Abstract
Introduction
Many patients with mild coronavirus disease 2019 (COVID-19) have symptoms requiring acute and follow-up care. The aims of this study were to assess (1) provider-reported use of medications and their perceived effectiveness and (2) degree of difficulty managing specific symptoms at episodic COVID-19 care sites and in a longitudinal monitoring program.
Methods
We sent an online survey to physicians, advanced practice providers, and registered nurses redeployed to COVID-19 care sites at an academic medical center from March to May 2020. We asked about the use of medications and perceived effectiveness of medications to treat symptoms of COVID-19 and the perceived challenge of symptom management. Comparison was made by provider type (episodic or longitudinal site of care).
Results
Responses from 64 providers were included. The most frequently used medications were acetaminophen (87.1% of respondents), benzonatate (83.9%), and albuterol metered dose inhalers (MDI) (80.6%). Therapies for lower respiratory tract symptoms were reported as more commonly used by longitudinal follow-up providers compared to episodic providers including guaifenesin (90.6% vs 60.0%, p = 0.007), benzonatate (93.8% vs 73.3%, p = 0.04), nebulized albuterol for patients with asthma (75.0% vs 43.3%, p = 0.019), and albuterol MDIs for patients without asthma (90.6% vs 66.7%, p = 0.029). Medications found to have the highest perceived efficacy by respondents using the therapy (> 80% reporting “very efficacious”) included albuterol, acetaminophen for fever, non-sedating antihistamines, nasal steroid spray, and non-steroidal anti-inflammatory drugs (NSAIDs) for myalgia, arthralgia, or headache. Lower respiratory symptoms and anxiety were rated as the most challenging symptoms to manage.
Conclusions
Providers reported that clinical care of mild COVID-19 with medications in common use for other respiratory infections is effective, both at episodic care and longitudinal sites of care, but that specific symptoms are still challenging to manage.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40121-021-00432-8.
Keywords: COVID-19, SARS-CoV-2, Outpatient, Telemedicine, Medication, Symptom, Survey
Key Summary Points
| Why carry out this study? |
| Coronavirus disease 2019 (COVID-19) has required a new paradigm in the outpatient management of viral pneumonia through telemedicine and in-person encounters. |
| Treatment guidelines have focused on inpatient pharmacotherapy and not on outpatient symptom management despite high numbers of patients requesting advice. |
| This study asked if providers assigned to COVID-19 sites (March–May 2020) found specific therapies beneficial for patient symptoms and if the perceived benefit differed by the site of care (episodic providers versus longitudinal providers who called patients until symptoms improved). |
| What was learned from the study? |
| Many therapies used for nonspecific acute respiratory infections are perceived as beneficial by medical providers for the respiratory and systemic symptoms of COVID-19. |
| Longitudinal providers who call patients until symptom resolution report higher use of medications for lower respiratory tract symptoms and high perceived efficacy of antitussives and inhaled albuterol, findings which merit future investigation. |
Digital Features
This article is published with digital features, including a summary slide, to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.14160761.
Introduction
In March 2020, healthcare systems in the USA rapidly shifted care structures to treat large numbers of patients with coronavirus disease 2019 (COVID-19) [1]. Early clinical guidelines addressed the supportive care of hospitalized patients [2, 3] and subsequent treatment guidelines [4, 5] have evolved with evidence about specific therapies in this patient population. While many patients require inpatient care, most patients are well enough to receive care at home for a broad range of symptoms [2]. Few therapeutic options exist for the outpatient management of COVID-19 and considerable variation in treatment guidelines and clinical practice have been reported [1, 6, 7].
For ambulatory patients with COVID-19, our institution created a telemedicine virtual outpatient management clinic (VOMC) and an in-person acute respiratory clinic (ARC). The purpose of this study was to assess healthcare provider perceptions regarding the care of ambulatory patients with COVID-19 as supported by institutional guidelines. The specific objectives of this study were (1) to characterize perceptions of difficulty in the outpatient management of symptoms associated with COVID-19, (2) to characterize the perceived efficacy of therapeutic agents used in symptom management, and (3) to assess the impact of provider care experience (episodic or longitudinal care) on these perceptions. At our center, lower respiratory tract symptoms were the longest-lasting symptoms [8] and most common reason for hospitalization (unpublished data). Therefore, we theorized that lower respiratory symptoms and associated therapies would be perceived as more difficult to manage and less effective, respectively, by survey respondents providing longitudinal care to patients with COVID-19.
Methods
Setting
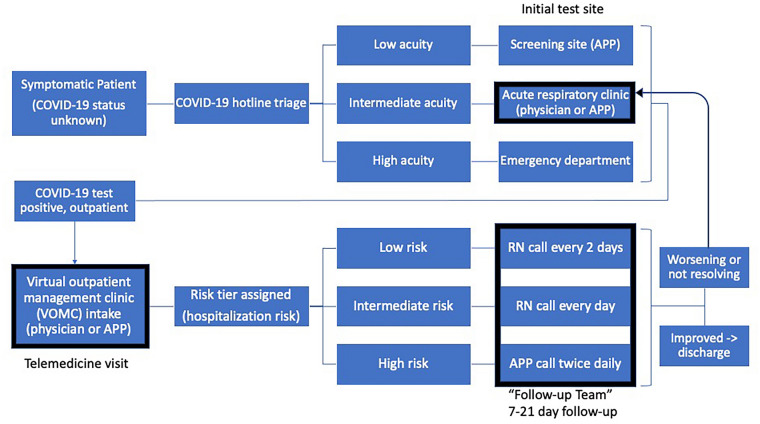
The study was conducted at the COVID-19 outpatient sites (VOMC and ARC) at Emory Healthcare, the largest academic health system in Georgia including four acute care hospitals and 120 primary care locations. The VOMC was a telemedicine clinic staffed by primary care physicians and advanced practice providers (APPs), available to any patient at home with acute COVID-19 [8, 9]. The VOMC care included an intake visit (synchronous audio/video) for initial triage and symptom advice followed by regular phone calls by a “follow-up” longitudinal team of APPs and registered nurses (RNs) until improvement (Fig. 1). A typical duration of follow-up was 19 days from symptom onset [8]. Patients requiring in-person evaluation for confirmed or possible COVID-19 (including VOMC patients) could schedule a visit at the ARC site, staffed by primary care and infectious disease physicians [10] (Fig. 1). During the study period, the majority of patients seen in VOMC and ARC were tested for COVID-19 at two outpatient testing sites (one clinic and one drive-through), with a smaller proportion tested in the emergency departments. At the time of survey administration, 560 patients had been cared for by the VOMC and 473 patients had been seen in the ARC.
Fig. 1.
Flowchart illustrating patient flow through outpatient COVID-19 care sites. Boxes in bold indicate the practice sites of the providers included in the survey including the ARC, VOMC telemedicine intake, and VOMC RN follow-up. Bold arrow indicates patient flow between the outpatient care sites. Not pictured: PCPs may refer patients with known COVID-19 to either the ARC in-person evaluation or VOMC for telemedicine intake. APP advanced practice provider, ARC acute respiratory clinic, COVID-19 coronavirus disease 2019, PCP primary care physician, RN registered nurse, VOMC virtual outpatient management clinic
To standardize care at these outpatient sites, clinical guidelines summarizing the available data and protocols from journals, medical societies, and institutions were created (see supplementary material). Since limited resources for COVID-19 symptom management were available [11, 12], the institutional guidelines included recommendations for management of nonspecific systemic, upper respiratory, and lower respiratory tract symptoms. We disseminated the guideline document at our institution’s COVID-19 care sites and posted it to a national physician resource [11].
This study was conducted as a quality improvement initiative and met criteria for determination of non-human subject research by the Emory University Institutional Review Board (IRB).
Survey Design
A survey was created to assess healthcare provider perceptions of the outpatient management of patients with COVID-19. A total of 21 symptoms and 19 therapeutic agents were assessed using multiple choice, free-response, and open-ended questions. The symptom-based survey questions included systemic, upper respiratory, lower respiratory, gastrointestinal, and other non-specific symptoms associated with COVID-19. Systemic symptoms were defined as fever, dizziness, fatigue, muscle aches, and joint pain. Upper respiratory symptoms were defined as sore throat, runny nose, postnasal drip, and nasal or sinus congestion or pain. Lower respiratory symptoms included shortness of breath at rest, shortness of breath on exertion, chest pressure, chest pain, and cough. Abdominal symptoms were defined as loss of appetite, nausea, diarrhea, and abdominal pain. Therapeutic-based survey questions assessed the perceived efficacy of medication and non-medication therapies for the management of systemic, upper respiratory, and lower respiratory symptoms in addition to sleep and anxiety. One open-ended question asked survey respondents to identify effective therapies used in the management of COVID-19-related symptoms to ensure the survey was comprehensive in the list of therapeutic agents provided. The survey was administered via SurveyMonkey (SurveyMonkey, Inc., San Mateo, California) from May 24, 2020 to June 4, 2020. Participants were identified from COVID-19 outpatient provider staff lists and were invited to complete the survey by email, with two additional reminder emails.
To assess the impact of patient care experience on perceptions, survey respondents were categorized as providing episodic or longitudinal care. Telemedicine VOMC intake providers and acute respiratory clinic providers were categorized as providing episodic care (single visit care). VOMC follow-up providers who contacted patients by telephone on a recurring basis for symptom management were categorized as longitudinal care providers. As the two licensed practical nurses (LPNs) in the study did not have a predefined care role, their responses were excluded from the symptom-based and therapeutic-based analyses.
Statistical Analysis
We computed descriptive statistics including inferential means, proportions, and standard deviations. We used multiple tests (Pearson chi-square and likelihood ratio) to compare data between provider groups (episodic vs longitudinal) and between provider license types (physician, APP, RN), between symptom categories, and to compare perceived difficulty of symptoms with perceived effectiveness of treatments. We applied a significance level p < 0.05 and performed analyses using IBM Statistical Package for the Social Sciences (SPSS, Armonk, NY).
Results
A total of 106 healthcare providers (42 physicians, 34 APPs, and 28 RNs, 2 LPNs) were invited to participate in the study, 64 (60.4%) of which completed the survey. Table 1 provides demographics of the survey respondents; 52 respondents (81.3%) indicated that they read the institutional guidelines. Among the 18 physician respondents, nine (50%) indicated that they had seen 25 or more patients for COVID-19, while the other half reported seeing 1–25 patients.
Table 1.
Demographics of healthcare providers and supporting personnel responding to survey about COVID-19 outpatient treatment (n = 64)
| Characteristics | n (% respondents) |
|---|---|
| Professional role | |
| MD | 18 (28.1) |
| APP | 23 (35.9) |
| RN | 20 (31.3) |
| LPN | 2 (3.1) |
| Not reported | 1 (1.6) |
| Practice specialty | |
| Primary care | 28 (43.8) |
| Medical specialty | 13 (20.3) |
| Surgical specialty | 11 (17.2) |
| Other | 7 (10.9) |
| Not reported | 5 (7.8) |
| Location of carea | |
| ARC | 21 (32.8) |
| VOMC | 45 (70.3) |
| Screening site | 10 (15.6) |
| Patient number | |
| 0–50 patients | 32 (50.0) |
| 51–100 patients | 12 (18.8) |
| > 100 patients | 18 (28.1) |
| Not reported | 2 (3.1) |
APP advanced practice provider, ARC acute respiratory clinic, LPN licensed practical nurse, MD medical doctor, RN registered nurse, VOMC virtual outpatient management clinic
aHealthcare providers could practice in multiple locations
The use of medications for the management of COVID-19-related symptoms by providers of episodic care and longitudinal care is described in Table 2. Over 80% of respondents indicated they used acetaminophen, benzonatate, and albuterol metered dose inhalers (MDI) for the management of COVID-19-related symptoms. Systemic symptoms were most commonly managed with acetaminophen. The management of upper respiratory symptoms was similar across both groups except for non-steroidal anti-inflammatory drug (NSAID) therapy, which was more likely to be used by episodic care providers for sinus pain (50.0% vs 21.9%, p = 0.033). Antitussive use was common across all survey respondents; however, guaifenesin and benzonatate use were more common in the longitudinal provider group compared to the episodic group (90.6% vs 60.0%, p = 0.007 and 93.8% vs 73.3%, p = 0.04, respectively). The majority of respondents reported use of albuterol for management of shortness of breath. Albuterol MDIs were used similarly in both provider groups in patients with a history of asthma. However, significantly more longitudinal care providers reported use of albuterol MDI in patients without a history of asthma (p = 0.029). Nebulized albuterol was also more commonly used by longitudinal care providers (p = 0.019). When medication use was analyzed by healthcare license type, no significant differences were found with the exception of honey for cough [n = 5 (27.8%), n = 19 (82.6%), and n = 10 (50.0%), for physician, APP, and RN, respectively; p = 0.002].
Table 2.
Therapy use and site of COVID-19 care
| COVID-19-related symptoms | Therapy | All respondents (n = 62) | Episodic carea (n = 30) | Longitudinal careb (n = 32) | Chi-square test p value |
|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | |||
| Systemic | |||||
| Fever | Acetaminophen | 54 (87.1) | 24 (80.0) | 30 (93.8) | 0.14 |
| NSAIDs | 24 (38.7) | 15 (50.0) | 9 (28.1) | 0.12 | |
| Headache | Acetaminophen | 54 (87.1) | 24 (80.0) | 30 (93.8) | 0.14 |
| NSAIDs | 30 (48.4) | 17 (56.7) | 13 (40.6) | 0.31 | |
| Fioricet | 13 (21.0) | 7 (23.3) | 6 (18.8) | 0.76 | |
| Myalgia, arthralgia | Acetaminophen | 50 (80.6) | 22 (73.3) | 28 (87.5) | 0.21 |
| NSAIDs | 25 (40.3) | 16 (53.3) | 9 (28.1) | 0.07 | |
| Upper respiratory | |||||
| Nasal or sinus pain | Acetaminophen | 43 (69.4) | 19 (63.3) | 24 (75.0) | 0.41 |
| NSAIDs | 22 (35.5) | 15 (50.0) | 7 (21.9) | 0.03* | |
| Runny nose, postnasal drip | Nasal saline spray | 44 (71.0) | 21 (70.0) | 23 (71.9) | 1.00 |
| Nasal saline lavage | 28 (45.2) | 15 (50.0) | 13 (40.6) | 0.61 | |
| Nasal steroid spray (no allergic rhinitis) | 38 (61.3) | 19 (63.3) | 19 (59.4) | 0.80 | |
| Nasal steroid spray (history of allergic rhinitis) | 41 (66.1) | 20 (66.7) | 21 (65.6) | 1.00 | |
| Non-sedating antihistamine (no history of allergic rhinitis) | 47 (75.8) | 20 (66.7) | 27 (84.4) | 0.14 | |
| Non-sedating antihistamine (history of allergic rhinitis) | 45 (72.6) | 21 (70.0) | 24 (75.0) | 0.78 | |
| Decongestant | 40 (64.5) | 19 (63.3) | 21 (65.6) | 1.00 | |
| Sedating antihistamine | 29 (46.8) | 13 (43.3) | 16 (50.0) | 0.62 | |
| Lower respiratory | |||||
| Cough | Honey | 33 (53.2) | 12 (40.0) | 21 (65.6) | 0.07 |
| Dextromethorphan | 46 (74.2) | 21 (70.0) | 25 (78.1) | 0.57 | |
| Guaifenesin | 47 (75.8) | 18 (60.0) | 29 (90.6) | 0.007* | |
| Benzonatate | 52 (83.9) | 22 (73.3) | 30 (93.8) | 0.04* | |
| Codeine, hydrocodone | 31 (50.0) | 15 (50.0) | 16 (50.0) | 1.00 | |
| OTC lozenge | 39 (62.9) | 15 (50.0) | 24 (75.0) | 0.07 | |
| Shortness of breath | Albuterol MDI (asthma history) | 50 (80.6) | 21 (70.0) | 29 (90.6) | 0.06 |
| Albuterol nebulized (asthma history) | 37 (59.7) | 13 (43.3) | 24 (75.0) | 0.02* | |
| Albuterol MDI (no asthma history) | 49 (79.0) | 20 (66.7) | 29 (90.6) | 0.03* | |
| Other | |||||
| Sleep | Sedating antihistamine | 31 (50.0) | 16 (53.3) | 15 (46.9) | 0.80 |
| Melatonin | 27 (43.5) | 12 (40.0) | 15 (46.9) | 0.62 | |
| Hypnotic | 10 (16.1) | 6 (20.0) | 4 (12.5) | 0.50 | |
| Anxiety | Benzodiazepines | 12 (19.4) | 6 (20.0) | 6 (18.8) | 1.00 |
| Various | Systemic steroids | 23 (37.1) | 10 (33.3) | 13 (40.6) | 0.61 |
COVID-19 coronavirus disease 2019, MDI metered-dose inhaler, NSAIDs non-steroidal anti-inflammatory drugs, OTC over-the-counter
aEpisodic care providers saw patients with COVID-19 for single visits by telemedicine or in-person
bLongitudinal care providers called patients regularly over time until symptom improvement
*Significant, p < 0.05
Perceived efficacy of therapeutic agents used in the management of COVID-19-related symptoms is described in Table 3. Albuterol, acetaminophen, non-sedating antihistamines, nasal steroid spray, and NSAIDs were perceived as very efficacious by 80% or more of survey respondents. Guaifenesin and nasal saline lavage were perceived as more effective by longitudinal care providers compared to those encountering patients episodically (p = 0.011 and p = 0.024, respectively).
Table 3.
Therapies perceived as moderately or very effective in management of COVID-19-related symptoms and site of COVID-19-related patient care
| All respondents | Episodic carea | Longitudinal careb | p value | |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| Albuterol MDI (history of asthma) (n = 50) | 47 (94.0) | 18 (85.7) | 29 (100.0) | 0.07 |
| Acetaminophen for fever (n = 54) | 50 (92.6) | 21 (87.5) | 29 (96.7) | 0.31 |
| Non-sedating antihistamine (no history of allergic rhinitis) (n = 47) | 33 (89.2) | 13 (65.0) | 20 (74.1) | 0.54 |
| Albuterol, nebulized (history of asthma) (n = 37) | 31 (83.8) | 9 (69.2) | 22 (91.7) | 0.16 |
| Nasal steroid spray (history of allergic rhinitis) (n = 41) | 34 (82.9) | 16 (80.0) | 18 (85.7) | 0.70 |
| Albuterol MDI (no history of asthma) (n = 49) | 40 (81.6) | 14 (70.0) | 26 (89.7) | 0.13 |
| Nasal steroid spray (no history of allergic rhinitis) (n = 38) | 31 (81.6) | 14 (73.7) | 17 (89.5) | 0.41 |
| NSAIDs myalgia, arthralgia (n = 25) | 20 (80.0) | 13 (81.3) | 7 (77.8) | 1.00 |
| NSAIDs for headache (n = 30) | 24 (80.0) | 14 (82.4) | 10 (76.9) | 1.00 |
| Non-sedating antihistamine (history allergic rhinitis) (n = 45) | 36 (80.0) | 17 (81.0) | 19 (79.2) | 1.00 |
| Acetaminophen for myalgia, arthralgia (n = 50) | 39 (78.0) | 17 (77.3) | 22 (78.6) | 1.00 |
| Codeine, hydrocodone for cough (n = 31) | 24 (77.4) | 10 (66.7) | 14 (87.5) | 0.22 |
| NSAIDs for nasal or sinus pain (n = 22) | 16 (72.7) | 11 (73.3) | 5 (71.4) | 1.00 |
| Decongestant for nasal congestion or postnasal drip (n = 40) | 29 (72.5) | 13 (68.4) | 16 (76.2) | 0.72 |
| Benzonatate for cough (n = 52) | 37 (71.2) | 13 (59.1) | 24 (80.0) | 0.13 |
| Sedating antihistamine for sleep (n = 31) | 22 (71.0) | 9 (56.3) | 13 (86.7) | 0.11 |
| NSAIDs for fever (n = 24) | 17 (70.8) | 9 (60.0) | 8 (88.9) | 0.19 |
| Hypnotic for sleep (n = 10) | 7 (70.0) | 4 (66.7) | 3 (75.0) | 1.00 |
| Fioricet for headache (n = 13) | 9 (69.2) | 5 (71.4) | 4 (66.7) | 1.00 |
| Acetaminophen for headache (n = 54) | 37 (68.5) | 16 (66.7) | 21 (70.0) | 1.00 |
| Dextromethorphan for cough (n = 46) | 31 (67.4) | 12 (57.1) | 19 (76.0) | 0.22 |
| Benzodiazepine for anxiety (n = 12) | 8 (66.7) | 3 (50.0) | 5 (83.3) | 0.55 |
| Guaifenesin for cough (n = 47) | 30 (63.8) | 7 (38.9) | 23 (79.3) | 0.01* |
| Nasal saline lavage (n = 28) | 17 (60.8) | 6 (40.0) | 11 (84.6) | 0.02* |
| Sedating antihistamine for nasal congestion or postnasal drip (n = 29) | 17 (58.6) | 7 (53.8) | 10 (62.5) | 0.72 |
| Nasal saline spray (n = 44) | 23 (52.3) | 9 (42.9) | 14 (60.9) | 0.37 |
| Systemic steroids (n = 23) | 12 (52.2) | 5 (50.0) | 7 (53.8) | 1.00 |
| Melatonin for sleep (n = 27) | 14 (51.9) | 4 (33.3) | 10 (66.7) | 0.13 |
| Honey for cough (n = 33) | 17 (51.5) | 6 (50.0) | 11 (52.4) | 1.00 |
| OTC lozenge for cough (n = 39) | 20 (51.3) | 7 (46.7) | 13 (54.2) | 0.75 |
| Acetaminophen for nasal or sinus pain (n = 43) | 21 (48.8) | 10 (52.6) | 11 (45.8) | 0.76 |
COVID-19 coronavirus disease 2019, MDI metered-dose inhaler, NSAIDs non-steroidal anti-inflammatory drugs, OTC over-the-counter
aEpisodic care providers saw patients with COVID-19 for single visits by telemedicine or in-person
bLongitudinal care providers called patients regularly over time until symptom improvement
*Significant, p < 0.05
Lower respiratory symptoms (such as shortness of breath and cough) in addition to anxiety were rated as most challenging to manage (Table 4). No significant differences (p = 0.17–1.00 across 21 symptoms) were found between provider groups in symptoms perceived as very challenging. When grouped into symptom categories, there were no significant differences (p = 0.29–1.00 across 4 systems) between provider groups in perceived difficulty of symptom management.
Table 4.
Symptoms perceived as very challenging and site of COVID-19-related patient care
| All respondents | Episodic carea | Longitudinal careb | p value | |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| Shortness of breath at rest (n = 52) | 28 (53.8) | 15 (65.2) | 13 (44.8) | 0.17 |
| Constant chest pain or chest pressure (n = 52) | 27 (51.9) | 14 (60.9) | 13 (44.8) | 0.28 |
| Chest tightness with breathing or coughing (n = 53) | 27 (50.9) | 13 (56.5) | 14 (46.7) | 0.58 |
| Anxiety (n = 54) | 26 (48.1) | 11 (47.8) | 15 (48.4) | 1.00 |
| Shortness of breath on exertion (n = 54) | 23 (42.6) | 13 (54.2) | 10 (33.3) | 0.17 |
| Cough (n = 54) | 21 (38.9) | 12 (50.0) | 9 (30.0) | 0.17 |
| Fatigue (n = 52) | 11 (21.2) | 5 (22.7) | 6 (20.0) | 1.00 |
| Headache (n = 53) | 11 (20.8) | 4 (17.4) | 7 (23.3) | 0.74 |
| Myalgia (n = 53) | 7 (13.2) | 3 (13.0) | 4 (13.3) | 1.00 |
| Fever (n = 43) | 5 (11.6) | 3 (15.8) | 2 (8.3) | 0.64 |
| Dizziness (n = 46) | 5 (10.9) | 2 (16.7) | 2 (7.1) | 0.37 |
| Abdominal pain (n = 46) | 2 (4.3) | 1 (5.9) | 1 (3.4) | 1.00 |
| Anosmia, ageusia (n = 49) | 2 (4.1) | 1 (4.3) | 1 (3.8) | 1.00 |
| Joint pain (n = 50) | 2 (4.0) | 1 (4.8) | 1 (3.4) | 1.00 |
| Loss of appetite (n = 51) | 2 (3.9) | 0 (0.0) | 2 (6.7) | 0.51 |
| Sinus congestion or pain (n = 53) | 2 (3.8) | 1 (4.2) | 1 (3.4) | 1.00 |
| Nausea (n = 51) | 1 (2.0) | 0 (0.0) | 1 (3.4) | 1.00 |
| Sore throat (n = 52) | 1 (1.9) | 1 (4.2) | 0 (0.0) | 0.46 |
| Diarrhea (n = 52) | 1 (1.9) | 0 (0.0) | 1 (3.3) | 1.00 |
| Weakness (n = 52) | 8 (15.4) | 4 (17.4) | 4 (13.8) | 1.00 |
| Runny nose, postnasal drip | 0 (0.0) | 0 (0.0) | 0 (0.0) | – |
aEpisodic care providers saw patients with COVID-19 for single visits by telemedicine or in-person
bLongitudinal care providers called patients regularly over time until symptom improvement
Responses to the open-ended question in the survey did not identify any additional medications used by more than one provider that were missing from the survey of therapeutics. The highest frequency medications reported for fever, cough, and shortness of breath were acetaminophen, benzonatate, and albuterol, respectively. Only two medications were reported by more than one respondent for an indication that we did not query in the structured portion of the survey: (1) guaifenesin for nasal or sinus congestion and (2) albuterol for cough. Additional responses by single providers included inhaled fluticasone and budesonide/formoterol, both used for cough.
Discussion
Frequency of Use
To our knowledge, this is the first survey to explore the therapies used in the home management of COVID-19 as well as the perceived challenge of treating specific symptoms. As expected, we found high use of medications indicated for the treatment of upper respiratory infections, such as acetaminophen for fever and over-the-counter (OTC) antitussives for cough. The high level of albuterol use was remarkable, because this was not a first-line medication in our clinical guideline except for patients with history of asthma or obstructive lung disease. Reports of albuterol shortages in the USA during the first wave of the pandemic suggested that this agent was being used for COVID-19 management [13].
Interestingly, some additional therapies were perceived as very effective, but were used less frequently than other therapies. NSAIDs, for example, were considered effective by survey respondents for body aches and headaches but were subject to scrutiny early in the pandemic from the World Health Organization as possibly increasing risk for severe COVID-19 [14]. Similarly, nebulized albuterol received negative attention with reported increase aerosol generation and risk for COVID-19 transmission [15] and was used less frequently than albuterol in an MDI formulation in this study. Note of this risk associated with nebulized albuterol was included in the institutional guidelines. Nasal steroid spray use may have been less frequent by survey respondents because of uncertain efficacy in other respiratory infections [16]. Finally, opiates were the least utilized antitussive agent in this study. While prominent in British treatment guidelines [17], opiates are considered high-risk prescriptions in the USA, potentially decreasing their use for COVID-19-related cough [18].
Differences by Phase of Care
The care structure for outpatient COVID-19 management (Fig. 1) presented a unique opportunity to compare the experience of providers practicing in a more traditional, episodic care role to a team of continuity providers that followed patients by telephone through the course of their acute illness. Our finding that longitudinal providers used therapies for lower respiratory tract symptoms (i.e., cough and shortness of breath) more than episodic providers may reflect a frequent need for add-on therapies as lower respiratory tract symptoms develop later in the natural history of COVID-19 [8]. This finding should inform expectant symptom management advice given to patients early in their illness to include the possibility of needing additional treatments. Other notable differences between groups include reports of higher efficacy of guaifenesin and nasal saline lavage by the follow-up teams. The latter is remarkable because instructions for saline lavage were included in our clinical guideline related to evidence base [16] but usage is low, perhaps because of patient factors (i.e., hesitancy about technique or burden of making and using lavage solution) or provider factors (i.e., limited knowledge) [19]. The higher level of perceived effectiveness of these therapies noted by longitudinal providers should reassure episodic providers that these therapies may be recommended in the current management of COVID-19.
Lower Respiratory Symptom Management
The increased perceived difficulty of management of lower respiratory symptoms reported by all providers may explain the high reported usage of medications for lower respiratory symptoms such as cough and shortness of breath, particularly by follow-up providers. Despite reporting high efficacy of these medications (albuterol, opioid-containing cough medication, and benzonatate), respondents still reported high difficulty, highlighting the overall challenge of managing lower respiratory infections at home. With the rapid expansion of home monitoring programs, including remote physiologic monitoring technologies, it is likely that patients will continue to be monitored at home for dyspnea due to COVID-19 (and other diseases) [20]. Our center deployed home pulse oximetry devices for patients seen at the ARC site and our VOMC telephone team was given monitoring parameters, but we did not investigate the effect of physiologic monitoring on provider experience in this study. It is possible the remote patient monitoring technology eases the difficulty of management by providing clinical data, but also possible that it increases the provider burden by permitting home care of highly symptomatic patients who might otherwise be observed in a hospital setting.
For comparison, systemic symptoms are reported to be easier to manage with acetaminophen and its use was reported as highly effective, making the need for additional antipyretic or analgesic treatment options appear less immediately necessary. It is notable that a prior survey of healthcare providers found a low percentage (21.6%) finding paracetamol effective, but this study was subject to bias as the comparison options included antiviral medications (not antipyretics) and less than 50% of respondents worked in dedicated COVID-19 settings [7].
Notably, anxiety is the only symptom that approaches the same difficulty as lower respiratory symptoms. This corresponds to a narrative report by a COVID-19-specific clinic that anxiety often produces dyspnea that can be difficult to differentiate from primary respiratory symptoms [21].
Limitations
The primary limitation of this study is the use of self-reported practice, a subjective measure, as an indicator of medication use and effectiveness. Directly observed practice data for medication advice for COVID-19 symptoms are not available at our center. Similarly, we report perceived effectiveness, which is subject to the placebo effect and multiple biases (e.g., patient response bias, provider confirmation bias). An additional limitation is the small sample size from a single institution and therefore external validation of our findings is necessary. The number of providers with experience in COVID-19 outpatient care was limited at the time of the study. The low response rate (e.g., 18 of 42 invited physicians) may be due to the variation in experience among providers; those who completed the survey reported high numbers of patient encounters and it is possible that volunteer physicians who were not scheduled for direct COVID-19 patient care would be less likely to complete a survey about treatment experience.
The use of a standard clinical guideline at our sites, introduced in late March 2020, provided the basis for similar medication and symptom terminology used for this survey, but also may have introduced bias towards our recommended treatments. As an additional consideration, nonpharmacologic treatments (e.g., prone position, relaxation, and breathing exercises) may have a role and were not assessed in this study.
Despite these limitations, this study provides early insight into the symptom management of COVID-19. We note that data are limited for the treatment of symptoms of respiratory infections in general, such as cough [22] and fever [23]. We are not aware of any clinical trials directed at cough or dyspnea symptom management. At the time of writing, 3009 studies are registered on ClinicalTrials.gov for COVID-19 treatment; we find trials of hypertonic saline lavage, but none testing albuterol or benzonatate.
Conclusions
This study provides information about the management of COVID-19 in ambulatory patients including the perceived difficulty of symptom management and the provider-reported use and perceived effectiveness of common medications. Leveraging the novel care structures created early in the pandemic, we compare the experience of providers who see patients for single encounters with a follow-up longitudinal care team. The results provide limited validation for our symptom management guideline used in COVID-19 care sites, while suggesting an increased role for specific therapies (e.g., nasal saline lavage, albuterol in patients without asthma) that may be best addressed in future studies.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors wish to acknowledge Denise A. Moultrie RN, MSN and Maanowo Ventii RN, MSN, MBA for distributing the survey to the registered nurses and Kajal Vora FNP-C for distributing the survey to the advanced practice providers. We also would like to acknowledge the contributors to the symptom management guidelines, listed in the supplementary material.
Funding
James O’Keefe is funded by the Georgia Geriatrics Workforce Enhancement Program (GA-GWEP) COVID-19 Telehealth award, supported by the Health Resources and Services Administration (HRSA) of the U.S. Department of Health and Human Services (HHS) as part of Award Number T1MHP39056 totaling $90,625 with 0% percentage financed with nongovernmental sources. The contents are those of the author and do not necessarily represent the official views of, nor an endorsement, by HRSA, HHS, or the U.S. Government. The journal’s Rapid Service Fee was funded by the Paul W. Seavey Internal Medicine Clinic research fund. The funder/sponsor had no direct role in study planning and conduct, reporting, or authorship.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
James B. O’Keefe, Lydia C. Newsom and Thomas H. Taylor Jr have nothing to disclose.
Compliance with Ethics Guidelines
This study was conducted as a quality improvement initiative and met criteria for determination of non-human subject research by the Emory University Institutional Review Board.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Sahu KK, Kumar R. Current perspective on pandemic of COVID-19 in the United States. J Family Med Prim Care. 2020;9(4):1784–1791. doi: 10.4103/jfmpc.jfmpc_424_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Interim clinical guidance for management of patients with confirmed coronavirus disease (COVID-19). 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html. Accessed 18 Mar 2020.
- 3.World Health Organization. Novel coronavirus (2019-nCoV) technical guidance. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance. Accessed 18 Mar 2020.
- 4.COVID-19 Treatment Guidelines Panel. Coronavirus disease 2019 (COVID-19) treatment guidelines. National Institutes of Health. 2020. https://www.covid19treatmentguidelines.nih.gov/. Accessed 14 Aug 2020. [PubMed]
- 5.Bhimraj A, Morgan RL, Shumaker AH, et al. Infectious Diseases Society of America guidelines on the treatment and management of patients with coronavirus disease 2019 (COVID-19) Clin Infect Dis. 2020;382:1708–1720. doi: 10.1093/cid/ciaa478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dagens A, Sigfrid L, Cai E, et al. Scope, quality, and inclusivity of clinical guidelines produced early in the covid-19 pandemic: rapid review. BMJ. 2020;369:1936. doi: 10.1136/bmj.m1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dima A, Balaban DV, Jurcut C, Berza I, Jurcut R, Jinga M. Physicians' perspectives on COVID-19: an international survey. Healthcare (Basel) 2020;8:3. doi: 10.3390/healthcare8030250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Keefe JB, Tong EJ, O'Keefe GD, et al. Description of symptom course in a telemedicine monitoring clinic for acute symptomatic covid-19 COVID-19: a retrospective cohort study. BMJ Open. 2021;2021:1–13. doi: 10.1136/bmjopen-2020-044154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bergquist SH, Partin C, Roberts DL, et al. Non-hospitalized adults with COVID-19 differ noticeably from hospitalized adults in their demographic, clinical, and social characteristics. SN Compreh Clin Med. 2020;2020:1–9. doi: 10.1007/s42399-020-00453-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramakrishnan A, Zreloff J, Moore MA, et al. Prolonged symptoms after COVID-19 infection in outpatients. Open Forum Infect Dis. 2021;2021:2. doi: 10.1093/ofid/ofab060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chick, Davoren. COVID-19: an ACP physician’s guide + resources. American College of Physicians. 2020. https://assets.acponline.org/coronavirus/scormcontent/#/. Accessed 20 Apr 2020.
- 12.Beeching NJ, Fletcher TE, Fowler R. BMJ best practice: coronavirus disease 2019 (COVID-19). 2020. https://bestpractice.bmj.com/topics/en-gb/3000168. Accessed 20 Apr 2020.
- 13.US Food and Drug Administration. FDA drug shortages: current and resolved drug shortages and discontinuations reported to FDA. 2020. https://www.accessdata.fda.gov/scripts/drugshortages/default.cfm. Accessed 30 May 2020.
- 14.Little P. Non-steroidal anti-inflammatory drugs and covid-19. BMJ. 2020;2020:368. doi: 10.1136/bmj.m1185. [DOI] [PubMed] [Google Scholar]
- 15.Gandhi RT, Lynch JB, del Rio C. Mild or moderate Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMcp2009249. [DOI] [PubMed] [Google Scholar]
- 16.Farrell NF, Klatt-Cromwell C, Schneider JS. Benefits and safety of nasal saline irrigations in a pandemic-washing COVID-19 away. JAMA Otolaryngol Neck Surg. 2020;2020:1–2. doi: 10.1001/jamaoto.2020.1622. [DOI] [PubMed] [Google Scholar]
- 17.National Institute for Health and Care Excellence (NICE). COVID-19 rapid guideline: managing symptoms (including at the end of life) in the community. London: NICE; 2020. pp. 1–24. https://www.nice.org.uk/guidance/ng163. Accessed 15 Aug 2020 [PubMed]
- 18.US Food and Drug Administration. FDA drug safety communication: FDA requires labeling changes for prescription opioid cough and cold medicines to limit their use to adults 18 years and older. 2018. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-requires-labeling-changes-prescription-opioid-cough-and-cold. Accessed 29 Aug 2020.
- 19.Werning JW, Preston TW, Khuder S. Physician specialty is associated with differences in the evaluation and management of acute bacterial rhinosinusitis. Arch Otolaryngol Neck Surg. 2002;128(2):123–130. doi: 10.1001/archotol.128.2.123. [DOI] [PubMed] [Google Scholar]
- 20.Ilowite J, Lisker G, Greenberg H. Digital health technology and telemedicine-based hospital and home programs in pulmonary medicine during the COVID-19 pandemic. Am J Ther. 2021;28(2):e217–e223. [DOI] [PubMed]
- 21.Cohen PA, Hall LE, John JN, Rapoport AB. The early natural history of SARS-CoV-2 infection: clinical observations from an urban, ambulatory COVID-19 clinic. Mayo Clin Proc. 2020;95(6):1124–1126. doi: 10.1016/j.mayocp.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith SM, Schroeder K, Fahey T. Over-the-counter (OTC) medications for acute cough in children and adults in community settings. Cochrane Database Syst Rev. 2014;10(6):248–263. doi: 10.1002/14651858.CD001831.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park S, Brassey J, Heneghan C, Mahtani K. Managing fever in adults with possible or confirmed COVID-19 in primary care. Cent Evid Based Med. 2020;2020:1–4. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.