Abstract
Background:
Trauma is the leading cause of death in children. Resuscitative endovascular balloon occlusion of the aorta (REBOA) provides temporary hemorrhage control, but its potential benefit has not been assessed in children. We hypothesized that there are pediatric patients who may benefit from REBOA.
Methods:
Trauma patients <18 years old at a level 1 pediatric trauma center between 2009–2019 were queried for deaths, pre-hospital cardiac arrest, massive transfusion protocol activation, transfusion requirement, or hemorrhage control surgery. These patients defined the cohort of severely injured patients. From this cohort, patients with intraabdominal injuries for which REBOA may provide temporary hemorrhage control were identified, including solid organ injury necessitating intervention, vascular injury, or pelvic hemorrhage.
Results:
There were 239 severely injured patients out of 6538 pediatric traumas. Of these, 38 had REBOA-amenable injuries (15.9%) with 34.2% mortality, accounting for 10.2% of all pediatric trauma deaths at one center. Eleven patients with REBOA-amenable injuries had TBI (28.9%). Patients with REBOA-amenable injuries represented 0.6% of all pediatric traumas.
Conclusion:
Nearly twenty percent of severely injured patients could potentially benefit from REBOA. The overall proportion of pediatric patients with REBOA-amenable injuries is similar to adult studies.
Keywords: pediatric trauma, REBOA, resuscitative endovascular balloon occlusion of the aorta, gap analysis, pediatric REBOA
Introduction:
Trauma is the leading cause of death for children, with most children dying within 24 hours of injury [1]. Hemorrhage and traumatic brain injury are the most common causes of pediatric trauma mortality, and nearly half of hemorrhagic deaths are thought to be preventable [2]. Resuscitative endovascular balloon occlusion of the aorta (REBOA) has emerged as a viable intervention for the patient in hemorrhagic shock [3–5], but most reports of its use have been in adult patients, with a few small case series reporting on REBOA use in adolescent patients [6–9].
REBOA is a minimally invasive form of aortic occlusion used for non-compressible torso hemorrhage [10] and significant pelvic trauma [4]. REBOA is placed by obtaining common femoral access and inserting an aortic balloon catheter into the intrathoracic or infrarenal aorta to occlude distal blood flow, thus limiting ongoing hemorrhage. Although more than one thousand cases have been reported in the adult literature [11,12], it has only rarely been used in pediatric trauma patients [6,7]. Gap analyses have been performed to identify patients with injuries for which REBOA may provide temporary hemorrhage control in adult combat casualties [13], civilian adult trauma patients in the United Kingdom [14] and the United States [15], and through autopsy reports from patients undergoing resuscitative thoracotomy [16]. Estimates of adult patients with injuries amenable to REBOA range from 0.6% of all civilian adult trauma patients in the US [15] to 18% of combat casualties [13] and 45% of trauma patients deceased following resuscitative thoracotomy [16]. While there is emerging interest in the application of REBOA in children [17], no such study has been performed evaluating the proportion of pediatric trauma patients with injuries that may be amenable to hemorrhage control with REBOA.
We hypothesized that there would be a population of severely injured civilian pediatric trauma patients with injury patterns that may benefit from temporary aortic occlusion with REBOA and that this population would have a higher mortality than other pediatric trauma patients.
1. Methods
1.1. Study Design and Setting
Institutional Review Board approval was obtained for this study (IRB # 1582638). We queried our institution’s prospectively collected trauma registry for all pediatric trauma admissions < 18 years old at a level 1 pediatric trauma center between January 1, 2009 and March 31, 2019. We included patients who died, had pre-hospital cardiac arrest, activation of massive transfusion protocol (MTP), received any transfusions, or underwent hemorrhage control surgery as coded by the registry. This group of patients was defined as the cohort of severely injured patients. Patients declared dead on arrival without imaging or surgical intervention were excluded. Patients who had the MTP activated but did not receive any transfusions were excluded if they did not meet one of the other inclusion criteria. Hemorrhage control surgery became a variable in the database in 2014, and activation of MTP and pre-hospital cardiac arrest became variables in the database in 2015 and thus are missing for patients prior to those dates. Chart review of these severely injured patients was performed to identify patients with injuries for which REBOA may have provided temporary hemorrhage control. This was our primary outcome and was defined as solid organ injury necessitating intervention, intraabdominal vascular injury, or pelvic hemorrhage. These injuries were identified by review of imaging and operative records. Patients were not considered to have REBOA-amenable injuries if they had any of the following: solid organ injury managed without intervention, abdominal exploration with no evidence of hemorrhage, or injuries which were hemostatic at the time of laparotomy. Secondary outcomes included presence of traumatic brain injury (TBI), injury severity score (ISS), admission value for Shock Index, Pediatric Age-Adjusted (SIPA), mortality, length of stay (LOS), and intensive care unit (ICU) LOS. Admission SIPA was calculated by dividing the admission heart rate (HR) by the admission systolic blood pressure (SBP). SIPA is validated in patients 4–16 years old and was only calculated for those patients [18]. SIPA was not calculated for any patient with an admission HR or SBP of zero. We calculated the sensitivity, specificity, positive predictive value, and negative predictive value for SIPA in identifying REBOA-amenable injuries. Any missing data from the registry were collected by chart review.
Our institution is a level one pediatric and adult trauma center, with 400–500 pediatric trauma admissions per year from 2009 to 2013 and 700–900 per year from 2014 to 2019. Trauma patients < 6 years old are admitted to the pediatric surgical service and patients ≥ 6 years old are admitted to the adult trauma surgical service. Data on all trauma activations are collected prospectively into an institutional trauma database, including demographics, injury mechanism, injury severity, admission vital signs, interventions performed, and outcomes. Standardized protocols exist to guide initial management of trauma patients upon arrival to the emergency department (ED) but decisions regarding imaging, transfusion, and surgical intervention are ultimately up to the attending pediatric or trauma surgeon caring for the patient.
1.2. Statistical analysis
Descriptive statistics were performed for baseline characteristics. Categorical data are presented as proportion and percentage. All continuous data were non-parametric and are presented as median and interquartile range (IQR). Categorical variables were compared using chi-square tests or Fisher’s exact test when appropriate. Continuous variables were compared with Mann-Whitney-U tests. Demographics and outcomes of patients with REBOA-amenable injuries were compared with those of severely injured patients without REBOA-amenable injuries, as well as those of severely injured patients to non-severely injured patients. Sensitivity, specificity, positive and negative predictive values are presented with 95% confidence intervals (CI). The level of significance was set at p< 0.05. Analysis was conducted using SAS (SAS, version 9.45; SAS Institute Inc).
2. Results:
2.1. Overall results
From 2009 – 2019 there were 6538 pediatric trauma admissions. The median age of all patients was 8 years old (IQR 3–13 years old). They were mostly male (63.6%) and the vast majority sustained blunt trauma (91.0%). The median ISS was 5 (IQR 4–10) and the median hospital LOS was 2 days (IQR 1–3 days), with a median of one ICU day for those with ICU admissions (IQR 1–3 days). SIPA was elevated in 794 of 4267 patients (18.6%) for which it was able to be calculated. SIPA was unable to be calculated in 39 patients with admission HR or SBP of zero and in 2232 patients who were out of the age range for which SIPA has been validated.
2.2. Severely Injured Patients Compared to the Remainder of the Trauma Cohort
There were 282 patients who met one of more of the following criteria for severely injured patients: 128 deaths (2.0%), 42 pre-hospital cardiac arrests (0.6%), 122 MTP activations (1.9%), 135 patients who received transfusions (2.1%), and 38 hemorrhage control operations (0.6%). There were 27 patients who were declared dead on arrival without imaging or surgical intervention, 9 patients who had MTP activated but did not receive any transfusion and did not meet other inclusion criteria and 7 patients who sustained no trauma, leaving 239 unique severely injured patients for analysis. These were compared to 6,265 trauma patients who were not severely injured, after excluding patients who were declared dead on arrival and who sustained no trauma (Figure 1).
Figure 1: Determination of cohort of patients with REBOA-amenable injury from all pediatric trauma admissions.
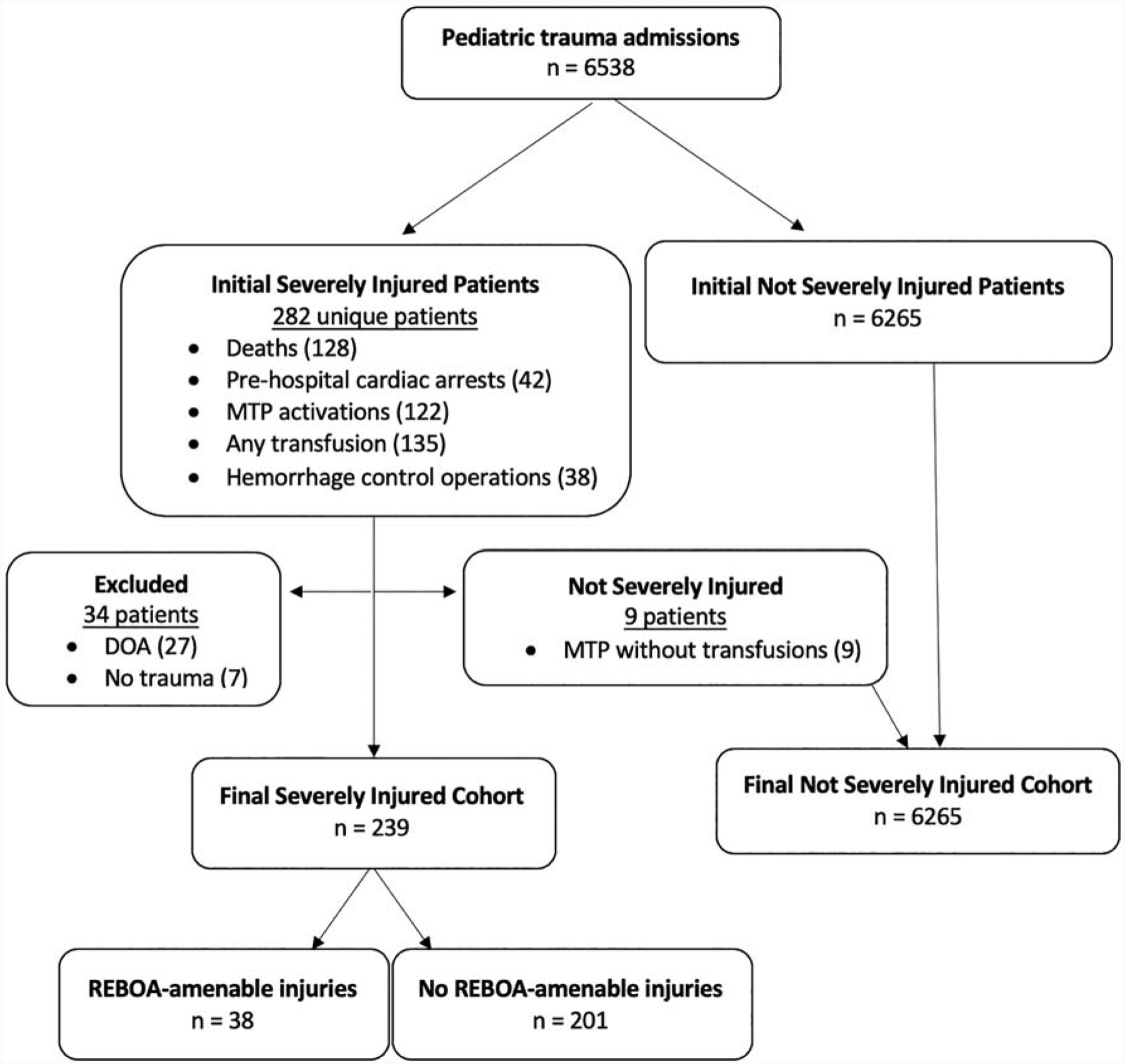
MTP: Massive transfusion protocol; DOA: declared dead on arrival.
For these severely injured patients, the median age was 9 years old (IQR 3–15) and the majority were male (70.3%). Most patients sustained blunt trauma (74.5%, n = 178). The median ISS was 25 (IQR 16–30). The median hospital LOS was 6 days (IQR 2–13 days) and median ICU LOS for patients admitted to the ICU was 4 (IQR 1.8–8 days). SIPA upon ED arrival was elevated in 72 of 135 (53.3%). SIPA was unable to be calculated for 8 patients due to lack of obtainable vital signs on admission and in 96 patients who were out of the validated SIPA age range. Two-thirds of severely injured patients had a TBI (61.5%). The mortality rate of severely injured patients was 41.4%. When comparing severely injured patients (Table 1) to the remainder of the trauma patients (non-severely injured, n = 6265), severely injured patients were more likely to be male (70.3% vs. 63.3%, p = 0.03) and more likely to sustain penetrating trauma (25.5% vs. 8.4%, p < 0.0001). They were more severely injured (median ISS 25 vs. 5, p < 0.0001), and were more likely to have an elevated SIPA on admission (53.3% vs. 17.5%, p < 0.0001).
Table 1:
Comparison of Severely Injured Patients to Not Severely Injured Patients
| Variable | Severely Injured (n = 239) | Not Severely Injured (n = 6265) | p-value |
|---|---|---|---|
| Age, years: median (IQR) | 9 (3–15) | 8 (3–13) | 0.11 |
| Sex: % male (n) | 70.3% (168) | 63.3% (3966) | 0.03* |
| Blunt trauma: % blunt (n) | 74.5% (178) | 91.6% (5739) | < 0.0001* |
| ISS: median (IQR) | 25 (16–30) | 5 (4–10) | < 0.0001* |
| Hospital LOS, days: median (IQR) | 5 (1.5–9) | 1 (1–3) | < 0.0001* |
| Elevated SIPA: % (n) | 53.3% (72/135) | 17.5% (722/4129) | < 0.0001* |
IQR: interquartile range; ISS: injury severity score; LOS: length of stay; SIPA: Shock Index, Pediatric Age-Adjusted.
denotes statistical significance at p < 0.05.
2.3. Severely Injured Patients with REBOA-Amenable Injuries Compared to Severely Injured Patients Without REBOA-Amenable Injuries
Of the severely injured patients, 38 were identified with injuries for which REBOA may have provided temporary hemorrhage control (15.9% of severely injured patients and 0.6% of all pediatric trauma admissions, Table 2). REBOA-amenable injuries identified on review of operative reports and imaging included high-grade solid organ injuries which required intervention, intraabdominal vascular injuries, retroperitoneal hemorrhage, and pelvic hemorrhage (Table 3). Interventions included splenectomy, nephrectomy, hepatic packing, angioembolization, and vascular repair. Patients with REBOA-amenable injuries were more likely than those without to undergo any surgical intervention (97.4% vs. 67.7%, OR 17.7, 95% CI 3.0–183.3, p < 0.0001, Table 4). They were also significantly more likely to undergo laparotomy (84.2% vs. 12.4%, OR 37.6, 95% CI 13.9–94.8, p < 0.0001) and interventional radiology procedures (23.7% vs. 0.5%, OR 59.7, 95% CI 8.9–657, p < 0.0001). Of the 38 patients with REBOA-amenable injuries, 36 required immediate operative interventions for hemorrhage control (94.7%). Severely injured patients without REBOA-amenable injuries were more likely to have a TBI (67.7% vs. 29.0%, p < 0.0001) and more likely to undergo neurosurgical intervention (34.8% vs. 15.8%, OR 2.9, 95% CI 1.2–6.8, p = 0.03). In the REBOA-amenable injury cohort, there were three patients with subdural hematomas, one patient with an epidural hematoma, and the remaining seven patients had multiple intracranial injuries including subarachnoid hemorrhage (n=5), intraparenchymal hemorrhage/contusion (n=2), subdural hematoma (n=2), anoxic brain injury (n=2), and diffuse cerebral edema (n=3).
Table 2:
Comparison of Severely Injured Patients with REBOA-Amenable Injuries to Severely Injured Patients Without REBOA-Amenable Injuries
| Variable | REBOA-amenable injuries (n = 38) | No REBOA-amenable injuries (n = 201) | p-value |
|---|---|---|---|
| Age, years: median (IQR) | 11 (8–16) | 8 (2–14) | 0.02* |
| Sex: % male (n) | 57.9% (22) | 72.6% (146) | 0.08 |
| Mechanism of injury: % blunt trauma (n) | 71.1% (27) | 75.1% (151) | 0.7 |
| ISS: median (IQR) | 29.5 (22–43) | 25 (14–29) | 0.0002* |
| Hospital LOS, days: median (IQR) | 9.5 (2–18) | 5 (2–11) | 0.07* |
| Elevated SIPA: % (n) | 76.9% (20/26) | 47.7% (52/109) | 0.009* |
| TBI: % (n) | 29.0% (11) | 67.7% (136) | <0.0001* |
| Mortality: % (n) | 34.2% (13) | 42.8% (86) | 0.4 |
IQR: interquartile range; ISS: injury severity score; LOS: length of stay; SIPA: Shock Index, Pediatric Age-Adjusted; TBI: traumatic brain injury; REBOA: Resuscitative Endovascular Balloon Occlusion of the Aorta.
denotes statistical significance at p < 0.05.
Table 3:
Details of REBOA-Amenable Injuries in 38 Severely Injured Pediatric Trauma Patients
| Injury Type* | Details |
|---|---|
| Solid organ injury (n = 35) | Liver lacerations (n = 20) Splenic lacerations (n = 11) Renal lacerations (n = 4) |
| Intraabdominal vascular injury (n = 10) | External iliac artery Internal iliac vein Ileocolic artery Adrenal artery Mesenteric arteries Inferior vena cava (n = 3) |
| Retroperitoneal hemorrhage (n = 7) | |
| Pelvic hemorrhage (n = 7) |
Numbers add up to greater than 38 as some patients had multiple injuries. REBOA: Resuscitative Endovascular Balloon Occlusion of the Aorta.
Table 4:
Interventions Required in Severely Injured Patients
| Interventions: n (%) | REBOA-amenable injury (n=38) | No REBOA-amenable injury (n=201) | p-value |
|---|---|---|---|
| Any intervention: n (%) | 37 (97.4) | 136 (67.7) | <0.0001* |
| Resuscitative thoracotomy: n (%) | 3 (7.9) | 6 (3.0) | 0.16 |
| Sternotomy: n (%) | 2 (5.3) | 1 (0.5) | 0.07 |
| Laparotomy: n (%) | 32 (84.2) | 25 (12.4) | <0.0001* |
| Neurosurgical intervention: n (%) | 6 (15.8) | 70 (34.8) | 0.03* |
| Orthopedic intervention: n (%) | 9 (23.7) | 28 (13.9) | 0.14 |
| Interventional radiology: n (%) | 9 (23.7) | 1 (0.5) | <0.0001* |
| Any transfusion: n (%) | 35 (89.5) | 151 (75.1) | 0.06 |
| Massive transfusion: n (%) | 24 (64.9) | 55 (27.6) | <0.0001* |
| Transfusion volume per kg of body weight: median (IQR) | 69.1 (24.6–123) | 18.1 (0–42.6) | <0.0001* |
REBOA: Resuscitative Endovascular Balloon Occlusion of the Aorta; IQR: interquarterile range.
Although there was no statistically significant difference in the proportion of patients receiving any transfusion (89.5% of patients with REBOA-amenable injuries vs. 75.1% of those without, p = 0.06, Table 4), patients with REBOA-amenable injuries were transfused more blood products per kilogram of body weight (median 69.1 ml/kg vs. 18.1 ml/kg, p < 0.0001) and more likely to receive massive transfusion, defined as > 40 ml/kg of blood products (63.2% vs. 27.8%, OR 4.8, 95% CI 2.3–9.8, p < 0.0001).
When compared to severely injured patients without REBOA-amenable injuries (n = 201), they were slightly older (median age 11 vs. 8 years old, p = 0.02). Children with REBOA-amenable injuries ranged from 11 months to 17 years old, and 26.3% (n = 10) were < 9 years old. The majority of the patients in the REBOA-amenable injury group sustained blunt trauma (71.1%), and the most common blunt mechanisms were motor vehicle collisions (n = 13), and pedestrian struck by vehicle (n = 7). Eleven patients sustained penetrating trauma, all of which were firearm injuries. There was a similar rate of pre-hospital cardiac arrest in both groups (21.1% of REBOA-amenable injuries vs. 20.9% of patients without REBOA-amenable injuries, p = 1).
Patients with REBOA-amenable injuries had higher injury severity scores (median ISS 29 vs. 25, p = 0.0005). SIPA was more commonly elevated in severely injured patients with REBOA-amenable injuries than those without (76.9% vs. 47.7%, p = 0.009). Nearly all patients who survived out of the ED were admitted to the ICU (100% of patients with REBOA-amenable injuries and 91.4% of patients without REBOA-amenable injuries), with a median ICU LOS of 5 days (IQR 3–9 days) and 3 days (IQR 1–8) respectively (p = 0.06). The vast majority of deaths overall were due to TBI (88.9%), with 7 deaths due to hemorrhage. Of the 13 deaths in patients with REBOA-amenable injuries, 4 of these deaths were due to hemorrhage (30.8%), 2 were due to hemorrhage and TBI (15.4%), 6 were due to TBI (46.2%), and 1 was due to respiratory arrest on hospital day 33. In total, hemorrhage contributed to or caused 46.2% of deaths in patients with REBOA-amenable injuries.
2.4. Utility of SIPA in Identifying Patients with REBOA-Amenable Injuries
In the population of severely injured patients for whom SIPA was able to be calculated (n=145), the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of an elevated SIPA in identifying REBOA-amenable injuries was calculated (Table 5). The sensitivity was 76.9% (95% CI 56.4%−91.0%) and the specificity was 52.3% (95% CI 42.5%−62.0%). The PPV was 27.8% (91% CI 17.9%−39.6%), and the NPV was 90.5% (95% CI 80.4% – 96.4%).
Table 5:
Utility of SIPA in Identifying Patients with REBOA-Amenable Injuries
| REBOA-amenable injury | No REBOA-amenable injury | Total patients | |
|---|---|---|---|
| SIPA elevated | 20 | 52 | 72 |
| SIPA not elevated | 6 | 57 | 63 |
| Total patients | 27 | 108 | 135 |
SIPA: Shock Index, Pediatric Age-Adjusted; REBOA: Resuscitative Endovascular Balloon Occlusion of the Aorta.
3. Discussion:
This is the first study evaluating the potential utility of REBOA in the pediatric trauma population. In this review of all pediatric trauma admissions at a single level one pediatric trauma center over ten years, 0.6% of patients were identified with injuries for which REBOA may provide temporary hemorrhage control. Furthermore, patients with REBOA-amenable injuries accounted for 16% of severely injured pediatric trauma patients and made up 10% of all pediatric trauma deaths. Hemorrhage was a rare cause of death in severely injured pediatric trauma patients (4.9% of patients) but contributed to 46.2% of deaths in patients with REBOA-amenable injuries. Patients with REBOA-amenable injuries were more likely have an elevated SIPA on admission, to sustain penetrating trauma, and were more severely injured. SIPA had a high sensitivity (76.9%) and a high negative predictive value (90.5%), indicating a non-elevated SIPA may be useful in identifying patients at low risk for REBOA-amenable injuries. TBI was present in one-third of patients with REBOA-amenable injuries.
We found that the proportion of children with REBOA-amenable injuries was comparable to that reported in adult gap analysis studies, 0.6% of all pediatric trauma patients [15]. In studies of combat-injured adult patients, the proportion with REBOA-amenable injuries was much higher at 18.5% [13]. Potentially preventable battlefield deaths are nearly all due to hemorrhage [19] and pediatric patients accounted for 1 in 10 admissions to U.S. and coalition military hospitals in Iraq and Afghanistan between 2007–2016 [20]. The proportion of combat-injured children with REBOA-amenable injuries is likely much higher than the 0.6% that we found in a civilian population, due to higher rates of penetrating trauma [21]. Importantly, REBOA has been used as a successful bridge to definitive care in the resource-limited environment of the battlefield [22]. Given similar rates of REBOA amenable injuries in adults and children, our work provides evidence of the potential utility of pediatric-sized REBOA devices in the stabilization of severely injured children in need of definitive hemorrhage control both in the civilian and combat environments. However, the overall proportion of pediatric trauma admissions sustaining REBOA-amenable injuries is still very low, and it may be necessary to maintain proficiency with the procedure through cadaveric or simulation-based training sessions.
The largest reported use of REBOA in pediatric trauma patients comes from one retrospective cohort study of 54 Japanese patients younger than 18 years old, of which 72% were between the ages of 15 and 18 years [6]. The data from the rest of the world is even more limited. One case series from the United States reported 7 cases of REBOA use in adolescent trauma patients between 14–18 years old with a 43% survival rate [8]. There are also three additional case reports of balloon occlusion of the aorta in children for a blunt aortic laceration [9], an intraabdominal vascular injury [7], and an aorto-esophageal fistula following foreign body removal [23]. Although pediatric trauma patients infrequently require emergency intervention for traumatic injuries [24–28], we have demonstrated that a small population of severely injured pediatric trauma patients with hemorrhagic injuries exists and they have a high mortality rate. Although there is controversy surrounding the ideal indications for REBOA use [29], it is an additional tool for physicians in the care of these severely injured patients. In adult patients, REBOA has been successfully used to limit blood loss following severe intraabdominal trauma [30], patients with pre-hospital arrest due to trauma [31], non-traumatic abdominal hemorrhage [32], and obstetrical hemorrhage [33].
Concern has been raised regarding the use of REBOA in patients with TBI, as proximal hypertension may contribute to worsening intracranial hypertension and worsening TBI [34]. This is a major concern in pediatric trauma patients, as TBI is the leading cause of morbidity and mortality in children [35]. In our cohort of children with REBOA-amenable injuries, 29% had a TBI as well. Thus, the safety of aortic occlusion in pediatric patients with TBI must be established. Although the primary purpose of balloon occlusion of the aorta is to limit exsanguination from points of injury distal to the balloon, it also augments proximal blood flow to the heart and brain. REBOA has been shown to reduce blood loss in both adult [36] and pediatric [37] animal models, but it may also cause proximal hypertension [38], which may result in cardiac injury [39] and intracranial pressure elevations [40]. However, in adult swine models of aortic occlusion, progression of TBI with REBOA use was not evident on serial head imaging [34]. The true effects of REBOA on pediatric patients with a TBI remain unknown.
Access for REBOA is obtained through the common femoral artery (CFA) and access vessel size must be considered for the use of REBOA in pediatric patients. The diameter of the CFA ranges from 2.1–4.4 mm in children weighing less than 12 kg and 3.1–7.1 mm in children weighing greater than 12 kg. In pediatric patients undergoing cardiac catheterization, there is an increased risk of loss of distal pulses in that extremity when the catheter outer diameter is > 50% of the diameter of the arterial lumen [41]. The most common device in use is the commercially available ER-REBOA catheter which is inserted through a 7 French introducer sheath which has a 2.95 mm outer diameter. This suggests that a common femoral artery diameter of 5.9 mm would permit REBOA access without increasing the risk of arterial injury or limb ischemia, which corresponds to a minimum patient weight of 30 kg [42]. This is the weight of a 9–10-year-old child at the 50th percentile of weight [43,44], which is slightly younger than the youngest reported REBOA patients [7,9]. In this study, we found that the median age of a patient with a REBOA-amenable injury was 11 years, but 26.3% of patients with REBOA-amenable injuries were younger than 9 years old, indicating an ongoing need for a REBOA catheter that can be deployed through a smaller sheath.
The use of REBOA is not without risk. One of the potential risks of REBOA use is vascular access site complications, including arterial dissection, thrombosis, or occlusion resulting in distal ischemia. These risks have been found to be minimized by use of a 7 French introducer sheath when compared to older, larger-bore sheaths [45]. Due to the smaller size of pediatric blood vessels, it is possible that the risk of vascular complications may be higher. Even with smaller introducer sheaths and aortic occlusion devices, providers who use REBOA in pediatric patients must be vigilant in monitoring for potential complications. Additionally, inadvertent balloon inflation distal to aortic bifurcation can result in injury to or rupture of the iliac arteries. Although rare, over-inflation of the balloon may injure the aorta. Inflation of the balloon for too long a duration may result in irreversible distal ischemia, and REBOA should only be used as an immediate bridge to hemorrhage control, and not as a prolonged intervention. During balloon deflation, recirculation of blood from temporarily ischemic regions of the body can result in reperfusion injury with significant hypotension and thus must be coordinated carefully with the anesthesia team [46]. Additionally, there is some evidence of worsening of cardiac injury with complete aortic occlusion via REBOA [39]. These potential adverse events must be considered with each case, and care taken to minimize these risks.
Lastly, identifying the pediatric patient who may have injuries for which REBOA may be of use is an additional challenge. SIPA, or Shock Index, Pediatric Age-Adjusted, has been found to be more accurate than age-adjusted hypotension [47] or shock index not adjusted for age [48] in identifying severely injured pediatric patients. Our findings add support to the use of SIPA as one tool in helping to identify pediatric trauma patients at high risk for intra-abdominal injury, as 81% of patients with injuries amenable to REBOA use had an elevated SIPA on admission, compared to 44% of severely injured children without REBOA-amenable injuries and 17.7% of non-severely injured patients. The high sensitivity and high NPV indicate that a non-elevated SIPA may be used to indicate a lower risk of REBOA-amenable injury in severely injured children, but that an elevated SIPA is poorly predictive of a REBOA-amenable injury due to its low specificity (53.9%) and PPV (29.3%). In adult patients, suspicion of non-compressible torso hemorrhage and hemodynamic instability are used as indications for REBOA placement, but pediatric-specific guidelines must be developed.
3.1. Future Directions
Further research is needed to guide the use of REBOA in pediatric trauma patients. Given the size discrepancies of adult and pediatric vasculature, smaller-profile REBOA catheters are needed to be used in patients younger than adolescents. Identifying the appropriate indications for REBOA use in the pediatric population is needed to accurately identify patients who may benefit from REBOA. In our cohort, elevated SIPA was not predictive of REBOA-amenable injuries, but other pediatric risk predictors were not evaluated. Although REBOA was not shown to worsen progression of TBI in a previously performed adult animal model, the monitoring period of the study was short, and long-term effects of REBOA on TBI progression are currently unknown. Additionally, it is unknown if these results can be applied to a pediatric population. As TBI is a leading cause of morbidity and mortality in children [35], further research is indicated to establish the effects of aortic occlusion specifically on pediatric patients with traumatic brain injury. Specifically, the effect of aortic occlusion on intracranial hypertension and worsening of brain injury, as well as neurologic functional outcomes, should be studied in pediatric animal models prior to the use of aortic occlusion in children with TBI. Additionally, partial REBOA is an emerging technology which has been shown in animal models [49] to reduce blood loss without causing increases in carotid arterial flow, lending support to its potential use in the exsanguinating trauma patient with TBI. Pediatric animal models of partial REBOA should be compared to complete REBOA to determine if either mode of aortic occlusion is feasible in severely injured children.
3.2. Limitations
Our study has several limitations. This is a single-center retrospective review and may not be reflective of the broader pediatric trauma population. Additionally, these conclusions are drawn from 239 patients we identified as severely injured, and 38 patients in whom we identified injuries that may benefit from REBOA. As such, the population is very limited, and these patients reflect a very small percent of the pediatric trauma population. Although the information in the trauma database at our institution is prospectively collected, there may be coding inaccuracies. The use of surgery for hemorrhage control was not added to the database until 2013, and activation of MTP and pre-hospital cardiac arrest were not variables in the database until 2015, which means there are likely patients from 2009–2015 who met those criteria for inclusion but were not able to be identified; thus, the true incidence of severely injured pediatric trauma patients is likely higher than stated here.
4. Conclusion:
Nearly 20% of severely injured pediatric patients had injuries for which REBOA may provide temporary hemorrhage control, and half of deaths in this patient population were attributable to hemorrhage. Although the rate of children with REBOA-amenable injuries was low overall at 0.6% of all pediatric trauma activations, this is comparable to estimates from adult gap analyses. Further research should focus on accurately identifying children at risk for death from hemorrhage and adapting currently available REBOA catheters for use in pediatric patients.
Funding Information:
The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant number UL1 TR001860 for author C.M.T. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abbreviations:
- REBOA
Resuscitative Endovascular Balloon Occlusion of the Aorta
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of Interest: None.
References:
- [1].Mclaughlin C, Zagory JA, Fenlon M, Park C, Lane CJ, Meeker D, et al. Timing of Mortality in Pediatric Trauma Patients: A National Trauma Databank Analysis. J Pediatr Surg 2019;53:344–51. 10.1016/j.jpedsurg.2017.10.006.Timing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Drake SA, Holcomb JB, Yang Y, Thetford C, Myers L, Brock M, et al. Establishing a regional pediatric trauma preventable/potentially preventable death rate. Pediatr Surg Int 2020;36:179–89. 10.1007/s00383-019-04597-9. [DOI] [PubMed] [Google Scholar]
- [3].Biffl WL, Fox CJ, Moore EE. The role of REBOA in the control of exsanguinating torso hemorrhage. J Trauma Acute Care Surg 2015;78:1054–8. 10.1097/TA.0000000000000609. [DOI] [PubMed] [Google Scholar]
- [4].Brenner M, Teeter W, Hoehn M, Pasley J, Hu P, Yang S, et al. Use of resuscitative endovascular balloon occlusion of the aorta for proximal aortic control in patients with severe hemorrhage and arrest. JAMA Surg 2018;153:130–5. 10.1001/jamasurg.2017.3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Aso S, Matsui H, Fushimi K, Yasunaga H. Resuscitative endovascular balloon occlusion of the aorta or resuscitative thoracotomy with aortic clamping for noncompressible torso hemorrhage: A retrospective nationwide study. J Trauma Acute Care Surg 2017;82:910–4. 10.1097/TA.0000000000001345. [DOI] [PubMed] [Google Scholar]
- [6].Norii T, Miyata S, Terasaka Y, Guliani S, Lu SW, Crandall C. Resuscitative endovascular balloon occlusion of the aorta in trauma patients in youth. J Trauma Acute Care Surg 2017;82:915–20. 10.1097/TA.0000000000001347. [DOI] [PubMed] [Google Scholar]
- [7].Sadeghi M, Mcgreevy DT, Lindgren R, Ågren K, Hörer TM. Endovascular Resuscitation with Aortic Balloon Occlusion in Pediatric Trauma. J Endovasc Resusc Trauma Manag 2019;3:139–42. 10.26676/jevtm.v3i3.99. [DOI] [Google Scholar]
- [8].Smith AD, Wasicek P, Teeter W, Hudson JA, Moore LJ, Brenner M. Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) for Temporization of Hemorrhage in Pediatric Trauma Patients. Pediatr Trauma Soc Annu Meet 2017. [DOI] [PubMed] [Google Scholar]
- [9].Kobayashi T, Matsuda K, Iwase F, Miyazaki Y, Amenomori S, Kikuchi H, et al. Blunt abdominal aortic injury in a child: a case report. Nihon Kyukyu Igakukai Zasshi 2010;21:343–50. 10.3893/jjaam.21.343. [DOI] [Google Scholar]
- [10].Ordoñez CA, Manzano-Nunez R, del Valle AM, Rodriguez F, Burbano P, Naranjo MP, et al. Current use of Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) in trauma. Colomb J Anesthesiol 2017;45:30–8. 10.1016/j.rcae.2017.09.007. [DOI] [Google Scholar]
- [11].Petrone P, Pérez-Jiménez A, Rodríguez-Perdomo M, Brathwaite CEM, Joseph DK. Resuscitative endovascular balloon occlusion of the aorta (REBOA) in the management of trauma patients: A systematic literature review. Am Surg 2019;85:654–62. 10.1177/000313481908500631. [DOI] [PubMed] [Google Scholar]
- [12].Morrison JJ, Galgon RE, Jansen JO, Cannon JW, Rasmussen TE, Eliason JL. A systematic review of the use of resuscitative endovascular balloon occlusion of the aorta in the management of hemorrhagic shock. J Trauma Acute Care Surg 2016;80:324–34. 10.1097/TA.0000000000000913. [DOI] [PubMed] [Google Scholar]
- [13].Morrison JJ, Ross JD, Rasmussen TE, Midwinter MJ, Jansen JO. Resuscitative endovascular balloon occlusion of the aorta: A gap analysis of severely injured UK Combat casualties. Shock 2013;41:388–93. 10.1097/SHK.0000000000000136. [DOI] [PubMed] [Google Scholar]
- [14].Barnard EBG, Morrison JJ, Madureira RM, Lendrum R, Fragoso-Iñiguez M, Edwards A, et al. Resuscitative endovascular balloon occlusion of the aorta (REBOA): A population based gap analysis of trauma patients in England and Wales. Emerg Med J 2015;32:926–32. 10.1136/emermed-2015-205217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Dumas RP, Holena DN, Smith BP, Jafari D, Seamon MJ, Reilly PM, et al. Resuscitative endovascular balloon occlusion of the aorta: assessing need in an urban trauma center. J Surg Res 2019;233:413–9. 10.1016/j.jss.2018.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Joseph B, Ibraheem K, Haider AA, Kulvatunyou N, Tang A, O’Keeffe T, et al. Identifying potential utility of resuscitative endovascular balloon occlusion of the aorta: An autopsy study. J. Trauma Acute Care Surg, vol. 81, Lippincott Williams and Wilkins; 2016, p. S128–32. 10.1097/TA.0000000000001104. [DOI] [PubMed] [Google Scholar]
- [17].Campagna GA, Cunningham ME, Hernandez JA, Chau A, Vogel AM, Naik-Mathuria BJ. The utility and promise of Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) in the pediatric population: An evidence-based review. J Pediatr Surg 2020:1–6. 10.1016/j.jpedsurg.2020.01.052. [DOI] [PubMed] [Google Scholar]
- [18].Nordin A, Coleman A, Shi J, Wheeler K, Xiang H, Acker S, et al. Validation of the age-adjusted shock index using pediatric trauma quality improvement program data. J Pediatr Surg 2018;53:130–5. 10.1016/j.jpedsurg.2017.10.023. [DOI] [PubMed] [Google Scholar]
- [19].Eastridge BJ, Mabry RL, Seguin P, Cantrell J, Tops T, Uribe P, et al. Death on the battlefield (2001–2011): Implications for the future of combat casualty care. J Trauma Acute Care Surg 2012;73:431–7. 10.1097/TA.0b013e3182755dcc. [DOI] [PubMed] [Google Scholar]
- [20].Gale HL, Borgman MA, April MD, Schauer SG. Pediatric Trauma Patient Intensive Care Resource Utilization in U.S. Military Operations in Iraq and Afghanistan. Crit Care Explor 2019;1:e0062. 10.1097/cce.0000000000000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Villamaria CY, Morrison JJ, Fitzpatrick CM, Cannon JW, Rasmussen TE. Wartime vascular injuries in the pediatric population of Iraq and Afghanistan: 2002–2011. J Pediatr Surg 2014;49:428–32. 10.1016/j.jpedsurg.2013.10.002. [DOI] [PubMed] [Google Scholar]
- [22].Manley JD, Mitchell BJ, DuBose JJ, Rasmussen TE. A Modern Case Series of Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) in an Out-of-Hospital, Combat Casualty Care Setting. J Spec Oper Med 2017;17:1–8. [DOI] [PubMed] [Google Scholar]
- [23].Hill SJ, Zarroug AE, Ricketts RR, Veeraswamy R. Bedside placement of an aortic occlusion balloon to control a ruptured aorto-esophageal fistula in a small child. Ann Vasc Surg 2010;24:822.e7–822.e9. 10.1016/j.avsg.2009.12.016. [DOI] [PubMed] [Google Scholar]
- [24].Nicolson NG, Schwulst S, Esposito TA, Crandall ML. Resuscitative thoracotomy for pediatric trauma in Illinois, 1999 to 2009. Am J Surg 2015;210:720–3. 10.1016/j.amjsurg.2015.05.007. [DOI] [PubMed] [Google Scholar]
- [25].Moore HB, Moore EE, Bensard DD. Pediatric emergency department thoracotomy: A 40-year review. J Pediatr Surg 2016;51:315–8. 10.1016/j.jpedsurg.2015.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Flynn-O’Brien KT, Stewart BT, Fallat ME, Maier RV, Arbabi S, Rivara FP, et al. Mortality after emergency department thoracotomy for pediatric blunt trauma: Analysis of the National Trauma Data Bank 2007–2012. J. Pediatr. Surg, vol. 51, W.B. Saunders; 2016, p. 163–7. 10.1016/j.jpedsurg.2015.10.034. [DOI] [PubMed] [Google Scholar]
- [27].Stephens CQ, Boulos MC, Connelly CR, Gee A, Jafri M, Krishnaswami S. Limiting thoracic CT: a rule for use during initial pediatric trauma evaluation. J Pediatr Surg 2017;52:2031–7. 10.1016/j.jpedsurg.2017.08.039. [DOI] [PubMed] [Google Scholar]
- [28].Boatright DH, Byyny RL, Hopkins E, Bakes K, Hissett J, Tunson J, et al. Validation of rules to predict emergent surgical intervention in pediatric trauma patients. J Am Coll Surg 2013;216. 10.1016/j.jamcollsurg.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Joseph B, Zeeshan M, Sakran JV, Hamidi M, Kulvatunyou N, Khan M, et al. Nationwide Analysis of Resuscitative Endovascular Balloon Occlusion of the Aorta in Civilian Trauma. JAMA Surg 2019;154:500–8. 10.1001/jamasurg.2019.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Aoki M, Abe T, Hagiwara S, Saitoh D, Oshima K. Resuscitative endovascular balloon occlusion of the aorta may contribute to improved survival. Scand J Trauma Resusc Emerg Med 2020;28. 10.1186/s13049-020-00757-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hilbert-Carius P, McGreevy DT, Abu-Zidan FM, Hörer TM, McGreevy DT, Hilbert-Carius P, et al. Pre-hospital CPR and early REBOA in trauma patients-results from the ABOTrauma Registry. World J Emerg Surg 2020;15. 10.1186/s13017-020-00301-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hatchimonji JS, Chipman AM, McGreevy DT, Hörer TM, Burruss S, Han S, et al. REBOA Use in Nontrauma Emergency General Surgery: A Multi-institutional Experience. J Surg Res 2020;256:149–55. 10.1016/j.jss.2020.06.034. [DOI] [PubMed] [Google Scholar]
- [33].Manzano-Nunez R, Escobar-Vidarte MF, Orlas CP, Herrera-Escobar JP, Galvagno SM, Melendez JJ, et al. Resuscitative endovascular balloon occlusion of the aorta deployed by acute care surgeons in patients with morbidly adherent placenta: A feasible solution for two lives in peril. World J Emerg Surg 2018;13:1–6. 10.1186/s13017-018-0205-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Johnson MA, Williams TK, Ferencz SAE, Davidson AJ, Russo RM, O’Brien WT, et al. The effect of resuscitative endovascular balloon occlusion of the aorta, partial aortic occlusion and aggressive blood transfusion on traumatic brain injury in a swine multiple injuries model. J Trauma Acute Care Surg 2017;83:61–70. 10.1097/TA.0000000000001518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Centers for Disease Control and Prevention. Report to congress on the management of traumatic brain injury in children. 2018.
- [36].Russo RM, Neff LP, Lamb CM, Cannon JW, Galante JM, Clement NF, et al. Partial Resuscitative Endovascular Balloon Occlusion of the Aorta in Swine Model of Hemorrhagic Shock. J. Am. Coll. Surg, vol. 223, Elsevier Inc.; 2016, p. 359–68. 10.1016/j.jamcollsurg.2016.04.037. [DOI] [PubMed] [Google Scholar]
- [37].Yamashiro KJ, Wishy AM, Beyer CA, Kashtan HW, Galganski LA, Grayson JK, et al. Resuscitative endovascular balloon occlusion of the aorta (REBOA) in a pediatric swine liver injury model: A pilot study. J Pediatr Surg 2020;55:346–52. 10.1016/j.jpedsurg.2019.10.013. [DOI] [PubMed] [Google Scholar]
- [38].Tibbits EM, Hoareau GL, Simon MA, Davidson AJ, Desoucy ES, Robert Faulconer E, et al. Location is everything: The hemodynamic effects of REBOA in Zone 1 versus Zone 3 of the aorta. J. Trauma Acute Care Surg, vol. 85, Lippincott Williams and Wilkins; 2018, p. 101–7. 10.1097/TA.0000000000001858. [DOI] [PubMed] [Google Scholar]
- [39].Beyer CA, Hoareau GL, Tibbits EM, Davidson AJ, Desoucy ED, Simon MA, et al. Resuscitative endovascular balloon occlusion of the aorta induced myocardial injury is mitigated by endovascular variable aortic control. J Trauma Acute Care Surg 2019;87:590–8. 10.16309/j.cnki.issn.1007-1776.2003.03.004. [DOI] [PubMed] [Google Scholar]
- [40].Krishnamoorthy V, Chaikittisilpa N, Kiatchai T, Vavilala M. Hypertension after Severe Traumatic Brain Injury: Friend or Foe? J Neurosurg Anesthesiol 2017;29:382–7. 10.1097/ANA.0000000000000370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Alexander J, Yohannan T, Abutineh I, Agrawal V, Lloyd H, Zurakowski D, et al. Ultrasound-guided femoral arterial access in pediatric cardiac catheterizations: A prospective evaluation of the prevalence, risk factors, and mechanism for acute loss of arterial pulse. Catheter Cardiovasc Interv 2016;88:1098–107. 10.1002/ccd.26702. [DOI] [PubMed] [Google Scholar]
- [42].DeSoucy ES, Trappey AF, Wishy AM, Simon MA, Davidson AJ, DuBose JJ, et al. Approximation of Pediatric Morphometry for Resuscitative Endovascular Balloon Occlusion of the Aorta. J Endovasc Resusc Trauma Manag 2019;3:97–103. 10.26676/jevtm.v3i3.95. [DOI] [Google Scholar]
- [43].Centers for Disease Control Growth Chart, Girls aged 2–20 years n.d. cdc.gov/growthcharts/data/set1clinical/cj41l022.pdf (accessed April 15, 2020).
- [44].Centers for Disease Control Growth Chart, Boys aged 2–20 years n.d. https://www.cdc.gov/growthcharts/data/set1clinical/cj41l021.pdf (accessed April 15, 2020).
- [45].Matsumura Y, Matsumoto J, Kondo H, Idoguchi K, Ishida T, Kon Y, et al. Fewer REBOA complications with smaller devices and partial occlusion: Evidence from a multicentre registry in Japan. Emerg Med J 2017;34:793–9. 10.1136/emermed-2016-206383. [DOI] [PubMed] [Google Scholar]
- [46].Davidson AJ, Russo RM, Reva VA, Brenner ML, Moore LJ, Ball C, et al. The pitfalls of resuscitative endovascular balloon occlusion of the aorta: Risk factors and mitigation strategies. J Trauma Acute Care Surg 2018;84:192–202. 10.1097/TA.0000000000001711. [DOI] [PubMed] [Google Scholar]
- [47].Acker SN, Bredbeck B, Partrick DA, Kulungowski AM, Barnett CC, Bensard DD. Shock index, pediatric age-adjusted (SIPA) is more accurate than age-adjusted hypotension for trauma team activation. Surg (United States) 2017;161:803–7. 10.1016/j.surg.2016.08.050. [DOI] [PubMed] [Google Scholar]
- [48].Acker SN, Ross JT, Partrick DA, Tong S, Bensard DD. Pediatric specific shock index accurately identifies severely injured children. J Pediatr Surg 2015;50:331–4. 10.1016/j.jpedsurg.2014.08.009. [DOI] [PubMed] [Google Scholar]
- [49].Russo RM, Williams TK, Grayson JK, Lamb CM, Cannon JW, Clement NF, et al. Extending the golden hour: Partial resuscitative endovascular balloon occlusion of the aorta in a highly lethal swine liver injury model. J Trauma Acute Care Surg 2016;80:372–80. 10.1097/TA.0000000000000940. [DOI] [PubMed] [Google Scholar]


