Abstract
Background:
Non-vitamin K antagonist oral anticoagulants (NOACs) have been widely used for stroke prevention in atrial fibrillation (AF) and the treatment and prevention of venous thromboembolism. There is an issue with safety, especially in clinically relevant bleeding. We performed a network meta-analysis to evaluate the risk of major gastrointestinal (GI) bleeding associated with NOACs.
Methods:
Interventions were warfarin, enoxaparin, apixaban, dabigatran, edoxaban, and rivaroxaban. The primary outcome was the incidence of major GI bleeding. A subgroup analysis was performed according to the following indications: AF, deep venous thrombosis/pulmonary embolism, and postsurgical prophylaxis.
Results:
A total of 29 randomized controlled trials (RCTs) and 4 large observation population studies were included. Compared with warfarin, apixaban showed a decreased the risk of major GI bleeding (relative risk [RR] 0.54, 95% confidence interval [CI] 0.25–0.76), and rivaroxaban tended to increase this risk (RR 1.40, 95% CI 1.06–1.85). Dabigatran (RR 1.25, 95% CI 0.98–1.60), edoxaban (RR 1.07, 95% CI 0.69–1.65), and enoxaparin (RR 1.24, 95% CI 0.63–2.43) did not significantly increase the risk of GI bleeding than did warfarin. In the subgroup analysis, according to indications, apixaban showed a decreased risk of major GI bleeding (RR 0.50, 95% CI 0.34–0.74) than did warfarin in AF studies. Dabigatran (RR 2.36, 95% CI 1.55–3.60, and rivaroxaban (RR 1.75, 95% CI 1.10–6.41) increased the risk of major GI bleeding than did apixaban. An analysis of studies on venous thromboembolism or pulmonary embolism showed that no individual NOAC or enoxaparin was associated with an increased risk of major GI bleeding compared to warfarin.
Conclusion:
Individual NOACs had varying profiles of GI bleeding risk. Results of analyses including only RCTs and those including both RCTs and population studies showed similar trends, but also showed several differences.
Keywords: apixaban, direct factor Xa inhibitor, edoxaban, network meta-analysis, novel oral anticoagulants, rivaroxaban, warfarin
1. Introduction
Non-vitamin K antagonist oral anticoagulants (NOACs) that inhibit thrombin or activated factor X were developed and approved for stroke prevention in atrial fibrillation (AF) and for the treatment or prevention of venous thromboembolism.[1] Recently, NOACs are also safe to use in patients with cancer-associated VTE and also AF-associated HF.[2–6] These drugs, including apixaban, dabigatran, edoxaban, and rivaroxaban, were shown to be as effective as traditional anticoagulation therapy.[7–11] In addition, unlike the vitamin K antagonists (VKAs), NOACs have rapid onset and termination of action, fewer drug interactions, lack of dietary vitamin K intake interaction, and no need for drug monitoring, which led to rapid adoption in clinical practice worldwide.[12]
Although NOACs have been widely used due to efficacy and compliance, the issue of safety has arisen, especially with respect to clinical relevant bleeding.[13,14] For decades, gastrointestinal bleeding has been a serious medical condition that causes considerable morbidity and mortality (5%–15%).[15] In addition, GI bleeding in patients with anticoagulants has significant impacts,[16] including the requirement to alter or discontinue anticoagulant agents, activation of inflammation states, and paradoxical thromboembolic events. Furthermore, in contrast with the traditional VKA, no clinically tested antidote is currently available for NOACs, except for dabigatran. Various randomized controlled trials and large population studies have been carried out, and some studies reported an increased GI bleeding risk in patients with NOACs. Recently, population based observation studies reported that individual NOACs are associated with various risks of GI bleeding compared to warfarin. Traditional pair-wise meta-analysis can only answer questions about pairs of drugs; therefore, they cannot determine which among several drugs is the safest. A network meta-analysis is a useful statistical method for comparing the GI bleeding risk of multiple drugs.
Therefore, we evaluate the risk of major GI bleeding associated with NOACs using a network meta-analysis of randomized controlled trials (RCTs) and observation studies.
2. Methods
This study was exempted from institutional review board review because it did not involve human subjects.
2.1. Search strategy
This systematic review and network meta-analysis was conducted and reported based on the guidelines and recommendations for network meta-analysis. PubMed-Medline, EMBASE, Cochrane Library and Web of Science searches were performed on July 1, 2018 using key terms (“apixaban,” “rivaroxaban,” “dabigatran,” “edoxaban,” and “bleeding”). The detailed search strategies in each database are presented in Supplemental Table 1. All trials had to be randomized, double-blinded, and controlled to ensure a minimum high level of quality. We checked the reference lists of all potentially eligible studies and reviewed papers to find additional relevant publications.
2.2. Study selection
We considered all full-text RCTs and population studies that investigated patients treated with NOACs or conventional coagulation therapy (vitamin K antagonist and low molecular weighted heparin) for approved indications such as the prevention or treatment of venous thromboembolism (VTE) and the prevention of stroke or systemic embolism in patients with AF, reporting major GI bleeding, without limitation of study size. Among observational studies, we included studies of clear comparisons with propensity scoring matched controls or nested case controls to minimize bias.
The exclusion criteria included the following:
-
1.
non-English publications;
-
2.
abstract-only publications or unpublished studies inclusion of nonhuman subjects;
-
3.
failure to include major GI bleeding as a specified outcome; and
-
4.
trials assessing NOACs for unapproved indications.
Concerning population studies, we excluded single-arm observational studies without comparisons, including case series, case reports, and medical chart review studies. Studies were also excluded if there were insufficient data for determining the hazard risks, relative risks (RR) or odds ratios with 95% confidence intervals (CIs).
Two reviewers (HJO and BHY) independently evaluated the studies for eligibility and resolved any disagreements through discussion and consensus. If no agreement could be reached, a third reviewer (KHR) determined eligibility. The Cochrane risk of bias assessment tool was used for assessing the risk of bias individual studies.
2.3. Data extraction and outcome measure
Two reviewers (HJO and BHY) independently classified the data from included studies as indications. NOACs was classified as apixaban, rivaroxaban, dabigatran, and edoxaban. Conventional anticoagulation was divided by vitamin K antagonist, and low molecular weighted heparin or heparin. The primary outcome was major GI bleeding as defined by the International Society on Thrombosis and Hemostasis.[17] Other GI bleeding events, not referred or classified as major bleeding, were not included.
2.4. Subgroup and sensitivity analysis
The subgroup analysis was done according to the indication: AF, deep venous thrombosis/pulmonary embolism, and postsurgical prophylaxis. There are several limitations in studies based on large observational data such as nonstandardized follow-up and outcome evaluation. Therefore, we performed a sensitivity analysis by excluding 4 large cohort observational studies.
2.5. Statistical analysis
Direct meta-analysis was conducted to calculate pooled RRs with 95% CIs for each pairwise comparison across NOACs and conventional anticoagulant therapy. Taking a conservative approach, we used a random-effects model, which produces wider CIs than a fixed effect model. Statistical heterogeneity was assessed using I2 statistics, with values >50% suggestive of significant heterogeneity. P values <.05 were assumed to indicate statistical significance. The tests for funnel plot asymmetry were not conducted when there were fewer than 10 included studies for each pair-wise comparison.
In order to combine indirect and direct comparisons, we performed a network meta-analysis to determine comparative safety among the 6 treatments. This type of analysis allowed us to utilize results from 2 drugs compared to the same third drug for indirect comparisons. For example, 2 treatments (apixaban and dabigatran) had trial data compared to warfarin. The network meta-analysis application allows for the comparison between these 2 treatments using the evidence for each non-operative treatment and provides indirect evidence of the comparative effects between the treatment modalities. All analyses were performed using the “mvmeta” command of STATA (version 14.0; Stata Corporation, College Station, TX, USA).[18] Corresponding 95% credible intervals (CrIs) were obtained using the 2.5th and 97.5th percentiles of the posterior distribution.
2.6. Quality of evidence
Two investigators (HJO and BHY) independently performed quality assessments using the risk of bias assessment tool, which was described in the Cochrane Handbook for Systematic Reviews of Interventions.
3. Results
3.1. Included studies
A total 29 of RCTs and 4 of large observation population studies were included. The flowchart of study selection is shown in Figure 1. All included studies evaluated the risk of major GI bleeding associated with NOACs, including dabigatran, rivaroxaban, apixaban, and edoxaban, warfarin, and enoxaparin. Twenty nine RCTs included a total of 121,246 patients with indication of AF (n = 8),[7–9,11,19–22] venous thromboembolism (VTE) or pulmonary embolism (n = 11),[23–31] and postsurgical prophylaxis of VTE[32–42] (n = 11) (Fig. 2). Table 1 summarizes the characteristics of included studies. We analyzed an additional 4 population studies including 265,948 patients with AF.[43–46] All population observational studies were retrospective cohort studies with propensity matching. Regarding comparative efficacy for early postpolypectomy bleeding, there was no inconsistency between direct and indirect estimates in all 6 comparisons (Table 2).
Figure 1.
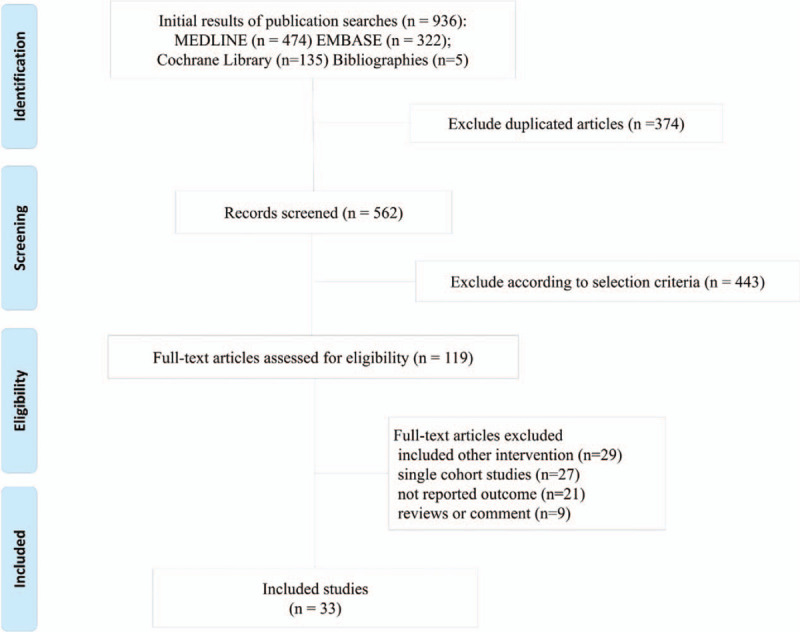
PRISMA Flow diagram details the process of relevant clinical study selection.
Figure 2.
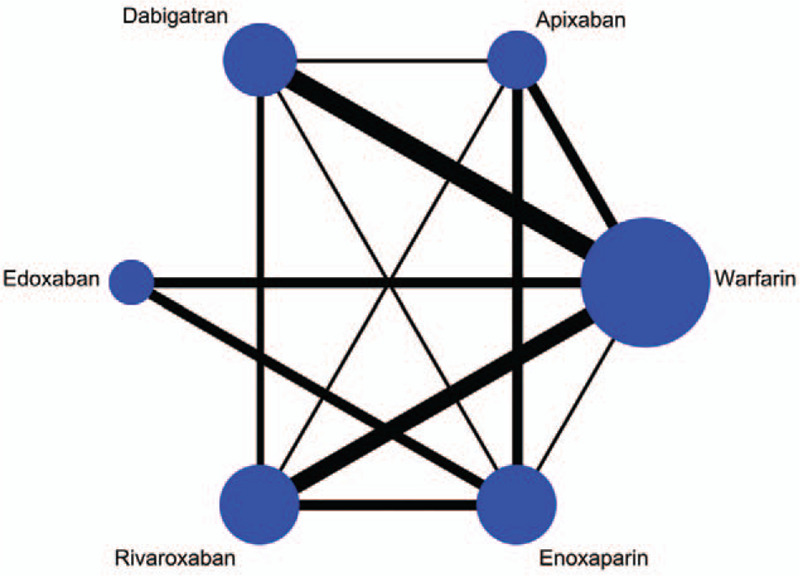
Network plot depicting the direct evidence used in the network meta-analysis. The widths of lines for each connection in the evidence network are proportional to the number of randomized controlled trials comparing each pair of treatments and the circle size is proportional to the number of patients.
Table 1.
Baseline characteristics and results of included trials.
| Study (patients) | Study design | Source of participant | Study Period | Intervention | Conventional treatment | Major GI bleeding, n/N NOAC vs n/N conventional treatment group |
| Atrial Fibrillation (11 studies) | ||||||
| Granger2011 | Double blind randomized | North America, South America, Europe, Asian Pacific | 2006–2010 | Apixaban (2.5 mg twice) | Warfarin | 150/9120 vs 119/9081 |
| Giugliano2013 | Double blind randomized | North America, South America, Europe, Asian Pacific | 2008–2010 | Edoxaban (60 mg, 30mg) | Warfarin | 361/14069 vs 190/7036 |
| Hori2013 | Double blind randomized | Asia | 2007–2010 | Rivaroxaban (15mg) | Warfarin | 8/639 vs 15/639 |
| Connolly2009 | Open-label randomized | North America, South America, Europe, Asian Pacific | 2005–2007 | Dabigatran (150 mg, 110mg) | Warfarin | 315/1291 vs 120/6022 |
| Patel 2011 | Double blind randomized | North America, South America, Europe, Asian Pacific | 2006–2009 | Rivaroxaban (20mg) | Warfarin | 224/7131 vs 154/7133 |
| Connolly2011 | Double blind randomized | North America, South America, Europe, Asian Pacific, South Africa | 2007–2009 | Apixaban (2.5 mg twice) | Aspirin (81–324mg) | 12/2808 vs 14/2791 |
| Chung 2011 | Double blind randomized | Asian countries (Taiwan, South Korea, Hong Kong and Singapore) | 2007–2008 | Edoxaban (60 mg, 30mg) | Warfarin | 0/159 vs 1/75 |
| Ogawa 2011 | Double blind randomized | Japan | Apixaban (2.5 mg twice,5 mg twice) | Warfarin | 0/75 vs 2/143 | |
| Abraham 2017 | Retrospective, propensity matched cohort study. | US, Optum Data Warehouse | 2010–2015 | Apixaban Dabigatran Rivaroxaban | None | 33/6542 222/15787 215/15787 |
| Abraham 2015 | Retrospective, propensity matched cohort study. | US, Optum Data Warehouse | 2010–2013 | Dabigatran Rivaroxaban | Warfarin | 18/7749 vs 22/7749 15/5166 vs 16/5166 |
| Graham 2016 | Retrospective, propensity matched cohort study. | US, fee-for-service Medicare | 2011–2014 | Dabigatran, 150 mg, twice daily; Rivaroxaban, 20 mg, once daily. | None | 362/52 240 656/66 651 |
| Yao 2016 | Retrospective, propensity matched cohort study. | US insurance database | 2010–2015 | Apixaban Dabigatran Rivaroxaban | Warfarin | 14/7695 vs 23/7695 28/14307 vs 28/14307 53/16175 vs 40/16175 |
| Venous thromboembolism or Pulmonary embolism (11 studies) | ||||||
| Bauersachs 2010 | Double blind randomized | 2007–2009 | Rivaroxaban (20mg) | Placebo | 3/598 vs 0/590 | |
| Buller 2012 | Open-label, randomized | 2007–2011 | Rivaroxaban (15 mg twice daily for 3 weeks, followed by 20 mg once daily | Enoxaparin | 1/2420 vs 2/2413 | |
| Yamada 2015 | Double blind randomized | Japan | 2012–2013 | Rivaroxaban (15 or 10 mg twice) | warfarin | 0/77 vs 0/19 |
| Agnelli 2013 (acute VTE) | Double blind randomized | North America, South America, Europe, Asian Pacific | 2008–2012 | Apixaban (5 mg twice) | Enoxaparin (1 mg/kilogram of body weight, 12 h) | 8/2676 vs 19/2689 |
| Agnelli 2013Ext | Double blind randomized | North America, South America, Europe, Asian Pacific | 2008–2011 | Apixaban (2.5 mg, 5mg) | Placebo | 1/1635 vs 1/829 |
| Nakamura 2015 | Double blind randomized | Japan | NA | Apixaban (10 mg twice) | Warfarin | 0/40 vs 0/40 |
| Buller 2013 | Double blind randomized | Europe | 2010–2012 | Edoxaban (60mg) | Warfarin | 27/4118 vs 18/4122 |
| Schulman 2009 | Double blind randomized | Europe, North America | 2006–2008 | Dabigatran (150mg) | Warfarin | 53/1274 vs 35/1265 |
| Schulman 2014 | Double blind randomized | Europe, North America | 2008–2010 | Dabigatran (150 mg twice) | Warfarin | 48/1279 vs 33/1289 |
| Schulman 2013 (RE-MEDY) | Double blind randomized | North America, South America, Europe, Asian Pacific | 2006–2010 | Dabigatran (150 mg twice) | Warfarin | 5/1430 vs 8/1426 |
| Schulman 2013 (RE-SONATE) | Double blind randomized | North America, South America, Europe, Asian Pacific | 2007–2010 | Dabigatran (150 mg twice) | Placebo | 2/681 vs 0/662 |
| Post-surgical prophylaxis of venous thromboembolism (11 studies) | ||||||
| Lassen 2009 | Double blind randomized | Europe | NA | Apixaban (2.5 mg twice) | Enoxaparin (30mg) | 1/1596 vs 6/1588 |
| Lassen 2010K | Double blind randomized | Europe | 2007–2008 | Apixaban (2.5 mg twice) | Enoxaparin (40mg) | 2/1501 vs 2/1508 |
| Lassen 2010H | Double blind randomized | Europe | 2007–2009 | Apixaban (2.5 mg twice) | Enoxaparin (40mg) | 4/2673 vs 0/2659 |
| Fuji 2014K | Double blind randomized | Japan | 2009 | Edoxaban (30mg) | Enoxaparin (2000IU) | 1/354 vs 0/349 |
| Fuji 2015 | Double blind randomized | Japan | 2009–2010 | Edoxaban (30mg) | Enoxaparin (2000IU) | 0/303 vs 2/301 |
| Fuji 2014H | Double blind randomized | Japan | 2008–2009 | Edoxaban (30mg) | Enoxaparin (2000IU) | 1/59 vs 0/29 |
| Eriksson 2007 | Double blind randomized | Europe | 2004–2006 | Dabigatran (150 mg or 220mg) | Enoxaparin | 1/2309 vs 0/1154 |
| Eriksson 2008 | Double blind randomized | Europe | 2006–2007 | Rivaroxaban (10mg) | Enoxaparin (40mg) | 2/2209 vs 1/2224 |
| Kakkar 2008 | Double blind randomized | Europe | 2006–2007 | Rivaroxaban (10mg) | Enoxaparin (40mg) | 1/1228 vs 0/1229 |
| Turpie 2009 | Double blind randomized | North America, Europe | 2006–2007 | Rivaroxaban (10mg) | Enoxaparin (30mg) | 1/1526 vs 0/1508 |
| Lassen 2008 | Double blind randomized | Europe | 2006 | Rivaroxaban 10mg | Enoxaparin (40mg) | 7/12,20 vs 6/1,239 |
NA = non-available.
Table 2.
Inconsistency test between direct and indirect treatment comparisons in mixed treatment comparison.
| Direct | Indirect | Difference | |||||
| Side | Coef | Std. Err | Coef | Std. Err | Coef | Std. Err | P > |z| |
| AvsB | −0.2207917 | 0.1778605 | −0.9997044 | 0.189685 | 0.7789127 | 0.2594087 | .103 |
| AvsC | 0.1685377 | 0.1590577 | 0.3348726 | 0.2327751 | −0.1663349 | 0.2814689 | .555 |
| AvsD | 0.0609293 | 0.2311838 | 0.123441 | 1.011456 | −0.0625117 | 1.038363 | .952 |
| AvsE | 0.1339615 | 0.1858956 | 0.6116441 | 0.2010711 | −0.4776826 | 0.2742042 | .081 |
| AvsF | 0.8642481 | 0.4970639 | −0.3319522 | 0.4595372 | 1.1962 | 0.6769394 | .077 |
| BvsC | 1.312897 | 0.2981637 | 0.62859 | 0.2021851 | 0.6843069 | 0.3602505 | .057 |
| BvsE | 1.300795 | 0.3325848 | 0.7915953 | 0.2337526 | 0.5092002 | 0.4065131 | .21 |
| BvsF | 0.2263967 | 0.67782 | 1.066951 | 0.4201928 | −0.840554 | 0.7972694 | .292 |
| CvsE | 0.1977302 | 0.2088035 | 0.0180269 | 0.2178189 | 0.1797033 | 0.3016484 | .551 |
| CvsF | −0.4056816 | 1.655209 | 0.0088333 | 0.3622032 | −0.4145148 | 1.694376 | .807 |
| DvsF | 0.0977506 | 0.9423688 | 0.1602654 | 0.4362663 | −0.0625148 | 1.038369 | .952 |
| EvsF | −0.9106376 | 0.8555769 | 0.0362069 | 0.3859908 | −0.9468445 | 0.9386117 | .313 |
A = wafarin, B = apixaban, C = dabigatran, Coef = coeffiiciency, D = edoxaban, E = ribaroxaban, F = enoxaparin, Std. Err = standard error.
3.2. The risk of GI bleeding
Compared with warfarin, apixaban showed a lower risk of major GI bleeding (RR 0.54, 95% CI 0.25–0.76, P < .001), and rivaroxaban showed a higher risk (RR 1.40, 95% CI 1.06–1.85, P = .017). The other 2 NOACs, dabigatran (RR 1.25, 95% CI 0.98–1.60, P = .076) and edoxaban (RR 1.07, 95% CI 0.69–1.65, P = .776), and enoxaparin (RR 1.24, 95% CI 0.63–2.43, P = .536) did not significantly increase the risk of GI bleeding than that with warfarin (Fig. 3). Compared to apixaban, the remaining NOACs (dabigatran, edoxaban, and rivaroxaban) and enoxaparin were associated with increased risk of major GI bleeding.
Figure 3.
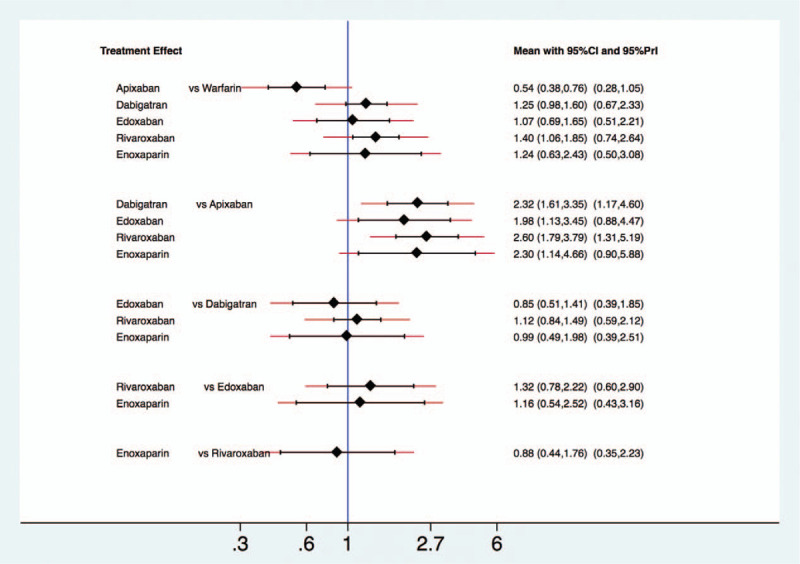
The interval plot of the relative risk for the major gastrointestinal bleeding in network meta-analysis including all studies.
3.3. Subgroup analysis according to the indications
Eight RCTs and 4 population observation cohort studies with AF were analyzed for risk of major GI bleeding associated with individual NOACs and warfarin in 352,058 patients. Compared with warfarin, apixaban showed a lower risk of major GI bleeding (RR 0.50, 95% P = .001), and the other individual NOACs showed no differences in the risk Compared to apixaban, dabigatran (RR 2.36, 95% CI 1.55–3.60, P = .037) and rivaroxaban (RR 1.75, 95% CI 1.10–6.41, P = .014) were associated with greater risk of major GI bleeding (Fig. 4A).
Figure 4.
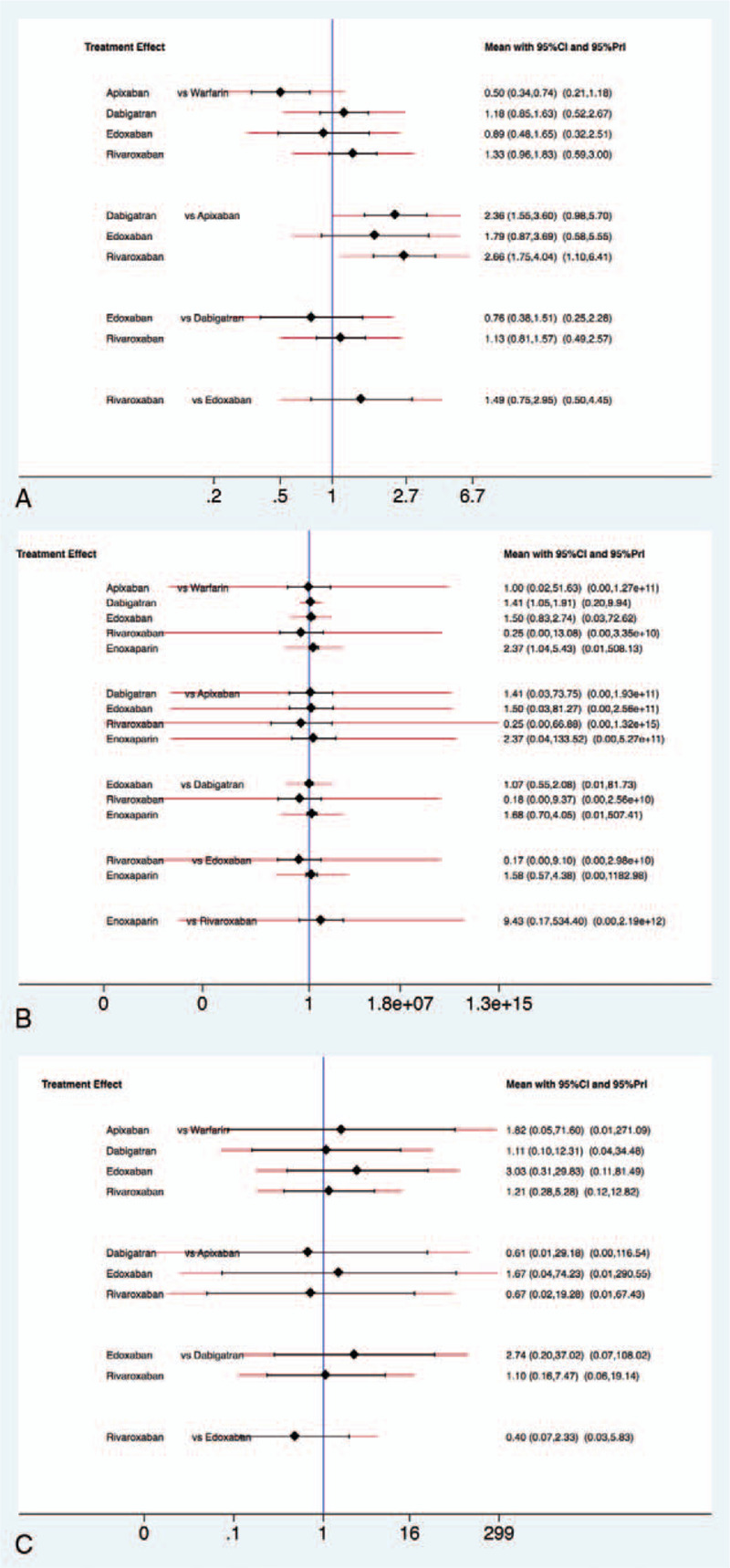
The interval plot of the relative risk for the major gastrointestinal bleeding according to the indication (A) atrial fibrillation, (B) deep venous thrombosis/pulmonary embolism, and (C) post-surgical prophylaxis.
Analysis of studies of VTE or PE (11 RCTs, 26,739 patients) revealed that no individual NOAC or enoxaparin was associated with increased risk of major GI bleeding compared to warfarin. Overall, there was no difference in major GI bleeding rates comparing each individual NOAC (Fig. 4B).
Analysis of studies of post-surgical prophylaxis of VTE (11 RCTs, 28,766 patients) showed no significant differences of major GI bleeding risk among individual NOACs and enoxaparin (Fig. 4C).
3.4. Sensitivity analysis and quality assessment
Most of the studies included in the analysis were classified as having an overall low risk of bias. However, a few studies (the EINSTEIN acute deep vein thrombosis, the EINSTEIN-PE and the RE-LY study) [7,30,47] were open-label RCTs, and consequently allocation concealment procedures and blinding of participants and study personnel items were considered to be of high risk of bias (Supplemental Fig. 1). As with the sensitivity analysis, we performed meta-analysis of 29 RCTs after excluding 4 large observation population studies; the plot showed a similar trend with the plot of analysis including all RCTs and population study biases (Supplemental Fig. 2).
4. Discussion
GI bleeding is a representative complication of NOAC use; this complication has been a matter of controversy in many RCTs and observation studies. Although NOACs have favorable safety profiles, efficacy, compliance, and convenience compared to conventional anticoagulation, GI bleeding is a fatal disadvantage. Previous RCTs and meta-analyses showed increased risk of GI bleeding with NOACs such as rivaroxaban or dabigatran, than that with conventional therapy.[7,11,13,48–50] Recently, many large RCTs and meta-analyses addressed the risk of increased GI bleeding with NOACs than with conventional therapy.[51,52] Recently published population-based observational studies demonstrated variable risk of GI bleeding among each individual NOACs through direct and indirect comparisons [43,45,46]; Graham et al reported that, in patients with AF, rivaroxaban increased risk of GI bleeding than did dabigatran. Other studies by Abraham showed equivalent risks between rivaroxaban and dabigatran, and apixaban decreased the risk of GI bleeding compared to rivaroxaban and dabigatran. A recent propensity matched cohort study (YAO) in patients with AF reported increased, equivalent, and decreased GI bleeding risks associated with rivaroxaban, dabigatran, and apixaban, respectively, compared to warfarin.[44,53]
In this network meta-analysis, we assessed major GI bleeding data of 30 RCTs and 4 observation population studies with updated and approved indications, including nonvalvular AF, VTE or PE, and postsurgical prophylaxis of VTE comparing individual NOACs and conventional anticoagulation therapy. We found that each individual NOAC had a different profile of GI bleeding. Overall, apixaban significantly decreased the risk of GI bleeding, and rivaroxaban increased the risk than those with other individual NOACs and conventional therapy. Among other individual NOACS (dabigatran, edoxaban, and rivaroxaban) and conventional therapy, there were no significant associations with major GI bleeding. When we analyzed only RCTs, the plot showed a similar trend. However, some differences were noted:
-
1.
Compared to warfarin, dabigatran significantly increased the risk of major GI bleeding, rather than rivaroxaban.
-
2.
Among individual NOACs, dabigatran showed increased risk of major bleeding, and the other individual NOACs showed no difference between one another.
In subgroup analysis according to indications, in cases of AF, apixaban significantly decreased the risk of GI bleeding than did other individual NOACs, and warfarin. Indirect comparison showed that, with respect to VTE or PE, no individual NOAC, enoxaparin, and warfarin were associated with increased risk of major GI bleeding. In cases of postsurgical prophylaxis of VTE, no significant difference in major GI bleeding was shown among individual NOACs and enoxaparin.
Taken together, analysis including only RCTs and analysis including both RCTs and population studies showed similar trends, but several differences. According to indications, there were several differences regarding results as well. We found that rivaroxaban increased the risk of GI bleeding in the analysis of RCTs and population studies, while dabigatran increased the risk of GI bleeding in the analysis of only the RCTs. This might be due to study-related differences, including patient criteria and baseline demographic characteristics. Compared with the population cohort study, RCT studies included patients with high CHADs scores who had risk factors for bleeding, including old age and diabetes mellitus. By contrast, population studies included patients with renal dysfunction or liver dysfunction who were excluded from RCTs. Plasma levels of dabigatran and rivaroxaban are elevated in renal dysfunction patients because of their prolonged excretion rates. The recommendation of creatinine clearance (CrCl) range for dabigatran and rivaroxaban were different, 15 to 30 ml/minute/1.73 m2 and 15 to 50 ml/minute/1.73 m2, respectively.[54,55] Different ranges of CrCl could affect the results of analysis of the 2 agents. In addition, concerning CrCl, off-label under-dosing of NOAC occurs in about 40% of real-world practice, possibly leading to different results between RCTs and population studies.[56,57]
Ethnicity could be another factor. The population cohort studies were conducted with Western countries cohorts, while RCT studies included those conducted with Asian groups. In addition, RCTs for postsurgical prophylaxis of VTE included Asian trials. These ethnic differences may be related to VKORC1 gene variation or factor V Leiden mutation, which is common or exceedingly rare in Asians, and may play role in varying outcomes of the analyses.[19,58,59]
The pathophysiology of different GI bleeding risk with individual NOACs is uncertain. One possible explanation is different and lower oral bioavailability of NOACs. Dabigatran, apixaban, and rivaroxaban had 6%, 50%, and 60% to 80% oral bioavailability, respectively.[60,61] In addition, incomplete absorption of the NOACs across the GI mucosa could lead to activation of intra-luminal anticoagulant activity. [62,63] This theory may explain the increased risk of major GI bleeding with dabigatran or rivaroxaban compared to that with warfarin, and might explain the increased risk of GI bleeding rather than bleeding at sites such as the brain. Nevertheless, this is not sufficient to explain the decreased, equivalent, and increased risk of GI bleeding with apixaban and edoxaban compared to warfarin, respectively. The biological differences for GI bleeding of individual NOACs need further study.
Despite the fact that individual NOACs are associated with distinct profiles of GI bleeding, there are no specific screening guidelines and no established risk factor grading system for GI bleeding for individual NOACs. Current guidelines have not definitely addressed the risk of major bleeding risk of GI bleeding with individual NOACs [64]. Although based on insufficient data, American Gastroenterological Association recommended lowering dose of dabigatran and rivaroxaban depending on creatinine clearance. Other risk stratification tools for GI bleeding or specific recommendations for preventing GI bleeding for individual NOACs have not been defined. We found individual NOACs had various GI bleeding profiles, and the results of RCTs and population studies showed slight differences. In addition, patients with NOACs had various comorbidities that are known to increase risk of GI bleeding. Therefore, creation and validation of individual NOAC-specific scoring tools for GI bleeding, such as the HAS-BLED score [65] are needed, as are recommendations of patient screening, selection, and changing of NOACs.
This study has several limitations. First, we could perform network analysis only on studies with AF patients. Other studies with VTE or PE and postsurgery VTE prophylaxis were not subjected to network analysis, because a closed loop was not formed. Second, there was heterogeneity because RCTs as well as observation studies were analyzed. Nevertheless, major confounding factors (including age, use of gastroprotective agents, and ulcerogenic agents (including antiplatelet agents, NSAIDs, steroids, and selective serotonin reuptake inhibitors), as well as indications for anticoagulation were evaluated using subgroup and sensitivity analysis. Through analysis of not only RCTs but also observation studies, we recognized differences between them which make this study to be relevant. These differences suggest that further studies are needed. Last, all the included studies in this meta-analysis investigated the warfarin and enoxaparin as VKA and low molecular weight heparin group respectively. Further studies accessed other various types of conventional anticoagulation therapies employed in real clinical world, especially such as dalteparin and nadroparin, are needed.
In conclusion, this network meta-analysis showed that individual NOACs had distinct profiles of GI bleeding risk. Overall, apixaban significantly decreased the risk of GI bleeding, and rivaroxaban increased the risk of GI bleeding than did individual NOACs and conventional therapy. According to the meta-analysis of RCTs, dabigatran increased the risk of GI bleeding. To confirm the clinical relevance and to establish the practical clinical guidelines for tailored therapy, high-quality head-to-head comparison studies are needed.
Author contributions
Conceptualization: Hyun Jin Oh, Kum Hei Ryu, Bum Jun Park, Byung-Ho Yoon.
Data curation: Hyun Jin Oh, Bum Jun Park, Byung-Ho Yoon.
Formal analysis: Hyun Jin Oh, Kum Hei Ryu, Bum Jun Park, Byung-Ho Yoon.
Funding acquisition: Hyun Jin Oh, Byung-Ho Yoon.
Investigation: Hyun Jin Oh, Kum Hei Ryu, Bum Jun Park, Byung-Ho Yoon.
Methodology: Hyun Jin Oh, Kum Hei Ryu, Bum Jun Park, Byung-Ho Yoon.
Project administration: Hyun Jin Oh, Kum Hei Ryu, Bum Jun Park, Byung-Ho Yoon.
Resources: Hyun Jin Oh, Kum Hei Ryu, Bum Jun Park, Byung-Ho Yoon.
Software: Hyun Jin Oh, Kum Hei Ryu, Bum Jun Park, Byung-Ho Yoon.
Supervision: Hyun Jin Oh, Bum Jun Park, Byung-Ho Yoon.
Validation: Hyun Jin Oh, Kum Hei Ryu, Bum Jun Park, Byung-Ho Yoon.
Visualization: Hyun Jin Oh, Kum Hei Ryu, Bum Jun Park, Byung-Ho Yoon.
Writing – original draft: Hyun Jin Oh, Kum Hei Ryu, Bum Jun Park, Byung-Ho Yoon.
Writing – review & editing: Hyun Jin Oh, Kum Hei Ryu, Bum Jun Park, Byung-Ho Yoon.
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: AF = atrial fibrillation, CI = confidence intervals, GI = gastrointestinal, NOAC = non-vitamin K antagonist oral anticoagulant, RCT = randomized controlled trial, RR = relative risks, VKA = vitamin K antagonist, VTE = venous thromboembolism.
How to cite this article: Oh HJ, Ryu KH, Park BJ, Yoon BH. The risk of gastrointestinal hemorrhage with non-vitamin K antagonist oral anticoagulants: a network meta-analysis. Medicine. 2021;100:11(e25216).
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
This meta-analysis summaries previously published data and does not include new human data or tissue that require ethical approval and consent. The authors assume that the studies reviewed were conducted after ethical approval and consent, and in accordance with the Declaration of Helsinki.
The authors have no conflicts of interests to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Supplemental digital content is available for this article.
References
- [1].Weitz JI, Eikelboom JW, Samama MM. New antithrombotic drugs: antithrombotic therapy and prevention of thrombosis: American college of chest physicians evidence-based clinical practice guidelines. Chest 2012;141:e120S–51S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Gui YY, Zou S, Yang WL, et al. The impact of renal function on efficacy and safety of new oral anticoagulant in atrial fibrillation patients: A systemic review and meta-analysis. Medicine (Baltimore) 2019;98:e18205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lv S, Liu Y, Wei G, et al. The anticoagulants rivaroxaban and low molecular weight heparin prevent PICC-related upper extremity venous thrombosis in cancer patients. Medicine (Baltimore) 2019;98:e17894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Demelo-Rodriguez P, Galeano-Valle F, Garcia-Fernandez-Bravo I, et al. Rivaroxaban for the treatment of venous thromboembolism in real life: a single-center prospective study. Medicine (Baltimore) 2019;98:e14093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Iorga RA, Bratu OG, Marcu RD, et al. Venous thromboembolism in cancer patients: still looking for answers. Exp Ther Med 2019;18:5026–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Otilia AT, Ovidiu Ţ, Liana A, et al. Modern oral anticoagulant treatment in patients with atrial fibrillation and heart failure: insights from the clinical practice. Farmacia 2018;66:972–6. [Google Scholar]
- [7].Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139–51. [DOI] [PubMed] [Google Scholar]
- [8].Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013;369:2093–104. [DOI] [PubMed] [Google Scholar]
- [9].Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981–92. [DOI] [PubMed] [Google Scholar]
- [10].Miller CS, Grandi SM, Shimony A, et al. Meta-analysis of efficacy and safety of new oral anticoagulants (dabigatran, rivaroxaban, apixaban) versus warfarin in patients with atrial fibrillation. Am J Cardiol 2012;110:453–60. [DOI] [PubMed] [Google Scholar]
- [11].Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883–91. [DOI] [PubMed] [Google Scholar]
- [12].Cho MR, Jun CM, Choi WK. Preoperative temporary discontinuation of aspirin medication does not increase the allogeneic transfusion rate and blood loss in primary total hip arthroplasty. Hip Pelvis 2019;31:82–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014;383:955–62. [DOI] [PubMed] [Google Scholar]
- [14].Yoon BH, Lee BS, Won H, et al. Preoperative iron supplementation and restrictive transfusion strategy in hip fracture surgery. Clin Orthop Surg 2019;11:265–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Van Leerdam M, Vreeburg E, Rauws E, et al. Acute upper GI bleeding: did anything change?: time trend analysis of incidence and outcome of acute upper GI bleeding between 1993/1994 and 2000. Am J Gastroenterol 2003;98:1494–9. [DOI] [PubMed] [Google Scholar]
- [16].Gómez-Outes A, Lagunar-Ruíz J, Terleira-Fernández A-I, et al. Causes of death in anticoagulated patients with atrial fibrillation. J Am Coll Cardiol 2016;68:2508–21. [DOI] [PubMed] [Google Scholar]
- [17].Schulman S, Kearon C, Subcommittee on Control of Anticoagulation of the S, et al. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005;3:692–4. [DOI] [PubMed] [Google Scholar]
- [18].Shim S, Yoon BH, Shin IS, et al. Network meta-analysis: application and practice using Stata. Epidemiol Health 2017;39:e2017047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hori M, Connolly SJ, Zhu J, et al. Dabigatran versus warfarin: effects on ischemic and hemorrhagic strokes and bleeding in Asians and non-Asians with atrial fibrillation. Stroke 2013;44:1891–6. [DOI] [PubMed] [Google Scholar]
- [20].Ogawa S, Shinohara Y, Kanmuri K. Safety and efficacy of the oral direct factor Xa inhibitor apixaban in Japanese patients with non-valvular atrial fibrillation. Circ J 2011;75:1852–9. [DOI] [PubMed] [Google Scholar]
- [21].Connolly SJ, Eikelboom J, Joyner C, et al. Apixaban in patients with atrial fibrillation. N Engl J Med 2011;364:806–17. [DOI] [PubMed] [Google Scholar]
- [22].Chung N, Jeon H-K, Lien L-M, et al. Safety of edoxaban, an oral factor Xa inhibitor, in Asian patients with non-valvular atrial fibrillation. Thromb Haemost 2011;105:535–45. [DOI] [PubMed] [Google Scholar]
- [23].Yamada N, Hirayama A, Maeda H, et al. Oral rivaroxaban for Japanese patients with symptomatic venous thromboembolism–the J-EINSTEIN DVT and PE program. Thrombos J 2015;13:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Nakamura M, Nishikawa M, Komuro I, et al. Apixaban for the treatment of Japanese subjects with acute venous thromboembolism (AMPLIFY-J Study). Circ J 2015;CJ-15-0195. [DOI] [PubMed] [Google Scholar]
- [25].Schulman S, Kakkar AK, Goldhaber SZ, et al. Treatment of acute venous thromboembolism with dabigatran or warfarin and pooled analysis. Circulation 2014;129:764–72. [DOI] [PubMed] [Google Scholar]
- [26].Schulman S, Kearon C, Kakkar AK, et al. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med 2013;368:709–18. [DOI] [PubMed] [Google Scholar]
- [27].Investigators H-V. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med 2013;369:1406–15. [DOI] [PubMed] [Google Scholar]
- [28].Agnelli G, Buller HR, Cohen A, et al. Apixaban for extended treatment of venous thromboembolism. N Engl J Med 2013;368:699–708. [DOI] [PubMed] [Google Scholar]
- [29].Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med 2013;369:799–808. [DOI] [PubMed] [Google Scholar]
- [30].Investigators E. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010;363:2499–510. [DOI] [PubMed] [Google Scholar]
- [31].Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009;361:2342–52. [DOI] [PubMed] [Google Scholar]
- [32].Fuji T, Fujita S, Kawai Y, et al. Efficacy and safety of edoxaban versus enoxaparin for the prevention of venous thromboembolism following total hip arthroplasty: STARS JV. Thromb J 2015;13:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Fuji T, Wang C-J, Fujita S, et al. Safety and efficacy of edoxaban, an oral factor Xa inhibitor, versus enoxaparin for thromboprophylaxis after total knee arthroplasty: the STARS E-3 trial. Thromb Res 2014;134:1198–204. [DOI] [PubMed] [Google Scholar]
- [34].Fuji T, Fujita S, Kawai Y, et al. Safety and efficacy of edoxaban in patients undergoing hip fracture surgery. Thromb Res 2014;133:1016–22. [DOI] [PubMed] [Google Scholar]
- [35].Lassen MR, Raskob GE, Gallus A, et al. Apixaban versus enoxaparin for thromboprophylaxis after knee replacement (ADVANCE-2): a randomised double-blind trial. Lancet 2010;375:807–15. [DOI] [PubMed] [Google Scholar]
- [36].Lassen MR, Gallus A, Raskob GE, et al. Apixaban versus enoxaparin for thromboprophylaxis after hip replacement. N Engl J Med 2010;363:2487–98. [DOI] [PubMed] [Google Scholar]
- [37].Turpie AG, Lassen MR, Davidson BL, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty (RECORD4): a randomised trial. Lancet 2009;373:1673–80. [DOI] [PubMed] [Google Scholar]
- [38].Lassen MR, Raskob GE, Gallus A, et al. Apixaban or enoxaparin for thromboprophylaxis after knee replacement. N Engl J Med 2009;361:594–604. [DOI] [PubMed] [Google Scholar]
- [39].Lassen MR, Ageno W, Borris LC, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med 2008;358:2776–86. [DOI] [PubMed] [Google Scholar]
- [40].Kakkar AK, Brenner B, Dahl OE, et al. Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double-blind, randomised controlled trial. Lancet 2008;372:31–9. [DOI] [PubMed] [Google Scholar]
- [41].Eriksson BI, Borris LC, Friedman RJ, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med 2008;358:2765–75. [DOI] [PubMed] [Google Scholar]
- [42].Eriksson BI, Dahl OE, Rosencher N, et al. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomised, double-blind, non-inferiority trial. Lancet 2007;370:949–56. [DOI] [PubMed] [Google Scholar]
- [43].Abraham NS, Noseworthy PA, Yao X, et al. Gastrointestinal safety of direct oral anticoagulants: a large population-based study. Gastroenterology 2017;152:1014–22. e1011. [DOI] [PubMed] [Google Scholar]
- [44].Yao X, Abraham NS, Sangaralingham LR, et al. Effectiveness and safety of dabigatran, rivaroxaban, and apixaban versus warfarin in nonvalvular atrial fibrillation. J Am Heart Assoc 2016;5:e003725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Graham DJ, Reichman ME, Wernecke M, et al. Stroke, bleeding, and mortality risks in elderly Medicare beneficiaries treated with dabigatran or rivaroxaban for nonvalvular atrial fibrillation. JAMA Intern Med 2016;176:1662–71. [DOI] [PubMed] [Google Scholar]
- [46].Abraham NS, Singh S, Alexander GC, et al. Comparative risk of gastrointestinal bleeding with dabigatran, rivaroxaban, and warfarin: population based cohort study. BMJ 2015;350:h1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Investigators EP. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med 2012;366:1287–97. [DOI] [PubMed] [Google Scholar]
- [48].Hylek EM, Held C, Alexander JH, et al. Major bleeding in patients with atrial fibrillation receiving apixaban or warfarin: The ARISTOTLE Trial (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation): Predictors, Characteristics, and Clinical Outcomes. J Am Coll Cardiol 2014;63:2141–7. [DOI] [PubMed] [Google Scholar]
- [49].Majeed A, Hwang H-G, Connolly S. Management and outcomes of major bleeding during treatment with dabigatran or warfarin. Circulation 2013;128:2325–32. [DOI] [PubMed] [Google Scholar]
- [50].Holster IL, Valkhoff VE, Kuipers EJ, et al. New oral anticoagulants increase risk for gastrointestinal bleeding: a systematic review and meta-analysis. Gastroenterology 2013;145:105–12. e115. [DOI] [PubMed] [Google Scholar]
- [51].Caldeira D, Barra M, Ferreira A, et al. Systematic review with meta-analysis: the risk of major gastrointestinal bleeding with non-vitamin K antagonist oral anticoagulants. Aliment Pharmacol Ther 2015;42:1239–49. [DOI] [PubMed] [Google Scholar]
- [52].Miller CS, Dorreen A, Martel M, et al. Risk of gastrointestinal bleeding in patients taking non–vitamin K antagonist oral anticoagulants: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2017;15:1674–83. e1673. [DOI] [PubMed] [Google Scholar]
- [53].Noh KC, Liu XN, Zhuan Z, et al. Leukocyte-poor platelet-rich plasma-derived growth factors enhance human fibroblast proliferation in vitro. Clin Orthop Surg 2018;10:240–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Diener H-C, Aisenberg J, Ansell J, et al. Choosing a particular oral anticoagulant and dose for stroke prevention in individual patients with non-valvular atrial fibrillation: part 2. Eur Heart J 2016;38:860–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Heidbuchel H, Verhamme P, Alings M, et al. Updated European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist anticoagulants in patients with non-valvular atrial fibrillation. EP Europace 2015;17:1467–507. [DOI] [PubMed] [Google Scholar]
- [56].Steinberg BA, Shrader P, Pieper K, et al. Frequency and outcomes of reduced dose non–vitamin K antagonist anticoagulants: results from ORBIT-AF II (the outcomes registry for better informed treatment of atrial fibrillation II). J Am Heart Assoc 2018;7:e007633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Yu HT, Yang P-S, Kim T-H, et al. Impact of renal function on outcomes with edoxaban in real-world patients with atrial fibrillation: a nationwide cohort study. Stroke 2018;49:2421–9. [DOI] [PubMed] [Google Scholar]
- [58].Gregg JP, Yamane AJ, Grody WW. Prevalence of the factor V-Leiden mutation in four distinct American ethnic populations. Am J Med Genet 1997;73:334–6. [DOI] [PubMed] [Google Scholar]
- [59].Yasuda S, Zhang L, Huang SM. The role of ethnicity in variability in response to drugs: focus on clinical pharmacology studies. Clin Pharmacol Ther 2008;84:417–23. [DOI] [PubMed] [Google Scholar]
- [60].Stangier J, Rathgen K, Stähle H, et al. The pharmacokinetics, pharmacodynamics and tolerability of dabigatran etexilate, a new oral direct thrombin inhibitor, in healthy male subjects. Br J Clin Pharmacol 2007;64:292–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Kubitza D, Becka M, Roth A, et al. Dose-escalation study of the pharmacokinetics and pharmacodynamics of rivaroxaban in healthy elderly subjects. Curr Med Res Opin 2008;24:2757–65. [DOI] [PubMed] [Google Scholar]
- [62].Desai J, Kolb JM, Weitz JI, et al. Gastrointestinal bleeding with the new oral anticoagulants–defining the issues and the management strategies. Thromb Haemost 2013;110:205–12. [DOI] [PubMed] [Google Scholar]
- [63].Kashyap S, Diwan Y, Mahajan S, et al. The majority of corona mortis are small calibre venous blood vessels: a cadaveric study of North Indians. Hip Pelvis 2019;31:40–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Abraham NS. Prevention of gastrointestinal bleeding in patients receiving direct oral anticoagulants. Am J Gastroenterol Suppl 2016;3:2–12. [Google Scholar]
- [65].Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010;138:1093–100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


