Abstract
Aims
There is inconsistent evidence on the relation of alcohol intake with incident atrial fibrillation (AF), in particular at lower doses. We assessed the association between alcohol consumption, biomarkers, and incident AF across the spectrum of alcohol intake in European cohorts.
Methods and results
In a community-based pooled cohort, we followed 107 845 individuals for the association between alcohol consumption, including types of alcohol and drinking patterns, and incident AF. We collected information on classical cardiovascular risk factors and incident heart failure (HF) and measured the biomarkers N-terminal pro-B-type natriuretic peptide and high-sensitivity troponin I. The median age of individuals was 47.8 years, 48.3% were men. The median alcohol consumption was 3 g/day. N = 5854 individuals developed AF (median follow-up time: 13.9 years). In a sex- and cohort-stratified Cox regression analysis alcohol consumption was non-linearly and positively associated with incident AF. The hazard ratio for one drink (12 g) per day was 1.16, 95% CI 1.11–1.22, P < 0.001. Associations were similar across types of alcohol. In contrast, alcohol consumption at lower doses was associated with reduced risk of incident HF. The association between alcohol consumption and incident AF was neither fully explained by cardiac biomarker concentrations nor by the occurrence of HF.
Conclusions
In contrast to other cardiovascular diseases such as HF, even modest habitual alcohol intake of 1.2 drinks/day was associated with an increased risk of AF, which needs to be considered in AF prevention.
Keywords: Alcohol consumption, Atrial fibrillation, Epidemiology, Biomarkers
Graphical Abstract
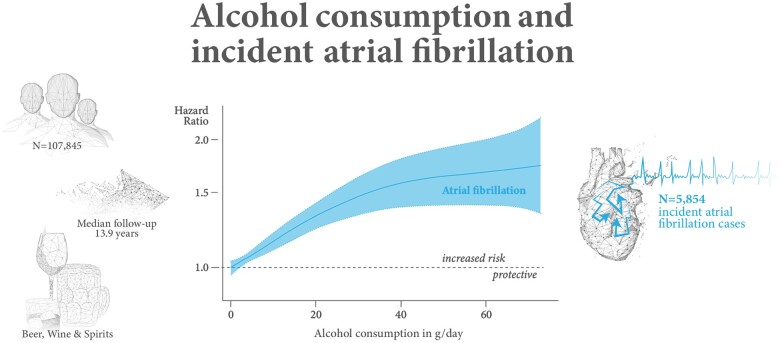
Hazard ratio for incident atrial fibrillation for alcohol consumption in gram per day by non-linear Cox regression plotted on the log-scale (N = 92 452). The model uses age as time scale and is sex and cohort stratified. The reference value is 0 g/day.
See page 1178 for the editorial comment on this article (doi: 10.1093/eurheartj/ehaa955)
Introduction
Atrial fibrillation (AF) is an arrhythmia with a major impact on public health due to its increasing prevalence in ageing populations and its association with adverse outcomes, including stroke and heart failure (HF), with more than a doubling of mortality risk.1 , 2 The effect of alcohol on AF risk has remained ambiguous. For diseases predisposing to AF such as coronary artery disease3 or HF4 , 5 low to moderate alcohol consumption seems to be related to a lower incidence, while higher levels of consumption are associated with an increased risk.6 The reported associations with AF range from null associations at lower regular alcohol intake,5 , 7 rather linearly increasing in large meta-analyses8 , 9 to a more J-shaped relation in women.1 In particular, the association at low levels of alcohol consumption is less clear.
From a pathophysiological perspective, alcohol may exhibit direct effects on arrhythmogenesis as observed for the holiday heart syndrome.10–13 Acute alcohol consumption induces autonomic imbalance reflected by sinus tachycardia, predisposing to arrhythmia.10 Electrolyte disturbance and alterations of the acid-base balance are further pro-arrhythmic triggers. Chronic alcohol consumption is known to be correlated with changes in cardiac structure and function including cardiomyopathy.4 , 11–13
Habitual alcohol intake has been related to atrial remodelling as an intermediate AF phenotype in the community.12 , 13 At the same time, alcohol intake is also associated with the most prevalent risk factors of AF. Increased alcohol intake is accompanied by higher frequency of hypertension and obesity.14 In younger individuals with low-risk factor burden and heart disease, acute excessive alcohol consumption was not associated with higher AF burden.10 Furthermore, alcohol consumption is predictive of incident HF, which itself is a risk factor for new-onset AF4 , 6 and may help explain known associations.
Circulating cardiac biomarkers are quantitative measures which shed light on current cardiac pathophysiology. Troponin reflects myocardial injury, while N-terminal pro-B-type natriuretic peptide (NT-proBNP) indicates often chronic, subclinical wall stress.15 A recent study demonstrated that both biomarkers showed distinct patterns in relation to alcohol consumption.15 Whereas troponin concentrations decreased with higher alcohol consumption, NT-proBNP increased.15 Whether this pattern is related to AF risk remains to be shown.
A strong controversy remains for the relation of alcohol consumption with AF in individuals with low alcohol consumption. Therefore, we examined the association of alcohol consumption with incident AF while accounting for classical risk factors, HF and cardiac biomarkers across European cohorts.
Methods
Study design
The present study comprises five community-based cohorts from the Monica Risk, Genetics, Archiving and Monograph (MORGAM) (https://www.thl.fi/morgam/)/ Biomarker for Cardiovascular Risk Assessment across Europe (BiomarCaRE) (http://biomarcare.eu/) projects with available information on AF status at baseline and follow-up (DAN-MONICA, FINRISK, Moli-sani, Tromsø, and Northern Sweden), totalling 107 845 individuals with baseline examinations between 1982 and 2010.
We excluded 7753 individuals with self-reported and/or physician-diagnosed history of AF/atrial flutter and/or prior ICD-8-9- or -10 coding for AF/atrial flutter from the analyses. Details on enrolment and follow-up procedures by study are provided in the Supplementary material online. In total, 100 092 participants free of AF at baseline entered our analyses. Where appropriate, we stratified cohorts by the period of recruitment and study area, as shown in Supplementary material online, Table S1. Cohort stratification was possible because we did not observe an interaction for association of alcohol and incident AF by cohort.
Local Ethics Committees have approved all participating studies. Participants signed written informed consent. The authors had full access to the data and take responsibility for its integrity. All authors have read and agreed to the manuscript as written.
Risk factors and follow-up
Risk factor information was available from the baseline visits. Information on body mass index (BMI), hypertension, systolic blood pressure, diabetes, total cholesterol, current smoking, anti-hypertensive medication, history of HF, myocardial infarction or stroke, employment status, education level, and habitual alcohol intake were collected and measured locally. These data were centrally harmonized by the MORGAM project16 and defined AF in a way consistent with previous risk prediction algorithms.14
Average alcohol consumption was assessed in gram per day and categorized according to the World Health Organization average volume drinking categories.17 The quantity of average alcohol consumption in gram per day was a harmonized variable derived from different questionnaires across cohorts as a component of local food frequency questionnaires. Participants were asked to indicate how often they consumed beer, wine, and spirits as well as the drinking pattern.
For the calculation of the amount of alcohol consumed, it was assumed that 120 mL of wine, 330 mL of beer, or 40 mL of spirits contained 12 g of ethanol.5
Continuous alcohol variables were average alcohol consumption in gram per day and alcohol in gram per day consumed from beer, spirits, and wine, respectively. These continuous variables were right winsorized at the level of 98.9% for the analysis. We further examined categorical variables. Drinking pattern had six categories: lifelong abstainer (reference group), ex-drinker, less than once a week, 1–2, 3–5, and 6–7 days/week. We created a new categorical variable from initial average alcohol consumption in gram per day by subdividing the population into seven groups as defined5: former drinker, never drinker (reference group), occasional drinker (<1 g/day), 1–12 g/day (<1 drink/day), 12.1–24 g/day (>1 drink/day), 24.1–48 g/day (2–4 drinks/day) and more than 48 g/day. The ‘never drinker’ group comprised those who consume 0 g of alcohol and who were lifelong abstainers. Individuals with missing values for alcohol variables were excluded from the analyses. Analyses using the continuous variables had the reference level at 0 g/day. Meta-analyses have shown that former drinkers have sustained higher risk of cardiovascular diseases and higher mortality even compared with never drinkers. The interpretation of findings in this group remains difficult. Therefore, we excluded former drinkers with 0 g in average daily alcohol consumption from the group of non-drinkers.18
Analyses using categorical variables comprise the category of former drinkers. The reference of these analyses is the category of never drinkers.
The outcome diagnosis of AF and HF was based on questionnaire information, national hospital discharge registry data including data on ambulatory visits and comorbidities available from causes of death registry data to identify incident AF or HF. Atrial fibrillation was defined as either of AF/atrial flutter. Further information on the definition of the outcome of AF is provided in the Supplementary material online. The last available follow-up was between 2010 and 2011 in different cohorts.
Biomarker measurement
In the subgroup of 23 205 participants from FINRISK and Moli-sani studies, NT-proBNP was measured from stored blood samples, in 31 129 participants from DAN-MONICA, FINRISK and Moli-sani, serum high-sensitivity troponin I (hsTnI) was determined. Measurement details are provided in the Supplementary material online.
Statistical analysis
Continuous variables are presented as median (25th, 75th percentile) and binary variables as absolute and relative frequencies. Missing data were handled by available case analyses.
To examine the association of alcohol consumption and incident AF, sex and cohort-stratified Cox regressions were performed. Multivariable Cox models were adjusted for cardiovascular risk factors and stratified by sex and cohort. Age was used as time scale in all models studying incident AF. Covariates were BMI, hypertension, systolic blood pressure, diabetes, current daily smoker, anti-hypertensive medication, history of HF, myocardial infarction or stroke, defined as classical cardiovascular risk factors. Furthermore, employment status and education level were used as covariates. The association of AF with alcohol consumption was quantified by hazard ratios (HRs). The term ‘risk’ in this paper is used in the epidemiological sense of probability measured by the occurrence of new AF cases during follow-up. It does not confer causality.
Since Cox regression assumes that association of the covariate with the endpoint is linear, data transformation of alcohol consumption was required. The transformation of the alcohol consumption variable via penalized splines showed an almost linear association with the endpoint.19 In all statistical models, 0 g/day alcohol consumption, excluding former drinkers, served as reference group having HR = 1.5 To substantiate the conclusion of the association of incident AF and alcohol consumption, we carried out cause-specific Cox regression with incident AF as the outcome, accounting for the competing risk of all-cause mortality.
We used Spearman correlations to examine bivariate correlations between alcohol consumption and hsTnI and Nt-proBNP. We also investigated whether there were interactions of biomarkers and alcohol consumption on incident AF or interactions of the biomarker associations by sex. Additionally, we investigated the interaction between incident HF as time-dependent covariate and alcohol consumption with AF as an outcome. Due to high prevalence of possibly undetected AF in individuals aged ≥80 years, we performed a sensitivity analysis excluding the oldest old.20
All statistical models were implemented in R statistical software version 3.3.3 (www.R-project.org). A two-tailed P-value <0.05 was considered statistically significant. A more detailed description of the statistical methods is provided in the Supplementary material online.
Results
Baseline characteristics
The characteristics of 100 092 participants without AF at baseline are shown in Table 1. The study cohort had a median age of 47.8 years, age range 24–97 years at baseline, 51.7% women. Study participants were slightly overweight (median BMI 25.7 kg/m2). Median alcohol consumption was 3 g/day (mean 8.7 g/day). During the follow-up time with a median of 13.9 years, N = 5854 incident AF cases were documented. The baseline characteristics by cohort are shown in Supplementary material online, Table S1. Supplementary material online, Table S2 shows missing variables across the individual cohorts and explains the different sample sizes of the variables listed in Table 1.
Table 1.
Baseline characteristics of the study cohort
| Variables | Total cohort (N = 100 092) | Men (N = 48 354) | Women (N = 51 738) |
|---|---|---|---|
| Age at baseline (years) | 47.8 (37.8, 58.6) | 48.1 (38.1, 58.9) | 47.6 (37.5, 58.4) |
| Body mass index (kg/m²) | 25.7 (23.2, 28.8) | 26.3 (24.0, 28.9) | 25.1 (22.5, 28.7) |
| Average daily alcohol consumption (g) | 3.0 (0, 10.0) | 6.0 (1.0, 16.0) | 2.0 (0, 5.0) |
| Beer consumption (g of ethanol/day) | 1.7 (0, 5.0) | 3.0 (0.8, 7.0) | 0.8 (0, 2.0) |
| Spirits consumption (g of ethanol/day) | 0.1 (0, 2.0) | 0.7 (0, 3.0) | 0 (0, 1.0) |
| Wine consumption (g of ethanol/day) | 2.0 (0, 5.7) | 1.6 (0, 6.6) | 2.0 (0, 5.1) |
| Alcohol category, No. (%) | |||
| Never drinker | 8546 (9.1) | 1949 (4.2) | 6597 (13.7) |
| Former drinker | 1374 (1.5) | 719 (1.6) | 655 (1.4) |
| Occasional drinker (<1 g of alcohol/day) | 21 849 (23.2) | 7737 (16.8) | 14 112 (29.3) |
| 1–12 g/day (up to 1 drink/day) | 43 598 (46.3) | 21 337 (46.4) | 22 261 (46.2) |
| 12.1–24 g/day (up to 2 drinks/day) | 9682 (10.3) | 6486 (14.1) | 3196 (6.6) |
| 24.1–48 g/day (2–4 drinks/day) | 6345 (6.7) | 5125 (11.1) | 1220 (2.5) |
| >48 g/day (>4 drinks/day) | 2790 (3.0) | 2646 (5.8) | 144 (0.3) |
| Drinking pattern, No. (%) | |||
| Lifelong abstainer | 12 048 (16.7) | 3082 (8.9) | 8966 (24.0) |
| Ex-drinker | 1410 (2.0) | 745 (2.2) | 665 (1.8) |
| <Once a week | 30 297 (42.1) | 12 868 (37.1) | 17 429 (46.7) |
| 1–2 days/week | 13 964 (19.4) | 8216 (23.7) | 5748 (15.4) |
| 3–5 days/week | 5955 (8.3) | 3955 (11.4) | 2000 (5.4) |
| 6–7 days/week | 8337 (11.6) | 5850 (16.9) | 2487 (6.7) |
| NT-proBNP (pg/mL) | 48.7 (25.8, 89.5) | 36.6 (19.4, 74.1) | 59.1 (34.6, 98.7) |
| HsTnI (ng/L) | 2.4 (1.5, 3.8) | 2.9 (2,0, 4.5) | 1.9 (1.2, 3.0) |
| Systolic blood pressure (mmHg) | 133 (121, 148) | 136 (125.5, 149) | 129 (118, 145.5) |
| Hypertension, No. (%) | 42 415 (42.7) | 23 343 (48.6) | 19 072 (37.1) |
| Anti-hypertensive medication, No. (%) | 12 717 (13.2) | 6147 (13.2) | 6570 (13.1) |
| Heart failure, No. (%) | 1425 (1.9) | 790 (2.2) | 635 (1.7) |
| History of myocardial infarction or stroke, No. (%) | 3959 (4.0) | 2723 (5.6) | 1236 (2.4) |
| Diabetes, No. (%) | 3939 (3.9) | 2041 (4.2) | 1898 (3.7) |
| Current smoker, No. (%) | 31 529 (31.6) | 17 209 (35.6) | 14 320 (27.7) |
| Education level, No. (%) | |||
| University or college degree | 16 324 (16.5) | 7953 (16.7) | 8371 (16.4) |
| Intermediate degree | 21 331 (21.6) | 9800 (20.5) | 11 531 (22.6) |
| Secondary school | 28 558 (28.9) | 14 065 (29.5) | 14 493 (28.4) |
| Primary school or less | 32 588 (33.0) | 15 915 (33.4) | 16 673 (32.7) |
| Employment status, No. (%) | |||
| Full- or part-time job | 64 486 (66.1) | 33 198 (70.8) | 31 288 (61.8) |
| Working in household | 7901 (8.1) | 223 (0.5) | 7678 (15.2) |
| Unemployed | 4395 (4.5) | 2433 (5.2) | 1962 (3.9) |
| Retired or long-term disabled | 19 045 (19.5) | 10 308 (22.0) | 8737 (17.3) |
| Full-time student | 1676 (1.7) | 720 (1.5) | 956 (1.9) |
Baseline characteristics of participants were described by median values for continuous variables (25th, 75th percentile) and proportions for categorical variables.
hsTnI, high-sensitivity troponin I; NT-proBNP, N-terminal pro-B-type natriuretic peptide.
Alcohol consumption and incident atrial fibrillation
In Cox regression analyses, alcohol consumption was positively associated with incident AF, the HR for one drink (12 g) per day was 1.16, 95% CI 1.11–1.22, P < 0.001 (Take home figure). The cut-off for statistically significantly increased risk for AF was observed with regular alcohol consumption of 2 g/day (Table 2).
Table 2.
Hazard ratio for incident atrial fibrillation by number of drinks/day
| No. of drinks/day | Alcohol (g/day) | Hazard ratio (95% confidence interval) |
|---|---|---|
| 0.08 | 1 | 1.01 (0.99–1.04) |
| 0.17 | 2 | 1.02 (1.0–1.04) |
| 0.25 | 3 | 1.04 (1.02–1.05) |
| 0.33 | 4 | 1.05 (1.03–1.07) |
| 0.42 | 5 | 1.06 (1.04–1.08) |
| 0.5 | 6 | 1.07 (1.05–1.1) |
| 1 | 12 | 1.16 (1.11–1.22) |
| 2 | 24 | 1.36 (1.25–1.47) |
| 3 | 36 | 1.52 (1.35–1.7) |
| 4 | 48 | 1.59 (1.37–1.85) |
| ≥5 | 60 | 1.61 (1.35–1.92) |
Presented are HRs and 95% confidence intervals. The models use age as time scale and are sex and cohort stratified. A drink contains 12 g of alcohol. N = 91 980.
Additional adjustment for classical cardiovascular risk factors did not change the association markedly (Supplementary material online, Figure S1). The HR at one drink per day was 1.18, 95% CI 1.12–1.25, P < 0.001. There was some heterogeneity in associations across cohorts (Supplementary material online, Table S3). Association patterns were similar across different types of alcohol as shown in Supplementary material online, Table S4. Additional adjustments did not change the associations markedly.
The HRs were fairly similar for women and men. We did not observe an interaction for alcohol consumption and incident AF by sex, P = 0.07.
The association of incident AF and alcohol consumption did not change markedly after having accounted for all-cause mortality as competing risk.
After excluding individuals over 80 years, the dataset was reduced to N = 99 352 with 5728 cases of incident AF. The HR at one drink per day was 1.17, 95% CI 1.11–1.22, P < 0.001.
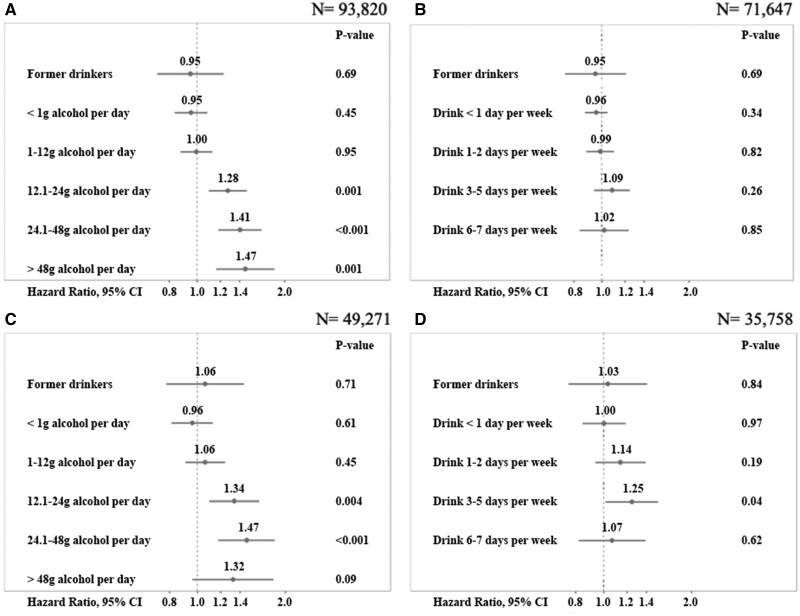
Associations for categories of alcohol consumption and drinking pattern are shown in Figure 1A–D. In former drinkers, occasional drinkers and individuals with alcohol consumption up to one drink per day, there was no statistically significant association (Figure 1A). Higher categories of alcohol intake were associated with 28% (consumption of more than one up to two drinks) to 47% (consumption of more than four drinks) increased risk for developing AF (Figure 1A). No statistically significant association was found with drinking patterns (Figure 1B). These associations did not change markedly by further multivariable adjustment (Figure 1C and D).
Figure 1.
Association of alcohol consumption in gram per day and incident atrial fibrillation by categories (A, C) and drinking pattern (B, D). Figures A-B show results from Cox regression models which use age as time scale and are sex and cohort stratified. Figures C-D are additionally adjusted for classical cardiovascular risk factors, employment status, and education level. The overall association of incident atrial fibrillation and alcohol categories was significant, P < 0.0001 (A), but not significant with drinking pattern, P = 0.53 (B). Via augmented models, the overall association of incident atrial fibrillation and alcohol categories was significant, P < 0.0001 (C) and also significant with drinking pattern, P < 0.0001 (D). Hazard ratios are shown on the log-scale, 95% confidence intervals and P-values. One drink = 12 g of alcohol. Never drinkers were the reference category.
Association of alcohol consumption with incident atrial fibrillation and incident heart failure
From the joint dataset with available follow-up for AF and HF, we observed 4995 incident HF cases from N = 69 084 individuals. A sex- and cohort-stratified Cox regression analysis exhibited a J-shaped relationship with incident HF, P < 0.0001. The lowest HRs were observed at levels up to 20 g/day, or 1.6 drinks/day (Supplementary material online, Figure S2). The HR curves for AF or HF revealed different patterns at lower daily alcohol consumption. The interaction between incident HF as time-dependent covariate and alcohol consumption with AF as outcome was not statistically significant, P = 0.07.
Alcohol consumption, cardiac biomarkers, and incident atrial fibrillation
We observed weak correlations between circulating biomarkers and alcohol consumption for NT-proBNP (r = −0.10) or hsTnI (r = 0.12), or for biomarkers with different types of alcohol (Supplementary material online, Table S5). Levels of cardiac biomarker concentrations did not significantly modify the relationship between alcohol consumption and incident AF (Supplementary material online, Table S6).
Discussion
In our large pooled dataset, we observed a positive association between alcohol consumption and incident AF across different types of alcoholic beverages and drinking patterns, evident even in individuals with low alcohol consumption. The association was explained neither by cardiac biomarker concentrations nor by the occurrence of HF during follow-up. Interaction terms between cardiac biomarker concentrations or HF with alcohol consumption were not statistically significant.
Alcohol consumption amounts and patterns have been related to higher incidence of AF, in particular since the description of the ‘holiday heart’ syndrome in 1978. Chronic heavy alcohol consumption is strongly associated with increased AF risk even in the absence of alcoholic cardiomyopathy as shown in multiple epidemiological studies.1–3 Chronic alcohol consumption usually is related to a higher burden of other classical risk factors, such as hypertension, diabetes, overweight, and obesity, which are strong predictors of AF, and may help explain the observed associations.13 In several observational studies, chronic alcohol consumption, in particular two or more drinks per day, resulted in an up to 30% higher risk for incident AF.2 , 3 The relationship for light alcohol consumption and risk of AF is less consistent.1 , 21–23 In the Women’s Health Study consuming up to two alcoholic beverages per day was not associated with increased risk of AF, though this observation was based on a comparatively small number of cases.1 In the PREDIMED study, a Mediterranean alcohol consumption pattern in high cardiovascular risk population was not associated with higher risk of AF.22 In contrast, two large meta-analyses of heterogeneous studies suggested that even light alcohol consumption may increase the incidence of AF.2 , 9 In a meta-analysis of seven prospective studies, even one alcoholic drink per day increased AF incidence by a relative risk of 1.08.9 Kodama et al.2 meta-analysed 14 studies concluding that alcohol abstinence is most favourable for AF risk reduction. But the estimates, particularly in the range of low amounts of alcohol intake, remained inconclusive. Our large community-based study clearly shows that low alcohol consumption is already associated with increased risk of incident AF. We observed a non-linearly increasing relationship between alcohol consumption and incident AF, independent of common confounders. The test for non-linearity was statistically significant. Due to large sample size, we had power to detect associations even at low doses of daily alcohol intake. Alcohol is a modifiable risk factor and change in drinking behaviour may affect AF risk. Importantly, in the ARIC study, it could be demonstrated that every decade of abstinence from alcohol in former drinkers was related to a 20% lower rate of AF development.24 In a randomized trial, abstinence from alcohol reduced AF recurrences in regular drinkers.25 We did not observe large differences in the associations by type of alcoholic beverages and AF risk. All were related to an increased risk of AF.
We further observed that the association of alcohol consumption with incident AF is independent of HF. As known from other studies, alcohol intake is a predictor of HF, but with reduced risk for HF events for moderate alcohol consumption and a relative protection up to 20 g/day resulting in a J-shaped risk curve.5 , 6 Similarly, prior studies demonstrated an association of alcohol consumption with myocardial wall stress reflected by NT-proBNP, and hsTnI concentrations as indicator of myocardial damage.15 In our study, we observed a fairly weak correlation between alcohol consumption and concentrations of NT-proBNP and hsTnI, and no interaction with AF incidence or adverse events after AF onset.
Limitations and strengths
Our study has some limitations: first, we determined type and quantity of alcohol intake by self-report, which may have underestimated the related AF risk due to alcohol underreporting.26 We know that information on alcohol intake provided by study participants is imprecise and may be subject to social desirability bias though the latter probably is less relevant in the groups with lower alcohol intake. In addition, our data did not permit the evaluation of binge drinking.
Only single biomarker measurements were available at baseline, whereas AF often occurred years after the measurement.
Atrial fibrillation episodes can be asymptomatic and thus remain clinically undetected.27 Atrial fibrillation cases identified during follow-up are more likely to be symptomatic or more persistent types of AF. Unfortunately, baseline electrocardiograms were not available systematically in all cohorts, which may be related to under diagnosis of AF. Follow-up information on AF derived from hospital discharge data, including data on ambulatory visits to specialized hospitals may be related to misclassification of AF cases, in particular intermittent AF. Our dataset is limited to adult participants across Europe.
Strengths of our study include the large sample size, harmonized outcome assessment, and the availability of two contemporary cardiac biomarkers, both of which have previously been shown to be predictors of AF.15 , 28 Our rigorously harmonized covariate definitions and multivariable adjustments in pooled analyses provide a robust basis for evaluating the shape of the relationship at the lower end of the alcohol consumption spectrum.
Conclusions
Alcohol consumption is positively associated with risk of AF independent of classical pathophysiological pathways including those related to myocardial wall stress and injury reflected by NT-proBNP and hsTnI. Although light alcohol consumption may reduce the risk of other cardiovascular diseases,6 , 29 we observed a clear linear increase in AF incidence starting at very low levels of alcohol consumption. Alcohol consumption across common types of alcoholic beverages and drinking patterns was associated with an increased AF risk even at low doses. Given recent trials among moderate drinkers showing reduced episodes of AF recurrence after periods of abstinence,30 , 31 and the fact that we found that even low levels of alcohol intake may confer risk, a strategy of reduction of alcohol consumption might have the potential to prevent a substantial number of cases of AF.
Supplementary material
Supplementary material is available at European Heart Journal online.
Supplementary Material
Acknowledgements
We thank the participants and the staff of the cohorts for their continuing dedication and efforts.
Funding
R.B.S. has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement no. 648131), from the European Union’s Horizon 2020 research and innovation programme under the grant agreement no. 847770 (AFFECT-EU), and German Center for Cardiovascular Research (DZHK e. V.) (81Z1710103); German Ministry of Research and Education (BMBF 01ZX1408A) and ERACoSysMed3 (031L0239).
The BiomarCaRE Project was funded by the European Union Seventh Framework Programme (FP7/2007–2013) under grant agreement no. HEALTH-F2-2011 to 278913. The activities of the MORGAM Data Centre have also been sustained by recent funding from the European Union Seventh Framework Programme projects ENGAGE (HEALTH-F4-2007-2014113) and CHANCES (HEALTH-F3-2010-242244). A part of the biomarker determinations in the population cohorts was funded by the Medical Research Council London (G0601463, identification no. 80983: Biomarkers in the MORGAM Populations).
The FINRISK surveys were mainly funded by budgetary funds of the National Institute for Health and Welfare, Finland (THL). Additional funding has been obtained from numerous non-profit foundations. Dr V.S. (principal investigator) has been supported by the Finnish Foundation for Cardiovascular Research and the Academy of Finland (grant no. 139635). Dr T.N. has been funded by the Emil Aaltonen Foundation, Paavo Nurmi Foundation, Finnish Medical Foundation, and the Academy of Finland (grant no. 321351).
The NSW MONICA study was mainly funded by the Norr- and Västerbotten county councils. Dr S.S. (principal investigator) has been supported by the Västerbotten county council (ALF) and by the Swedish Heart and Lung Foundation.
G.d.G. and S.Co. were supported by a grant from the Ministero della Salute, Italy. Bando Ricerca Finalizzata 2018 RF-2018-12367074.
Conflict of interest: R.B.S. reports consulting and lecture fees from BMS/Pfizer outside the submitted work. V.S. reports consulting and honoraria from Novo Nordisk and Sanofi. He also has research collaboration with Bayer Ltd (All unrelated to the present study). S.S. reports consulting and lecture fees from Actelion Pharmaceuticals Ltd. W.K. reports personal fees (consulting) from AstraZeneca, Novartis, Pfizer, The Medicines Company, DalCor, Kowa, and Amgen; personal fees (honorarium for lectures) from AstraZeneca, Sanofi and Berlin-Chemie; grants and non-financial support (provision of reagents for biomarker measurements free of charge) from Roche Diagnostics, Beckmann, Singulex, and Abbott, outside the submitted work. S.B. reports honoraria from Abbott, Siemens, Thermo Fisher, and Roche, outside of the submitted work. S.C. and A.D.C. are the principal investigator and the co-applicant, respectively, of an ongoing a study supported by a research grant from ERAB (the European Foundation for Alcohol Research; id. EA1767, 2018-2020), outside the submitted work. A.D.C. reports personal fees as member of the Organizing Committee for the 7th European Beer and Health Symposium (2014), Beer and Health Initiative (The Dutch Beer Institute Foundation—The Brewers of Europe), outside the submitted work. S.C. reports personal fees as member of the Organizing Committee and speaker for the 9th European Beer and Health Symposium (Bruxelles 2019) and for given lecture at the 13th European Nutrition Conference FENS 2019 (Dublin), sponsored by the Beer and Health Initiative (The Dutch Beer Institute foundation—The Brewers of Europe), outside the submitted work. G.d.G. is a member of the International Scientific Forum on Alcohol Research, an independent organization of scientists that prepares critiques of emerging research reports on alcohol and health. The members of the Forum donate their time and effort in the review of papers and receive no financial support. The Forum itself receives no support from any organization or company in the alcoholic beverage industry. G.d.G. was also a consultant to the Web Newsletter of Assobirra, the Italian Association of the Beer and Malt Industries and is a corresponding member of the non-profit Accademia Italiana della Vite e del Vino. G.d.G. reports personal fees for given lectures at the 8th European Beer and Health Symposium (2017), Beer and Health Initiative (The Dutch Beer Institute Foundation—The Brewers of Europe), outside the submitted work. The other authors report no conflicts of interest.
Contributor Information
Dora Csengeri, Department of Cardiology, University Heart & Vascular Center Hamburg, Martinistraße 52, 20246 Hamburg, Germany.
Ngoc-Anh Sprünker, Department of Cardiology, University Heart & Vascular Center Hamburg, Martinistraße 52, 20246 Hamburg, Germany.
Augusto Di Castelnuovo, Mediterranea Cardiocentro, Via Orazio 2, 80122 Napoli, Italy.
Teemu Niiranen, Division of Medicine, Turku University Hospital and University of Turku, Kiinamyllynkatu 4-8, 20521 Turku, Finland; Department of Public Health Solutions, Finnish Institute for Health and Welfare, POB 30, Mannerheimintie 166, 00271 Helsinki, Finland.
Julie Kk Vishram-Nielsen, Center for Clinical Research and Prevention, Bispebjerg and Frederiksberg Hospital, The Capital Region of Denmark, Nordre Fasanvej 57, 2000 Frederiksberg, Denmark; Department of Cardiology, Righospitalet, University Hospital of Copenhagen, Blegdamsvej 9, 2100 Copenhagen Denmark.
Simona Costanzo, Department of Epidemiology and Prevention, IRCCS Neuromed, Via dell´ Elettronica, 86077 Pozzilli, Italy.
Stefan Söderberg, Department of Public Health and Clinical Medicine, and Heart Centre, Umeå University, SE-901 87 Umeå, Sweden.
Steen M Jensen, Department of Public Health and Clinical Medicine, and Heart Centre, Umeå University, SE-901 87 Umeå, Sweden.
Erkki Vartiainen, Department of Public Health Solutions, Finnish Institute for Health and Welfare, POB 30, Mannerheimintie 166, 00271 Helsinki, Finland.
Maria Benedetta Donati, Department of Epidemiology and Prevention, IRCCS Neuromed, Via dell´ Elettronica, 86077 Pozzilli, Italy.
Christina Magnussen, Department of Cardiology, University Heart & Vascular Center Hamburg, Martinistraße 52, 20246 Hamburg, Germany; German Centre for Cardiovascular Research (DZHK), partner site, Hamburg/Kiel/Luebeck, Potsdamer Straße 58, 10785 Berlin, Germany.
Stephan Camen, Department of Cardiology, University Heart & Vascular Center Hamburg, Martinistraße 52, 20246 Hamburg, Germany; German Centre for Cardiovascular Research (DZHK), partner site, Hamburg/Kiel/Luebeck, Potsdamer Straße 58, 10785 Berlin, Germany.
Francesco Gianfagna, Mediterranea Cardiocentro, Via Orazio 2, 80122 Napoli, Italy; Research Center in Epidemiology and Preventive Medicine (EPIMED), Department of Medicine and Surgery, University of Insubria, Via Rossi 9, 21100 Varese, Italy.
Maja-Lisa Løchen, Department of Community Medicine, UiT The Arctic University of Norway, Hansine Hansens veg 18, 9019 Tromsø, Norway.
Frank Kee, Center for Public Health, Institute of Clinical Sciences A, Queens University, Grosvenor Road, BT 12 6BJ Belfast, Ireland.
Jukka Kontto, Department of Public Health Solutions, Finnish Institute for Health and Welfare, POB 30, Mannerheimintie 166, 00271 Helsinki, Finland.
Ellisiv B Mathiesen, Brain and Circulation Research Group, Department of Clinical Medicine, UiT The Arctic University of Norway, Hansine Hansens veg 18, 9019 Tromsø, Norway.
Wolfgang Koenig, Deutsches Herzzentrum München, Lazarettstraße 36, 80636 Munich, Germany; DZHK (German Centre for Cardiovascular Research), partner site Munich Heart Alliance, Biedersteinerstraße 29, 80802 Munich, Germany; Institute of Epidemiology and Medial Biometry, University of Ulm, Helmholtzstraße 22, 89081 Ulm, Germany.
Stefan Blankenberg, Department of Cardiology, University Heart & Vascular Center Hamburg, Martinistraße 52, 20246 Hamburg, Germany; German Centre for Cardiovascular Research (DZHK), partner site, Hamburg/Kiel/Luebeck, Potsdamer Straße 58, 10785 Berlin, Germany.
Giovanni de Gaetano, Department of Epidemiology and Prevention, IRCCS Neuromed, Via dell´ Elettronica, 86077 Pozzilli, Italy.
Torben Jørgensen, Center for Clinical Research and Prevention, Bispebjerg and Frederiksberg Hospital, The Capital Region of Denmark, Nordre Fasanvej 57, 2000 Frederiksberg, Denmark; Department of Public Health, Faculty of Health and Medical Science, University of Copenhagen, Blegdamsvej 3B, 2200 Copenhagen, Denmark; Faculty of Medicine, Aalborg University, Niels Jernes Vej 10, 9220 Aalborg, Denmark.
Kari Kuulasmaa, Department of Public Health Solutions, Finnish Institute for Health and Welfare, POB 30, Mannerheimintie 166, 00271 Helsinki, Finland.
Tanja Zeller, Department of Cardiology, University Heart & Vascular Center Hamburg, Martinistraße 52, 20246 Hamburg, Germany.
Veikko Salomaa, Department of Public Health Solutions, Finnish Institute for Health and Welfare, POB 30, Mannerheimintie 166, 00271 Helsinki, Finland.
Licia Iacoviello, Department of Epidemiology and Prevention, IRCCS Neuromed, Via dell´ Elettronica, 86077 Pozzilli, Italy; Research Center in Epidemiology and Preventive Medicine (EPIMED), Department of Medicine and Surgery, University of Insubria, Via Rossi 9, 21100 Varese, Italy.
Renate B Schnabel, Department of Cardiology, University Heart & Vascular Center Hamburg, Martinistraße 52, 20246 Hamburg, Germany; German Centre for Cardiovascular Research (DZHK), partner site, Hamburg/Kiel/Luebeck, Potsdamer Straße 58, 10785 Berlin, Germany.
References
- 1. Conen D, Tedrow UB, Cook NR, Moorthy MV, Buring JE, Albert CM. Alcohol consumption and risk of incident atrial fibrillation in women. JAMA 2008;300:2489–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kodama S, Saito K, Tanaka S, Horikawa C, Saito A, Heianza Y, Anasako Y, Nishigaki Y, Yachi Y, Iida KT, Ohashi Y, Yamada N, Sone H. Alcohol consumption and risk of atrial fibrillation. J Am Coll Cardiol 2011;57:427–436. [DOI] [PubMed] [Google Scholar]
- 3. Whitman IR, Agarwal V, Nah G, Dukes JW, Vittinghoff E, Dewland TA, Marcus GM. Alcohol abuse and cardiac disease. J Am Coll Cardiol 2017;69:13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goncalves A, Claggett B, Jhund PS, Rosamond W, Deswal A, Aguilar D, Shah AM, Cheng S, Solomon SD. Alcohol consumption and risk of heart failure: the Atherosclerosis Risk in Communities Study. Eur Heart J 2015;36:939–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Di Castelnuovo A, Costanzo S, Bonaccio M, Rago L, De Curtis A, Persichillo M, Bracone F, Olivieri M, Cerletti C, Donati MB, de Gaetano G, Iacoviello L, Moli-sani Investigators. Moderate alcohol consumption is associated with lower risk for heart failure but not atrial fibrillation. JACC Heart Fail 2017;5:837–844. [DOI] [PubMed] [Google Scholar]
- 6. Bell S, Daskalopoulou M, Rapsomaniki E, George J, Britton A, Bobak M, Casas JP, Dale CE, Denaxas S, Shah AD, Hemingway H. Association between clinically recorded alcohol consumption and initial presentation of 12 cardiovascular diseases: population based cohort study using linked healt records. BMJ 2017;356:j909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gallagher C, Hendriks JML, Elliott AD, Wong CX, Rangnekar G, Middeldorp ME, Mahajan R, Lau DH, Sanders P. Alcohol and incident atrial fibrillation—a systematic review and meta-analysis. Int J Cardiol 2017;246:46–52. [DOI] [PubMed] [Google Scholar]
- 8. Mukamal KJ, Tolstrup JS, Friberg J, Jensen G, Grønbæk M. Alcohol consumption and risk of atrial fibrillation in men and women. Circulation 2005;112:1736–1742. [DOI] [PubMed] [Google Scholar]
- 9. Larsson SC, Drca N, Wolk A. Alcohol consumption and risk of atrial fibrillation. J Am Coll Cardiol 2014;64:281–289. [DOI] [PubMed] [Google Scholar]
- 10. Brunner S, Herbel R, Drobesch C, Peters A, Massberg S, Kääb S, Sinner MF. Alcohol consumption, sinus tachycardia, and cardiac arrhythmias at the Munich Octoberfest: results from the Munich Beer Related Electrocardiogram Workup Study (MunichBREW). Eur Heart J 2017;38:2100–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McManus DD, Yin X, Gladstone R, Vittinghoff E, Vasan RS, Larson MG, Benjamin EJ, Marcus GM. Alcohol consumption, left atrial diameter, and atrial fibrillation. J Am Heart Assoc 2016;5:e004060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bébarová M, Horáková Z, Kula R. Addictive drugs, arrhythmias, and cardiac inward rectifiers. Europace 2017;19:346–355. [DOI] [PubMed] [Google Scholar]
- 13. Voskoboinik A, Prabhu S, Ling L, Kalman JM, Kistler PM. Alcohol and atrial fibrillation: a sobering review. J Am Coll of Cardiol 2016;68:2567–2576. [DOI] [PubMed] [Google Scholar]
- 14. Mukamal KJ, Psaty BM, Rautaharju PM, Furberg CD, Kull LH, Mittleman MA, Gottdiener JS, Siscovick DS. Alcohol consumption and risk and prognosis of atrial fibrillation among older adults: the Cardiovascular Health Study. Am Heart J 2007;153:260–266. [DOI] [PubMed] [Google Scholar]
- 15. Lazo M, Chen Y, McEvoy JW, Ndumele C, Konety S, Ballantyne CM, Sharrett AR, Selvin E. Alcohol consumption and cardiac biomarkers: the Atherosclerosis Risk in Communities (ARIC) Study. Clin Chem 2016;62:1202–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zeller T, Hughes M, Tuovinen T, Schillert A, Conrads-Frank A, Ruijter H. D, Schnabel RB, Kee F, Salomaa V, Siebert U, Thorand B, Ziegler A, Breek H, Pasterkamp G, Kuulasmaa K, Koenig W, Blankenberg S. BiomarCaRE: rationale and design of the European BiomarCaRE project including 300,000 participants from 13 European countries. Eur J Epidemiol 2014;29:777–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization. Comparative Quantification of Health Risks. Geneva, Switzerland. https://www.who.int/publications/cra/chapters/volume1/0959-1108.pdf? ua=1 (4 December 2020). [Google Scholar]
- 18. Knott CS, Coombs N, Stamatakis E, Biddulph JP. All cause mortality and the case for age specific alcohol consumption guidelines: pooled analyses of up to 10 population based cohorts. BMJ 2015;350:h384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Harrell FE Jr. Regression Modeling Strategies: with Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. 2nd ed. Cham: Switzerland: Springer International Publishing; 2015. [Google Scholar]
- 20. Boriani G, Laroche C, Diemberger I, Fantecchi E, Popescu MI, Rasmussen LH, Sinagra G, Petrescu L, Tavazzi L, Maggioni AP, Lip GY. Asymptomatic atrial fibrillation: clinical correlates, management and outcomes in the EORP-AF Pilot General Registry. Am J Med 2015;128:509–518.e2. [DOI] [PubMed] [Google Scholar]
- 21. Gémes K, Malmo V, Laugsand LE, Loennechen JP, Ellekjaer H, László KD, Ahnve S, Vatten LJ, Mukamal KJ, Janszky I. Does moderate drinking increase the risk of atrial fibrillation? The Norwegian HUNT (Nord‐Trøndelag Health) Study. J Am Heart Assoc 2017;6:e007094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bazal P, Gea A, Martínez-González MA, Salas-Salvadó J, Asensio EM, Muñoz-Bravo C, Fiol M, Muñoz MA, Lapetra J, Serra-Majem LL, Pintó X, González JI, Becerra-Tomás N, Fitó M, Ros E, Alonso-Gómez A, Ruiz-Canela M. Mediterranean alcohol-drinking pattern, low to moderate alcohol intake and risk of atrial fibrillation in the PREDIMED study. Nutr Metab Cardiovasc Dis 2019;29:676–683. [DOI] [PubMed] [Google Scholar]
- 23. Kim YG, Han KD, Choi JI, Boo KY, Kim DY, Lee KN, Shim J, Kim JS, Kim YH. Frequent drinking is a more important risk factor for new-onset atrial fibrillation than binge drinking: a nationwide population-based study. Europace 2020;22:216–224. [DOI] [PubMed] [Google Scholar]
- 24. Dixit S, Alonso A, Vittinghoff E, Soliman E, Chen LY, Marcus GM. Past alcohol consumption and incident atrial fibrillation: the Atherosclerosis Risk in Communities (ARIC) Study. PLoS One 2017;12:e0185228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Voskoboinik A, Kalman JM, De Silva A, Nicholls T, Costello B, Nanayakkara S, Prabhu S, Stub D, Azzopardi S, Vizi D, Wong G, Nalliah C, Sugumar H, Wong M, Kotschet E, Kaye D, Taylor AJ, Kistler PM. Alcohol abstinence in drinkers with atrial fibrillation. N Engl J Med 2020;382:20–28. [DOI] [PubMed] [Google Scholar]
- 26. Di Castelnuovo A, Costanzo S, Bagnardi V, Donati MB, Iacoviello L, de Gaetano G. Alcohol dosing and total mortality in men and women: an updated meta-analysis of 34 prospective studies. Arch Intern Med 2006;166:2437–2445. [DOI] [PubMed] [Google Scholar]
- 27. Diederichsen SZ, Haugan KJ, Brandes A, Lanng MB, Graff C, Krieger D, Kronborg C, Holst AG, Køber L, Højberg S, Svendsen JH. Natural history of subclinical atrial fibrillation detected by implanted loop recorders. J Am Coll Cardiol 2019;74:2771–2781. [DOI] [PubMed] [Google Scholar]
- 28. Rienstra M, Yin X, Larson MG, Fontes JD, Magnani JW, Mcmanus DD, McCabe EL, Coglianese EE, Amponsah M, Ho JE, Januzzi JL Jr, Wollert KC, Fradley MG, Vasan RS, Ellinor PT, Wang TJ, Benjamin EJ. Relation between soluble ST2, growth differentiation factor-15, and high-sensitivity troponin I and incident atrial fibrillation. Am Heart J 2014;167:109–115.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wood AM, Kaptoge S, Butterworth AS, Willeit P, Warnakula S, Bolton T, Paige E, Paul DS, Sweeting M, Burgess S, Bell S, Astle W, Stevens D, Koulman A, Selmer RM, Verschuren WMM, Sato S, Njølstad I, Woodward M, Salomaa V, Nordestgaard BG, Yeap BB, Fletcher A, Melander O, Kuller LH, Balkau B, Marmot M, Koenig W, Casiglia E, Cooper C, Arndt V, Franco OH, Wennberg P, Gallacher J, de la Cámara AG, Völzke H, Dahm CC, Dale CE, Bergmann MM, Crespo CJ, van der Schouw YT, Kaaks R, Simons LA, Lagiou P, Schoufour JD, Boer JMA, Key TJ, Rodriguez B, Moreno-Iribas C, Davidson KW, Taylor JO, Sacerdote C, Wallace RB, Quiros JR, Tumino R, Blazer DG, Linneberg A, Daimon M, Panico S, Howard B, Skeie G, Strandberg T, Weiderpass E, Nietert PJ, Psaty BM, Kromhout D, Salamanca-Fernandez E, Kiechl S, Krumholz HM, Grioni S, Palli D, Huerta JM, Price J, Sundström J, Arriola L, Arima H, Travis RC, Panagiotakos DB, Karakatsani A, Trichopoulou A, Kühn T, Grobbee DE, Barrett-Connor E, van Schoor N, Boeing H, Overvad K, Kauhanen J, Wareham N, Langenberg C, Forouhi N, Wennberg M, Després J-P, Cushman M, Cooper JA, Rodriguez CJ, Sakurai M, Shaw JE, Knuiman M, Voortman T, Meisinger C, Tjønneland A, Brenner H, Palmieri L, Dallongeville J, Brunner EJ, Assmann G, Trevisan M, Gillum RF, Ford I, Sattar N, Lazo M, Thompson SG, Ferrari P, Leon DA, Smith GD, Peto R, Jackson R, Banks E, Di Angelantonio E, Danesh J, Wood AM, Kaptoge S, Butterworth A, Willeit P, Warnakula S, Bolton T, Paige E, Paul DS, Sweeting M, Burgess S, Bell S, Astle W, Stevens D, Koulman A, Selmer RM, Verschuren M, Sato S, Njølstad I, Woodward M, Veikko S, Nordestgaard BG, Yeap BB, Flecther A, Melander O, Kuller LH, Balkau B, Marmot M, Koenig W, Casiglia E, Cooper C, Arndt V, Franco OH, Wennberg P, Gallacher J, Gómez de la Cámara A, Völzke H, Dahm CC, Dale CE, Bergmann M, Crespo C, van der Schouw YT, Kaaks R, Simons LA, Lagiou P, Schoufour JD, Boer JMA, Key TJ, Rodriguez B, Moreno-Iribas C, Davidson KW, Taylor JO, Sacerdote C, Wallace RB, Quiros JR, Rimm EB, Tumino R, Blazer Iii DG, Linneberg A, Daimon M, Panico S, Howard B, Skeie G, Salomaa V, Strandberg T, Weiderpass E, Nietert PJ, Psaty BM, Kromhout D, Salamanca-Fernandez E, Kiechl S, Krumholz HM, Grioni S, Palli D, Huerta JM, Price J, Sundström J, Arriola L, Arima H, Travis RC, Panagiotakos DB, Karakatsani A, Trichopoulou A, Kühn T, Grobbee DE, Barrett-Connor E, van Schoor N, Boeing H, Overvad K, Kauhanen J, Wareham N, Langenberg C, Forouhi N, Wennberg M, Després J-P, Cushman M, Cooper JA, Rodriguez CJ, Sakurai M, Shaw JE, Knuiman M, Voortman T, Meisinger C, Tjønneland A, Brenner H, Palmieri L, Dallongeville J-P, Brunner EJ, Assmann G, Trevisan M, Gillumn RF, Ford IF, Sattar N, Lazo M, Thompson S, Ferrari P, Leon DA, Davey Smith G, Peto R, Jackson R, Banks E, Di Angelantonio E, Danesh J, Emerging Risk Factors Collaboration/EPIC-CVD/UK Biobank Alcohol Study Group. Risk thresholds for alcohol consumption: combined analysis of individual-participant data for 599 912 current drinkers in 83 prospective studies. Lancet 2018;391:1513–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener H-C, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Van Vardas P; ESC Scientific Document Group. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016;37:2893–2962. [DOI] [PubMed] [Google Scholar]
- 31. Lau DH, Nattel S, Kalman JM, Sanders P. Modifiable risk factors and atrial fibrillation. Circulation 2017;136:583–596. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.