Abstract
Background
COVID-19 can lead to anxiety due to its high mortality rate. Patients with COVID-19 may suffer from muscle pain. This study aimed to determine the effect of guided imagery on anxiety, muscle pain, and vital signs in patients with COVID-19.
Methods
110 patients with COVID-19 were recruited and randomly assigned to two control and intervention groups. Data were collected using the Spielberger Anxiety Inventory, the McGill Pain Questionnaire, and the Visual Analogue Scale. The intervention group received ten training sessions of guided imagery.
Results
The results indicated a significant difference in the mean scores of state (t = -3.829, p < .001), trait anxiety (t = -2.946, p = .004), pain quality (t = -4.223, p < .001), pain intensity (t = -3.068, p = .003), and heart rate, systolic blood pressure, and oxygen saturation (p < .001) between the two groups after the intervention.
Conclusions
Guided imagery as a cost-effective method of complementary medicine is recommended to manage anxiety and pain in patients with COVID-19.
Keywords: Guided imagery, Anxiety, Pain, COVID-19, Clinical trial
1. Introduction
In December 2019, a cluster of pneumonia cases of unknown causes emerged in Wuhan, China, with clinical symptoms similar to viral pneumonia [1]. The new viral pneumonia was named COVID-19 (Coronavirus Disease), which has spread from the People's Republic of China to almost all countries, and on January 30, 2020, the World Health Organization (WHO) declared that the outbreak constitutes a public health emergency of international concern [2]. The WHO recently classified six clinical syndromes associated with COVID-19, including uncomplicated disease, mild pneumonia, severe pneumonia, Acute Respiratory Distress Syndrome (ARDS), sepsis, and septic shock. In uncomplicated cases, sufferers may have nonspecific symptoms such as fever, cough, sore throat, nasal congestion, weakness, headache, and muscle pain [3]. Chen et al. (2020) found that patients with COVID-19 suffer from clinical manifestations of fever (83%), cough (82%), dyspnea (31%), muscle pain (11%), confusion (9%), headache (8%), sore throat (5%), rhinorrhea (4%), chest pain (2%), diarrhea (2%), and nausea/vomiting (1%) [4]. Pimentel et al. (2020), in a study on patients with COVID-19, showed that during the disease course, blood pressure and fever remained within the normal range, and heart and respiration rates increased, and oxygen saturation decreased [5]. Moreover, Han et al. (2020) indicated that 44% of COVID-19 patients had muscle pain [6]. Regarding the lack of effective treatment for COVID-19, supportive therapies are mostly used as treatments, including oxygen therapy, antiviral drugs, and corticosteroid therapy [7].
However, to increase patient safety and prevent cross-infection in infectious respiratory patients (such as influenza, COVID-19), the contact, airborne, and droplet precautions should be used, which preferably require an isolation room [8]. Therefore, these patients should be quarantined or isolated for treatment, and most of them experience post-isolation anxiety as the clinical symptoms appear [9]. In a study by Wang et al. (2020), 28.8% of patients reported moderate to severe anxiety [10]. Other causes of anxiety in COVID-19 patients include stigma, social isolation, fear of death, uncertainty throughout the disease crisis, and immunological mechanisms [11,12].
As a type of psychological stress, anxiety causes a series of physiological and endocrine events, and ultimately weakens the immune system [9]. Ongoing anxiety causes the body to increase metabolism and consume more oxygen. As a result, the body responds to anxiety by increasing the depth of respiration [13]. Failure to relieve the anxiety causes cardiac, pulmonary, gastrointestinal, endocrine, and immune complications, while effective management of the pain and anxiety speeds up patients' recovery [14].
There are appropriate treatment strategies to alleviate anxiety's physical and psychological symptoms, including pharmacological and non-pharmacological interventions [15]. Pharmacological intervention is often time-consuming, leads to adverse effects, and increases healthcare costs [16]. Therefore, using non-pharmacological intervention as a complementary and not an alternative method is recommended to be used. There are methods in complementary medicine through which nurses can help patients [17]. In this regard, relaxation techniques are the most important non-pharmacological pain management methods [18]. Relaxation can reduce muscle tension and the destructive physiological effects of stress, such as high blood pressure, tachycardia, and muscle spasm by balancing the anterior and posterior hypothalamus' function, reducing the activity of the sympathetic nervous system, and releasing catecholamines [19]. Relaxation is performed in various methods such as progressive muscle relaxation, meditation, rhythmic breathing, etc. [20]. In this regard, guided imagery as a technique of mind-body medicine is based on the interconnection between body and mind as they can influence and strengthen each other in causing illness or being healthy. In guided imagery, the brain activated in the same area when experiencing an event is re-activated. In other words, a person creates exactly a stream of thoughts through which he/she would able to see, hear, feel, or smell what he/she desires or imagines in his/her imagination at the time [16]. Guided imagery is a relaxation technique that focuses on pleasant mental events and images rather than stressful emotions [21]. In this method, the client is involved with mental imagery to the extent that the body responds to it as a real experience to cause profound physiological consequences [22]. This method, as a complementary medicine technique, can be learned through either an instructor or self-study materials and used in cases of pain and anxiety to reduce pain and psychological stress [16]. Regarding the high levels of anxiety in COVID-19 patients [9,10], they suffer from severe muscle pain [6] as both anxiety and muscle pain can cause hemodynamic instability, delay the patient's recovery, and put the patient at risk. As a result, effective complementary medicine techniques are needed to help these patients. Regarding that, it is the nurses' duty to implement complementary therapies. The use of relaxation techniques such as guided imagery can be easily taught and performed by nurses, and the patients can learn these methods and perform them independently. Most importantly, these measures can establish deeper communication between the nurse and the client. This study aimed to determine the effect of guided imagery on anxiety, muscle pain, and vital signs in patients with COVID-19. Our hypotheses were:
-
1
Patients who receive guided imagery will have greater reduction in pain intensity than patients in the control group.
-
2
Patients who receive guided imagery will have greater reduction in pain quality than patients in the control group.
-
3
The mean anxiety scores of the patients who receive guided imagery will be higher than patients in the control group.
-
4
Guided imagery may affect patients' vital signs.
2. Methods
2.1. Research design
A single-blinded, parallel, randomized controlled trial was designed to achieve the research objectives.
2.2. Participants
After obtaining approval from the Faculty Research Committee and the Ethics Committee of Urmia University of Medical Sciences (with the ethics number of IR.UMSU.REC.1399.102), the researcher first referred to the research setting (temporary accommodations for COVID-19 patients in Urmia) and then received permission from the authorities to conduct the study. Inclusion criteria consisted of (a) signing written informed consent, (b) being literate, being conscious and oriented to time, place, and person, (c) having no severe visual and auditory disorders, (d) having no mental disorders, (e) having no history of hospitalization with a diagnosis of COVID-19, (f) having an oxygen saturation level of above 90%, and (g) being in the 18–60 age range. Exclusion criteria consisted of (a) withdrawal from the study because of any reason, (b) having oxygen saturation level of below 90%, (c) being seriously ill for any reason (such as ARDS), (d) departure from the temporary accommodation or being transfer to the hospital, (e) being discharged with personal consent or death, and (f) performing any procedure out of the routine program.
Eligible patients were selected using convenience sampling, and written informed consent was then obtained from those who achieved the inclusion criteria. The sample size was calculated using STATA software (StataCorp LP, College Station, TX, USA). According to the study by Shamekhi et al. [23] in which the mean score and standard deviation in the intervention and control groups were 82.67 ± 14.03 and 90.17 ± 11.73, respectively. The minimum sample size for each group was calculated 48 patients, based on the confidence interval of 95% and the power of 80%. The final sample size was 55 patients for each group and 110 patients for both intervention and control groups to consider a 10% attrition rate.
2.3. Intervention
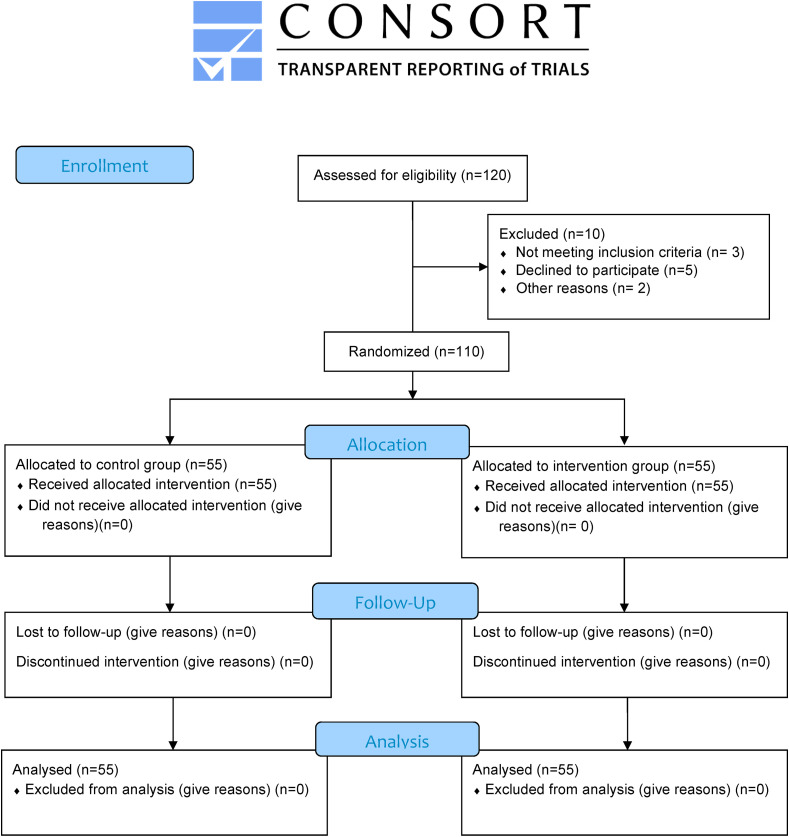
The participants were randomly allocated to two groups of intervention and control. To this end, the sealed envelope system was utilized as a total of 110 cards were prepared, and the letter G (Guided imagery group) was written on half of the cards (n = 55), and the letters C (control group) was written on another half (n = 55). The cards were then mixed, and at the patient's bedside, one of the cards was randomly selected by the patients. Then they were divided into one of two groups based on the letter of the cards. In the first session and after observing safety points (hand washing and sanitizing before and after the guided imagery sessions) and using personal protective equipment (N95 respirator, and medical gloves, gown, disposable cap, and shoe cover), the researcher introduced himself to the patients and explained the study methodology and objective. Then written informed consent was taken from all the participants. Moreover, preliminary data, including demographic characteristics, vital signs, and oxygen saturation were collected in the first session, and the questionnaires were filled in by the participants in both intervention and control groups. The researcher also completed the vital signs flow sheet before the intervention. The researcher recruitment and randomization until the target sample size (n = 110) was reached in a month period. Recruitment started on June 15, 2020 and ended on July 07, 2020. Among 120 consecutive qualified patients, 5 patients declined to participate in the study, 3 patients did not meet the inclusion criteria and 2 patients were transferred to other medical centers due to exacerbation of the disease (see Fig. 1 ). The patients were placed in separate rooms to prevent the probability of data contamination between the two intervention and control groups. The staff was also justified in this regard. Patients in both groups received routine care. However, in the intervention group, guided imagery protocol was conducted under a psychiatrist's supervision. In the intervention group, in parallel with routine care, each patient attended ten sessions of guided imagery for five consecutive days, twice a day (once from 9:00 to 9:30 a.m. and once from 6:00 to 6:30 p.m.). In each session, five different audio tracks were administered by the nurse, and the patient listened to the instructional guided imagery audio tracks using a headphone for about 25 min. Each session had five different guided imagery audio tracks from other sessions. During each session, the patient closed his/her eyes, took deep breaths, and relaxed his/her muscles to keep completely calm. Then, he/she moved towards the relevant imagery using the mind power and imagination [24]. The research team provided adequate standard headphones to conduct the intervention. Prepared audio tracks [25], which contained guided imagery training for the power of mind and visualization to control horrific events, were approved by a psychiatrist and the Ethics Committee members. The researcher himself had also been trained in guided imagery by a psychiatrist. During each session, it was tried to reduce any distractions such as background noises and help the patients concentrate upon the intervention. In addition, all phases of the study were performed under the supervision of an infectious disease specialist to tackle the problems. After the completion of training sessions, the questionnaires and the vital signs flow sheet were completed again. Finally, the data obtained from the two groups were compared.
Fig. 1.
Research flow diagram based on Consort statement 2010.
2.4. Data collection
In the present study, data collection tools consisted of the demographic questionnaire, the Spielberger State-Trait Anxiety Inventory (STAI), the Short-Form McGill Pain Questionnaire (SF-MPQ), the Visual Analogue Scale (VAS), and the Vital Signs Flow Sheet. The demographic questionnaire included age, gender, education, marital status, occupation, residence, and smoking.
The Spielberger State-Trait Anxiety Inventory (STAI) is a 40-item self-report tool and consists of two parts. The first part measures state anxiety and includes 20 questions. This part is scored on a 4-point Likert scale from “Not at all = 1″ to “Very much so = 4".The second part measures trait anxiety and also includes 20 questions. This part is also scored on a 4-point Likert scale from “Almost never = 1″ to “Almost always = 4". The overall score of each part ranges from 20 to 80, so that low scores indicate a mild form of anxiety, whereas median scores indicate a moderate form of anxiety, and high scores indicate a severe form of anxiety [26]. In the study by Spielberger et al. (1983), the Cronbach's alpha coefficient for the state and the trait anxiety scale was reported to be 0.92 and 0.90, respectively. Moreover, the test-retest coefficient for the state and the trait anxiety scale was reported to be 0.62 and 0.68, respectively [27]. In Iran, the validity of this questionnaire has been confirmed, and its internal consistency has been corroborated as having a Cronbach's alpha of .91 [28,29].
The Short-Form McGill Pain Questionnaire (SF-MPQ) is a shorter version of the original MPQ and was developed by Melzak in 1987 [30]. This tool is made up of 15 descriptors in two subscales (11 sensory; 4 affective), and the Pain Rating Index has six rank values (from “No pain” to “Excruciating.” This scale has been used in numerous studies for assessing different types of pain. Stephenson and Herman (2000) found a good correlation between the SF-MPQ and the original MPQ (r = 0.86). Moreover, for internal consistency reliability, they reported Cronbach's alpha of 0.90 [31]. In Iran, Khosravi et al. (2013) developed the Persian version of this questionnaire using the cross-cultural adaptation with preserving the original structure. They also confirmed its reliability with Cronbach's alpha of above 0.8 for overall and all subscales of the questionnaire [32].
The Visual Analogue Scale is a psychometric instrument used to measure pain intensity. This scale consists of a 100 mm horizontal line with the left side signifying “no pain = 0″ and the right side signifying “worst pain = 100". The participants were asked to mark a spot on the line indicating their current level of pain or report its numerical value to the researcher. The pain intensity was divided into three levels of mild (1–30), moderate (40–70), and severe pain (80–100). This scale has been widely used, and its validity and reliability have been confirmed in case of acute pain [33]. The scale's reliability was assessed using Cronbach's alpha, which was found to be 0.94 [34]. The CONSORT 2010 checklist was used to ensure quality reporting in this study [35] (see Supplementary File).
2.5. Statistical analysis
After data collection, the Shapiro-Wilk test was used to determine the normality of data distribution. The researcher who was blinded to the data, conducted the analysis. All data were entered into IBM SPSS Statistics for Windows, version 25 (IBM Corp., Armonk, N.Y., USA) and analyzed using descriptive (frequency and percentage for analyzing qualitative variables, mean and standard deviation for normal quantitative variables, and median and Interquartile Range for non-normal quantitative variables) and analytical statistics (Chi-square and Fisher's exact test for examining the group homogeneity). The independent-samples t-test was also used to compare the normally distributed parameters between the two groups. Finally, the paired-samples t-test was used for in-group comparisons of normally distributed parameters. All analyses were performed by a researcher who was blind to the data.
3. Results
The results showed no statistically significant difference between the two groups in terms of gender, education, marital status, employment, residence, and smoking (p > .05). However, there was a statistically significant difference in terms of age between the two groups (p = .01) (Table 1 ).
Table 1.
Comparison of demographic characteristics of the patients in the study groups.
| Variable | Group |
Result | ||
|---|---|---|---|---|
| Control |
Intervention |
|||
| n (%) | n (%) | |||
| Gender | Male | 32 (58.2) | 30 (54.5) | x = .148 |
| Female | 23 (41.8) | 25 (45.5) | df = 1ap-value = .701 | |
| Elementary | 4 (7.3) | 4 (7.3) | ||
| Secondary | 10 (18.2) | 13 (23.6) | x = 4.055 | |
| Education level | High school | 12 (21.8) | 19 (34.5) | df = 3 |
| Higher education | 29 (52.7) | 19 (34.5) | ap-value = .256 | |
| Marital status | Single | 18 (32.7) | 13 (23.6) | bp-value = .397 |
| Married | 37 (67.3) | 42 (76.4) | ||
| Occupation | Employed | 35 (63.6) | 35 (63.6) | x = .784 |
| Unemployed | 18 (32.7) | 16 (29.1) | df = 2 | |
| Inactive | 2 (3.6) | 4(7.3) | ap-value = .676 | |
| Residence | Rural | 13 (23.6) | 18 (32.7) | x = 1.123 |
| Urban | 42 (76.4) | 37 (67.3) | df = 1ap-value = .289 | |
| Smoking | Yes | 32 (58.2) | 36 (65.5) | bp-value = .278 |
| No | 23 (41.8) | 19 (34.5) | ||
| Age | Control | Intervention | t = 2.610 | |
| Mean ± SDd | Mean ± SD | df = 108 | ||
| 37.32 ± 11.12 | 43.14 ± 12.22 | cp-value = .010 | ||
Chi-squared test.
Fisher's exact test.
Independent-samples t-test.
SD: "Standard Deviation
The results of the independent-samples t-test indicated no statistically significant difference in the mean score of the state (t = -0.627, p = .532) and trait anxiety (t = 0.360, p = .719) between the two groups. Moreover, Cohen's d showed that the difference in the mean score of the state (d = 0.11) and trait anxiety (d = 0.06) between the two groups was small (Table 2 ). However, after the intervention, the difference in the mean scores of the state (t = -3.829, p < .001) and trait anxiety (t = -2.946, p = .004) were statistically significant between the two groups. In addition, Cohen's d showed that after the intervention, the difference in the mean score of the state (d = 0.73) and trait anxiety (d = 0.56) between the two groups was fairly large and medium, respectively (Table 3 ).
Table 2.
Comparison of the anxiety mean scores in the study groups before the intervention.
| Variable | Group | N | Mean ± SD | Result | Effect size(Cohen's d) |
|---|---|---|---|---|---|
| State anxiety | Guided imagery | 55 | 45.03 ± 13.38 | t = -.627 df = 108 | 0.11 |
| Control | 55 | 46.72 ± 14.86 | ap = .532 | ||
| Trait anxiety | Guided imagery | 55 | 47.34 ± 13.16 | t = .360 df = 108 | 0.06 |
| Control | 55 | 46.47 ± 12.21 | ap = .719 |
Independent-samples t-test.
Table 3.
Comparison of the anxiety mean scores in the study groups after the intervention.
| Variable | Group | N | Mean ± SD | Result | Effect size(Cohen's d) |
|---|---|---|---|---|---|
| State anxiety | Guided imagery | 55 | 38.27 ± 10.04 | t = -3.829 df = 108 | 0.73 |
| Control | 55 | 47.21 ± 14.11 | ap < .001 | ||
| Trait anxiety | Guided imagery | 55 | 39.58 ± 10.05 | t = -2.946 df = 108 | 0.56 |
| Control | 55 | 46.00 ± 12.64 | ap = .004 |
Independent-samples t-test.
The results of the paired-samples t-test revealed that the mean scores of the state (t = 8.161, p < .001) and the trait anxiety (t = 7.962, p < .001) significantly differed in the intervention group after the intervention compared to before the intervention. Moreover, it was found that the mean scores of the state (t = -1.259, p = .214) and the trait anxiety (t = 0.487, p = .629) did not differ significantly in the control group after the intervention compared to before the intervention. Moreover, according to Cohen's d, the difference in the mean score of the state (d = 1.10) and trait anxiety (d = 1.07) in the guided imagery group after the intervention compared to before the intervention was large. But the difference in the mean score of the state (d = 0.16) and trait anxiety (d = 0.06) in the control group was small. (Table 4 ).
Table 4.
Comparison of the anxiety mean scores of the patients within the study groups before and after the intervention.
| Variable | Group | Anxiety mean score before the intervention |
Anxiety mean score after the intervention |
Result | Effect size(Cohen's d) |
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | ||||
| State anxiety | Guided imagery | 45.03 ± 13.38 | 38.27 ± 10.04 | t = 8.161 | 1.10 |
| df = 54 | |||||
| ap < .001 | |||||
| Control | 46.72 ± 14.86 | 47.21 ± 14.11 | t = -1.259 | 0.16 | |
| df = 54 | |||||
| ap = .214 | |||||
| Trait anxiety | Guided imagery | 47.34 ± 13.16 | 39.58 ± 10.05 | t = 7.962 | 1.07 |
| df = 54 | |||||
| ap < .001 | |||||
| Control | 46.47 ± 12.21 | 46.00 ± 12.64 | t = .487 | 0.06 | |
| df = 54 | |||||
| ap = 0.628 |
Paired-samples t-test.
The results of the independent-samples t-test indicated no statistically significant difference between the two groups in terms of pain quality (t = 0.457, p = .649) and pain intensity (t = -0.852, p = .398) before the intervention. Moreover, Cohen's d indicated that the difference in the mean score of the pain quality (d = 0.08) and pain intensity (d = 0.16) between the two groups was fairly small (Table 5 ). However, after the intervention, there was a statistically significant difference between the two groups in terms of pain quality (t = -4.223, p < .001) and pain intensity (t = -3.068, p = .003) after the intervention. Also, Cohen's d revealed that after the intervention, the difference in the mean score of the pain quality (d = 0.80) and pain intensity (d = 0.58) between the two groups was large and fairly medium, respectively (Table 6 ).
Table 5.
Comparison of the pain mean scores of the patients in the study groups before the intervention.
| Variable | Group | N | Mean ± SD | Result | Effect size(Cohen's d) |
|---|---|---|---|---|---|
| Quality of pain | Guided imagery | 55 | 30.90 ± 5.51 | t = .457 df = 108 | 0.08 |
| Control | 55 | 30.43 ± 5.34 | ap = .649 | ||
| Intensity of pain | Guided imagery | 55 | 41.67 ± 10.75 | t = -.852 df = 108 | 0.16 |
| Control | 55 | 43.45 ± 11.17 | ap = .398 |
Independent-samples t-test.
Table 6.
Comparison of the pain mean scores of the patients in the study groups after the intervention.
| Variable | Group | N | Mean ± SD | Result | Effect size(Cohen's d) |
|---|---|---|---|---|---|
| Quality of pain | Guided imagery | 55 | 26.47 ± 3.67 | t = - 4.223 df = 108 | 0.80 |
| Control | 55 | 30.12 ± 5.26 | ap < .001 | ||
| Intensity of pain | Guided imagery | 55 | 37.49 ± 9.99 | t = -3.068 df = 108 | 0.58 |
| Control | 55 | 43.41 ± 10.26 | ap = .003 |
Independent-samples t-test.
The results of the paired-samples t-test revealed that the mean scores of pain quality (t = 6.651, p < .001) and pain intensity (t = 6.069, p < .001) before and after the intervention were significantly different in the intervention group. The results also showed that the mean scores of pain quality (t = 1.041, p = .302) and pain intensity (t = 0.065, p = .948) before and after the intervention were not statistically significant in the control group. Moreover, Cohen's d showed that the difference in the mean score of the pain quality (d = 0.89) and pain intensity (d = 0.81) in the guided imagery group after the intervention compared to before the intervention was large. But the difference in the mean score of the pain quality (d = 0.14) and pain intensity (d = 0.01) in the control group was small (Table 7 ).
Table 7.
Comparison of the pain mean scores of the patients within the study groups before and after the intervention.
| Variable | Group | Mean score of pain before the intervention |
Mean score of pain after the intervention |
Result | Effect size(Cohen's d) |
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | ||||
| Quality of pain | Guided imagery | 30.90 ± 5.51 | 26.47 ± 3.67 | t = 6.651 df = 54 | 0.89 |
| ap < .001 | |||||
| Control | 30.43 ± 5.34 | 30.12 ± 5.26 | t = 1.041 df = 54 | 0.14 | |
| ap = .302 | |||||
| Intensity of pain | Guided imagery | 41.67 ± 10.75 | 37.49 ± 9.99 | t = 6.069 df = 54 | 0.81 |
| ap < .001 | |||||
| Control | 43.45 ± 11.17 | 43.41 ± 10.26 | t = .065 df = 54 | 0.01 | |
| ap = .948 |
Paired-samples t-test.
The results of the paired-samples t-test showed that, in the intervention group, there was a statistically significant difference in heart rate (t = 6.672, p < .001), systolic blood pressure (t = 5.493, p < .001), and oxygen saturation (t = -14.99, p < .001) before and after the guided imagery, although the difference in terms of respiratory rate (t = -0.331, p = .742), body temperature (t = -0.705, p = .484), and diastolic blood pressure was not statistically significant (t = 0.230, p = .819). Furthermore, in the control group, the results showed no statistically significant difference in heart rate, respiratory rate, body temperature, systolic and diastolic blood pressure, and oxygen saturation before and after the guided imagery (Table 8 ).
Table 8.
Comparison of the mean vital sign score of the patients within the study groups before and after the intervention.
| Vital signs | Group | Mean score before the intervention |
Mean score after the intervention |
Result | Effect size(Cohen's d) |
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | ||||
| Pulse Rate(beat per minute) | Guided imagery | 79.29 ± 11.10 | 74.41 ± 9.07 | t = 6.672 df = 54 | 0.90 |
| ap < .001 | |||||
| Control | 78.94 ± 10.28 | 79.40 ± 10.43 | t = -1.360 df = 54 | 0.18 | |
| ap = .179 | |||||
| Respiratory Rate(breaths per minute) | Guided imagery | 16.72 ± 1.77 | 16.76 ± 1.79 | t = -.331 df = 54 | 0.04 |
| ap = .742 | |||||
| Control | 17.12 ± 2.20 | 16.96 ± 2.05 | t = 1.176 df = 54 | 0.15 | |
| ap = .245 | |||||
| Temperature(Celsius scale) | Guided imagery | 37.03 ± 0.26 | 37.06 ± 0.26 | t = -.705 df = 54 | 0.10 |
| ap = .484 | |||||
| Control | 37.07 ± 0.29 | 37.05 ± 0.28 | t = 0.459 df = 54 | 0.06 | |
| ap = .648 | |||||
| Systolic Pressure(mm Hg) | Guided imagery | 124.91 ± 8.44 | 119.89 ± 9.59 | t = 5.493 df = 54 | 0.74 |
| ap < .001 | |||||
| Control | 123.91 ± 7.02 | 123.65 ± 6.94 | t = .581 df = 54 | 0.07 | |
| ap = .564 | |||||
| Diastolic Pressure(mm Hg) | Guided imagery | 80.44 ± 7.21 | 80.40 ± 7.00 | t = .230 df = 54 | 0.03 |
| ap = .819 | |||||
| Control | 80.78 ± 6.51 | 80.69 ± 6.72 | t = .510 df = 54 | 0.06 | |
| ap = .612 | |||||
| O2 Saturation(percent) | Guided imagery | 95.61 ± 1.40 | 98.07 ± 0.93 | t = -14.99 df = 54 | 2.03 |
| ap < .001 | |||||
| Control | 96.52 ± 1.92 | 96.41 ± 1.68 | t = .375 df = 54 | 0.05 | |
| ap = .709 |
Paired-samples t-test.
4. Discussion
The present study aimed to determine the effect of guided imagery on anxiety, muscle pain, and vital signs in patients with COVID-19. The results showed a moderate level of anxiety among participants. It was also found that guided imagery can reduce the level of anxiety in COVID-19 patients. The results also showed that COVID-19 patients experienced a moderate level of the quality and intensity of pain, and guided imagery reduced the intensity and quality of pain among COVID-19 patients. Guided imagery can also affect heart rate, systolic blood pressure, and oxygen saturation in patients with COVID-19.
Guo et al. (2020) reported moderate anxiety levels in quarantined COVID-19 patients [36]. Peloso et al. (2020) showed that most patients with COVID-19 did not feel calm during the quarantine, and 28.6% of patients experienced anxiety, and 23.2% showed fear of the disease [37].
In a study by Liu et al. (2020), COVID-19 patients had high anxiety levels. In their study, progressive muscle relaxation was utilized to relieve the patients' anxiety. They revealed that this method of complementary medicine is effective in reducing anxiety [38]. Zhang et al. (2020) also showed that the COVID-19 patients suffered from high levels of anxiety, so that a high level of anxiety was associated with being female since females would experience more psychological symptoms [39]. Wang et al. (2020) stated that 28.8% of patients with COVID-19 had moderate to high anxiety [10]. Of course, fear of death increases anxiety in patients with COVID-19, as seen in the SARS (Severe Acute Respiratory Syndrome) outbreak [40]. Besides, when the level of anxiety is higher than normal, it weakens the immune system, which, in turn, can cause the novel coronavirus to attack the body. The results of the above studies are consistent with the results of the present study.
Alam et al. (2016) showed that guided imagery reduces anxiety during excisional procedures. However, in contrast to our study results, it did not affect the patients' pain and vital signs, i.e., pulse rate and blood pressure [41]. Beizaee et al. (2018) indicated that guided imagery was effective in reducing patients' anxiety and also had an effect on patients' heart rate. However, in contrast to our study results, they found that guided imagery also affected patients' respiratory rate [42]. Inconsistent results may be due to differences in the number of statistical population and pathophysiology of the type of diseases in the two studies and the underlying factors affecting the vital signs of patients during hospitalization.
It has recently been found that COVID-19 is associated with symptoms, including myalgia, arthralgia, abdominal pain, headache, and chest pain. Even patients who are not hospitalized in Intensive Care Units (ICUs) may suffer from pain so that they require strategies to reduce their pain intensity [43]. Han et al. (2020) showed that patients with COVID-19 suffer from muscle pain [6]. Delfani et al. (2016) also revealed that guided imagery reduces patients' pain intensity [44]. The results of the above studies are also consistent with the results of our study.
Guided imagery is a spiritual method that navigates the participant's imagination to a calm and positive condition that reduces anxiety and promote well-being. With this method, a nurse uses a script to deliberately guide one's imagination to bring up positive and relax mental images [45]. Although guided imagery can be simple to implement in practice, nurses play a fundamental role in the implementation of guided imagery because patients can easily diverge from the train of thought during the implementation.
One of the limitations of this study is the participants’ differences and psychological conditions, which can affect their anxiety and pain. The second limitation was the effect of the research setting on the intervention as the high contagious nature of the COVID-19 can cause anxiety for the researcher. To tackle this limitation, the researcher was selected from the health team of the COVID-19 units and equipped with personal protective equipment to reduce his anxiety to some extent. In regards to the variables examined in this study being psychological and likely changing over time, the cross-sectional nature and small sample size of this study could present limitations. Therefore, it is recommended that similar studies should be conducted on larger populations over a longer period of time. Thus, it is recommended that similar studies should be conducted on larger populations over a longer time period.
5. Conclusions
Novel coronavirus has caused global concern due to its emergence and being unknown as well as its high mortality rate. In this situation, the main responsibility of the medical staff is to implement strategies to adapt to these stressful conditions. Nurses have a fundamental and important role in promoting the patients’ mental and physical health and consequently reducing patients' anxiety and pain. The results showed that the implementation of guided imagery by nurses reduces anxiety and pain in COVID-19 patients, which indicates the effectiveness of this complementary method. Therefore, the guided imagery as a cost-effective, easy-to-use, and uncomplicated complementary medicine method is recommended for managing anxiety and pain in COVID-19 patients, which can increase relaxation and boost patients' immune systems to fight against COVID-19.
Ethics approval and consent to participate
The ethics committee of Urmia University of Medical Sciences approved the study (Ethical code: IR.UMSU.REC.1399.102). This study was registered in the Iranian Registry of Clinical Trials (Registration code: IRCT20131112015390N5) on September 26, 2020. The participants were fully informed about the purpose of the study. Each participant provided written consent prior to participation. They were explained regarding their voluntary nature of participation and that they can stop cooperation at any given time. They also assured about their privacy and confidentiality of their information.
Authors’ contributions
NP, RG, NF, RM, MMQ, RB, NGH, HF, MMH: Conceptualization, Methodology, Software. NP, RG, NF, RM, MMQ: Data curation, Writing- Original draft preparation. NP, RG, NF, RM, MMQ, RB, NGH, HF: Visualization, Investigation NP, RG: Supervision: NP, RG, NF, RM, MMQ, RB, NGH, HF, MMH: Software, Validation: RB, NGH, HF, MMH: Writing- Reviewing and Editing, all authors read and approved the final manuscript before submission.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Acknowledgment
This study is derived from a research project approved by the Ethics Committee of Urmia University of Medical Sciences (with a registration number of 10185 and ethics number of IR.UMSU.REC.1399.102). This study is also registered in the Iranian Registry of Clinical Trials (with registry code of IRCT20131112015390N5). The researchers express their gratitude to the Vice-Chancellor for Research of Urmia University of Medical Sciences and all patients who helped us conduct this study. We also thank the medical staff, especially nurses, working at Taleghani Hospital and temporary accommodations of patients with COVID-19 in Urmia.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ctcp.2021.101335.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Lu H., Stratton C.W., Tang Y.W. Outbreak of pneumonia of unknown etiology in Wuhan, China: the mystery and the miracle. J. Med. Virol. 2020;92(4):401–402. doi: 10.1002/jmv.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. 10223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo Y.R., Cao Q.D., Hong Z.S., Tan Y.Y., Chen S.D., Jin H.J., et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak–an update on the status. Military Medical Research. 2020;7(1):1. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. Jama. 2020;323(11):1061–1069. doi: 10.1101/2020.02.11.20022053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pimentel M.A., Redfern O.C., Hatch R., Young J.D., Tarassenko L., Watkinson P.J. Trajectories of vital signs in patients with COVID-19. Resuscitation. 2020;156:99–106. doi: 10.1016/j.resuscitation.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han R., Huang L., Jiang H., Dong J., Peng H., Zhang D. Early clinical and CT manifestations of coronavirus disease 2019 (COVID-19) pneumonia. Am. J. Roentgenol. 2020;215(2):338–343. doi: 10.2214/AJR.20.22961. [DOI] [PubMed] [Google Scholar]
- 7.Chen X., Shang Y., Yao S., Liu R., Liu H. Perioperative care provider's considerations in managing patients with the COVID-19 infections. Transl Perioper Pain Med. 2020;7:216–224. [Google Scholar]
- 8.Sommerstein R., Fux C.A., Vuichard-Gysin D., Abbas M., Marschall J., Balmelli C., et al. Risk of SARS-CoV-2 transmission by aerosols, the rational use of masks, and protection of healthcare workers from COVID-19. Antimicrob. Resist. Infect. Contr. 2020;9(1):1–8. doi: 10.1186/s13756-020-00763-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rajeswari S., SanjeevaReddy N. Efficacy of progressive muscle relaxation on pregnancy outcome among anxious Indian primi mothers. Iran. J. Nurs. Midwifery Res. 2020;25(1):23–30. doi: 10.4103/ijnmr.IJNMR_207_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang C., Pan R., Wan X., Tan Y., Xu L., Ho C.S., et al. Immediate psychological responses and associated factors during the initial stage of the 2019 coronavirus disease (COVID-19) epidemic among the general population in China. Int. J. Environ. Res. Publ. Health. 2020;17(5):1729. doi: 10.3390/ijerph17051729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Medeiros Carvalho P.M., Moreira M.M., de Oliveira M.N., Landim J.M., Neto M.L. The psychiatric impact of the novel coronavirus outbreak. Psychiatr. Res. 2020;286:112902. doi: 10.1016/j.psychres.2020.112902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brooks S.K., Webster R.K., Smith L.E., Woodland L., Wessely S., Greenberg N., Rubin G.J. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet. 2020;395:912–920. doi: 10.1016/S0140-6736(20)30460-8. 10227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor C.R. Lippincott Wiliam &Wilkins; 2010. Pain Management, Fundamental of Nursing. [Google Scholar]
- 14.Hinkle J.L., Cheever K.H. Wolters kluwer india Pvt Ltd; 2018 Aug 30. Brunner and Suddarth's Textbook of Medical-Surgical Nursing. [Google Scholar]
- 15.Phipps W.J., Sands J.K., Marek J.F., Neighbors M. seventh ed. Mosby; London: 2010. Medical Surgical Nursing; pp. 364–382. [Google Scholar]
- 16.Tadayonfar M., Mohebbi M., Koushan M., Rakhshani M.H. The effects of guided imagery on anxiety level of the patients undergoing appendectomy. Journal of Sabzevar University of Medical Sciences. 2014;20(5):681–688. [Google Scholar]
- 17.Ball J.W., DrPH R.N., Bindler R.M., Cowen K.J., Shaw M.R. Prentice Hall; Upper Saddle River: 2013. Child Health Nursing. [Google Scholar]
- 18.LeMone P., Burke K., Dwyer T., Levett-Jones T., Moxham L., Reid-Searl K. Pearson Higher Education AU; New York: 2015. Medical Surgical Nursing. [Google Scholar]
- 19.Brown R.P., Gerbarg P.L., Muench F. Breathing practices for treatment of psychiatric and stress-related medical conditions. Psychiatr. Clin. 2013;36(1):121–140. doi: 10.1016/j.psc.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 20.Papathanassoglou E., Park T. To put the patient in the best condition: integrating integrative therapies in critical care. Nurs. Crit. Care. 2016;21(3):123–126. doi: 10.1111/nicc.12243. [DOI] [PubMed] [Google Scholar]
- 21.Bozorg-Nejad M., Azizkhani H., Mohaddes Ardebili F., Mousavi S.K., Manafi F., Hosseini A.F. The effect of rhythmic breathing on pain of dressing change in patients with burns referred to ayatollah mousavi hospital. World J. Plast. Surg. 2018;7(1):51–57. PMID: 29651392; PMCID: PMC5890366. [PMC free article] [PubMed] [Google Scholar]
- 22.Coelho A., Parola V., Fernandes O., Querido A., Apóstolo J. Development of a guided imagery program for patients admitted to palliative care units. Journal of Nursing Referência (Revista de Enfermagem Referência) 2018;4(17):23–32. [Google Scholar]
- 23.Shamekhi A., Tadayonfar M., Rastaghi S., Molavi M. Comparison of the effect of video education and guided imagery on patient anxiety before endoscopy. Biomed. Res. 2019;30(1) doi: 10.35841/biomedicalresearch.30-19-036. [DOI] [Google Scholar]
- 24.Afshar M., Mohsenzadeh A., Gilasi H., Sadeghi-Gandomani H. The effects of guided imagery on state and trait anxiety and sleep quality among patients receiving hemodialysis: a randomized controlled trial. Compl. Ther. Med. 2018;40:37–41. doi: 10.1016/j.ctim.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 25.Foji S., Tadayonfar M.A., Mohsenpour M., Rakhshani M.H. The study of the effect of guided imagery on pain, anxiety and some other hemodynamic factors in patients undergoing coronary angiography. Compl. Ther. Clin. Pract. 2015;21(2):119–123. doi: 10.1016/j.ctcp.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 26.Spielberger C.D. State‐Trait anxiety inventory. The Corsini encyclopedia of psychology. 2010 Jan 30:1. doi: 10.1002/9780470479216.corpsy0943. [DOI] [Google Scholar]
- 27.Spielberger C., Gorsuch R., Lushene R., Vagg P., Jacobs G. Consulting Psychologists Press; Palo Alto, CA: 1983. Manual for State-Trait Anxiety Inventory. [Google Scholar]
- 28.Abolhassani S. Effects of sensuous stimulation on anxiety in the patients hospitalized in coronary care unit. SJKU. 2007;12(2) [Google Scholar]
- 29.Mahram B. Allameh Tabatabaee University; Tehran: 1994. Standardization of Spielberger Inventory in Mashhad. MA Thesis. [Google Scholar]
- 30.Melzack R., Raja S.N. The McGill pain questionnaire: from description to measurement. The Journal of the American Society of Anesthesiologists. 2005;103(1):199–202. doi: 10.1097/00000542-200507000-00028. [DOI] [PubMed] [Google Scholar]
- 31.Stephenson N.L., Herman J. Pain measurement: a comparison using horizontal and vertical visual analogue scales. Appl. Nurs. Res. 2000;13(3):157–158. doi: 10.1053/apnr.2000.7658. [DOI] [PubMed] [Google Scholar]
- 32.Khosravi M., Sadighi S., Moradi S., Zendehdel K. Persian-McGill pain questionnaire; translation, adaptation and reliability in cancer patients: a brief report. Tehran Univ. Med. J. 2013;71(1):49–59. [Google Scholar]
- 33.Breivika H. Fifty years on the Visual Analogue Scale (VAS) for pain-intensity is still good for acute pain. But multidimensional assessment is needed for chronic pain. Scandinavian journal of pain. 2016;11(1):150–152. doi: 10.1016/j.sjpain.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 34.Phadke A., Bedekar N., Shyam A., Sancheti P. Effect of muscle energy technique and static stretching on pain and functional disability in patients with mechanical neck pain: a randomized controlled trial. Hong Kong Physiother. J. 2016;35:5–11. doi: 10.1016/j.hkpj.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schulz K.F., Altman D.G., Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMC Med. 2010;8(1):18. doi: 10.1186/1741-7015-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo Q., Zheng Y., Shi J., Wang J., Li G., Li C., et al. Immediate psychological distress in quarantined patients with COVID-19 and its association with peripheral inflammation: a mixed-method study. Brain Behav. Immun. 2020;80:17–27. doi: 10.1016/j.bbi.2020.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peloso R.M., Pini N.I., Sundfeld Neto D., Mori A.A., Oliveira R.C., Valarelli F.P., et al. How does the quarantine resulting from COVID-19 impact dental appointments and patient anxiety levels? Braz. Oral Res. 2020;34:1–11. doi: 10.1590/1807-3107bor-2020.vol34.0084. [DOI] [PubMed] [Google Scholar]
- 38.Liu K., Chen Y., Wu D., Lin R., Wang Z., Pan L. Effects of progressive muscle relaxation on anxiety and sleep quality in patients with COVID-19. Compl. Ther. Clin. Pract. 2020;39:101132. doi: 10.1016/j.ctcp.2020.101132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang J., Yang Z., Wang X., Li J., Dong L., Wang F., Li Y., Wei R., Zhang J. The relationship between resilience, anxiety and depression among patients with mild symptoms of COVID‐19 in China: a cross‐sectional study. J. Clin. Nurs. 2020;29(21–22):4020–4029. doi: 10.1111/jocn.15425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu K.K., Chan S.K., Ma T.M. Posttraumatic stress, anxiety, and depression in survivors of severe acute respiratory syndrome (SARS) J. Trauma Stress: Official Publication of The International Society for Traumatic Stress Studies. 2005;18(1):39–42. doi: 10.1002/jts.20004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alam M., Roongpisuthipong W., Kim N.A., Goyal A., Swary J.H., Brindise R.T., et al. Utility of recorded guided imagery and relaxing music in reducing patient pain and anxiety, and surgeon anxiety, during cutaneous surgical procedures: a single-blinded randomized controlled trial. J. Am. Acad. Dermatol. 2016;75(3):585–589. doi: 10.1016/j.jaad.2016.02.1143. [DOI] [PubMed] [Google Scholar]
- 42.Beizaee Y., Rejeh N., Heravi-Karimooi M., Tadrisi S.D., Griffiths P., Vaismoradi M. The effect of guided imagery on anxiety, depression and vital signs in patients on hemodialysis. Compl. Ther. Clin. Pract. 2018;33:184–190. doi: 10.1016/j.ctcp.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 43.Lovell N., Maddocks M., Etkind S.N., Taylor K., Carey I., Vora V., et al. Characteristics, symptom management and outcomes of 101 patients with COVID-19 referred for hospital palliative care. J. Pain Symptom Manag. 2020;60(1):77–81. doi: 10.1016/j.jpainsymman.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Delfani F., Zakerimoghadam M., Mohammadaliha J. Comparative study of the effects of muscle relaxation and mental imagery techniques on pain intensity in patients with the second-degree burn wounds. Nursing Practice Today. 2016;3(1):5–10. [Google Scholar]
- 45.Boehm L., Alice M. Application of guided imagery to facilitate the transition of new graduate registered nurses. J. Cont. Educ. Nurs. 2013;44(3):113–116. doi: 10.3928/00220124-20130115-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.