Abstract
Background:
Postoperative atrial fibrillation (POAF) occurs commonly after cardiac surgery. Studies suggest that corticosteroid can reduce the incident of POAF. However, the results remain controversial. This meta-analysis aimed to evaluate the efficacy and safety corticosteroid on the prevention of POAF following cardiac surgery.
Methods:
Randomized controlled trials were identified through a systematic literature search. Two investigators independently searched articles, extracted data, and assessed the quality of included studies. Primary outcome was the incidence of POAF as well as length of hospital stay and intensive care unit stay, wound and other infection, mortality, duration of ventilation, myocardial infarction, gastrointestinal complications, high blood sugar, stroke, and postoperative bleeding.
Results:
Fourteen studies with 13,803 patients were finally involved in the present study. Overall, corticosteroid significantly decreased the risk of POAF (relative risk [RR], 0.7; 95% confidence interval [CI], 0.55–0.89; P = .003). There were no significant differences in the incidence of length of intensive care unit stay (RR, −2.32; 95% CI, −5.44 to 0.80; P = .14) and hospital stay (RR, −0.43; 95% CI, −0.84 to −0.02; P = .04), infections (RR, 1.01; 95% CI, 0.83–1.23; P = .9), mortality (RR, 0.87; 95% CI, 0.71–1.06; P = .16), duration of ventilation (RR, −0.29; 95% CI, −0.65 to 0.07; P = .12), gastrointestinal complications (RR, 1.26; 95% CI, 0.91–1.76; P = .16), high blood sugar (RR, 1.98; 95% CI, 0.91–4.31; P = .09), stroke (RR, 0.9; 95% CI, 0.69–1.18; P = .45), postoperative bleeding (RR −44.54; 95% CI, −115.28 to 26.20; P = .22) and myocardial infarction (RR, 1.71; 95% CI, 0.96–1.43; P = .12).
Conclusion:
Our review suggests that the efficacy of corticosteroid might be beneficial to POAF development in patients undergoing cardiac surgery. The strength of this association remains uncertain because of statistical and clinical heterogeneity among the included studies.
Keywords: atrial fibrillation, cardiac surgery, complication, corticosteroid
1. Introduction
Postoperative atrial fibrillation has (POAF) been reported in 20% to 50% of patients following coronary artery bypass grafting (CABG) and is even higher after combined CABG and valve surgery.[1] New-onset atrial fibrillation after cardiac surgery also associates with numerous postoperative complications, including stroke, increased inotropic support, congestive heart failure, acute kidney injury, and death.[2,3] These in turn lead to prolonged intensive care and hospital length of stay.[3] However, the cause of POAF and its associated adverse outcomes is still not well defined.[4] Different students have illustrated that systemic inflammatory response and local inflammation of the atrium are believed to contribute to the pathogenesis of atrial fibrillation after cardiac surgery. In addition, complex inflammatory reaction may contribute to postoperative complications such as ventricular dysfunction and organ failure.[5] The relationship between inflammation and atrial fibrillation after cardiac surgery is further strengthened by studies that showed that corticosteroid (CS) prophylaxis can reduce the occurrence of atrial fibrillation after cardiac surgery. However, the potential risks of CS remain controversial and inconclusive in terms of several side effects of CS such as hyperglycemia, gastrointestinal disturbances, and postoperative infections.[6] Although previous meta-analyses of 50 small randomized controlled trials (RCTs) showed that CSs could reduce POAF when compared with placebo.[1,7] After publication of these meta-analyses, some recent reports from large RCTs of CSs in cardiac surgery showed no difference in POAF rates between the treatment group and the control group.[8,9] Taken together, use of intravenous injection steroids to prevent POAF in the cardiac surgical population still remain unclear. We performed a meta-analysis to assess determine the clinical benefits and risks of CS use in adult cardiac surgery.
2. Materials and methods
2.1. Search strategy and selection criteria
Two investigators independently searched the literatures collected in PubMed, MEDLINE, and Cochrane databases up to March 1, 2020. Search terms included: “glucocorticoid,” “steroid,” “hydrocortisone,” “dexamethasone,” “methylprednisolone,” and “cardiac surgery,” “cardiothoracic surgery,” “heart surgery,” “cardiopulmonary bypass,” “CPB,” “coronary artery bypass grafting,” “CABG,” “CAB,” and clinical trial. We also sought additional studies by reviewing the reference lists of included articles, conference abstracts, and the bibliographies of expert advisors. The searches were limited to English publications in humans. This search strategy was performed iteratively until no new potential citations could be found on review of the reference lists of retrieved articles.
Studies were included if they met all of the following criteria:
-
(1)
RCTs about comparison of steroids with a control group;
-
(2)
adult patients undergoing CABG surgery (off-pump or on-pump) alone or combined with valvular surgery or other cardiac surgery;
-
(3)
reporting outcome at least including incidence of POAF;
-
(4)
incidence of other postoperative complications according to our review-checklist.
Exclusion criteria included:
-
(1)
not clearly define the incident of atrial fibrillation was new-onset;
-
(2)
duplicate publication;
-
(3)
ongoing/unpublished study.
In addition, if the same author published multiple studies reporting outcomes at different follow-up points, we extracted patient characteristics from the first study, with data for outcomes of interest at subsequent follow-up times extracted from the later studies. When 2 studies by the same institution reported the same outcomes at similar follow-up periods, we included in our analysis either the better quality or the most informative publication. Ethical approval is unnecessary due to it is a review of previously reported articles and does not involve any processing of individual patient data.
2.2. Data extraction
The qualities of each contributing evidence were evaluated following the recommended Cochrane risk of bias tool respecting to 7 parts about selection (random and allocation), performance, detection, attrition, reporting, and other bias, and each study was assessed to be of low, unclear, or high risk of bias.
All data were extracted from article texts, tables, and figures. Two individual investigators independently extracted data on patient and study characteristics, outcomes, and study quality for each trial using a standardized protocol and reporting form. Disagreements were resolved by consensus with a third reviewer.
2.3. Study outcomes
The end points of this meta-analysis were as follow:
-
(1)
incident of POAF;
-
(2)
length of hospital stay;
-
(3)
length of intensive care unit (ICU) stay;
-
(4)
wound infection;
-
(5)
other infection (urinary infect, pulmonary infection, pericarditis, mediastinitis, intravenous line infection, bacteremia, or any other infection);
-
(6)
mortality;
-
(7)
duration of;
-
(8)
myocardial infarction or injury;
-
(9)
gastrointestinal complications (upper gastrointestinal bleeding, gastrointestinal disturbance, gastritis, acute pancreatitis, perforated gastric ulcer);
-
(10)
high blood sugar;
-
(11)
stroke;
-
(12)
postoperative bleeding;
-
(13)
delirium.
2.4. Statistical Methods
We used fixed-effects or random-effects models to produce across-study summary relative risk (RR) with 95% confidence interval (CI). The pooled effects were calculated using fixed-effect model with the Mantel–Haenszel method when there was no significant heterogeneity or with DerSimonian–Laird weights for the random effects model when there was significant heterogeneity. The Chi-square test was used to study heterogeneity between trials, and the I2 statistic was used to estimate the percentage of total variation across studies. I2 value greater than 50% was considered as significant heterogeneity. Sensitivity analyses were performed to compare the treatment effects obtained from different subgroups with the overall treatment effects. Publication bias was explored through visual inspection of funnel plots and assessed by applying the Egger weighted regression statistic with a P-value < .05 indicating significant publication bias among the included studies. Correction for publication bias was performed using trim-and-fill methods. A P-value < .05 was regarded as significant. All statistical analyses were performed using Review Manager (version 5.3, Cochrane Collaboration, Oxford, UK).
3. Results
3.1. Characteristics of Included Studies
The literature search identified relative references. After selection according to the inclusion/exclusion criteria, 14 studies were eligible for meta-analysis finally.[2,8–20] A total of 13803 patients were involved, of whom 6892 patients undergoing CS group and 6911 patients undergoing control group, as summarized in Table 1. Figure 1 shows the risk of bias of the included studies. A funnel plot was generated to aid in interpretation of potential publication bias (Supplemental Figure 1).
Table 1.
Characteristics of randomized controlled trials.
| N | Mean age | |||||||
| Study | Year | Regimen | CS | C | CS | C | Type of surgery | Study design |
| Halonen et al[2] | 2007 | Hydrocortisone | 120 | 127 | 64.4 ± 8.4 | 66.1 ± 9.5 | On-pump CABG combined valvular surgery | RCT |
| Halvorsen et al[10] | 2003 | Dexamethasone | 147 | 147 | 63 ± 11 | 64 ± 10 | On-pump CABG | RCT |
| Abbaszadeh et al[11] | 2012 | Dexamethasone | 92 | 92 | 60.7 ± 8.7 | 59.4 ± 10 | On-pump CABG | RCT |
| Yared et al[12] | 2007 | Dexamethasone | 37 | 34 | 69.2 (62,78) | 74.2 (64,79) | On-pump CABG combined valvular surgery | RCT |
| Yared et al[13] | 2000 | Dexamethasone | 106 | 110 | 62.6 ± 11.4 | 63.2 ± 11.3 | On-pump CABG combined valvular surgery | RCT |
| Whitlock et al[14] | 2006 | Methylprednisolone | 30 | 30 | 67 ± 10 | 66 ± 11 | On-pump CABG combined valvular surgery | RCT |
| Suezawa et al[15] | 2013 | Methylprednisolone | 15 | 15 | 64.8 ± 5 | 60.7 ± 9.1 | On-pump CABG | RCT |
| Prasongsukarn et al[16] | 2005 | Methylprednisolone | 43 | 43 | 67.2 (64.5–70) | 61.7 (58.6–64.8) | On-pump CABG | RCT |
| Mirhosseini et al[17] | 2011 | Methylprednisolone | 60 | 60 | 63 ± 11 | 61 ± 13 | Off-pump CABG | RCT |
| Al-Shawabkeh et al[18] | 2017 | Methylprednisolone, Hydrocortisone | 170 | 170 | 65.7 ± 9.2 | 64.2 ± 8.9 | CABG orCABG+ valvular surgery | RCT |
| Gomez Polo et al[19] | 2017 | Methylprednisolone, Dexamethasone | 52 | 52 | 65 | 63 | CABG, valve replacement or combined surgery | RCT |
| Jacob et al[20] | 2015 | Dexamethasone | 30 | 32 | 70.4 ± 9.1 | 68.9 ± 9.0 | CABG or CABG+ valvular surgery | RCT |
| Dieleman et al[8] | 2015 | Dexamethasone | 2235 | 2247 | 66.2 ± 11.0 | 66.1 ± 10.7 | CABG, valvular surgery, CABG+ valvular surgery, Other cardiac surgery | RCT |
| Whitlock et al[9] | 2015 | Methylprednisolone | 3755 | 3752 | 67 5 ± 13 6 | 67 3 ± 13.8 | valvular surgery | RCT |
CABG = coronary artery bypass grafting, CS = corticosteroid, RCTs = randomized controlled trials.
Figure 1.
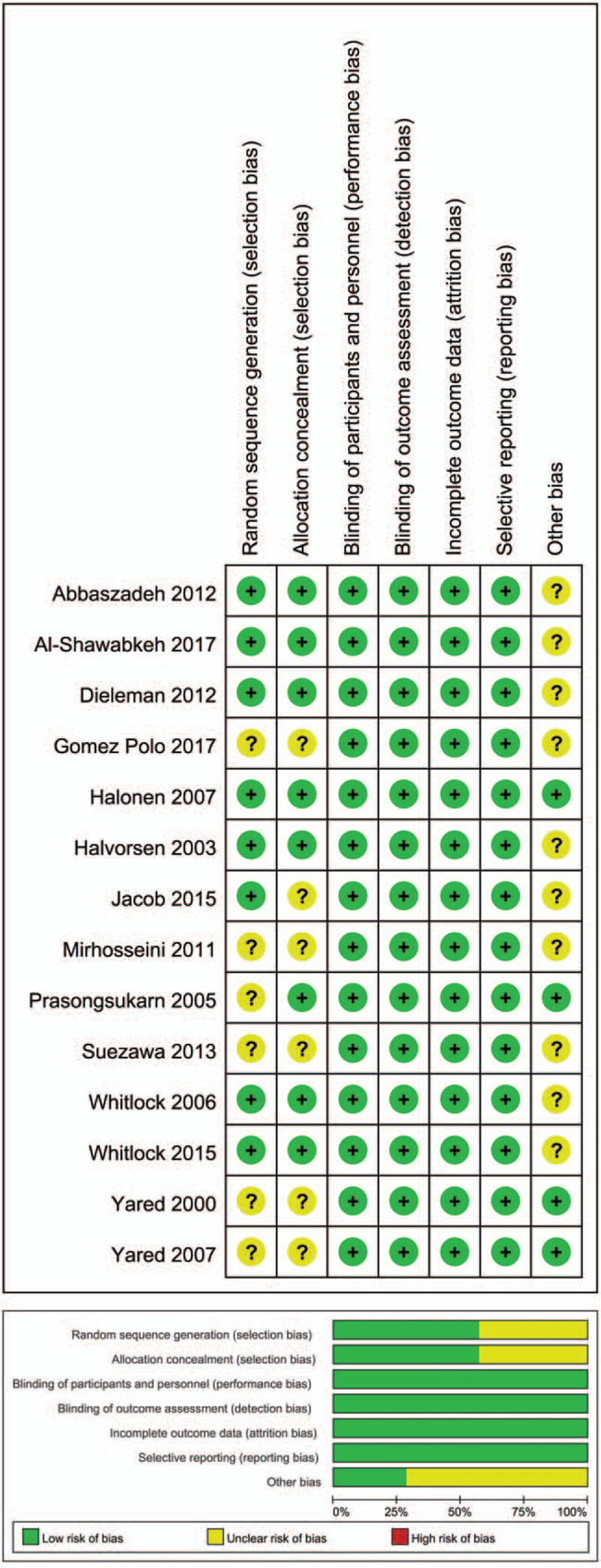
Risk of bias assessment. Authors’ judgments about risk of bias graph for each included study (above); authors’ judgments about risk of bias summary across all included studies (below).
3.2. POAF
A total of 13,803 patients were included from 14 RCTs, which reported data on POAF. Of these patients, 6892 cases were allocated to CS, and 6911 cases to the control group. POAF occurred in 24% in the CS group, and 26.48% in the control group. Pooled treatment effect analysis revealed that CS therapy significantly reduced the incidence of POAF (RR, 0.7; 95% CI, 0.55–0.89; P = .003; Fig. 2) using a random model. We found a moderate level of heterogeneity (I2 = 65%, P = .0003) for the pooled results for mortality.
Figure 2.
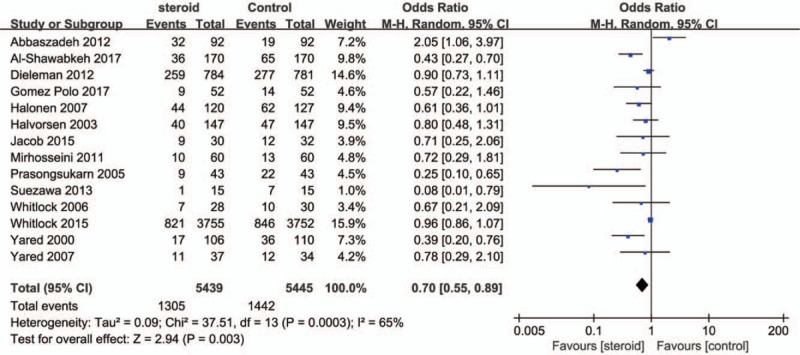
Forest plot for the meta-analysis of postoperative atrial fibrillation.
3.3. Postoperative length of ICU and hospital stay
Pooled analysis revealed that CS was not associated with a reduction in length of ICU (RR, −2.32; 95% CI, −5.44 to 0.80; P = .14; Fig. 3A) and hospital stay (RR, −0.43; 95% CI, −0.84 to −0.02; P = .04; Fig. 3B) using a random effect model. Significant heterogeneity was observed among the RCTs (I2 = 66% and I2 = 78%, respectively).
Figure 3.
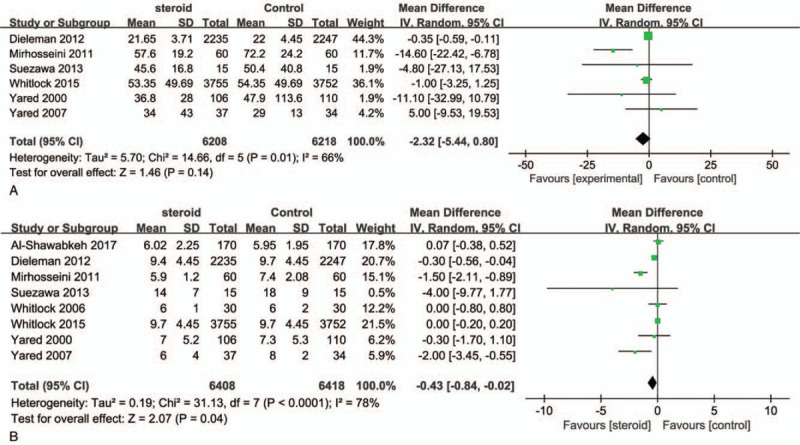
Forest plot for the meta-analysis of postoperative length of ICU (A) and hospital stay (B). ICU = intensive care unit.
3.4. Postoperative wound and other infection
A total 12 RCTs reported data on postoperative infectious complications. After removing 3 RCTs with no events in 2 arms, a total of 9219 patients from 9 studies were enrolled in the meta-analysis. In the pooled analyses, no significant difference was observed in wound complications (RR, 1.01; 95% CI, 0.83–1.23; P = .9; I2 = 0%; Fig. 4A) and other infectious complications (RR, 0.94; 95% CI, 0.65–1.38; I2 = 64% Fig. 4B) which were defined as urinary infect, pulmonary infection, pericarditis, mediastinitis, intravenous line infection, bacteremia, or any other infection.
Figure 4.
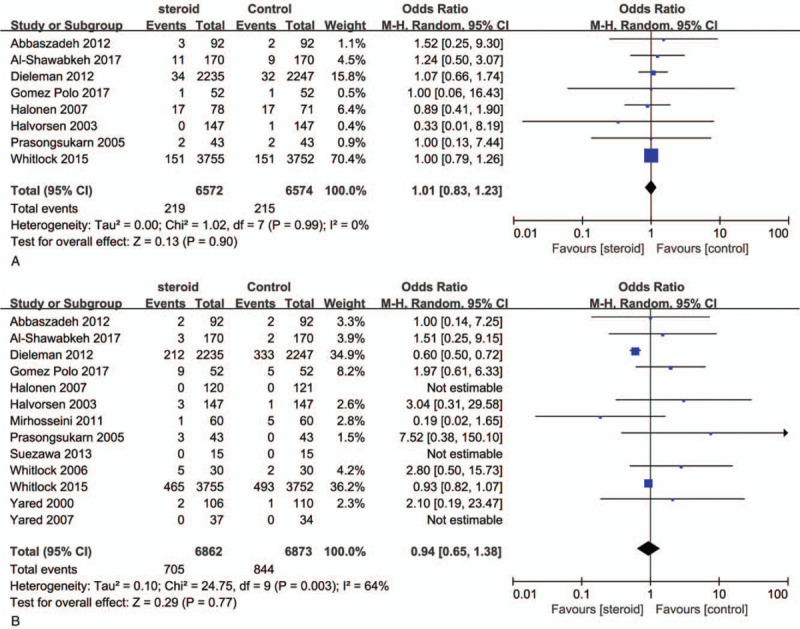
Forest plot for the meta-analysis of postoperative wound (A) and other infection (B).
3.5. Postoperative mortality
As shown in Figure 5A, Pooled treatment effect analysis revealed that CS therapy could not reduce incidence of postoperative mortality (RR, 0.87; 95% CI, 0.71–1.06; P = .16). No heterogeneity was observed among the RCTs (I2 = 0%, P = .98)
Figure 5.
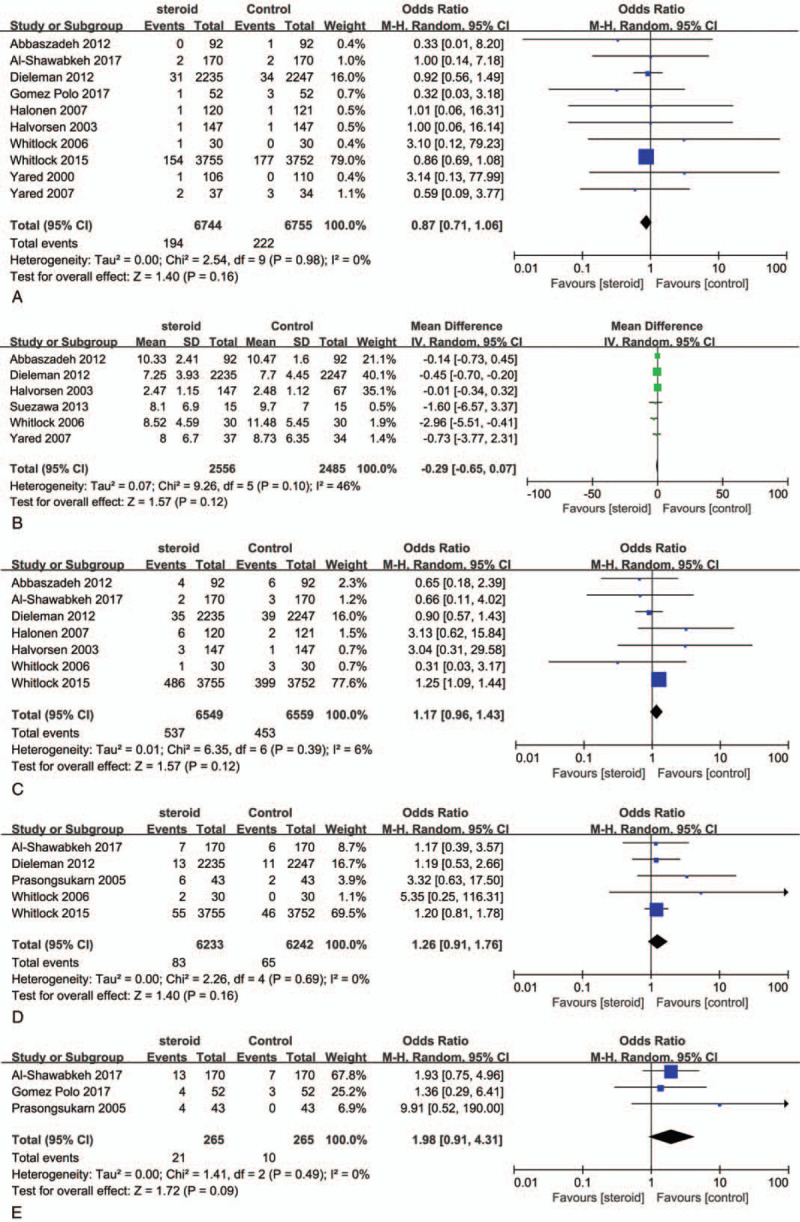
Forest plot for the meta-analysis of postoperative mortality (A), duration of ventilation (B), myocardial infarction (C), gastrointestinal complications (D), and high blood sugar (E).
3.6. Postoperative duration of ventilation
The pooled results showed no significant difference in the duration of mechanical ventilation in hours between the treatment groups (RR, −0.29; 95% CI, −0.65 to 0.07; P = .12, Fig. 5B). We found a medium level of heterogeneity (I2 = 46%, P = .10) in the pooled results.
3.7. Postoperative myocardial infarction
The analysis indicated the risk of Myocardial infarction rates were not significant difference between CS group and placebo group (RR, 1.71; 95% CI, 0.96–1.43; P = .12; Fig. 5C) and found a low level heterogeneity (I2 = 6%, P = .39) in the pooled results for myocardial infarction (MI).
3.8. Postoperative gastrointestinal complications
The rate of gastrointestinal complications was similar in both groups (RR, 1.26; 95% CI, 0.91–1.76; P = .16; Fig. 5D). And no heterogeneity was found (I2 = 0%, P = .68) for the pooled results.
3.9. Postoperative high blood sugar
CS treatment did not increase the risk of high blood sugar after cardiac surgery compared with control groups (RR, 1.98; 95% CI, 0.91–4.31; P = .09; Fig. 5E). There was no heterogeneity across the trials (I2 = 0%, P = .49).
3.10. Postoperative stroke
Figure 6A shows the overall RR as well as the RRs of individual trials regarding stroke. No heterogeneity across the trials was observed regarding this event (I2 = 0%, P = 1.00). There was no significant difference in the risk of stroke between CS groups and control groups (RR, 0.9; 95% CI, 0.69–1.18; P = .45).
Figure 6.
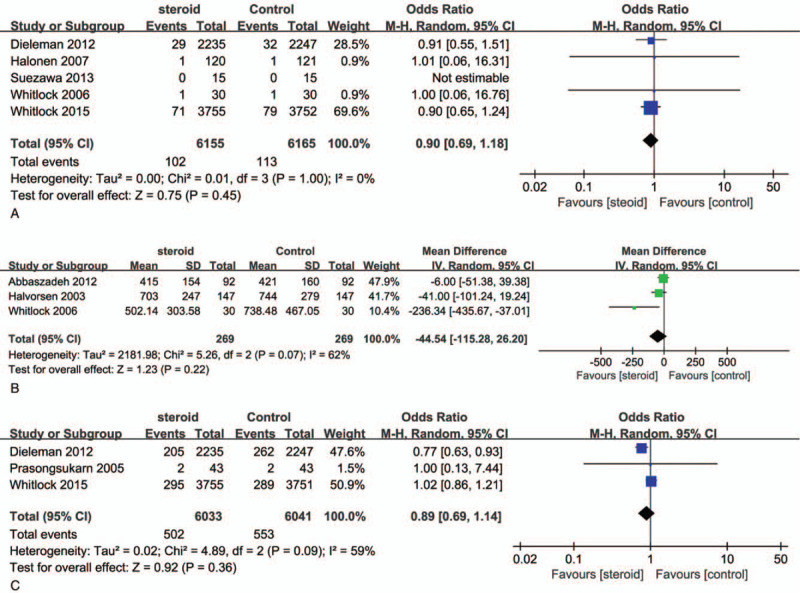
Forest plot for the meta-analysis of postoperative stroke (A), bleeding (B), and delirium (C).
3.11. Postoperative bleeding
Pooled effects showed no significant difference in blood lose after cardiac surgery (RR −44.54; 95% CI, −115.28 to 26.20; P = .22; Fig. 6B). There was significant heterogeneity among the studies (I2 = 62%; P = .07).
3.12. Postoperative delirium
Figure 6C shows the overall RR as well as the RRs of individual trials regarding delirium. There was a medium level of heterogeneity across the trials (I2 = 59%; P = .09). The analysis indicated that there was no significant difference in the risk of delirium between CS groups and control groups (8.32% vs 9.15%; RR, 0.89; 95% CI, 0.69–1.41; P = .36).
3.13. Subgroup analyses of POAF
The effect of steroids on incidence of new-onset atrial fibrillation did not differ based on steroid type (Fig. 7). However, trials of low dose steroids (3 studies, 725 patients) were associated with a smaller clinical benefit (RR = 0.96; 95% CI, 0.50–1.86; P = .92; I2 = 66%), whereas trials of medium dose steroids (11 studies, 10,159 patients) were associated with a greater clinical benefit (RR = 0.63; 95% CI, 0.48–0.83; P = .001; I2 = 76%). Trials of isolated CABG (13 studies, 1208 patients) were not associated with a benefit (RR = 0.64; 95% CI, 0.29–1.41; P = .27; I2 = 77%).
Figure 7.
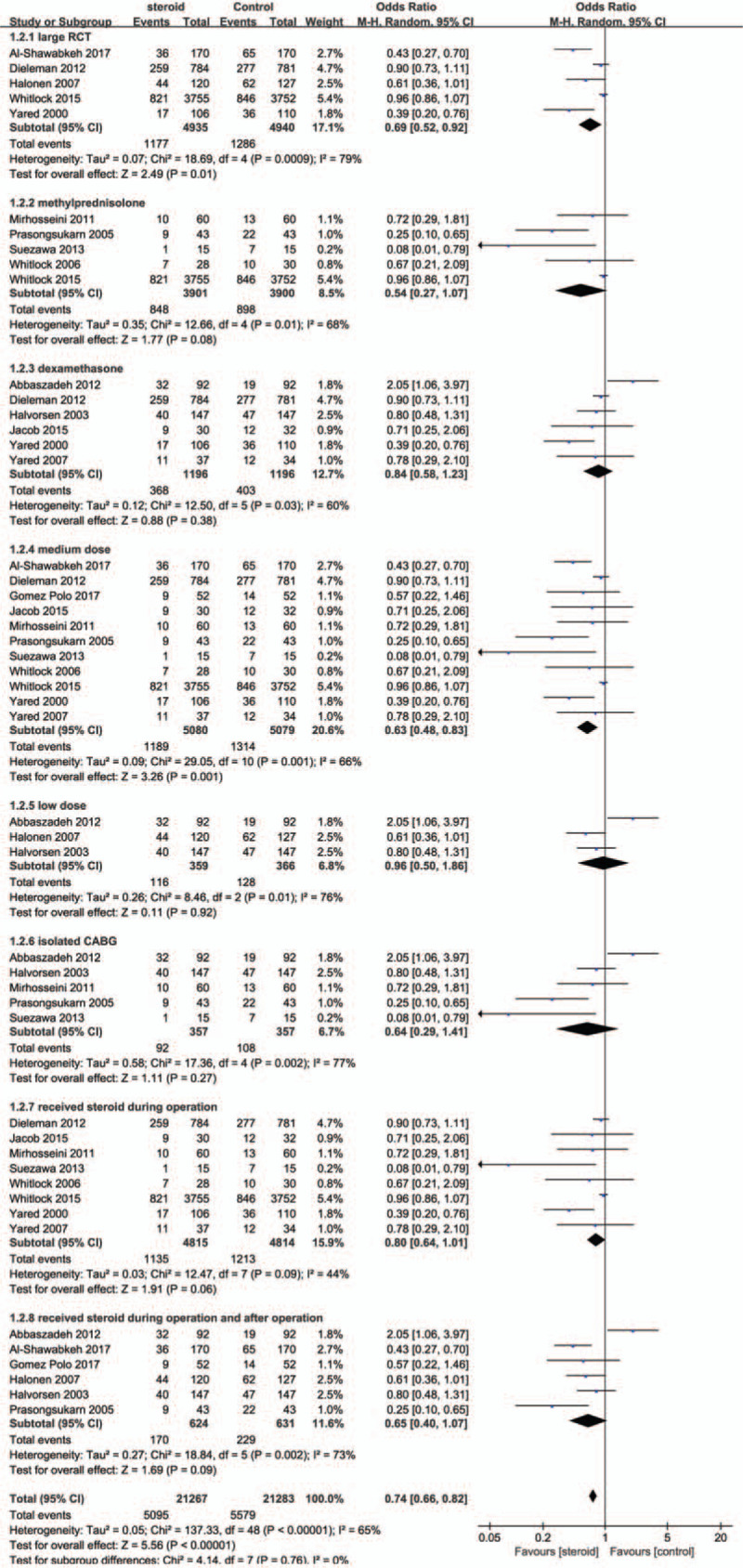
Forest plot for subgroup analyses of postoperative atrial fibrillation.
4. Discussion
Atrial fibrillation is a very common complication after cardiac surgery. Previous small RCTs and meta-analyses demonstrate that CSs could reduce the incidence of POAF when compared with placebo. However, some recent reports from large RCTs conclude that there is no protective effect of CSs on the incidence of new-onset AF after cardiac surgery.[8,9] Our meta-analysis revealed that using steroids both intraoperatively and postoperatively proved to be safe and effective in reducing the incidence of POAF without increasing the incidence of postoperative complications and adverse effects due to CS therapy.
Considering the contradictious conclusions of different RCTs, we included 5 large trials (n > 200) into subgroup analysis, which also suggesting that perioperative steroid use may decrease the incidence of atrial fibrillation after cardiac surgery. The mechanism behind the beneficial effects of steroid on the development of AF is not well known. The anti-inflammatory activity of CSs may play the vital role of preventing POAF. All markers of increased inflammatory reaction concentration, such as complement C-reactive protein complex, white blood cells, inflammatory cytokines, are higher in patients with POAF than in patients who remain in sinus rhythm after cardiac surgery.[2] The concentration of C-reactive protein was significantly lower postoperatively in the steroid group than in the placebo group.[2,12,15,18] Another possible effect of steroids is that CSs reduce postoperative nausea, vomiting, and anorexia. Thus, CS therapy may improve absorption of oral medications, such as -blockers, and thereby reduce the incidence of AF.[16]
Interestingly, the subgroup analysis shows that a medium dose of CSs was associated with reducing the incidence of POAF compared with low doses. A possible explanation for this might be that fewer studies using low or high doses of CS, so that low and high doses of steroid in these studies did not have prophylactic effects on AF. Another reason is the response of CS therapy, which is in normal distribution (bell curve) where the optimum effective dose is the medium. Furthermore, the anti-inflammatory effects are dose-dependent, according to the pharmacology.[21] However, high dose of steroid has the potential risks of increasing side effects. Some previous meta-analyses also investigating that low- and high-dose CSs were ineffective in preventing AF in contrast to moderate doses.[22,23] This fact was confirmed in our meta-analysis that optimum dose was the medium one.
Compared with different types of CSs, maybe, methylprednisolone could be better candidate to reduce the incidence of POAF according to this meta-analysis, although there was not statistically significant difference. Therefore, no firm conclusions can be drawn here, because of the limitations of the subgroup analysis.
There are several reasons that might explain why some studies could not demonstrate any protective effect of CSs on postoperative AF. One possible reason is that the moment and duration of steroid administration. Previous studies which demonstrating a protective effect of steroid treatment on POAF were designed to administer steroid not only preoperatively, but also on the following days of surgery. Other trials just gave a single shot shortly after the induction of anesthesia or during the surgery process. In our subgroup analysis, we did not see the significant difference between administration at variety perioperative moments. The optimal timing of drug delivery and the frequency of administration remain unclear.
One large RCT and a recent systematic review indicate that prophylactic administration of steroid is associated with an increase in myocardial infarction or injury.[9,24] However, the increase in myocardial injury with steroid in these studies was not reported in other previous trials. The reason of this contradicted result may mainly because of differing MI definitions which can impacted the event incidence between trials. Taking the 2 large clinical trials (Steroids In caRdiac Surgery study [SIRS] and Dexamethasone for Cardiac Surgery Study [DECS]) as examples, in the SIRS trial that mandated postoperative creatinine kinase-fraction myocardial band and Electrocardiograph monitoring, the overall incidence of myocardial injury was 11.8%.[8,9] In contrast, in the DECS trial, MIs were defined by a biomarker elevation in association with new Q-waves or left bundle branch block on Electrocardiograph, which resulted in a much lower incidence of 1.7%. our meta-analysis did not see the evidence that steroid administration can impact the incidence of myocardial injury after cardiac surgery.
The findings of our study demonstrated that CS receivers had a statistically reduction on the length of hospital stay, which indeed depends on reduction of surgical complications and improvement in clinical outcomes after CABG. There are other meta-analysis supporting our finding.[1,25] However, steroid prophylaxis had no effect on reducing the length of ICU stay and did not increase the time of need for mechanical ventilation. In agreement with our study, Cappabianca et al also reported that steroids could reduce morbidity, surgical complications, and Hospital Length of Stay.[6]
One of the potential risks of applying CS perioperatively is that steroid-induced suppression could significantly increase the risk of infection. Our study indicated that administration of steroid did not increase any infections at all. In addition, there was no statistical difference in the rate of major complication between the steroid and the placebo groups on high blood sugar level, gastrointestinal complications, postoperative bleeding, and delirium. According to our meta-analysis, we believe that using steroid in perioperative moments with low or medium doses are safety in patients undergoing CABG alone or combined with valve surgery.
Our analysis included 2 large clinical trials (SIRS and DECS) which contribute about 12,000 patients to the total 13,803 patients.[8,9] Therefore, our results are mostly based on these 2 large high quality RCTs, and more multicentric large clinical trials are needed to confirm our meta-analysis findings.
There are several limitations in our study. First, a major limitation of this meta-analyses is the heterogeneity of the included studies. Some results of our meta-analysis have significant heterogeneities. Most of these studies were small in sample size (18–294 patients) and investigated various types of CSs in multiple doses at different time points of administration. Second, definitions of end points were different across included studies, such as myocardial infarctions, which threatens the validity of our results. The low event rates and the small proportion of trials reporting outcomes limit our ability to draw conclusions about the effect of steroids on these outcomes. Moreover, we did not have access to further propensity analysis or stratified analysis to better define differences between treatment groups. Finally, we would also like to point out the publication bias exaggerating the positive effects when meta-analysis was based on previously published studies, due to positive results are more tendency to be published than negative results.
In conclusion, the present meta-analysis suggests a beneficial effect of steroids to prevent new-onset atrial fibrillation after cardiac surgery. Steroid prophylaxis in patients undergoing CABG or combine with valve surgery could significantly reduce the incidence of new-onset POAF, and the length of hospital stay. On the other hand, it is an effective safe treatment that does not increase the incidence of infection or other side effects of steroids compared with the placebo. The strength of this relationship should be interpreted with caution because of statistical and clinical heterogeneity among the included studies.
Author contributions
Conceptualization: Xiao-Wen Wang.
Data curation: Lu Liu, Xiao-Wen Wang.
Formal analysis: Lu Liu, Fu-Yu Jing, Lin-Jun Li.
Methodology: Lu Liu, Fu-Yu Jing, Lin-Jun Li, Rui-Qin Zhou, Cheng Zhang.
Project administration: Chen Zhang, Rui-Qin Zhou, Xiao-Wen Wang.
Software: Fu-Yu Jing, Lin-Jun Li, Lu Liu, Rui-Qin Zhou, Cheng Zhang.
Supervision: Xiao-Wen Wang, Qing-Chen Wu.
Validation: Cheng Zhang.
Writing – original draft: Lu Liu.
Writing – review & editing: Xiao-Wen Wang, Qing-Chen Wu.
Supplementary Material
Footnotes
Abbreviations: CABG = coronary artery bypass grafting, CI = confidence interval, CS = corticosteroid, POAF = postoperative atrial fibrillation, RCTs = randomized controlled trials, RR = relative risk.
How to cite this article: Liu L, Jing FY, Wang XW, Li LJ, Zhou RQ, Zhang C, Wu QC. Effects of corticosteroids on new-onset atrial fibrillation after cardiac surgery: a meta-analysis of randomized controlled trials. Medicine. 2021;100:11(e25130).
This research received no specific grant from the National Natural Science Foundation of China (NSFC81700320) and Chongqing Science and Technical Commission Research Funding (CSTC2019-MSXMX0827).
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Supplemental digital content is available for this article.
References
- [1].Ali-Hassan-Sayegh S, Mirhosseini SJ, Haddad F, et al. Protective effects of corticosteroids in coronary artery bypass graft surgery alone or combined with valvular surgery: an updated and comprehensive meta-analysis and systematic review. Interact Cardiovasc Thorac Surg 2015;20:825–36. [DOI] [PubMed] [Google Scholar]
- [2].Halonen J, Halonen P, Jarvinen O, et al. Corticosteroids for the prevention of atrial fibrillation after cardiac surgery: a randomized controlled trial. JAMA 2007;297:1562–7. [DOI] [PubMed] [Google Scholar]
- [3].Yadava M, Hughey AB, Crawford TC. Postoperative atrial fibrillation: incidence, mechanisms, and clinical correlates. Cardiol Clin 2014;32:627–36. [DOI] [PubMed] [Google Scholar]
- [4].Ha AC, Mazer CD, Verma S, et al. Management of postoperative atrial fibrillation after cardiac surgery. Curr Opin Cardiol 2016;31:183–90. [DOI] [PubMed] [Google Scholar]
- [5].Teoh KH, Bradley CA, Gauldie J, et al. Steroid inhibition of cytokine-mediated vasodilation after warm heart surgery. Circulation 1995;92:I347–53. [DOI] [PubMed] [Google Scholar]
- [6].Cappabianca G, Rotunno C, de Luca Tupputi Schinosa L, et al. Protective effects of steroids in cardiac surgery: a meta-analysis of randomized double-blind trials. J Cardiothorac Vasc Anesth 2011;25:156–65. [DOI] [PubMed] [Google Scholar]
- [7].Whitlock RP, Chan S, Devereaux PJ, et al. Clinical benefit of steroid use in patients undergoing cardiopulmonary bypass: a meta-analysis of randomized trials. Eur Heart J 2008;29:2592–600. [DOI] [PubMed] [Google Scholar]
- [8].Dieleman JM, Nierich AP, Rosseel PM, et al. Intraoperative high-dose dexamethasone for cardiac surgery: a randomized controlled trial. JAMA 2012;308:1761–7. [DOI] [PubMed] [Google Scholar]
- [9].Whitlock RP, Devereaux PJ, Teoh KH, et al. Methylprednisolone in patients undergoing cardiopulmonary bypass (SIRS): a randomised, double-blind, placebo-controlled trial. Lancet 2015;386:1243–53. [DOI] [PubMed] [Google Scholar]
- [10].Halvorsen P, Raeder J, White PF, et al. The effect of dexamethasone on side effects after coronary revascularization procedures. Anesth Analg 2003;96:1578–83. [DOI] [PubMed] [Google Scholar]
- [11].Abbaszadeh M, Khan ZH, Mehrani F, et al. Perioperative intravenous corticosteroids reduce incidence of atrial fibrillation following cardiac surgery: a randomized study. Rev Bras Cir Cardiovasc 2012;27:18–23. [DOI] [PubMed] [Google Scholar]
- [12].Yared JP, Bakri MH, Erzurum SC, et al. Effect of dexamethasone on atrial fibrillation after cardiac surgery: prospective, randomized, double-blind, placebo-controlled trial. J Cardiothorac Vasc Anesth 2007;21:68–75. [DOI] [PubMed] [Google Scholar]
- [13].Yared JP, Starr NJ, Torres FK, et al. Effects of single dose, postinduction dexamethasone on recovery after cardiac surgery. Ann Thorac Surg 2000;69:1420–4. [DOI] [PubMed] [Google Scholar]
- [14].Whitlock RP, Young E, Noora J, et al. Pulse low dose steroids attenuate post-cardiopulmonary bypass SIRS; SIRS I. J Surg Res 2006;132:188–94. [DOI] [PubMed] [Google Scholar]
- [15].Suezawa T, Aoki A, Kotani M, et al. Clinical benefits of methylprednisolone in off-pump coronary artery bypass surgery. Gen Thorac Cardiovasc Surg 2013;61:455–9. [DOI] [PubMed] [Google Scholar]
- [16].Prasongsukarn K, Abel JG, Jamieson WR, et al. The effects of steroids on the occurrence of postoperative atrial fibrillation after coronary artery bypass grafting surgery: a prospective randomized trial. J Thorac Cardiovasc Surg 2005;130:93–8. [DOI] [PubMed] [Google Scholar]
- [17].Mirhosseini SJ, Forouzannia SK, Sayegh AH, et al. Effect of prophylactic low dose of methylprednisolone on postoperative new atrial fibrillation and early complications in patients with severe LV dysfunction undergoing elective off-pump coronary artery bypass surgery. Acta Med Iran 2011;49:288–92. [PubMed] [Google Scholar]
- [18].Al-Shawabkeh Z, Al-Nawaesah K, Anzeh RA, et al. Use of short-term steroids in the prophylaxis of atrial fibrillation after cardiac surgery. J Saudi Heart Assoc 2017;29:23–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gomez Polo JC, Vilacosta I, Martin-Garcia AC, et al. Use of corticosteroids in the prophylaxis of atrial fibrillation after cardiac surgery (ECOFA study). Eur Heart J 2017;38:580–1. [Google Scholar]
- [20].Jacob KA, Dieleman JM, Nathoe HM, et al. The effects of intraoperative dexamethasone on left atrial function and postoperative atrial fibrillation in cardiac surgical patients. Neth Heart J 2015;23:168–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Liu C, Wang J, Yiu D, et al. The efficacy of glucocorticoids for the prevention of atrial fibrillation, or length of intensive care unite or hospital stay after cardiac surgery: a meta-analysis. Cardiovasc Ther 2014;32:89–96. [DOI] [PubMed] [Google Scholar]
- [22].Marik PE, Fromm R. The efficacy and dosage effect of corticosteroids for the prevention of atrial fibrillation after cardiac surgery: a systematic review. J Crit Care 2009;24:458–63. [DOI] [PubMed] [Google Scholar]
- [23].Baker WL, White CM, Kluger J, et al. Effect of perioperative corticosteroid use on the incidence of postcardiothoracic surgery atrial fibrillation and length of stay. Heart Rhythm 2007;4:461–8. [DOI] [PubMed] [Google Scholar]
- [24].Dvirnik N, Belley-Cote EP, Hanif H, et al. Steroids in cardiac surgery: a systematic review and meta-analysis. Br J Anaesth 2018;120:657–67. [DOI] [PubMed] [Google Scholar]
- [25].Dieleman JM, van Paassen J, van Dijk D, et al. Prophylactic corticosteroids for cardiopulmonary bypass in adults. Cochrane Database Syst Rev 2011;11:CD005566. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


