ABSTRACT
Wound Bed Preparation is a paradigm to optimize chronic wound treatment. This holistic approach examines the treatment of the cause and patient-centered concerns to determine if a wound is healable, a maintenance wound, or nonhealable (palliative). For healable wounds (with adequate blood supply and a cause that can be corrected), moisture balance is indicated along with active debridement and control of local infection or abnormal inflammation. In maintenance and nonhealable wounds, the emphasis changes to patient comfort, relieving pain, controlling odor, preventing infection by decreasing bacteria on the wound surface, conservative debridement of slough, and moisture management including exudate control. In this fourth revision, the authors have reformulated the model into 10 statements. This article will focus on the literature in the last 5 years or new interpretations of older literature. This process is designed to facilitate knowledge translation in the clinical setting and improve patient outcomes at a lower cost to the healthcare system.
GENERAL PURPOSE
To present the 2021 update of the Wound Bed Preparation paradigm.
TARGET AUDIENCE
This continuing education activity is intended for physicians, physician assistants, nurse practitioners, and nurses with an interest in skin and wound care.
LEARNING OBJECTIVES/OUTCOMES
After participating in this educational activity, the participant will: 1. Apply wound assessment strategies. 2. Identify patient concerns about wound care. 3. Select management options for healable, nonhealable, and maintenance wounds.
KEYWORDS: debridement, exudate, healability, infection, inflammation, moisture management, pain, paradigm, patient-centered care, wound bed preparation, wound healing
INTRODUCTION
Wound Bed Preparation (WBP) is a structured approach to wound healing. Now entering its third decade of widespread use, the WBP paradigm was first published in 2000, with periodic updates in 2003, 2006, 2011, 2015, and now 2021. This article lists 10 statements formulated from previous versions of the WBP model, reports the results of a survey of current wound care practitioners conducted to achieve consensus on those principles, and summarizes related evidence supporting each statement. This latest iteration features the following key changes:
Audible handheld Doppler (AHHD) of dorsalis pedis or posterior tibial artery as an alternative to the traditional ankle-brachial pressure index (ABPI) for the clinical assessment of adequate arterial supply to heal and ability to apply compression therapy safely
Updated approaches to topical and systemic pain management for persons with wounds
An update on the management of maintenance and nonhealable wounds
New enablers to facilitate knowledge dissemination for the other eight components of WBP
Sackett and colleagues1 define evidence-based medicine as “integrating individual clinical expertise and the best external evidence.” Specifically, the three pillars of evidence-based medicine include scientific evidence, expert knowledge, and patient preference. These are incorporated into the 10 statements included in the WBP 2021 paradigm (Figure 1).
Figure 1.
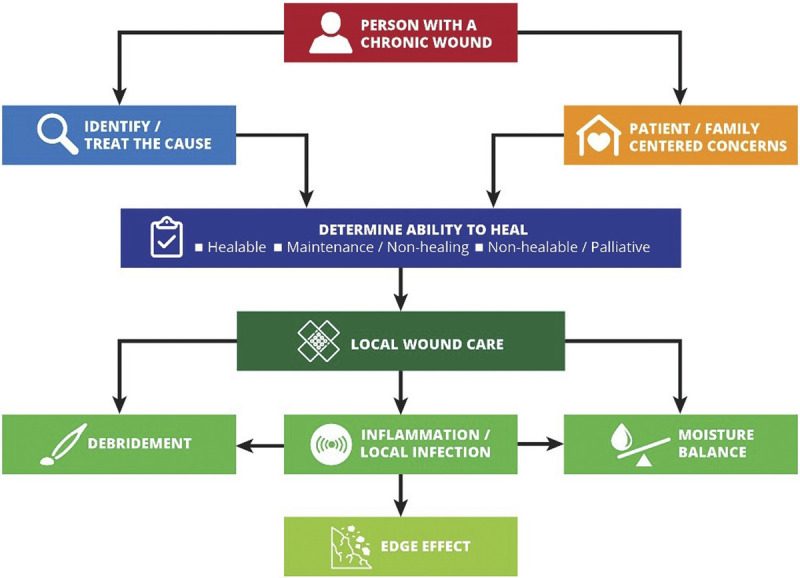
WOUND BED PREPARATION 2021 PARADIGM
©WoundPedia 2021.
Engagement of stakeholders in the evaluation process has been suggested as strategy to bridge the “translation gap.”2 Wound healing experts and active wound care practitioners were involved in the evaluation process of the 10 statements to enhance dissemination.3,4 Further, the authors conducted a comprehensive search of recent literature, findings from which are included throughout this document. Finally, WBP 2021 includes a set of enablers for translation of knowledge into practice. These enablers are tools intended for use at the point of care to enhance implementation of the WBP statements.
METHODS
Ten statements were initially developed by the authors based on previous versions of the WBP paradigm and informed by a review of recent literature. These initial statements were used to create an online survey and a set of visual “enablers” that added further detail to each statement. Some of the 10 statements were further subdivided into lettered substatements (1A, 1B, 1C, etc). The survey was iteratively reviewed and assessed for face and content validity by a total of 20 developers and external wound care stakeholders over a 6-month period and finalized for send-out.
The survey (Supplemental Table 1, http://links.lww.com/NSW/A58) was sent to a purposive sample of wound healing key opinion leaders (KOLs). The authors chose at least one KOL from each continent and from each key wound healing profession: physicians, nurses, and allied health practitioners. For each statement, respondents stated whether they strongly agreed, somewhat agreed, somewhat disagreed, or strongly disagreed. The desired consensus level for statement acceptance was 80% of respondents somewhat agreeing or strongly agreeing with each statement. The survey was also sent to graduating classes of the International Interprofessional Wound Care Courses (IIWCC) in Abu Dhabi and Canada. The respondents were completing a yearlong KOL training with a certificate of completion. Most (but not all) class members voluntarily participated.
RESULTS
Surveys were requested from KOLs (n = 21) and students of the IIWCC 2020 class of Abu Dhabi (n = 66) and Canada (n = 65). The 21 KOLs’ consensus for each statement was between 86% and 100% (Supplemental Table 2, http://links.lww.com/NSW/A59). The 2020 IIWCC class in Abu Dhabi demonstrated 98% to 100% consensus (Supplemental Table 3, http://links.lww.com/NSW/A60), and the class in Canada reached an 85% to 100% consensus (Supplemental Table 4, http://links.lww.com/NSW/A61). The most notable result, beyond the generally high level of consensus, was the comparatively low KOL agreement with Statement 5 (still 86%; discussed later) and the high agreement with all statements among the Abu Dhabi students. This could be because the students in Abu Dhabi (from several West Asian countries and a small number of students from Africa) had less wound care experience than the other groups.
The final 10 statements are listed in Table 1. Each statement will now be expanded upon in more detail in a narrative summary and with accompanying visuals for translation to practice.
Table 1.
WOUND BED PREPARATION 2021: 10 FINAL STATEMENTS
| No. | Statement | Substatements |
|---|---|---|
| 1 | Treatment of the cause | A. Determine if there is sufficient blood supply to heal/adequate perfusion. |
| B. Identify the cause(s) as specifically as possible or make appropriate referrals | ||
| C. Review cofactors/comorbidities (systemic disease, previous surgery, nutrition, medications, fragile skin) that may delay or inhibit healing | ||
| 2 | Patient-centered concerns | A. Manage pain (diagnosis and treatment) |
| B. Evaluate activities of daily living, mobility/exercise, eating habits, psychological wellbeing (mental health), and support system (patient circle of care, access to care, and financial constraints) | ||
| C. Evaluate habits: smoking, alcohol, substance use, personal hygiene | ||
| D. Empower patients with education and support to increase treatment adherence (coherence) | ||
| 3 | Determine ability to heal (status may change) | A. Healable: adequate blood supply to heal and treated the cause |
| B. Maintenance: adequate blood supply to heal where the patient either cannot or will not adhere to the plan of care/healthcare system does not have appropriate resources | ||
| C. Nonhealable: inadequate blood supply and/or a cause that cannot be corrected (eg, terminal cancer, negative protein balance) | ||
| 4 | Local wound care: monitor wound history and clinical examination | A. Document wound(s): location, longest length × widest width at right angles, wound shape, wound bed, exudate, margin, undermining, tunneling, surrounding skin condition, and photoimaging when available |
| B. Cleansing: gently with water, saline, or low-toxicity antiseptic agents | ||
| C. Reassess and document wounds at appropriate, regular intervals | ||
| 5 | When appropriate, debride wounds with adequate pain control | A. Consider sharp surgical debridement (to bleeding tissue) for healable wounds and conservative surgical debridement for maintenance/nonhealable wounds |
| B. Evaluate the need for alternative debridement modalities: autolytic with dressings, enzymatic, mechanical, or biologic | ||
| 6 | Assess and treat wounds for infection/inflammation | A. Treat local infection (three or more NERDS criteria) with topical antimicrobials (silver, iodine, PHMB/chlorhexidine, methylene blue/crystal violet, surfactants) |
| B. Consider treating deep and surrounding infection (three or more STONEES criteria) with systemic antimicrobials | ||
| C. Evaluate and alleviate persistent inflammation including consideration of anti-inflammatory agents (topical dressings, systemic medication) | ||
| 7 | Moisture management | A. Healable, moisture balance, and autolytic debridement: alginates, hydrogels, hydrocolloids, acrylics, films |
| B. Moisture balance alone: super absorbents, foams, calcium alginates, hydrofibers, hydrocolloids, films, hydrogels | ||
| C. Nonhealable and maintenance wounds and moisture reduction: if antibacterial needed, low toxicity topical anesthetics: chlorhexidine/PHMB, iodine, acetic acid | ||
| D. Wound packing: saline wet (donate moisture) or dry (absorb moisture) but not antibacterial; PHMB gauze: antibacterial, nonrelease—above the wound (stays in the gauze) only not in the wound surface; povidone iodine or other antiseptic soaked gauze: antibacterial above and on wound surface | ||
| 8 | Evaluate the rate of healing | A healable wound should be at least 20% to 40% smaller by week 4 to heal by week 12 A. Stalled (healable) wounds should be reevaluated for alternate diagnoses; consider wound biopsy, further investigation, and/or referral to an interprofessional assessment team to optimize management |
| 9 | Edge effect: use active therapies for stalled but healable wounds | A. Some active modalities have weak to mixed evidence and should be only used after interprofessional assessment of the patient and with regular reevaluations |
| B. Skin grafts have variable but positive evidence, and cellular and/or tissue-based products may or may not be cost effective at this time | ||
| 10 | Organizational support | A. Organizational support may include a culture conducive to interprofessional education and patient-centered care, standardized evidence-informed protocols, adequate staffing, and established quality improvement programs that may include audits, prevalence and incidence studies, patient navigation |
Abbreviations: NERDS, Nonhealing, Exudate increase, Red friable granulation, Debris or dead cells, and Smell; PHMB, polyhexamethylenebiguanide; STONEES, Size enlargement, Temperature increase of ≥3° F versus the opposite limb mirror image temperature, Os (bone exposed or direct probing), New areas of break down on the wound margin, Exudate increase, Erythema and/or Edema, and Smell.
STATEMENT 1
Treatment of the Cause
Optimal, timely diagnosis and treatment of the wound cause are the most important aspects of chronic wound care.
Substatement 1A. Determine if there is sufficient blood supply to heal/adequate perfusion. Clinicians should assess vascular supply for leg and foot ulcers to identify if there is adequate blood supply to heal. A palpable dorsalis pedis pulse usually indicates there is at least 80 mm Hg pressure in the foot (Table 2).
Table 2.
VASCULAR ASSESSMENT METHODS
| Method | Indication for Healability5,6 |
|---|---|
| Palpable pulse—dorsalis pedis, posterior tibial | >80 mm Hg |
| Ankle-brachial pressure index (ABPI) | >0.6 and <1.4 |
| Transcutaneous O2 tension | >30 mm Hg |
| Toe pressure | >30–55 mm Hg |
| Audible handheld Doppler | Triphasic or biphasic sound (represents ABPI ≥0.9) |
©WoundPedia 2021.
The ABPI is a ratio of the ankle systolic BP over the brachial systolic BP obtained using an 8-MHz portable Doppler. Approximately 8% of individuals may have an aberrant dorsalis pedis pulse, and the posterior tibial or peroneal pulse should be palpated as an alternative. The ABPI has been the standard for assessment of blood supply in the foot. A normal value is usually equal to or greater than 0.9 and less than 1.4;5,6 under 0.9, there may be some arterial disease, and over 1.4, the foot vessels are calcified and the value is inaccurate.
Ideally, the ABPI should be obtained after the patient has been recumbent for 20 minutes. A BP cuff is placed over the gaiter area of the lower leg. The clinician locates an audible arterial signal on the foot, and the cuff is inflated until the sound disappears. The cuff is deflated, and when the sound reappears, the systolic BP is recorded. The same procedure is repeated over the brachial artery.
Often edema and inflammation (including congestive heart failure, acute or subacute lipodermatosclerosis, or thrombophlebitis), along with infection, may result in pain. Acute pain may make occlusion of the lower leg artery impossible. In addition, up to 80% of persons with diabetes or 20% of older adults will have calcified vessels, providing an artificially high ABPI, rendering the test inaccurate.
An alternative test is the AHHD evaluation. This test can be performed with the patient sitting or recumbent, and the BP cuff is not necessary around the gaiter area. An adequate amount of gel is placed over the dorsum of the foot, and the audible waveform elicited (Table 2; see the following video for the test procedure: https://journals.lww.com/aswcjournal/Pages/videogallery.aspx?videoId=20). A monophasic or absent audible signal indicates the need for a full vascular assessment. The presence of an audible multiphasic (biphasic/triphasic) wave indicates there is no significant peripheral vascular disease in the lower extremity, and compression therapy can be instituted. The foot should be checked for normal temperature and the absence of dependent rubor (dusky red color) that blanches with elevation. This physical examination can be used to rule out an angiosomal defect (local or segmental artery occlusion). The dorsalis pedis or posterior tibial pulse should also be palpable.
A 2015 study documented the results of AHHD readings performed on 379 legs in 200 patients, which were compared with sequential lower-leg Doppler readings in a certified vascular laboratory.7 The test is specific for excluding arterial disease (posterior tibial, 98.6%; dorsalis pedis, 97.8%) but is not sensitive for a diagnosis of arterial disease (posterior tibial, 37.5%; dorsalis pedis, 30.2%). This test is a reliable, simple, rapid, inexpensive bedside exclusion test for peripheral vascular disease among patients with or without diabetes. The results are independent of vascular calcification.
Again, a monophasic Doppler result or absent pulses should trigger segmental lower leg duplex Doppler studies of the arterial blood supply. In some cases, venous studies may be warranted, especially if there is a possibility of surgical or other venous intervention. This test can avoid delays in applying compression therapy when traditional ABPI studies are not possible (lack of access to a Doppler, pain, noncompressible vessels, or time constraints).
For ulcers elsewhere on the body, there is a need for adequate perfusion: check the temperature of the surrounding skin. Examine the regional skin for dependent rubor of the arm or leg distally. On the central body, check the area for edema or necrosis along with circulation time (a white area from a depressed finger on the skin should return in 3 seconds or less; otherwise, there may be compromise). Compromised circulation may indicate a maintenance or nonhealable wound until the underlying defect is corrected.
Substatement 1B. Identify the cause(s) as specifically as possible or make appropriate referrals. Often the cause of a nonhealing wound is an “inadequate diagnosis.”4 Practitioners must identify the wound cause as precisely as possible, considering vascular leg ulcers (venous, mixed, arterial, lymphatic, or combinations), diabetic foot ulcers (neuropathic, ischemic, or mixed), and pressure injuries (which must be distinguished from moisture-associated skin damage); each has specific management considerations (Table 3). Other diagnoses include inflammatory ulcers (pyoderma gangrenosum, vasculitis), malignant ulcers (primary skin, other secondary malignancies), trauma/previous surgeries, medications, and congenital or acquired coexisting diseases. Some coexisting conditions put skin at risk. As skin ages, it becomes thinner. Photodamage and hereditary (eg, epidermolysis bullosa, Ehlers-Danlos syndrome) or acquired (eg, bullous pemphigoid, toxic epidermal necrolysis) dermatologic disease increase susceptibility to trauma including skin tears. Further, areas of moisture-associated skin damage may be more susceptible to pressure injuries or infection.
Table 3.
TREATMENT OF WOUND CAUSE BY TYPE
| Wound Type | Treatment |
|---|---|
| All wounds | Aim for optimal nutrition, moisture management, pain control |
| Venous ulcers | • Compression bandages for healing |
| • Stockings for healing and to prevent recurrence | |
| • High compression in absence of arterial disease (ankle-brachial pressure index [ABPI] >0.9 or audible multiphasic signal with handheld Doppler) | |
| • Modified compression with mixed venous/arterial disease (ABPI 0.6–0.9) | |
| Pressure injuries | • Redistribute pressure over bony prominences and areas under pressure |
| • Reduce shear forces | |
| • Optimize physical activity and mobility | |
| • Manage incontinence and moisture | |
| Diabetic foot ulcers | V = vascular: confirm adequate vascular supply |
| I = infection: control superficial critical colonization/deep and surrounding infection | |
| P = pressure: redistribute plantar/dorsal foot pressure (neuropathy) | |
| S = sharp: surgical serial debridement |
©WoundPedia 2021.
Substatement 1C. Review cofactors/comorbidities (systemic disease, previous surgery, nutrition, medications, fragile skin) that may delay or inhibit healing. Addressing modifiable cofactors is important for all persons with chronic wounds (Figure 2). Appropriate referrals for optimal management can often facilitate wound healing.
Figure 2.
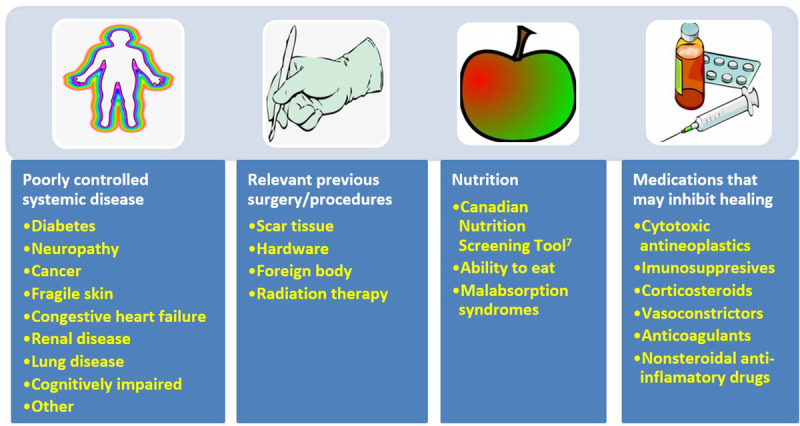
COFACTORS AND COMORBIDITIES TO REVIEW FOR WOUND HEALING
©WoundPedia 2021.
Nutrition assessment can be facilitated with the validated two-question Canadian Nutritional Screening Tool:8
Have you lost weight in the past 6 months without trying to lose this weight? (If the patient reports a weight loss but gained it back, consider it as no weight loss.)
Have you been eating less than usual for more than a week?
This tool has many advantages; no blood tests or diagnostic procedures are required, it is simple and rapid to administer, and it is reliable.9 Any healthcare professional can quickly identify a potential nutrition deficiency and the need for referral to a dietitian.
STATEMENT 2
Patient-Centered Concerns
Substatement 2A. Manage pain (diagnosis and treatment). Pain is often the foremost concern of patients, whereas it is rarely the top concern of healthcare providers. Pain must also be quantified. The numeric rating scale (0–10) is typically used (Table 4). Reported pain levels of 5 or greater require intervention.
Table 4.
MANAGEMENT OF WOUND-RELATED PAIN
| Simplified Pain Component | Therapeutic Action |
|---|---|
| Measurement tool | • Numeric Rating Scale, 0–10 (11-point scale; 0 = no pain, 5 = bee sting, 10 = slam the car door on your thumb; most people can live with a 3 or 4 out of 10) |
| • Faces scale: cognitively challenged, young children, older persons | |
| Neuropathic pain | • Burning, stinging, shooting, stabbing (see Supplemental Figure 1, http://links.lww.com/NSW/A62) |
| • Gabapentin/pregabulin, tricyclics, medical marijuana, selective serotonin reuptake inhibitors | |
| Nociceptive pain | • Gnawing, aching, tender, throbbing |
| • Acetaminophen, aspirin, nonsteroidal anti-inflammatory drugs, narcotics (short/long acting) | |
| Dressing removal | • Pull laterally to release adhesive bond and rotate like the hands of a clock before lifting up |
| • Avoid strong adhesives (acrylates, etc) and use silicone adhesives or nonadhesive dressings | |
| Wound cleansing (sterile only required with immune compromise, deep postsurgical wounds) | • Use saline or (potable) water solutions at room temperature |
| • Compresses or soaks are less traumatic than irrigation (make sure all solution is retrieved and you can visualize the base of the wound with no procedure-induced bleeding or unnecessary trauma) | |
| Debridement | • Topical eutectic mixture of local anesthetics is superior to other topical pain modalities |
| • Use a thick layer and occlude with film type dressing for 10–30 min (shorter period for genitalia, face, folds; longer times on back or thick skin) | |
| • Can supplement topical agents with intralesional xylocaine with adrenaline (if not end artery and no other contraindication) |
©WoundPedia 2021.
There are two major types of pain: nociceptive and neuropathic (Supplemental Figure 1, http://links.lww.com/NSW/A62). Nociceptive pain is related to injury; stimulus dependent; and typically associated with aching, gnawing, tender, or throbbing sensations. Neuropathic pain is often spontaneous and described as burning, shooting, stinging, or stabbing. Each type has a different physiologic basis, necessitating different pharmacologic treatment.
A recent systematic review on topical analgesics associated with pain in chronic leg ulcers demonstrated a topical cream (eutectic mixture of local anesthetics) was superior to other formulations for people living with chronic leg ulcers.10 There are other topical modalities that may be associated with pain relief and strategies including the use of silicone adhesives to replace other, more traumatic acrylic adhesives at dressing removal.
Inadequate pain control can occur during many components of local wound care.11 For painful dressing changes, oral medication must be administered at an appropriate time prior to the change. Between dressing changes, pain is often linked to the cause of the wound or its complications. Consider nonpharmacologic measures (music therapy, meditation, acupuncture, transcutaneous electrical nerve stimulation, homeopathy, naturopathy, and spiritual healing).
In summary, a patient’s rights in terms of pain involve the six C’s: every patient deserves to be Checked, the Cause determined, the Consequences of treatment explained (with adverse effects), adequate Control, the ability to Call time-outs during procedures, and Comfort. Finally, providers must remember that pain management not documented is equivalent to no pain management.
Substatement 2B. Evaluate activities of daily living, mobility/exercise, eating habits, psychological well-being (mental health), and support system (patient circle of care, access to care, and financial constraints). Patient-centered concerns often involve inadequate support structures. They can also involve a lack of healthcare system agency impairing access to appropriate healthcare. Personal mental health may impair the patient’s ability to cope with the management of a chronic wound, and he or she may require help. There is a need for social workers, discharge coordinators, and clinical psychologists to support systems in the community.
Substatement 2C: Evaluate habits: smoking, alcohol, substance use, personal hygiene. Every cigarette will decrease local oxygenation 30% for an hour.12 Cigarettes and other tobacco products can be a major factor preventing healing of chronic wounds or act as a proinflammatory stimulus for persons with hidradenitis suppurativa. Opiate use alone (especially >10 mg/d) was associated with an increase in wound size and reduced likelihood of healing in a 2017 study of 450 patients.13
Substatement 2D. Empower patients with education and support to increase treatment adherence (coherence). Aujoulat et al14 examined patient empowerment in relation to chronic disease education. They determined that “the goals and outcomes… should neither be predefined by the health-care professions, nor restricted to some disease and treatment-related outcomes but should be discussed and negotiated with every patient according to his/her own particular situation and life priorities.”14 Moore et al15 outlined four steps to increase patient involvement in their care:
Seek patient views/understanding of their condition
Identify fears/concerns
Establish what is important for the patient
Assess willingness for involvement in their care
STATEMENT 3
Determine Ability to Heal
One of the first steps providers must take after diagnosis is to determine healability, with the knowledge that the wound status may change. Generally, chronic wounds fall into one of three categories: healable, maintenance, and nonhealable. Local wound care strategies will vary by classification (Table 5).
Table 5.
SUMMARY OF LOCAL WOUND CARE STRATEGIES
| Wound Healability Classification | Considerations | Surgical Debridement | Inflammation/Infection Management | Moisture Management |
|---|---|---|---|---|
| Healable | Provide moist environment | Active | Treat inflammation/infection (topically or systemic) | Moisture balance |
| Promote granulation | ||||
| Maintenance | Decrease moisture and bacteria | Conservative (no bleeding) | Bacterial reduction Topical antisepsis / systemic antimicrobial | Moisture reduction |
| Prevent deterioration | ||||
| Nonhealable | Decrease moisture and bacteria | Comfort removal of slough | Bacterial reduction Topical antisepsis / systemic antibiotics | Moisture reduction |
| Prevent infection | ||||
| Enhance comfort |
Adapted from Sibbald et al.16
©WoundPedia 2021.
Substatement 3A. Healable: adequate blood supply to heal and treated the cause. A healable wound has enough blood supply to heal, and the cause has been corrected. As a rule, approximately two-thirds of wounds in the community are healable.
Substatement 3B. Maintenance: adequate blood supply to heal where the patient either cannot or will not adhere to the plan of care/healthcare system does not have appropriate resources. A quarter of wounds are maintenance wounds, either because of patient issues (eg, refusal to wear compression bandages) and/or health system factors that prevent healing (eg, cannot afford plantar pressure redistribution devices and the system will not supply the footwear).
Substatement 3C. Nonhealable: inadequate blood supply and/or a cause that cannot be corrected (eg, terminal cancer, negative protein balance). Approximately 5% to 10% of wounds are nonhealable, often because of inadequate blood supply that cannot be treated or corrected, advanced chronic disease, or the dying process. For patients with nonhealable wounds, the paramount points of care to address are pain, infectious complications, exudate, and odor control as well as activities of daily living.
Thirteen KOLs from the Wound Healing Society of South Africa conducted a recent systematic integrative review of nonhealable and maintenance wounds.17 This 13-member panel sourced 13 reviews, 6 best practice guidelines, 3 consensus studies, and 6 original nonexperimental studies. The three main conclusions were the need for patient-centered care, timely intervention by skilled healthcare providers, and an interprofessional referral pathway.17
STATEMENT 4
Local Wound Care: Monitor Wound History and Clinical Examination
Substatement 4A. Document wound(s): location, longest length × widest width at right angles, wound shape, wound bed, exudate, margin, undermining, tunneling, surrounding skin condition, and photoimaging when available. Wound documentation is important (Table 6). Document the wounds’ location and size. These authors recommend using the longest length and widest width perpendicular to one another, although head-to-toe alignment is also common. Pick the method of measurement that aligns with institutional policy; consistency is most important. Note and monitor undermining, tunneling, tissue type in the wound bed, wound margins, and periwound skin characteristics.
Table 6.
WOUND ASSESSMENT
| Criterion | Details |
|---|---|
| Location | Identify using accepted medical terminology |
| Measurement (or alternate method head to toe, depending on facility policy) | Longest length in any direction (in cm) |
| Widest width at right angle to longest length (in cm) | |
| Total surface area by longest length × widest width (in cm2) | |
| Shape | Circular, oval, triangular, square, other |
| Undermining/tunneling | Measure and describe (in cm) |
| Describe the direction by clock (patient’s head is 12 o’clock) | |
| Wound base color | Percent of tissue; pink, yellow, black, or friable red |
| Exudate amount | None, scant, moderate, heavy |
| Margin | Normal, rolled/irregular/advancing edge, cribriform |
| Periwound skin | Normal, erythema, indurated, satellite lesions |
©WoundPedia 2021.
Substatement 4B. Cleansing: gently with water, saline, or low-toxicity antiseptic agents. For healable wounds, local wound care may include sharp surgical debridement, treatment of infection (local infections, deep and surrounding infection), and moisture management. For nonhealable wounds, optimal care may be aimed at conservative debridement of slough, bacterial reduction, and moisture reduction. In these cases, antiseptic agents that may have some tissue toxicity may be preferable to allowing bacterial proliferation to cause further tissue damage leading to infection.
There is extraordinarily little high-quality evidence on the topic of wound cleansing (Table 7); accordingly, it is hard to draw any conclusions. The topic of wound cleansing is one that requires further research.19 When irrigating, note the amount of solution that was used going into and out of the wound bed. Caution should be used when the entire wound bed is not clearly visualized or intact. Be careful not to harm the wound bed through excess trauma.
Table 7.
METHODS OF WOUND CLEANSING
| Method | Description | Purpose | Risks |
|---|---|---|---|
| Compress | Use sterile saline or potable water | Astringent action (coagulate protein) to remove surface debris from the wound bed surface | • Compresses can stick to the wound surface or there may be local pain from application or removal |
| No cavities/tunneling: gently pressing excess moisture from a moistened gauze/cloth applied to the wound, removed, repeated | • Faulty technique can introduce infection | ||
| For cavities/tunneling: moistened ribbon gauze may be applied similarly by gently packing into tunnel, removed and repeated | • Remember to leave external remnant of gauze packing above the wound to facilitate removal | ||
| Irrigation | Steady gentle flow of solution across wound surface when the base of wound is clearly visualized | Hydrate the wound | • Retained irrigation solution may collect in pocket if wound base is not visible |
| Remove deeper debris | • Trauma if pressure is too high | ||
| Assist with visual examination of wound base | • Splash back | ||
| • High pressure may drive bacteria into deeper compartments | |||
| Soaking | Immersion of wound in solution by applying an overhydrated gauze/cloth to the wound surface (no removal of excess moisture prior to application) | Hydrate the wound | • Disruption of moisture balance |
| Allow for physical removal of surface debris | • Maceration of surrounding skin | ||
| • Impaired healing with introduction or redistribution of bacteria from immersion fluid |
Adapted from Nicks et al.18
©WoundPedia 2021.
Substatement 4C. Reassess and document wounds at appropriate, regular intervals.
STATEMENT 5
When Appropriate, Debride Wounds with Adequate Pain Control
Debridement is a way to remove slough, debris, or foreign substances that may facilitate infection or act as a proinflammatory stimulus, prolonging the inflammatory stage of wound healing and delaying the proliferative reparative process. Sharp surgical debridement requires assessment of the blood supply to be sure it is adequate for healing. Before starting, providers who are considering even conservative debridement methods must ensure they have appropriate competency, scope of practice, the required equipment, and support in the event of bleeding, as well as alignment with their facility’s policies and procedures.
Although it did achieve consensus, the relatively lower agreement levels among KOLs for this statement were likely attributable to facility-related limitations on sharp debridement. This procedure requires clinical experience, appropriate scope of practice, and availability of equipment to perform the procedure and stop bleeding if required.
Substatement 5A. Consider sharp surgical debridement (to bleeding tissue) for healable wounds and conservative surgical debridement for maintenance/nonhealable wounds. For healable wounds, this means sharp surgical debridement; autolytic debridement with dressings; or enzymatic, biologic (medical maggots), or mechanical debridement. For nonhealable and maintenance wounds, this means conservative surgical or other methods of nonviable slough removal.
Patient empowerment can be modeled on the 4-Step Clinical Decision-Making Debridement Guide20 for a mutual agreement between patients and clinicians. First, ask whether the wound is capable of healing. If the answer is yes, select the appropriate method based on patient concerns and wound characteristics. Next, investigate which wound characteristics influence debridement choice, such as secondary infection, pain, wound size, and exudate. Ascertain how selective a debridement method is needed; determine if there is any risk to healthy tissue when necrotic tissue is being debrided. Finally, consider the care setting. Some clinicians and/or types of resources may not be available in all care settings. Government regulation and facility policy may also be factors.20
Substatement 5B. Evaluate the need for alternative debridement modalities: autolytic with dressings, enzymatic, mechanical, or biologic. Autolytic debridement can be accomplished via calcium alginate, hydrogel, and hydrocolloid dressings. This type of debridement is often relatively painless, but it may be slower than surgical methods. Enzymatic debridement (collagenase) is often used where surgical debridement or autolytic dressings are not available. It is a relatively slow method, and the treatment requires a prescription.
Mechanical debridement may be accomplished using advanced technologies such as ultrasound that require clean or sterile conditions with protection from bacterial contamination and airborne bacterial pathogens or particulate matter. Whirlpool systems may contaminate areas of emersed skin and may cause cross-contamination between patients. Saline wet-to-dry gauze is nursing time-intensive, painful on dressing removal, and can remove healthy viable tissue from the wound surface.
Moya-López et al21 recently published a review of maggot debridement therapy for chronic wounds. Maggot therapy can be faster than some other nonsurgical debridement methods, and it is selective for devitalized tissue. The authors concluded that more data were needed by wound type, frequency of application, and the efficacy of treatment. Maggots are not indicated for ischemic wounds and when deep and surrounding infection has not been treated systemically.
STATEMENT 6
Assess and Treat Wounds for Infection/Inflammation
Wound infections have two compartments: one superficial and the other deep.10,12 Wounds can be thought of as a bowl of soup: the thin layer on the surface of a wound is analogous to the superficial compartment, and the sides and bottom of the soup bowl are equivalent to the surrounding and deep components of a chronic wound.
Substatement 6A. Treat local infection (three or more NERDS criteria) with topical antimicrobials (silver, iodine, polyhexamethylenebiguanide [PHMB]/chlorhexidine, methylene blue/crystal violet, surfactants). The superficial compartment of a chronic wound is a thin layer of cells that can be treated topically. Any three or more NERDS (Nonhealing, Exudate increase, Red friable granulation, Debris or dead cells, and Smell) criteria are signs of local infection, for which topical antimicrobials may be indicated. If the wound is healable and the cause treated, it should take 4 weeks or less to improve. Clinicians should know that treating the superficial wound compartment requires dressings to release antimicrobial agents onto the surface of the wound. Nonrelease dressings will work above the wound surface but cannot penetrate the superficial compartment. This may prevent bacterial growth above the wound, but another agent may be needed to target the surface wound compartment. For example, antiseptic sprays such as chlorhexidine mouthwashes often have less available alcohol with decreased local burning and stinging compared with some presurgical antiseptics designed for intact skin. Some topical agents release silver or iodine in various concentrations to penetrate the surface compartment and treat local infection.
Substatement 6B. Consider treating deep and surrounding infection (three or more STONEES criteria) with systemic antimicrobials. Topical antimicrobial agents penetrate only a few millimeters. Deep and surrounding infections may require systemic antimicrobials (Supplemental Table 5, http://links.lww.com/NSW/A63). Four of the seven STONEES criteria represent the wounds’ surrounding features (the sides of the soup bowl): increased Size, elevated Temperature of 3° F over a mirror image of the surrounding wound skin, New or satellite areas of involvement, and a surrounding cellulitis (Erythema or Edema). Cellulitis is not always present when chronic wounds are associated with deep and surrounding infection, and erythema is not easily recognized in skin of color or the presence of edema. The three remaining STONEES signs in the wound bed include probing to bone (Os [Latin for bone]), increased Exudate, and Smell.
Substatement 6C. Evaluate and alleviate persistent inflammation including consideration of anti-inflammatory agents (topical dressings, systemic medication). Factors other than infectious organisms can play a role in a persistent inflammatory response. These factors include invading cells (neutrophils, macrophages, lymphocytes), immune complexes (vasculitis), granulomatous inflammation (sarcoidosis, etc), and others. Consider these factors when picking a topical or systemic therapy. There are some topical antimicrobials that are proinflammatory, such as iodine. There are other agents that may be anti-inflammatory, including silver, and some that are neutral, such as PHMB gauze/foam and gentian violet/methylene blue foam.
Inflammation can also lead to delayed wound healing in both compartments. Protease tests are not always available in the clinical setting and may only measure surface rather than deep changes. Some of the signs of infection may also be part of the clinical presentation for persistent inflammation. Supplemental Figure 2 (http://links.lww.com/NSW/A64), The Sibbald Cube, outlines where high proteases in wounds with and without infection may prevent healing in both the superficial and deep compartments. Recently published data indicate biomarkers may predict the healing trajectory of venous leg ulcers.22 The right therapy at the right time could more effectively control proteases, bacterial contamination, debridement and moisture control with optimal timing of growth factors, matrix constructs, and cellular components.
In regard to topical therapies, silver and honey-based products have reported anti-inflammatory effects. These agents should only be used with local infection and inflammation for short periods of time. Systemically, several antibacterial agents have anti-inflammatory action. Commonly recommended antimicrobials (some with anti-inflammatory effects) for wounds and related skin infections are listed in Supplemental Table 5 (http://links.lww.com/NSW/A63).
STATEMENT 7
Moisture Management
Providers must select an appropriate dressing to match the wound characteristics and individual patient needs (Figure 3). Ideal moisture management depends on a wound’s healability.
Figure 3.
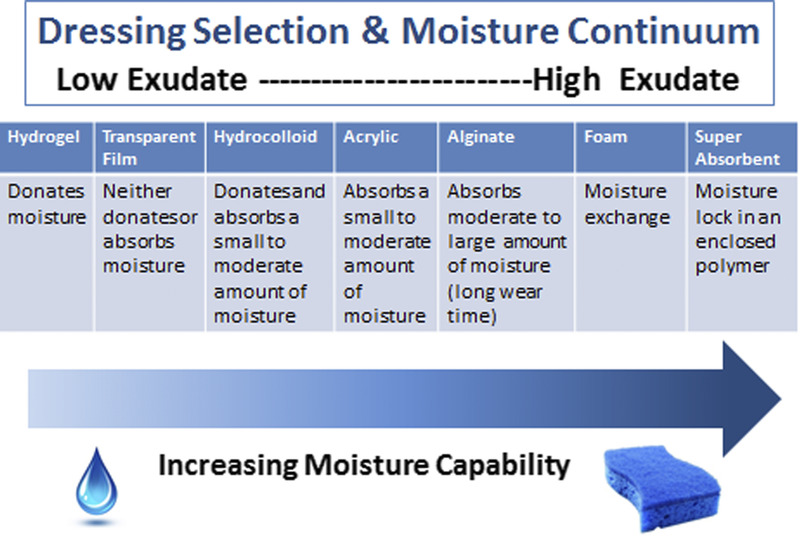
OPTIMIZING MOISTURE MANAGEMENT
Adapted from Sibbald et al.16 ©WoundPedia 2021.
Substatement 7A. Healable, moisture balance, and autolytic debridement: alginates, hydrogels, hydrocolloids, acrylics, films. In healable wounds, moisture balance can be achieved by choosing the appropriate dressing from the moisture continuum in the enabler (Supplemental Table 6, http://links.lww.com/NSW/A65) that lists dressings for low to highly exudative wounds.
Substatement 7B. Moisture balance alone: super absorbents, foams, calcium alginates, hydrofibers, hydrocolloids, films, hydrogels.
Substatement 7C: Nonhealable and maintenance wounds and moisture reduction: if antibacterial needed, low toxicity topical anesthetics: chlorhexidine/PHMB, iodine, acetic acid. For individuals with maintenance or nonhealable wounds, target moisture and bacteria reduction. Wounds need to be constantly reassessed for healing or deterioration, and dressing choices may need to be altered based on presentation.
For these wounds, providers need to balance patient preference and comfort to avoid pain, as well as prevent overdrying of wounds. Tulle dressings are often most appropriate; they are a combination of gauze or fabric with a petrolatum or paraffin coating. They may also contain an antiseptic (eg, chlorhexidine, iodine).
However, several dressings can optimize moisture management.16 Chlorhexidine (0.5% in white paraffin impregnated into a tulle sheet) is active against Gram-positive and negative bacteria; PHMB is a nonrelease foam, gauze/packing ribbon formulation. Iodine dressings (either in cadexomer molecule or as povidone iodine) have a broad spectrum of activity, albeit decreased effectiveness in the presence of pus or exudate. Note that these dressings may be toxic with prolonged use over large areas (as povidone iodine). Finally, acetic acid (0.5% to 1%, eg, diluted white vinegar) should be placed using gauze on the wound bed usually for about 5 to 10 minutes, often as a rotating compress. These dressings have a low pH and are effective against Pseudomonas species; however, they may select out other organisms.16
Substatement 7D: Wound packing: saline wet (donate moisture) or dry (absorb moisture) but not antibacterial; PHMB gauze: antibacterial, nonrelease-above the wound (stays in the gauze) only not in the wound surface; povidone iodine or other antiseptic soaked gauze: antibacterial above and on wound surface. Saline packing may be used in healable wounds without critical colonization. It is not the purpose of these dressings to stick to the wound bed so that there will be trauma with dressing removal. If a dry saline gauze sticks to the wound bed, the gauze should be moistened before application and, if it sticks, moistened again prior to removal. Alternate dressings should then be chosen to maintain moist, interactive healing.
STATEMENT 8
Evaluate the Rate of Healing
If a wound is not at least 20% to 40% smaller by week 4, it is unlikely to heal by week 12 (Figure 4).
Figure 4.
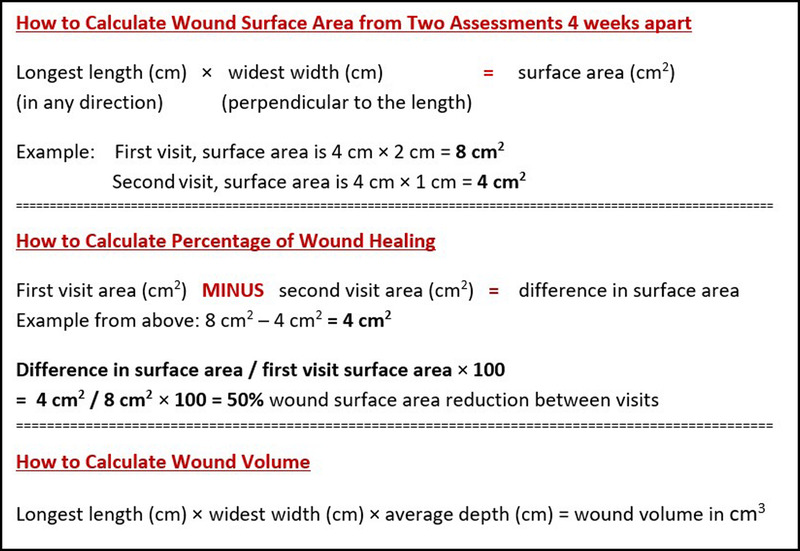
HOW TO CALCULATE WOUND SURFACE AREA
Substatement 8A. Stalled (healable) wounds should be reevaluated for alternate diagnoses; consider wound biopsy, further investigation, and/or referral to an interprofessional assessment team to optimize treatment. Healing trajectory can be assessed in the first 4 to 8 weeks to predict if a wound is likely to heal by week 12, provided there are no new complicating factors.9 Stalled but healable wounds often need a comprehensive interprofessional assessment to optimize treatment and improve the healing trajectory. This may necessitate the reclassification of a wound to the maintenance or nonhealable category.
STATEMENT 9
Edge Effect
Use active therapies for stalled but healable wounds. See Supplemental Table 7 (http://links.lww.com/NSW/A66) for evidence on adjunctive therapies: negative-pressure wound therapy, electrical stimulation, cellular and/or tissue-based products, skin grafts, ultrasound, and hyperbaric oxygen therapy (Table 8).
Table 8.
ADJUNCTIVE THERAPIES
| Recommendation | Therapy |
|---|---|
| Benefit in carefully selected patients | Skin grafts: split-thickness, full-thickness |
| Negative-pressure wound therapy | |
| Hyperbaric oxygen | |
| Uncertain evidence for routine clinical practice | Electrical stimulation |
| Ultrasound | |
| Neuromuscular stimulation | |
| Not recommended for clinical practice at this time | Light therapy (lasers and UV-C) |
| Topical oxygen |
Substatement 9A. Some active modalities have weak to mixed evidence and should be used only after interprofessional assessment of the patient and with regular reevaluations.
Substatement 9B. Skin grafts have variable but positive evidence, and cellular and/or tissue-based products may or may not be cost-effective at this time.
Many active therapies have appeared and disappeared in the wound healing toolbox. Not only do these therapies need to stimulate healing, but also they must be cost-effective within the context of the local health system. Some of these therapies have better evidence for acute wounds than with chronic, nonhealing wounds (eg, negative-pressure wound therapy after diabetic foot surgery, split-thickness skin grafts), particularly where the cause is not or cannot be corrected. If an active therapy is selected, it is imperative that a consistent and accurate wound assessment be conducted so that wound progression in either direction may be determined and the therapy discontinued in a timely manner if the wound is not on a healing trajectory. More high-quality randomized controlled trials on these therapies are needed before definitive recommendations on their use can be made.
STATEMENT 10
Organizational Support
Substatement 10A. Organizational support may include a culture conducive to interprofessional education and patient-centered care, standardized evidence-informed protocols, adequate staffing, and established quality improvement programs that may include audits, prevalence and incidence studies, and patient navigation. Elements of an effective organizational plan for guideline implementation are as follows:23
Assess organizational readiness and barriers to implementation, considering local circumstances.
Involve all members (whether in a direct or indirect supportive function) in the implementation process.
Provide ongoing educational opportunities to reinforce best practice.
One or more qualified individual(s) should provide the support needed for the education and implementation process.
Provide opportunities for reflection on personal and organizational experience in implementing guidelines.
Often the barriers to successful wound healing are related to the health system and not a lack of provider knowledge. Better coordination of care is needed across the continuum, from acute to chronic care, as well as standardization of formularies and best practices. This could be accomplished through situational learning, changing healthcare systems to facilitate interprofessional assessment of complex patient problems, and breaking down barriers within and across healthcare organizations. This requires organizations to invest in resources for interprofessional education on wound care practices, as well as collection and regular review of wound care data outcomes in the form of an ongoing quality initiative.
Patients with chronic wounds often have limited resources and come from lower socioeconomic backgrounds. Using patient navigation models to facilitate referrals and link homecare providers with care coordinators to access system resources is one way forward.24,25 However, this is only successful when team members are linked together as part of a coordinated interprofessional model. These health system changes can increase value.
The Porter model of healthcare links the voice of the patient with the provider, payer, policy maker, and even the politician to provide value for the healthcare dollar.26 For systems to change, policymakers and politicians must be aware of inconsistencies and inequities facing wound care patients and providers as the first step toward improving patient-centered wound care.


CONCLUSIONS
These 10 evidence-informed statements have received consensus from KOLs in repeated surveys. The provision of enablers is intended to assist with dissemination of the WBP paradigm into practice. A concerted effort has been made to emphasize the importance of early, proactive assessment of the wound healing trajectory. By intervening before wounds become chronic, there are benefits for the patient, providers, payors, and policy makers. This is more important now than ever in the face of mounting healthcare costs and aging populations.
PRACTICE PEARLS
The AHHD is a quicker alternative to the ABPI that does not require the patient to be recumbent, does not cause pain from a BP cuff, and is not influenced by arterial calcification.
The Canadian Nutritional Screening Tool has two simple questions that can be performed by any clinician without blood work or special investigations to identify persons in need of a dietitian consult.
A healable wound should have the cause corrected and must have adequate blood supply to heal. A maintenance or nonhealable wound should be treated to decrease moisture and bacteria to prevent local wound infection.
A wound should be at least 20% to 40% smaller at week 4 to heal at week 12.
Footnotes
Dr LeBlanc has disclosed that she is a speaker for Hollister, Coloplast, 3M, and Mölnlycke. Dr Ayello has disclosed that she has received educational/research grants from Sage/Stryker and Calmoseptine. Dr Sibbald has received grants from Mölnlycke, Calmoseptine, and the Government of Ontario for Project ECHO Skin and Wound. The remaining authors, faculty, staff, and planners, including spouses/partners (if any), in any position to control the content of this CME/NCPD activity have disclosed that they have no financial relationships with, or financial interests in, any commercial companies relevant to this educational activity.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.ASWCjournal.com).
To earn CME credit, you must read the CME article and complete the quiz online, answering at least 7 of the 10 questions correctly. This continuing educational activity will expire for physicians on March 31, 2023, and for nurses March 3, 2023. All tests are now online only; take the test at http://cme.lww.com for physicians and www.NursingCenter.com/CE/ASWC for nurses. Complete NCPD/CME information is on the last page of this article.
Supplemental digital content is available in the text.
REFERENCES
- 1.Sackett D, Rosenberg W, Gray J, Haynes R, WS R. Evidence based medicine: what it is and what it isn’t. BMJ 1996;312(7023):71–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brownson RC, Eyler AA, Harris JK, Moore JB, Tabak RG. Getting the word out: new approaches for disseminating public health science. J Public Health Manage Pract 2018;24(2):102–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keown K, Van Eerd D, Irvin E. Stakeholder engagement opportunities in systematic reviews: knowledge transfer for policy and practice. J Continuing Educ Health Prof 2008;28(2):67–72. [DOI] [PubMed] [Google Scholar]
- 4.Minkler M, Salvatore A. Participatory approaches for study design and analysis in dissemination and implementation research. In: Dissemination and Implementation Research in Health: Translating Science to Practice. New York, NY: Oxford University Press; 2012:192–212. [Google Scholar]
- 5.Gerhard-Herman MD Gornik HL Barrett C, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017;135(12):e726–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beaumier M Murray BA Despatis MA, et al. Best Practice Recommendations for the Prevention and Management of Peripheral Arterial Ulcers. Toronto, Ontario, Canada: Wounds Canada; 2020:1–75. [Google Scholar]
- 7.Alavi A, Sibbald RG, Nabavizadeh R, Valaei F, Coutts P, Mayer D. Audible handheld Doppler ultrasound determines reliable and inexpensive exclusion of significant peripheral arterial disease. Vascular 2015;23(6):622–9. [DOI] [PubMed] [Google Scholar]
- 8.Canadian Nutrition Society, Canadian Malnutrition Task Force . Canadian Nutritional Screening Tool (CNST). 2014. http://nutritioncareincanada.ca/sites/default/uploads/files/CNST.pdf. Last accessed January 14, 2021.
- 9.Laporte M Keller HH Payette H, et al. Validity and reliability of the new Canadian Nutrition Screening Tool in the ‘real-world’ hospital setting. Eur J Clin Nutr 2015;69(5):558–64. [DOI] [PubMed] [Google Scholar]
- 10.Purcell A, Buckley T, King J, Moyle W, Marshall A. Topical analgesic and local anesthetic agents for pain associated with chronic leg ulcers: a systematic review. Adv Skin Wound Care 2020;33(5):240–51. [DOI] [PubMed] [Google Scholar]
- 11.Woo KY, Coutts PM, Price P, Harding K, Sibbald RG. A randomized crossover investigation of pain at dressing change comparing 2 foam dressings. Adv Skin Wound Care 2009;22(7):304–10. [DOI] [PubMed] [Google Scholar]
- 12.Jensen J, Goodson W, Hopf H, Hunt T. Cigarette smoking decreases tissue oxygen. Arch Surg 1991;126(9):1131–4. [DOI] [PubMed] [Google Scholar]
- 13.Shanmugam VK, Couch KS, McNish S, Amdur RL. Relationship between opioid treatment and rate of healing in chronic wounds: opioids in chronic wounds. Wound Repair Regen 2017;25(1):120–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aujoulat I, d’Hoore W, Deccache A. Patient empowerment in theory and practice: polysemy or cacophony? Patient Educ Couns 2007;66(1):13–20. [DOI] [PubMed] [Google Scholar]
- 15.Moore Z Butcher G Corbett LQ, et al. Exploring the concept of a team approach to wound care: Managing wounds as a team. J Wound Care 2014;23 Suppl 5b:S1–S38. [DOI] [PubMed] [Google Scholar]
- 16.Sibbald RG, Elliott JA, Ayello EA, Somayaji R. Optimizing the moisture management tightrope with Wound Bed Preparation 2015. Adv Skin Wound Care 2015;28(10):466–76. [DOI] [PubMed] [Google Scholar]
- 17.Boersema GC Smart H Giaquinto-Cilliers MGC, et al. Management of nonhealable and maintenance wounds: a systematic integrative review and referral pathway. Adv Skin Wound Care 2021;34(1):11–22. [DOI] [PubMed] [Google Scholar]
- 18.Nicks BA, Ayello EA, Woo K, Nitzki-George D, Sibbald RG. Acute wound management: revisiting the approach to assessment, irrigation, and closure considerations. Int J Emerg Med 2010;3(4):399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moulin D Boulanger A Clark A, et al. Pharmacological management of chronic neuropathic pain: revised consensus statement from the Canadian Pain Society. Pain Res Manage 2014;19(6):328–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sibbald R, Niezgoda J, Ayello E. Debridement. In: Wound Care Essentials: Practice Principles. Baranoski S, Ayello EA, eds. ed 5th. Philadelphia, PA: Wolters Kluwer; 2020. [Google Scholar]
- 21.Moya-López J, Costela-Ruiz V, García-Recio E, Sherman RA, De Luna-Bertos E. Advantages of maggot debridement therapy for chronic wounds: a bibliographic review. Adv Skin Wound Care 2020;33(10):515–25. [DOI] [PubMed] [Google Scholar]
- 22.Stacey MC. Biomarker directed chronic wound therapy—a new treatment paradigm. J Tissue Viability 2020;29(3):180–3. [DOI] [PubMed] [Google Scholar]
- 23.Registered Nurses Association of Ontario . Assessment and Management of Foot Ulcers for People with Diabetes. 2nd ed.Toronto, Ontario, Canada: Registered Nurses’ Association of Ontario; 2013. [Google Scholar]
- 24.Freeman HP. The origin, evolution, and principles of patient navigation. Cancer Epidemiol Biomarkers Prev 2012;21(10):1614–7. [DOI] [PubMed] [Google Scholar]
- 25.Freund KM. Implementation of evidence-based patient navigation programs. Acta Oncol 2017;56(2):123–7. [DOI] [PubMed] [Google Scholar]
- 26.Porter ME, Lee TH. The Strategy That Will Fix Health Care. 2013. Harvard Business Review. https://hbr.org/2013/10/the-strategy-that-will-fix-health-care. Last accessed January 14, 2021.


