Abstract
The aim of the study was to study sonoelastographic features of thesartorius muscle, and its relation to the demographic factors.
The study included 70 muscles in 35 healthy subjects. High-resolution ultrasound and shearwave elastography were used to evaluate the sartorius muscle. Stiffness values were measured.
The mean shear elastic modulus of the sartorius muscle was 21.96 ± 5.1 kPa. Demographic factors showed no relation to the elastic modulus of the left sartorius muscle. Positive statistical correlation was noted between the elastic modulus of the right sartorius muscle, weight, and body mass index.
Our results could be a reference point for evaluating sartorius muscle stiffness in future research considering different pathologies.
Keywords: elastography, muscle, Sartorius, shear wave, ultrasound
1. Introduction
Radiology plays an important role in the diagnosis of different muscular diseases. Diagnostic imaging allows evaluation of the morphologic, qualitative, and metabolic status of skeletal muscles by revealing fatty atrophy, edema, and focal lesions. Despite these contributions, conventional radiologic techniques remain unable to assess biomechanical properties of muscles related to contractile properties, an important feature of skeletal muscles.[1] Conventional ultrasound has gained popularity as a complimentary tool for the examination of the musculoskeletal system. In addition to shorter acquisition time and less discomfort, advantages of ultrasound include wide availability, cheap price, and noninvasive nature. An important limitation of conventional ultrasound is its inability to show the biomechanical properties of tissues and to assess the relationship between different clinical symptoms and structural disorganization. Elastography is a relatively new technique introduced in the early 1990s to provide information about tissue stiffness. In addition to morphologic data, elastography can obtain data about the biomechanical properties of the musculoskeletal system. There are 2 main types of elastography. The first is strain elastography, where tissue displacement is evaluated by probe compression to estimate tissue stiffness, resulting in color scaled qualitative and semiquantitative evaluation of elasticity. The other type is shear wave elastography (SWE), where the probe induces a pulse, which then propagates through the tissue of interest in a shear manner and presented in kilopascals (kPa, Young modulus).[2–9] Advantages of SWE include the ability to display quantitative results, user-friendliness, and reproducibility. SWE is now widely used in evaluating hepatic fibrosis, thyroid gland, and breast masses, in addition to muscles, tendons, and ligaments.[2] SWE can also evaluate the mechanical properties of muscles in different age groups. Stiffness of muscles tends to increase with age. This could influence health and activity of the adult population.[3] Since muscles are anisotropic, probe orientation relative to muscle fibers would be an important factor to determine shear wave velocity.[10] Measurement of muscle elasticity at rest provides important physiologic baseline, although measuring the stiffness of a contracted muscle is more likely to differentiate between normal and pathologic muscle.[11–15]
The sartorius muscle is the longest muscle in the body and is the most superficial muscle in the anterior compartment of the thigh. The word sartorius is derived from the Latin word sartor which means tailor. It travels obliquely in an inferomedial direction from its origin at the anterior superior iliac spine to its insertion at the pes anserine at the superior medial side of the proximal tibia near the medial tubercle. It spans both the knee and hip joints, and acts synergistically with muscles of the thigh, hip, and knee. Its main function is flexion of the knee and hip joints. In addition, it assists in the lateral rotation and abduction of the hip joint. More than half of the arterial supply of the sartorius muscle comes from the muscular branches of the femoral artery, but collateral flow could come from other arteries. The nerve supply comes from the femoral nerve.[11–18] Chronic overuse of the sartorius muscle could cause inflammation at the insertion of the conjoint tendon. This inflammation could cause local irritation of the surrounding tissues leading to pes anserine bursitis. Another clinically significant condition is avulsion injury of the origin at the anterior superior iliac spine. These injuries usually occur in young athletes secondary to sudden indirect trauma with forceful contraction of the sartorius and tensor fascialata.[13] The Sartorius muscle can be used as a donor flap to cover complicated wounds in reconstruction surgeries and is considered ideal considering its length and high vascularity.[14] Knowledge of the stiffness of the muscle before this kind of surgery is crucial for the selection of the proper candidate. Numerous studies were conducted using SWE for different muscles in healthy subjects, and some muscle pathologies. These studies revealed informative but heterogenous results.[6,7,19] The aim of this work is to study the sonoelastographic features of the sartorius muscle.
2. Methods
2.1. Participants
Seventy muscles were evaluated in 35 healthy adult subjects. After institutional review board approval, participants of the study were recruited between September 2019 and October 2019, and written consent was obtained. Inclusion criteria included healthy subjects, male or female, asymptomatic with normal physical activity (age range 20–46). Exclusion criteria were: history of history of anabolic steroid use, lower limb pain, weakness, lower limb surgery, and lower limb muscle injury, history of limb overuse. For each participant, data including sex, age, weight, body mass index (BMI), and height were recorded.
2.1.1. Technique
Ultrasound examinations were performed by using an 18 to 5 MHz linear-array transducer (Aixplorer; Mach 30, AixenProvence, France). A radiologist (M.B, 19 years of experience) performed all examinations, images were reviewed by neurologist (AAE 10 years of experience). Participants were examined at rest with no preceding exercise. Ultrasound was performed in the supine position, with semi-flexed knee, and slight external rotation of the hip. The ultrasound probe was positioned in the middle third of the thigh where the femoral artery was identified at its medial part. The sartorius muscle was recognized as an elliptical structure in the short axis. Each subject was scanned 3 times with the removal of the probe from the skin between measurements. Large amount of gel was used with light touch of the probe to decrease pressure effect on the skin. In each examination, and after identifying the muscle, the probe was held stationary for 3 to 4 seconds and a 2 mm diameter region of interest circle was placed within the muscle where stiffness measurements were taken in the resting position (short axis). Quantitative SWE measurements show display of the mean elasticity (Mean), minimum elasticity (Min), and maximum elasticity (Max) with standard deviation (SD) and values were reported in kilopascals, kPa. The spectrum of scale colors range from blue for softer tissues, to red for stiffer tissues (Fig. 1).
Figure 1.
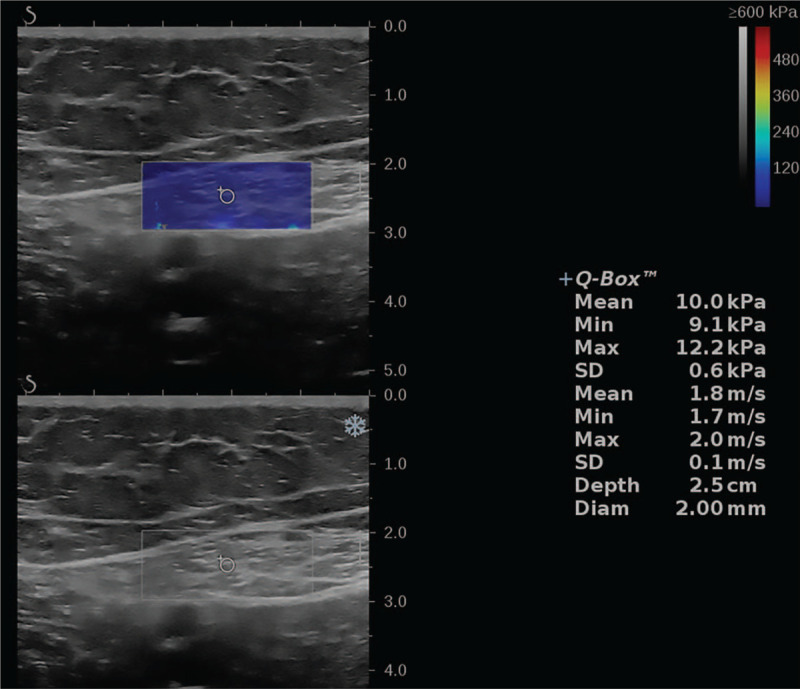
Short-axis view shearwave elastography of the sartorius muscle, with minimum, maximum, and mean stiffness in kPa.
2.2. Statistical analysis
Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS) version 21 software (SPSS Inc, Chicago, IL). Data were presented as mean ± standard deviation and range. Intra-observer variability was measured using Kohen Kappatest. Independent sample test was used to assess the differences between mean elasticity of the right and left tibial nerves. The correlations between the mean elasticity bilaterally and age, weight, height, and BMI were calculated by Pearson correlation coefficient test.
3. Results
The study included 70 muscle in 35 healthy adult subjects, with a mean age of 33.09 ± 6.59 [range 20–46], mean height 160.49 ± 10.10 [range 149–193], mean weight 64.09 ± 14.81[range 45–125], mean BMI 25.09 ± 3.60 [range 18.50–33.60]. The mean shear elastic modulus of the sartorius muscle was 21.96 ± 5.1 kPa [range 9–38.3 kPa]. Table 1 shows the demographic characteristics of study participants. Table 2 shows correlation between demographic factors and elasticity of the sartorius muscle. The intra-observer reliability calculations resulted in an overall intraclass correlation coefficient of 0.78. No statistical differences were noted between the right and left sides (P = .072). No significant statistical difference between both sexes could be found at our study. Age, height, weight, and BMI showed no correlation with elastic modulus of the left sartorius muscle. Positive statistical correlation was noted between the elastic modulus of the right sartorius muscle, weight, and BMI, P = (.05).
Table 1.
The demographic characteristics of study participants (mean ± standard deviation).
| (n = 35) | Range | |
| Age (yr) | 33.09 ± 6.59 | 20–46 |
| Weight (kg) | 64.09 ± 14.81 | 45–125 |
| Height (cm) | 160.49 ± 10.10 | 149–193 |
| BMI | 25.09 ± 3.60 | 18.50–33.60 |
BMI = body mass index.
Table 2.
Correlation between demographic factors and elasticity of the sartorius muscle.
| RT SART | LT SART | |
| Age | 0.204 | 0.275 |
| Sig | 0.240 | 0.109 |
| Weight/kg | −0.016 | 0.355∗ |
| Sig | 0.925 | 0.037 |
| Height/cm | 0.186 | 0.294 |
| Sig | 0.284 | 0.087 |
| BMI | −0.113 | 0.399∗ |
| Sig | 0.519 | 0.018 |
BMI = body mass index, LT = left, RT = right, SART = sartorius muscle, Sig = significance.
Correlation is significant at the 0.05 level.
4. Discussion
We studied the sartorius muscle in healthy adult subjects by SWE. The relationship between elasticity and height, weight, body mass index, gender was also studied. Conventional ultrasound is widely used in the diagnosis of musculoskeletal disorders, and it can clearly depict the echotexture. In addition to morphologic data, SWE can now obtain data about the biomechanical properties of the musculoskeletal system. Also SWE can assess the absolute elasticity values of muscles and obtain important information about the mechanical properties related to injury, degeneration, and healing.[6,20] Muscle injuries could occur via various mechanisms, like contusions, lacerations. They could also occur via indirect mechanisms like neurologic dysfunction and ischemia. This relationship between muscle function and mechanical properties might help clinicians to understand the pathophysiology and background of many muscular disorders. Effective evaluation of skeletal muscles could lead to improvement in management of these disorders, understanding the healing process, and speeding up methodology development important for recovery of the muscle function. Understanding practice guidelines is important to enhance reproducibility. In the short axis, mild probe angulation can cause considerable changes in elasticity measurements, and every attempt was made to be steady during image acquisition.[19–22] The mean stiffness of the sartorius muscle in our study was 21.96 ± 5.1 kPa [range 9–38.3 kPa]. Side-to-side analysis revealed no significant difference of stiffness, and showed no difference between both sexes matching with our results. Age, height, weight, and BMI showed no correlation with elastic modulus of the left sartorius muscle in accordance with previous studies.[23] Positive statistical correlation was noted between the elastic modulus of the right sartorius muscle, weight, and BMI, P = (.05). This could be attributed to the dominance of the right leg muscles in several sports. Only 1 study (Dubois et al) considered the sartorius muscle by SWE in the literature, he reported a mean elasticity at rest of 5.3 ± 1.1 kPa. This value is low compared with our results. This could be attributed to different techniques, like probe orientation, and axis of examination.[24] Several studies considered SWE of the lower limb muscles. Akagi and Takahashi reported mean elasticity of the gastrocnemius at rest (medial head 27.0 ± 59.0 kPa, lateral head 32.0 ± 6.3 kPa), this is comparable to our results, especially considering that measurements were taken in short axis like our study.[24,25] Sato et al also reported comparable results to our study, reporting a mean elasticity of the medial head of the gastrocnemius at rest to be 19.5 ± 14 kPa.[26] Arda et al[27] studied the mean elasticity for the gastrocnemius muscle at rest 11.4 ± 4.1 kPa [range 2–28 kPa] which is lower than our results. Shinohara et al[28] showed a slightly higher mean elasticity for the gastrocnemius muscle at rest to be 16.5 kPa, for the soleus muscle [14.5 kPa], and for the tibialis anterior muscle [40.6 kPa]. Koo et al[29] reported a mean elasticity for the tibialis anterior muscle in the resting state as low as 7 kPa. Statistically significant difference is observed between different skeletal muscles. Elasticity values in the resting condition in vivo range between 3.1 and 42.8 kPa.[30] This diversity in shear modulus measurements between studies could be attributed to several factors. First, the anisotropic physical properties of skeletal muscles. These properties are maintained as a result of parallel arrangement of collagen, myofibrils, muscular fibers, elastic fibers, fascicles. This could lead to the fact that shear waves travel faster along the direction of fibers than they do when perpendicular to them, meaning that stiffness measurements are sensitive to the angle between the transducer axis and the orientation of muscle fibers. Consequently, measuring stiffness is more difficult in muscles with complex multiorientation fibers. Second, the fact that a muscle is deformable, and is sensitive to transducer pressure, mandates the use of large amount of coupling gel so that the transducer does not compress the underlying muscle. Third, different measurement techniques like limb positions, and examination during rest or contraction, could result in significant variability of elasticity measurements.[22] A contracted muscle is stiffer than a “relaxed” or “resting” muscle.[31] SWE could detect stiffness changes related to muscle stretching, contraction, and manipulation. SWE could also detect these changes. Muscle stiffness is linearly related to passive and active forces, induced by hyperemia, actomyosin bridges, and changes related to extracellular matrix during contraction. Inside each muscle, the number, type of fibers, the spatial arrangement of fascicles, amount of connective tissue, fat, isoforms of actin-myosin, and capillary supply, vary depending on the use and function of a particular muscle.[1] This study has several limitations. First, the sample size is small, which could decrease the validity of our measurements. Second, there is lack of comparison to pathological tissues. Third, we evaluated muscle stiffness during rest only. Fourth, we only measured the muscle elasticity on short axis. Future studies should be conducted with larger sample size, considering different pathologies, measuring elasticity in both axes, during rest, contraction, and with load.
5. Conclusion
The results obtained in our study could be a reference point for evaluating sartorius muscle stiffness in future research considering different pathologies.[32,33]
Acknowledgments
The authors are grateful to the deanship of scientific research at Prince Sattam bin Abdulaziz University.
Author contributions
Conceptualization: Mohamed Abdelmohsen Bedewi, Ayman A Elsifey, Tariq Alfaifi, Ayman K Saleh, Sherine M Swify, Kholoud J Sandougah.
Data curation: Mohamed Abdelmohsen Bedewi.
Formal analysis: Mohamed Abdelmohsen Bedewi.
Investigation: Mohamed Abdelmohsen Bedewi, Ayman A Elsifey, Tariq Alfaifi, Ayman K Saleh.
Methodology: Mohamed Abdelmohsen Bedewi, Ayman A Elsifey, Tariq Alfaifi.
Project administration: Mohamed Abdelmohsen Bedewi, Sherine M Swify, Kholoud J Sandougah.
Resources: Mohamed Abdelmohsen Bedewi.
Supervision: Mohamed Abdelmohsen Bedewi, Sherine M Swify.
Validation: Mohamed Abdelmohsen Bedewi.
Visualization: Mohamed Abdelmohsen Bedewi, Ayman A Elsifey.
Writing – original draft: Mohamed Abdelmohsen Bedewi.
Writing – review & editing: Mohamed Abdelmohsen Bedewi.
Footnotes
Abbreviations: BMI = body mass index, SWE = shearwave elastography.
How to cite this article: Bedewi MA, Elsifey AA, Alfaifi T, Saleh AK, Swify SM, Sandougah KJ. Shearwave elastography of the Sartorius muscle. Medicine. 2021;100:11(e25196).
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Creze M, Nordez A, Soubeyrand M, et al. Shear wave sonoelastography of skeletal muscle: basic principles, biomechanical concepts, clinical applications, and future perspectives. Skeletal Radiol 2018;47:457–71. [DOI] [PubMed] [Google Scholar]
- [2].Ryu JA, Jeong WK. Current status of musculoskeletal application of shear wave elastography. Ultrasonography 2017;36:185–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Phan A, Lee J, Gao J. Ultrasound shear wave elastography in assessment of skeletal muscle stiffness in senior volunteers. Clin Imaging 2019;58:22–6. [DOI] [PubMed] [Google Scholar]
- [4].Winn N, Lalam R, Cassar-Pullicino V. Sonoelastography in the musculoskeletal system: current role and future directions. World J Radiol 2016;8:868–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Davis LC, Baumer TG, Bey MJ, et al. Clinical utilization of shear wave elastography in the musculoskeletal system. Ultrasonography 2019;38:2–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Klauser AS, Miyamoto H, Bellmann-Weiler R, et al. Sonoelastography: musculoskeletal applications. Radiology 2014;272:622–33. [DOI] [PubMed] [Google Scholar]
- [7].Taljanovic MS, Gimber LH, Becker GW, et al. Shear-wave elastography: basic physics and musculoskeletal applications. Radiographics 2017;37:855–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Paluch Ł, Nawrocka-Laskus E, Wieczorek J, et al. Use of ultrasound elastography in the assessment of the musculoskeletal system. Pol J Radiol 2016;81:240–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hobson-Webb LD. Emerging technologies in neuromuscular ultrasound. Muscle Nerve 2020;61:719–25. [DOI] [PubMed] [Google Scholar]
- [10].Wang M, Byram B, Palmeri M, et al. Imaging transverse isotropic properties of muscle by monitoring acoustic radiationforce induced shear waves using a 2-D matrix ultrasound array. IEEE Trans Med Imaging 2013;32:1671–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gao Z, Wu S, Liu Z, et al. Learning the implicit strain reconstruction in ultrasound elastography using privileged information. Med Image Anal 2019;58:101534. [DOI] [PubMed] [Google Scholar]
- [12].Botanlioglu H, Kantarci F, Kaynak G, et al. Shear wave elastography properties of vastus lateralis and vastus medialis obliquus muscles in normal subjects and female patients with patellofemoral painsyndrome. Skeletal Radiol 2013;42:659–66. [DOI] [PubMed] [Google Scholar]
- [13].Walters BB, Varacallo M. Anatomy, Bony Pelvis and Lower Limb, Thigh Sartorius Muscle. In: StatPearls. Treasure Island (FL): 2020 Sep 17; PMID: 30422484. Review. [PubMed] [Google Scholar]
- [14].Manjunath KN, Venkatesh MS, Shivaprasad A. Distal major pedicle of sartorius muscle flap: anatomical study and its clinical implications. Indian J Plast Surg 2018;51:40–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Dziedzic D, Bogacka U, Ciszek B. Anatomy of sartorius muscle. Folia Morphol (Warsz) 2014;73:359–62. [DOI] [PubMed] [Google Scholar]
- [16].Malherbe K. A rare ultrasound case report: intramuscular tear of the sartorius muscle. Ultrasound J 2019;11:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wang J, Jiao J, Zhao G, et al. Case report: a rare case of focal myositis presenting as Sartorius muscle contracture: a case report and review of literature. Medicine (Baltimore) 2018;97:e10766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Eby S, Zhao H, Song P, et al. Quantitative evaluation of passive muscle stiffness in chronicstroke. Am J Phys Med Rehabil 2016;95:899–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Shimizu M, Ito Y. Change in shear elastic modulus of thigh muscle by changing muscle length using ultrasound shear wave elastography in beagle dogs. Vet Comp OrthopTraumatol 2019;32:454–9. [DOI] [PubMed] [Google Scholar]
- [20].Lv F, Tang J, Luo Y, et al. Muscle crush injury of extremity: quantitative elastographywith supersonic shear imaging. Ultrasound Med Biol 2012;38:795–802.20. [DOI] [PubMed] [Google Scholar]
- [21].Du LJ, He W, Cheng LG, et al. Ultrasound shearwave elastography in assessment of muscle stiffness in patientswith Parkinson's disease: a primary observation. Clin Imaging 2016;40:1075–80. [DOI] [PubMed] [Google Scholar]
- [22].Ichihashi N, Umegaki H, Ikezoe T, et al. The effects of a 4-week static stretching programmeon the individual muscles comprising the hamstrings. J Sports Sci 2016;34:2155–9. [DOI] [PubMed] [Google Scholar]
- [23].Dubois G, Kheireddine W, Vergari C, et al. Reliable protocol for shear wave elastography of lower limb muscles at rest andduring passive stretching. Ultrasound Med Biol 2015;41:2284–91. [DOI] [PubMed] [Google Scholar]
- [24].Akagi R, Takahashi H. Effect of a 5-week static stretching program on hardness of the gastrocnemius muscle. Scand J Med Sci Sports 2014;24:950–7. [DOI] [PubMed] [Google Scholar]
- [25].Akagi R, Takahashi H. Acute effect of static stretching on hardness of the gastrocnemius muscle. Med Sci Sports Exerc 2013;45:1348–54. [DOI] [PubMed] [Google Scholar]
- [26].Sato S, Kiyono R, Takahashi N, et al. The acute and prolonged effects of 20-s static stretching on muscle strength and shear elastic modulus. PLoS One 2020;15:e0228583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Arda K, Ciledag N, Aktas E, et al. Quantitative assessment of normal soft-tissue elasticity using shear-wave ultrasound elastography. AJR Am J Roentgenol 2011;197:532–6. [DOI] [PubMed] [Google Scholar]
- [28].Shinohara M, Sabra K, Gennisson JL, et al. Real-time visualization of muscle stiffness distribution with ultrasound shear wave imaging during muscle contraction. Muscle Nerve 2010;42:438–41. [DOI] [PubMed] [Google Scholar]
- [29].Koo TK, Guo JY, Cohen JH, et al. Quantifying the passive stretching response of human tibialis anterior muscle using shear wave elastography. Clin Biomech (Bristol, Avon) 2014;29:33–9. [DOI] [PubMed] [Google Scholar]
- [30].Nakamura M, Hasegawa S, Umegaki H, et al. The difference in passive tension applied to the muscles composing the hamstrings—comparison among muscles using ultrasound shear wave elastography. Man Ther 2016;24:1–6. [DOI] [PubMed] [Google Scholar]
- [31].Nordez A, Hug F. Muscle shear elastic modulus measured usingsupersonic shear imaging is highly related to muscle activity level. J Appl Physiol 2010;108:1389–94. [DOI] [PubMed] [Google Scholar]
- [32].Ahmed R, Ye J, Gerber SA, et al. Preclinical imaging using single track location shear wave elastography: monitoring the progression of murine pancreatic tumor liver metastasis in vivo. IEEE Trans Med Imaging 2020;39:2426–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Huard J, Li Y, Fu FH. Muscle injuries andrepair: current trends in research. J Bone Joint Surg Am 2002;84-A:822–32. [PubMed] [Google Scholar]


