Abstract
A recent report demonstrated that the prevalence of obstructive sleep apnea (OSA) is 67.6% among Caucasian and Chinese patients with primary aldosteronism (PA). Moreover, the report showed a significant association between plasma aldosterone concentration (PAC) and the severity of OSA in Caucasian patients. However, no studies have examined the prevalence of OSA with PA or the association of its severity with PAC in the Japanese population. We retrospectively evaluated the prevalence and severity of OSA in 71 newly diagnosed Japanese patients with PA. Thirty-nine (55%) of the 71 patients were diagnosed with OSA, and 69% of PA patients with OSA reported snoring. No correlation was found between the respiratory event index (REI), snoring index, and PAC and plasma renin activity (PRA). In contrast, REI correlated significantly with body mass index (BMI), which was significantly correlated with PRA. In conclusion, although the severity of OSA did not correlate with PAC and PRA, there was a high prevalence of OSA among Japanese patients with PA. Moreover, the severity of OSA was strongly affected by BMI. Thus, the examination of OSA in patients with PA and the proper management of OSA might be important for the Japanese population.
Keywords: aldosterone, hypertension, obstructive sleep apnea, primary aldosteronism
1. Introduction
Primary aldosteronism (PA) has an estimated prevalence of 17% to 23% among patients with resistant hypertension, and it is regarded as a major cause of secondary hypertension.[1,2] Obstructive sleep apnea (OSA) is also thought to contribute to the development of resistant hypertension,[3] and among patients with this condition, the prevalence of OSA is 60% to 80%.[4] Therefore, there is a high likelihood of co-occurrence of PA and OSA. Although there are limited data on the prevalence of OSA in patients with PA, 2 independent reports have shown that OSA occurs in 78.1% and 84% of patients with PA, respectively.[4,5] Recently, Buffolo et al[6] demonstrated that the prevalence of OSA was 67.6% in Caucasian and Chinese patients with PA, suggesting some discrepancies in the prevalence of OSA among patients with PA in different cohorts. Given the high co-occurrence of PA and OSA, it has been hypothesized that there might be a bidirectional relationship between aldosterone and OSA.[7] For example, aldosterone has been shown to exacerbate OSA by promoting the accumulation of fluid, which shifts to the neck in the supine position and contributes to increased upper airway resistance.[8–10] Indeed, Buffolo et al[6] showed a positive association between aldosterone levels and the apnea-hypopnea index (AHI) in Caucasian patients with PA but not in Chinese patients. This study suggested that the association between PA and OSA varies between racial groups. However, little is known about the association between PA and OSA in patients with PA, and the prevalence of OSA in the Japanese PA population. In this study, we evaluated the prevalence of OSA in Japanese patients with PA and the association between the clinical characteristics and severity of OSA in the Japanese PA population.
2. Materials and methods
2.1. Setting and patients
This study was performed in the Division of Nephrology, Hypertension and Endocrinology, Department of Internal Medicine, Nihon University School of Medicine, Tokyo, Japan. The study protocol was approved by the Ethics Committee of the hospital and conducted in accordance with the Declaration of Helsinki. This study was registered in the UMIN Clinical Trial Registry (UMIN-ID; UMIN 000040258). Seventy-one patients who were admitted to the hospital for examination of the cause of hypertension and diagnosed with PA between April 2017 and March 2020 were retrospectively reviewed.
2.2. Diagnosis of PA
PA was diagnosed according to the Japanese Endocrine Society guidelines.[11] Hypertensive patients with an elevated aldosterone/renin ratio (>200 with a plasma aldosterone concentration [PAC] in pg/mL and plasma renin activity [PRA] in ng/mL/h) in the PA screening test underwent loading tests, including the captopril challenge test, furosemide upright test, and post-saline infusion test. Medications that affect the loading test (e.g., angiotensin-converting enzyme inhibitors, angiotensin II receptor antagonists, β-blockers, and diuretics) were replaced with calcium channel antagonists or α-blockers, at least 4 weeks prior to testing. Patients with hypokalemia were allowed to take oral potassium supplementation before confirmatory tests. The captopril challenge test was considered positive if the aldosterone/renin ratio at 90 minutes after taking 50 mg captopril were ≥200. The furosemide upright test was considered positive if PRA after 2 hours of being in an upright position following furosemide infusion were <2.0 ng/mL/h. The post-saline infusion test was considered positive if the aldosterone level at 4 hours after 2 L saline infusion were ≥60.0 pg/mL. PA was diagnosed if ≥1 of the 3 tests were positive.
2.3. Reagents and measurement of variable parameters
Medications, including angiotensin-converting enzyme inhibitors, angiotensin II receptor antagonists, β-blockers, and diuretics, were stopped at least 4 weeks before admission. During hospitalization, all 71 patients completed bedside tests, including the serum electrolyte panel and the adrenocortical hormone test. The levels of peripheral PAC, plasma cortisol concentration (PFC), and PRA were measured in blood samples obtained at 00:00, 06:00, 12:00, and 18:00 hours. In addition, the aldosterone urinary level was measured in 24-hours urine samples. PAC was measured by a radioimmunoassay using a commercial kit (SPAC-S Aldosterone Kit; Fuji Lebio, Tokyo, Japan). PRA was determined in vitro by the generation of angiotensin I, using a commercially available kit (PRA Kit; Fuji Lebio, Tokyo, Japan). PRA values <0.1 ng/mL/h were reported as 0.09 ng/mL/h. All 71 patients were equipped with portable polysomnography devices as follows: 56 patients used a Smart Watch PMP-300E (Pacific Medico, Tokyo, Japan) and 15 patients used an Apnomonitor Mini (Chest MI, Inc., Tokyo, Japan). However, the number of snores was measured only with a Smart Watch PMP-300E. Apnea was defined as a ≥90% reduction of nasal flow for ≥10 seconds, and hypopnea was defined as a ≥30% reduction in nasal flow for ≥10 seconds with a decrease of ≥3% in oxygen saturation. The number of apneas and hypopneas per hour of measurement time is defined as the respiratory event index (REI). OSA was diagnosed by an REI ≥15 or REI ≥5 together with symptoms such as daytime somnolence.[12] Patients diagnosed with OSA were classified into mild (5 ≤ REI < 15), moderate (15 ≤ REI < 30), and severe (30 ≤ REI) groups. The snore index was defined as the number of snores detected by the pressure sensor per hour.
2.4. Statistical analysis
Statistical analysis was performed using SPSS version 22 (SPSS, Inc., Chicago, IL). Variables are presented as the median with interquartile range (25–75%). Differences in measured parameters between groups were evaluated using the Mann–Whitney U test (for skewed variables). Fisher exact test was used to compare categorical variables. Correlations were evaluated using Spearman correlation coefficients. Statistical significance was set at P < .05.
3. Results
3.1. Prevalence of OSA in patients with PA
Seventy-one patients who were admitted to the hospital for a diagnosis of the cause of secondary hypertension, and diagnosed with PA were included. Among them, 16 underwent adrenal venous sampling and 8 were diagnosed with unilateral hyperaldosteronism. According to the REI, 55% (39/71) of PA patients were diagnosed with OSA, of which 12 were identified with mild (17%), 16 with moderate (23%), and 11 with severe OSA (15%). The clinical and biochemical parameters of the included patients are reported in Table 1.
Table 1.
Clinical characteristics of the study participants.
| Clinical characteristic | Non-OSA (n = 32) | OSA (n = 39) | P-value |
| Age, y | 47 (41–55) | 49 (39–54) | .93 |
| Sex (male, %) | 16% | 56% | <.001 |
| Self-reported snoring (%) | 31% | 69% | <.001 |
| Daytime somnolence (%) | 16% | 28% | .26 |
| BMI, kg/m2 | 23.1 (20.0–25.4) | 27.7 (24.2–30.2) | <.001 |
| Systolic blood pressure, mm Hg | 137 (129–148) | 139 (128–146) | .97 |
| Diastolic blood pressure, mm Hg | 89 (84–100) | 90 (82–93) | .54 |
| REI, times/h | 4.6 (3.2–5.8) | 21.6 (11.1–34.3) | <.001 |
| Snoring index, times/h | 2.8 (0.8–7.2) | 4.2 (1.3–26.5) | .070 |
| Serum sodium, mmol/L | 141 (140–142) | 142 (140–143) | .069 |
| Serum potassium, mmol/L | 4.0 (3.8–4.2) | 4.0 (3.7–4.2) | .57 |
| HbA1c (%) | 5.5 (5.4–5.7) | 5.7 (5.4–6.0) | .024 |
| Total cholesterol, mg/dL | 201 (169–224) | 205 (183–228) | .43 |
| LDL, mg/dL | 113 (89–132) | 120 (106–140) | .15 |
| HDL, mg/dL | 65 (54–76) | 48 (40–61) | .001 |
| Triglycerides, mg/dL | 70 (56–124) | 165 (89–206) | <.001 |
| eGFR, mL/min/1.73 m2 | 81.5 (65.8–90.1) | 80.0 (67.1–88.3) | .65 |
| Urinary aldosterone, μg/day | 10 (7–15) | 11 (6–16) | .67 |
| ACR, mg/g | 14.3 (6.7–21.2) | 19.9 (7.7–49.3) | .18 |
| PAC at diagnosis, pg/mL | 197 (153–278) | 193 (144–259) | .64 |
| PAC 00:00 h, pg/mL | 103 (82–149) | 116 (83–162) | .42 |
| PAC 06:00 h, pg/mL | 186 (150–280) | 152 (107–270) | .13 |
| PAC 12:00 h, pg/mL | 212 (155–280) | 200 (155–238) | .80 |
| PAC 18:00 h, pg/mL | 161 (115–222) | 136 (104–192) | .22 |
| PFC 00:00 h, μg/dL | 1.59 (1.07–3.28) | 1.80 (0.95–2.81) | .91 |
| PFC 06:00 h, μg/dL | 13.05 (10.53–15.30) | 11.10 (9.22–14.40) | .14 |
| PFC 12:00 h, μg/dL | 5.37 (4.24–7.57) | 6.58 (5.27–9.32) | .054 |
| PFC 18:00 h, μg/dL | 3.85 (2.35–5.38) | 3.82 (2.81–4.82) | .91 |
| PRA at diagnosis, ng/mL/h | 0.4 (0.2–0.7) | 0.5 (0.3–0.9) | .14 |
| PRA 00:00 h, ng/mL/h | 0.3 (0.2–0.4) | 0.3 (0.2–0.7) | .30 |
| PRA 06:00 h, ng/mL/h | 0.3 (0.2–0.6) | 0.4 (0.3–0.7) | .22 |
| PRA 12:00 h, ng/mL/h | 0.5 (0.3–0.9) | 0.7 (0.4–1.1) | .14 |
| PRA 18:00 h, ng/mL/h | 0.3 (0.3–0.5) | 0.5 (0.3–0.7) | .50 |
Data are presented as the median (interquartile range). ACR = urine albumin to creatinine ratio, BMI = body mass index, eGFR = estimated glomerular filtration rate, HbA1c = glycated hemoglobin, HDL = high-density lipoprotein cholesterol, LDL = low-density lipoprotein cholesterol, OSA = obstructive sleep apnea, PAC = plasma aldosterone concentration, PFC = plasma cortisol concentration, PRA = plasma renin activity, REI = respiratory event index.
3.2. Comparison between PA patients with and without OSA
PA patients with OSA were predominantly men, and displayed a significantly higher proportion of self-reported snoring, body mass index (BMI), glycated hemoglobin (HbA1c), and triglycerides (P < .001, P < .001, P = .024, and P < .001, respectively), and lower high-density lipoprotein cholesterol (HDL) levels (P < .001), compared with those without OSA. Multivariate logistic regression analysis revealed that BMI and triglycerides content were associated with OSA (odds ratio = 1.27, P = .0063; odds ratio = 1.01, P = .019, respectively) (Table 2). There were no significant differences in PAC, PRA, and PFC values between PA patients with and without OSA at 00:00, 06:00, 12:00, and 18:00 hours. Similar associations were observed in patients with mild (REI < 15) and moderate-to-severe OSA (15 ≤ REI) (Supplementary Table 1). Spearman rank correlation analysis indicated that BMI, HDL, triglycerides, urine albumin to creatinine ratio (ACR), and PAC at 06:00 hours, correlated significantly with the REI (P < .001, P < .001, P < .001, P = .037, and P = .017, respectively) (Supplementary Table 2). This analysis further showed that BMI was most strongly associated with OSA (Fig. 1). Therefore, we suspected BMI was a strong contributor to the REI, and accordingly, adjusted the Spearman rank correlation analysis for BMI. Only triglycerides and the ACR were significant after adjusting for BMI (r = 0.24, P = .047; r = 0.25, P = .038, respectively). Thus, there was no significant association between PAC and the REI, contrary to previous studies. However, BMI had the strongest association with the REI in our study. Table 3 shows the relationship between the BMI and hormone levels. Importantly, BMI was significantly positively correlated with PRA at all time points.
Table 2.
Multivariable logistic regression model for obstructive sleep apnea and other clinical factors.
| Characteristic | Odds ratio (95% CI) | P-value |
| Body mass index | 1.27 (1.07–1.50) | .0063 |
| Triglycerides | 1.01 (1.00–1.02) | .019 |
Sex, body mass index, glycated hemoglobin, triglycerides, and high-density lipoprotein cholesterol were included in the multivariable model. CI = confidence interval.
Figure 1.
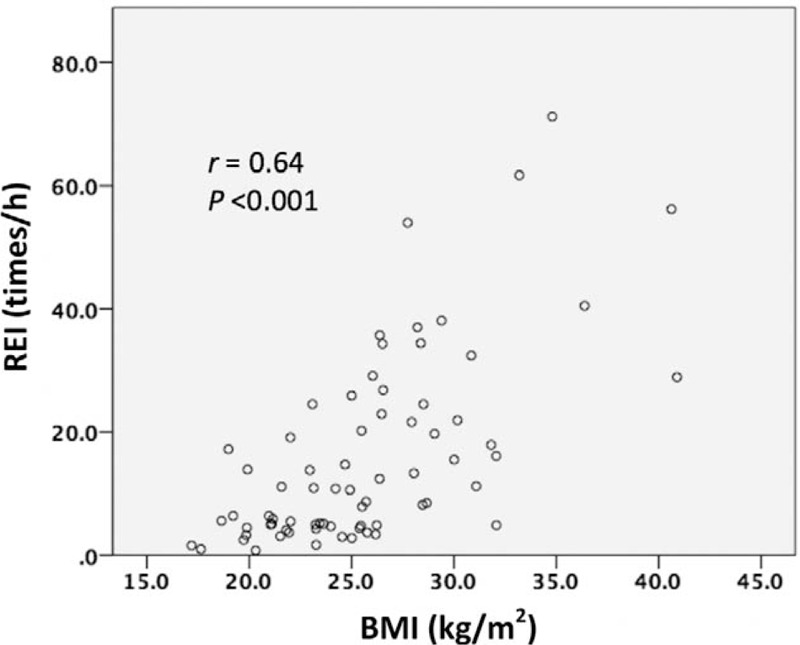
Correlation between the respiratory event index (REI) and body mass index (BMI).
Table 3.
Spearman correlation between the body mass index and hormone levels.
| BMI | ||
| Characteristic | r | P-value |
| PAC at diagnosis | −0.008 | .95 |
| PAC at 00:00 h | −0.067 | .58 |
| PAC at 06:00 h | −0.21 | .087 |
| PAC at 12:00 h | −0.021 | .86 |
| PAC at 18:00 h | −0.12 | .34 |
| PFC at 00:00 h | −0.15 | .22 |
| PFC at 06:00 h | −0.32 | .0062 |
| PFC at 12:00 h | −0.093 | .44 |
| PFC at 18:00 h | −0.18 | .13 |
| PRA at diagnosis | 0.35 | .0025 |
| PRA at 00:00 h | 0.40 | <.001 |
| PRA at 06:00 h | 0.33 | .0050 |
| PRA at 12:00 h | 0.34 | .0042 |
| PRA at 18:00 h | 0.35 | .0033 |
| Urinary aldosterone | −0.056 | .64 |
BMI = body mass index, PAC = plasma aldosterone concentration, PFC = plasma cortisol concentration, PRA = plasma renin activity.
3.3. PA patients with and without snoring
Previously, it was demonstrated that patients with PA who self-report snoring tend to have increased BMI and diastolic blood pressure and decreased estimated glomerular filtration rate and aldosterone to renin activity ratio.[13] Therefore, we evaluated the association between the prevalence of snoring and clinical characteristics in patients with PA. Except for triglycerides, there were no significant differences in clinical indicators of OSA between PA patients with and without self-reported snoring (Table 4). Notably, there was no significant difference in the snoring index, as measured by portable polysomnography, between PA patients with and without self-reported snoring, implying that self-reported snoring is not a reliable indicator of OSA. Spearman rank correlation analysis showed that the snoring index was correlated with REI, BMI, HDL, triglycerides, and PFC at 06:00 hours (r = 0.39, P = .0031; r = 0.35, P = .0077; r = −0.40, P = .0020; r = 0.36, P = .0063; r = −0.39, P = .0027, respectively), and with PFC at 12:00 hours (r = −0.26, P = .049) (Table 5). Only HDL and PFC at 06:00 hours were significant after adjusting for BMI (r = −0.27, P = .047; r = −0.29, P = .029, respectively).
Table 4.
Differences in clinical characteristics between patients with and without self-reported snoring.
| Clinical characteristic | Without snoring (n = 35) | With snoring (n = 36) | P-value |
| Age, y | 49 (42–56) | 46 (38–53) | .21 |
| Sex (male, %) | 29% | 47% | .14 |
| BMI, kg/m2 | 24.5 (21.0–27.9) | 25.6 (23.2–28.4) | .13 |
| Systolic blood pressure, mm Hg | 135 (128–145) | 140 (130–148) | .63 |
| Diastolic blood pressure, mm Hg | 86 (82–93) | 91 (85–95) | .27 |
| REI, times/h | 8.5 (4.8–17.2) | 11.1 (4.4–31.0) | .24 |
| Snoring index, times/h | 3.4 (1.3–10.0) | 3.5 (1.1–23.1) | .62 |
| Serum sodium, mmol/L | 141 (140–142) | 142 (140–143) | .09 |
| Serum potassium, mmol/L | 4.0 (3.8–4.2) | 4.0 (3.7–4.1) | .77 |
| HbA1c (%) | 5.6 (5.4–5.7) | 5.7 (5.4–6.0) | .21 |
| Total cholesterol, mg/dL | 205 (169–224) | 201 (186–227) | .70 |
| LDL, mg/dL | 113 (90–140) | 120 (105–133) | .64 |
| HDL, mg/dL | 61 (48–73) | 55 (43–62) | .13 |
| Triglycerides, mg/dL | 82 (57–140) | 138 (89–202) | .003 |
| eGFR, mL/min/1.73 m2 | 80.2 (65.4–87.8) | 83.0 (67.5–90.9) | .47 |
| Urinary aldosterone, μg/day | 10 (7–17) | 11 (7–14) | .80 |
| PAC at diagnosis, pg/mL | 202 (154–261) | 193 (145–278) | .96 |
| PAC at 00:00 h, pg/mL | 109 (85–161) | 108 (82–148) | .96 |
| PAC at 06:00 h, pg/mL | 180 (138–277) | 167 (125–273) | .45 |
| PAC at 12:00 h, pg/mL | 218 (161–303) | 195 (154–237) | .24 |
| PAC at 18:00 h, pg/mL | 164 (113–227) | 132 (105–180) | .11 |
| PFC at 00:00 h, μg/dL | 1.67 (1.02–3.14) | 1.90 (0.94–2.92) | .87 |
| PFC at 06:00 h, μg/dL | 12.30 (9.64–14.90) | 11.70 (10.35–14.33) | .95 |
| PFC at 12:00 h, μg/dL | 5.69 (4.42–8.44) | 6.50 (5.04–8.70) | .57 |
| PFC at 18:00 h, μg/dL | 3.78 (3.19–5.36) | 3.87 (2.44–4.66) | .49 |
| PRA at diagnosis, ng/mL/h | 0.3 (0.2–0.7) | 0.5 (0.3–0.8) | .40 |
| PRA at 00:00 h, ng/mL/h | 0.3 (0.2–0.7) | 0.3 (0.1–0.6) | .44 |
| PRA at 06:00 h, ng/mL/h | 0.4 (0.2–0.7) | 0.4 (0.2–0.6) | .93 |
| PRA at 12:00 h, ng/mL/h | 0.5 (0.3–1.1) | 0.6 (0.3–0.9) | .79 |
| PRA at 18:00 h, ng/mL/h | 0.4 (0.3–0.8) | 0.4 (0.2–0.6) | .20 |
Data are presented as the median (interquartile range). ACR = urine albumin to creatinine ratio, BMI = body mass index, eGFR = estimated glomerular filtration rate, HbA1c = glycated hemoglobin, HDL = high-density lipoprotein cholesterol, LDL = low-density lipoprotein cholesterol, PAC = plasma aldosterone concentration, PFC = plasma cortisol concentration, PRA = plasma renin activity, REI = respiratory event index.
Table 5.
Spearman correlation between the snoring index and other clinical factors.
| Snoring index | ||||
| Univariate | Adjusted for BMI | |||
| Characteristic | r | P-value | r | P-value |
| REI | 0.39 | .0031 | 0.22 | .10 |
| Age | 0.10 | .46 | ||
| BMI | 0.35 | .0077 | ||
| Systolic blood pressure | −0.10 | .45 | ||
| Diastolic blood pressure | −0.12 | .36 | ||
| Serum potassium | −0.061 | .65 | ||
| HbA1c | 0.069 | .61 | ||
| LDL | 0.029 | .83 | ||
| HDL | −0.40 | .0020 | −0.27 | .047 |
| Triglycerides | 0.36 | .0063 | 0.23 | .094 |
| eGFR | −0.074 | .59 | ||
| ACR | 0.012 | .93 | ||
| PAC at diagnosis | −0.078 | .56 | ||
| PAC at 00:00 h | −0.001 | .99 | ||
| PAC at 06:00 h | −0.22 | .095 | ||
| PAC at 12:00 h | −0.20 | .15 | ||
| PAC at 18:00 h | −0.20 | .14 | ||
| PFC at 00:00 h | −0.16 | .24 | ||
| PFC at 06:00 h | −0.39 | .0027 | −0.29 | .029 |
| PFC at 12:00 h | −0.26 | .049 | −0.23 | .088 |
| PFC at 18:00 h | −0.14 | .32 | ||
| PRA at diagnosis | −0.17 | .22 | ||
| PRA at 00:00 h | −0.044 | .750 | ||
| PRA at 06:00 h | −0.16 | .24 | ||
| PRA at 12:00 h | −0.14 | .30 | ||
| PRA at 18:00 h | −0.031 | .820 | ||
| Urinary aldosterone | −0.036 | .79 | ||
ACR = urine albumin to creatinine ratio, BMI = body mass index, eGFR = estimated glomerular filtration rate, HbA1c = glycated hemoglobin, HDL = high-density lipoprotein cholesterol, LDL = low-density lipoprotein cholesterol, PAC = plasma aldosterone concentration, PFC = plasma cortisol concentration, PRA = plasma renin activity; REI, respiratory event index.
4. Discussion
A recent study demonstrated that OSA is 67.6% prevalent in Caucasian and Chinese patients with PA.[6] However, it has been observed that PAC and AHI are significantly correlated only in Caucasian PA patients, but not in Chinese patients. This result suggests that the relationship between PA and OSA varies between racial groups. However, little is known about the association between PAC and the severity of OSA in Japanese patients with PA or the prevalence of OSA in the Japanese PA population. In this study, we demonstrated that the prevalence of OSA in Japanese patients with PA was 55%. We also showed that there was no significant correlation between hormonal markers of hypertension and the REI, and that BMI was most strongly associated with the REI in the Japanese population.
Our findings are consistent with those of other studies which showed that OSA is a highly prevalent disorder in the general population in Japan, with a reported prevalence of 9% in men and 3% in women.[14,15] Additionally, it has been observed that ∼30% of hypertensive patients suffer from OSA.[16] Therefore, our results suggest that hyperaldosteronism itself may affect the prevalence of OSA. One possible explanation for this relationship that aldosterone might induce the accumulation of fluid, which shifts to the neck in the supine position. Indeed, Gaddam et al[17] showed that when spironolactone was administered for 8 weeks to 12 patients with resistant hypertension and moderate-to-severe OSA, there was a significant improvement in the severity of OSA. Furthermore, AHI was found to decrease in all patients.[17] It has also been speculated that OSA can cause hyperaldosteronism. Saarelainen et al[18] demonstrated that the treatment of untreated hypertensive patients with OSA with 3 months of continuous positive airway pressure decreases aldosterone levels. In agreement with this bidirectional relationship, aldosterone levels and the AHI have been found to correlate in Caucasian patients with PA, but not in the Japanese cohort.[6] It has been suggested that these disparities might be explained by anthropometric differences in craniomandibular anatomy, body fat distribution, and salt intake between Caucasians and Asians.[19–21] Given the fact that the prevalence of OSA was slightly lower in Japanese PA patients (55%) than in Caucasian patients (64.4%),[6] we speculate that the role of aldosterone might be less relevant in the Japanese population, due to predisposing anatomic factors and high salt intake. There was no significant relationship between hormonal markers and the REI, but the REI was significantly positively correlated with BMI, which was also significantly positively correlated with PRA. The strong correlation between BMI and PRA can be explained by 2 hypotheses. Firstly, visceral fat in obese subjects might compresses the thin loops of Henle and vasa recta, decreasing renal tubule flow and medullary blood flow, and increasing fractional NaCl reabsorption in the loops of Henle. Consequently, increased sodium reabsorption could contribute indirectly to increased renin secretion in obese subjects.[22] Alternatively, hyperinsulinemia and cytokines, such as leptin, which are released from adipocytes, might stimulate the sympathetic nervous system, which in turn increases renin secretion.[22,23] Therefore, the effect of BMI should be accounted for when considering the association between hormonal markers and AHI.
In this study, we demonstrated that neither self-reported snoring nor snoring index were associated with aldosterone to renin activity ratio. In addition, there was no significant association between self-reported snoring and snoring index, as measured by portable polysomnography. However, it was previously demonstrated that PA patients with self-reported snoring tend to have a decreased snoring index.[13] Presently, the specific mechanisms of snoring, OSA, and PA, are unknown. Given the high prevalence of OSA in the PA population and the bidirectional relationship between OSA and aldosterone, it might be limiting to examine OSA only in PA patients with self-reported snoring. Therefore, the examination of OSA in all PA patients and the proper management of OSA may be important in the Japanese population.
One of the strengths of our study was that all patients who were diagnosed with PA underwent portable polysomnography. In addition, PAC, PFC, and PRA were evaluated at different time points. However, our study was limited by a relatively small sample size and was retrospective by design. Therefore, a large-scale, multicenter, prospective study is required to confirm the inferred role of aldosterone in OSA. In addition, we did not examine the prevalence of PA in patients with OSA. To ascertain whether all OSA patients should be screened for PA in the Japanese population, the confirmation of the prevalence of PA in patients with OSA is necessary. Furthermore, this study included PA patients who were diagnosed according to Japanese guidelines. Since screening and confirmatory tests for PA differ by country, the different criteria might have biased our results. The usage of a portable polysomnography device to assess OSA might have resulted in the measurement of fewer physiologic variables than in laboratory polysomnography, and affected our conclusions. However, assuming no other suspected sleep disorders, the diagnostic performance appears to be good in populations which are at high risk of moderate-to-severe OSA.[24,25]
In this study, sleep tests were performed only for patients strongly suspected of aldosteronism. However, the absence of a control group makes the interpretation of the results somewhat difficult. However, other studies have reported the prevalence of sleep apnea in the general or hypertensive or hyper aldosteronism population.[6,14,15,16] Therefore, the results of our study can be interpreted by examining the differences with the results of those reports.
In conclusion, we observed a high prevalence of OSA in Japanese patients with PA. Notably, BMI, rather than PAC or PRA, strongly affected the severity of OSA in the Japanese PA population. Therefore, the examination of OSA in patients with PA and the proper management of OSA might be important in the Japanese population.
Author contributions
Formal analysis: Hiroki Kobayashi.
Investigation: Yoshihiro Nakamura, Sho Tanaka, Yoshinari Hatanaka.
Project administration: Hiroki Kobayashi.
Supervision: Yoshinobu Fuke, Noboru Fukuda, Masanori Abe.
Writing – original draft: Yoshihiro Nakamura, Hiroki Kobayashi.
Writing – review & editing: Yoshihiro Nakamura, Hiroki Kobayashi.
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: ACR = albumin to creatinine ratio, AHI = apnea-hypopnea index, BMI = body mass index, eGFR = estimated glomerular filtration rate, HbA1c = glycated hemoglobin, HDL = high-density lipoprotein cholesterol, LDL = low-density lipoprotein cholesterol, OSA = obstructive sleep apnea, PA = primary aldosteronism, PAC = plasma aldosterone concentration, PFC = plasma cortisol concentration, PRA = plasma renin activity, REI = respiratory event index.
How to cite this article: Nakamura Y, Kobayashi H, Tanaka S, Hatanaka Y, Fuke Y, Fukuda N, Abe M. Primary aldosteronism and obstructive sleep apnea: a single-center cross-sectional study of the Japanese population. Medicine. 2021;100:11(e25049).
Disclosure: None of the authors have any potential conflicts of interest associated with this research. This work has not been published previously in any other form.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplemental digital content is available for this article.
References
- [1].Willenberg HS. How to escape from primary aldosteronism? News and views on an adrenal disorder of salt retention. Horm Metab Res 2017;49:151–63. [DOI] [PubMed] [Google Scholar]
- [2].Funder JW, Carey RM, Mantero F, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2016;101:1889–916. [DOI] [PubMed] [Google Scholar]
- [3].Chahal CA, Somers VK. Secondary hypertension: obstructive sleep apnea. J Am Soc Hypertens 2015;9:244–7. quiz 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Florczak E, Prejbisz A, Szwench-Pietrasz E, et al. Clinical characteristics of patients with resistant hypertension: the RESIST-POL study. J Hum Hypertens 2013;27:678–85. [DOI] [PubMed] [Google Scholar]
- [5].Gonzaga CC, Gaddam KK, Ahmed MI, et al. Severity of obstructive sleep apnea is related to aldosterone status in subjects with resistant hypertension. J Clin Sleep Med 2010;6:363–8. [PMC free article] [PubMed] [Google Scholar]
- [6].Buffolo F, Li Q, Monticone S, et al. Primary aldosteronism and obstructive sleep apnea: a cross-sectional multi-ethnic study. Hypertension 2019;74:1532–40. [DOI] [PubMed] [Google Scholar]
- [7].Prejbisz A, Kołodziejczyk-Kruk S, Lenders JWM, et al. Primary aldosteronism and obstructive sleep apnea: is this a bidirectional relationship? Horm Metab Res 2017;49:969–76. [DOI] [PubMed] [Google Scholar]
- [8].Friedman O, Bradley TD, Chan CT, et al. Relationship between overnight rostral fluid shift and obstructive sleep apnea in drug-resistant hypertension. Hypertension 2010;56:1077–82. [DOI] [PubMed] [Google Scholar]
- [9].Acelajado MC, Calhoun DA. Aldosteronism and resistant hypertension. Int J Hypertens 2011;2011:837817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Egan BM, Li J. Role of aldosterone blockade in resistant hypertension. Semin Nephrol 2014;34:273–84. [DOI] [PubMed] [Google Scholar]
- [11].Nishikawa T, Omura M, Satoh F, et al. Guidelines for the diagnosis and treatment of primary aldosteronism--the Japan Endocrine Society 2009. Endocr J 2011;58:711–21. [DOI] [PubMed] [Google Scholar]
- [12].Kushida CA, Morgenthaler TI, Littner MR, et al. Practice parameters for the treatment of snoring and obstructive sleep apnea with oral appliances: an update for 2005. Sleep 2006;29:240–3. [DOI] [PubMed] [Google Scholar]
- [13].Li M, Ge Q, Sheng CS, et al. Clinical characteristics of snoring patients with primary aldosteronism and obstructive sleep apnea-hypopnea syndrome. J Hum Hypertens 2019;33:693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Tanigawa T, Tachibana N, Yamagishi K, et al. Relationship between sleep—disordered breathing and blood pressure levels in community—based samples of Japanese men. Hypertens Res 2004;27:479–84. [DOI] [PubMed] [Google Scholar]
- [15].Cui R, Tanigawa T, Sakurai S, et al. Associations of sleep—disordered breathing with excessive daytime sleepiness and blood pressure in Japanese women. Hypertens Res 2008;31:501–6. [DOI] [PubMed] [Google Scholar]
- [16].Fletcher EC, DeBehnke RD, Lovoi MS, et al. Undiagnosed sleep apnea in patients with essential hypertension. Ann Intern Med 1985;103:190–5. [DOI] [PubMed] [Google Scholar]
- [17].Gaddam K, Pimenta E, Thomas SJ, et al. Spironolactone reduces severity of obstructive sleep apnoea in patients with resistant hypertension: a preliminary report. J Hum Hypertens 2010;24:532–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Saarelainen S, Hasan J, Siitonen S, et al. Effect of nasal CPAP treatment on plasma volume, aldosterone and 24-h blood pressure in obstructive sleep apnoea. J Sleep Res 1996;5:181–5. [DOI] [PubMed] [Google Scholar]
- [19].Li KK, Powell NB, Kushida C, et al. A comparison of Asian and white patients with obstructive sleep apnea syndrome. Laryngoscope 1999;109:1937–40. [DOI] [PubMed] [Google Scholar]
- [20].Yamagishi K, Ohira T, Nakano H, et al. Cross-cultural comparison of the sleep-disordered breathing prevalence among Americans and Japanese. Eur Respir J 2010;36:379–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kario K, Chen CH, Park S, et al. Consensus document on improving hypertension management in Asian patients, taking into account Asian characteristics. Hypertension 2018;71:375–82. [DOI] [PubMed] [Google Scholar]
- [22].Hall JE, do Carmo JM, da Silva AA, et al. Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. Circ Res 2015;116:991–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Leggio M, Lombardi M, Caldarone E, et al. The relationship between obesity and hypertension: an updated comprehensive overview on vicious twins. Hypertens Res 2017;40:947–63. [DOI] [PubMed] [Google Scholar]
- [24].Collop NA, Anderson WM, Boehlecke B, et al. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med 2007;3:737–47. [PMC free article] [PubMed] [Google Scholar]
- [25].El Shayeb M, Topfer LA, Stafinski T, et al. Diagnostic accuracy of level 3 portable sleep tests versus level 1 polysomnography for sleep-disordered breathing: a systematic review and meta-analysis. CMAJ 2014;186:E25–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


