with aggressive osmotherapy, decompressive craniectomy, and other interventional therapies currently employed to reduce edema and herniation.[11,18,31-33]
Given that glibenclamide for the application of ischemic stroke is indicated on an emergent basis, it is important to screen for obvious contraindications before initiating treatment. However, as with any therapy, the benefits of life-saving treatment must be weighed against contraindications that may result in undesirable sequalae but are more concerning with long-term use.
Pharmacology
SUR1-TRPM4 channels, previously referred to as SUR1-regulated NCCa-ATP channels, have been shown to play a critical role in the formation of cytotoxic edema. Normally, such channels regulate against a pathological rise in intracellular calcium during brain injury; SUR1-TRPM4 channels also are sensitive to the intracellular concentration of ATP.[44] In cerebral vascular accidents, extreme depletion of ATP can result in persistent activation of SUR1-TRPM4 channels, allowing for unregulated sodium entry into the cell, resulting in depolarization, which can lead to necrotic cell death and cytotoxic edema.[35,37] The SUR1-TRPM4 channel is not constitutively expressed and thus is not expressed in healthy tissues.[44] SUR1-TRPM4 channels become transcriptionally upregulated in neurovascular structures and central nervous system (CNS) following cerebral ischemia or trauma.[1] The SUR1 protein has been shown to be transcriptionally upregulated in neurons, astrocytes, oligodendrocytes, and microvascular endothelial cells after focal ischemia.[24,34,36] Upregulation of SUR1 is paralleled by upregulation of TRPM4[21] and is associated with expression of increased SUR1-TRPM4 channels.[34] However, transcriptional upregulation of the SUR1-TRPM4 channels alone in CNS injury does not directly induce cell death.[35] Twelve glibenclamide acts as a potent inhibitor of SUR1-regulated channel activity.
In ischemic or traumatic brain cells, binding of glibenclamide to SUR1-TRPM4 reduces depolarization which reduced blood-brain barrier (BBB) leakage and the formation of cerebral edema.[35] Glibenclamide inhibits these channels by specifically targeting the regulatory subunit SUR1.[27] SUR1 receptors contain binding sites for first and second generation sulfonylurea drugs like glibenclamide and related compounds such as meglitinides, commonly used to treat diabetes mellitus type 2 and other related diseases.[35] In addition to SUR1-TRPM4 channels, glibenclamide has been known to inhibit four molecularly distinct KATP channels, SUR1-KIR6.2, SUR2A-KIR6.2, SUR2B-KIR6.2, and SUR2BKIR6.1, which are expressed by various cell types.[21,27,40,41] Most commonly, SUR1-KIR6.2 potassium channels regulate insulin secretion in pancreatic β cells.[12] The affinity of binding and the efficacy of inhibition are highest for SUR1-regulated channels than in SUR2-regulated channels.[27] SUR1 in SUR1-TRPM4 channels and in the KATP SUR1–Kir6.2 channels has the same pharmacological properties.[3] In addition, glibenclamide also inhibits the NOD-like receptor pyrin domain containing 3 (NLRP3) inflammasome, an effect that is independent of the potassium ATP channels or SUR which may further add to neuroinflammation.[47]
Physiologically, glibenclamide and other sulfonylureas do not accumulate in the brain, even though there are neurons that express KATP channels in the CNS.[42] Although the penetration of glibenclamide into the CNS is aided due to increased permeability after an ischemic insult, glibenclamide is a weak acid with high lipid solubility which increases its ability to penetrate the BBB at low pH.[25,34,35] This pharmacologic property allows for relatively low doses of glibenclamide, when administered intravenously, to obtain a therapeutic effect without major effects to the insulin section in the pancreas.[27,35]
MATERIALS AND METHODS
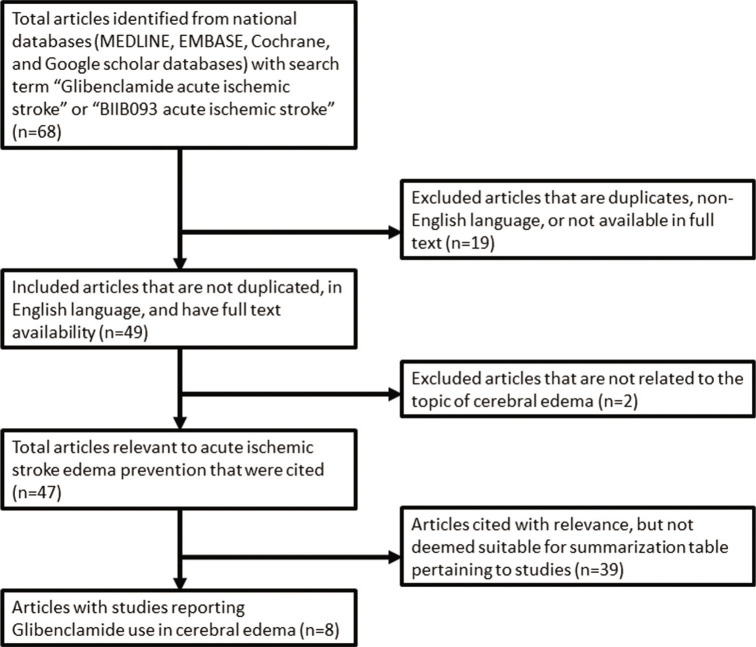
To identify investigational use of glibenclamide, a literature search of national databases was performed to identify studies that reported objective outcomes when used in the setting of stroke. Studies were also identified that could provide insight into the clinical use and specific indications of glibenclamide. Eight studies published over the past 6 years were identified as having meaningful data that looked at clinical outcomes of glibenclamide use in acute ischemic stroke [Figure 1]. Most studies identified were subgroup analyses of outcomes of patients enrolled in previous or ongoing clinical trials, listed in [Table 1].[32,31,17,33,18,43,10] A complete summary of studies included in this review may be seen in [Table 2]. Primary outcomes of interest in the literature surrounding cerebral edema, specifically in the setting of ischemic stroke, was (1) MMP-9 serum levels, (2) midline shift, (3) quality of life scores, and (4) mortality.
Figure 1:
PRISMA flowchart with the criteria for inclusion and exclusion for this review.
Table 1:
Clinical trials investigating use of glibenclamide in ischemic stroke.

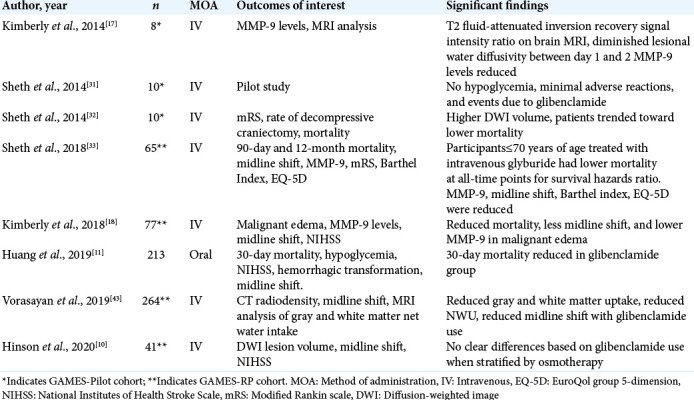
Table 2:
Studies reporting glibenclamide use in cerebral edema.
Trials and outcomes
Previous clinical studies have demonstrated that IV glibenclamide has resulted in reduction in plasma MMP-9 levels, which are typically elevated following stroke and indicate several stroke-related complications, particularly brain edema after stroke.[5,29,37] Exploratory analyses of the GAMES-Pilot study have suggested that IV glibenclamide reduces MMP-9 antigen levels, which can be measured in the serum and correlated with favorable clinical profile.[17] Thus, lower MMP-9 levels in the setting of stroke may be correlated with lower cerebral swelling and for this reason; MMP-9 was an important serum measure in the study of stroke patients treated with glibenclamide.[17,18,29,37] In a retrospective analysis of the GAMES-Pilot trial, Kimberly et al. compared levels of MMP-9 in patients treated with IV glibenclamide to a control cohort of similar large ischemic infarction. At 48 h post-stroke onset, the IV glibenclamide group had significantly reduced levels of MMP-9 in plasma samples using a quantitative sandwich ELISA (54 ± 17 ng/mL vs. 212 ± 151 ng/mL, P < 0.01). Kimberly et al. also noted that the levels of tissue inhibitor of metalloproteinase 1 (TIMP-1), a protein that circulates with MMP-9 to regulate it, were unchanged in the presence of IV glibenclamide.[17] This was suggestive of direct mechanism of lowering MMP-9 that did not depend on physiologic inhibitors. A similar analysis was completed by Kimberly et al. on the patient outcomes of the GAMES-PR trial.[18] The plasma MMP-9 was measured during the infusion period of 24–72 h and analyzed using a Wilcoxon rank sum test. Among those patients who experienced malignant edema (n = 36), IV glibenclamide was also associated with approximately 50% lower plasma level of MMP-9 (161 ng/mL vs. 335 ng/mL, P = 0.001) compared to matched controls. Another analysis of outcomes of the GAMES-PR patients by Sheth et al., specifically of subjects less than 70 years old at 12 months post-treatment, showed that treatment with IV glibenclamide resulted in lower concentrations of total serum MMP-9 compared to patients who were given placebo (189 ng/mL vs. 367 ng/mL, P = 0.001).[33] Interestingly, subgroup analysis of patient less than 70 years of age showed treatment with IV glibenclamide resulted in a 51% reduction in plasma MMP-9 levels.[33] This was noticeably larger than the 39% reduction observed in the overall cohort in the GAMES-RP trial which included patients from the age of 18–80, suggesting possible higher efficacy of glibenclamide in younger patients, specifically with respect to MMP-9 levels.
In further analyzing the changes in the brain with IV glibenclamide in the GAMES-RP cohort, Vorasayan et al. conducted a post-hoc exploratory analysis using a modified intention-to-treat sample.[43] This study was unique in that the primary outcomes of interest also included computed tomography (CT) radiodensity changes of gray and white matter to identify quantitative changes in radiodensity in addition to midline shift. The data considered in this analysis consisted of analyzing the CT scans of patients presenting with ischemic stroke over the first 7 days of hospital admission and considered radiodensity changes with a CT-derived water-uptake ratio measured at multiple portions gray and white matter sections of the brain.[43] Treatment with IV glibenclamide was associated with reduced water uptake ratio (β = −2.80; 95% CI, −5.07 to −0.53, P = 0.016) and reduced midline shift (β=−1.50; 95% CI, −2.71 to −0.28, p=0.016). Furthermore, Vorasayan et al. demonstrated that in the treatment arm of the study, this was evident in both gray and white matter (β = 0.15; 95% CI, 0.11–0.20; P < 0.001), however, gray matter water uptake ratio was correlated with more significant midline shift and mass effect than white matter (β = 0.08; 95% CI, 0.03–0.13; P = 0.001).[43] Thus, the finding of this analysis confirmed the utility of reduced cerebral edema due to IV glibenclamide CT radiodensity change demonstrating reduced water uptake ration in addition to midline shift.
Another important direct result of cerebral edema is reflected in the quantitative measure of midline shift, which describes displacement of cerebral structures laterally and has been correlated with diminished and often irreversible poor mental status, especially when measures such as decompressive craniectomy are not pursued.[4,20,39] Four studies were identified having been published on the degree of midline shift reduction in the setting of glibenclamide.[11,19,43] Three studies were published on patients included in the GAMES-RP trial and reviewed it using a post-hoc analysis. Kimberly et al. showed IV glibenclamide had approximately 50% reduction of midline shift compared with the placebo in all patients (4.6 mm vs. 12.4 mm, P = 0.001).[18] Another post-hoc analysis by Sheth et al. compared midline shift in the IV glibenclamide group with placebo groups, finding statistical reduction of midline shift in the treatment group at 72–96 h specifically in patient less than 70 years of age (4.7 mm vs. 9.0 mm, P = 0.001).[33] Finally, Vorasayan et al. considered the degree of midline shift in association with other factors such as CT-derived water uptake ratio of both white and gray matter regions and progressive degree of change over initial time points leading up to 96 h. When analyzed over time, patients treated with IV glibenclamide demonstrated statistical reduction over six equally distributed time points over the four initial days following ischemic stroke (β = −1.50; 95% CI, −2.71 to −0.28, P = 0.016).[43]
Another study was identified investigating the use of glibenclamide similarly in acute ischemic stroke, however, administered orally, not IV.[11] Notably, this did not include patient from either the GAMES-Pilot or GAMES-RP clinical trials, rather a new exploratory analysis.[11] Although Huang et al. failed to find statistical difference in treatment arms in midline shift, the mean reported midline shift was lower in the Huang study (2.4 mm and 2.9 mm in the control and treatment, respectively) than reported values in the GAMES trial cohorts. The degree of seemingly different values of midline shift was not attributable to the methods of measurement, as both studies used deviation of the septum pellucidum to characterize shift. Thus, it is possible that the degree of cerebral swelling may have been less severe the Huang study compared with the GAMES trials. Thus, less severe cerebral edema in the Huang study may have contributed to lack of adequate detection of significant effect. Thus, while a comparison of oral and IV methods of administration would be helpful, differences and heterogeneity between the studies would not be meaningful. Some of the self-reported weaknesses of this study included the small number of subjects, time window of administration that glibenclamide was administered as the oral form has been reported to have roughly 9-h delay due to delay in gastrointestinal absorption of the oral form, and dosing that was adapted from IV preparations.[11] Thus, while their study failed to demonstrate statistical significance in terms of midline shift, the study was found to have significant contribution understanding the efficacy glibenclamide, in terms of oral preparation.[11] To our knowledge and review of literature, this is the only study to report outcomes of oral glibenclamide in the setting of acute ischemic stroke.
In the discussion of long-term outcomes and quality of life differences that may be apparent due to treatment with glibenclamide, four studies investigated the functional outcomes of patients who suffered ischemic stroke and were subsequently treated with glibenclamide compared with placebo controls. Outcomes of interest included a modified Rankin Scale (mRS), Barthel index, EuroQol group 5-dimension (EQ-5D), NIHSS, and finally mortality. Sheth et al. described the outcomes of patients included in the GAMES-Pilot cohort of ten patients in terms of 90-day mRS compared with matched controls, finding that patient’s treatment with glibenclamide has a higher proportion of 0–4 mRS (P = 0.049, Fisher’s exact test).[31,32] However, changes in NIHSS and other intervals of mRS were not found to be statistically significant. In analyzing GAMES-RP patients, functional outcome was more likely among patients who received IV glibenclamide compared to the placebo group at 90 days (COR, 2.49; 95% CI, 1.02–6, P = 0.05).[33] At the 12-month interval, there was minimal reduction of the effect size (COR, 2.24; 95% CI, 0.92–5.46; P = 0.08).[33] After adjusting for age, the mRS scores favored the use of IV glibenclamide at 90 days (adjusted COR, 2.31; 95% CI, 0.93–5.72, P = 0.07) and 12 months (adjusted COR, 2.11; 95% CI, 0.86–5.18, P = 0.10).[33] Furthermore, patients in the glibenclamide group had higher Barthel index scores (95% CI, 2.1–37; P = 0.03) and higher EQ-5D scores 5D (95% CI, 0.001–0.249; P = 0.05) at 90-day, 6-month, and 12-month time points.[33] In a secondary analysis of the GAMES-RP cohort, Kimberly et al. considered NIHSS scores while correcting the potential confounders of decompressive craniectomy.[18] In the GAMES-RP cohort, there were more decompressive craniectomy cases than the placebo group. Despite correction of decompressive surgical intervention, fewer patients treated with IV glibenclamide still had an increase in NIHSS score ≥4 before craniectomy or day 3 (n = 5 [12%] vs. n = 11 [31%] in placebo, P = 0.048).[18] Of patients who were defined as having malignant edema, the rates of clinical deterioration based on NIHSS were lower based in those treated with IV glibenclamide (P < 0.01).[18] Finally, in the analysis of the 213 patients suffering acute stroke by Huang et al., mRS of 0–4 at 6 months was shown to be statistically significant in both a total cohort and propensity matched analysis (P = 0.001 and P = 0.044, respectively).[11]
With regard to mortality, glibenclamide was not shown to reduce mortality in the GAMES-Pilot study; however, statistically significant difference in mortality was achieved in analysis of the GAMES-RP cohort.[18,31,32] Sheth et al. showed that mortality at 12 months 5/35 (14%) in the IV glibenclamide treatment group and 12/30 (40%) in the placebo group.[33] In a different analysis, Kimberly et al. considered edema related deaths at 30 days finding that IV glibenclamide treatment had lower mortality compared with placebo (1 vs. 8 deaths, P = 0.010, fishers exact test).[18] When adjusting for patients receiving decompressive craniectomy, IV glibenclamide remained independently associated with lower mortality (odds ratio = 0.09, 95% CI 0.01–0.74, P = 0.026).[18] Furthermore, a Kaplan–Meier survival analysis showed that IV glibenclamide was effective in reducing likelihood of edema-related deaths compared to placebo groups within 30 days of suffering stroke (P = 0.009).[18] In the Huang study, 30-day mortality was shown to be reduced in the glibenclamide treatment group in the unmatched cohort (P = 0.011) but failed to show statistical significance in the propensity matched cohort.[11]
CONCLUSION
This review provides, to date, the largest and most comprehensive review of the use of glibenclamide for use in acute ischemic stroke with analysis of measurable outcomes of MMP-9, midline shift, mRS, NIHSS, and mortality. Literature not included in this analysis was minimal and reported on the investigational use of glibenclamide in the setting of traumatic brain injury, which was not reviewed in depth.[13,16] The collection of studies in this review promotes the notion of glibenclamide as an effective addition to current therapies that may be used to reduce cerebral edema. Although there are extensive subgroup analyses of the different aspects of glibenclamide use in ischemic stroke as evidenced by the studies included in this review, the limited number of patients certainly remains a limiting factor in drawing meaningful conclusion and demonstrating broad clinical efficacy. As future studies are published on the results of glibenclamide use in ongoing phase III trials (CHARM), current results from the GAMES-Pilot and GAMES-PR trials suggest that glibenclamide has a moderate, however, measurable effect on intermediate biomarker and clinical endpoints.
Footnotes
How to cite this article: Griepp DW, Lee J, Moawad CM, Davati C, Runnels J, Fiani B. BIIB093 (intravenous glibenclamide) for the prevention of severe cerebral edema. Surg Neurol Int 2021;12:80.
Contributor Information
Daniel W. Griepp, Email: dgriepp@nyit.edu.
Jason Lee, Email: jlee98@nyit.edu.
Christina M. Moawad, Email: cmoawad2@illinois.edu.
Cyrus Davati, Email: cdavati@nyit.edu.
Juliana Runnels, Email: jmrunnels@salud.unm.edu.
Brian Fiani, Email: bfiani@outlook.com.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Arundine M, Tymianski M. Molecular mechanisms of calcium-dependent neurodegeneration in excitotoxicity. Cell Calcium. 2003;34:325–37. doi: 10.1016/s0143-4160(03)00141-6. [DOI] [PubMed] [Google Scholar]
- 2.da Costa BB, Windlin IC, Koterba E, Yamaki VN, Rabelo NN, Solla DJ, et al. Glibenclamide in aneurysmatic subarachnoid hemorrhage (GASH): Study protocol for a randomized controlled trial. Trials. 2019;20:413. doi: 10.1186/s13063-019-3517-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ding J, Yuan F, Guo JY, Chen H, Tian HL. Influence of glibenclamide on outcome in patients with Type 2 diabetes and traumatic brain injury. Clin Neurol Neurosurg. 2013;115:2166–9. doi: 10.1016/j.clineuro.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 4.Eichler AF, Chung E, Kodack DP, Loeffler JS, Fukumura D, Jain RK. The biology of brain metastases-translation to new therapies. Nat Rev Clin Oncol. 2011;8:344–56. doi: 10.1038/nrclinonc.2011.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foerch C, Montaner J, Furie KL, Ning MM, Lo EH. Invited article: Searching for oracles? Blood biomarkers in acute stroke. Neurology. 2009;73:393–9. doi: 10.1212/WNL.0b013e3181b05ef9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gangji AS, Cukierman T, Gerstein HC, Goldsmith CH, Clase CM. A systematic review and meta-analysis of hypoglycemia and cardiovascular events: A comparison of glyburide with other secretagogues and with insulin. Diabetes Care. 2007;30:389–94. doi: 10.2337/dc06-1789. [DOI] [PubMed] [Google Scholar]
- 7.Gerzanich V, Kwon MS, Woo SK, Ivanov A, Simard JM. SUR1-TRPM4 channel activation and phasic secretion of MMP-9 induced by tPA in brain endothelial cells. PLoS One. 2018;13:e0195526. doi: 10.1371/journal.pone.0195526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glibenclamide: A review. Drugs. 1971;1:116–40. doi: 10.2165/00003495-197101020-00002. [DOI] [PubMed] [Google Scholar]
- 9.Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–29. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 10.Hinson HE, Sun E, Molyneaux BJ, von Kummer R, Demchuk A, Romero J, et al. Osmotherapy for malignant cerebral edema in a phase 2 prospective, double blind, randomized, placebo-controlled study of IV glibenclamide. J Stroke Cerebrovasc Dis. 2020;29:104916. doi: 10.1016/j.jstrokecerebrovasdis.2020.104916. [DOI] [PubMed] [Google Scholar]
- 11.Huang K, Hu Y, Wu Y, Ji Z, Wang S, Lin Z, et al. Exploratory analysis of oral glibenclamide in acute ischemic stroke. Acta Neurol Scand. 2019;140:212–8. doi: 10.1111/ane.13134. [DOI] [PubMed] [Google Scholar]
- 12.Jha RM, Desai SM, Zusman BE, Koleck TA, Puccio AM, Okonkwo DO, et al. Downstream TRPM4 polymorphisms are associated with intracranial hypertension and statistically interact with ABCC8 polymorphisms in a prospective cohort of severe traumatic brain injury. J Neurotrauma. 2019;36:1804–17. doi: 10.1089/neu.2018.6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jha RM, Puccio AM, Chou SH, Chang CH, Wallisch JS, Molyneaux BJ, et al. Sulfonylurea receptor-1: A novel biomarker for cerebral edema in severe traumatic brain injury. Crit Care Med. 2017;45:e255–64. doi: 10.1097/CCM.0000000000002079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jha SK. Cerebral edema and its management. Med J Armed Forces India. 2003;59:326–31. doi: 10.1016/S0377-1237(03)80147-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawoos U, McCarron RM, Auker CR, Chavko M. Advances in intracranial pressure monitoring and its significance in managing traumatic brain injury. Int J Mol Sci. 2015;16:28979–97. doi: 10.3390/ijms161226146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khalili H, Derakhshan N, Niakan A, Ghaffarpasand F, Salehi M, Eshraghian H, et al. Effects of oral glibenclamide on brain contusion volume and functional outcome of patients with moderate and severe traumatic brain injuries: A randomized double-blind placebo-controlled clinical trial. World Neurosurg. 2017;101:130–6. doi: 10.1016/j.wneu.2017.01.103. [DOI] [PubMed] [Google Scholar]
- 17.Kimberly WT, Battey TW, Pham L, Wu O, Yoo AJ, Furie KL, et al. Glyburide is associated with attenuated vasogenic edema in stroke patients. Neurocrit Care. 2014;20:193–201. doi: 10.1007/s12028-013-9917-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kimberly WT, Bevers MB, von Kummer R, Demchuk AM, Romero JM, Elm JJ, et al. Effect of IV glyburide on adjudicated edema endpoints in the GAMES-RP Trial. Neurology. 2018;91:e2163–9. doi: 10.1212/WNL.0000000000006618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kimber-Trojnar Z, Marciniak B, Patro-Malysza J, SkorzynskaDziduszko K, Poniedzialek-Czajkowska E, Mierzynski R, et al. Is glyburide safe in pregnancy? Curr Pharm Biotechnol. 2014;15:100–12. doi: 10.2174/1389201015666140330200254. [DOI] [PubMed] [Google Scholar]
- 20.Liang D, Bhatta S, Gerzanich V, Simard JM. Cytotoxic edema: Mechanisms of pathological cell swelling. Neurosurg Focus. 2007;22:E2. doi: 10.3171/foc.2007.22.5.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loh KP, Ng G, Yu CY, Fhu CK, Yu D, Vennekens R, et al. TRPM4 inhibition promotes angiogenesis after ischemic stroke. Pflugers Arch. 2014;466:563–76. doi: 10.1007/s00424-013-1347-4. [DOI] [PubMed] [Google Scholar]
- 22.Mannucci E, Monami M, Candido R, Pintaudi B, Targher G. SID-AMD Joint Panel for Italian Guidelines on Treatment of Type 2 Diabetes, Effect of insulin secretagogues on major cardiovascular events and all-cause mortality: A meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis. 2020;30:1601–8. doi: 10.1016/j.numecd.2020.05.032. [DOI] [PubMed] [Google Scholar]
- 23.Mehta AB. Glucose-6-phosphate dehydrogenase deficiency. Postgrad Med J. 1994;70:871–7. doi: 10.1136/pgmj.70.830.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mehta RI, Ivanova S, Tosun C, Castellani RJ, Gerzanich V, Simard JM. Sulfonylurea receptor 1 expression in human cerebral infarcts. J Neuropathol Exp Neurol. 2013;72:871–83. doi: 10.1097/NEN.0b013e3182a32e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ortega FJ, Gimeno-Bayon J, Espinosa-Parrilla JF, Carrasco JL, Batlle M, Pugliese M, et al. ATP-dependent potassium channel blockade strengthens microglial neuroprotection after hypoxia-ischemia in rats. Exp Neurol. 2012;235:282–96. doi: 10.1016/j.expneurol.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 26.Pallan TV, Ahmed I. Glyburide in treating malignant cerebral edema, blocking sulfonyl urea one (SUR1) receptors. J Vasc Interv Neurol. 2014;7:23–5. [PMC free article] [PubMed] [Google Scholar]
- 27.Pergakis M, Badjatia N, Chaturvedi S, Cronin CA, Kimberly WT, Sheth KN, et al. BIIB093 (IV glibenclamide): An investigational compound for the prevention and treatment of severe cerebral edema. Expert Opin Investig Drugs. 2019;28:1031–40. doi: 10.1080/13543784.2019.1681967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raee MR, Nargesi AA, Heidari B, Mansournia MA, Larry M, Rabizadeh S, et al. All-cause and cardiovascular mortality following treatment with metformin or glyburide in patients with Type 2 diabetes mellitus. Arch Iran Med. 2017;20:141–6. [PubMed] [Google Scholar]
- 29.Serena J, Blanco M, Castellanos M, Silva Y, Vivancos J, Moro MA, et al. The prediction of malignant cerebral infarction by molecular brain barrier disruption markers. Stroke. 2005;36:1921–6. doi: 10.1161/01.STR.0000177870.14967.94. [DOI] [PubMed] [Google Scholar]
- 30.Sheth KN, Elm JJ, Molyneaux BJ, Hinson H, Beslow LA, Sze GK, et al. Safety and efficacy of intravenous glyburide on brain swelling after large hemispheric infarction (GAMES-RP): A randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2016;15:1160–9. doi: 10.1016/S1474-4422(16)30196-X. [DOI] [PubMed] [Google Scholar]
- 31.Sheth KN, Kimberly WT, Elm JJ, Kent TA, Mandava P, Yoo AJ, et al. Pilot study of intravenous glyburide in patients with a large ischemic stroke. Stroke. 2014;45:281–3. doi: 10.1161/STROKEAHA.113.003352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sheth KN, Kimberly WT, Elm JJ, Kent TA, Yoo AJ, Thomalla G, et al. Exploratory analysis of glyburide as a novel therapy for preventing brain swelling. Neurocrit Care. 2014;21:43–51. doi: 10.1007/s12028-014-9970-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sheth KN, Petersen NH, Cheung K, Elm JJ, Hinson HE, Molyneaux BJ, et al. Long-term outcomes in patients aged ≤70 years with intravenous glyburide from the Phase II GAMES-RP study of large hemispheric infarction: An exploratory analysis. Stroke. 2018;49:1457–63. doi: 10.1161/STROKEAHA.117.020365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simard JM, Chen M, Tarasov KV, Bhatta S, Ivanova S, Melnitchenko L, et al. Newly expressed SUR1-regulated NC(Ca-ATP) channel mediates cerebral edema after ischemic stroke. Nat Med. 2006;12:433–40. doi: 10.1038/nm1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simard JM, Sheth KN, Kimberly WT, Stern BJ, del Zoppo GJ, Jacobson S, et al. Glibenclamide in cerebral ischemia and stroke. Neurocrit Care. 2014;20:319–33. doi: 10.1007/s12028-013-9923-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simard JM, Tsymbalyuk N, Tsymbalyuk O, Ivanova S, Yurovsky V, Gerzanich V. Glibenclamide is superior to decompressive craniectomy in a rat model of malignant stroke. Stroke. 2010;41:531–7. doi: 10.1161/STROKEAHA.109.572644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simard JM, Woo SK, Gerzanich V. Transient receptor potential melastatin 4 and cell death. Pflugers Arch. 2012;464:573–82. doi: 10.1007/s00424-012-1166-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sola D, Rossi L, Schianca GP, Maffioli P, Bigliocca M, Mella R, et al. Sulfonylureas and their use in clinical practice. Arch Med Sci. 2015;11:840–8. doi: 10.5114/aoms.2015.53304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sorby-Adams AJ, Marcoionni AM, Dempsey ER, Woenig JA, Turner RJ. The role of neurogenic inflammation in blood-brain barrier disruption and development of cerebral oedema following acute central nervous system (CNS) injury. Int J Mol Sci. 2017;18:1788. doi: 10.3390/ijms18081788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Szeto V, Chen NH, Sun HS, Feng ZP. The role of KATP channels in cerebral ischemic stroke and diabetes. Acta Pharmacol Sin. 2018;39:683–94. doi: 10.1038/aps.2018.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tinker A, Aziz Q, Li Y, Specterman M. ATP-sensitive potassium channels and their physiological and pathophysiological roles. Compr Physiol. 2018;8:1463–511. doi: 10.1002/cphy.c170048. [DOI] [PubMed] [Google Scholar]
- 42.Tomiyama Y, Brian JE, Jr, Todd MM. Cerebral blood flow during hemodilution and hypoxia in rats: Role of ATP-sensitive potassium channels. Stroke. 1999;30:1942–7. doi: 10.1161/01.str.30.9.1942. discussion 1947-8. [DOI] [PubMed] [Google Scholar]
- 43.43. Vorasayan P, Bevers MB, Beslow LA, Sze G, Molyneaux BJ, Hinson HE, et al. Intravenous glibenclamide reduces lesional water uptake in large hemispheric infarction. Stroke. 2019;50:3021–7. doi: 10.1161/STROKEAHA.119.026036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Woo SK, Kwon MS, Ivanov A, Gerzanich V, Simard JM. The sulfonylurea receptor 1 (Sur1)-transient receptor potential melastatin 4 (Trpm4) channel. J Biol Chem. 2013;288:3655–67. doi: 10.1074/jbc.M112.428219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu S, Yuan R, Wang Y, Wei C, Zhang S, Yang X, et al. Early prediction of malignant brain edema after ischemic stroke. Stroke. 2018;49:2918–27. doi: 10.1161/STROKEAHA.118.022001. [DOI] [PubMed] [Google Scholar]
- 46.Yang Y, Rosenberg GA. Blood-brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke. 2011;42:3323–8. doi: 10.1161/STROKEAHA.110.608257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang G, Lin X, Zhang S, Xiu H, Pan C, Cui W. A protective role of glibenclamide in inflammation-associated injury. Mediators Inflamm. 2017;2017:3578702. doi: 10.1155/2017/3578702. [DOI] [PMC free article] [PubMed] [Google Scholar]