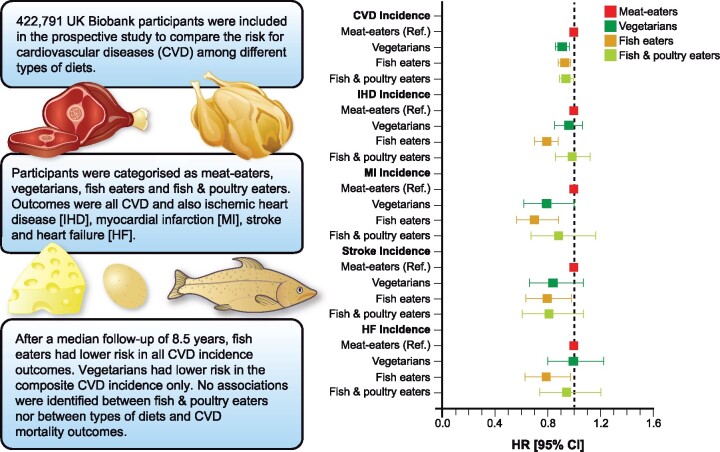
Abstract
Aims
To compare the incidence and mortality risk for cardiovascular diseases (CVD) [CVD and also ischaemic heart disease (IHD), myocardial infarction (MI), stroke, and heart failure (HF)] among people with different types of diets—including vegetarians, fish eaters, fish and poultry eaters, and meat-eaters—using data from UK Biobank.
Methods and results
A total of 422 791 participants (55.4% women) were included in this prospective analysis. Using data from a food frequency questionnaire, four types of diets were derived. Associations between types of diets and health outcomes were investigated using Cox proportional hazard models. Meat-eaters comprised 94.7% of the cohort and were more likely to be obese than other diet groups. After a median follow-up of 8.5 years, fish eaters, compared with meat-eaters, had lower risks of incident CVD {hazard ratios (HR): 0.93 [95% confidence intervals (CI): 0.88–0.97]}, IHD [HR: 0.79 (95% CI: 0.70–0.88)], MI [HR: 0.70 (95% CI: 0.56–0.88)], stroke [HR: 0.79 (95% CI: 0.63–0.98)] and HF [HR: 0.78 (95% CI: 0.63–0.97)], after adjusting for confounders. Vegetarians had lower risk of CVD incidence [HR: 0.91 (95% CI: 0.86–0.96)] relative to meat-eaters. In contrast, the risk of adverse outcomes was not different in fish and poultry eaters compared with meat-eaters. No associations were identified between types of diets and CVD mortality.
Conclusion
Eating fish rather than meat or poultry was associated with a lower risk of a range of adverse cardiovascular outcomes. Vegetarianism was only associated with a lower risk of CVD incidence.
Keywords: Cardiovascular diseases, Vegetarians, Meat, Incidence, Mortality
Graphical Abstract
See page 1144 for the editorial comment on this article (doi: 10.1093/eurheartj/ehaa1088)
Introduction
Cardiovascular diseases (CVD) remain one of the top 10 causes of death worldwide.1 Although there are several behavioural risk factors for CVD, a poor diet accounts for ∼10 million deaths worldwide.2 Of these, 3.8 million deaths have been attributable to a diet low in fruit and vegetables, 1.4 million to a diet low in seafood intake, and 150 000 to high red and processed meat intake.2 With current dietary guidelines encouraging people to limit their intake of red and processed meat2 , 3 and increase their intake of fruit and vegetable as well as fish,4 alternative diets, which restrict the intake of either meats or animal products, have become more popular in recent years.
Due to the health benefits of plant-based diets, as well as concerns over animal protein (animal welfare and apprehension over antibiotics use) and the environmental protection, some of the most popular diets are vegetarian and vegan.5 Vegetarian diets have been associated with lower CVD6 and cancer7 risk in comparison to all nonvegetarian diets due to their higher content of fibre, vitamins, and minerals, and lower content of saturated fat.8 , 9 However, the relative merits of vegetarianism compared with other alternative diets have been less well studied.
Previous studies have shown heterogeneous findings when comparing the risk of CVD associated with vegetarian, vegan, and pescatarian diets to diets containing meat.6 , 10 , 11 For instance, some have reported a higher risk of CVD among fish eaters compared with meat-eaters, while others have demonstrated that despite vegetarians having a lower risk of ischaemic heart diseases (IHD), they had a higher risk of stroke.6 , 10 , 11 However, although these studies had a long follow-up, smaller sample sizes (<50 000), as well as the multifactorial nature of CVD,1 may explain some of the discrepancies in previous studies. Therefore, data from larger prospective studies are still needed. Hence, this study aimed to compare the incidence and mortality risk for a range of CVD outcomes among people with different types of diets—vegetarians, fish eaters, fish and poultry eaters, and meat-eaters—using data from UK Biobank.
Methods
Between 2006 and 2010, UK Biobank recruited over 500 000 participants (5.5% response rate), aged 37–73 years from the general population.12 Participants attended one of the 22 assessment centres across England, Wales, and Scotland13 , 14 where they completed a touch-screen questionnaire, had physical measurements taken, and provided biological samples, as described in detail elsewhere.13 , 14
UK Biobank was approved by the North West Multi-Centre Research Ethical Committee (REF: 11/NW/03820). This study complies with the Declaration of Helsinki.
Outcomes
The outcomes in the current study were incident (hospitalization or death) and fatal events due to: CVD [International Classification of Diseases 10 revision (ICD10) codes I00–I99, i.e. all diseases from the circulatory system], IHD (ICD10 I20–I25), myocardial infarction (MI) (ICD10 I21–I23), stroke (I60, I61, I63, or I64), and heart failure (HF) (I50.0, I50.1, I50.9). Date of death was obtained from death certificates held by the National Health Service (NHS) Information Centre (England and Wales) and the NHS Central Register Scotland (Scotland). Dates and causes of hospital admission were identified via record linkage to Health Episode Statistics (England and Wales) and the Scottish Morbidity Records (SMR01) (Scotland). Details of the linkage procedure can be found at http://content.digital.nhs.uk/services. Death data were available up to June 2020. Follow-up for mortality outcomes was censored on this date or the date of death if that occurred earlier. Hospital admissions were available up to June 2020 in England and March 2017 in Wales and Scotland. Follow-up for incident events was censored on this date or the date of death if this occurred earlier.
Definitions of types of diets
The touch-screen questionnaire, self-completed at baseline, was used to collect the frequency of consumption of the following items: cheese, milk, fish (oily and non-oily), poultry, and red meat (beef, pork, lamb, and processed red meat) over the previous year. All food items were dichotomized into consumed or not consumed.
Using these variables, participants were categorized into four types of diets: vegetarians (consumption of cheese and/or milk but not fish, poultry, or red meat, i.e. lacto-ovo-vegetarian); fish eaters (consumption of cheese, milk, and fish but not poultry or red meat); fish and poultry eaters (consumption of cheese, milk, fish, and poultry but not red meat); and meat-eaters (consumption of cheese, milk, fish, poultry, and red meat). People with missing data for any of the dietary variables were excluded [n = 9011 (1.8%)]. In addition, we excluded vegan participants as the sample size was not sufficient for conducting the analyses (n = 57, 0.01%). To take account of people changing their dietary pattern, we excluded people who self-reported at baseline that their diet often varied (n = 45 028, 8.99%). Therefore, 448 396 participants had available information for the different types of diets (89.2%). The groups were mutually exclusive (Supplementary material online, Figure S1).
Covariates
Age was calculated from dates of birth and baseline assessment. Area-based socioeconomic status (deprivation) was derived from the postcode of residence, using the Townsend score (more details in Supplementary material online).15 Self-reported smoking status was categorized as never, former, or current smoker. Total time spent in discretionary sedentary behaviours was derived from the sum of self-reported time spent driving, using a computer and watching television during leisure time. Body mass index (BMI) was calculated from weight/height2, and the WHO criteria were applied.16 Medical history was also self-reported. Frequency of alcohol intake was self-reported at baseline via touch-screen questionnaire and categorized as daily/almost daily, 3–4 times a week, once/twice a week, 1–3 times a month, special occasions only, and never. Prevalent morbidity was ascertained during a nurse-led interview at baseline. We calculated morbidity count based on 43 long-term conditions developed initially for a large epidemiological study in Scotland and subsequently adapted for UK Biobank17 (Table 1).
Table 1.
Sociodemographic characteristics of the study population by types of diets.
| Vegetarians | Fish eaters | Fish and poultry eaters | Meat-eaters | |
|---|---|---|---|---|
| Socio-demographics | ||||
| Total, n (%) | 7537 (1.8) | 9951 (2.4) | 4883 (1.1) | 400 470 (94.7) |
| Age (years), mean (SD) | 53.1 (7.9) | 54.0 (8.0) | 56.3 (8.1) | 56.5 (8.1) |
| Sex (female), n (%) | 5042 (66.9) | 7197 (72.3) | 3721 (77.0) | 218 307 (54.1) |
| Deprivation, n (%) | ||||
| Lower | 1965 (26.0) | 2893 (29.1) | 1381 (28.6) | 139 264 (34.8) |
| Middle | 2425 (32.2) | 3315 (33.3) | 1558 (32.2) | 135 436 (33.8) |
| Higher | 3147 (41.8) | 3743 (37.6) | 1894 (39.2) | 125 770 (31.4) |
| Ethnicity, n (%) | ||||
| White | 6150 (81.6) | 9372 (94.2) | 4377 (90.6) | 382 551 (95.5) |
| Mixed | 118 (1.6) | 158 (1.6) | 103 (2.1) | 5360 (1.3) |
| South Asian | 1231 (16.3) | 285 (2.9) | 213 (4.4) | 5550 (1.4) |
| Black | 29 (0.4) | 126 (1.2) | 135 (2.8) | 5784 (1.5) |
| Chinese | 9 (0.1) | 10 (0.1) | 5 (0.1) | 1225 (0.3) |
| Obesity-related markers | ||||
| BMI, mean (SD) | 25.6 (4.6) | 25.2 (4.2) | 25.5 (4.5) | 27.4 (4.7) |
| BMI categories, n (%) | ||||
| Underweight (<18.5 kg/m 2) | 128 (1.7) | 162 (1.6) | 76 (1.5) | 1867 (0.4) |
| Normal weight (18.5–24.9 kg/m 2) | 3730 (49.5) | 5262 (52.9) | 2423 (50.1) | 131 241 (32.8) |
| Overweight (25.0–29.9 kg/m2) | 2629 (34.9) | 3354 (33.7) | 1631 (33.8) | 172 577 (43.1) |
| Obese (≥30.0 kg/m2) | 1050 (13.9) | 1173 (11.8) | 703 (11.6) | 94 785 (23.7) |
| Fitness and lifestyle | ||||
| Total PA (MET-min/week), mean (SD) | 2811.1 (2930.2) | 2884.2 (2900.6) | 3196.9 (3152.4) | 2818.7 (3019.9) |
| Sedentary behaviour (h/day), mean (SD) | 4.3 (2.2) | 4.3 (2.1) | 4.5 (2.3) | 5.0 (2.2) |
| Smoking status, n (%) | ||||
| Never | 4825 (64.0) | 5696 (57.3) | 2895 (59.9) | 223 054 (55.7) |
| Previous | 2197 (29.2) | 3564 (35.8) | 1571 (32.5) | 137 220 (34.3) |
| Current | 515 (6.8) | 691 (6.9) | 367 (7.6) | 40 196 (10.0) |
| Alcohol intake frequency, n (%) | ||||
| Daily or almost daily | 1069 (14.2) | 1905 (19.1) | 685 (14.2) | 82 898 (20.7) |
| 3–4 times a week | 1347 (17.9) | 2414 (24.3) | 862 (17.8) | 95 137 (23.8) |
| Once or twice a week | 1514 (20.1) | 2299 (23.1) | 1112 (23.0) | 105 521 (26.4) |
| 1–3 times a month | 885 (11.7) | 1162 (11.7) | 547 (11.3) | 44 535 (11.1) |
| Special occasions only | 1059 (14.0) | 1143 (11.5) | 866 (17.9) | 44 214 (11.0) |
| Never | 1663 (22.1) | 1028 (10.3) | 761 (15.8) | 28 165 (7.0) |
| Health status | ||||
| Multimorbidity, n (%) | ||||
| None | 3122 (41.4) | 4172 (41.9) | 1770 (36.6) | 143 910 (35.9) |
| ≥1 | 4415 (58.6) | 5779 (58.1) | 3063 (63.4) | 256 560 (64.1) |
BMI, body mass index; MET, metabolic equivalent; PA, physical activity; SD, standard deviation.
Other diet variables
Water and fruit and vegetable intake were collected through the touch-screen questionnaire at baseline. In turn, dietary information for macro- and micro-nutrients—as well as other food items (such as fast food intake)—was collected via the Oxford WebQ, a web-based 24-h recall questionnaire (more details in Supplementary material online).18 For this study, the average of five 24-h recalls was used. However, as the average of the 24-h recalls was only available for about 200 000 individuals, the number of individuals with data available for each variable is shown in Table 2.
Table 2.
Dietary characteristics of the study population by types of diets
| Dietary intakes, mean (SD) | Data available ina | Vegetarians | Fish eaters | Fish and poultry eaters | Meat-eaters |
|---|---|---|---|---|---|
| Total energy intake (kcal/day) | 183 318 | 2117 (725) | 2126 (674) | 2032 (701) | 2170 (657) |
| CHO intake (% of TE) | 183 310 | 52.1 (8.1) | 50.1 (8.1) | 50.2 (8.6) | 47.0 (8.0) |
| Sugar intake (% of TE) | 183 310 | 24.1 (7.4) | 23.7 (7.0) | 25.1 (7.6) | 22.4 (6.9) |
| Fibre intake (g/day) | 188 318 | 20.4 (8.0) | 19.3 (7.2) | 18.5 (7.6) | 16.2 (6.4) |
| Protein intake (% of TE) | 183 310 | 12.4 (2.3) | 13.6 (2.7) | 15.1 (3.5) | 15.7 (3.6) |
| Fat intake (% of TE) | 183 310 | 31.8 (7.1) | 31.9 (7.0) | 30.9 (7.3) | 32.1 (6.7) |
| Polyunsaturated fat intake (% of TE) | 183 310 | 6.2 (2.4) | 6.2 (2.3) | 6.0 (2.3) | 5.9 (2.2) |
| Saturated fat intake (% of TE) | 183 310 | 12.0 (3.6) | 11.8 (3.4) | 11.4 (3.5) | 12.4 (3.3) |
| Fruit and vegetables intake (g/day) | 422 791 | 403.9 (234.8) | 406.3 (216.3) | 418.7 (241.7) | 323.6 (187.5) |
| Water intake (glasses/day) | 391 410 | 3.5 (2.6) | 3.4 (2.4) | 3.6 (2.6) | 2.8 (2.2) |
| Vegetarian alternatives intake, n (%), yes | 183 305 | 2158 (53.8) | 2174 (40.2) | 470 (22.2) | 6788 (4.0) |
| Crisp intake (amount/day), n (%) | |||||
| Half small bag | 55 180 | 244 (18.7) | 398 (25.3) | 131 (27.1) | 12 271 (23.7) |
| One small bag | 919 (70.6) | 1023 (65.1) | 308 (63.6) | 34 865 (67.3) | |
| Two or more small bags | 140 (10.7) | 151 (9.6) | 45 (0.3) | 4685 (9.0) | |
| Pizza intake (amount/day), n (%) | |||||
| ≤ one medium slice | 13 130 | 145 (31.3) | 167 (33.7) | 48 (33.1) | 4642 (38.6) |
| Two to three medium slices | 220 (47.5) | 218 (44.0) | 67 (46.2) | 5184 (43.1) | |
| Four or more medium slices | 98 (21.2) | 111 (22.3) | 30 (20.7) | 2200 (18.3) | |
| Sugary drinks intake (amount/day), n (%)b | 59 353 | ||||
| ≤1 glass/can | 863 (72.1) | 1034 (69.3) | 387 (70.8) | 40 319 (71.8) | |
| >1 glass/can | 334 (27.9) | 458 (30.7) | 160 (29.2) | 15 798 (28.2) | |
| Smoothie drinks intake (amount/day), n (%)b | |||||
| ≤1 glass/bottle/250 mL | 21 235 | 504 (88.0) | 634 (90.4) | 323 (90.7) | 17 924 (91.4) |
| >1 glass/bottle/250 mL | 69 (12.0) | 67 (9.6) | 33 (9.3) | 1681 (8.6) | |
| Type of meals eaten, n (%) | |||||
| Takeaway meals | 178 168 | 43 (1.1) | 47 (0.9) | 30 (1.5) | 2929 (1.8) |
| Restaurant meals | 295 (7.6) | 445 (8.4) | 152 (7.4) | 14 755 (8.8) | |
| Bought sandwiches | 408 (10.5) | 554 (10.5) | 165 (8.1) | 17 167 (10.3) | |
| Ready meals | 888 (22.8) | 1353 (25.7) | 431 (21.1) | 37 429 (22.4) | |
| Home-cooked meals | 2258 (58.0) | 2874 (54.5) | 1265 (61.9) | 94 690 (56.7) | |
The average of five 24-h recall was used for this study (except for water and fruit and vegetable intake).
Data available for the different subcomponents of diet in the dataset.
Sugary drinks were derived from fizzy and squash drinks. Smoothie drinks were derived from fruit and dairy smoothie drinks.
CHO, total carbohydrates; TE, total energy.
Further details of these measurements can be found in the UK Biobank online protocol (http://www.ukbiobank.ac.uk).
Statistical analyses
Associations between types of diets and cardiovascular events (CVD, HF, IHD, MI, and stroke) were investigated using Cox proportional hazard models with the time of follow-up used as the timeline variable. Individuals who self-reported being meat-eaters were used as the reference group. The results are reported as hazard ratios (HR) and their 95% confidence intervals (CI). The proportional hazard assumptions were checked using Schoenfeld residuals. Participants with MI and/or stroke at baseline were also excluded from all analyses (n = 15 737, 3.6%). For CVD incidence (outcome with the highest numbers of events), the Kaplan–Meier survival estimate was also calculated.
All Cox proportional analyses were performed using a 2-year landmark analysis, excluding participants who experienced events within the first 2 years of follow-up: 24 343 for overall CVD incidence (4504 IHD, 1026 MI, 689 strokes and 600 HF) and 538 for overall CVD mortality (258 IHD, 97 MI, 82 strokes and 47 HF).
We ran four incremental models for each outcome: ‘model 1’ included sociodemographic covariates (age, sex, deprivation, and ethnicity); ‘model 2’ additionally included multimorbidity (based on 43 diseases and coded as ordinal 1, 2, 3, 4, and ≥5); ‘model 3’ additionally included lifestyle factors (smoking, total discretionary sedentary time, alcohol intake, and total physical activity); and ‘model 4’ additionally included BMI at baseline.
In addition, to investigate whether the associations between the different types of diets and cardiovascular outcomes differed by subgroups, the models were re-run stratified by sex, age category (<60 and ≥60 years), BMI (normal/overweight and obese), and deprivation (below and above median).
Finally, we created a propensity score based in all the relevant covariates included in the study to investigate the associations between types of diets and the outcome using a matched propensity score design (see Supplementary material online, pages 14–16).
STATA 16 statistical software (StataCorp LP) was used to perform all analyses.
Results
A total of 422 791 participants (55.4% women) had data available for the types of diets and covariates of this study (Supplementary material online, Figure S1). Excluding the 2-year landmark period, the median follow-up period was 8.5 (interquartile range: 7.0–9.5) years for CVD incidence and 9.3 (interquartile range: 8.6–10.0) years for CVD mortality. Over the follow-up period, 106 690 (24.3%) developed CVD (24 794 IHD, 6770 MI, 5946 stroke, and 7685 HF) and 6580 (1.5%) died from CVD (2767 IHD, 885 MI, 1088 stroke, and 965 HF).
Sociodemographic and diet characteristics
The characteristics of the population by type of diet are presented in Table 1. The large majority of the participants were meat-eaters (94.7%) while fish and poultry eaters only constituted 1.1%. In comparison to meat-eaters, vegetarian, fish, and fish and poultry eaters were younger, more likely to be women, south Asian, and to have a lower BMI. Meat-eaters, in turn, were more likely to have more than one multimorbidity, and to be current smokers (Table 1). Similar characteristics by event occurrence are shown in Supplementary material online, Table S1.
Dietary intake characteristics by types of diets are shown in Table 2. In general, meat-eaters had a higher protein and total fat and lower carbohydrates and sugar intake, compared with the other diets. Meat-eaters showed the lowest consumption of fibre, polyunsaturated fat (PUFA), water, and fruit and vegetables. As expected, vegetarians were more likely to eat and buy vegetarian alternatives in comparison to meat-eaters (53.7% vs. 3.9%). However, vegetarians reported consuming more crisps, slices of pizza, and smoothie drinks than meat-eaters. Fish eaters were more likely to drink more than one glass/can of sugary drinks compared with the other groups and had the highest prevalence of ready meal consumption but also reported the lowest prevalence of takeaways. Fish and poultry eaters were more likely to eat home-cooked meals, followed by vegetarians (Table 2). Diet characteristics by the different types of diets and BMI (<25 and ≥25 kg/m2) and by event occurrence (develop or no develop the event) are shown in Supplementary material online, Tables S2 and S3, respectively.
Associations between types of diets and cardiovascular disease incidence and mortality
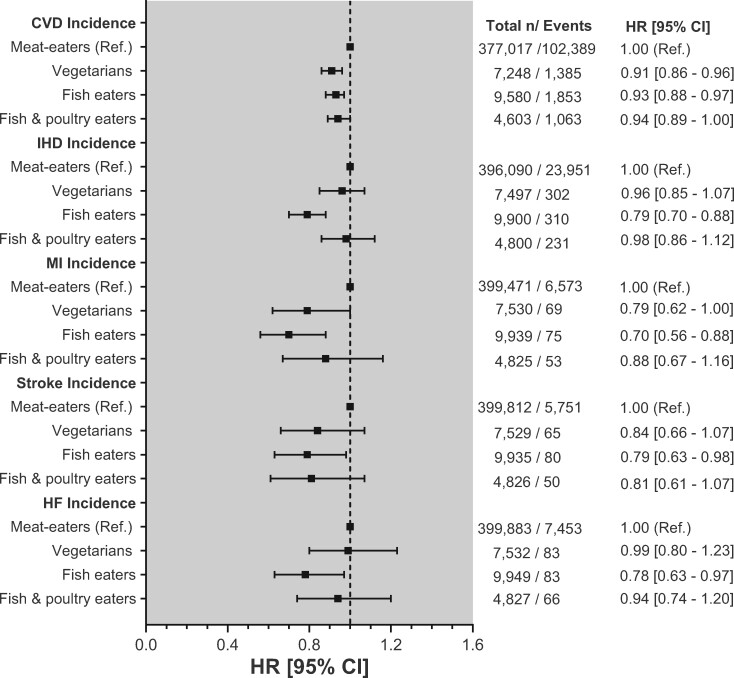
The associations of types of diets with incident CVD are shown in Figure 1 and Supplementary material online, Table S4. In the minimally adjusted model (Model 1), fish eaters had lower incident risk for CVD [HRCVD: 0.81 (95% CI: 0.78–0.85)], IHD [HRIHD: 0.68 (95% CI: 0.61–0.76)], MI [HRMI: 0.63 (95% CI: 0.50–0.79)], stroke [HRstroke: 0.73 (95% CI: 0.59–0.91)] and HF [HRHF: 0.62 (95% CI: 0.50–0.76)] compared with meat-eaters. After adjusting for multimorbidity, lifestyle, and BMI (Models 2–4), the associations were attenuated but remained significant [HRCVD: 0.93 (95% CI: 0.88–0.97), HRIHD: 0.79 (95% CI: 0.70–0.88), HRMI: 0.70 (95% CI: 0.56–0.88), HRstroke: 0.79 (95% CI: 0.63–0.98), and HRHF: 0.78 (95% CI: 0.63–0.97)]. Vegetarians, in contrast, showed a lower risk of MI in Models 1–3; however, this association was attenuated after adjusting for BMI [HRModel 4: 0.79 (95% CI: 0.62–1.00)]. For CVD incidence, vegetarians showed an association across the four models studied [HRModel 4: 0.91 (95% CI: 0.86–0.96)] (Figure 1). Although fish and poultry eaters were associated with CVD incidence, this association fully attenuated after the adjustments. No other associations were observed. The adjusted Kaplan–Meir survival estimated also showed that, compared with meat-eaters, the other types of diets had a higher probability of survival in terms of CVD incidence (Supplementary material online, Figure S2).
Figure 1.
Associations between types of diets and incident cardiovascular diseases. Data presented as adjusted hazard ratio and its 95% confidence interval by types of diets. Meat-eaters were used as the reference group. All analyses were performed using a 2-year landmark analysis, excluding participants who experienced events within the first 2 years of follow-up: 24 343 for overall cardiovascular disease incidence (4504 ischaemic heart disease, 1026 myocardial infarction, 689 strokes, and 600 heart failure). Analyses were adjusted by age, sex, deprivation, ethnicity, comorbidities, smoking, alcohol intake, total sedentary time, physical activity, and body mass index.
Take home figure.
Associations between types of diets and incident cardiovascular diseases.
In terms of mortality, fish eaters, compared with meat-eaters, had 30% and 41% lower risk of mortality from CVD and IHD, respectively (Supplementary material online, Table S5, Model 1). However, when the analyses were further adjusted, these associations fully attenuated. No other associations between the different types of diets and CVD mortality outcomes were observed (Supplementary material online, Figure S3 and Table 5).
When we investigated whether the association between the different types of diets and cardiovascular outcomes differed by subgroups, significant interactions were identified for CVD incidence between sex and vegetarians (P-interaction = 0.041) and fish and poultry eaters (P-interaction = 0.048); age and vegetarians (P-interaction < 0.001); BMI and vegetarians (P-interaction = 0.004), and fish eaters (P-interaction = 0.004). There was also an interaction between age and fish eaters for IHD; age and vegetarians for MI; and among fish eaters, sex and age for stroke (Supplementary material online, Table S6). In terms of mortality, a significant interaction was observed only between sex and CVD mortality for fish eaters and fish and poultry eaters (Supplementary material online, Table S7).
Finally, when the cox proportional analyses were restricted to participants who were matched by the propensity score, similar trends of associations were observed between types of diets and CVD incidence (Supplementary material online, Table S8). After matching, there was no imbalance in all included covariates (Supplementary material online, Figures S4–S6).
Discussion
In the current study, we have demonstrated that, compared with meat-eaters, fish eaters had a lower risk of several cardiovascular outcomes—incident CVD, IHD, MI, stroke, and HF—independent of confounders. People who ate poultry, as well as fish, did not have a lower risk, and vegetarians showed only lower risk of CVD incidence. However, previous studies have shown an inverse association between CVD and white meat-eaters (poultry and fish).19 , 20
Overall, the beneficial associations we demonstrated between types of diets and cardiovascular outcomes were strongest in men and individuals who were not obese. A systematic review and meta-analysis showed that Seventh Day Adventists (also vegetarians) had 40% lower risk of IHD in both sexes, but the associations on mortality and cerebrovascular disease were significant in men only.21 On the other hand, in our study, the associations were different according to the outcome studied for deprivation and age. In terms of age, taking into account that the risk of CVD incidence increases with age—and that older adults are more vulnerable to malnutrition—it was expected that older adults with a higher intake of fish could have a lower incidence risk. In addition, more deprived individuals who had a higher intake of fish had a lower risk of CVD incidence, although no interaction was observed. We previously demonstrated that the association between an unhealthy lifestyle and CVD mortality became stronger with increasing levels of deprivation.22 Therefore, individuals in our study who were more deprived but adopted a healthier lifestyle, such as fish intake, could have a greater protective effect.
In the UK, the associations between different types of diets and CVD have shown mixed results. For instance, Key et al.11 did not find any differences between vegetarians and meat-eaters for circulatory mortality, neither did Appleby et al.10 using participants from both the EPIC-Oxford study and the Oxford Vegetarian Study. More recently, Tong et al.6 demonstrated that despite vegetarians having a 22% lower risk of IHD compared with meat-eaters, they had 43% higher risk of haemorrhagic stroke and 20% higher risk of total stroke. In this line, other studies have shown that vegetarians from the Adventist Health Study had a lower risk of IHD and CVD compared with nonvegetarians.23 However, studies carried out outside the Adventist community did not show the same findings.21 In our study, we identified that vegetarians had a lower risk of incident CVD, but no association was identified for IHD as in previous studies. These heterogeneous results could be due to smaller numbers of incident IHD events among vegetarians in our study (n = 302); therefore, this analysis was probably underpowered [HRIHD: 0.96 (95% CI: 0.85–1.07)].
Fish eaters had a lower risk of incident CVD (for all outcomes included). Other studies have also shown an inverse relationship between fish and HF,19 cerebrovascular diseases,24 coronary heart disease,25 and IHD.6 Perhaps this association is not surprising considering that fish is an essential source of PUFA (mainly n-3), vitamin D, and selenium, nutrients that are cardioprotective. n-3 PUFA has been demonstrated to be cardioprotective, and oily fish is one of its rich sources.26 In our study, we did not find a significant difference in the overall PUFA intake of vegetarians and fish eaters, but we did not have data on specific categories of PUFA intake (n-3, n-6, or n-9). Despite this lack of information, it is likely that fish eaters had a higher intake of cardioprotective nutrients and, therefore, could explain the lower risk association between fish eaters and CVD outcomes in our study. However, in contrast to our results, Appleby et al.10 identified that fish eaters had 26% and 45% higher risk of circulatory and other circulatory diseases than meat-eaters after adjusting by BMI. The disparity between Appleby and our results could be explained by the general characteristic of the UK Biobank population, who—as reported by Fry et al.—have healthier lifestyles than the general UK population.27
Strength and limitations
UK Biobank is a large, prospective, general population cohort with data available on diet and a wide range of potential confounders and health outcomes. As a result, the analyses could be adjusted for multiple confounders and stratified by different subgroups. Among the limitations, our study used a single measure of diet at baseline, and diet may change over time. However, we attempted to mitigate this limitation by excluding those who reported changes in their diet. In addition, the association found was of modest absolute risk difference as shown in the adjusted survival curve. Owing to insufficient statistical power, we were unable to study vegan diets. On the other hand, while 94.7% of the population was classified as meat-eater, only 1.8% was classified as vegetarian. Although the National Diet and Nutrition Survey 2008–2012 reported a similar prevalence (∼2%),28 UK Biobank is not representative of the UK population in terms of lifestyle; therefore, the summary statistics should not be generalizable to the general population.27 In addition, the Vegan Society has reported a higher prevalence of vegans and vegetarians in the last years.29 Finally, the Oxford WebQ was not available for the whole population included in this study; therefore, dietary intake characteristics across types of diets may not represent the full UK Biobank cohort.
Conclusion
Compared with meat-eaters, fish eaters had a lower risk of a range of adverse cardiovascular outcomes, supporting its promotion as a healthy diet that should be encouraged. Vegetarianism was only associated with a lower risk of incident CVD. However, as a group, vegetarians consumed more unhealthy foods, such as crisps, than meat-eaters. Therefore, vegetarians should not be considered a homogeneous group, and avoidance of meat will not be sufficient to reduce health risk if the overall diet is not healthy.
Supplementary material
Supplementary material is available at European Heart Journal online.
Supplementary Material
Acknowledgements
We are grateful to UK Biobank participants. This research has been conducted using the UK Biobank resource under application number 7155.
Author contributions
F.P.-R. contributed to the conception and design of the study. C.C.-M., F.K.H., and J.P.P. advised on all statistical aspects. F.P.-R. performed the literature search, the analyses, and interpreted the data with support from C.C.-M., F.K.H., and J.P.P. All authors critically reviewed this and previous drafts. All authors approved the final draft for submission. F.K.H., C.C.-M., and J.P.P. contributed equally to this work and are joint senior authors. F.P.-R., C.C.-M., F.K.H., and J.P.P. are the guarantor.
Data availability
The data of this study can be requested from the UK Biobank (https://www.ukbiobank.ac.uk/).
Funding
UK Biobank was established by the Wellcome Trust medical charity, Medical Research Council, Department of Health, Scottish Government, and the Northwest Regional Development Agency. It has also had funding from the Welsh Assembly Government and the British Heart Foundation. All authors had final responsibility for submission for publication. F.P.-R. and S.P.-S. receive financial support from the Chilean Government for doing their PhD (ANID-Becas Chile).
Conflict of interest: N.S. has consulted for Amgen, Inc., Sanofi, and Astra Zeneca. J.P.P. has received funding from the Medical Research Council and Chief Scientist Office and has sat on the Medical Research Council Strategy Board and UK Biobank Scientific Advisory Board. None of these disclosures are directly related to the study, nor its conception, analyses, or interpretation.
Contributor Information
Fanny Petermann-Rocha, Institute of Health and Wellbeing, University of Glasgow, Glasgow G12 8RZ, UK; British Heart Foundation Glasgow Cardiovascular Research Centre, Institute of Cardiovascular and Medical Sciences, University of Glasgow, Glasgow, UK.
Solange Parra-Soto, Institute of Health and Wellbeing, University of Glasgow, Glasgow G12 8RZ, UK; British Heart Foundation Glasgow Cardiovascular Research Centre, Institute of Cardiovascular and Medical Sciences, University of Glasgow, Glasgow, UK.
Stuart Gray, British Heart Foundation Glasgow Cardiovascular Research Centre, Institute of Cardiovascular and Medical Sciences, University of Glasgow, Glasgow, UK.
Jana Anderson, Institute of Health and Wellbeing, University of Glasgow, Glasgow G12 8RZ, UK.
Paul Welsh, British Heart Foundation Glasgow Cardiovascular Research Centre, Institute of Cardiovascular and Medical Sciences, University of Glasgow, Glasgow, UK.
Jason Gill, British Heart Foundation Glasgow Cardiovascular Research Centre, Institute of Cardiovascular and Medical Sciences, University of Glasgow, Glasgow, UK.
Naveed Sattar, British Heart Foundation Glasgow Cardiovascular Research Centre, Institute of Cardiovascular and Medical Sciences, University of Glasgow, Glasgow, UK.
Frederick K Ho, Institute of Health and Wellbeing, University of Glasgow, Glasgow G12 8RZ, UK.
Carlos Celis-Morales, Institute of Health and Wellbeing, University of Glasgow, Glasgow G12 8RZ, UK; British Heart Foundation Glasgow Cardiovascular Research Centre, Institute of Cardiovascular and Medical Sciences, University of Glasgow, Glasgow, UK; Centre of Exercise Physiology Research (CIFE), Universidad Mayor, Santiago, Chile; Research Group in Education, Physical Activity and Health, University Catolica del Maule, Talca, Chile.
Jill P Pell, Institute of Health and Wellbeing, University of Glasgow, Glasgow G12 8RZ, UK.
Listen to the audio abstract of this contribution.
References
- 1. Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney M-T, Corrà U, Cosyns B, Deaton C, Graham I, Hall MS, Hobbs FDR, Løchen M-L, Löllgen H, Marques-Vidal P, Perk J, Prescott E, Redon J, Richter DJ, Sattar N, Smulders Y, Tiberi M, van der Worp HB, van Dis I, Verschuren WMM, Binno S; Group ESD. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 2016;37:2315–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GD Collaborators. Health effects of dietary risks in 195 countries, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019;393:1958–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Willett W, Rockström J, Loken B, Springmann M, Lang T, Vermeulen S, Garnett T, Tilman D, DeClerck F, Wood A, Jonell M, Clark M, Gordon LJ, Fanzo J, Hawkes C, Zurayk R, Rivera JA, De Vries W, Majele Sibanda L, Afshin A, Chaudhary A, Herrero M, Agustina R, Branca F, Lartey A, Fan S, Crona B, Fox E, Bignet V, Troell M, Lindahl T, Singh S, Cornell SE, Srinath Reddy K, Narain S, Nishtar S, Murray CJL. Food in the Anthropocene: the EAT-Lancet Commission on healthy diets from sustainable food systems. Lancet 2019;393:447–492. [DOI] [PubMed] [Google Scholar]
- 4. Johnston BC, Zeraatkar D, Han MA, Vernooij RWM, Valli C, El Dib R, Marshall C, Stover PJ, Fairweather-Taitt S, Wójcik G, Bhatia F, de Souza R, Brotons C, Meerpohl JJ, Patel CJ, Djulbegovic B, Alonso-Coello P, Bala MM, Guyatt GH. Unprocessed red meat and processed meat consumption: dietary guideline recommendations from the Nutritional Recommendations (NutriRECS) consortium. Ann Intern Med 2019;171:756–764. [DOI] [PubMed] [Google Scholar]
- 5. Rosenfeld DL, Burrow AL. Vegetarian on purpose: understanding the motivations of plant-based dieters. Appetite 2017;116:456–463. [DOI] [PubMed] [Google Scholar]
- 6. Tong TYN, Appleby PN, Bradbury KE, Perez-Cornago A, Travis RC, Clarke R, Key TJ. Risks of ischaemic heart disease and stroke in meat eaters, fish eaters, and vegetarians over 18 years of follow-up: results from the prospective EPIC-Oxford study. BMJ 2019;366:l4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tantamango-Bartley Y, Jaceldo-Siegl K, Fan J, Fraser G. Vegetarian diets and the incidence of cancer in a low-risk population. Cancer Epidemiol Biomarkers Prev 2013;22:286–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Craig WJ. Health effects of vegan diets. Am J Clin Nutr 2009;89:1627s–1633s. [DOI] [PubMed] [Google Scholar]
- 9. Kahleova H, Levin S, Barnard N. Cardio-metabolic benefits of plant-based diets. Nutrients 2017;9:848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Appleby PN, Crowe FL, Bradbury KE, Travis RC, Key TJ. Mortality in vegetarians and comparable nonvegetarians in the United Kingdom. Am J Clin Nutr 2016;103:218–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Key TJ, Appleby PN, Spencer EA, Travis RC, Roddam AW, Allen NE. Mortality in British vegetarians: results from the European Prospective Investigation into Cancer and Nutrition (EPIC-Oxford). Am J Clin Nutr 2009;89:1613s–1619s. [DOI] [PubMed] [Google Scholar]
- 12. Collins R. What makes UK Biobank special? Lancet 2012;379:1173–1174. [DOI] [PubMed] [Google Scholar]
- 13. Palmer LJ. UK Biobank: bank on it. Lancet 2007;369:1980–1982. [DOI] [PubMed] [Google Scholar]
- 14. Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, Downey P, Elliott P, Green J, Landray M, Liu B, Matthews P, Ong G, Pell J, Silman A, Young A, Sprosen T, Peakman T, Collins R. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med 2015;12:e1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Townsend PP, Beattie A. Health and deprivation. Inequality and the North. Health Policy (New York) 1988;10. [Google Scholar]
- 16.WHO. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. In: World Health Organization Technical Report Series, i–xii; 2000. p1–253. [PubMed]
- 17. Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet 2012;380:37–43. [DOI] [PubMed] [Google Scholar]
- 18. Galante J, Adamska L, Young A, Young H, Littlejohns TJ, Gallacher J, Allen N. The acceptability of repeat Internet-based hybrid diet assessment of previous 24-h dietary intake: administration of the Oxford WebQ in UK Biobank. Br J Nutr 2016;115:681–686. [DOI] [PubMed] [Google Scholar]
- 19. Li YH, Zhou CH, Pei HJ, Zhou XL, Li LH, Wu YJ, Hui RT. Fish consumption and incidence of heart failure: a meta-analysis of prospective cohort studies. Chin Med J (Engl) 2013;126:942–948. [PubMed] [Google Scholar]
- 20. Etemadi A, Sinha R, Ward MH, Graubard BI, Inoue-Choi M, Dawsey SM, Abnet CC. Mortality from different causes associated with meat, heme iron, nitrates, and nitrites in the NIH-AARP Diet and Health Study: population based cohort study. BMJ 2017;357:j1957–j1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kwok CS, Umar S, Myint PK, Mamas MA, Loke YK. Vegetarian diet, Seventh Day Adventists and risk of cardiovascular mortality: a systematic review and meta-analysis. Int J Cardiol 2014;176:680–686. [DOI] [PubMed] [Google Scholar]
- 22. Foster HME, Celis-Morales CA, Nicholl BI, Petermann-Rocha F, Pell JP, Gill JMR, O'Donnell CA, Mair FS. The effect of socioeconomic deprivation on the association between an extended measurement of unhealthy lifestyle factors and health outcomes: a prospective analysis of the UK Biobank cohort. Lancet Public Health 2018;3:e576–e585. [DOI] [PubMed] [Google Scholar]
- 23. Orlich MJ, Singh PN, Sabaté J, Jaceldo-Siegl K, Fan J, Knutsen S, Beeson WL, Fraser GE. Vegetarian dietary patterns and mortality in Adventist Health Study 2. JAMA Intern Med 2013;173:1230–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chowdhury R, Stevens S, Gorman D, Pan A, Warnakula S, Chowdhury S, Ward H, Johnson L, Crowe F, Hu FB, Franco OH. Association between fish consumption, long chain omega 3 fatty acids, and risk of cerebrovascular disease: systematic review and meta-analysis. BMJ 2012;345:e6698–e6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Streppel MT, Ocké MC, Boshuizen HC, Kok FJ, Kromhout D. Long-term fish consumption and n-3 fatty acid intake in relation to (sudden) coronary heart disease death: the Zutphen study. Eur Heart J 2008;29:2024–2030. [DOI] [PubMed] [Google Scholar]
- 26. Hall WL. The future for long chain n-3 PUFA in the prevention of coronary heart disease: do we need to target non-fish-eaters? Proc Nutr Soc 2017;76:408–418. [DOI] [PubMed] [Google Scholar]
- 27. Fry A, Littlejohns TJ, Sudlow C, Doherty N, Adamska L, Sprosen T, Collins R, Allen NE. Comparison of sociodemographic and health-related characteristics of UK Biobank participants with those of the general population. Am J Epidemiol 2017;186:1026–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Public, Health, England. National Diet and Nutrition Survey: Results from Years 1,2,3,4 (Combined) of the Rolling Programme (2008/2009–2011/2012). https://www.gov.uk/government/statistics/national-diet-and-nutrition-survey-results-from-years-1-to-4-combined-of-the-rolling-programme-for-2008-and-2009-to-2011-and-2012.
- 29.The, Vegan, Society. Statistics. https://www.vegansociety.com/news/media/statistics.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.