Abstract
The platelet-albumin-bilirubin (PALBI) grade plays critical role in evaluating liver function. However, the change of PALBI grade from the preoperative to postoperative period in predicting patient outcomes after hepatectomy remains unclear.
A total of 489 HCC patients who underwent hepatectomy in West China Hospital between January, 2010 and June, 2016 were analyzed retrospectively.ΔPALBI grade was calculated by PALBI grade at the first postoperative month - preoperative PALBI grade.ΔPALBI >0 was considered as stable; otherwise, worse PALBI grade was considered. Kaplan– Meier method and Cox proportional hazard regression analyses were performed for survival analysis. Prognostic model was constructed by nomogram method.
Three hundred forty two patients and 147 patients were classified into training group and validation group, respectively. In the training group, results from Cox model suggested that worse PALBI grade (HR 1.328, 95% CI 1.010–1.746, P = .042), tumor size (HR 1.460, 95% CI 1.058–2.015, P = .021), microvascular invasion (MVI, HR 1.802, 95% CI 1.205–2.695, P < .001), and high alpha-fetoprotein level (AFP, HR 1.364, 95% CI 1.044–1.781, P = .023) negatively influenced postoperative recurrence. Similarly, worse PALBI grade (HR 1.403, 95% CI 1.020–1.930, P = .038), tumor size (HR 1.708, 95% CI 1.157–2.520, P = .007), MVI (HR 1.914, 95% CI 1.375–2.663, P < .001), and presence of cirrhosis (HR 1.773, 95% CI 1.226–2.564, P = .002) had negatively impacts on overall survival. Patients with worse PALBI grade had worse recurrence free (RFS) and overall survival (OS). The prognostic model incorporating the change of PALBI grade constructed in training group and tested in the validation group could perform well in predicting the outcomes.
Postoperative change of PALBI grade was independently risk factor related with prognosis. Prognostic model incorporating the change of PALBI grade might be a useful index to predict the prognosis of HCC patients following hepatectomy.
Keywords: hepatectomy, hepatocellular carcinoma, nomogram, PALBI grade, prognosis
1. Introduction
Hepatocellular carcinoma (HCC) is the sixth most common cancer and the fourth leading cause of cancer-related mortalities worldwide.[1] More than half of newly diagnosed HCC cases occurred in China, leading to a heavy economic burden.[2] Hepatectomy, as a radical therapy, is the mostly widely used for HCC. However, the majority of HCC patients will develop HCC recurrence, compromising the long-term survival.[3,4] Different from some other tumors, prognosis of HCC patient is determined not only by tumor stage but also by the status of liver function.
The Child–Pugh(C-P) scoring system was originally designed to estimate liver function in cirrhotic patients.[5] Recently, it has become a potential prognostic factor for HCC after liver resection. However, in clinical practice, the accuracy of the Child–Pugh scoring system limits due to it involves subjective variable, such as ascites and encephalopathy.[6] In addition, the majority of HCC patients who underwent hepatectomy were classified as Child–Pugh A grade. Since the liver function reserve in C-P grade A HCC patients varies widely, Child–Pugh(C-P) scoring was unable to further discriminate liver function and clinical outcomes.
In 2015, Johnson et al. put forward the albumin–bilirubin (ALBI) grade, which consists of 2 laboratory parameters, namely total bilirubin and albumin.[7] Numerous studies have validated the prognostic accuracy of this ALBI grade in HCC patients with underlying liver cirrhosis.[8–10] As we known, HCC commonly occurred in cirrhotic patients. Portal hypertension is a crucial factor when planning surgical method for cirrhotic patients.[11] Giving to the impact of portal hypertension, platelet count as a marker of portal hypertension was incorporated into ALBI and constructed as the platelet-albumin-bilirubin grade (PALBI).[7] For HCC patients underwent surgery, it seems that PALBI was better to evaluate the liver function.[12] Some study even confirmed that the PALBI grade was an objective measurement to predict postoperative liver complication and overall survival for HCC patients after hepatectomy.[13,14] Previous studies mainly focused on preoperative liver function when assessed the role of liver function in the outcome of HCC patients, and ignored the impacts of changed status of liver function after surgery on the prognosis of HCC. The dynamic liver function related index might be more precise to reflect the liver status, especially when we analyzed the impact of liver function on survival. Unfortunately, there was few studies to discuss the role of the change of PALBI grade in predicting patient outcomes after hepatectomy.
Therefore, the current study aimed to assess the prognostic significance of the change of PALBI grade for HCC patients following hepatectomy.
2. Material and methods
2.1. Patients
Between January 2010 and June 2016, a total of 489 patients with HBV-related HCC within the Milan criteria who underwent liver resection in West China Hospital were prospectively collected. All enrolled patients were diagnosed with HCC according to radiological or histological criteria as recommended by the American Association for the Study of Liver Diseases guidelines. Clinical features, including demographic data, routine blood test, liver function test, AFP, tumor number, maximum tumor diameter, satellite lesions, microvascular invasion (MVI) were examined. Meanwhile, routine blood test and liver function was collected at first postoperative month. Inclusion criteria were as follows:
-
1.
HCC within the Milan criteria;
-
2.
Patients underwent hepatectomy.
Exclusion criteria were as follows:
-
1.
recurrent HCC;
-
2.
ruptured HCC;
-
3.
hepatitis C virus infection;
-
4.
preoperative antitumor treatment;
-
5.
positive surgical margin;
-
6.
concurrent other malignancy;
-
7.
incomplete follow-up data.
PALBI grade was calculated: PALBI: 2.02 × log10 bilirubin (μmol/L) − 0.37 × (log10 bilirubin)2 − 0.04 × albumin (g/L) − 3.48 × log10 platelets + 1.01 × (log10 platelets).[2] PALBI grade was defined as PALBI grade 1 (score ≤−2.53), PALBI grade 2 (>−2.53 and ≤−2.09), and PALBI grade 3 (>−2.09). ΔPALBI grade: PALBI grade at first postoperative month–preoperative PALBI grade. If ΔPALBI ≥0, the stable PALBI grade was defined; otherwise, the worse PALBI grade was defined. All patients were randomly separated into training group and validation group with the ratio of 7:3. The study was approved by the institutional review board of West China Hospital.
2.2. Follow-up
Preoperative laboratory examinations were performed 2 days before the operation. After hepatectomy, patients were regularly followed up at the first postoperative month, every 3 months for the first 3 years, and every 6 months in subsequent years. At each investigation, blood tests, liver function tests, serum AFP measurement, HBV-DNA tests, liver ultrasonography and chest radiography were performed for all patients. When HCC recurrence was suspected, enhanced computed tomography or magnetic resonance imaging or biopsy, and bone scintigraphy was performed. Overall survival (OS) was defined as the time between the date of surgery and the date of death for any cause. Recurrence-free survival (RFS) was defined as the time from the date of surgery to any recurrence.
2.3. Statistical analysis
All statistical analyses were performed using SPSS 21.0 (SPSS Company, Chicago, IL) and R 3.6.1 for Windows. All continuous variables were displayed as the median ± interquartile range (IQR) and compared using Mann–Whitney tests. Categorical variables were analyzed using X2 test or Fisher exact test. The survival analysis was performed by Kaplan–Meier (K–M) tests and the differences were evaluated by log-rank tests. Independent risk factors for RFS and OS were identified using the stepwise forward Cox regression model. Potential risk factors with a P value <.1 in the univariate analysis were included in the multivariate analysis. Based on the results of Cox model, a nomogram model was built up to predict 3-year and 5-year survival rates in the training group. The model was evaluated by calibration plots and C-index (concurrence index) in both groups. A P value <.05 was considered statistically significant.
3. Results
3.1. Demographic characteristics
As shown in Table 1, there were 416 male and 73 female patients. The mean age was 50 years. With regard to tumor characteristics, 461 HCC patients (94.3%) had single tumor and 28 patients had 2 or 3 nodules. The tumor diameter ≥3.0 accounted for 337(68.9) patients. High serum AFP levels (>400 ng/ml) were observed in 308 patients (63.0%). HBV-DNA load was positive in 241 patients (49.3%). MVI was detected in 106 patients (21.7%). All patients had a liver function with Child–Pugh A. Before surgery, 377 patients were classified as PALBI grade 1, 103 patients were classified as PALBI grade 2, and 9 patients were classified as PALBI grade 3. At postoperative first month, the PALBI grade deteriorated in 120 patients and 369 patients remained stable. All patients were randomly categorized into training cohort and validation cohort. Among the training group, there were differences in age, tumor size, tumor differentiation and platelet count between stable and worse PALBI groups. Other differences in both groups were not observed (Table 2).
Table 1.
Demographic features of patients with hepatocellular carcinoma following liver resection.
| Variable | All subjects (N = 489) | Training cohort (N = 342) | Validation cohort (N = 147) | P value |
| Median age at diagnosis (IQR) | 50 (17) | 50 (18) | 50 (15) | .924 |
| Gender, n (%) | .094 | |||
| Male | 416 (85.1) | 297 (86.8) | 119 (81.0) | |
| Female | 73 (14.9) | 45 (13.2) | 28 (19.0) | |
| HBsAg, n (%) | .789 | |||
| Yes | 438 (89.6) | 305 (89.2) | 133 (90.5) | |
| No | 51 (10.4) | 37 (10.8) | 14 (9.5) | |
| Tumor number, n (%) | .413 | |||
| 1 | 461 (94.3) | 324 (94.7) | 137 (93.2) | |
| 2 | 22 (4.5) | 13 (3.8) | 9 (6.1) | |
| 3 | 6 (1.2) | 5 (1.5) | 1 (0.7) | |
| Differentiation, n (%) | .462 | |||
| Well | 149 (30.5) | 110 (32.2) | 39 (26.5) | |
| Moderate | 327 (66.9) | 223 (65.2) | 104 (70.7) | |
| Poor | 13 (2.7) | 9 (2.6) | 4 (2.7) | |
| Maximum diameter of tumor, cm, n (%) | <.001 | |||
| <3.0 | 152 (31.1) | 87 (25.4) | 65 (44.2) | |
| ≥3.0 | 337 (68.9) | 255 (74.6) | 82 (55.8) | |
| Satellite lesion, n (%) | .446 | |||
| Yes | 429 (87.7) | 297 (86.8) | 132 (89.8) | |
| No | 60 (12.3) | 45 (13.2) | 15 (10.2) | |
| Presence of MVI, n (%) | <.001 | |||
| Yes | 106 (21.7) | 94 (27.5) | 12 (8.2) | |
| No | 383 (78.3) | 248 (72.5) | 135 (91.8) | |
| Presence of cirrhosis, n (%) | .595 | |||
| Yes | 393 (80.4) | 277 (81.0) | 116 (78.9) | |
| No | 96 (19.6) | 65 (19.0) | 31 (21.1) | |
| Hb, n (%) | .995 | |||
| <LLN | 20 (4.1) | 14 (4.1) | 6 (4.1) | |
| ≥LLN | 469 (95.9) | 328 (95.9) | 141 (95.9) | |
| PLT (∗10^9/L) | 129.33 (58.52) | 130.51 (60.16) | 126.59 (54.62) | .498 |
| NLR | 2.44 (1.84) | 2.47 (1.71) | 2.37 (2.13) | .597 |
| TBIL (umol/L) | 15.11 (6.77) | 15.05 (6.84) | 15.23 (6.63) | .791 |
| ALT (U/L) | 37.00 [27.00, 52.00] | 37.00 [27.25, 53.00] | 39.00 [27.00, 52.00] | .982 |
| AST (U/L) | 36.00 [28.00, 47.00] | 36.00 [28.00, 48.00] | 34.00 [27.50, 45.50] | .26 |
| ALB (g/L) | 41.93 (4.19) | 41.92 (4.18) | 41.96 (4.23) | .908 |
| CREA (umol/L) | 77.28 (21.29) | 78.38 (23.57) | 74.73 (14.40) | .083 |
| WBC, n (%) | .860 | |||
| <10 | 483 (98.8) | 338 (98.8) | 145 (98.6) | |
| ≥10 | 6 (1.2) | 4 (1.2) | 2 (1.4) | |
| AFP, n (%) | .622 | |||
| <400 | 308 (63.0) | 213 (62.3) | 95 (64.6) | |
| ≥400 | 181 (37.0) | 129 (37.7) | 52 (35.4) | |
| HBV DNA, n (%) | .913 | |||
| Negative | 248 (50.7) | 174 (50.9) | 74 (50.3) | |
| Positive | 241 (49.3) | 168 (49.1) | 73 (49.7) | |
| Preoperative PALBI grade, n (%) | .371 | |||
| 1 | 377 (77.1) | 265 (77.5) | 112 (76.2) | |
| 2 | 103 (21.1) | 69 (20.2) | 34 (23.1) | |
| 3 | 9 (1.8) | 8 (2.3) | 1 (0.7) | |
| Postoperative PALBI grade, n (%) | .065 | |||
| 1 | 322 (65.8) | 214 (62.6) | 108 (73.5) | |
| 2 | 131 (26.8) | 100 (29.2) | 31 (21.1) | |
| 3 | 36 (7.4) | 28 (8.2) | 8 (5.4) | |
| Change of PALBI grade, n (%) | .003 | |||
| Stable | 369 (75.5) | 245 (71.6) | 124 (84.4) | |
| Worse | 120 (24.5) | 97 (28.4) | 23 (15.6) |
AFP = alpha-fetoprotein, Hb = hemoglobin, IQR = interquartile range, MVI = microvascular invasion, PALBI = platelet-albumin-bilirubin, WBC = white blood cell.
Table 2.
Demographic features of patients in between stable and worse PALBI group.
| All subjects (N = 342) | stable PALBI (N = 245) | worse PALBI (N = 97) | P value | |
| Age | ||||
| ≤60 | 170 (49.7) | 132 (53.9) | 38 (39.2) | .020 |
| >60 | 172 (50.3) | 113 (46.1) | 59 (60.8) | |
| Gender | ||||
| female | 45 (13.2) | 31 (12.7) | 14 (14.4) | .794 |
| male | 297 (86.8) | 214 (87.3) | 83 (85.6) | |
| HBsAg | ||||
| No | 37 (10.8) | 27 (11.0) | 10 (10.3) | 1.000 |
| Yes | 305 (89.2) | 218 (89.0) | 87 (89.7) | |
| HBeAg | ||||
| No | 282 (82.5) | 206 (84.1) | 76 (78.4) | .272 |
| Yes | 60 (17.5) | 39 (15.9) | 21 (21.6) | |
| HBV-DNA | ||||
| Negative | 174 (50.9) | 127 (51.8) | 47 (48.5) | .657 |
| Positive | 168 (49.1) | 118 (48.2) | 50 (51.5) | |
| Tumor number | ||||
| 1 | 324 (94.7) | 233 (95.1) | 91 (93.8) | .658 |
| 2 | 13 (3.8) | 8 (3.3) | 5 (5.2) | |
| 3 | 5 (1.5) | 4 (1.6) | 1 (1.0) | |
| Tumor size (cm) | 5.64 (3.52) | 5.88 (3.80) | 5.03 (2.59) | .045 |
| Satellite lesion | ||||
| No | 297 (86.8) | 209 (85.3) | 88 (90.7) | .247 |
| Yes | 45 (13.2) | 36 (14.7) | 9 (9.3) | |
| MVI | ||||
| No | 248 (72.5) | 178 (72.7) | 70 (72.2) | 1.000 |
| Yes | 94 (27.5) | 67 (27.3) | 27 (27.8) | |
| Differentiation | ||||
| Well | 110 (32.2) | 80 (32.7) | 30 (30.9) | .036 |
| Moderate | 223 (65.2) | 162 (66.1) | 61 (62.9) | |
| Poor | 9 (2.6) | 3 (1.2) | 6 (6.2) | |
| liver cirrhosis | ||||
| No | 65 (19.0) | 50 (20.4) | 15 (15.5) | .369 |
| Yes | 277 (81.0) | 195 (79.6) | 82 (84.5) | |
| PLT (∗10^9/L) | 130.51 (60.16) | 136.82 (62.51) | 114.56 (50.66) | .002 |
| NLR | 2.47 (1.71) | 2.47 (1.57) | 2.46 (2.02) | .947 |
| TBIL (umol/L) | 15.05 (6.84) | 14.97 (7.14) | 15.26 (6.04) | .731 |
| ALT (U/L) | 37.00 [27.25, 53.00] | 37.00 [28.00, 54.00] | 37.00 [27.00, 52.00] | .998 |
| AST (U/L) | 36.00 [28.00, 48.00] | 36.00 [27.00, 48.00] | 37.00 [31.00, 46.00] | .240 |
| ALB (g/L) | 41.92 (4.18) | 41.91 (4.31) | 41.92 (3.88) | .990 |
| CREA (umol/L) | 78.38 (23.57) | 79.16 (21.57) | 76.41 (28.03) | .331 |
| AFP | ||||
| <400 | 213 (62.3) | 150 (61.2) | 63 (64.9) | .605 |
| ≥400 | 129 (37.7) | 95 (38.8) | 34 (35.1) | |
Based on the survival analysis, the 1-, 3-, 5-year RFS rates for all patients were 75.1%, 52.8%, and 40.5%, respectively (Fig. 1A). The 1-, 3-, 5-year OS rates for all patients were 94.6%, 76.8%, and 62.9%, respectively (Fig. 1B). Based on the change of PALBI grade, the 1-, 3-, 5-year RFS rates were 67.9%, 41.9% and 33.2% for patients with stable PALBI grade, and 63.4%, 34.5%, and 19.4% for patients with worse PALBI grade, respectively. The 1-, 3-, 5-year OS rates were 93.6%, 66.1%, and 49.3% for patients with stable PALBI grade, and 88.3%, 65.0% and 39.2%for patients with worse PALBI grade, respectively. There were significant differences between both groups (RFS: P < .001; OS: P = .001). (Fig. 1C and 1D).
Figure 1.
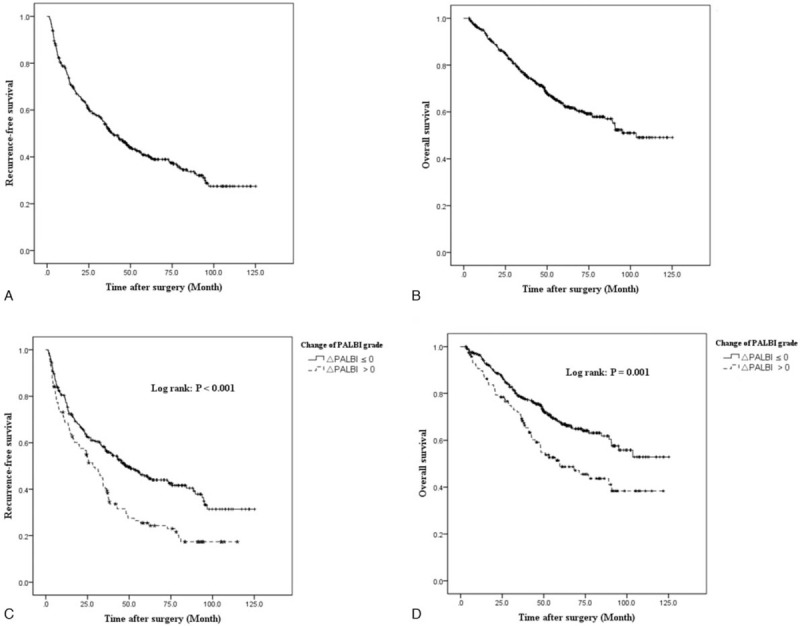
Kaplan–Meier analysis of RFS and OS on all cohorts and categorized by postoperative ΔPALBI. Survival analysis. Recurrence-free survival (A) and overall survival (B) plots of all included subjects. Comparison of recurrence-free survival (C) and overall survival (D) between stable and worse PALBI group.
3.2. Factors associated with RFS and OS
In the training cohort, multivariate analyses revealed that postoperative worse PALBI grade (HR 1.328, 95% CI 1.010–1.746, P: 0.042), tumor size (HR 1.460, 95% CI 1.058–2.015, P:.021), MVI (HR 1.802, 95% CI 1.205–2.695, P < .001), and high AFP level (HR 1.364, 95% CI 1.044–1.781, P:.023) were associated with postoperative recurrence. As to risk factors related to OS, worse PALBI grade (HR 1.403, 95% CI 1.020–1.930, P:.038), large tumor size (HR 1.708, 95% CI 1.157–2.520, P:.007), MVI (HR 1.914, 95% CI 1.375–2.663, P < .001) and presence of cirrhosis (HR 1.773, 95% CI 1.226–2.564, P:.002) were confirmed in the Cox model. Moreover, patients with postoperative worsening of PALBI grade exhibited increased incidence of recurrence and worse long-term survival (Table 3).
Table 3.
Univariate and multivariate analyses of prognostic factors for OS and RFS in patients with hepatocellular carcinoma following liver resection.
| OS (month) | RFS (month) | |||||||
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||
| Variable | Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value |
| Age | 0.886 (0.655–1.200) | .435 | 0.829 (0.641–1.072) | .153 | ||||
| Gender | 1.506 (0.886–2.561) | .131 | 0.956 (0.647–1.411) | .819 | ||||
| Number | 0.584 (0.655–2.120) | .584 | 1.413 (0.837–2.386) | .195 | ||||
| Differentiation | 0.744 (0.540–1.024) | .069 | 0.764 (0.553–1.056) | .103 | 1.034 (0.782–1.367) | .817 | ||
| Maximum diameter of tumor | 1.982 (1.353–2.902) | <.001 | 1.708 (1.157–2.520) | .007 | 1.696 (1.242–2.316) | .001 | 1.460 (1.058–2.015) | .021 |
| Presence of MVI | 2.050 (1.486–2.829) | <.001 | 1.914 (1.375–2.663) | <.001 | 1.815 (1.374–2.398) | <.001 | 1.642 (1.235–2.184) | .001 |
| Presence of cirrhosis | 1.673 (1.163–2.407) | .006 | 1.773 (1.226–2.564) | .002 | 1.302 (0.944–1.796) | .107 | ||
| Hb | 0.801 (0.409–1.570) | .518 | 1.428 (0.705–2.892) | .323 | ||||
| WBC | 1.975 (0.487–8.011) | .341 | 0.810 (0.201–3.266) | .767 | ||||
| AFP | 1.214 (0.889–1.658) | .221 | 1.479 (1.138–1.922) | .003 | 1.364 (1.044–1.781) | .023 | ||
| HBV DNA | 0.975 (0.720–1.321) | .870 | 1.230 (0.950–1.593) | .116 | ||||
| Preoperative PALBI grade | ||||||||
| 1 | Reference | .478 | Reference | .436 | ||||
| 2 | 0.784 (0.527–1.166) | .229 | 0.805 (0.579–1.120) | .198 | ||||
| 3 | 1.035 (0.423–2.529) | .941 | 0.936 (0.415–2.113) | .874 | ||||
| Postoperative PALBI grade | ||||||||
| 1 | Reference | .133 | Reference | .016 | Reference | .137 | ||
| 2 | 1.366 (0.982–1.900) | .064 | 1.491 (1.129–1.969) | .005 | 1.405 (0.905–2.180) | .13 | ||
| 3 | 1.359 (0.829–2.227) | .223 | 1.009 (0.636–1.600) | .969 | 0.978 (0.523–1.829) | .944 | ||
| Change of PALBI grade | 1.309 (0.953–1.798) | .097 | 1.403 (1.020–1.930) | 0.038 | 1.316 (1.002–1.729) | .049 | 1.328 (1.010–1.746) | .042 |
AFP = alpha-fetoprotein, CI = confidence interval, Hb = hemoglobin, MVI = microvascular invasion, OS = overall survival, PALBI = platelet-albumin-bilirubin, RFS = recurrence-free survival, WBC = white blood cell.
3.3. Prognostic model construction and validation
Independent prognostic factors for OS including tumor size, presence of MVI, presence of cirrhosis and postoperative change of PALBI, were used to construct the nomogram model to predict the personalized probability of survival of HCC patients after hepatectomy (Fig. 2). The C-index was 0.652 (95%CI: 0.566–0.738) in the training group and 0.737 (95% CI: 0.549–0.925) in the validation group. Calibration plots based on the 5 predictors were performed for the training group in Figures 3A and B. There was good agreement between actual- and nomogram-predicted probabilities after resection for 3-year and 5-year HCC-related death. Similarly, in the validation group, the calibration curves showed great consistency of 3- and 5-year survival probabilities between predicted results and actual observations (Fig. 3C-D).
Figure 2.
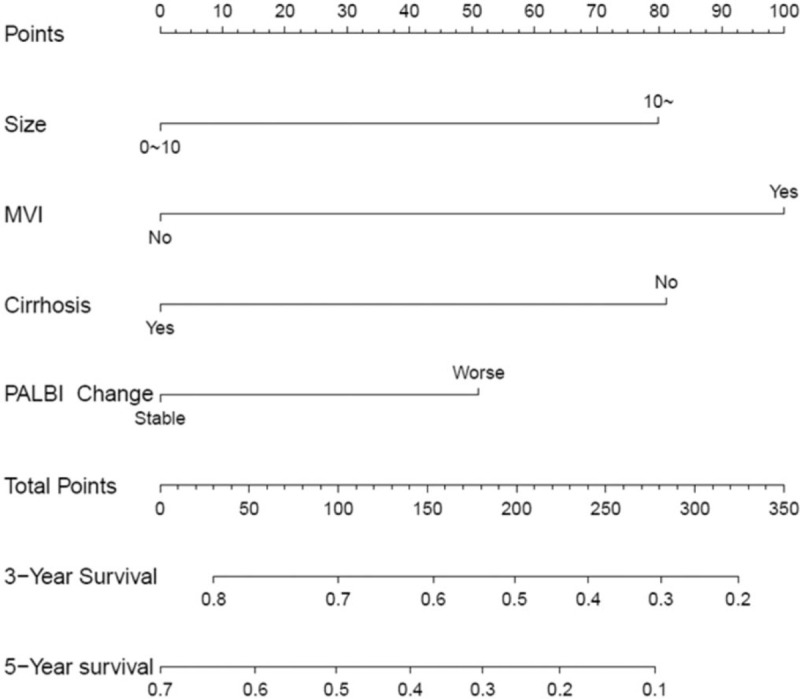
Developed nomogram. The nomogram was developed according to the training cohort, with the tumor size, presence of MVI, presence of cirrhosis, and change of postoperative PALBI grade incorporated.
Figure 3.
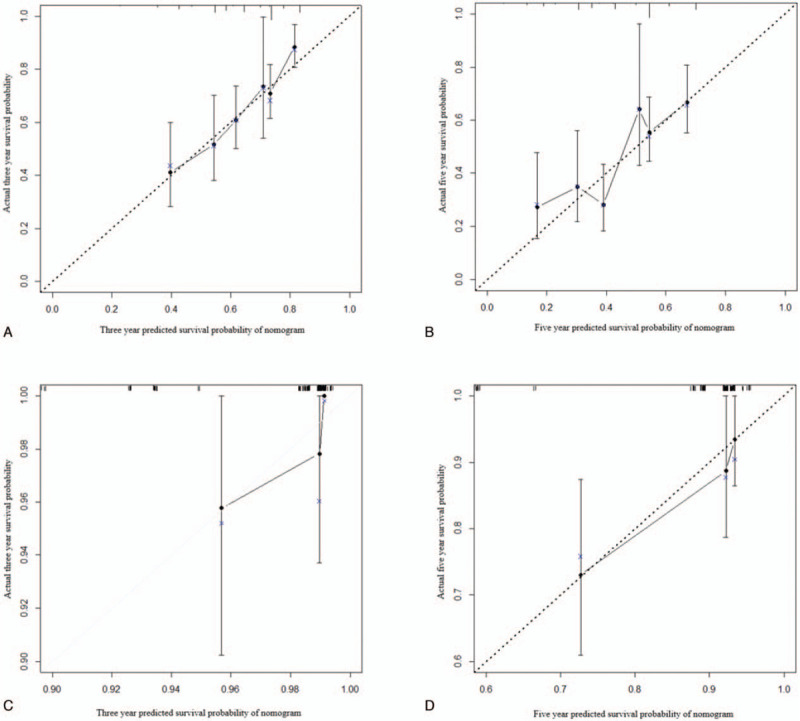
Calibration curves of the nomogram predicting 3-year overall survival and 5-year overall survival for both training and validation cohorts. (A) Calibration curve of nomogram predicting 3-year OS for the training cohort. (B) Calibration curve of nomogram predicting 5-year OS for the training cohort. (C) Calibration curve of nomogram predicting 3-year OS for the validation cohort. (D) Calibration curve of nomogram predicting 5-year OS for the validation cohort. Calibration curves describe the calibration of developed nomogram in terms of the agreement between the predicted 3-year and 5-year OS and observed 3-year and 5-year OS. The y-axis represents the actual OS. The x-axis represents the predicted OS. The line derived from the function y=x represents a perfect prediction by an ideal model. The dotted line represents the prediction performed by this nomogram, which indicate a better prediction when it fits closer to the diagonal line.
4. Discussion
With respect to clinical factors, liver function is a significant factor associated with prognosis of HCC patients after hepatectomy.[8,15,16] The Child–Pugh grade was widely used to evaluated the liver function. However, there were 2 subjective indicators, namely ascites and hepatic encephalopathy, which might lead to an inaccuracy for liver function assessment. Meanwhile, the C-P score were rarely identified as risk factor for HCC patients after hepatectomy. The role of liver function in prognosis remained to be further clarified. Therefore, based on the ALBI grade, the PALBI grades were recently proposed. PALBI grade was a simple, objective and discriminatory method for prognostic predictor in Child–Pugh grade A HCC patients following hepatectomy.[13,17] The majority of previous studies mainly paid attention to the role preoperative PALBI grade in HCC, however, few studies focused on change of PALBI grade between preoperative and postoperative phase. In this study, we mainly focused on and addressed this issue. As the result from our study, liver function (assessed by PALBI Grade) worsened in approximately 24.5% of patients after hepatectomy. As the result of survival analysis, we found out that the change of PALBI grade was an independently prognostic factor in patients with HCC following hepatectomy. Patients with worse PALBI after surgery had a poor prognosis.
Assessment of liver function was crucially important because it might be a competing cause of death of HCC patients. In clinical practice, the majority of HCC patients undergoing hepatectomy had Child-Pugh A liver function. Actually, C-P score was difficult to further discriminate liver function. In 2015, Philip J. Johnson proposed the Albumin-Bilirubin (ALBI) grade could simply, objectively, and effectively assess liver function in HCC.[7] In ALBI grade system, albumin and bilirubin were particularly key factors for assessment of chronic liver cirrhosis and could represent the impact of the underlying liver function on survival.[18] In addition, portal hypertension (PH) for HCC patients with underlying liver disease also related with liver function and was closely related with prognosis of HCC patients. The simply index platelet (PLT) count could reflect the status of PH and extensive experimental evidence suggested that PLT supported tumor metastasis.[19] In HCC patients, PLT changes are more complex than those in other malignancies and are modulated by various factors, including tumor related thrombocytosis and PH.[20] In current study, the PLT was significant higher in stable PALBI group. Therefore, it was necessary to take PLT into account for prediction of prognosis. Recent study showed that incorporating PLT into ALBI, namely PALBI, appeared to increase the predictive power.[17] Some other study also suggested PALBI containing PLT could better define the distinguished prognosis of patients with HCC.[21] This complex index could simply reflect the liver function and PH. In the current study, there were 377 (77.1%) patients with PALBI I, 103 (21.1) with PALBI II and 9 (1.8) with PALBI III. After hepatectomy, 100 (24.5%) had deteriorated PALBI grade. As we all known, liver resection could possibly lead to insufficient liver function of the remnant liver after surgery. The change of PALBI might better represent the ability of liver function recovery. Currently, our study provided the evidence that the stable PALBI reflecting well preserved liver function had better prognosis. Interestingly, as the result of multivariate analysis, the dynamic PALIB of HCC patients after hepatectomy was the independent prognostic factor (HR: 1.403, P = .038), rather than the preoperative PALBI or postoperative PALBI. It seemed that the dynamic PALIB was more significant to assess the impact of underly liver function on the prognosis of HCC patients compared with preoperative or postoperative PALBI. High PALBI could result in stable PALBI. As the definition of PALBI, high albumin and PLT count would lead to high PALBI grade. Recent study show that high albumin could suppresses tumor cell proliferation and invasion through its effects on growth-controlling kinases.[22] From the perspective of clinical practice, we also identified that high preoperative serum albumin level is associated with better long-term survival in ICC patients.[23] As to PLT, high PLT commonly indicated less rate of PH and was associated with better outcomes.[24] All these evidences suggested stable PALBI was related with low incidence of HCC recurrence and long-term survival of patients with HCC.
In this study, 489 patients with HCC were included, and all patients were randomly classed into training group and validation group. In the training group, postoperative worse PALBI grade was associated with HCC recurrence and long-term survival. Besides, tumor size, the presence of MVI and liver cirrhosis were related with the prognosis. These commonly risk factors were identified as previous study reported.[25] As shown Table 1, patients with worsen PALBI had larger tumor size and poorly tumor differentiation. This also could explain why the worsen PALBI patients had worse prognosis. It in turn suggested the aggressive tumor characteristics could negatively affect the change of PALBI, indicating the interaction between liver function recovery and tumor characteristics. Meanwhile, the PLT count was lower in the worse PALBI. Previous study revealed thrombocytopenia, as an indirect indicator for portal hypertension, is significantly associated with poor outcomes after hepatectomy.[24] Another phenomenon was that the worsen PALBI was more likely to occur in elderly patients (60.8% in patients with age >60 years old). A report of a Japanese nationwide survey, the cumulative incidence of other causes of death in elderly patients was significantly high in HCC-related death.[26] These might be partial reason for the poor prognosis of HCC patients with worsen PALBI.
Based on the change of PALBI grade, we constructed a prognostic model. The c-index of this model reached up to 0.737. Moreover, in the training and validation group, the result showed great consistency of 3- and 5-year survival probabilities between predicted and actual observations. This finding indicated that the model consisting of the change of PALBI grade could accurately and objectively predict the prognosis of patients with HCC after liver resection. As we can see, this model represented 2 aspects, namely tumor characteristic and liver function. Importantly, the liver function index was evaluated dynamically. It was reasonable that the model had good performance. In detail, the deterioration of liver function (worsen PALBI) may be due to perioperative factors (the extent of surgery and liver cirrhosis)[27] and postoperative factors (postoperative complications, malnutrition and transient hyperbilirubinemia).[28,29] All these negative impacts could affect the change of PALBI and liver function recovery, thus influencing the prognosis of HCC patients. These results also told us that postoperative management, such as infection prevention and nutrition support, was crucial and it could bring survival benefits for HCC patients with underly liver disease.[30] On the other hand, as previous studies reported, the clinicopathological factors, such as large tumor size, MVI, cirrhosis and high AFP level were associated with a high incidence of recurrence or worse long-term survival.[31–33] Therefore, the model including these clinicopathological factors could comprehensively represent the status of HCC patients and better predict the prognosis.
There are some limitations of this study. Firstly, this study was a single-center and retrospective study. External and prospective validation of the PALBI-based model need to should be performed. Secondly, we only included patients with HBV-related HCC within the Milan criteria. Whether this could be applied to HCC caused by other etiology should be further validated.
5. Conclusion
The current study identified that the postoperative change of PALBI grade could predict the prognosis of HCC patients after hepatectomy. Incorporation the change of PALBI grade into prognostic model might perform well to predict the individual prognosis of HCC patients after hepatectomy.
Author contributions
Conceptualization: Zheng-Xia Wang, Tianfu wen.
Formal analysis: Wei Peng.
Investigation: Wei Peng.
Methodology: Wei Peng.
Project administration: Chuan Li.
Supervision: Tianfu wen, Chuan Li.
Validation: Xiao-Yun Zhang.
Visualization: Xiao-Yun Zhang.
Writing – original draft: Zheng-Xia Wang.
Writing – review & editing: Xiao-Yun Zhang, Tianfu wen, Chuan Li.
Footnotes
Abbreviations: AFP = alpha-fetoprotein, ALB = albumin, CI = confidence interval, HBV = hepatitis B virus, HCC = hepatocellular carcinoma, HR = hazard ratio, IQR = interquartile range, MVI = microvascular invasion, OS = overall survival, PALBI = platelet-albumin-bilirubin, PLT = platelet count, RFS = recurrence free survival.
How to cite this article: Wang ZX, Peng W, Zhang XY, Wen TF, Li C. Prognostic significance of postoperative change of PALBI grade for patients with hepatocellular carcinoma after hepatectomy. Medicine. 2021;100:11(e24476).
This study was supported by grants from the State Key Scientific and Technological Research Programs (2017ZX10203207-003-0020), the National Natural Science Foundation of China:81900463 and 81900576, the Science and Technological Supports Project of Sichuan Province (2018SZ0204, 2018JY0544 and 2019YJ0149), the Science and Technology Project of Chengdu (2018-YF05-01460- SN).
The authors have no conflicts of interests to disclose.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
References
- [1].Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- [2].Wang FS, Fan JG, Zhang Z, et al. The global burden of liver disease: the major impact of China. Hepatology 2014;60:2099–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cheng YC, Chen TW, Fan HL, et al. Transarterial chemoembolization for intrahepatic multiple recurrent HCC after liver resection or transplantation. Ann Transplant 2014;19:309–16. [DOI] [PubMed] [Google Scholar]
- [4].Poon RT, Fan ST, Lo CM, et al. Long-term survival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function: implications for a strategy of salvage transplantation. Ann Surg 2002;235:373–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Child CG, Turcotte JG. Surgery and portal hypertension. Major Probl Clin Surg 1964;1:1–85. [PubMed] [Google Scholar]
- [6].Durand F, Valla D. Assessment of the prognosis of cirrhosis: child-pugh versus MELD. J Hepatol 2005;42:S100–107. [DOI] [PubMed] [Google Scholar]
- [7].Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol 2015;33:550–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Harimoto N, Yoshizumi T, Sakata K, et al. The prognostic significance of ALBI(Albumin-bilirubin)-T score in patient who underwent hepatic resection for hepatocellular carcinoma. Hepatol Res 2017;47:1289–98. [DOI] [PubMed] [Google Scholar]
- [9].Chan AW, Kumada T, Toyoda H, et al. Integration of albumin-bilirubin (ALBI) score into Barcelona clinic liver cancer (BCLC) system for hepatocellular carcinoma. J Gastroenterol Hepatol 2016;doi: 10.1111/jgh.1329. [DOI] [PubMed] [Google Scholar]
- [10].Chan AWH, Leung HHW, Chong CCN, et al. Validating the ALBI grade: it is current and future use in HCC prognostication. J Hepatol 2017;66:661–3. [DOI] [PubMed] [Google Scholar]
- [11].Takemura N, Aoki T, Hasegawa K, et al. Hepatectomy for hepatocellular carcinoma after perioperative management of portal hypertension. Br J Surg 2019;106:1066–74. [DOI] [PubMed] [Google Scholar]
- [12].Sonohara F, Yamada S, Tanaka N, et al. Comparison of non-invasive liver reserve and fibrosis models: implications for surgery and prognosis for hepatocellular carcinoma. Hepatol Res 2019;49:1305–15. [DOI] [PubMed] [Google Scholar]
- [13].Liu PH, Hsu CY, Hsia CY, et al. ALBI and PALBI grade predict survival for HCC across treatment modalities and BCLC stages in the MELD Era. J Gastroenterol Hepatol 2017;32:879–86. [DOI] [PubMed] [Google Scholar]
- [14].Wu B, Hu X, Jin H, et al. Albumin-bilirubin and platelet-albumin-bilirubin grades for hepatitis B-associated hepatocellular carcinoma in Child-Pugh A patients treated with radical surgery: a retrospective observational study. Medicine (Baltimore) 2019;98:e17394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Shah SA, Cleary SP, Wei AC, et al. Recurrence after liver resection for hepatocellular carcinoma: risk factors, treatment, and outcomes. Surgery 2007;141:330–9. [DOI] [PubMed] [Google Scholar]
- [16].Imamura H, Matsuyama Y, Tanaka E, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol 2003;38:200–7. [DOI] [PubMed] [Google Scholar]
- [17].Lu LH, Zhang YF, Mu-Yan C, et al. Platelet-albumin-bilirubin grade: risk stratification of liver failure, prognosis after resection for hepatocellular carcinoma. Dig Liver Dis 2019;51:1430–7. [DOI] [PubMed] [Google Scholar]
- [18].Fox R, Berhane S, Teng M, et al. Biomarker-based prognosis in hepatocellular carcinoma: validation and extension of the BALAD model. Br J Cancer 2014;110:2090–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer 2011;11:123–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chauhan A, Adams DH, Watson SP, et al. Platelets: no longer bystanders in liver disease. Hepatology 2016;64:1774–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wang B, Zhu J, Ma X, et al. Platelet activation status in the diagnosis and postoperative prognosis of hepatocellular carcinoma. Clin Chim Acta 2019;495:191–7. [DOI] [PubMed] [Google Scholar]
- [22].Bağirsakçi E, Şahin E, Atabey N, et al. Role of albumin in growth inhibition in hepatocellular carcinoma. Oncology 2017;93:136–42. [DOI] [PubMed] [Google Scholar]
- [23].Shen J, Wen T, Li C, et al. The prognostic prediction role of preoperative serum albumin level in patients with intahepatic cholangiocarcinoma following hepatectomy. Dig Dis 2018;36:306–13. [DOI] [PubMed] [Google Scholar]
- [24].Venkat R, Hannallah JR, Krouse RS, et al. Preoperative thrombocytopenia and outcomes of hepatectomy for hepatocellular carcinoma. J Surg Res 2016;201:498–505. [DOI] [PubMed] [Google Scholar]
- [25].Liu PH, Hsu CY, Hsia CY, et al. Prognosis of Hepatocellular Carcinoma: Assessment of Eleven Staging Systems. J Hepatol 2016;64:601–8. [DOI] [PubMed] [Google Scholar]
- [26].Kaibori M, Yoshii K, Yokota I, et al. Impact of advanced age on survival in patients undergoing resection of hepatocellular carcinoma: report of a Japanese nationwide survey. Ann Surg 2019;269:692–9. [DOI] [PubMed] [Google Scholar]
- [27].Asencio JM, García Sabrido JL, Olmedilla L. How to expand the safe limits in hepatic resections? J Hepatobiliary Pancreat Sci 2014;21:399–404. [DOI] [PubMed] [Google Scholar]
- [28].Wrighton LJ, O’Bosky KR, Namm JP, et al. Postoperative management after hepatic resection. J Gastrointest Oncol 2012;3:41–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hallet J, Karanicolas PJ, Zih FS, et al. Hypophosphatemia and recovery of post-hepatectomy liver insufficiency. Hepatobiliary Surg Nutr 2016;5:217–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Li C, Zhang XY, Peng W, et al. Postoperative albumin-bilirubin grade change predicts the prognosis of patients with hepatitis b-related hepatocellular carcinoma within the milan criteria. World J Surg 2018;42:1841–7. [DOI] [PubMed] [Google Scholar]
- [31].Shen J, Liu J, Li C, et al. The prognostic significance of serum HBeAg on the recurrence and long-term survival after hepatectomy for hepatocellular carcinoma: a propensity score matching analysis. J Viral Hepat 2018;25:1057–65. [DOI] [PubMed] [Google Scholar]
- [32].Nault JC, Nkontchou G, Nahon P, et al. Percutaneous treatment of localized infiltrative hepatocellular carcinoma developing on cirrhosis. Ann Surg Oncol 2016;23:1906–15. [DOI] [PubMed] [Google Scholar]
- [33].Farinati F, Vitale A, Spolverato G, et al. Development and validation of a new prognostic system for patients with hepatocellular carcinoma. PLoS Med 2016;13:e1002006. [DOI] [PMC free article] [PubMed] [Google Scholar]


