Abstract
Background
Severe acute respiratory syndrome due to coronavirus 2 has rapidly spread worldwide in an unprecedented pandemic. Patients with an ongoing COVID-19 infection requiring surgery have higher risk of mortality and complications. This study describes the mortality and morbidity in patients with perioperative COVID-19 infection undergoing elective and emergency surgeries.
Methods
Prospective cohort of consecutive patients who required a general, gastroesophageal, hepatobiliary, colorectal, or emergency surgery during COVID-19 pandemic at an academic teaching hospital. The primary outcome was 30-day mortality and major complications. Secondary outcomes were specific respiratory mortality and complications.
Results
A total of 701 patients underwent surgery, 39 (5.6%) with a perioperative COVID-19 infection. 30-day mortality was 12.8% and 1.4% in patients with and without COVID-19 infection, respectively (p < 0.001). Major surgical complications occurred in 25.6% and 6.8% in patients with and without COVID-19 infection, respectively (p < 0.001). Respiratory complications occurred in 30.8% and 1.4% in patients with and without COVID-19 infection, respectively (p < 0.001). Mortality due to a respiratory complication was 100% and 11.1% in patients with and without COVID-19 infection, respectively (p < 0.006).
Conclusions
30-day mortality and surgical complications are higher in patients with perioperative COVID-19 infection. Indications for elective surgery need to be reserved for non-deferrable procedures in order to avoid unnecessary risks of non-urgent procedures.
Introduction
Due to coronavirus 2 (SARS-CoV-2), a severe acute respiratory syndrome has rapidly spread globally since the beginning of 2020, affecting most countries in what has become an unprecedented pandemic. The World Health Organization (WHO) declared the COVID-19 pandemic on March 11, 2020. A few days later, Chile declared phase 4 of the pandemic, establishing measures designed to limit the spread of the virus. To focus on human resources and infrastructure, public and private health institutions suspended or postponed elective surgeries, prioritizing patients with surgical emergencies or oncological diseases [1]. By the end of August in Chile, 447,527 COVID-19 cases have been diagnosed, with a rate of 2300 per 100,000 inhabitants [2]. In our center, from March to August, a total of 1098 hospitalized patients have tested positive for COVID-19, with 925 discharges (84%) and 38 patients that remained hospitalized (3.5%) on August 31. In our institution, a total of 135 patients have died due to COVID-19 (12.3%), of which 67% (n = 90) were older than 70 years. Of the hospitalized patients, 23 patients remained in the critical care unit.
Patients undergoing surgery have a greater risk of becoming infected with COVID-19 due to the surgery's pro-inflammatory nature and its associated immunosuppressive response. Mechanical ventilation's deleterious effect facilitates the greater risk of respiratory complications during surgery [3, 4]. Studies have shown an increased risk of complications and mortality in patients with COVID-19 infection undergoing surgery throughout this pandemic. To date, the international collaborative group CovidSurg has the most extensive available data on complications in patients undergoing surgery during this pandemic. The results from 1128 patients from 235 hospitals in 24 countries have shown a high 30-day mortality rate of 23.8%. Respiratory complications occurred in 51.2% of the patients, with a mortality of 38%, which represents 82.6% of all deaths in the study [5]. Other series have also shown high mortality rates between 20 and 25%, with respiratory complications been reported in up to 40–50% of patients [6–15].
The objective of this study is to report 30-day mortality early morbidity, pulmonary complications, and mortality in patients who underwent emergency or elective surgery in our Department of General and Digestive Surgery, including gastroesophageal, HBP, and colorectal surgeries, since the beginning of the pandemic outbreak in Chile.
Methods
Study design
A prospective observational cohort study was conducted at the Hospital Clínico Pontificia Universidad Católica, Chile. The institutional ethics committee approved the present study.
Patient selection
All patients ≥ 15 years old who underwent elective or emergency abdominal surgeries consecutively from March to August of 2020 were included. Esophagogastric, hepatobiliary, colorectal, and general surgery procedures were included.
COVID diagnostic test
COVID-19 infection was determined based on the results of viral SARS-CoV-2 RNA detection by quantitative RT-PCR. Samples were obtained through nasopharyngeal swabs according to the standardized technique. COVID-19 PCR has a specificity close to 100%; however, the sensitivity varies depending on the viral load, the site of sample collection, and the moment that the sample is obtained in the evolution of the disease, it means a lower sensitivity in the first days of infection and higher after seven days of infection [16].
Perioperative infection was defined in all patients with SARS-CoV-2 disease diagnosed within seven days before and 30 days after surgery. For elective patients, the sample was obtained up to 48 h before surgery. A sample was obtained immediately before the transfer to the OR for patients undergoing emergency surgery with a previous negative PCR test or unknown COVID status. During the 30-day follow-up, PCR tests were indicated if the patient developed fever or respiratory symptoms.
Clinical variables and follow-up
Relevant clinical variables included age, sex, comorbidities, body mass index, type of surgery (elective vs. emergency), surgical approach (open vs. laparoscopic), type of anesthesia, hospital length of stay, morbidity, and mortality. Morbidity was categorized according to the Clavien-Dindo classification [17]. Postoperative follow-up was carried out until 30 days after surgery and included outpatient visits, telemedicine, and telephone calls [18].
Primary and secondary outcomes
The primary outcomes were 30-day mortality and major complications. Major complications were defined as Clavien-Dindo score ≥ III. Secondary outcomes included specific respiratory mortality and respiratory complications, defined as pneumonia, respiratory distress, need for non-invasive mechanical ventilation, or invasive mechanical ventilation (IMV). Reoperations and readmissions rates during the 30-days follow-up were also analyzed.
A relative risk (RR) analysis was performed for both primary outcomes, with a 95% confidence interval.
Institutional protocols
The institutional protocols adopted for the care of patients undergoing emergency or elective surgical procedures with unknown or confirmed COVID-19 infection, from arrival to the emergency service, ward, and postoperative recovery, are detailed in Table 1 [19]. The protocol includes COVID-19 sample collection, admission to a specific ward for COVID-19 (+) patients, restriction of healthcare personnel, avoiding unnecessary flow in the ward, pneumoperitoneum evacuation protocol, and differentiated sample transport. For COVID-19 (+) patients or patients with pending PCR results at the time of surgery, they were transferred to a special recovery unit and then to a differentiated basic ward. After surgery, patients with ICU requirements were transferred to a differentiated ICU unit exclusive for patients with COVID-19 infection.
Table 1.
Institutional protocols adopted for the care of patients undergoing surgical procedures with unknown or confirmed COVID-19 infection
| Operation room (OR) preparation |
|
Use the same assigned OR Minimum of people in the OR Only necessary items for surgery The use of cell phones is prohibited inside the OR Maintain the OR with positive pressure Handling of supplies outside the OR Ensure a filter presence in the expiratory tract and between the tube and the circuit Use signs to indicate contact and droplet caution Ask for the patient once the OR is ready for expedited access Patients' transfer should always be with a mask |
| Patient management in the OR |
|
Environmental management during anesthetic induction: doors closed, access limitation, preferential use of disposable materials Surfaces cleaning using quaternary ammonium The anesthesia team must use an N95 mask, face shield or googles, disposable cap and shoe covers, disposable plastic long sleeve bib, and procedure gloves The surgery team must use scrub clothing, disposable plastic bib, N95 mask, face shield or googles, disposable cap and shoe covers |
| Ending the procedure |
|
Patients confirmed or suspected with COVID-19 destined to low complexity services must accomplish recovery time in the OR Patients with ICU requirements are immediately transferred with the same personal team to a previously reserved bed and with the respective isolation Surgery, anesthesia and nursing teams must remove their PPE inside the OR using procedure gloves and a subsequent hand wash Disinfection of face shields and googles with quaternary ammonium |
| Minimally invasive procedures |
|
Skin incision according to trocar diameter to avoid CO2 leakage by counter-opening Intraabdominal pressure as minimum as possible. Recommended 10–12 mmHg Use of a safe filter aspiration/exufflation system with a Luer-lock connection to the pneumoperitoneum Pneumoperitoneum safe evacuation and at the moment of piece extraction To avoid the retrograde passage of gas into the system, the insufflating trocar valve was closed before disconnection or turning off the insufflation at the end of the surgery Pneumoperitoneum should be wholly evacuated through the filter safe aspiration/exsufflation system with all the trocars in position and before its removal Extraction of the piece following the complete evacuation of CO2 and gas Use of surgical drainage only if mandatory Aponeurosis closure after exufflation Avoid using automatic closing devices Consider the open approach if any of the above requirements cannot be accomplished |
Statistical analysis
Statistical analysis was performed with SPSS 25 (IBM Corp., Amonk, New York). Patients with COVID-19 infection and without COVID-19 were compared with Student's t-test or Mann–Whitney test depending on data distribution, and chi-square test for comparison of proportions. All data were expressed as mean and standard deviation or median and interquartile range. Differences were considered significant when p value < 0.05.
Results
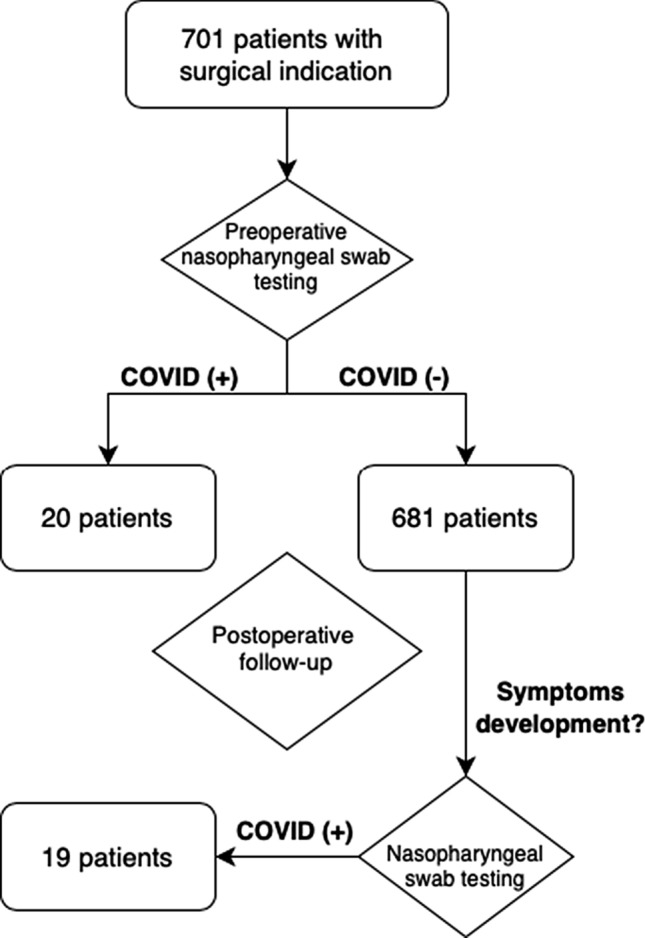
From March to August of 2020, during the ongoing COVID-19 outbreak in Chile, 701 patients underwent a general, esophagogastric, hepatobiliary, or coloproctological surgery at our university teaching affiliated hospital. The mean age of patients was 51 ± 18 years, and 343 (49%) were women. At least one comorbidity at the time of the surgery was found in 50.1% of patients (Table 2). 124 out of 701 surgeries were oncological procedures (17.7%, Table 2). Perioperative SARS-CoV-2 infection was detected in 39 patients (5.6%), in 20 of them (51.3%) were detected before surgery (Fig. 1).
Table 2.
Demographic variables
| Total | COVID-19 (+) | COVID-19 (−) | p value | |
|---|---|---|---|---|
| Patients | 701 | 39 | 662 | – |
| Age; mean ± SD | ||||
| Years | 51 ± 18.4 | 46 ± 19.4 | 52 ± 18.4 | 0.124 |
| Sex (%) | ||||
| Male | 358 (51%) | 19 (48.7%) | 339 (51.2%) | 0.890 |
| Female | 343 (49%) | 20 (51.3%) | 323 (48.8%) | |
| ASA; n (%) | ||||
| I–II | 649 (92.6%) | 34 (87.2%) | 615 (92.9%) | 0.308 |
| ≥ III | 52 (7.4%) | 5 (12.8%) | 47 (7.1%) | |
| BMI; n (%) | ||||
| ≤ 18,5 | 16 (2.3%) | 3 (7.7%) | 13 (2%) | 0.043 |
| 18,5–24,9 | 257 (36.7%) | 15 (38.5%) | 242 (36.6%) | |
| 25–29,9 | 275 (39.2%) | 10 (25.6%) | 265 (40%) | |
| 30–39,9 | 139 (19.8%) | 11 (28.2%) | 128 (19.3%) | |
| ≥ 40 | 14 (2%) | 0 (0%) | 14 (2.1%) | |
| Comorbidities; n (%) | ||||
| None | 350 (49.9%) | 19 (48.7%) | 331 (50%) | 0.876 |
| One or more | 351 (50.1%) | 20 (51.3%) | 331 (50%) | |
| Hypertension | 174 (24.8%) | 11 (28.2%) | 163 (24.6%) | 0.615 |
| Metabolic disease* | 96 (13.7%) | 5 (12.8%) | 91 (13.7%) | 0.870 |
| Respiratory disease | 45 (6.4%) | 3 (7.7%) | 42 (6.3%) | 0.738 |
| Cardiovascular disease | 46 (6.6%) | 3 (7.7%) | 43 (6.5%) | 0.769 |
| Neurological disease | 17 (2.4%) | 1 (2.6%) | 16 (2.4%) | 0.953 |
| Oncological** or immunosuppression | 57 (8.1%) | 5 (12.8%) | 52 (7.9%) | 0.271 |
| Cancer***, n (%) | ||||
| Yes | 124 (17.7%) | 8 (20.5%) | 116(17.5%) | 0.634 |
| No | 577 (82.3%) | 31 (79.5%) | 546 (82.5%) | |
SD standard deviation, ASA American society of anesthesia classification, BMI body mass index.
*Includes diabetes, hypothyroidism, obesity, or dyslipidemia. **Includes cancer diagnosis not related to the surgical indication. ***Includes cancer diagnosis related to the surgical indication
Fig. 1.
The COVID-19 detection process
Most of the procedures (368, 52.5%) were surgical emergencies, which were more frequent in patients with COVID-19 infection (71.8% COVID-19 (+) vs. 51.4% COVID-19 (−), p = 0.03) (Table 3). General anesthesia was used in 653 (93.2%) of surgeries. A total of 463 (66%) surgeries were performed by a minimally invasive approach, either laparoscopic and/or endoscopic, under general anesthesia (ERCP, rendezvous procedure, others). The three most common surgeries performed were 191 (27.2%) cholecystectomies, 129 (18.4%) appendectomies, and 90 (12.8%) colorectal surgeries (Table 3).
Table 3.
Operative variables
| Total | COVID-19 (+) | COVID-19 (−) | p value | |
|---|---|---|---|---|
| Patients | 701 | 39 | 662 | – |
| Urgency of surgery; n (%) | ||||
| Emergency | 368 (52.5%) | 28 (71.8%) | 340 (51.4%) | 0.030 |
| Elective | 333 (47.5%) | 11 (28.2%) | 322 (48.6%) | |
| More frequent surgeries; n (%) | ||||
| Cholecystectomy | 191 (27.2%) | 10 (25.6%) | 181 (27.3%) | 0.625 |
| Appendectomy | 129 (18.4%) | 9 (23.1%) | 120 (18.1%) | |
| Colorectal surgery | 90 (12.8%) | 3 (7.7%) | 87 (13.1%) | |
| Proctological surgery | 68 (9.7%) | 3 (7.7%) | 65 (9.8%) | |
| Hepatobiliary surgery | 57 (8.1%) | 4 (10.3%) | 53 (8%) | |
| Exploratory laparotomy | 49 (7%) | 5 (12.8%) | 44 (6.6%) | |
| Surgical approach; n (%) | ||||
| Open | 238 (34%) | 11 (28.2%) | 227 (34.3%) | 0.435 |
| Minimally invasive* | 463 (66%) | 28 (71.8%) | 435 (65.7%) | |
| Anesthesia; n (%) | ||||
| General | 653 (93.2%) | 36 (92.3%) | 617 (93.2%) | 0.744 |
| Local/regional | 48 (6.8%) | 3 (7.7%) | 45 (6.8%) | |
| Length of stay; median (IQR) | ||||
| Days | 2 (1–4) | 2 (1–10) | 2 (1–4) | 0.020 |
IQR interquartile range.
*Laparoscopic and/or endoscopic under general anesthesia
Overall 30-day mortality was 2% (n = 14). However, in patients with COVID-19 infection, 30-day mortality was higher (12.8% (5/39) COVID-19 (+) versus. 1.4% (9/662) COVID-19 (−), p < 0.001) (Table 4). Among patients with COVID-19 (+) infection who died, in all of them, the cause of death was a consequence of a respiratory complication. In contrast, in patients without COVID-19 infection, a respiratory complication was the cause of death only in 11.1% (1/9) of patients (100% (5/5) COVID-19 (+) vs. 11.1% (1/9) COVID-19 (−) , p = 0.006). Baseline characteristics of the five patients with mortality in the COVID-19 (+) group are described in Table 5.
Table 4.
Postoperative outcomes
| Total | COVID-19 (+) | COVID-19 (−) | p value | |
|---|---|---|---|---|
| Patients | 701 | 39 | 662 | – |
| 30-day mortality; n (%) | ||||
| Yes | 14 (2%) | 5 (12.8%) | 9 (1.4%) | < 0.001 |
| No | 687 (98%) | 34 (87.2%) | 653 (98.6%) | |
| Respiratory mortality; n (%) | ||||
| Yes | 6 (42.9%) | 5 (100%) | 1 (11.1%) | 0.006 |
| No | 8 (57.1%) | 0 (0%) | 8 (88.9%) | |
| Postoperative complications; n (%) | ||||
| Without complications | 601 (85.7%) | 21 (53.9%) | 580 (87.6%) | < 0.001 |
| Clavien-Dindo I-II | 45 (6.4%) | 8 (20.5%) | 37 (5.6%) | |
| Clavien-Dindo ≥ III | 55 (7.9%) | 10 (25.6%) | 45 (6.8%) | |
| Respiratory morbidity; n (%) | ||||
| Yes | 21 (3%) | 12 (30.8%) | 9 (1.4%) | < 0.001 |
| No | 680 (97%) | 27 (69.2%) | 653 (98.6%) | |
| IMV requirement | ||||
| Yes | 8 (38.1%) | 6 (50%) | 2 (22.2%) | 0.401 |
| No | 13 (61.9%) | 6 (50%) | 7 (77.8%) | |
| Readmission | ||||
| Yes | 34 (4.9%) | 8 (20.5%) | 26 (3.9%) | < 0.001 |
| No | 667 (95.1%) | 31 (79.5%) | 636 (96.1%) | |
| Readmission by cause | ||||
| Medical | 16 (47.1%) | 5 (62.5%) | 11 (42.3%) | 0.551 |
| Surgical | 18 (52.9%) | 3 (37.5%) | 15 (57.7%) | |
| Reoperation | ||||
| Yes | 31 (4.4%) | 2 (5.1%) | 29 (4.4%) | 0.825 |
| No | 670 (95.6%) | 37 (94.9%) | 633 (95.6%) | |
IMV invasive mechanical ventilation
Table 5.
Baseline characteristics of patients with mortality in the COVID-19 (+) group
| Patients | Sex | Age | BMI | Comorbidities | Diagnosis | ASA | Anesthesia | Surgery | Approach | LOS | COVID-19 (+) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A | M | 71 | 30–40 | Hypertension, metabolic | Perforated hollow viscus | II | General | Exploratory laparotomy | Open | 20 | Preoperative |
| B | M | 84 | 30–40 | Hypertension, metabolic | Acute cholecystitis | II | General | Cholecystectomy | MIS | 6 | Postoperative |
| C | M | 76 | 18,5–25 | Hypertension, neurological | Acute cholecystitis | III | General | Cholecystectomy | MIS | 2 | Postoperative |
| D | M | 80 | < 18,5 | Hypertension, cardiovascular | Gastric cancer | III | General | Exploratory laparoscopy | MIS | 2 | Postoperative |
| E | M | 69 | 25–30 | Hypertension, cardiovascular, metabolic | Acute cholecystitis + | ||||||
| Choledocholithiasis | II | General | Cholecystectomy | MIS | 16 | Preoperative |
BMI body mass index, ASA American society of anesthesia classification, MIS minimally invasive surgery, LOS lenght of stay
Major complications occurred in 7.9% (n = 55) of patients and were more common in patients with COVID-19 infection (25.6% (10/39) COVID-19 (+) versus 6.8% (45/662) COVID-19 (−), p < 0.001). Respiratory complications were more frequent in patients with COVID-19 infection (30.8% (12/39) COVID-19 (+) versus 1.4% (9/662) COVID-19 (−), p < 0.001). No difference was observed in terms of need of invasive mechanical ventilation (Table 4).
In the subgroup analysis of the COVID-19 (+) patients, 20 had a preoperative diagnosis and 19 were diagnosed after surgery. Among the 20 patients diagnosed before surgery, seven were symptomatic and 13 asymptomatic. Five patients in the symptomatic group developed complications. No morbidity was reported in the asymptomatic patients. Of the 19 patients diagnosed after surgery, all were symptomatic and 13 developed postoperative complications. Postoperative outcomes in the COVID-19 (+) group are detailed in Table 6.
Table 6.
Subgroup analysis of COVID-19 (+) patients
| Outcome | Preoperative COVID-19 (n = 20) | Postoperative COVID-19 (n = 19) | ||
|---|---|---|---|---|
| Symptomatic (n = 7) | Asymptomatic (n = 13) | Symptomatic (n = 19) | Asymptomatic (n = 0) | |
| 30-day mortality | 2 (10%) | 0 (0%) | 3 (15.8%) | – |
| 30-day morbidity | 5 (25%) | 0 (0%) | 13 (68.4%) | – |
| Major complications* | 2 (10%) | 0 (0%) | 8 (42.1%) | – |
| Respiratory complications | 4 (20%) | 0 (0%) | 8 (42.1%) | – |
| IMV requirement | 2 (10%) | 0 (0%) | 4 (21.1%) | – |
IMV invasive mechanical ventilation
*Clavien-Dindo ≥ III
In the whole series, 31 patients (4.4%) required an unplanned reoperation, without differences between both groups (5.1% (2 of 39) COVID-19 (+) vs 4.4% (29 of 662) COVID-19 (−), p = 0.825)) (Table 4). The causes of reoperations in the COVID-19 (+) group were an exploratory laparoscopy after adhesiolysis in a patient with a small bowel obstruction and a postoperative abscess drainage.
During 30-day follow-up after hospital discharge, 34 (4.9%) patients were readmitted. The cause of hospital readmission was surgical in 18 (52.9%) and medical in 16 (47.1%) patients. No differences were observed between patients with and without COVID-19 infection (Table 4).
One hundred and twenty-four patients (17.7%) had an oncological diagnosis; among them, SARS-CoV-2 infection was detected in eight patients (20.5%). One patient (0.8%) with a sigmoid adenocarcinoma and severe COVID pneumonia got his surgery delayed.
In the RR analyses, patients with COVID-19 infection had a 9.4 times higher risk of dying after surgery than patients without COVID-19 infection (RR 9.43 [95% CI 3.31–26.79], p < 0.001). Excluding patients with postoperative COVID-19 infection, patients with preoperative COVID-19 infection had 7.3 times the risk of dying after surgery than patients without COVID-19 infection (RR 7.35 [95% CI 1.69–31.86], p = 0.007) (Table 7). Concerning surgical complications, patients with COVID-19 infection had 3.7 times the risk to develop a major complication after surgery than patients without COVID-19 infection (RR 3.77 [95% CI 2.06–6.90], p < 0.001). However, when patients with postoperative COVID-19 infection are excluded from the analysis, no differences are observed regarding to risk of major complication between patients with and without COVID-19 infection (RR 1.47 [95% CI 0.38–5.64], p = 0.573) (Table 7).
Table 7.
Relative risk analysis
| Outcome | COVID-19 (+) | COVID-19 (−) | RR | 95% CI | p value |
|---|---|---|---|---|---|
| 30-day mortality | 5/39 | 9/662 | 9.43 | 3.31–26.79 | < 0.001 |
| 30-day mortality, excluding postoperative COVID | 2/20 | 9/662 | 7.35 | 1.69–31.86 | 0.007 |
| Major complications* | 10/39 | 45/662 | 3.77 | 2.06–6.90 | < 0.001 |
| Major complications*, excluding postoperative COVID | 2/20 | 45/662 | 1.47 | 0.38–5.64 | 0.573 |
*Clavien-Dindo ≥ III
Discussion
It has been reported that patients undergoing surgery during the ongoing COVID-19 pandemic are at increased risk of complications and mortality. This study sought to report 30-day perioperative outcomes in patients undergoing emergency and oncological surgeries during the COVID-19 outbreak at our university hospital center in Chile. We found that patients with SARS-CoV-2 infection had higher mortality and severe surgical complications. Patients with COVID-19 infection also had a higher risk of respiratory complications, which was the cause dead in all patients with COVID-19 infection who died. Our results confirmed the high risk of mortality and complications in patients undergoing surgery during the COVID-19 pandemic.
Our results are in concordance with those reported in other studies [5–15], indicating that patients with COVID-19 infection undergoing surgery are at higher risk of mortality, mainly due to respiratory complications. In this study, 30-day mortality was 12.8% in patients with COVID-19 infection, which is a rate lower mortality than the report of other reviews [5, 6, 8, 9, 13]. Respiratory complications occurred in 30.8% of patients with COVID-19 infection, whereas patients without COVID-19, only appeared in 1.4%. This higher respiratory complication rate compares with other reports that have observed higher respiratory complications in patients with COVID-19 infection [5, 6, 8, 9, 13]. In the most significant study published to date by the CovidSurg group, the overall 30-day mortality was 23.8%, with 51.2% of respiratory complications and a mortality rate from respiratory complications of 38% [5]. Other studies report general mortality rates ranging from 12.5 to 83.3%, with respiratory morbidity even up to 100% [6–15]. An Italian series of 41 patients infected with COVID-19 who underwent surgery describe overall mortality of 19.5% and respiratory morbidity of 59% [8]. A retrospective study from Spain analyzed morbidity and mortality in patients operated on until the pandemic's peak in that country, reports mortality of 20% in COVID-19 patients and severe respiratory morbidity of 27% [13].
Similarly, a Chinese series that included 34 patients with a perioperative diagnosis of COVID-19 infection undergoing elective surgery reports a mortality rate of 20.5%, requiring ICU management for respiratory failure in 44.1% of patients [6]. The available literature is summarized in Table 8. The observed differences across studies can be attributed to a variety of factors such as the implemented protocols, the period and severity of the pandemic, variations in healthcare policies implemented by different countries, individuals between hospital centers and resources, to name a few.
Table 8.
Summary of available evidence of surgical outcomes during COVID-19 pandemic
| Author | Period (2020) | Total | COVID-19 (+) | COVID-19 mortality | Respiratory morbidity | Major complications* |
|---|---|---|---|---|---|---|
| COVIDSurg collaborative | January-March | 1128 | 1128 | 23.8% (n = 268) | 51.2% (n = 577) | – |
| Doglietto et al | February-April | 333 | 41 | 19.5% (n = 8) | 58.5% (n = 24) | 41.5% (n = 17) |
| Seretis et al | March–May | 100 | 3 | 0% | 66.6% (n = 2) | – |
| Lei et al | January–February | 34 | 34 | 20.5% (n = 7) | 44.1% (n = 15) | – |
| Di Martino et al | February–March | 213 | 15 | 20% (n = 3) | 33% (n = 5) | 26.6% (n = 4) |
| Aminian et al | February | 4 | 4 | 50% (n = 2) | 100% (n = 4) | – |
| Shrikhande et al | March–April | 494 | 6 | 0% | – | – |
| Seeliger et al | March–May | 127 | 13 | 23% (n = 3) | 61.5% (n = 8) | 53.8% (n = 7) |
| Carpio Colmenares et al | March–June | 59 | 1 | 0% | – | 5.1% (n = 3) |
| González-Catalayud et al | April–July | 42 | 36 | 42.8% (n = 18) | – | – |
*Clavien-Dindo ≥ III
Compared to the results reported in other series, our study's lower mortality and complication rates could be attributed to the fast and strict implementation of preventive measures within our institution from the beginning of the pandemic outbreak. In our opinion, the development of a multidisciplinary COVID-19 committee was essential for the development, implementation, and surveillance of protocols aimed at minimizing the risk of in-hospital contagion between patients and healthcare professionals. Other measures included preventive isolation of patients, continuous training of personnel, mandatory use of personal protective equipment, rotating preventive quarantines of health teams, and restriction of visits to patients. Likewise, in all patients with or without respiratory symptoms, samples were obtained to detect COVID-19 upon admission. While the PCR result was pending, they were isolated and managed as COVID-19 (+) patients until confirmed or discarded.
There are few experiences reported in Latin America regarding surgical outcomes during the COVID-19 pandemic. Carpio Colmenares et al. published a Peruvian experience including 59 patients who underwent emergency abdominal laparoscopic surgery during 2020, and they compared this group of patients with a control group of patients who underwent surgery in 2019 [20]. They found no statistically significant differences regarding age, sex, duration of symptoms, the severity of surgical illness, and postoperative morbidity rate. Patients who underwent surgery during the COVID-19 pandemic had the most prolonged postoperative stay, and only one patient had a perioperative COVID-19 infection. The postoperative morbidity rate was 45.7% in the 2020 group, far higher than the 14.3% reported in our series; nevertheless, in our study, patients with both elective and emergency procedures were included, so both samples are not comparable. Gonzalez-Calatayud et al. [21] described their experience in a tertiary center in Mexico, including all patients with suspected or confirmed SARS-CoV-2 infection who underwent surgery. They report a mortality rate of 42.8%, but they included patients undergoing a vast range of surgical procedures like C-section, tracheostomy, and general surgery procedures.
Finally, many Latin American countries had to face a lack of resources and deficient infrastructure during this pandemic. In Peru, for example, there is only one center that has rooms with negative pressure equipment [22]. As a result, Latin American countries had difficulties achieving the requirements and guidelines set forth by professional societies from the rest of the world.
Concerning in-hospital COVID-19 infection, a study by Luong-Nguyen et al. reported that the risk augmented as the length of hospitalization increases has shown by 80% of in-hospital infection of COVID-19 in patients with hospital stay higher than 14 days (15–63 days), with general mortality of 13.3% [7]. These patients' infection could be directly related to hospital contagion, both by health personnel, support personnel, or patients accompanying them. In our study, COVID-19 infection was diagnosed during postoperative follow-up in 19 patients, 6 (31.5%) of which occurred in patients with prolonged hospital stay between 20 to 87 days.
The cohort analyzed in this study included emergency operations as well as selected oncologic ones. None of the oncological patients in this cohort was deferred in their surgical treatment due to the pandemic. We report the case of a patient with severe COVID pneumonia, with thromboembolic events including a stroke during ICU hospitalization, who developed a first episode of rectorrhagia in the context of anticoagulation therapy. A colonoscopy detected a sigmoid adenocarcinoma (pT3N1bM0, IIIB staging). The oncological committee decided to defer the surgery until the patient was in better conditions during the hospital stay. A week later, the patient presented a second episode of lower GI bleeding with hemodynamic compromise. An emergency Hartmann surgery was performed. The patient recovered satisfactorily from surgery. Due to COVID-19 sequels and neurological sequels had prolonged hospitalization. The adjuvant treatment was deferred initially until the patient achieved a better performance status.
According to a subgroup analysis in the COVID-19 (+) group, those asymptomatic patients diagnosed prior to surgery (13 patients) had better outcomes, with no morbidity or mortality reported. In contrast, in symptomatic patients with preoperative infection (seven patients), five morbidities were diagnosed, four of them with respiratory morbidity, including two with IMV requirement who subsequently died by respiratory cause. In the group of COVID-19 (+) patients diagnosed in the postoperative follow-up (19 patients), all were symptomatic. Thirteen patients developed morbidities, eight had respiratory morbidities, four with IMV requirement, and three of them died by respiratory cause. Taking all this into account, we can say that asymptomatic patients with preoperative COVID-19 infection were the ones with better outcomes, in contrast with those symptomatic in the preoperative or postoperative period that had the worst outcomes.
Our study has limitations and strengths. One limitation is that our cohort only included patients with esophagogastric, hepatobiliary, colorectal, or general abdominal surgeries, excluding procedures performed by other surgical specialties. Although this could lead to a selection bias, it allows us to analyze the perioperative results of a specific group of commonly performed procedures in surgical practice. Another limitation is the low prevalence of patients with COVID-19 infection compared to other series, which could also influence the lower rates of mortality and complications. The lower mortality and complications could also be attributed to the fact that our center is a private high complexity hospital. The availability of operation rooms, personal protection supplies, highly trained health care professionals, and specific isolation areas was only slightly compromised. Thus, critical care bed availability was maintained despite the high transitory demand during the pandemic's most critical periods.
Our results summarize the clinical outcomes of consecutive patients operated who underwent surgery during the first six months of the COVID-19 pandemic outbreak period in Chile. Various surgical services worldwide have adapted to minimize the risks in their patients [23, 24]. In this context, we provide our experience that can help others center to establish a decision-making process on surgical patients management during the pandemic. New studies and other experimental designs will be necessary to reliably determine the behavior and risk factors related to perioperative infection by COVID-19.
In conclusion, 30-day mortality, specific respiratory mortality, and major surgical complications are higher in patients with perioperative COVID-19 infection. The observed lower rate of mortality and complications than the reported in other studies could be explained to the implementation and fulfillment of strict systematized protocols for preventive isolation, protection of personnel, preventing isolation of patients, and PCR screening of all patients admission to discharge. As more countries are facing the second wave of COVID-19 infection, we hope that the lessons learned during the first wave will help to improve surgical outcomes during the ongoing current COVID-19 pandemic.
Funding
This study did not receive any funding.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The institutional ethics committee approved the present study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Surgeons (2020) ACo local resumption of elective surgery guidance. American College of Surgeons
- 2.Departamento de Epidemiología MdS 45º Informe Epidemiológico Enfermedad por COVID-19, Santiago de Chile, MINSAL, 2020
- 3.Besnier E, Tuech JJ, Schwarz L. We asked the experts: covid-19 outbreak: is there still a place for scheduled surgery? "Reflection from pathophysiological data". World J Surg. 2020;44:1695–1698. doi: 10.1007/s00268-020-05501-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collaborative C. Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. Lancet. 2020;396:27–38. doi: 10.1016/S0140-6736(20)31182-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lei S, Jiang F, Su W, et al. Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID-19 infection. EClinicalMedicine. 2020;21:100331. doi: 10.1016/j.eclinm.2020.100331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luong-Nguyen M, Hermand H, Abdalla S, et al. Nosocomial infection with SARS-Cov-2 within departments of digestive surgery. J Visc Surg. 2020;157:S13–S18. doi: 10.1016/j.jviscsurg.2020.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doglietto F, Vezzoli M, Gheza F, et al. Factors associated with surgical mortality and complications among patients with and without coronavirus disease 2019 (COVID-19) in Italy. JAMA Surg. 2020;155(8):691–702. doi: 10.1001/jamasurg.2020.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aminian A, Safari S, Razeghian-Jahromi A, et al. COVID-19 outbreak and surgical practice: unexpected fatality in perioperative period. Ann Surg. 2020;272:e27–e29. doi: 10.1097/SLA.0000000000003925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cai M, Wang G, Zhang L, et al. Performing abdominal surgery during the COVID-19 epidemic in Wuhan, China: a single-centred, retrospective, observational study. Br J Surg. 2020;107:e183–e185. doi: 10.1002/bjs.11643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu D, Zhang P, Wang L, et al. Emergency abdominal surgery in COVID-19 patients: a note of caution from Wuhan. Br J Surg. 2020;107:e262. doi: 10.1002/bjs.11722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McDermott A, O'Kelly J, de Barra E, et al. Perioperative outcomes of urological surgery in patients with SARS-CoV-2 infection. Eur Urol. 2020;78:118–120. doi: 10.1016/j.eururo.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Martino M, Septiem JG, González RM, Nova JL, Rodríguez Hoz, Bonito AC, Martín-Pérez E. Elective surgery during the SARS-CoV-2 pandemic (COVID-19): morbidity analysis and recommendations on patient prioritization and safety measures. Span Surg. 2020;98(9):525–32. doi: 10.1016/j.ciresp.2020.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallego MÁ, de las Casas SG, Migueláñez IP, Rubio-Pérez I, Serrano CB, Peña EÁ, Domínguez JD. Impacto de la pandemia por SARS-CoV-2 sobre la actividad y profesionales de un Servicio de Cirugía General y del Aparato Digestivo en un hospital terciario. Cir Esp. 2020;98(6):320–7. doi: 10.1016/j.ciresp.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pérez-Rubio Á, Sebastián Tomás JC, Navarro-Martínez S, et al. Incidence of surgical abdominal emergencies during SARS-CoV-2 pandemic. Cir Esp. 2020;98(10):618–24. doi: 10.1016/j.ciresp.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang Y, Yang M, Shen C, et al. Evaluating the accuracy of different respiratory specimens in the laboratory diagnosis and monitoring the viral shedding of 2019-nCoV infections. MedRxiv. 2020;78(3):241. [Google Scholar]
- 17.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Irarrázaval MJ, Inzunza M, Muñoz R, et al. Telemedicine for postoperative follow-up, virtual surgical clinics during COVID-19 pandemic. Surg Endosc. 2020 doi: 10.1007/s00464-020-08130-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pattillo JC, Rabagliati R (2020) Atención de pacientes confirmados COVID-19, casos sospechosos COVID-19 o contacto de alto riesgo en pabellón. Santiago de Chile, Red Salud UC-CHRISTUS
- 20.Carpio Colmenares YT, Ruiz Cárdenas, de Castilla D, García Barrionuevo LA, et al. Emergency abdominal laparoscopic surgery during the coronavirus disease 2019 pandemic: experience in a private center in Peru. J Laparoendosc Adv Surg Tech A. 2020;31(3):261–265. doi: 10.1089/lap.2020.0917. [DOI] [PubMed] [Google Scholar]
- 21.González-Calatayud DM, Vargas-Ábrego DB, Gutiérrez-Uvalle DGE, et al. Observational study of the suspected or confirmed cases of sars COV-2 infection needing emergency surgical intervention during the first months of the pandemic in a third level hospital: case series. Ann Med Surg (Lond) 2020;60:149–154. doi: 10.1016/j.amsu.2020.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Panduro-Correa V, Arteaga-Livias K, Rodríguez-Morales AJ. Coronavirus disease 2019 (COVID-19) and surgical recommendations in latin America. Am Surg. 2020;86:596–598. doi: 10.1177/0003134820927313. [DOI] [PubMed] [Google Scholar]
- 23.Elizabeth Brindle M, Gawande A. Managing COVID-19 in surgical systems. Ann Surg. 2020;272:e1–e2. doi: 10.1097/SLA.0000000000003923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heffernan DS, Evans HL, Huston JM, et al. Surgical infection society guidance for operative and peri-operative care of adult patients infected by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) Surg Infect (Larchmt) 2020;21:301–308. doi: 10.1089/sur.2020.101. [DOI] [PubMed] [Google Scholar]


