Supplemental Digital Content is available in the text.
Keywords: fractional flow reserve, percutaneous coronary intervention
Background:
Fractional flow reserve (FFR)-guided treatment has been demonstrated to improve percutaneous coronary intervention (PCI) results. However, little is known on the long-term impact of low post-PCI FFR.
Methods:
This is a large prospective all comers study evaluating the impact of post-PCI FFR on clinical outcomes. All patients undergoing successful PCI were eligible for enrollment. FFR measurements were performed immediately after PCI when the operator considered the angiographic result acceptable and final. No further action was undertaken based on the post-PCI result. Suboptimal post-PCI FFR was defined as FFR<0.90. The primary end point was major adverse cardiac events, a composite of cardiac death, any myocardial infarction, or any revascularization at 2-year follow-up. Secondary end points were target vessel revascularizations and stent thrombosis and the separate components of the primary end point.
Results:
A total of 1000 patients were enrolled. Post-PCI FFR was successfully measured in 1165 vessels from 959 patients. A poststenting FFR<0.90 was observed in 440 vessels (37.8%). A total of 399 patients had at least 1 vessel with FFR<0.90 post-PCI. At 2-year follow-up, a patient level analysis showed no association between post-PCI FFR and major adverse cardiac event (hazard ratio [HR], 1.08 [95% CI, 0.73–1.60], P=0.707), cardiac death (HR, 1.55 [95% CI, 0.72–3.36], P=0.261), any myocardial infarction (HR, 1.53 [95% CI, 0.78–3.02], P=0.217). A vessel level analysis showed a higher rate of target vessel revascularization (HR, 1.91 [95% CI, 1.06–3.44], P=0.030) and a tendency toward higher rate of stent thrombosis (HR, 2.89 [95% CI, 0.88–9.48], P=0.081) with final post-PCI FFR<0.90.
Conclusions:
Suboptimal post-PCI FFR has only a moderate impact on major adverse cardiac event but coronary arteries with a post-PCI FFR<0.90 have a higher rate of target vessel revascularization.
What Is Known
Fractional flow reserve (FFR) is a reliable index of functional severity for epicardial vessel stenosis.
FFR immediately after stenting to assess the impact of the treatment on coronary flow and the possible residual stenosis has been poorly investigated, and data on this specific FFR application are sparse.
The impact of microcatheter-based post–percutaneous coronary intervention FFR has been not evaluated in large prospective studies.
What the Study Adds
Microcatheter-based suboptimal post–percutaneous coronary intervention FFR has only a moderate impact on major adverse cardiac events but is associated with a higher rate of target vessel revascularizations.
See Editorial by Lee and Koo
Fractional flow reserve (FFR) is a reliable index of functional severity for epicardial vessel stenosis.1 This diagnostic tool facilitates the correct identification of hemodynamically significant coronary artery disease, translating into increased intervention appropriateness and improved clinical outcomes.2,3
Therefore, the European Society for Cardiology (ESC)/The European Association for Cardio-Thoracic Surgery (EACTS) guidelines on myocardial revascularization formulated strong recommendations toward FFR guidance for percutaneous coronary intervention (PCI).4
Conversely, the significance of FFR immediately after stenting to assess the impact of the treatment on coronary flow and the possible residual stenosis has been poorly investigated and data on this specific FFR application are sparse.5
In particular, a relationship between post-PCI FFR and clinical outcomes has mainly been derived from retrospective studies and post hoc analyses of randomized studies, with unclear results in terms of optimal cutoff values for the identification and definition of suboptimal poststenting FFR.6–8
Given this background, we performed the FFR-SEARCH (Fractional Flow Reserve Stent Evaluated at Rotterdam Cardiology Hospital) prospective study, to investigate the clinical impact of post-PCI FFR values on long-term clinical outcomes using a cutoff value for the definition of suboptimal FFR (FFR<0.90) already hypothesized in the FAME 1 (Fractional Flow Reserve Versus Angiography for Guiding Percutaneous Coronary Intervention) and FAME 2 (Fractional Flow Reserve-Guided PCI Versus Medical Therapy in Stable Coronary Disease) trials7,9 and supported by large meta-analyses10 but never evaluated in a prospective fashion.
Methods
Patient Population
The FFR-SEARCH is a large prospective, open label, all comers study evaluating the impact of poststenting FFR on long-term clinical outcomes.
Consecutive patients undergoing coronary intervention with stent implantation, irrespectively of the clinical presentation were considered for the study. Culprit lesions in patients presenting with ST-elevation or non ST-elevation acute coronary syndromes were included in the analysis. Exclusion criteria comprised age <18 years, cardiogenic shock, high-risk PCI with mechanical circulatory support, vessel size <2.25 mm by visual estimation, uncertain neurological outcome after cardiopulmonary resuscitation, planned coronary artery bypass graft as a staged procedure (hybrid) within 12 months of the index procedure. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Poststenting FFR Measurements and Analysis
Functional assessments were performed at the end of the procedure when the operator considered the angiographic result acceptable and final.
Guidewire access to the vessel was maintained and was used to advance a monorail microcatheter with an optical pressure FFR sensor technology (Navvus RXi, ACIST Medical Systems, Eden Prairie, MN).11
For poststenting FFR values the microcatheter sensor was positioned in the mid-distal segment of the investigated vessel and at least 20 mm distal of the most distal stent edge and hyperemia was induced with a continuous intravenous infusion of adenosine at 140 μg/kg per minute for at least 2 minutes.
As per study protocol and in order not to bias the predictive value of post-PCI FFR, no additional interventions were performed regardless of the final post-PCI FFR value.12
Based on previous reports, comparisons in terms of long-term clinical outcomes were made using a post-PCI FFR cutoff value of 0.90.7,10 In the patient-level analysis, patients were stratified based on the presence at least 1 post-PCI FFR value <0.90. Therefore, for the patient-level analysis, the patients were divided into 2 groups: (1) at least 1 FFR<0.90 and (2) no any FFR<0.90.
In addition, a vessel level analysis was performed.
The study was performed in accordance with the Declaration of Helsinki. The study protocol was approved by the local ethics committee. All patients provided written informed consent for the procedure and the use of anonymous data-sets for research purposes in alignment with the Dutch Medical Research Act.
Quantitative Coronary Angiography
Two-dimensional quantitative coronary angiography was performed for descriptive purposes, pre- and post-stent implantation in all treated lesions, using angiographic projections with minimal foreshortening of the lesion and minimal overlap with others coronary vessels. Analyses were performed with a dedicated quantitative coronary angiography analysis software (CAAS Workstation, Pie Medical Imaging, Maastricht, the Netherlands). Quantitative coronary angiography measurements included lesion length, reference diameter, minimal lumen diameter, and diameter stenosis. In case of totally occluded vessels, either acutely or chronically, the minimal lumen diameter value was considered 0% and diameter stenosis 100% in the prestenting analysis and reference vessel diameter and lesion length were calculated from the first angiographic view with restored flow.
Clinical Follow-Up and Definitions
Clinical follow-up was obtained for each patient from electronic medical records of the hospital, general practitioner, and the municipal civil records databases. In addition, all patients were contacted personally by letter or telephone. Clinical events including all-cause mortality, cardiac mortality, any spontaneous myocardial infarction (MI), target vessel revascularization (TVR), any revascularization and stent thrombosis (ST), were collected.
The primary end point was major adverse cardiac event (MACE), defined as a composite of cardiac death, any spontaneous MI, or any revascularization. The secondary end points were TVRs, target vessel MI, ST, and the separate components of the primary end point. Cardiac death was defined as any death in which a cardiac cause could not be excluded.13 MI was defined according to the fourth universal definition of MI.14 TVR was defined as a reintervention driven by any lesion located in the same epicardial vessel. Target vessel MI was defined as a re-MI driven by any lesion located in the same epicardial vessel. ST was defined according to the academic research consortium 2 definitions.13 Event adjudication was performed by 2 independent cardiologists unaware of the final physiological assessment.
Statistical Analysis
Baseline, categorical variables are reported as counts and percentages and compared using the χ2 test on patient level and generalized linear mixed models with random intercepts on vessel level. Baseline, continuous data are presented as mean with SD for normally distributed variables and as medians with interquartile range (IQR) for variable that were not normally distributed. Differences between both groups for continuous data were assessed using the independent t test on patient level and generalized linear mixed models with random intercepts on vessel level.
The Kaplan-Meier method was applied to show the cumulative incidence of clinical end points. The association between post-PCI FFR and clinical end points was analyzed by Cox proportional hazard regression analysis. First, the analysis was performed univariably. Then, all models were adjusted for a set of potential confounders, which were chosen based on clinical relevance. Specifically. in the patient level analyses, the associations of post-PCI FFR with MACE, cardiac death, MI, and any revascularization were adjusted for sex, hypertension, dyslipidaemia, diabetes, smoking, peripheral arty disease, prior PCI, prior infarction, prior coronary artery bypass graft, ST-segment–elevation myocardial infarction (STEMI), non–ST-segment–elevation myocardial infarction (NSTEMI), and stable angina.
For the analysis on a vessel level, Cox regression with robust standard errors was used to account for the correlation between the vessels in case multiple vessels were assessed within one patient. In these analyses, the associations of post-PCI FFR with TVR and ST were adjusted for bifurcation, severe calcification, in-stent restenosis, thrombotic culprit lesion in STEMI, chronic total occlusion, and stented region located in the left anterior descending artery. Data are presented as hazard ratios (HRs) with 95% CIs.
All tests were 2-tailed, and a P<0.05 was considered statistically significant. Statistical analyses were performed using SPSS statistics for Windows, version 24.0 (SPSS, Chicago, IL) and R (version 3.4.1).
Results
A total of 1512 patients were screened and 1000 patients with 1207 treated vessels were included. Post-PCI FFR measurement was successfully performed in 959 patients and 1165 vessels (Tables 1 and 2, Figure 1). No complications related to the use of the FFR microcatheter were observed (Table 3). A post-PCI FFR <0.90 was reported in 440 vessels (37.8%), and ≤0.80 in 90 (7.7%) vessels (Figure 2). Baseline clinical characteristics are reported in Table 1. In brief, the mean age was 64.6±11.8 years, 20% of patients had diabetes, 70% of the coronary lesions were B2 (33%), or C (37%) with a median stent length of 23 mm (IQR, 15–36) and a median poststenting minimum luminal diameter of 2.6 mm (IQR, 2.25–2.93). Patients with a final poststenting FFR<0.90 more frequently had hypertension (57% versus 51%, P=0.005), hypercholesterolemia (52% versus 44%, P=0.001), diabetes (24% versus 17%, P=0.001). Patients with a final poststenting FFR ≥0.90 presented more often with a STEMI (20% versus 44%, P<0.001; Table 1). Vessels with a final poststenting FFR<0.90 were more often calcified (45% versus 28%, P<0.001) and less frequently thrombotic (11% versus 23%, P<0.001).
Table 1.
Baseline Clinic Characteristics
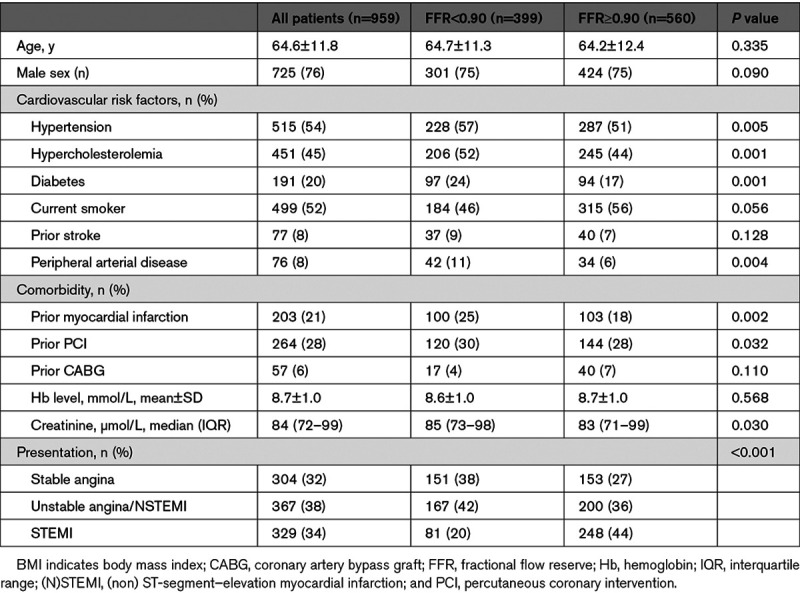
Table 2.
Procedural Characteristics
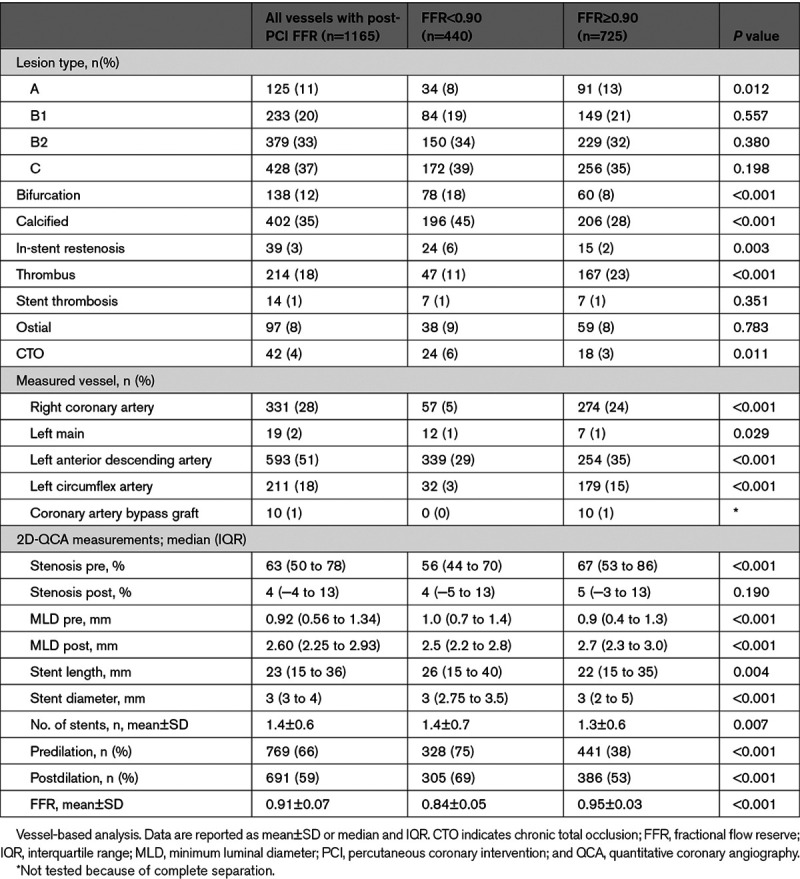
Figure 1.
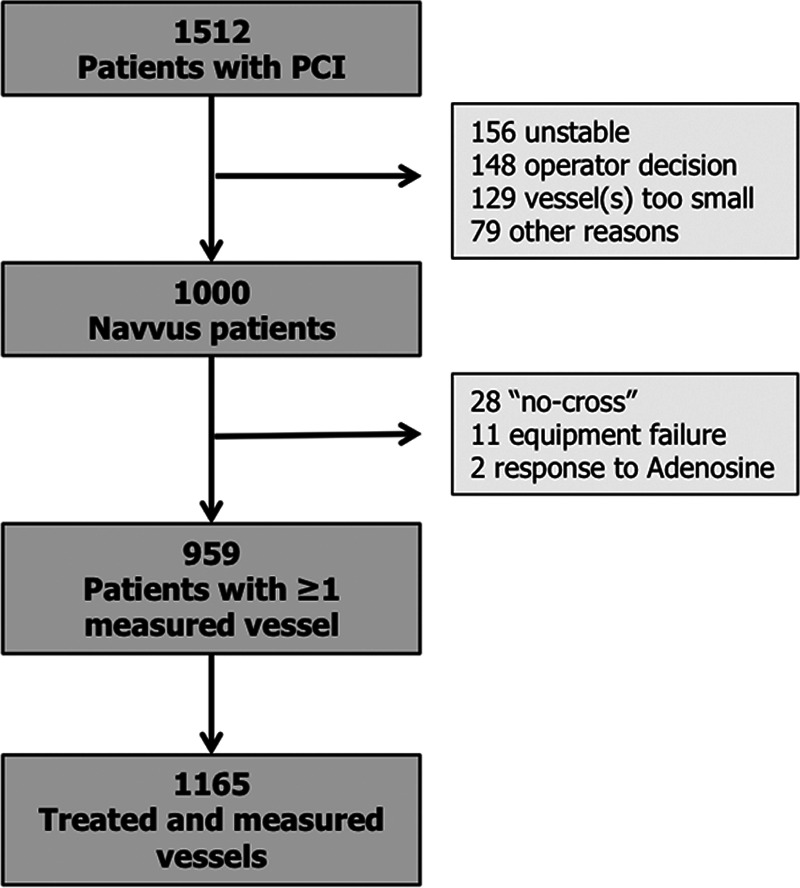
Study flow chart. PCI indicates percutaneous coronary intervention.
Table 3.
Poststenting FFR Measurements and Microcatheter Performance
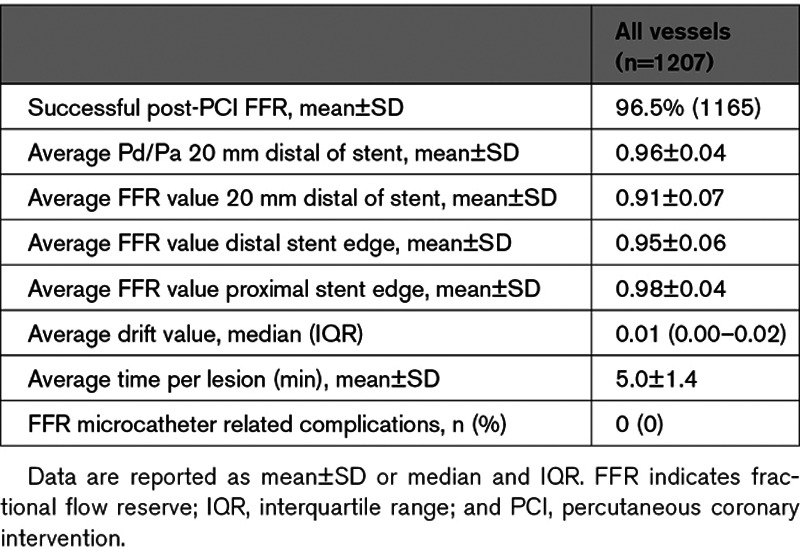
Figure 2.
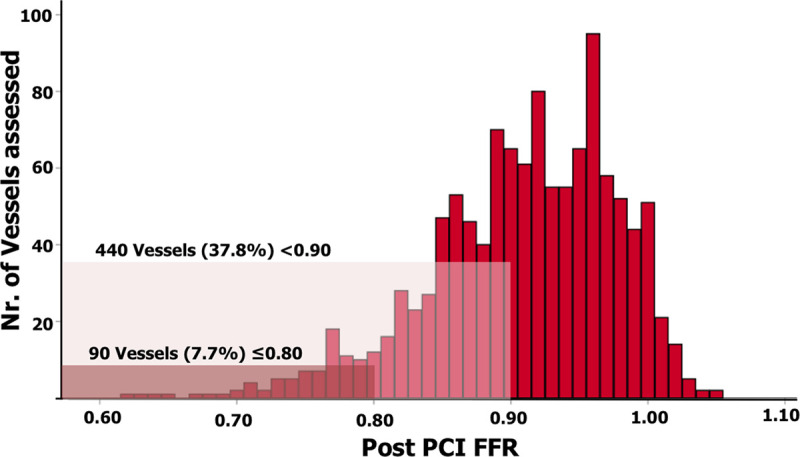
Vessels distribution per 0.01 fractional flow reserve (FFR) increment. PCI indicates percutaneous coronary intervention.
Vessels with poststenting FFR≥0.90 showed a smaller preintervention minimum luminal diameter of 0.9 mm (IQR, 0.4–1.3) versus 1.0 (IQR, 0.7–1.4), P<0.001 by quantitative coronary angiography, but a larger post-procedure minimum luminal diameter (median 2.7 versus 2.5 mm, P<0.001).
Mean follow-up was 655±183 days. Complete 2-year follow-up was available in 849 patients (88.5%), 39 had at least 1-year follow-up, 59 patients had follow-up between 1 and 365 days, 12 patients were lost at follow-up.
At the univariate analysis and after adjustment for confounders, in the patient level analysis, no associations were found between post-PCI FFR and MACE (HR, 1.08 [95% CI, 0.73–1.60]; P=0.707), cardiovascular death (HR, 1.55 [95% CI, 0.72–3.36]; P=0.261), and any MI (HR, 1.53 [95% CI, 0.78–3.02]; P=0.217; Table 4, Figure 3).
Table 4.
Clinical Outcomes at 2-Year Follow-Up
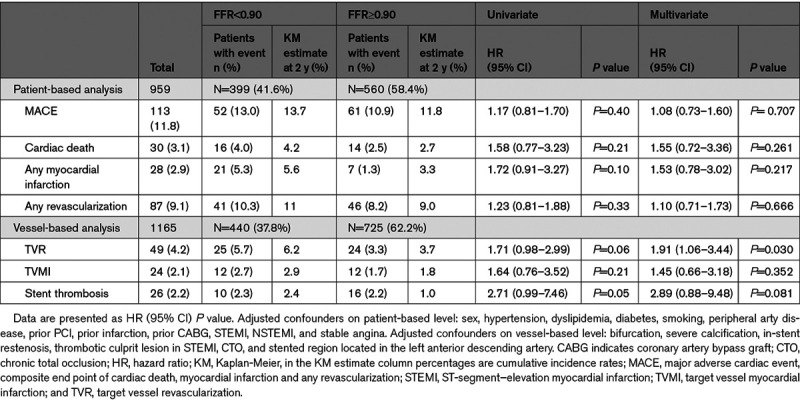
Figure 3.
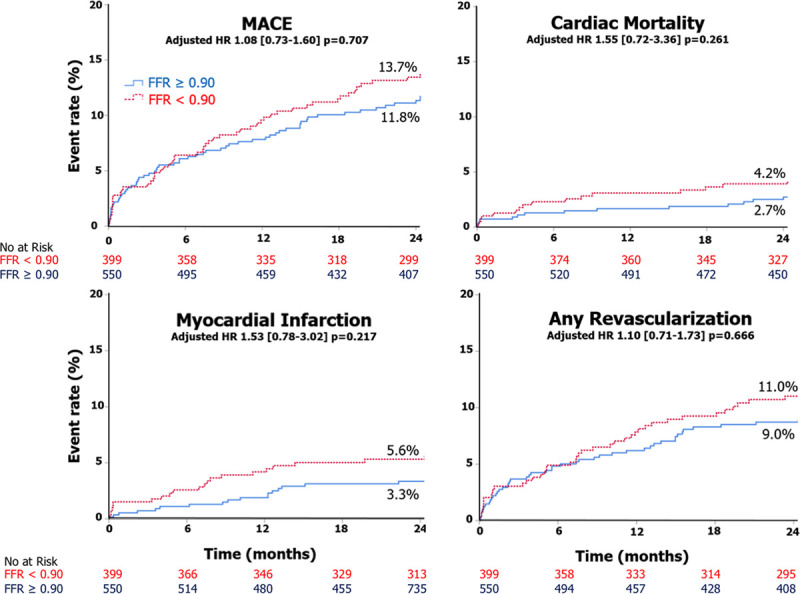
Kaplan-Meier curves for major adverse cardiac event (MACE), cardiac mortality, myocardial infarction, and any revascularization. Patient-based analysis. FFR indicates fractional flow reserve; and HR, hazard ratio.
In the individual vessel level analysis, a higher rate of TVR (HR, 1.91 [95% CI, 1.06–3.44]; P=0.030) and a tendency toward higher rate of ST (HR, 2.89 [95% CI, 0.88–9.48]; P=0.081) was observed with a final poststenting FFR<0.90 (Table 4, Figure 4).
Figure 4.
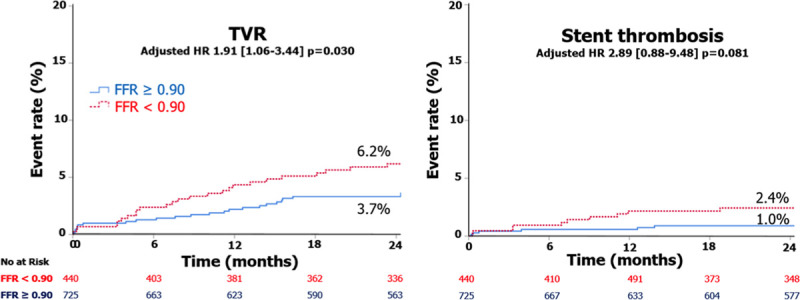
Kaplan-Meier curves for target vessel revascularization (TVR) and stent thrombosis. Vessel-level analysis. FFR indicates fractional flow reserve; and HR, hazard ratio.
After performing the predefined analysis, we evaluated in an exploratory fashion several different cutoff values including post-PCI FFR 0.85. However, results on overall MACE did not change in terms of significant differences between groups.
In addition, a separate analysis was performed excluding patients presenting with STEMI. After adjusting for confounders, no differences between groups were observed in both patient and vessel level analyses (Tables I through III in the Data Supplement).
Discussion
FFR SEARCH is a large prospective study evaluating the impact of microcatheter-based poststenting FFR on long-term clinical outcomes. The main findings of our study are the following: (1) poststenting FFR is safe, feasible, and can be easily performed when using a rapid exchange microcatheter maintaining wire access. (2) Impaired coronary physiology expressed by FFR<0.90 was common (37.8% of patients). (3) Post-PCI FFR <0.90 was not associated with an increase in overall MACE, but on a vessel level analysis, resulted into a higher rate of TVRs and a trend toward higher rate of ST during a follow-up of 2 years.
A large body of evidence has cemented FFR as the standard for invasive ischemia detection in the catheterization laboratory and both American and European clinical guidelines have formulated strong recommendations for FFR evaluation in intermediate coronary stenosis.4,15 Conversely, not much is known about the relevance of coronary physiology to address PCI results.
Post-PCI FFR with a rapid exchange microcatheter appeared safe and easy to execute over the coronary guidewire that was previously used for PCI mitigating the need for additional wire manipulations and concomitant risk of wire passage behind stent struts and coronary dissections.11
The FFR cutoff <0.90 was derived from a post hoc analysis of the FAME Trials9 and was supported by a large meta-analysis,10 however, never tested in a prospective fashion. Using this threshold, more than one-third of the final results judged as acceptable by angiography were categorized as suboptimal, highlighting its clinical relevance. On the other hand, we cannot exclude that lower cutoff values might have a similar or even higher association with clinical events.
The comparison of clinical outcomes on a patient-level analysis showed a consistent numerical, although nonstatistically significant, increase in clinical events in subjects with suboptimal post-PCI FFR values. Such results are in line with previous retrospective studies or post hoc analyses suggesting a moderate impact of suboptimal post-PCI FFR on hard clinical end points and a more relevant impact on vessel-specific end points.7,8 Piroth et al7 comparing the 2-year outcome of lower and upper tertiles of post-PCI FFR reported a significant increase of the vessel oriented composite end point, defined as the composite of vessel-related cardiovascular death, vessel-related spontaneous (nonperiprocedural) MI, and ischemia-driven TVR (9.2% vs 3.8%, P=0.037). Lee et al8 showed in patients with low post-PCI FFR a higher risk of 2-year TVF compared with those with high post-PCI FFR (9.1% versus 2.6%, P. 0.006). Similarly, in the DKCRUSH prospective registry, Post-DES FFR strongly correlated with TVF rate.16 Importantly, in the present study, lesions with FFR<0.90 post-PCI were associated with more TVR and a numerical increase of definite ST, this analysis might be able to better capture the real impact of a single poststenting FFR values on a specific vessel.
From a mechanistic point of view, poststenting FFR indicates residual flow impairment during maximal hyperemia.7 Various factors that may not be appreciated by conventional angiography might contribute to the flow impairment, such as proximal or distal residual focal stenosis, stent underexpansion, or diffuse atherosclerotic disease.17–19 Invasive coronary imaging may help elucidate the pathophysiologic mechanism of impaired coronary flow and guide corrective measures including high-pressure balloon post-dilation, additional stenting, or drug-eluting balloon therapy.
Optimization of a suboptimal post-PCI FFR may be challenging, and FFR pullbacks can help identifying focal drops or more gradual decreases.1,20 Still, FFR pullbacks are not devoid of limitations, such as the absence of a clear threshold,21 the need for prolonged adenosine infusion, with possible patients discomfort and unstable hyperemia, the occurrence of pressure recovery affecting pressure gradients and often increasing the proportion of focal lesion identification,20,22 and finally cases with no clear FFR drop but a diffuse pressure loss indicating a diffuse disease, particularly challenge to treat with local therapies21; therefore, intravascular coronary imaging may complement post-PCI FFR.
In this context, the currently on-going FFR REACT Trial (https://www.trialregister.nl; Unique identifier: NTR6711)23 is randomizing patients with a post-PCI FFR <0.90 to either standard of care (no additional intervention) or intravascular ultrasound directed optimization. The primary end point is the composite of cardiac death, target vessel MI, and clinically driven target vessel revascularisation (target vessel failure) at 1 year.
Limitations
FFR SEARCH is a single-center study. The sample size is limited and might be not sufficient to highlight differences in terms of hard clinical outcomes. The present study was performed in an all-comers population with a relevant number of patients presenting with acute MI. The occurrence of microvascular dysfunction in the myocardial territory supplied by the infarct-related artery might often results in higher post-PCI FFR values. Given the observational nature of the analysis, the results do not evaluate the clinical benefit of additional intervention in vessels with suboptimal post-PCI FFR. No direct comparisons between microcatheter-based and wire-based FFR was performed in terms of stented lesion crossability. Further, large randomized trials are needed to fully investigate the relation between suboptimal post-PCI FFR and clinical events and to elucidate PCI optimization strategies.
Conclusions
Poststenting FFR<0.90 occurs in approximately one-third of patients. Suboptimal post-PCI FFR has only a moderate impact on MACE. Post-PCI FFR <0.90 is associated with a higher rate of TVRs.
Sources of Funding
The FFR (Fractional Flow Reserve) Search Study was based on an unrestricted institutional research grant provided by ACIST Medical Systems.
Disclosures
Dr Diletti reports institutional research grant to Erasmus University Medical Center, consultant to ACIST Medical Systems. The other authors report no conflicts.
Supplementary Material
Nonstandard Abbreviations and Acronyms
- FFR
- fractional flow reserve
- FFR-SEARCH
- Fractional Flow Reserve Stent Evaluated at Rotterdam Cardiology Hospital
- HR
- hazard ratio
- IQR
- interquartile range
- MACE
- major adverse cardiac event
- MI
- myocardial infarction
- PCI
- percutaneous coronary intervention
- ST
- stent thrombosis
- TVMI
- target vessel myocardial infarction
- TVR
- target vessel revascularization
R. Diletti and K. Masdjedi contributed equally.
The Data Supplement is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCINTERVENTIONS.120.009681.
For Sources of Funding and Disclosures, see page 321.
Contributor Information
Kaneshka Masdjedi, Email: k.masdjedi@erasmusmc.nl.
Joost Daemen, Email: j.daemen@erasmusmc.nl.
Tara Neleman, Email: t.neleman@erasmusmc.nl.
Jeroen Wilschut, Email: j.wilschut@erasmusmc.nl.
Wijnand K. Den Dekker, Email: w.dendekker@erasmusmc.nl.
Rutger J. van Bommel, Email: rjvanbommel@hotmail.com.
Miguel Lemmert, Email: mlemmert@gmail.com.
Isabella Kardys, Email: i.kardys@erasmusmc.nl.
Paul Cummins, Email: p.cummins@erasmusmc.nl.
Peter de Jaegere, Email: p.dejaegere@erasmusmc.nl.
Felix Zijlstra, Email: f.zijlstra.1@erasmusmc.nl.
Nicolas M. Van Mieghem, Email: n.vanmieghem@erasmusmc.nl.
References
- 1.Pijls NH, De Bruyne B, Peels K, Van Der Voort PH, Bonnier HJ, Bartunek J Koolen JJ, Koolen JJ. Measurement of fractional flow reserve to assess the functional severity of coronary-artery stenoses. N Engl J Med. 1996;334:1703–1708. doi: 10.1056/NEJM199606273342604 [DOI] [PubMed] [Google Scholar]
- 2.Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, van’ t Veer M, Klauss V, Manoharan G, Engstrøm T, Oldroyd KG, et al. ; FAME Study Investigators. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360:213–224. doi: 10.1056/NEJMoa0807611 [DOI] [PubMed] [Google Scholar]
- 3.Xaplanteris P, Fournier S, Pijls NHJ, Fearon WF, Barbato E, Tonino PAL, Engstrøm T, Kääb S, Dambrink JH, Rioufol G, et al. ; FAME 2 Investigators. Five-year outcomes with PCI guided by fractional flow reserve. N Engl J Med. 2018;379:250–259. doi: 10.1056/NEJMoa1803538 [DOI] [PubMed] [Google Scholar]
- 4.Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ, et al. ; ESC Scientific Document Group. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40:87–165. doi: 10.1093/eurheartj/ehy394 [DOI] [PubMed] [Google Scholar]
- 5.Hakeem A, Uretsky BF. Role of postintervention fractional flow reserve to improve procedural and clinical outcomes. Circulation. 2019;139:694–706. doi: 10.1161/CIRCULATIONAHA.118.035837 [DOI] [PubMed] [Google Scholar]
- 6.Agarwal SK, Kasula S, Hacioglu Y, Ahmed Z, Uretsky BF, Hakeem A. Utilizing post-intervention fractional flow reserve to optimize acute results and the relationship to long-term outcomes. JACC Cardiovasc Interv. 2016;9:1022–1031. doi: 10.1016/j.jcin.2016.01.046 [DOI] [PubMed] [Google Scholar]
- 7.Piroth Z, Toth GG, Tonino PAL, Barbato E, Aghlmandi S, Curzen N, Rioufol G, Pijls NHJ, Fearon WF, Juni P, et al. Prognostic value of fractional flow reserve measured immediately after drug-eluting stent implantation. Circ Cardiovasc Interv. 2017;10:e005233. doi: 10.1161/CIRCINTERVENTIONS.116.005233 [DOI] [PubMed] [Google Scholar]
- 8.Lee JM, Hwang D, Choi KH, Rhee TM, Park J, Kim HY, Jung HW, Hwang JW, Lee HJ, Jang HJ, et al. Prognostic implications of relative increase and final fractional flow reserve in patients with stent implantation. JACC Cardiovasc Interv. 2018;11:2099–2109. doi: 10.1016/j.jcin.2018.07.031 [DOI] [PubMed] [Google Scholar]
- 9.Pijls NH, Klauss V, Siebert U, Powers E, Takazawa K, Fearon WF, Escaned J, Tsurumi Y, Akasaka T, Samady H, et al. ; Fractional Flow Reserve (FFR) Post-Stent Registry Investigators. Coronary pressure measurement after stenting predicts adverse events at follow-up: a multicenter registry. Circulation. 2002;105:2950–2954. doi: 10.1161/01.cir.0000020547.92091.76 [DOI] [PubMed] [Google Scholar]
- 10.Rimac G, Fearon WF, De Bruyne B, Ikeno F, Matsuo H, Piroth Z, Costerousse O, Bertrand OF. Clinical value of post-percutaneous coronary intervention fractional flow reserve value: a systematic review and meta-analysis. Am Heart J. 2017;183:1–9. doi: 10.1016/j.ahj.2016.10.005 [DOI] [PubMed] [Google Scholar]
- 11.Diletti R, Van Mieghem NM, Valgimigli M, Karanasos A, Everaert BR, Daemen J, van Geuns RJ, de Jaegere PP, Zijlstra F, Regar E. Rapid exchange ultra-thin microcatheter using fibre-optic sensing technology for measurement of intracoronary fractional flow reserve. EuroIntervention. 2015;11:428–432. doi: 10.4244/EIJY15M05_09 [DOI] [PubMed] [Google Scholar]
- 12.van Bommel RJ, Masdjedi K, Diletti R, Lemmert ME, van Zandvoort L, Wilschut J, Zijlstra F, de Jaegere P, Daemen J, van Mieghem NM. Routine fractional flow reserve measurement after percutaneous coronary intervention. Circ Cardiovasc Interv. 2019;12:e007428. doi: 10.1161/CIRCINTERVENTIONS.118.007428 [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Garcia HM, McFadden EP, Farb A, Mehran R, Stone GW, Spertus J, Onuma Y, Morel MA, van Es GA, Zuckerman B, et al. ; Academic Research Consortium. Standardized end point definitions for coronary intervention trials: the Academic Research Consortium-2 Consensus Document. Eur Heart J. 2018;39:2192–2207. doi: 10.1093/eurheartj/ehy223 [DOI] [PubMed] [Google Scholar]
- 14.Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD; Group ESCSD. Fourth universal definition of myocardial infarction (2018). Eur Heart J. 2019;40:237–269. [DOI] [PubMed] [Google Scholar]
- 15.Patel MR, Calhoon JH, Dehmer GJ, Grantham JA, Maddox TM, Maron DJ, Smith PK. ACC/AATS/AHA/ASE/ASNC/SCAI/SCCT/STS 2017 appropriate use criteria for coronary revascularization in patients with ss ischemic heart disease: a report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2017;69:2212–2241. doi: 10.1016/j.jacc.2017.02.001 [DOI] [PubMed] [Google Scholar]
- 16.Li SJ, Ge Z, Kan J, Zhang JJ, Ye F, Kwan TW, Santoso T, Yang S, Sheiban I, Qian XS, et al. Cutoff value and long-term prediction of clinical events by FFR measured immediately after implantation of a drug-eluting stent in patients with coronary artery disease: 1- to 3-year results from the DKCRUSH VII Registry Study. JACC Cardiovasc Interv. 2017;10:986–995. doi: 10.1016/j.jcin.2017.02.012 [DOI] [PubMed] [Google Scholar]
- 17.van Zandvoort LJC, Masdjedi K, Witberg K, Ligthart J, Tovar Forero MN, Diletti R, Lemmert ME, Wilschut J, de Jaegere PPT, Boersma E, et al. Explanation of postprocedural fractional flow reserve below 0.85. Circ Cardiovasc Interv. 2019;12:e007030. doi: 10.1161/CIRCINTERVENTIONS.118.007030 [DOI] [PubMed] [Google Scholar]
- 18.Baranauskas A, Peace A, Kibarskis A, Shannon J, Abraitis V, Bajoras V, Bilkis V, Aidietis A, Laucevicius A, Davidavicius G. FFR result post PCI is suboptimal in long diffuse coronary artery disease. EuroIntervention. 2016;12:1473–1480. doi: 10.4244/EIJ-D-15-00514 [DOI] [PubMed] [Google Scholar]
- 19.Wolfrum M, De Maria GL, Benenati S, Langrish J, Lucking AJ, Channon KM, Kharbanda RK, Banning AP. What are the causes of a suboptimal FFR after coronary stent deployment? Insights from a consecutive series using OCT imaging. EuroIntervention. 2018;14:e1324–e1331. doi: 10.4244/EIJ-D-18-00071 [DOI] [PubMed] [Google Scholar]
- 20.Collet C, Sonck J, Vandeloo B, Mizukami T, Roosens B, Lochy S, Argacha JF, Schoors D, Colaiori I, Di Gioia G, et al. Measurement of hyperemic pullback pressure gradients to characterize patterns of coronary atherosclerosis. J Am Coll Cardiol. 2019;74:1772–1784. doi: 10.1016/j.jacc.2019.07.072 [DOI] [PubMed] [Google Scholar]
- 21.Tonino PA, Johnson NP. Why is fractional flow reserve after percutaneous coronary intervention not always 1.0? JACC Cardiovasc Interv. 2016;9:1032–1035. doi: 10.1016/j.jcin.2016.04.001 [DOI] [PubMed] [Google Scholar]
- 22.Davies JE, Sen S, Dehbi HM, Al-Lamee R, Petraco R, Nijjer SS, Bhindi R, Lehman SJ, Walters D, Sapontis J, et al. Use of the instantaneous wave-free ratio or fractional flow reserve in PCI. N Engl J Med. 2017;376:1824–1834. doi: 10.1056/NEJMoa1700445 [DOI] [PubMed] [Google Scholar]
- 23.van Zandvoort LJC, Masdjedi K, Tovar Forero MN, Lenzen MJ, Ligthart J, Diletti R, Lemmert ME, Wilschut J, de Jaegere PPT, Zijlstra F, et al. Fractional flow reserve guided percutaneous coronary intervention optimization directed by high-definition intravascular ultrasound versus standard of care: Rationale and study design of the prospective randomized FFR-REACT trial. Am Heart J. 2019;213:66–72. doi: 10.1016/j.ahj.2019.03.017 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


