Abstract
Background:
It has been revealed that CD109 expression is associated with prognosis in cancer patients, but it remains unclear thus far. Therefore, we performed a meta-analysis in the present study for a better assessment of the prognostic role of CD109 expression in cancer patients.
Methods:
Eligible studies were collected through a search of the PubMed, Embase, Cochrane Library, and Scopus databases. The pooled hazard ratio (HR) with 95% confidence interval (CI) was evaluated to reveal the association between CD109 expression and overall survival (OS) in cancer patients.
Results:
Seven studies with 1583 patients were enrolled. The pooled HR with 95% CI was calculated as 2.31 (95% CI 1.93–2.76, P < .001), suggesting an association between high expression of CD109 and unfavorable OS in cancer patients.
Conclusion:
This analysis indicated that CD109 expression could be used as a prognostic biomarker in cancer patients. This is the first meta-analysis to report the relationship between CD109 expression and prognosis in cancer patients.
Keywords: cancer, CD109, meta-analysis, prognosis
1. Introduction
Cancer poses a serious threat to human health and remains the leading cause of death worldwide.[1] Despite advances in diagnosis and treatment, the prognosis of cancer remains poor.[2] Therefore, it is essential to develop prognostic biomarkers for identifying an accurate prognosis.[2] Prognostic biomarkers could enable a more accurate prediction of cancer prognosis and can help the clinician to preselect patients with a poor prognosis for a more aggressive treatment.[3]
CD109 is a glycosylphosphatidylinositol-anchored glycoprotein that is a member of the α2-macroglobulin/complement family.[4,5] CD109 is a coreceptor for transforming growth factor (TGF)-β, and it regulates TGF-β receptor endocytosis and degradation and suppresses TGF-β signaling.[5,6] Dysregulation of TGF-β signaling is related to malignancies because TGF-β plays a critical role in cell proliferation, differentiation, and migration.[6,7] The process of TGF-β associated with CD109 is thought to contribute to the development of cancer.
Recent studies have reported that CD109 expression is increased in various cancers, including squamous cell carcinoma of the lung, vulva and uterine cervix, adenosquamous carcinoma of the gallbladder, and ductal adenocarcinoma of the pancreas.[4,7–10] Moreover, some studies have suggested that high expression of CD109 is associated with unfavorable prognosis in cancer patients.[5,11–16] Thus, for a better assessment of the prognostic role of CD109 expression in cancer patients, we performed a meta-analysis in the present study.
2. Materials and methods
2.1. Search strategy
According to the PRISMA guidelines, articles published up to January 2021 were searched in the PubMed, Embase, Cochrane library, and Scopus databases using the following terms: (CD109) and (cancer or tumor or carcinoma or neoplasm or malignancy) and (prognostic or predict or prognosis or survival or outcome). All articles were screened for exclusion, and review articles were screened to identify eligible studies. As all analyses were based on previously published studies, ethical approval and informed consent were not needed for this study.
2.2. Inclusion and exclusion criteria
The eligible studies were required to meet the following criteria: CD109 expression was determined in human cancer tissue; the association between CD109 expression and overall survival (OS) was evaluated; and the hazard ratio (HR) and 95% confidence interval (CI) data calculated using Cox regression analysis were provided. Studies were excluded from further consideration if they were duplicate studies; and conference abstracts, case reports, reviews, letters, and non-English articles.
2.3. Data extraction and quality assessment
Data of the following variables were collected from the full texts of the included articles: first author, publication year, country, cancer type, sample size, sex and median age of patients, study period, follow-up period, endpoints, CD109 expression associated with poor prognosis, cutoff value of CD109 expression, HR, and 95% CI for OS. Any disagreements were resolved by consensus.
The quality of the included articles was assessed using the Newcastle–Ottawa Scale (NOS). The article that was scored greater than 5 was considered to have a high quality. Two authors independently performed quality assessment of each study.
2.4. Statistical analysis
Cochran Q and I2 statistics were used to analyze the heterogeneity among the included studies. The pooled HR and 95% CI were calculated to evaluate the prognostic role of CD109 expression in cancer patients. Funnel plots and Egger tests were also conducted to show publication bias. Sensitivity analysis was performed to assess the robustness of the pooled results. All data were analyzed using StataSE12 (Stata, College Station, TX). P < .05 was considered to indicate statistical significance.
3. Results
3.1. Characteristics of the included studies
Seven studies were selected from the 141 articles initially searched (Fig. 1). The basic characteristics of the included studies are summarized in Table 1. The included studies reported about 6 types of cancers with 1583 patients: ovarian epithelial cancer, diffuse large B-cell lymphoma, myxofibrosarcoma, breast cancer, soft tissue sarcoma, and lung adenocarcinoma. CD109 expression was assessed by immunohistochemistry analysis in all the included studies. The NOS score of the included studies was 6 or 7 or 8 [Supplemental Digital Content (table S1)].
Figure 1.
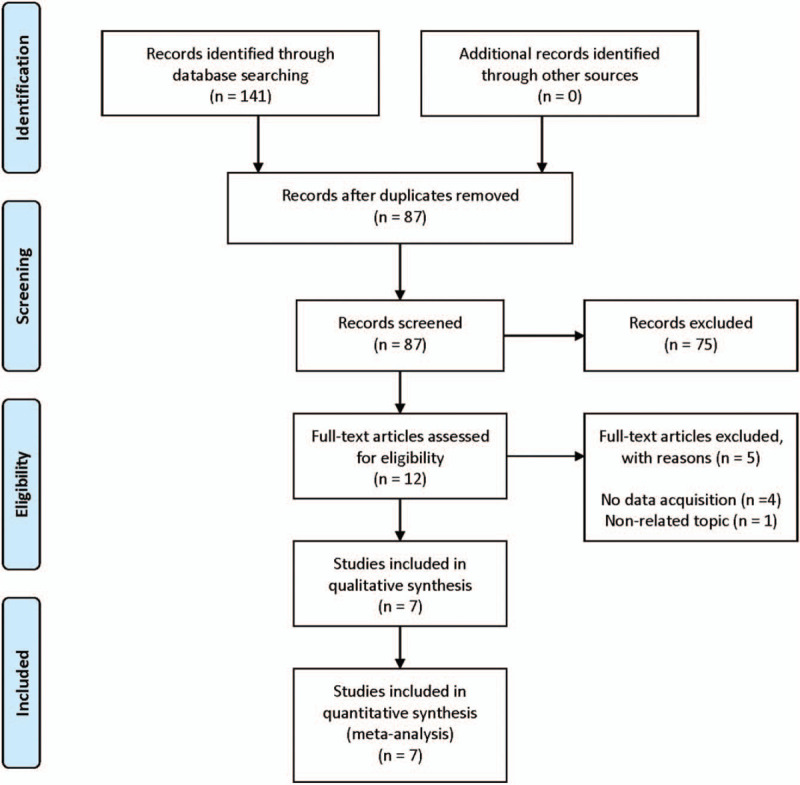
Flow diagram of the study selection process.
Table 1.
Basic characteristics of the included studies.
| Study | Country | Cancer type | Sample size | Sex (male/female) | Median or mean age, yr | Study period | Median follow-up, mo | Endpoints | CD109 detection | CD109 expression associated with poor prognosis | Cut-off value of CD109 expression | Survival analysis | NOS |
| Emori et al (2013)[12] | Japan | soft tissue sarcoma | 80 | NA | NA | 2004--2009 | NA | OS | IHC | positive expression | > 10% of the tumor cells and mild to strong reactivity | MVA | 7 |
| Tao et al (2014)[5] | China | breast cancer | 1032 | NA | NA | 2001--2008 | NA | OS | IHC | positive expression | > 10% of the tumor cells and mild to strong reactivity | MVA | 7 |
| Emori et al (2015)[11] | Japan | myxofibrosarcoma | 37 | 16/21 | 74 (46–89) | 1996--2013 | 39 (2–171) | OS, RFS | IHC | overexpression | > 10% of the tumor cells and mild to strong reactivity | MVA | 8 |
| Yokoyama et al (2017)[14] | Japan | diffuse large B-cell lymphoma | 84 | 47/37 | 64 (19–84) | 2005-2012 | 45 (CD109-low group) 31 (CD109-high group) | OS | IHC | high expression | immunoreactivity was almost equal to or stronger than that of endothelial cells | UVA | 7 |
| Kim et al (2019)[13] | South Korea | ovarian epithelial cancer | 120 | NA | 50 (15–82) | 1998-2009 | 50 (1–115) | OS, RFS | IHC | positive expression | > 10% of the tumor cells | MVA | 8 |
| Lee et al (2020)[15] | Taiwan | lung adenocarcinoma | 30 | NA | NA | NA | NA | OS, RFS | IHC | high expression | NA | UVA | 6 |
| Taki et al (2020)[16] | Japan | lung adenocarcinoma | 200 | 106/94 | 67.2 (26–84) | 2009--2011 | NA | OS | IHC | high expression | total scores more than 3 (total score = proportion score + intensity score) | MVA | 7 |
IHC = immunohistochemistry, MVA = multivariate analysis, NA = not available, NOS = Newcastle--Ottawa Scale, OS = overall survival, RFS = recurrence-free survival, UVA = univariate analysis.
3.2. Association between CD109 expression and OS
The heterogeneity among the included studies was evaluated using the fixed-effects model (I2 = = 0.0%, P = .490). A forest plot showed an association between high expression of CD109 and poor OS in cancer patients. The pooled HR was 2.31 (95% CI 1.93–2.76, P < .001) (Fig. 2).
Figure 2.
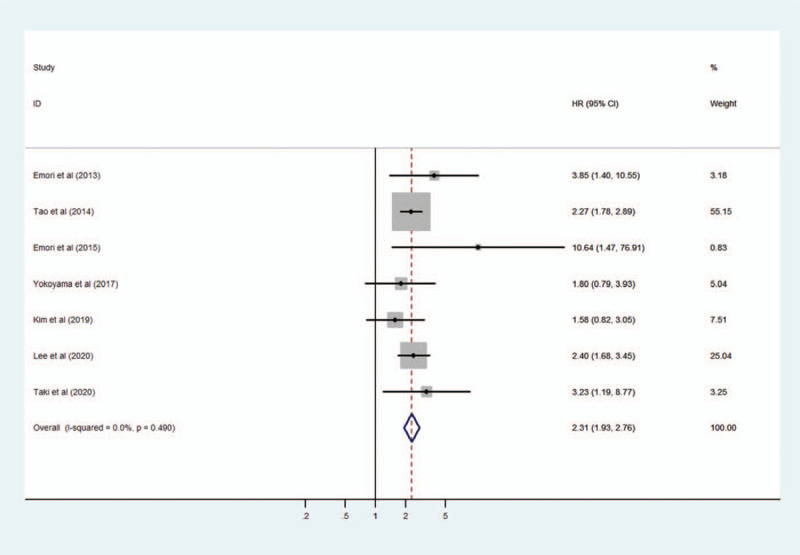
Forest plot of the association between CD109 expression and overall survival in cancer patients.
3.3. Sensitivity analysis
Sensitivity analysis was conducted to assess the robustness of the pooled results by omitting each study. As shown in Figure 3, the pooled results were stable on exclusion of any individual studies, suggesting that the pooled results were reliable (HR 2.31, 95% CI 1.93–2.76).
Figure 3.
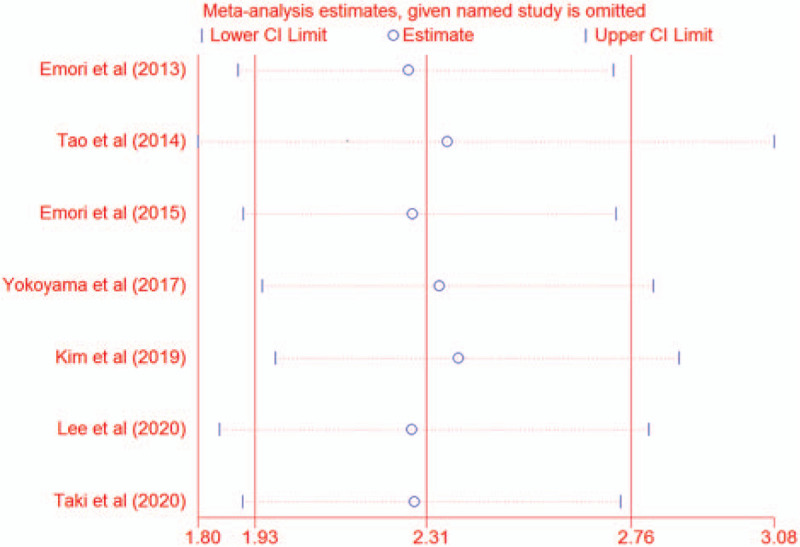
Sensitivity analysis.
3.4. Publication bias
The Funnel plot was constructed with pseudo 95% CI, and the Egger test was applied to evaluate publication bias. As shown in Figure 4A, the Funnel plot appeared asymmetrical, but it was not statistically proven (P = .321). Therefore, the filled Funnel plot was performed with pseudo 95% CI. The filled Funnel plot revealed that the pooled results were reliable (HR 2.24, 95% CI 1.88–2.67, P < .001) (Fig. 4B).
Figure 4.
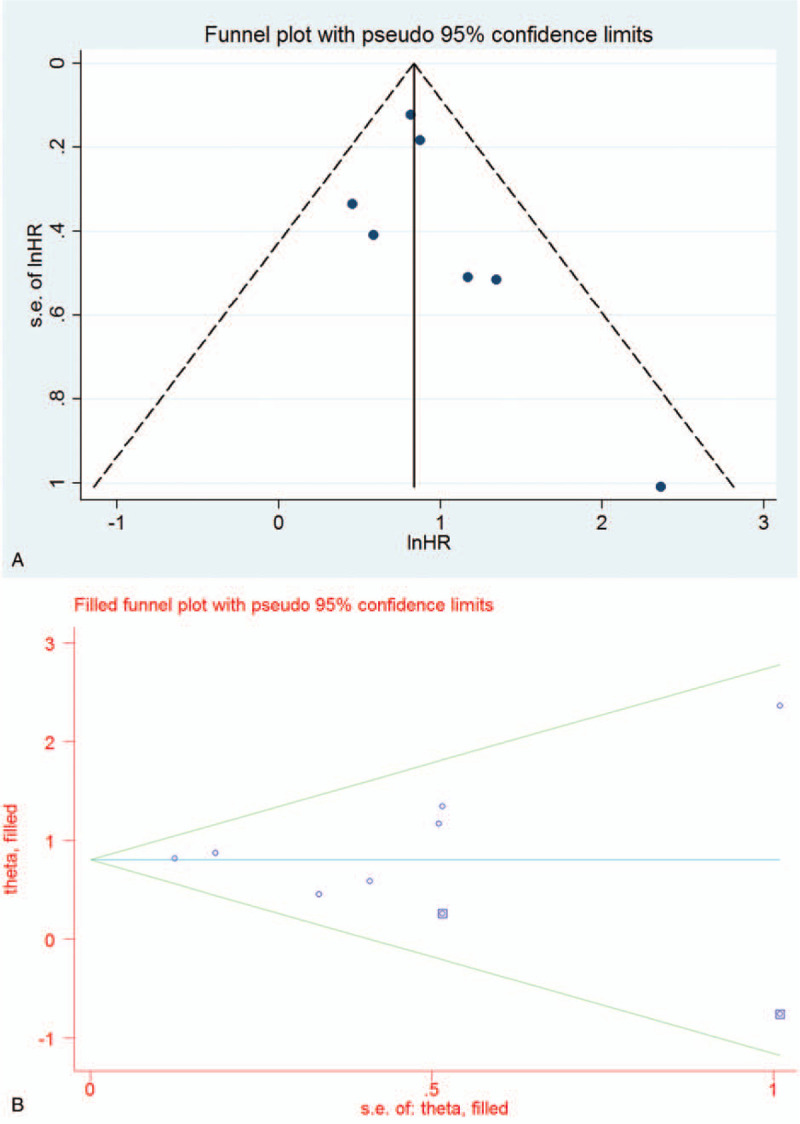
Funnel plot (A) and filled Funnel plot (B) for publication bias.
4. Discussion
This study revealed that there was an association between high expression of CD109 and poor OS in cancer patients and that the pooled results were reliable.
TGF-β controls various cellular processes such as cell proliferation, differentiation, migration, and apoptosis and plays an important role in development and homeostasis.[17,18] TGF-β signaling upregulates several inhibitors of kinases that mediate cell cycle arrest and anti-proliferative effects.[18] Thus, disruption of TGF-β signaling could result in enhanced cell proliferation and has been associated with pathological disorders including cancers.[17,18]
CD109 was originally reported as a cell surface antigen, identified in lymphoid and myeloid cells, and it has been recognized as a TGF-β coreceptor.[18] CD109 inhibits TGF-β signaling and responses by binding to TGF-β receptors and is involved in cancer development and progression.[17,18] Moreover, recent studies have indicated that CD109 is highly expressed in human cancers including squamous cell carcinoma of the lung, vulva and uterine cervix, adenosquamous carcinoma of the gallbladder, and ductal adenocarcinoma of the pancreas.[4,7–10] Furthermore, accumulating evidence suggests that high expression of CD109 is associated with poor prognosis in cancer patients.[5,11–16] Therefore, we conducted the present study to better evaluate the prognostic role of CD109 expression in cancer patients.
In the present study, we identified 7 relevant studies consisting of 1583 patients. Kim et al[13] revealed that CD109 expression was associated with OS, but not with recurrence-free survival in ovarian epithelial cancer. They demonstrated the relationship between CD109 expression and chemoresistance, suggesting the possibility of other underlying mechanisms stronger than the CD109-TGF-β signaling pathway. Yokoyama et al[14] reported the relationship between CD109 expression and survival, emphasizing the association with short-term survival in diffuse large B-cell lymphoma, suggesting that CD109 attenuates TGF-β signaling. Emori et al[11] demonstrated that CD109 overexpression was significantly associated with surgical stage, distant metastasis, and decreased survival, and CD109 overexpression was an independent risk factor for poor OS in patients with myxofibrosarcoma. These findings are in line with the research reported by Emori et al[12] that CD109 expression showed a strong correlation with poor prognosis and might be a cancer stem-like cell/cancer-initiating cell marker in epithelioid sarcoma. Tao et al[5] also reported that histologic grade and molecular type were significantly related to CD109 expression and that patients with high expression of CD109 had significantly worse survival than those with no or low expression in breast cancer. In addition, they found that CD109 expression is associated with the biological behavior of cancer stem cells and chemotherapeutic resistance. Lee et al[15] and Taki et al[16] showed that high expression of CD109 was associated with poor prognosis in patients with lung adenocarcinoma.
We demonstrated that CD109 expression is associated with unfavorable survival in cancer patients in the present study. To the best of our knowledge, the present study is the first to report the relationship between CD109 expression and prognosis in cancer patients through a meta-analysis.
5. Limitations
There are some limitations to the present study. First, only 7 studies were included, and different types of epithelial and mesenchymal cancers were included in the analysis. Thus, our findings might have been overestimated. Second, we did not reveal the relationship between CD109 expression and clinicopathological parameters and perform subgroup analysis. Third, in some studies, we found that CD109 expression is related to treatment, but we could not analyze the prognosis associated with the treatment. We hope that an increasing number of diverse research will be carried out in the future to clarify these issues.
6. Conclusion
The present study showed that CD109 expression could be a prognostic marker in cancer patients.
Author contributions
Conceptualization: Hyun Min Koh, Dong Chul Kim.
Data curation: Hyun Min Koh, Dong Chul Kim.
Formal analysis: Hyun Min Koh, Hyun Ju Lee, Dong Chul Kim.
Funding acquisition: Hyun Ju Lee.
Investigation: Hyun Min Koh, Dong Chul Kim.
Resources: Hyun Min Koh, Hyun Ju Lee, Dong Chul Kim.
Supervision: Hyun Ju Lee, Dong Chul Kim.
Validation: Hyun Min Koh, Hyun Ju Lee, Dong Chul Kim.
Writing – original draft: Hyun Min Koh.
Writing – review & editing: Hyun Min Koh, Hyun Ju Lee, Dong Chul Kim.
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, HR = hazard ratio, NOS = Newcastle–Ottawa Scale, OS = overall survival, TGF = transforming growth factor.
How to cite this article: Koh HM, Lee HJ, Kim DC. Usefulness of CD109 expression as a prognostic biomarker in patients with cancer: a systematic review and meta-analysis. Medicine. 2021;100:11(e25006).
HMK and HJL have contributed equally in this article.
This work was supported by the Soonchunhyang University Research Fund.
The authors declared no potential conflicts of interest.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Supplemental digital content is available for this article.
References
- [1].Zhong H, Qian Y, Fang S, et al. Prognostic value of plasma fibrinogen in lung cancer patients: a meta-analysis. J Cancer 2018;9:3904–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Song W, Tian C, Zhang RJ, et al. Meta-analysis of the prognostic value of lncRNA ZFAS1 in patients with solid tumors. Oncotarget 2017;8:90301–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wang W, Li J, Zhu W, et al. MicroRNA-21 and the clinical outcomes of various carcinomas: a systematic review and meta-analysis. BMC Cancer 2014;14:819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Dong F, Lu C, Chen X, et al. CD109 is a novel marker for squamous cell/adenosquamous carcinomas of the gallbladder. Diagn Pathol 2015;10:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Tao J, Li H, Li Q, et al. CD109 is a potential target for triple-negative breast cancer. Tumor Biol 2014;35:12083–90. [DOI] [PubMed] [Google Scholar]
- [6].Arias-Pinilla GA, Dalgleish AG, Mudan S, et al. Development of novel monoclonal antibodies against CD109 overexpressed in human pancreatic cancer. Oncotarget 2018;9:19994–20007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ozbay PO, Ekinci T, Yigit S, et al. Investigation of prognostic significance of CD109 expression in women with vulvar squamous cell carcinoma. Onco Targets Ther 2013;6:621–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sato T, Murakumo Y, Hagiwara S, et al. High-level expression of CD109 is frequently detected in lung squamous cell carcinomas. Pathol Int 2007;57:719–24. [DOI] [PubMed] [Google Scholar]
- [9].Zhang J, Hashimoto M, Kawai K, et al. CD109 expression in squamous cell carcinoma of the uterine cervix. Pathol Int 2005;55:165–9. [DOI] [PubMed] [Google Scholar]
- [10].Hatsuzawa Y, Yamaguchi K, Takanashi T, et al. CD109 promotes the tumorigenic ability and metastatic motility of pancreatic ductal adenocarcinoma cells. Pancreatology 2020;20:493–500. [DOI] [PubMed] [Google Scholar]
- [11].Emori M, Tsukahara T, Murata K, et al. Prognostic impact of CD109 expression in myxofibrosarcoma. J Surg Oncol 2015;111:975–9. [DOI] [PubMed] [Google Scholar]
- [12].Emori M, Tsukahara T, Murase M, et al. High expression of CD109 antigen regulates the phenotype of cancer stem-like cells/cancer-initiating cells in the novel epithelioid sarcoma cell line ESX and is related to poor prognosis of soft tissue sarcoma. PLoS One 2013;8:e84187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kim SY, Choi KU, Hwang C, et al. Prognostic significance of CD109 expression in patients with ovarian epithelial cancer. J Pathol Transl Med 2019;53:244–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yokoyama M, Ichinoe M, Okina S, et al. CD109, a negative regulator of TGF-( signaling, is a putative risk marker in diffuse large B-cell lymphoma. Int J Hematol 2017;105:614–22. [DOI] [PubMed] [Google Scholar]
- [15].Lee KY, Shueng PW, Chou CM, et al. Elevation of CD109 promotes metastasis and drug resistance in lung cancer via activation of EGFR-AKT-mTOR signaling. Cancer Sci 2020;111:1652–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Taki T, Shiraki Y, Enomoto A, et al. CD109 regulates in vivo tumor invasion in lung adenocarcinoma through TGF-beta signaling. Cancer Sci 2020;111:4616–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bizet AA, Liu K, Tran-Khanh N, et al. The TGF-β co-receptor, CD109, promotes internalization and degradation of TGF-β receptors. Biochim Biophys Acta 2011;1813:742–53. [DOI] [PubMed] [Google Scholar]
- [18].Hagiwara S, Murakumo Y, Mii S, et al. Processing of CD109 by furin and its role in the regulation of TGF-( signaling. Oncogene 2010;29:2181–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


