Supplemental Digital Content is available in the text.
Keywords: death, health status, heart failure, hospitalization, transcatheter aortic valve replacement
Background:
Health status assessment is essential for documenting the benefit of transcatheter aortic valve replacement (TAVR) or transcatheter mitral valve repair on patients’ symptoms, function, and quality of life. Health status can also be a powerful marker for subsequent clinical outcomes, but its prognostic importance around the time of both TAVR and transcatheter mitral valve repair has not been fully defined.
Methods:
Among 73 699 patients who underwent transfemoral TAVR or transcatheter mitral valve repair between 2011 and 2018 (mean age, 81.9±7.0 years, 53% men, 92% TAVR), we constructed sequential models examining the association of health status (as assessed with the Kansas City Cardiomyopathy Questionnaire–Overall Summary Score; KCCQ-OS) at baseline, 30 days, change from baseline to 30 days, and combinations of these assessments with death and heart failure (HF) hospitalization from 30 days to 1 year.
Results:
Although higher baseline KCCQ-OS and 30-day KCCQ-OS scores were each associated with lower risk of death and HF hospitalization (in individual models and in a model including both measures), the 30-day KCCQ-OS was most predictive (death: hazard ratio, 0.89 per 5-point increase [95% CI, 0.89–0.90]; HF hospitalization: hazard ratio, 0.91 [95% CI, 0.90–0.91]). The 30-day KCCQ-OS also was most predictive when included in a separate model with change in KCCQ from baseline to 30 days. Similar findings were noted for the outcomes of death and of HF hospitalization, unadjusted and adjusted for patient factors. All interaction terms between procedure type and KCCQ were not significant, suggesting that health status provided similar prognostic information in both procedures.
Conclusions:
The patient’s assessment of their health status immediately before and 30 days after TAVR and transcatheter mitral valve repair is associated with subsequent risk of death and HF hospitalization, with the 30-day assessment being most strongly associated with outcomes. Our findings support the routine use of KCCQ data as a prognostic tool.
What Is Known
Improvement in patient-reported health status in patients undergoing transcatheter valve therapies is an important outcome.
The Kansas City Cardiomyopathy Questionnaire has emerged as an important prognostic tool in patients undergoing transcatheter valve therapies.
What the Study Adds
We found that patient-reported health status collected before and 30 days after transcatheter valve therapies predict subsequent death and heart failure hospitalization.
The 30-day assessment is most strongly associated with subsequent outcomes.
We provide a practical guide for the application of patient-reported health status measures in patients undergoing transcatheter valve therapies.
Since the earliest days of both transcatheter aortic valve replacement (TAVR) and transcatheter mitral valve repair (TMVr), assessment of patient-reported health status before and after the procedure has been an integral component of both clinical trials and registries.1–7 Health status was felt to be so important to measure around the time of these procedures that it was mandated as part of the coverage decision from the Centers for Medicare and Medicaid Services (CMS)8 and is, therefore, included as a core data element within the Society of Thoracic Surgeons (STS)/American College of Cardiology (ACC) Transcatheter Valve Therapies (TVT) Registry.
Health status has 2 major functions in the care of patients undergoing TAVR or TMVr. First, as a reliable, responsive, and valid assessment of symptoms, functional status, and quality of life, disease-specific health status represents a key patient-centered outcome of these procedures, and improvement in health status has been recognized as one of the most important goals of these procedures.9 Second, disease-specific health status has emerged as a powerful prognostic marker. The Kansas City Cardiomyopathy Questionnaire (KCCQ) is a well-validated heart failure (HF)–specific tool.10 Low KCCQ scores before TAVR are strongly associated with increased risk of mortality over the following year,11 and more recently, a lack of improvement in KCCQ at 30 days after TMVr has been shown to be associated with increased risk of death or HF hospitalization over the following 23 months, independent of baseline clinical and anatomic factors.
Given that health status improvement is one of the key goals of these procedures, measuring health status around the time of TAVR and TMVr is essential to assessing the success of the procedure from patients’ perspectives. However, its role in predicting prognosis—both before and after the procedures—is less well defined. We, therefore, sought to use data from the STS/ACC TVT Registry to perform a comprehensive assessment of the prognostic importance of the KCCQ around the time of both TAVR and TMVr to better understand which assessments are most prognostically important and for which outcomes (survival or HF hospitalization). An additional goal was to determine the extent to which any such associations differ between patients who undergo TAVR or TMVr.12
Methods
Data Source and Study Protocol
We used data from the STS/ACC TVT Registry for this analysis.13,14 Requests to access the dataset from qualified researchers trained in human subject confidentiality protocols may be sent to the STS/ACC TVT Registry committee at www.sts.org/registries-research-center. CMS mandates participation in this registry for reimbursement for both TAVR and TMVr, which means the registry collects data on nearly all transcatheter valve procedures in the United States. The STS/ACC TVT Registry was established in 2011 with inclusion of patients who underwent TAVR and expanded to TMVr in 2013 after the Food and Drug Administration's approval of the MitraClip device.
Sites input data on patient demographics, comorbidities, hemodynamics, functional status, patient-reported health status, and clinical outcomes.15 Records are also linked to the CMS Medicare claims database through patient-specific identifiers, which allows for evaluation of subsequent mortality and rehospitalizations.16 Data-quality checks are implemented at the National Cardiovascular Data Registry data warehouse and the Duke Clinical Research Institute to optimize data completeness and accuracy with random third party sites conducting yearly audits to maintain data integrity. A central institutional review board approved all registry activities, and a waiver of informed consent was granted for this study by the Duke University School of Medicine Institutional Review Board. For this analysis, we included patients who underwent either TAVR or TMVr, survived 1 month, had health status data at both baseline and 1 month, and were able to be linked to CMS claims (age ≥65 years, participation in Medicare fee-for-service). We excluded patients who underwent TAVR via nontransfemoral access, as they often have delayed health status recovery compared with those undergoing transfemoral TAVR.1,5
Health Status Assessment
Disease-specific health status was assessed at baseline and 30 days after TAVR or TMVr with the 12-item KCCQ, which is a reliable, responsive, and valid assessment of the symptoms, functional limitations, and quality of life associated with HF.9 The 12-item KCCQ assesses 4 domains of health status related to HF (physical limitation, symptom frequency, quality of life, social limitation), which are combined into an overall summary score (KCCQ-OS). Scores for the KCCQ-OS range from 0 to 100 with higher values representing better health status.10,17 Changes in the KCCQ-OS of 5, 10, and 20 points correspond to small, moderate, or large clinical changes at the individual patient level, respectively.18
Statistical Analysis
The primary purpose of this study was to examine the association between KCCQ-OS scores at various time points (baseline, 30 days) and the risks of death and HF hospitalization 1 year after the 30-day assessment. To assess for potential bias in our study due to missing data, we first compared patients in the analytic cohort with those who survived 30 days but were missing KCCQ or CMS data. To examine our ability to potentially combine patients treated with different procedures, we compared patient factors among those treated with TAVR versus TMVr. Given the large sample size, patient factors were compared using standardized differences, where >10% is considered clinically significant.
To assess the association between the KCCQ-OS and the risks of death and HF hospitalization, we constructed 5 models: model 1: baseline KCCQ-OS; model 2: 30-day KCCQ-OS; model 3: baseline and 30-day KCCQ-OS; model 4: change in KCCQ-OS from baseline to 30 days; model 5: change in KCCQ-OS and 30-day KCCQ-OS. For each model, we assessed the association of KCCQ-OS (scaled per 5-points) with mortality using Cox proportional hazards models and with HF hospitalization using Fine and Gray proportional subdistribution hazards models (death as a competing risk). We accounted for clustering of patients within sites using robust sandwich variance estimates. In each model, we tested the interaction between KCCQ-OS and procedure type (TAVR or TMVr) to assess whether the association of health status with outcomes differed by procedure. We examined the linearity of the association of KCCQ with outcomes using restricted cubic splines, and if a nonlinear relationship existed, knots were selected based on visual inspection of the spline curves. Although the unadjusted results were our primary analysis to support interpretation of health status scores alone, we also assessed the independent association of KCCQ with outcomes by adjusting for patient factors associated with health status after TAVR and TMVr19,20: age, sex, race, body surface area, left ventricular ejection fraction, hemoglobin, creatinine, diabetes, severe lung disease, home oxygen, prior coronary artery bypass grafting, atrial fibrillation/flutter, pacemaker implantation, myocardial infarction, and stroke. For the last 4 covariates, we included events before- and within 30 days of the procedure (ie, before the 30-day KCCQ assessment). All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC), and statistical significance was defined as a 2-sided P value of <0.05.
Results
Study Sample
Between November 2011 and 2018, 175 113 patients from 593 sites underwent TAVR and 19 966 patients from 330 sites underwent TMVr in the STS-ACC TVT Registry. We excluded 2534 patients who had not yet reached 30 days of follow-up, 53 087 patients unable to be linked to CMS claims, and 66 759 patients with missing KCCQ data at either baseline or 30 days (KCCQ missing rate: 46% for TAVR and 60% for TMVr; Figure I in the Data Supplement). As such, our analytical cohort consisted of 73 699 patients from 575 sites (67 669 TAVR and 6030 TMVr). There were no meaningful differences between those in the analytical cohort versus those who survived 30 days but were missing data except included patients were more likely to be older and White (Table I in the Data Supplement).
Mean age of the cohort was 81.9±7.0 years, 53.3% were male, and 95.1% were White (Table). Mean STS predicted risk of surgical mortality was 6.8±5.1% for patients treated with TAVR and 7.5±6.1% for those treated with TMVr. Although patients treated with TAVR versus TMVr were generally similar, TAVR patients were more likely to have larger body surface areas and diabetes but less likely to have atrial fibrillation/flutter (Table II in the Data Supplement). KCCQ-OS scores were slightly higher at both baseline and 30 days in TAVR patients compared with TMVr patients (baseline: 46.3±24.4 versus 43.3±24.2, standardized difference, 12.3%; 30 days: 74.2±22.4 versus 67.2±24.6, standardized difference, 30.1%) and mean change from baseline to 30 days was also higher in TAVR patients (28.0±26.2 versus 23.9±26.0, standardized difference 15.4%), although notably, mean changes in both groups represent very large health status improvements. There were no significant interactions between KCCQ-OS and procedure type for any of the models, and the point estimates were similar between procedures (Figures II and III in the Data Supplement); as such, all analyses were performed using the combined cohort.
Table 1.
Characteristics of the Study Cohort
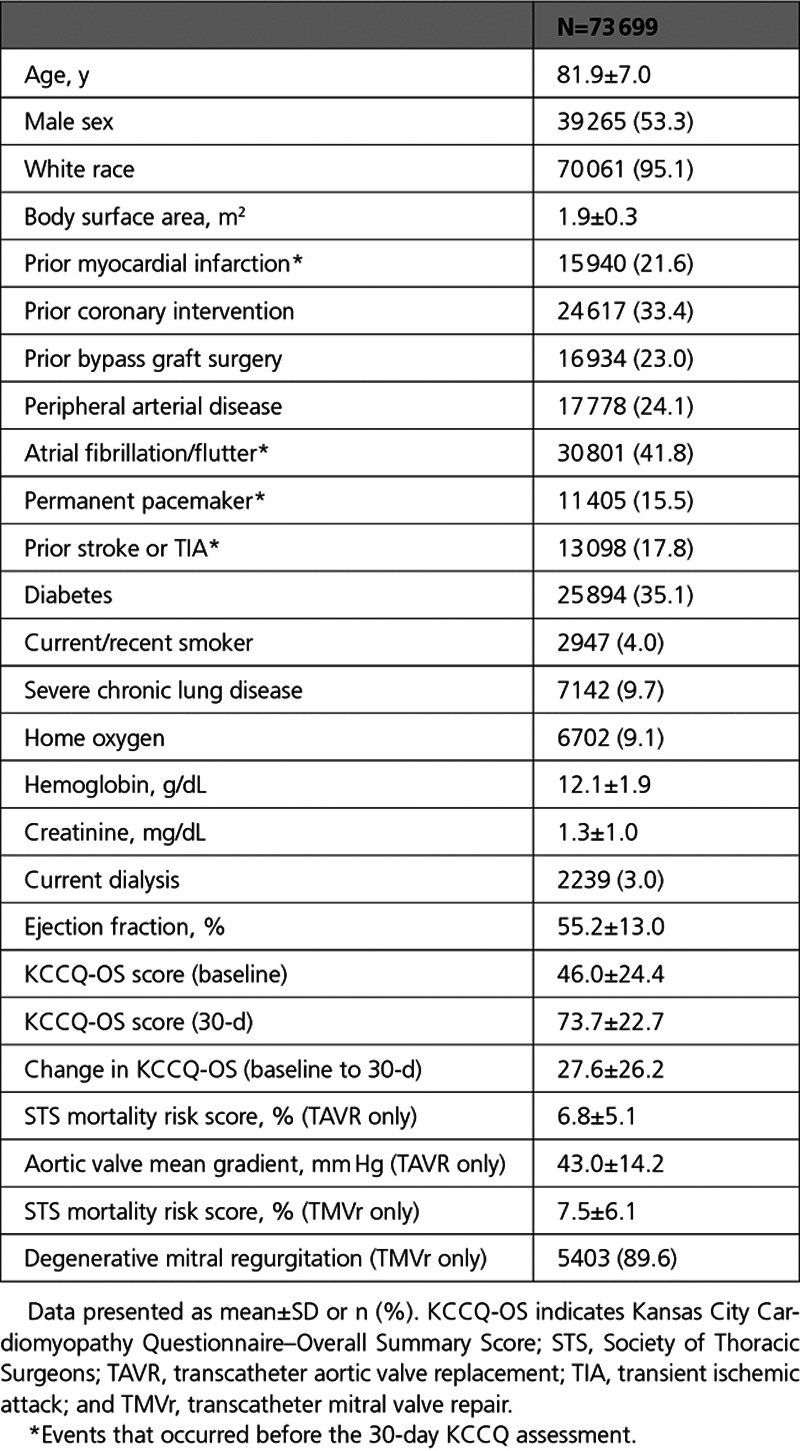
Association of KCCQ With Outcomes
Higher baseline KCCQ-OS (model 1) was strongly associated with a lower risk of death (hazard ratio [HR], 0.92/5-point higher score [95% CI, 0.92–0.93]) and HF hospitalization (HR, 0.92/5-point higher score [95% CI, 0.91–0.92]) 1 year after the 30-day assessment (estimates for death are shown in Figure 1 and HF hospitalization in Figure 2; all HRs are scaled per 5-point change in KCCQ-OS). Similarly, 30-day KCCQ (model 2) was also strongly associated with lower risk of death (HR, 0.88/5-point higher score [95% CI, 0.88–0.88]) and HF hospitalization (HR, 0.89/5-point higher score [95% CI, 0.89–0.90]). When both baseline and 30-day assessments were included (model 3), each was independently associated with subsequent outcomes, but the 30-day KCCQ-OS was more strongly associated with both death (30-day KCCQ-OS: HR, 0.89/5-point higher score [95% CI, 0.89–0.90]; baseline KCCQ-OS: HR, 0.97/5-point higher score [95% CI, 0.96–0.97]) and HF hospitalization (30-day KCCQ-OS: HR, 0.91/5-point higher score [95% CI, 0.90–0.91]; baseline KCCQ-OS: HR, 0.96/5-point higher score [95% CI, 0.95–0.96]).
Figure 1.
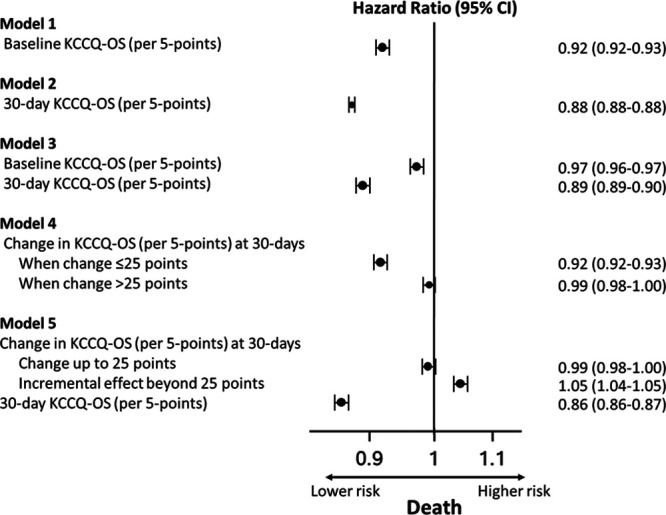
Association between Kansas City Cardiomyopathy Questionnaire–Overall Summary Score (KCCQ-OS) before and 30 day after transcatheter aortic valve replacement/transcatheter mitral valve repair and mortality 1 year after the 30-day assessment.
Figure 2.
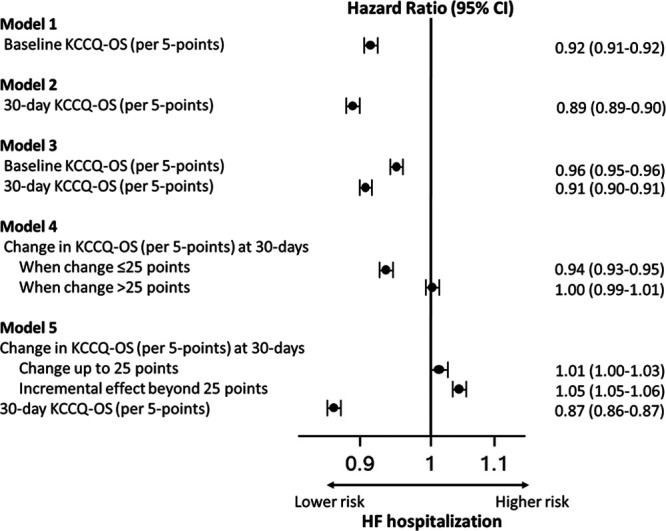
Association between Kansas City Cardiomyopathy Questionnaire–Overall Summary Score (KCCQ-OS) before and 30 day after transcatheter aortic valve replacement/transcatheter mitral valve repair and heart failure (HF) hospitalization 1 year after the 30-day assessment.
Improvement in KCCQ-OS from baseline to 30 days was associated with lower risk of death and of HF hospitalization (model 4). However, this association was statistically nonlinear (P value for cubic splines <0.001; Figure 3), with improvements up to 25 points associated with lower risk of death and lower risk of HF hospitalization but no further risk reduction beyond 25 points (ie, increase in KCCQ-OS of 25 points has similar risk reduction as an increase of 30 points). When both change in KCCQ-OS and 30-day assessments were included (model 5), higher 30-day KCCQ-OS was strongly associated with lower risk of death (HR, 0.86/5-point higher score [95% CI, 0.86–0.87]) and HF hospitalization (HR, 0.87/5-point higher score [95% CI, 0.86–0.87]) while any increase in KCCQ-OS score was associated with higher rates of death (HR, 1.05/5-point higher score [95% CI, 1.04–1.05]) and HF hospitalization (HR, 1.05/5-point higher score [95% CI, 1.05–1.06]) (ie, among two patients with the same 30-day KCCQ-OS, the one with the lower baseline KCCQ-OS has worse outcomes). There were no meaningful changes in the direction or magnitude of these associations after adjusting for patient factors (Figures IV and V in the Data Supplement).
Figure 3.
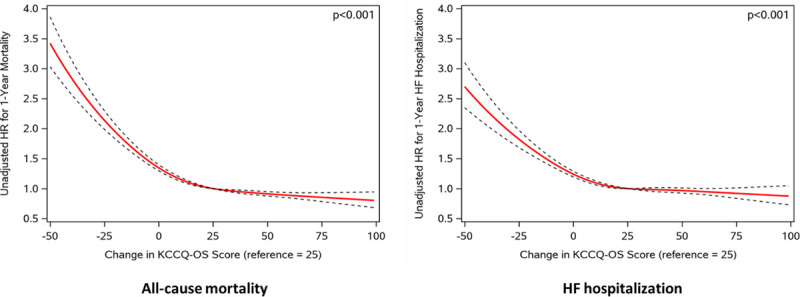
Association between change in Kansas City Cardiomyopathy Questionnaire–Overall Summary Score (KCCQ-OS) score and 1-y outcomes. HF indicates heart failure; and HR, hazard ratio.
Discussion
Health status assessment has a critical role in documenting the benefit of transcatheter valve procedures on patients’ symptoms, function, and quality of life—a key goal of treatment for many patients. However, few studies have examined how to optimally use these data to potentially also predict longer-term clinical outcomes after TAVR and TMVr. Using data from patients undergoing either TAVR or TMVr, we found 30-day KCCQ scores were most strongly associated with subsequent death and HF hospitalization. This association persisted after adjusting for baseline KCCQ-OS score, change in KCCQ-OS score, and patient characteristics. Higher baseline KCCQ-OS scores were also independently associated with lower death and HF hospitalization but to a much lesser extent. Collectively, these results add substantially to our understanding of the prognostic importance of patient-reported health status around the time of TAVR and TMVr and provide an additional role for the KCCQ to be used in clinical care.
TAVR Versus TMVr
Although TAVR and TMVr are fundamentally different procedures, the association between KCCQ-OS and outcomes did not differ by procedure type. We had hypothesized this, as the patients who undergo these procedures are relatively similar (eg, older adults with comorbidities), the underlying conditions produce similar HF symptoms and functional limitations, and the health status improvement is similarly large. However, what was unexpected is how similar our findings are to prior studies in patients with chronic HF. The prognostic importance of both current KCCQ-OS (HR, of 0.91/5-point increase21) and change in KCCQ-OS between 2 time points (HR, of 0.91–0.95/5-point increase21,22) were similar in chronic HF patients compared with those after TAVR or TMVr, although the typical improvement in health status is markedly larger following TAVR or TMVr. It is important to recognize that patients with chronic HF, aortic stenosis undergoing TAVR, and mitral regurgitation undergoing TMVr are inherently different, and thus the absolute survival and hospitalization rates will differ among these cohorts. However, our results demonstrate that the KCCQ has similar prognostic importance in regard to the relative change in risk of these adverse outcomes irrespective of HF or valvular heart disease, chronic disease or invasive procedure, TAVR or TMVr.
Implications
Although KCCQ has been used extensively in research and, to some degree, as a metric of favorable outcome after transcatheter valve procedures,23–25 our findings suggest health status data could also be a powerful tool for improving both patient selection and clinical management. The goals of treatment of severe valve disease are to improve patients’ health status, reduce HF hospitalizations, and prolong survival. Keeping these goals in mind and integrating current data with previous studies, we can envision a system where health status is used at multiple time points to establish a patient-centered approach to manage care around the time of TAVR/TMVr.
Preprocedure KCCQ—Patient Selection
We know from prior studies and the current analysis that KCCQ-OS before TAVR or TMVr is associated with long-term risk of death,11 HF hospitalization, and the composite of death or persistently poor health status18,19 (low pre-KCCQ→worse clinical outcomes24,25). Baseline KCCQ-OS is also associated with 30-day KCCQ-OS score19,20 (low pre-KCCQ→worse post-KCCQ) and change in KCCQ19,20 (low pre-KCCQ→greater improvement in KCCQ). The ideal patient to target for TAVR or TMVr may therefore be one who is symptomatic but have a high likelihood of achieving good postprocedure health status.
Patients with very poor preprocedural health status may still be reasonable candidates for transcatheter valve therapy, presuming they do not have other factors that substantially impair health status recovery.24 Integrating health status data with other clinical factors known to impair recovery (eg, severe lung disease, home oxygen, permanent pacemaker, atrial fibrillation, advanced frailty, or dementia19,20) may help clinicians identify patients most likely to benefit and provide targeted counseling before TAVR/TMVr (ie, setting reasonable expectations for recovery; Figure 3).
Postprocedure KCCQ—Response to Treatment
A vast majority of health status improvement has been observed by 30 days after TAVR or TMVr,1–7 at which time there are 2 pieces of health status data: change in KCCQ-OS from baseline and 30-day KCCQ-OS—both of which are informative. The first is an assessment of the patient’s response to treatment, in terms of symptoms and quality of life, and whether or not the key goal of making the patient feel better was accomplished. The second addresses longer-term prognosis and whether the patient has a reasonable likelihood of surviving without HF hospitalization.
In a patient with a suboptimal response in health status at 30 days (either no significant improvement or with persistently poor health status [eg, KCCQ-OS <60]), further investigation as to what can potentially be done to further improve their health status should be considered (Figure 3). This should first focus on the technical aspects of the procedure and consider factors that could be treated with further transcatheter or surgical intervention (eg, moderate/severe paravalvular leak [TAVR], residual moderate/severe regurgitation [TMVr]). In the absence of technical issues, the focus should shift toward addressing comorbidities, frailty, or procedural complications impeding health status recovery, in which case patients may benefit from prolonged rehabilitation or from referral for more intensive management of comorbidities. For example, persistently poor health status from an underlying cardiomyopathy might be best managed with referral for advanced HF therapies.
There is clearly no single solution or strategy, but the trajectory of recovery and long-term outcomes might be improved if we can understand how to better integrate health status data after TAVR or TMVr to inform treatment decisions or—in the absence of additional therapeutic options—conversations about prognosis. Although the KCCQ is currently collected before and after TAVR/TMVr in the United States, this serves mainly to fulfill CMS requirements. In our experience, health status data are rarely seen by treating physicians or integrated into treatment decisions, which is demonstrated by the high degree of missingness in our study. With the recent National Coverage Decision for TMVr that removes the requirement to participate in a national registry, such as STS/ACC TVT, for continued evidence generation, it is likely that collection of health status data will unfortunately decline. By quantifying the prognostic importance of health status in patients undergoing TAVR/TMVr, we are hopeful that our data encourage the continued routine collection of health status data before and after these procedures. Not only can these data inform both research and quality improvement efforts, but the KCCQ could also be a key component of a framework for improving clinical care (Figure 4). Further studies are needed to compare the predictive ability of the KCCQ to that of other commonly used prognostic tools.26,27
Figure 4.
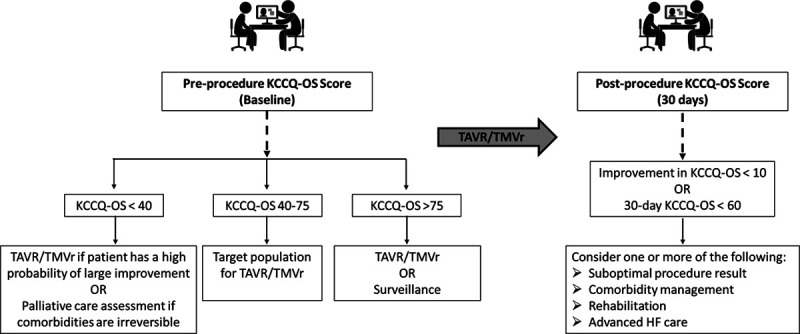
Conceptual model for clinical use of Kansas City Cardiomyopathy Questionnaire (KCCQ) data before and after transcatheter aortic valve replacement (TAVR)/transcatheter mitral valve repair (TMVr). HF indicates heart failure.
Limitations
With TAVR more commonly performed than TMVr over the time frame of our study, our analytical cohort included more TAVR patients. Notably, there were no significant interactions between KCCQ-OS and procedure type, and stratified results were also similar between TAVR and TMVr (Figures II and III in the Data Supplement). Second, due to the Food and Drug Administration approvals for the devices during our study, the TMVr cohort consisted mainly of patients with primary mitral regurgitation at prohibitive surgical risk, whereas TAVR patients were mostly high or intermediate surgical risk. Third, we only assessed the association of health status with subsequent death and HF hospitalizations but not with other outcomes, such as long-term health status or the outcome of alive and well. Fourth, nearly half of patients eligible for analysis were excluded due to missing KCCQ data. Patients excluded were generally similar to those in the analytic cohort in regard to demographics, clinical factors, and KCCQ scores, which likely limited the degree of bias due to missing data. We are hopeful that data such as ours will provide more support for the clinical use of the KCCQ at the time of collection and not just as a data element for regulatory purposes, thereby improving compliance with KCCQ completion over time. Finally, although patients with lower KCCQ scores had worse outcomes compared with those with higher KCCQ scores, there was no control group who did not get TAVR or TMVr.
Conclusions
In a large, contemporary, real-world database of patients undergoing TAVR or TMVr, health status immediately before and 30 days after TAVR/TMVr was associated with subsequent risk of death and HF hospitalization, with the 30-day KCCQ-OS assessment most strongly associated with outcomes. Although health status assessment has an established role in documenting the benefit of transcatheter valve procedures on patients’ symptoms, function, and quality of life, our findings suggest a broader role for these assessments. Specifically, our findings support the use of KCCQ data preprocedurally to counsel patients about goals of care and likelihood of success and postprocedurally to identify patients at high residual risk for poor outcomes who may benefit from further interventions to alter their recovery course. These findings provide additional support for serial collection of KCCQ in real-world practice and integration of these data into clinical decisions, both as a means to measure the symptomatic benefit of transcatheter valve procedures and potentially to also improve patients’ longer-term clinical outcomes.
Sources of Funding
The Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapies Registry is an initiative of the Society of Thoracic Surgeons and the American College of Cardiology. This research was supported by the American College of Cardiology’s National Cardiovascular Data Registry (NCDR). The views expressed in this article represent those of the authors and do not necessarily represent the official views of the NCDR or its associated professional societies identified at CVQuality.ACC.org/NCDR. The study sponsors were not involved in the design and conduct of the study; analysis and interpretation of the data; preparation of the article; or decision to submit the article for publication. Drs Hejjaji and Malik are supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number T32HL110837.
Disclosures
Dr Cohen has received grant support and consulting income from Edwards Lifesciences, Medtronic, Abbott, and Boston Scientific; Dr Carroll is an investigator for the Placement of Aortic Transcatheter Valve (PARTNER) trial sponsored by Edwards Lifesciences and the low risk transcatheter aortic valve replacement (TAVR) versus Surgical Aortic Valve Replacement trial sponsored by Medtronic; Dr Vemulapalli has received grants/contracts from the American College of Cardiology, Society of Thoracic Surgeons, National Institutes of Health, Patient-Centered Outcomes Research Institute, Food and Drug Administration (Nest cc), Boston Scientific & Abbott Vascular. Also received consulting fees from Boston Scientific, Janssen, HeartFlow; Dr Mack has served as a coprimary investigator for Edwards Lifesciences, study chair for Medtronic and trial coprimary investigator for Abbott; Dr Spertus has received consulting income from United Healthcare, Bayer, and Novartis; research grants from Abbott, Novartis, and American College of Cardiology. The other authors report no conflicts.
Supplemental Materials
Tables I–II
Figures I–V
Supplementary Material
Footnotes
The Data Supplement is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCOUTCOMES.120.007187.
Contributor Information
David J. Cohen, Email: djc795@gmail.com.
John D. Carroll, Email: john.carroll@ucdenver.edu.
Zhuokai Li, Email: zhuokaili95@gmail.com.
Pratik Manandhar, Email: pratik.manandhar@duke.edu.
Sreekanth Vemulapalli, Email: sreekanth.vemulapalli@duke.edu.
Adam J. Nelson, Email: a.nelson@duke.edu.
Ali O. Malik, Email: aomalik@saint-lukes.org.
Michael J. Mack, Email: michael.mack@bswhealth.org.
John A. Spertus, Email: spertusj@umkc.edu.
Suzanne V. Arnold, Email: suz.v.arnold@gmail.com.
References
- 1.Reynolds MR, Magnuson EA, Wang K, Thourani VH, Williams M, Zajarias A, Rihal CS, Brown DL, Smith CR, Leon MB, Cohen DJ; PARTNER Trial Investigators. Health-related quality of life after transcatheter or surgical aortic valve replacement in high-risk patients with severe aortic stenosis: results from the PARTNER (Placement of AoRTic TraNscathetER Valve) Trial (Cohort A). J Am Coll Cardiol. 2012;60:548–558. doi: 10.1016/j.jacc.2012.03.075 [DOI] [PubMed] [Google Scholar]
- 2.Reynolds MR, Magnuson EA, Lei Y, Leon MB, Smith CR, Svensson LG, Webb JG, Babaliaros VC, Bowers BS, Fearon WF, Herrmann HC, Kapadia S, Kodali SK, Makkar RR, Pichard AD, Cohen DJ; Placement of Aortic Transcatheter Valves (PARTNER) Investigators. Health-related quality of life after transcatheter aortic valve replacement in inoperable patients with severe aortic stenosis. Circulation. 2011;124:1964–1972. doi: 10.1161/CIRCULATIONAHA.111.040022 [DOI] [PubMed] [Google Scholar]
- 3.Baron SJ, Arnold SV, Wang K, Magnuson EA, Chinnakondepali K, Makkar R, Herrmann HC, Kodali S, Thourani VH, Kapadia S, Svensson L, Brown DL, Mack MJ, Smith CR, Leon MB, Cohen DJ; PARTNER 2 Investigators. Health status benefits of transcatheter vs surgical aortic valve replacement in patients with severe aortic stenosis at intermediate surgical risk: results from the PARTNER 2 Randomized Clinical Trial. JAMA Cardiol. 2017;2:837–845. doi: 10.1001/jamacardio.2017.2039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baron SJ, Thourani VH, Kodali S, Arnold SV, Wang K, Magnuson EA, Pichard AD, Babaliaros V, George I, Miller DC, Tuzcu EM, Greason K, Herrmann HC, Smith CR, Leon MB, Cohen DJ; PARTNER 2 Investigators. Effect of SAPIEN 3 transcatheter valve implantation on health status in patients with severe aortic stenosis at intermediate surgical risk: results from the PARTNER S3i Trial. JACC Cardiovasc Interv. 2018;11:1188–1198. doi: 10.1016/j.jcin.2018.02.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnold SV, Reynolds MR, Wang K, Magnuson EA, Baron SJ, Chinnakondepalli KM, Reardon MJ, Tadros PN, Zorn GL, Maini B, Mumtaz MA, Brown JM, Kipperman RM, Adams DH, Popma JJ, Cohen DJ; CoreValve US Pivotal Trial Investigators. Health status after transcatheter or surgical aortic valve replacement in patients with severe aortic stenosis at increased surgical risk: results from the corevalve US Pivotal Trial. JACC Cardiovasc Interv. 2015;8:1207–1217. doi: 10.1016/j.jcin.2015.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Osnabrugge RL, Arnold SV, Reynolds MR, Magnuson EA, Wang K, Gaudiani VA, Stoler RC, Burdon TA, Kleiman N, Reardon MJ, Adams DH, Popma JJ, Cohen DJ; CoreValve U.S. Trial Investigators. Health status after transcatheter aortic valve replacement in patients at extreme surgical risk: results from the CoreValve U.S. trial. JACC Cardiovasc Interv. 2015;8:315–323. doi: 10.1016/j.jcin.2014.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arnold SV, Chinnakondepalli KM, Spertus JA, Magnuson EA, Baron SJ, Kar S, Lim DS, Mishell JM, Abraham WT, Lindenfeld JA, Mack MJ, Stone GW, Cohen DJ; COAPT Investigators. Health status after transcatheter mitral-valve repair in heart failure and secondary mitral regurgitation: COAPT Trial. J Am Coll Cardiol. 2019;73:2123–2132. doi: 10.1016/j.jacc.2019.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Medicare & Medicaid Services. National Coverage Determination (NCD) for Transcatheter Aortic Valve Replacement (TAVR) (20.32). 2019. Accessed November 11, 2020. https://www.cms.gov/medicare-coverage-database/details/ncd-details.aspx?NCDid=355 [PubMed]
- 9.Arnold SV, Spertus JA, Lei Y, Allen KB, Chhatriwalla AK, Leon MB, Smith CR, Reynolds MR, Webb JG, Svensson LG, Cohen DJ. Use of the Kansas City Cardiomyopathy Questionnaire for monitoring health status in patients with aortic stenosis. Circ Heart Fail. 2013;6:61–67. doi: 10.1161/CIRCHEARTFAILURE.112.970053 [DOI] [PubMed] [Google Scholar]
- 10.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35:1245–1255. doi: 10.1016/s0735-1097(00)00531-3 [DOI] [PubMed] [Google Scholar]
- 11.Arnold SV, Spertus JA, Vemulapalli S, Dai D, O’Brien SM, Baron SJ, Kirtane AJ, Mack MJ, Green P, Reynolds MR, Rumsfeld JS, Cohen DJ. Association of patient-reported health status with long-term mortality after transcatheter aortic valve replacement: report from the STS/ACC TVT Registry. Circ Cardiovasc Interv. 2015;8:e002875. doi: 10.1161/CIRCINTERVENTIONS.115.002875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arnold SV, Stone GW, Mack MJ, Chhatriwalla AK, Austin BA, Zhang Z, Ben-Yehuda O, Kar S, Lim DS, Lindenfeld J, Abraham WT, Cohen DJ; COAPT Investigators. Health status changes and outcomes in patients with heart failure and mitral regurgitation: COAPT Trial. J Am Coll Cardiol. 2020;75:2099–2106. doi: 10.1016/j.jacc.2020.03.002 [DOI] [PubMed] [Google Scholar]
- 13.Carroll JD, Edwards FH, Marinac-Dabic D, Brindis RG, Grover FL, Peterson ED, Tuzcu EM, Shahian DM, Rumsfeld JS, Shewan CM, Hewitt K, Holmes DR, Jr, Mack MJ. The STS-ACC transcatheter valve therapy national registry: a new partnership and infrastructure for the introduction and surveillance of medical devices and therapies. J Am Coll Cardiol. 2013;62:1026–1034. doi: 10.1016/j.jacc.2013.03.060 [DOI] [PubMed] [Google Scholar]
- 14.Mack MJ, Brennan JM, Brindis R, Carroll J, Edwards F, Grover F, Shahian D, Tuzcu EM, Peterson ED, Rumsfeld JS, Hewitt K, Shewan C, Michaels J, Christensen B, Christian A, O’Brien S, Holmes D; STS/ACC TVT Registry. Outcomes following transcatheter aortic valve replacement in the United States. JAMA. 2013;310:2069–2077. doi: 10.1001/jama.2013.282043 [DOI] [PubMed] [Google Scholar]
- 15.Registry NCD. STS/ACC TVT Registry - data dictionary. Data Dictionary. 20202–42. [Google Scholar]
- 16.Holmes DR, Jr, Brennan JM, Rumsfeld JS, Dai D, O’Brien SM, Vemulapalli S, Edwards FH, Carroll J, Shahian D, Grover F, Tuzcu EM, Peterson ED, Brindis RG, Mack MJ; STS/ACC TVT Registry. Clinical outcomes at 1 year following transcatheter aortic valve replacement. JAMA. 2015;313:1019–1028. doi: 10.1001/jama.2015.1474 [DOI] [PubMed] [Google Scholar]
- 17.Spertus JA, Jones PG. Development and validation of a short version of the Kansas City Cardiomyopathy Questionnaire. Circ Cardiovasc Qual Outcomes. 2015;8:469–476. doi: 10.1161/CIRCOUTCOMES.115.001958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spertus J, Peterson E, Conard MW, Heidenreich PA, Krumholz HM, Jones P, McCullough PA, Pina I, Tooley J, Weintraub WS, Rumsfeld JS; Cardiovascular Outcomes Research Consortium. Monitoring clinical changes in patients with heart failure: a comparison of methods. Am Heart J. 2005;150:707–715. doi: 10.1016/j.ahj.2004.12.010 [DOI] [PubMed] [Google Scholar]
- 19.Arnold SV, Spertus JA, Vemulapalli S, Li Z, Matsouaka RA, Baron SJ, Vora AN, Mack MJ, Reynolds MR, Rumsfeld JS, Cohen DJ. Quality-of-life outcomes after transcatheter aortic valve replacement in an unselected population: a report from the STS/ACC Transcatheter Valve Therapy Registry. JAMA Cardiol. 2017;2:409–416. doi: 10.1001/jamacardio.2016.5302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arnold SV, Li Z, Vemulapalli S, Baron SJ, Mack MJ, Kosinski AS, Reynolds MR, Hermiller JB, Rumsfeld JS, Cohen DJ. Association of transcatheter mitral valve repair with quality of life outcomes at 30 days and 1 year: analysis of the transcatheter valve therapy registry. JAMA Cardiol. 2018;3:1151–1159. doi: 10.1001/jamacardio.2018.3359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pokharel Y, Khariton Y, Tang Y, Nassif ME, Chan PS, Arnold SV, Jones PG, Spertus JA. Association of Serial Kansas City Cardiomyopathy Questionnaire assessments with death and hospitalization in patients with heart failure with preserved and reduced ejection fraction: a secondary analysis of 2 randomized clinical trials. JAMA Cardiol. 2017;2:1315–1321. doi: 10.1001/jamacardio.2017.3983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kosiborod M, Soto GE, Jones PG, Krumholz HM, Weintraub WS, Deedwania P, Spertus JA. Identifying heart failure patients at high risk for near-term cardiovascular events with serial health status assessments. Circulation. 2007;115:1975–1981. doi: 10.1161/CIRCULATIONAHA.106.670901 [DOI] [PubMed] [Google Scholar]
- 23.Arnold SV, Spertus JA, Lei Y, Green P, Kirtane AJ, Kapadia S, Thourani VH, Herrmann HC, Beohar N, Zajarias A, Mack MJ, Leon MB, Cohen DJ. How to define a poor outcome after transcatheter aortic valve replacement: conceptual framework and empirical observations from the placement of aortic transcatheter valve (PARTNER) trial. Circ Cardiovasc Qual Outcomes. 2013;6:591–597. doi: 10.1161/CIRCOUTCOMES.113.000354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arnold SV, Afilalo J, Spertus JA, Tang Y, Baron SJ, Jones PG, Reardon MJ, Yakubov SJ, Adams DH, Cohen DJ; U.S. CoreValve Investigators. Prediction of poor outcome after transcatheter aortic valve replacement. J Am Coll Cardiol. 2016;68:1868–1877. doi: 10.1016/j.jacc.2016.07.762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arnold SV, Cohen DJ, Dai D, Jones PG, Li F, Thomas L, Baron SJ, Frankel NZ, Strong S, Matsouaka RA, Edwards FH, Brennan JM. Predicting quality of life at 1 year after transcatheter aortic valve replacement in a real-world population. Circ Cardiovasc Qual Outcomes. 2018;11:e004693. doi: 10.1161/CIRCOUTCOMES.118.004693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hermiller JB, Jr, Yakubov SJ, Reardon MJ, Deeb GM, Adams DH, Afilalo J, Huang J, Popma JJ; CoreValve United States Clinical Investigators. Predicting early and late mortality after transcatheter aortic valve replacement. J Am Coll Cardiol. 2016;68:343–352. doi: 10.1016/j.jacc.2016.04.057 [DOI] [PubMed] [Google Scholar]
- 27.Pilgrim T, Franzone A, Stortecky S, Nietlispach F, Haynes AG, Tueller D, Toggweiler S, Muller O, Ferrari E, Noble S, Maisano F, Jeger R, Roffi M, Grünenfelder J, Huber C, Wenaweser P, Windecker S. Predicting mortality after transcatheter aortic valve replacement: external validation of the transcatheter valve therapy registry model. Circ Cardiovasc Interv. 2017;10:e005481. doi: 10.1161/CIRCINTERVENTIONS.117.005481 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


