Abstract
As an international tourist center, Hainan province includes both imported and local COVID-19 cases. This study aimed to investigate the clinical characteristics and outcomes of COVID-19 patients in Hainan, China.
COVID-19 patients hospitalized in Hainan affiliated Hospital of Hainan Medical University in January to March 2020 were retrospectively assessed. Routine blood tests, blood gas analyses, and computed tomography imaging were performed within 24 hours. Virus nucleic acid was detected every other day. The patients were divided into local resident and traveler groups, and differences in clinical data as well as leukocyte, lymphocyte, and neutrophil levels were analyzed.
A total of 70 patients aged 51.23 ± 13.54 years were assessed, including 16 local residents and 54 travelers. Of these, 55 cases (78.6%) had fever, 47 (67.1%) had cough and sputum, and 9 (12.9%) had chest dyspnea; 60 and 10 cases were mild/common and severe/critical, respectively. Sex, basic diseases, smoking history and drinking history, Charlson Comorbidity Index, symptoms, time of onset to admission, clinical severity, white blood cell count, lymphocyte count, neutrophil count, oxygen inhalation, mechanical ventilation, glucocorticoid therapy, treatment, admission to ICU, hospital stay, and mortality were similar between the 2 groups.
The warm and humid climate of Hainan does not seem to significantly affect patient features and outcomes from COVID-19. Unnecessary travel to tourist areas should be avoided.
Keywords: COVID-19, hypertension, lymphocytes, nucleic acid negative conversion time
1. Introduction
The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) belongs to the coronavirus family, is often pleomorphic, with a diameter of 50 to 200 nm,[1] and is highly contagious.[2,3] At present, human-to-human transmission of the virus has been clearly documented.[4] The SARS-CoV2 associated disease, termed COVID-19, was declared a pandemic on March 12, 2020.[5–7] As of June 7, 2020, a total of 6,799,713 confirmed cases of COVID-19 have been reported globally, with 397,388 deaths.[8] As a respiratory disease, it is particularly important to identify the early characteristics of COVID-19 patients and the risk factors for severe transformation due to its rapid spread.
Currently, there is no specific antiviral drug or vaccine for COVID-19. Meanwhile, the time and location of sample collection are strongly associated with the accumulation of genetic diversity.[9] This indicates that virus infectivity and disease spread may significantly differ from one location to another.
The COVID-19 outbreak has negatively affected global health care systems with a ripple effect on virtually all aspects of human life.[10] Specifically, infectious disease outbreaks, such as COVID-19, substantially jeopardize the tourism industry that mostly relies on human mobility.[11] As an international tourist site, Hainan province, during the tourist season, deals with both imported and local COVID-19 cases. However, although some preliminary data are available on the general characteristics of this pandemic in Hainan province,[12] there has only been one other study into the specific characteristics of imported and local COVID-19 cases.[13]
Therefore, the present study aimed to assess the clinical features and outcomes of both imported and local COVID-19 patients in Hainan, China. Our findings provide a reference basis for the diagnosis and treatment of COVID-19.
2. Methods
2.1. Patients
This was a retrospective study of all confirmed COVID-19 patients hospitalized in Hainan affiliated Hospital of Hainan Medical University from January 20, 2020, to March 10, 2020. COVID-19 was diagnosed based on the novel coronavirus pneumonia diagnosis and treatment plan seventh edition issued by the national health commission of China.[14] The patients were divided into 2 groups according to residence, including the local resident and traveler groups.
The protocol was approved by the Medical Ethics Committee of Hainan General Hospital in China (Med-Eth-Re[2020]). Informed consent was waived due to the retrospective nature of this study.
2.2. Treatment
All hospitalized patients were administered oxygen therapy according to their conditions (mild/common types were treated with nasal catheters; severe cases were treated by mask or nasal high flow oxygen therapy, and critical cases were administered noninvasive or invasive auxiliary ventilation), and antiviral therapy (including Lopinavir/Ritonavir, Darunavir/Cobicistat, Arbidol, Chloroquine ,or Ribavirin). Some critical cases were treated with continuous renal replacement therapy (CRRT) and extracorporeal membrane oxygenation (ECMO).
2.3. Data collection and definition
The patients’ data were collected from medical records. After admission, the patient was asked for relevant data, including epidemiological history, symptoms, and previous disease history. Blood routine and blood gas analyses, and chest computed tomography (CT) imaging were performed within 24 hours, and virus nucleic acid detection in pharyngeal swabs was performed every other day during hospitalization. The clinical outcomes of the patients were recorded until discharge and included admission to intensive care unit (ICU), hospital stay, and mortality.
The clinical classification of disease severity was as follows: mild type, mild clinical symptoms, with no evidence of pneumonia on imaging; common type, symptoms such as fever and respiratory signs, with manifestations of pneumonia on imaging; severe type, any of the symptoms including respiratory distress (RR ≥30 times/min, oxygen saturation ≤93% in the resting state) and arterial partial oxygen pressure (PaO2)/oxygen absorption concentration (FiO2) ≤300 mm Hg; critical type, one of the conditions, including respiratory failure and the need for mechanical ventilation, shock, and combined with other organ failure requiring ICU care and treatment.
2.4. Statistical analysis
The SPSS 19.0 software (IBM, Armonk, NY) was used for data analysis. The normality assumption for continuous variables was assessed by the Kolmogorov--Smirnov test. Normally distributed continuous variables are presented as mean ± standard deviation (SD), and were compared by unpaired Student t test. Continuous variables with skewed distribution were presented as median (range) and assessed by the Mann--Whitney test. The Chi-square test or Fisher exact test was performed for categorical variables. P < .05 was considered statistically significant.
3. Results
3.1. Patient baseline characteristics
There were 70 patients included in this study. They were 51.23 ± 13.54 years old and included 41 males (58.6%) and 29 females (41.4%). A total of 5 patients (7.1%) had a long history of smoking, and 8 (11.4%) had a history of drinking. There were 12 cases of hypertension (17.1%), 3 of diabetes (4.3%), 5 of coronary heart disease (CHD) (7.1%), 3 of diabetes (4.3%), and 1 of chronic kidney disease requiring regular blood purification (1.4%).
There were 60 and 10 cases of mild/common and severe/critical types, respectively. Of the 70 diagnosed patients, 55 cases (78.6%) had fever, 47 (67.1%) had cough and sputum, 9 (12.9%) had dyspnea, 16 (22.9%) had fatigue, 6 (8.6%) had muscle aches, 4 (5.7%) had pharyngeal discomfort, 5 (7.1%) had digestive tract symptoms, and 5 (7.1%) had no symptoms. There were no statistically significant differences in sex, basic diseases, smoking history, and drinking history between the local resident and traveler groups (P > .05). However, age was elevated in the traveler group compared with the local resident group (53.69 ± 13.89 vs 42.94 ± 8.19 years; P = .049).
The total amounts of peripheral white blood cells, lymphocytes and neutrophils in all 70 cases were 5.20 ± 2.37 × 109/L, 1.17 ± 0.59 × 109/L, and 3.59 ± 2.36 × 109/L, respectively, and showed no significant differences between the local resident and traveler groups (P > .05). Baseline patient features are summarized in Table 1.
Table 1.
Characteristics of COVID-19 patients.
| Characteristics | All patients (n = 70) | Local resident group (n = 16) | Traveler group (n = 54) | P |
| Age, year, mean ± SD | 51.23 ± 13.54 | 42.94 ± 8.19 | 53.69 ± 13.89 | .049 |
| Male, n (%) | 41 (58.6) | 9 (56.3) | 32 (59.3) | .830 |
| Smoking, n (%) | 5 (7.1) | 2 (12.5) | 3 (5.6) | .343 |
| Drinking, n (%) | 8 (11.4) | 2 (12.5) | 6 (11.1) | .878 |
| Comorbidity, n (%) | ||||
| Hypertension | 12 (17.1) | 2 (12.5) | 10 (18.5) | .575 |
| Diabetes | 3 (4.3) | 0 | 3 (5.6) | – |
| CHD | 5 (7.1) | 1 (6.3) | 4 (7.4) | .875 |
| ESRD | 1 (1.4) | 1 (6.3) | 0 | – |
| Charlson Comorbidity Index, mean ± SD | 1.77 ± 1.84 | 1.44 ± 1.97 | 1.87 ± 1.81 | .854 |
| Symptoms, n (%) | ||||
| Fever | 55 (78.6) | 10 (62.5) | 45 (83.3) | .074 |
| Cough | 47 (67.1) | 11 (68.8) | 36 (66.7) | .876 |
| Dyspnea | 9 (12.9) | 2 (12.5) | 7 (13.0) | .961 |
| Fatigue | 16 (22.9) | 5 (31.3) | 11 (20.4) | .363 |
| Muscle aches | 6 (8.6) | 2 (12.5) | 4 (7.4) | .523 |
| Pharyngeal discomfort | 4 (5.7) | 2 (12.5) | 2 (3.7) | .183 |
| Digestive tract symptoms | 5 (7.1) | 2 (12.5) | 3 (5.6) | .343 |
| No symptoms | 5 (7.1) | 3 (18.8) | 2 (3.7) | .040 |
| Onset of symptom to admission, day, mean ± SD | 5.41 ± 3.91 | 5.06 ± 4.59 | 5.52 ± 3.73 | .197 |
| Clinical severity, n (%) | .163 | |||
| Mild/regular | 60 | 12 (75.0) | 48 (88.9) | |
| Severe/ critical | 10 | 4 (25.0) | 6 (11.1) | |
| White blood cell count, × 109/L, mean ± SD | 5.20 ± 2.37 | 5.22 ± 1.92 | 5.19 ± 2.50 | .479 |
| Lymphocyte count, × 109/L, mean ± SD | 1.17 ± 0.59 | 1.29 ± 0.53 | 1.13 ± 0.60 | .893 |
| Neutrophil count, × 109/L, mean ± SD | 3.59 ± 2.36 | 3.44 ± 1.94 | 3.64 ± 2.48 | .489 |
CHD = coronary heart disease, ESRD = end-stage renal disease, SD = standard deviation.
3.2. Treatments and outcomes of COVID-19 patients
For treatment, oxygen inhalation was administered in 38 (54.3%) cases, while 9 (12.9%) underwent mechanical ventilation. Glucocorticoid therapy was received by 12 (17.1%) cases. Of the 70 cases, 8 (11.4%) were treated in the ICU. The mean hospital stay was 13 (7, 60) days in the whole patient population, and there were 3 deaths recorded (4.3%). Antiviral therapeutics included Lopinavir/Ritonavir (n = 38, 54.3%), Darunavir/Cobicistat (n = 9, 12.9%), Lopinavir/Ritonavir and Arbidol (n = 12, 17.1%), Lopinavir/Ritonavir and Chloroquine (n = 4, 5.7%), Arbidol and Darunavir/Cobicistat (n = 5, 7.1%), and Arbidol and Ribavirin (n = 2, 2.9%). As summarized in Table 2, there were no significant differences in the above parameters between the local resident and traveler groups (all P > .05).
Table 2.
Treatments and outcomes of COVID-19 patients.
| All patients (n = 70) | Local resident group (n = 16) | Traveler group (n = 54) | P | |
| Oxygen inhalation, n (%) | 38 (54.3) | 10 (62.5) | 28 (51.9) | .453 |
| Mechanical ventilation, n (%) | 9 (12.9) | 3 (18.8) | 6 (11.1) | .423 |
| Glucocorticoid therapy, n (%) | 12 (17.1) | 3 (18.8) | 9 (16.7) | .846 |
| Anti-virus treatment, n (%) | ||||
| Lopinavir/Ritonavir | 38 (54.3) | 7 (43.8) | 31 (57.4) | .335 |
| Darunavir/Cobicistat | 9 (12.9) | 3 (18.8) | 6 (11.1) | .423 |
| Lopinavir/Ritonavir and Arbidol | 12 (17.1) | 2 (12.5) | 10 (18.5) | .575 |
| Lopinavir/Ritonavir and Chloroquine | 4 (5.7) | 2 (12.5) | 2 (3.7) | .183 |
| Arbidol and Darunavir/Cobicistat | 5 (7.1) | 1 (6.3) | 4 (7.4) | .875 |
| Arbidol and Ribavirin | 2 (2.9) | 1 (6.3) | 1 (1.9) | .354 |
| ICU admission, n (%) | 8 (11.4) | 3 (18.8) | 5 (9.3) | .295 |
| Hospital stay, day, median (range) | 13 (7, 60) | 11 (8, 34) | 14.5 (7, 60) | .749 |
| Mortality, n (%) | 3 (4.3) | 1 (6.3) | 2 (3.7) | .659 |
ICU = intensive care unit.
The longest nucleic acid positive time was 40 days. The nucleic acid negative conversion times after treatment with Lopinavir/Ritonavir, Lopinavir/Ritonavir, and Darunavir/Cobicistat, Lopinavir/Ritonavir and Arbidol, Lopinavir/Ritonavir, and Chloroquine, Arbidol and Darunavir/Cobicistat and Arbidol and Ribavirin were 11.23 ± 5.59, 18.89 ± 8.37, 17.29 ± 10.20, 24.00 ± 12.03, 13.00 ± 1.41, and 21.00 ± 5.66 days, respectively.
3.3. CT imaging findings
According to CT imaging, the typical features were as follows: multiple peripheral ground glass shadows; single ground glass shadow; patchy ground glass shadow in both lungs, accompanied by segmental lung consolidation; diffuse ground glass shadow in both lungs, accompanied by air bronchogram; large area consolidation in both lungs with lobular interstitial thickening; and diffuse lesions in both lungs mainly with consolidation shadow, combined with ground glass shadow, multiple cord-like shadows, and air bronchogram. Among all these signs, multiple peripheral ground glass shadows were the most common. With disease progression in critical cases, mostly diffuse lesions in both lungs and large consolidation shadows occurred. Representative images of the wide range of imaging results that were evident in patients with different COVID-19 severity are shown in Fig. 1.
Figure 1.
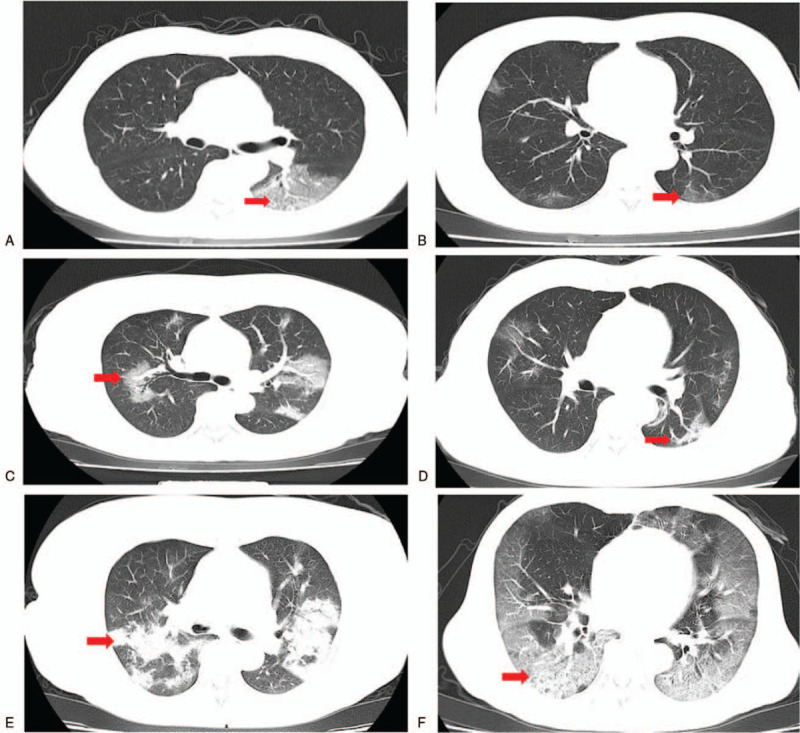
Typical imaging findings in COVID-19 patients. (A) Local ground glass lesion (arrow showed) in the left lung of a 59-year-old man. (B) Sub-pleural lamellar exudation (arrow) in a 69-year-old woman. (C) Multiple ground glass shadows (arrow) in both lungs in a 62-year-old woman. (D) Multiple plaques in both lungs (arrow) in a 29-year-old man. (E) Multiple consolidation and exudative shadows (arrow) in both lungs in a 64-year-old woman. (F) Diffuse ground glass shadows (arrow showed) in both lungs in a 73-year-old man.
4. Discussion
The current study showed that local residents and travelers in Hainan (China) had mostly similar outcomes and features, indicating that residence in the warm and humid climate of Hainan does not seem to remarkably affect patient features and outcomes in COVID-19.
The results of this study showed 60 (85.7%) were mild/common and 10 (14.3%) were severe/critical types of COVID-19 infection. The most common symptom was fever in 55 cases (78.6%), followed by cough and sputum in 47 (67.1%), while 5 (7.1%) had no symptoms. The traveler group were older compared with the local resident group, but treatment and outcomes were similar. Another study that also compared COVID-19 infection of travelers to Hainan with residents in 91 patients also found fever and cough were two main clinical manifestations.[13] In that study, 15 (16.5%) patients were severe. The imported cases were older and had a higher incidence of fever and concurrent infections than the residents, but there was no difference in outcomes.[13] Therefore, their results are similar to our study and we can conclude that local residents and travelers to Hainan had similar characteristics of COVID-19 infection. The 5 asymptomatic patients in present study were diagnosed by a nucleic acid test because they were close relatives of confirmed patients. Therefore, doctors should attach great importance to nonrespiratory symptoms and assess epidemiological history. In addition, comparison of the data in this study with other studies from regions of China with cooler climates also showed similar characteristics, such as a study from Wenzhou city, Zhejiang,[15] another from northeast Chongqing,[16] and one from Wuhan.[17] Therefore, the data, so far, suggest that there has been no change in the disease characteristics with the natural occurrence of genetic mutations in SARS-CoV2. Taken together, these results also suggest that residence in warm climates does not influence the progression of COVID-19.
After SARS-CoV2 enters the human body, lung ACE2 levels are reduced, while ACE is not affected, causing an imbalance between ACE2 and ACE in the lung. Meanwhile, overactivation of the AT1a receptor in the lung leads to increased capillary permeability in this organ, resulting in pulmonary edema and dry cough, which aggravate the inflammatory response, induce cell apoptosis, and accelerate lung injury.[18–21] In this study, most patients with COVID-19 had mild symptoms at onset. However, a rapid aggravation of the disease was found in some cases after diagnosis, which may be related to the “cytokine storm.”[22] The released factors may modulate lymphocyte apoptosis, which also played an important role in the pathogenesis of SARS in 2003.[23,24]
In terms of imaging features, most patients in this study had typical CT manifestations, including single or multiple patchy ground glass shadows at the early stage, accompanied by interlobular septa thickening. In the progressive stage, the lesions were increased and enlarged, and ground glass shadows coexisted with consolidation or striated shadows. Patients with severe and critical disease presented with diffuse lesions in both lungs, dominated by consolidation shadows, combined with ground glass shadows, strip shadows and air bronchogram. It is established that pleural effusions and lymphadenopathy are rare.[25,26] According to a lung pathology study on COVID-19, the terminal stage of the disease mainly consists of diffuse alveolar injury in both lungs with fibrous mucus-like exudate, accompanied by hyaline membrane formation and alveolar epithelial cell shedding (presenting as acute respiratory distress syndrome) and interstitial inflammatory cell infiltration dominated by lymphocytes.[27] The above pathological findings can explain the imaging characteristic changes in COVID-19 patients. When clinicians encounter patients with rapidly progressive pneumonia, COVID-19 can be preliminarily diagnosed with CT showing the above typical characteristics combined with epidemiological data. Although the final diagnosis cannot be made only according to the obtained imaging features, the combination of clinical and imaging data could greatly improve diagnostic accuracy in COVID-19.
In terms of antiviral therapy, effective antiviral drugs are still required against COVID-19. Patients were treated in this study according to guidance from the National Health Commission of the People's Republic of China. The main therapeutic drugs used included Lopinavir/Ritonavir, Darunavir/Cobicistat, Arbidol, Chloroquine, and Ribavirin. Different combinations of the above drugs were used in the treatment of the 70 COVID-19 patients admitted to our hospital. Preliminary observational data showed that compared with Lopinavir/Ritonavir alone, the use of combined antiviral drugs did not provide significant benefits to COVID-19 patients, nor did it reduce viral RNA load or nucleic acid negative conversion time. Therefore, in the treatment of COVID-19 patients, combining antiviral drugs does not seem to be a reasonable and effective option. In agreement, studies have reported that Lopinavir/Ritonavir fails to benefit COVID-19 patients.[28] However, a meta-analysis suggests it may improve outcomes in severe cases.[29] Redesivir is currently considered the most promising drug in the treatment for COVID-19, with strong in vitro antiviral activity against human and various bat-derived coronaviruses.[30] The efficacy of Redesivir in a COVID-19 patient in the United States has been recently reported.[31] However, the efficacy and safety of Redesivir in COVID-19 patients still needs to be confirmed by rigorous clinical studies.
The present study has some limitations. First, it was a retrospective study, with inherent shortcomings. Second, all patients were enrolled in the same center, and the sample size was relatively small making it difficult to compare any subtle differences between the two groups. Finally, there was no follow-up post-hospitalization, and whether differences could arise with time between the local resident and traveler groups remains undefined. Therefore, further large well-designed studies are required to confirm these findings.
In conclusion, overall, local residents and travelers with COVID-19 in Hainan had comparable outcomes and features, indicating that the warm and humid climate of Hainan does not seem to significantly affect patient COVID-19 characteristics. The above findings provide additional insights into the early diagnosis, evaluation, and treatment of COVID-19, revealing that unnecessary travel to tourist areas, even warm regions, should be avoided.
Author contributions
YZ and XC were involved in conception of study and drafting of manuscript. All the authors contributed to data analysis, critical revision of the manuscript, and final approval of manuscript.
Conceptualization: Yamei Zheng.
Data curation: Yamei Zheng, Yunsuo Gao, Biao Wu, Linhui Huang, Yongxing Chen, Xingjun Cai.
Formal analysis: Yamei Zheng, Yunsuo Gao, Biao Wu, Linhui Huang, Yongxing Chen, Xingjun Cai.
Investigation: Yamei Zheng, Yunsuo Gao, Biao Wu, Linhui Huang, Yongxing Chen, Xingjun Cai.
Methodology: Yamei Zheng, Xingjun Cai.
Project administration: Yamei Zheng, Xingjun Cai.
Writing – original draft: Yamei Zheng, Yunsuo Gao, Biao Wu, Linhui Huang, Yongxing Chen, Xingjun Cai.
Writing – review & editing: Yamei Zheng, Yunsuo Gao, Biao Wu, Linhui Huang, Yongxing Chen, Xingjun Cai.
Footnotes
Abbreviations: CT = computed tomography, SARS-CoV2 = severe acute respiratory syndrome coronavirus 2, SD = standard deviation.
How to cite this article: Zheng Y, Gao Y, Wu B, Huang L, Chen Y, Cai X. Characteristics and outcomes of patients with COVID-19 in Hainan, South China. Medicine. 2021;100:11(e24771).
This work was supported by“2019-ncov” Science and Technology Research Project of Hainan Medical University (No: XGZX2020001). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors report no conflicts of interest.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Gralinski LE, Menachery VD. Return of the coronavirus: 2019-nCoV. Viruses 2020;12:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020;382:727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wang C, Horby PW, Hayden FG, et al. A novel coronavirus outbreak of global health concern. Lancet (London, England) 2020;395:470–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med 2020;382:1199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hui DS, I Azhar E, Madani TA, et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health: the latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis 2020;91:264–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Stefana A, Youngstrom EA, Jun C, et al. The COVID-19 pandemic is a crisis and opportunity for bipolar disorder. Bipolar Disord 2020;22:641–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ha JF. The COVID-19 pandemic, personal protective equipment and respirator: a narrative review. Int J Clin Pract 2020;74:e13578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].World Health Organization. (2020). Coronavirus disease 2019 (COVID-19): situation report, 39. [Google Scholar]
- [9].Khailany RA, Safdar M, Ozaslan M. Genomic characterization of a novel SARS-CoV-2. Gene reports 2020;19:100682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Nicola M, Alsafi Z, Sohrabi C, et al. The socio-economic implications of the coronavirus pandemic (COVID-19): a review. Int J Surg 2020;78:185–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kraemer MUG, Yang CH, Gutierrez B, et al. The effect of human mobility and control measures on the COVID-19 epidemic in China. Science 2020;368:493–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yan S, Song X, Lin F, et al. Clinical characteristics of coronavirus disease 2019 in Hainan, China. medRxiv 2020;3:20038539. [Google Scholar]
- [13].Wu B, Lei ZY, Wu KL, et al. Compare the epidemiological and clinical features of imported and local COVID-19 cases in Hainan, China. Infect Dis Poverty 2020;9:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].China NHCotPsRo. Guidelines for the Diagnosis and Treatment of Novel Coronavirus (2019-nCoV) Infection by the National Health Commission (Trial Version 7). 2020. [DOI] [PubMed] [Google Scholar]
- [15].Yang W, Cao Q, Qin L, et al. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19): a multi-center study in Wenzhou city, Zhejiang, China. J Infect 2020;80:388–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wan S, Xiang Y, Fang W, et al. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J Med Virol 2020;92:797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395:507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Yan R, Zhang Y, Li Y, et al. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 2020;367:1444–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Imai Y, Kuba K, Rao S, et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature 2005;436:112–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wang H, Yang P, Liu K, et al. SARS coronavirus entry into host cells through a novel clathrin- and caveolae-independent endocytic pathway. Cell Res 2008;18:290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Glowacka I, Bertram S, Herzog P, et al. Differential downregulation of ACE2 by the spike proteins of severe acute respiratory syndrome coronavirus and human coronavirus NL63. J Virol 2010;84:1198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Nile SH, Nile A, Qiu J, et al. COVID-19: pathogenesis, cytokine storm and therapeutic potential of interferons. Cytokine Growth Factor Rev 2020;53:66–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chousterman BG, Swirski FK, Weber GF. Cytokine storm and sepsis disease pathogenesis. Semin Immunopathol 2017;39:517–28. [DOI] [PubMed] [Google Scholar]
- [24].Ding Y, Wang H, Shen H, et al. The clinical pathology of severe acute respiratory syndrome (SARS): a report from China. J Pathol 2003;200:282–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lei J, Li J, Li X, et al. CT imaging of the 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology 2020;295:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Koo HJ, Lim S, Choe J, et al. Radiographic and CT features of viral pneumonia. Radiographics 2018;38:719–39. [DOI] [PubMed] [Google Scholar]
- [27].Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020;8:420–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Cao B, Wang Y, Wen D, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med 2020;382:1787–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Verdugo-Paiva F, Izcovich A, Ragusa M, et al. Lopinavir-ritonavir for COVID-19: a living systematic review. Medwave 2020;20:e7967. [DOI] [PubMed] [Google Scholar]
- [30].Li H, Wang YM, Xu JY, et al. Potential antiviral therapeutics for 2019 Novel coronavirus. Zhonghua Jie He He Hu Xi Za Zhi 2020;43:170–2. [DOI] [PubMed] [Google Scholar]
- [31].Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med 2020;382:929–36. [DOI] [PMC free article] [PubMed] [Google Scholar]


