Abstract
Nickel remains the most commonly identified contact allergen. However, it has proven difficult to demonstrate significant skin-sensitizing activity for nickel in toxicology tests, which typically have indicated a weak skin sensitization potential. Information indicates that in vivo assays are not predictive of dermal sensitization hazard or potency for nickel due to a human-specific mechanistic route for nickel sensitization that animals lack. A similar rationale will apply to in vitro alternatives—although these currently have limited ability to determine intrinsic potency. Generally, in silico methods are not designed for metal allergens and cannot contribute to the analysis. For ethical reasons, human experimental work has been limited, with a single study suggesting moderate potency. Accordingly, it seems reasonable to conclude that the high frequency of contact allergy to nickel in humans is a function of both its intermediate potency coupled with a high level of dermal exposure, particularly to damaged/inflamed skin.
In the toxicological world of skin sensitizers and predictive tests for their identification and characterization, as well as the clinical world of contact allergy and the disease allergic contact dermatitis (ACD), no single cause has a longer and more abundant history than nickel. A good deal of this was captured about 3 decades ago in an impressive overview of current knowledge at that time.1 Recent publications confirm that nickel, despite legislation in some countries, remains at the top of the list of agents causing contact allergy—for example, approximately 16% (1 in 6) of patch-tested dermatology patients in Germany.2–4 The proportion with nickel contact allergy in the general population in Europe is not far short of this figure, at 14.5%.5 Of course, these high numbers contain a significant cohort who became nickel allergic before the European legislation, the purpose of which was to reduce exposure to metal objects in prolonged contact with the skin. The effectiveness of this legislation has been reviewed recently.6 However, the present work does not examine this history but rather offers a focus on 1 conundrum: the disparity between the frequency with which nickel induces contact allergy (and thereby the elicitation of ACD) versus its relative lack of skin-sensitizing potency in predictive tests.
Chemical substances causing skin sensitization (sensitizers) must make permanent changes to skin protein that can be recognized by the cellular immune system, triggering a response usually termed type IV delayed hypersensitivity. The immunobiology of this process has been reviewed recently and need not be reiterated here.2,7 However, pertinent to the current discussion is whether, and to what extent, skin sensitizers vary in their capacity to cause this outcome. This is typically referred to by the term “potency,” which refers to the ability to induce allergy. That skin sensitizers do have widely differing intrinsic induction potency is well described in animal models, as well as in humans.8–10 The central question addressed is where nickel fits into the potency spectrum; a secondary question considers thresholds.11,12 Prompted by this discussion is the question of whether there is a relationship between the potency of an allergen and its elicitation threshold.
For decades, substances that have the potential to behave as skin sensitizers have been identified by one or other in vivo methods, including the guinea pig maximization test, the Buehler occluded patch test (also using guinea pigs), or the murine local lymph node assay (LLNA).13–15 Recently, in vitro alternatives have come to the fore, although the interest in their use to identify novel metal allergens, for obvious reasons, has been very limited.16,17 In fact, some authors already have suggested that “metals are problematic to test” in in vitro methods.18 All of these methods are, at least in a regulatory sense, directed toward determining how a substance fits into the framework presented in Table 1. This shows how regulatory decisions are made using in vivo data to decide whether a substance is a significant skin sensitizer and, if so, whether it is stronger (category 1A) or weaker (category 1B). These determinations can be mapped to human potency categories; it is important to note that, for this purpose, each category is subdivided, with the lowest category “not classified” containing both true nonsensitizers and the very weakest skin sensitizers. Distinct categories introduce borders, which, by definition, introduce borderline substances (and decisions), always a cause of debate. The reality must be that skin sensitization potency represents a biological continuum, and this is how this article will endeavor to present how nickel fits into the picture.
TABLE 1.
Overview of Potency Categorization/Classification of Skin Sensitizers
| Human Category* | UN GHS Category† | LLNA EC3, %‡ | GPMT, % +ve‡ | Buehler, % +ve‡ | Examples* |
|---|---|---|---|---|---|
| 1 Extreme |
1A | ≤ 0.2% | ≥30% responding at ≤0.1% intradermal induction dose or ≥60% responding at >0.1% to ≤1% intradermal induction dose | ≥15% responding at ≤0.2% topical induction dose or ≥60% responding at >0.2% to ≤20% topical induction dose | Chloromethylisothiazolinone, 2,4-dinitrochlorobenzene, p-phenylenediamine, potassium dichromate |
| 2 Strong |
1A | ≤2% | Formaldehyde, isoeugenol, propyl gallate, p-aminophenol, thioglycerol | ||
| 3 Moderate |
1B | >2% | ≥30% to <60% responding at >0.1% to ≤1% intradermal induction dose or ≥30% responding at >1% intradermal induction dose | ≥15% to <60% responding at >0.2% to ≤20% topical induction dose or ≥15% responding at >20% topical induction dose | Citral, ethylenediamine, eugenol, imidazolidinyl urea, mercaptobenzothiazole, tetramethylthiuram disulfide |
| 4 Weak |
1B | >2% | Aniline, bronopol, carvone, hydroxycitronellal, resorcinol, ethyleneglycol dimethacrylate | ||
| 5 Very weak |
NC | Negative | <30% | <15% | p-Aminobenzoic acid, isopropanol, propyl paraben, propylene glycol |
| 6 Nonsensitizer |
NC | Negative | 0% | 0% | Ascorbic acid, benzene, dextran, glucose, hexane, phenol, sorbitol |
In the material that follows, the behavior of nickel in a range of predictive skin sensitization tests will be critically examined, alongside information on the behavior of nickel as a human allergen and insights that are provided concerning its relative sensitizing potency. This evidence will be weighed in the light of existing knowledge of the specific mechanism(s) by which nickel causes contact allergy but will exclude consideration of any toxicology beyond the classic skin sensitization pathways.
Predictive Toxicology Tests Using Nickel Salts
There exists a wide range of protocols, all using the guinea pig as the test species, developed for predictive identification of skin sensitizers.13 The methods follow a similar pattern—a primary phase in which a series of intradermal injections and/or topical applications were made, usually for 2 or 3 weeks, followed by a rest period of 1 or 2 weeks and, finally, a topical application (challenge) to uncover whether the primary phase had led to the induction of skin sensitization. The outcome of this type of work is presented in Table 2, excluding the (very limited amount of) data where challenge was conducted by intradermal injection, a procedure restricted to the evaluation of pharmaceuticals.21 Of these methods, only 2 survived for several decades as valid regulatory tests for skin sensitizer classification, namely, the occluded patch test of Buehler and the maximization test, with the latter being widely regarded as the more sensitive method.22,23
TABLE 2.
Results From the Testing of Nickel Salts in Guinea Pig Skin Sensitization Tests
| Method | Test Substance | Induction* | Elicitation | Positive | Reference |
|---|---|---|---|---|---|
| Maximization test | NiSO4 | 1%/5% | 0.5% | 55% | 20 |
| Maximization test | NiSO4 | 2%/2% | Not given | 50% | 58 |
| Maximization test | NiSO4 | 0.25%/0.7% | 0.15% | 10% | 23 |
| Maximization test | NiSO4 | 5%/25% | 5% | 23% | 21 |
| Maximization test | NiCl2 | 1% | 0.2% | 53% | 59 |
| Maximization test | NiCl2 | 0.25%/2% | 0.1% | 10% | 23 |
| Optimization test | NiSO4 | 0.1%/5% | 0.5% | 35% | 18 |
| Draize test | NiSO4 | Not given | Not given | 0% | 20 |
| Draize test | NiCl2 | 0.375% | 10% | 0% | 23 |
| Freund's complete adjuvant test | NiSO4 | 25% | 5% | 31% | 21 |
| Draize test | NiSO4 | 0.375% | 10% | 0% | 23 |
| Open epicutaneous test | NiSO4 | 25% | 5% | 50% | 21 |
| TINA test | NiSO4 | 1%/1% | 5% | 24% | 22 |
*Injection induction or topical induction concentrations.
In the various guinea pig skin sensitization assays, the frequency of positive results to either nickel chloride or nickel sulfate ranges from 0% to 55% positive. The highest value derives from the original work of Magnusson and Kligman23; 5 variations on this original work in terms of concentration often evinced similar positive frequencies, and as might be anticipated, the 2 studies at distinctly lower dose levels also gave a lower sensitization rate, 10%. Interestingly, an apparently higher dose study also delivered a lower sensitization rate of 23%.24 Maurer and colleagues'21 optimization test, designed to be highly sensitive, produced a positive frequency of 35%. The rarely used tierexperimenteller nachweistest (TINA) test showed 24% positive in 1 study, but collating all of the data in that publication showed 26 of 124 guinea pigs (21%) to be positive.25 The Draize test, recognized to be less sensitive than assays using Freund's complete adjuvant, failed to produce positive results upon exposure to either of the tested nickel salts; although not included in Table 2, injection challenge was also negative in both cases, indicating induction of allergy was wholly unsuccessful.26
Subsequent to the previously mentioned work, a Danish group conducted a dose-response guinea pig maximization test study, modifying the original protocol, such that, at any concentration, the maximum group size was only 6.27 At the highest induction concentration of 3% nickel sulfate, 8 of 18 animals (44%) were sensitized. Only when the induction concentration was reduced to 0.01% was no induction of sensitization observed. Combining the results at the highest induction concentration tested in this dose-response study with the results from 9 other guinea pig maximization tests summarized therein gives an average positive response frequency of only 36% (64/178), only a little higher than the 30% borderline for regulatory classification as a skin sensitizer (Table 1).
Several researchers have used nickel allergy in guinea pig models to examine various parameters associated with skin sensitization.28–30 Because such work is essentially research and typically uses nonstandard protocols, it is not considered further here, except to note that often authors such as those of previously mentioned research find it difficult to sensitize guinea pigs to nickel salts to any substantial degree.
How do these results map onto the regulatory framework shown in Table 1? Arguably, the definitive guinea pig maximization test is that of Magnusson and Kligman23 themselves, which would clearly lead to classification of nickel as a skin sensitizer in category 1B. Many of the other maximization tests lead to the same conclusion, but both of those by Goodwin and colleagues would lead to nonclassification, although this may simply reflect the lower concentrations used. None of the other tests have formal regulatory recognition, which means there are no criteria for evaluation of their results. However, it would be anticipated that, where there were clear positive responses, which was the case for several test types, this would lead to general classification as a skin sensitizer.
Taken together, the guinea pig results lead to the conclusion that nickel clearly has the potential to cause skin sensitization, but would only lead to categorization as a weak/moderate sensitizer.
The more recent in vivo method for the identification and characterization of potential skin sensitizers is the LLNA, which superseded the older guinea pig tests to become the preferred regulatory method before its own replacement by nonanimal alternatives.31 As part of the validation of the LLNA, the performance of the assay with a range of metal allergens and nonallergens was examined.32 In this study, there was a positive dose response to nickel chloride, but even at the highest response level, the result did not reach the level required for classification as a skin sensitizer. Had it been possible to conduct the LLNA using higher concentrations, it is possible that the threshold for positivity, a stimulation index of 3, would have been achieved. However, in a later study, using similar concentrations and vehicles, a clear positive result was obtained.32 This was then further substantiated in a nonvalidated LLNA variant using a nonradioactive endpoint.33 In addition, an LLNA study using a nonstandard aqueous vehicle gave a positive result with nickel sulfate at a concentration of 2.5%. Interpretation of the later Organisation for Economic Cooperation and Development regulatory standard LLNA results indicated an EC3 value of 3.5%.34 EC3 values are a well-recognized indicator of the relative potency of skin sensitizers.8,35 As indicated in Table 2, an EC3 figure of 3.5% places nickel into the lower subcategory, 1B, consistent with the conclusion from the guinea pig tests that this substance is not intrinsically a strong skin sensitizer.
Overall, in vivo skin sensitization tests suggest nickel is no more than a weakly/moderately potent allergen, but it is informative also to understand how it behaves in human experimental studies. Such investigations might be regarded as unethical in the 21st century, but results do exist from a pair of studies carried out in the middle of the last century. In 1963, on the basis of the clinical experience that “nickel is a weak sensitizer,” 172 subjects (previously proven not to be nickel allergic) were exposed to 5% nickel chloride on 3 test sites, variously involving freezing, irritation, occlusion, and repeated exposure. This treatment was repeated 3 times at 5-day intervals. Ultimately, 16 subjects (9%) were found to be positive to nickel upon challenge.36 Repeating the induction exposure regimen 4 months later on challenge-negative subjects sensitized a further 5 of 19. It is difficult to conclude much in terms of the potency of nickel, because no other allergens were used to provide points for comparison. All that can be said is that nickel is not a strong sensitizer—substances such as pentadecyl catechol (poison ivy), 2,4-dinitrochlorobenzene, and diphenylcyclopropenone (DPCP) would be expected to induce allergy in all subjects under similar exposure conditions.37 However, in this publication, the authors did note as part of their experimentation that nickel was rather slow to induce sensitization.36
A more interpretable human experimental study with nickel was reported a few years later by Prof Albert Kligman.38 In the human maximization test that he developed, nickel sulfate was tested at an induction concentration of 10% and a challenge concentration of 2.5%; in response to challenge, 48% (12/25) of the volunteers were shown to be sensitized. During his work to develop the final protocol for the assay, at an induction concentration of 5% and with challenge at 2.0%, 11 of 49 (22%) became sensitized to nickel sulfate.39 Unlike the earlier work mentioned previously, many other allergens were tested, meaning nickel could be ranked alongside them. To give this perspective, human maximization test results for selected and commonly understood contact allergens are shown in Table 3. Using only the “official” result of 48% positive, nickel sits toward the center of this selected group of marker allergens, being neither strong nor weak but seeming to be describable as a moderately potent allergen.
TABLE 3.
The Performance of Nickel in the Human Maximization Test
| Test Substance* | Induction/Challenge,† % | Positive |
|---|---|---|
| Potassium dichromate | 2.0/0.25 | 100% |
| p-Phenylenediamine | 10/0.5 | 100% |
| Mercuric chloride | 2.0/0.05 | 92% |
| Epoxy resin‡ | 25/15 | 84% |
| Butylglycidyl ether | 10/10 | 79% |
| Formalin | 5.0/1.0 | 72% |
| Gold chloride | 2.0/0.005 | 69% |
| Penicillin G | 25/10 | 58% |
| Nickel sulfate | 10/2.5 | 48% |
| Cobaltous sulfate | 25/2.5 | 40% |
| Mercaptobenzothiazole | 25/10 | 38% |
| Aniline | 20/10 | 28% |
| Benzocaine | 25/10 | 19% |
| Methyl paraben | 25/10 | 4% |
| Aluminum chloride | 25/10 | 0% |
| Propylene glycol | 25/10 | 0% |
Summarizing graphically where nickel fits within the sensitizing potency spectrum, the information from human, guinea pig, and mouse experimental studies has been positioned among a range of other well-known skin sensitizers (Fig. 1). As a further point of reference, the human potency categories previously defined for skin sensitizers are used, suggesting nickel fits toward the middle of this grouping.9,10
Figure 1.
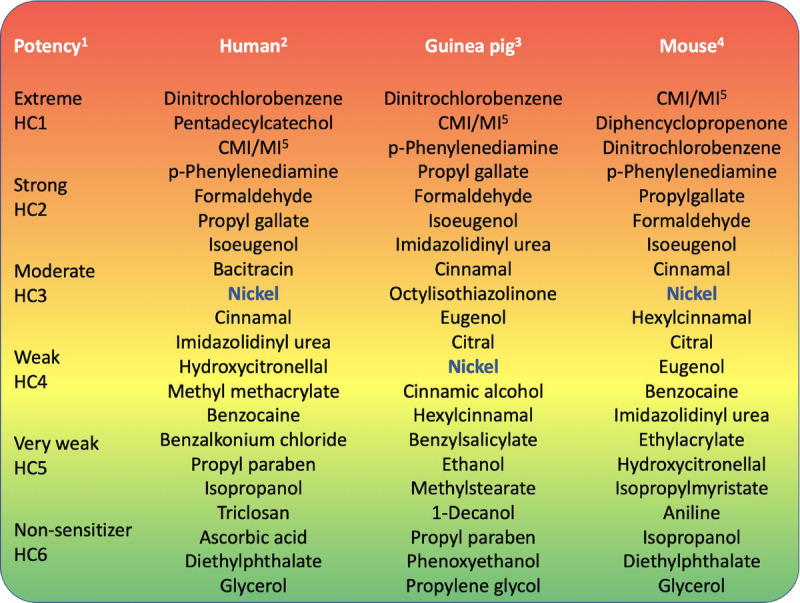
Nickel: its place in the potency spectrum. This figure presents an overview of where nickel seems to fit within the skin sensitization potency spectrum. The most potent sensitizers are at the red end (top); the weakest allergens and nonsensitizers are at the green end (bottom) of the figure. *The 6 groupings are an illustration based on Basketter et al.8,9 †This offers a rank order based on Basketter et al8,9 and Kimber and Basketter.35,63 ‡This list offers a rank order based on Buehler,22 Hicks et al,40 and Wahlberg and Boman.41 §The rank order presented is extracted from the listing previously published.42,62 CMI/MI indicates chloromethylisothiazolinone/methylisothiazolinone; HC, human category.
Nickel Salts and What the Clinical Picture Suggests Regarding Potency
Nickel remains a very common human contact allergen and an important cause of ACD. Evidence for this derives from use of nickel sulfate at either 2.5% or 5% in petrolatum in the baseline diagnostic patch test screening trays in North America, Europe, and many other countries.43–46 Results show the similarities and differences in prevalence rates for common contact allergens around the world. An example of data from 3 distinct locations is given in Table 4. Inevitably, such results embrace multiple variables, including differing patterns of exposure in these locations, as well as variation in local medical referral practices and the precise details of the diagnostic patch test process, combined with the impact of regional health and safety legislation. For example, whereas nickel has always a relatively high percentage of patients tested, the response to chromate varies, for example, in Europe, due to the use of ferrous sulfate chelating agent in cement, which sharply reduces the frequency of allergy.6 That same article notes also the positive impact of European legislation on lowering the frequency of nickel contact allergy, although room for improvement remains (eg, increased compliance with the legislation and adoption of similar legislation in other geographies) to decrease the prevalence further.
TABLE 4.
Prevalence of Contact Allergy to Selected Sensitizers in Clinical Populations in Europe and the United States
| Region | NiSO4 | Fragrance | MDGN* | MCI/MI† | Cr* |
|---|---|---|---|---|---|
| North America43 | 17.5% | 11.3% | 3.5% | 7.3% | 2.2% |
| Europe3 | 15.0% | 6.8% | 4.2% | 4.0% | 4.4% |
| Thailand45 | 28.1% | 13.2% | 2.9% | 16.8% | 11.3% |
*Tested as potassium dichromate.
MCI/MI indicates methylchloroisothiazolinone/methylisothiazolinone; MDGN, methyldibromo glutaronitrile.
Because of the wide range of variables associated with information on diagnostic patch test frequency, it is rarely possible to draw meaningful conclusions regarding the intrinsic sensitization induction potency of a substance from such data. For nickel, there exists a long history of testing for contact allergy by the application of nickel sulfate at 2.5% to 5% in petrolatum, but its interpretation in terms of intrinsic potency for induction is completely compromised by the very widespread and differing sources of exposure, whose relative impact is impossible to adequately characterize. Consequently, some have asked whether it may be possible to gain insights into potency from elicitation dose-response results.
Once an individual has become sensitized to a contact allergen, he/she will remain allergic to the material for the remainder of his/her life, which, subject to all necessary safety and ethical reviews, allows the possibility of recalling diagnosed patients to permit elicitation dose-response studies to be conducted. As a very common contact allergen, nickel has been featured prominently in such work.47,48 Indeed, not only have simple dose-response studies been conducted, but also experiments have been completed examining whether the slope of that dose response, or the elicitation threshold, is impacted by concomitant irritation or genotypes associated with atopic and/or allergic dermatitis.49–51 However, the essential question here is whether the elicitation dose response/threshold provides information on the intrinsic potency of a skin sensitizer. This formed the central subject of an authoritative review.52 These authors concluded, “Our results indicate that elicitation thresholds cannot be readily deduced from sensitization thresholds.” The opinion independently confirmed the earlier view from a European expert group that “elicitation thresholds correlate only poorly with induction potency.”53 Studies on elicitation dose response and thresholds are conducted on recalled allergy patients who are likely to vary greatly in their degree of sensitivity—highly allergic individuals will have lower elicitation thresholds and a steeper dose response; other subjects may be diagnosed as positive but be only very weakly allergic in practice. Thus, the makeup of a test panel has the potential to have a profound impact on the outcome of the study.
Ultimately, it is best to conclude that information from elicitation dose-response and threshold studies tells us only that nickel is a skin-sensitizing contact allergen but informs us not at all regarding its intrinsic potency.
Can anything else in the clinical picture of nickel allergy deliver insights into its potency as a contact allergen? Certainly, the fact that nickel has been associated with systemic contact dermatitis may have clinical importance, but this is not interpretable with respect to its intrinsic potency.54 Perhaps, the most obvious aspect is that, although not exclusively the cause of nickel allergy, nickel release from jewelry used in skin piercings is well recognized as a very important driver—the more piercings, the greater the risk of nickel allergy.5 It seems obvious that the intensity of exposure to compromised, probably inflamed skin (at least in the period immediately after the piercing procedure) is the major contributing factor to this reality. Were a similar process to be followed with a potent allergen, it is easy to imagine that the incidence of sensitization would be much higher. Even without the skin damage, powerful allergens such as 2,4-dinitrochlorobenzene and DPCP can induce sensitization after only a single contact.55,56 In the latter publication, 98% of those exposed to a 2% solution developed allergy to DPCP. In contrast, nickel seems to require prolonged and repeated, perhaps continuous, contact with the skin to produce a frequent incidence of contact allergy. This is evidenced by the (historic) importance of exposure to nickel via the metal clasps on suspenders—a well-recognized cause of contact allergy in women in the middle of the 20th century.57 In this setting, prolonged occlusion against the skin on a daily basis to a metal object capable of releasing nickel led to the clinical problem. A comparable situation arose in the later part of the 20th century, with nickel allergy being well recognized in association with the waistline button on denim jeans.58 It is “intimate” exposures such as these, particularly when they are prolonged and repeated, that have led to the high incidence of nickel contact allergy.
Summary and Conclusions
At the end of the last century, the very eminent research dermatologist Prof Jan Wahlberg came to the conclusion: “The basic question of why nickel is such a common cause of contact allergy in the female population, but not a potent contact allergen in experimental animals can probably be explained by exposure conditions.”59 Does anything we have learned since that time alter, or indeed substantiate, this perspective? In our view, the most significant new knowledge that has a bearing on this arises from new insights into the molecular mechanism of nickel allergy.60 The recognition that nickel allergy is mediated through a specific toll-like receptor present on human, but not other mammalian, cells was immediately perceived to provide a rationale for the less effective induction of sensitization in the in vivo test systems.61 Nevertheless, it does not explain the fact that nickel is clearly not intrinsically a strong sensitizer in human predictive tests. Thus, one is left with the obvious conclusion that the common clinical prevalence of nickel allergy is very largely a direct consequence of the extent of human skin exposure.
Footnotes
D.B. was compensated by NiPERA for the time spent in preparation of this article. Notwithstanding, the opinions expressed in this article are entirely those of the author.
REFERENCES
- 1.Maibach HI, Menné T. Nickel and the Skin: Immunology and Toxicology. Boca Raton, FL: CRC Press; 1989. [Google Scholar]
- 2.Ahlström MG Thyssen JP Wennervaldt M, et al. Nickel allergy and allergic contact dermatitis: a clinical review of immunology, epidemiology, exposure, and treatment. Contact Dermatitis 2019;81:227–241. [DOI] [PubMed] [Google Scholar]
- 3.Uter W. Prevalence of contact sensitization in the general population and patch tested patients. Hautarzt 2020;71(3):166–173. [DOI] [PubMed] [Google Scholar]
- 4.Johansen JD, Werfel T. Highlights in allergic contact dermatitis 2018/2019. Curr Opin Allergy Clin Immunol 2019;19:334–340. [DOI] [PubMed] [Google Scholar]
- 5.Schuttelaar MLA Ofenloch RF Bruze M, et al. Prevalence of contact allergy to metals in the European general population with a focus on nickel and piercings: the EDEN Fragrance Study. Contact Dermatitis 2018;79:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heim K, Basketter D. Metal exposure regulations and their effect on allergy prevention. In: Chen JK, Thyssen JP, eds. Metal Allergy. Cham, Switzerland: Springer; 2018:39–54. [Google Scholar]
- 7.Esser PR, Martin SF. Extended understanding of pathogenesis and treatment of contact allergy. Hautarzt 2020;71(3):174–181. [DOI] [PubMed] [Google Scholar]
- 8.Basketter DA, Gerberick GF, Kimber I. The local lymph node assay EC3 value: status of validation. Contact Dermatitis 2007;57:70–75. [DOI] [PubMed] [Google Scholar]
- 9.Basketter DA Alepee N Ashikaga T, et al. Categorisation of chemicals according to their relative human skin sensitising potency. Dermatitis 2014;25:11–21. [DOI] [PubMed] [Google Scholar]
- 10.Api AM Parakhia R O'Brien D, et al. Fragrances categorised according to their relative human skin sensitisation potency. Dermatitis 2017;28:299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kimber I Dearman RJ Basketter DA, et al. Dose metrics in the acquisition of skin sensitization: thresholds and importance of dose per unit area. Regul Toxicol Pharmacol 2008;52:39–45. [DOI] [PubMed] [Google Scholar]
- 12.Boukhman MP, Maibach HI. Thresholds in contact sensitization: immunologic mechanisms and experimental evidence in humans—an overview. Food Chem Toxicol 2001;39:1125–1134. [DOI] [PubMed] [Google Scholar]
- 13.Andersen KE, Maibach HI. Contact allergy: predictive tests in guinea pigs. Curr Probl Dermatol 1985;14:1–299. [PubMed] [Google Scholar]
- 14.Gerberick GF Ryan CA Kimber I, et al. Local lymph node assay validation assessment for regulatory purposes. Am J Contact Dermat 2000;11:3–18. [DOI] [PubMed] [Google Scholar]
- 15.Cockshott A Evans P Ryan CA, et al. The local lymph node assay in practice: a current regulatory perspective. Hum Exp Toxicol 2006;25:387–394. [DOI] [PubMed] [Google Scholar]
- 16.Ezendam J, Braakhuis HM, Vandebriel RJ. State of the art in non-animal approaches for skin sensitisation testing: from individual test methods towards testing strategies. Arch Toxicol 2016;90:2861–2883. [DOI] [PubMed] [Google Scholar]
- 17.Kleinstreuer NC Hoffmann S Alépée N, et al. Non-animal methods to predict skin sensitization (II): an assessment of defined approaches. Crit Rev Toxicol 2018;48:359–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gibbs S Kosten I Veldhuizen R, et al. Assessment of metal sensitizer potency with the reconstructed human epidermis IL-18 assay. Toxicology 2018;393:62–72. [DOI] [PubMed] [Google Scholar]
- 19.United Nations. 2015. Globally harmonized system of classification and labelling of chemicals (6th revised edition). Available at: www.unece.org/fileadmin/DAM/trans/danger/publi/ghs/ghs_rev06/English/03e_part3.pdf. Accessed November 10, 2018.
- 20.ECHA . Guidance on the application of the CLP criteria. Version 5. Dated July 2017. Available at: https://echa.europa.eu/documents/10162/23036412/clp_en.pdf/58b5dc6d-ac2a-4910-9702-e9e1f5051cc5. Accessed January 20, 2020.
- 21.Maurer T Thomann P Weirich EG, et al. Predictive evaluation in animals of the contact allergenic potential of medically important substances. II. Comparison of different methods of cutaneous sensitization with weak allergens. Contact Dermatitis 1979;5:1–10. [DOI] [PubMed] [Google Scholar]
- 22.Buehler EV. Delayed contact hypersensitivity in the guinea pig. Arch Dermatol 1965;91:171–177. [DOI] [PubMed] [Google Scholar]
- 23.Magnusson B, Kligman AM. Allergic Contact Dermatitis in the Guinea Pig. Springfield, IL: Charles C Thomas; 1970. [Google Scholar]
- 24.Lammintausta K, Kalimo K, Jansén CT. Experimental nickel sensitization in the guinea pig: comparison of different protocols. Contact Dermatitis 1985;12:258–262. [DOI] [PubMed] [Google Scholar]
- 25.Ziegler V, Suss E. The TINA test. In: Andersen KE, Maibach HI, Karger B, eds. Current Problems in Dermatology. Karger, Basel. Vol 14. 1985:172–192. [PubMed] [Google Scholar]
- 26.Goodwin BJ, Crevel RW, Johnson AW. A comparison of three guinea pig sensitization procedures for the detection of 19 reported human contact sensitizers. Contact Dermatitis 1981;7:248–258. [DOI] [PubMed] [Google Scholar]
- 27.Rohold AE, Nielsen GD, Andersen KE. Nickel-sulphate–induced contact dermatitis in the guinea pig maximization test: a dose-response study. Contact Dermatitis 1991;24:35–39. [DOI] [PubMed] [Google Scholar]
- 28.Wahlberg JE. Sensitization and testing of guinea pigs with nickel sulfate. Dermatologica 1976;152:321–330. [DOI] [PubMed] [Google Scholar]
- 29.Turk JL, Parker D. Sensitization with Cr, Ni and Zr salts and allergic type granuloma formation in the guinea pig. J Invest Dermatol 1977;68:341–345. [DOI] [PubMed] [Google Scholar]
- 30.van Hoogstraten IM de Groot J Boden D, et al. Development of a concomitant nickel and chromium sensitization model in the guinea pig. Int Arch Allergy Immunol 1992;97:258–266. [DOI] [PubMed] [Google Scholar]
- 31.Basketter DA McFadden JF Gerberick GF, et al. Nothing is perfect, not even the local lymph node assay. A commentary and the implications for REACH. Contact Dermatitis 2009;60:65–69. [DOI] [PubMed] [Google Scholar]
- 32.Basketter DA Lea L Cooper K, et al. The identification of metal allergens in the local lymph node assay. Am J Contact Dermat 1999;10:207–212. [DOI] [PubMed] [Google Scholar]
- 33.Basketter DA Kolle SN Schrage A, et al. Experience with local lymph node assay performance standards using standard radioactivity and non-radioactive cell count measurements. J Appl Toxicol 2012;32:590–596. [DOI] [PubMed] [Google Scholar]
- 34.Kolle S Basketter DA Casati S, et al. Performance standards and alternative assays: practical insights from skin sensitization. Regul Toxicol Pharmacol 2013;65:28–285. [DOI] [PubMed] [Google Scholar]
- 35.Kimber I, Basketter DA. Contact sensitization: a new approach to risk assessment. Hum Ecol Risk Assess 1997;3:385–395. [Google Scholar]
- 36.Vandenburg JJ, Epstein WL. Experimental nickel contact sensitization in man. J Invest Dermatol 1963;41:413–418. [DOI] [PubMed] [Google Scholar]
- 37.Kligman AM. Poison ivy (Rhus) dermatitis; an experimental study. AMA Arch Derm 1958;77:149–180. [DOI] [PubMed] [Google Scholar]
- 38.Kligman AM. The identification of contact allergens by human assay. III. The maximization test. J Invest Dermatol 1966;47:393–409. [DOI] [PubMed] [Google Scholar]
- 39.Kligman AM. The identification of contact allergens by human assay. II. Factors influencing the induction and measurment of allergic contact dermatitis. J Invest Dermatol 1966;47:375–392. [DOI] [PubMed] [Google Scholar]
- 40.Hicks R, Hewitt PJ, Lam HF. An investigation of the experimental induction of hypersensitivity in the guinea pig by material containing chromium, nickel and cobalt from arc welding fumes. Int Arch Allergy Appl Immunol 1979;59:265–272. [DOI] [PubMed] [Google Scholar]
- 41.Wahlberg JE, Boman A. Guinea pig maximization test. In: Andersen KE, Maibach HI, eds. Contact Allergy: Predictive Test in Guinea Pigs. Current Problems in Dermatology. Basel, Switzerland: Karger; 1985:59–106. [DOI] [PubMed] [Google Scholar]
- 42.Cronin MT, Basketter DA. Multivariate QSAR analysis of a skin sensitization database. SAR QSAR Environ Res 1994;2:159–179. [DOI] [PubMed] [Google Scholar]
- 43.Alikhan A Cheng LS Ale I, et al. Revised minimal baseline series of the International Contact Dermatitis Research Group: evidence-based approach. Dermatitis 2011;22:121–122. [PubMed] [Google Scholar]
- 44.DeKoven JG, Warshaw EM, Zug KA. North American Contact Dermatitis Group patch test results: 2015–2016. Dermatitis 2018;29:297–309. [DOI] [PubMed] [Google Scholar]
- 45.Wilkinson M Gonçalo M Aerts O, et al. The European baseline series and recommended additions: 2019. Contact Dermatitis 2019;80:1–4. [DOI] [PubMed] [Google Scholar]
- 46.Sukakul T Chaweekulrat P Limphoka P, et al. Changing trends of contact allergens in Thailand: a 12-year retrospective study. Contact Dermatitis 2019;81:124–129. [DOI] [PubMed] [Google Scholar]
- 47.Fischer LA, Johansen JD, Menné T. Nickel allergy: relationship between patch test and repeated open application test thresholds. Br J Dermatol 2007;157:723–729. [DOI] [PubMed] [Google Scholar]
- 48.Fischer LA, Menné T, Johansen JD. Experimental nickel elicitation thresholds—a review focusing on occluded nickel exposure. Contact Dermatitis 2005;52:57–64. [DOI] [PubMed] [Google Scholar]
- 49.Allenby CF, Basketter DA. An arm immersion model of compromised skin (II). Influence on minimal eliciting patch test concentrations of nickel. Contact Dermatitis 1993;28:129–133. [DOI] [PubMed] [Google Scholar]
- 50.Menné T, Calvin G. Concentration threshold of non-occluded nickel exposure in nickel-sensitive individuals and controls with and without surfactant. Contact Dermatitis 1993;29:180–184. [DOI] [PubMed] [Google Scholar]
- 51.Ross-Hansen K Johansen JD Vølund A, et al. The nickel dose-response relationship by filaggrin genotype (FLG). Contact Dermatitis 2014;71:49–53. [DOI] [PubMed] [Google Scholar]
- 52.Ezendam J Vermeulen JP de Klerk A, et al. A quantitative approach to assess the potency of skin sensitizers in the elicitation phase. Toxicology 2012;299:20–24. [DOI] [PubMed] [Google Scholar]
- 53.Basketter DA Andersen KE Lidén C, et al. Evaluation of the skin sensitising potency of chemicals using existing methods and considerations of relevance for elicitation. Contact Dermatitis 2005;52:39–43. [DOI] [PubMed] [Google Scholar]
- 54.Ricciardi L Arena A Arena E, et al. Systemic nickel allergy syndrome: epidemiological data from four Italian allergy units. Int J Immunopathol Pharmacol 2014;27:131–136. [DOI] [PubMed] [Google Scholar]
- 55.Rees JL, Friedmann PS, Matthews JN. The influence of area of application on sensitization by dinitrochlorobenzene. Br J Dermatol 1990;122:29–31. [DOI] [PubMed] [Google Scholar]
- 56.Chiang KS Mesinkovska NA Piliang MP, et al. Clinical efficacy of diphenylcyclopropenone in alopecia areata: retrospective data analysis of 50 patients. J Investig Dermatol Symp Proc 2015;17:50–55. [DOI] [PubMed] [Google Scholar]
- 57.Calnan CD, Wells GC. Suspender dermatitis and nickel sensitivity. Br Med J 1956;1:1265–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brandrup F, Larsen FS. Nickel dermatitis provoked by buttons in blue jeans. Contact Dermatitis 1979;5:148–150. [DOI] [PubMed] [Google Scholar]
- 59.Wahlberg JE. Nickel: animal sensitization assays. In: Maibach HI, Menné T, eds. Nickel and the Skin. Boca Raton, FL: CRC Press; 1989:65–73. [Google Scholar]
- 60.Saito M Arakaki R Yamada A, et al. Molecular mechanisms of nickel allergy. Int J Mol Sci 2016;17(2):E202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kimber I Basketter DA McFadden JP, et al. Characterisation of skin sensitising chemicals: a lesson learnt from nickel allergy. J Immunotoxicol 2011;8:1–2. [DOI] [PubMed] [Google Scholar]
- 62.Bourrinet P Puchault P Sarrazin G, et al. Etude comparée de quelques substance allergéniques chez l'homme et chez le cobaye. J Pharm Belg 1979;34:21–36. [PubMed] [Google Scholar]
- 63.Basketter DA, Kimber I. Predictive tests for irritants and allergens and their use in quantitative risk assessment. In: Johansen JD, Frosch PF, Lepoittevin J-P, eds. Contact Dermatitis. 5th ed. Chapter 13. Berlin, Germany: Springer; 229–240; 2011. [Google Scholar]


