Abstract
Objectives
The aim of this study was to investigate the relationship between M1 and M2 macrophage polarization and clinical stage in patients with medication-related osteonecrosis of the jaw (MRONJ) who underwent treatment with bisphosphonates or denosumab.
Materials and methods
M1 and M2 macrophage density and expression of interleukin (IL)-6 and IL-10 were assessed on biopsies of mucosal tissues surrounding necrotic bone in 30 MRONJ patients with stages 1–3 and controls. For identification of M1 and M2 macrophages, double CD68/iNOS and CD68/CD206 immunofluorescence staining was conducted, respectively. Computer-assisted immunofluorescence quantification of markers was performed.
Results
Early stage 1 MRONJ patients showed a switch toward the M2 phenotype, as indicated by the higher density of M2 macrophages, the decreased M1/M2 ratio and the upregulation of IL-10. MRONJ patients with advanced stages 2 and 3 showed a shift toward M1-polarized macrophages, as suggested by the higher density of M1 macrophages, the increased M1/M2 ratio and the overexpression of IL-6. The macrophage density of both M1 and M2 subsets was significantly enhanced in patients receiving bisphosphonates compared to those receiving denosumab.
Conclusions
The M1–M2 macrophage polarization status in mucosal tissues bordering necrotic bone correlates with clinical stage of MRONJ. Patients with early stage MRONJ show a switch toward M2-polarized macrophages, while MRONJ patients with advanced stage demonstrate a shift toward the M1 phenotype.
Clinical relevance
Therapeutic molecules targeting the inflammatory microenvironment via the regulation of either M1 or M2 macrophage polarization may represent a novel strategy for treatment of MRONJ.
Keywords: Jaw diseases, Denosumab, Bisphosphonate-Associated Osteonecrosis of the Jaw, Zoledronic acid, Alendronate, Macrophage, Phenotype
Introduction
Medication-related osteonecrosis of the jaw (MRONJ) is a serious adverse effect of antiresorptive agents, principally including bisphosphonates and denosumab, while angiogenic inhibitors have also been implicated [1]. Bisphosphonates are structural analogues of inorganic pyrophosphate that have a high affinity to bone matrix and inhibit bone resorption by suppressing osteoclast activity and inducing osteoclast apoptosis [2]. Denosumab is a human monoclonal antibody that binds to the receptor activator of nuclear factor kappa-B ligand (RANKL), thereby preventing RANKL from binding to RANK and inhibiting osteoclast formation, function and survival [3]. MRONJ is currently defined as exposed bone or bone that can be probed through an intraoral or extraoral fistula in the maxillofacial region and that has persisted for longer than 8 weeks in patients with current or previous treatment with antiresorptive agents and/or angiogenic inhibitors, while having no history of radiation therapy and no evidence of metastatic disease to the jaws [4].
Although the first MRONJ case was reported by Marx et al. in 2003, the pathophysiologic mechanisms of this disease have not yet been fully elucidated and its treatment remains empirical [5]. Proposed hypotheses for MRONJ pathogenesis include modulation of innate or acquired immunity, angiogenesis suppression, drug toxicity to oral mucosal tissues, inhibition of bone remodeling, as well as local inflammation and infection [6–8]. In recent years, several studies have emphasized the role of dentoalveolar infection in the pathophysiology of MRONJ [9–11]. Systemic risk factors for the development of MRONJ comprise chemotherapy, corticosteroid treatment, uncontrolled diabetes mellitus, and smoking, while local risk factors include poor oral health, dentoalveolar trauma and surgery [12–15]. There is a growing body of evidence indicating that surgery may be effective for the treatment of MRONJ at any disease stage, however, patients with early stage MRONJ may be successfully managed using a conservative approach [16–18].
Tissue macrophages are a heterogeneous cell population of both embryonic and hematopoietic origin with critical and diverse functions, such as response to inflammation and host defense against pathogens as well as restoration of tissue homeostasis [19]. Macrophages are polarized toward the M1 phenotype, when exposed to classical inflammatory activators such as lipopolysaccharide (LPS) and interferon. M1-polarized macrophages are characterized by the production of proinflammatory mediators, such as inducible nitric oxide synthase (iNOS) and interleukin (IL)-6 [20]. In M1 macrophages, iNOS uses L-arginine as a substrate to produce nitric oxide (NO), which plays an important role in many physiological processes including neuronal signaling, regulation of vascular tone, and immune response against pathogens [21]. IL-6 is a multifunctional cytokine that is involved in several biological functions, including regulation of the innate and adaptive immune system, development of the cardiovascular and neuronal systems, hematopoiesis, and modulation of bone metabolism, mainly acting as a positive regulator of osteoclastogenesis [22].
Macrophages are skewed toward the M2 phenotype when exposed to alternative activators such as IL-4, IL-13, IL-10 and transforming growth factor (TGF)-β [23]. Cluster of differentiation 206 (CD206) (or mannose receptor C type 1, MRC1) expressed predominantly by M2-polarized macrophages is a transmembrane glycoprotein that acts as a pattern recognition receptor (PRR) and serves as a specific marker for the identification of the M2 phenotype [24]. IL-10, produced by M2-polarized macrophages, is an anti-inflammatory cytokine that is associated with tissue repair and regeneration, control of immune responses, and regulation of bone homeostasis through suppression of osteoclastogenesis [25]. Drug- and inflammation-induced modulation of the M1–M2 macrophage polarization status has been suggested to play a critical role in the pathogenesis of MRONJ [26–28].
The objective of this study was to investigate the link between M1- and M2-polarized macrophages in mucosal tissues surrounding necrotic bone and the clinical stage of patients with MRONJ who underwent treatment with bisphosphonates or denosumab. Given that staging of MRONJ is determined by the progression and manifestations of clinical infection and inflammation in the maxillofacial region, we hypothesized a stage-dependent switch of macrophage polarization, predominantly toward the anti-inflammatory M2 phenotype in patients with early stage of MRONJ and toward the proinflammatory M1 phenotype in patients with advanced stage of disease.
Material and methods
Study population
Our study cohort comprised 30 patients with histologically confirmed MRONJ following therapy with either bisphosphonates (n =15) or denosumab (n = 15), who underwent surgical debridement and biopsy of mucosal tissue surrounding the osteonecrotic area in the maxilla or mandible at the Department of Oral and Maxillofacial Surgery of the School of Dentistry, National and Kapodistrian University of Athens (NKUA), Greece between 2016 and 2019. Inclusion criteria were 1) MRONJ patients who received: i) oral bisphosphonates for more than 4 years ii) intravenous bisphosphonates (e.g. 5 mg zoledronic acid once a year), iii) denosumab (Prolia 60 mg) administered subcutaneously every 6 months for osteoporosis [29–31], iiii) denosumab (Xgeva 120 mg) administered subcutaneously every 4 weeks for prevention of bone complications in cancer, 2) patients with sufficient clinical and radiographic data to determine stage of MRONJ, with 10 patients assigned to each clinical stage [stage 1 (n =10, 33.3%), stage 2 (n =10, 33.3%) and stage 3 (n =10, 33.3%)], and 3) availability of biopsies of sufficient quality for immunofluorescence studies. Exclusion criteria were: 1) history of head and neck radiotherapy, 2) patients with acute or chronic renal or hepatic insufficiency or any hematologic disorder, 3) patients who underwent cardiovascular operation within a year, and 4) recent use of local or systemic corticosteroids. Two groups of participants without MRONJ who underwent biopsy of inflamed oral mucosa adjacent to extraction socket of teeth with periodontal disease were assigned as controls: 1) 13 control group participants who received bisphosphonates (n = 8) or denosumab (n = 5) without presenting any clinical or radiographic findings of MRONJ, and 2) 6 control group participants who had never received any antiresorptive therapy.
For all eligible patients, clinical characteristics were recorded, including sex, age, primary disease, antiresorptive medication, administration period, site of lesion and MRONJ staging. Diagnosis and assignment of patients into clinical stages of MRONJ was based on clinical and radiographic examination and according to the proposed staging system of the American Association of Oral and Maxillofacial Surgeons (AAOMS) in 2014 (Supplementary Table 1) [4]. Radiographic evaluation took place at the Department of Oral Diagnosis and Radiology of the School of Dentistry, NKUA and included panoramic radiographs and cone-beam computed tomography (CBCT). Histopathologic examination of all biopsy specimens was performed for confirming the diagnosis; the type (chronic or mixed), distribution (focal or diffuse) and intensity (mild, moderate, or severe) of inflammation was assessed and recorded. Written informed consent was obtained from all the patients. The Strengthening the Reporting of Observational studies in Epidemiology (STROBE) guidelines were followed. The local Institutional Ethics Committee approved this study.
Immunofluorescence study and evaluation of staining
To study M1 and M2 macrophage density and the expression of IL-6 and IL-10, formalin-fixed, paraffin embedded (FFPE) biopsy specimens archived at the Department of Oral Medicine and Pathology of the School of Dentistry, NKUA, were subjected to standard immunofluorescence analysis. In brief, representative specimens were sectioned (4 μm thickness sections). One section was stained with haematoxylin and eosin and adjacent serial sections were used for immunofluorescence staining. Sections were dewaxed in xylene and then rehydrated in graded alcohols. Sections were washed in water before antigen retrieval with 10 mM sodium citrate buffer (pH 6.0) at 60 °C overnight. UltraCruz Blocking Reagent (Santa Cruz) was used for 1 hour at room temperature. In turn, tissue sections were incubated with the following primary antibodies overnight: CD68 1:200 (M00602, Boster), iNOS 1:100 (ab15323, Abcam), CD206 1:100 (sc-58986, Santa Cruz), IL-6 1:250 (sc-28343, Santa Cruz), IL-10 1:400 (ab34843, Abcam). Sections were incubated with the following secondary antibodies for 45 min: Boster BA1089 TRITC anti-mouse 1:500, Boster BA1101 FITC anti-mouse 1:500, Invitrogen Alexa Fluor 568 anti-rabbit 1:500. After incubation with secondary antibody and three washes with PBS/T, the slides were mounted using Fluoroshield with DAPI staining to detect nuclei (F6057, SIGMA).
Immunofluorescence stained slides were digitally scanned utilizing the Aperio AT automated slide scanner and automated image analysis was performed using the Aperio Image Scope software (Aperio Technologies, Inc., Vista, CA, USA). Digital imaging was performed at the Translational Pathology Core Laboratory (TPCL) at David Geffen School of Medicine, University of California, Los Angeles (UCLA), USA. The magnification of the digital images varied continuously as it could be controlled by the computer software. Computer-assisted immunofluorescence quantification of markers was performed. Stained slides were assessed independently by two separate investigators with more than 10 years of experience. The investigators evaluated at least two representative image fields at 20x followed by 40x magnification for further verification. For each slide, every marker was digitally evaluated for the intension of staining separately with Aperio ImageScope software and after the use of specific filters (threshold) for each antibody.
For identification of M1 and M2 macrophages, double CD68/iNOS and CD68/CD206 immunofluorescence staining was performed respectively. CD68 ab was labelled with secondary FITC ab, while iNOS and CD206 abs with secondary TRITC ab. Every cell expressing CD68, iNOS or CD206 above defined thresholds was considered positive. In turn, CD68 and iNOS as well as CD68 and CD206 images were fused to create the double staining image. All nucleated cells with double positive staining for the phenotype marker M1 (CD68+/iNOS+) or M2 (CD68+/CD206+) in each image were counted manually. Density of M1 and M2 macrophages was calculated as the number of positively stained cells per square millimeter (cells/mm2) in the region of interest. For IL-6 and IL-10 quantification, marker expression above defined thresholds in the region of interest was considered positive and the percentage of positive IL-6 and IL-10 staining was digitally calculated. The autofluorescence of erythrocytes was manually removed from all quantification. All investigators performing measurements were blinded to patient clinical data.
Statistical analysis
Categorical data were described with absolute and relative frequencies. Skew data were expressed as median and interquartile range (IQR) and group differences were tested by Mann–Whitney U test or Kruskal–Wallis H test as appropriate. Bonferroni correction was applied to protect from Type 1 error when conducting multiple comparison tests on the same dependent variable. Boxplots depicted the distribution of macrophage densities across MRONJ stages and controls. One-way ANOVA was used to assess whether there are differences among the means of two or more independent groups. A Tukey post hoc test was conducted for multiple comparisons. Inter-rater agreement was estimated using Cohen’s kappa statistic. Any discrepancies between investigators were resolved by consensus. Two-sided p-values <0.05 were considered statistically significant. Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS®, version 26.0; IBM Corp., Armonk, NY).
Results
Patient characteristics
The median age of the 30 MRONJ patients was 71, ranging from 45 to 82 years with a female predominance (70%, n = 21). The underlying disease was osteoporosis in 9 patients (30%) and malignancy in 21 patients (70%) including 9 patients with breast cancer, 3 with prostate cancer, 1 with lung cancer and 8 with multiple myeloma. Eleven patients (37%) received zoledronic acid (Zometa), 4 patients (13%) received alendronate (Fosamax) and 15 patients received denosumab [5 patients received Prolia (17%) and 10 patients received Xgeva (33%)]. The median administration period of antiresorptive therapy was 36 months, while 11 patients (37%) received antiresorptive treatment for more than 36 months. MRONJ was located in the mandible in 18 patients (60%) and the maxilla in 12 patients (40%). Demographic and clinical characteristics of MRONJ patients and control group participants are listed in Table 1.
Table 1.
Patient demographic and clinical characteristics
| MRONJ patients n = 30 |
Cohort antiresorptive n = 43 |
Control no antiresorptive n = 6 |
All patients n = 49 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Category | n | (%) | n | (%) | n | (%) | n | (%) |
| Sex | Male | 9 | (30) | 11 | (26) | 1 | (17) | 12 | (24) |
| Female | 21 | (70) | 32 | (74) | 5 | (83) | 37 | (76) | |
| Age | ≤ 60 years | 7 | (23) | 10 | (23) | 5 | (83) | 15 | (31) |
| > 60 years | 23 | (77) | 33 | (77) | 1 | (17) | 34 | (69) | |
| Primary disease | Osteoporosis | 9 | (30) | 19 | (44) | ||||
| Cancer | 21 | (70) | 24 | (56) | |||||
| Antiresorptive class | Bisphosphonates | 15 | (50) | 23 | (54) | ||||
| Denosumab | 15 | (50) | 20 | (46) | |||||
| Antiresorptive agents | Zoledronic acid (Zometa) | 11 | (37) | 12 | (28) | ||||
| Alendronate (Fosamax) | 4 | (13) | 11 | (25) | |||||
| Denosumab (Prolia) | 5 | (17) | 8 | (19) | |||||
| Denosumab (Xgeva) | 10 | (33) | 12 | (28) | |||||
| Administration period | ≤ 36 months | 19 | (63) | 24 | (56) | ||||
| > 36 months | 11 | (37) | 19 | (44) | |||||
| Site of MRONJ | Maxilla | 12 | (40) | ||||||
| Mandible | 18 | (60) | |||||||
| Staging of MRONJ | Stage 1 | 10 | (33.3) | ||||||
| Stage 2 | 10 | (33.3) | |||||||
| Stage 3 | 10 | (33.3) | |||||||
MRONJ=Medication-Related Osteonecrosis of the Jaw.
Microscopic features of the inflammatory infiltrate
Histopathologic examination of the soft tissue surrounding the osteonecrotic area was performed in MRONJ cases, revealing an inflammatory infiltrate of variable type, distribution and intensity in all cases (Supplementary Figure 1). More specifically, in the adjacent connective tissue, which showed different degrees of density (ranging from myxomatous to dense fibrous) and vascularity, inflammation was present, composed of chronic inflammatory cells (lymphocytes, macrophages and plasma cells) with or without a co-existing acute (neutrophilic) component. Comparing different stages of MRONJ, stage 1 group included more cases (7 out of 10) with an almost exclusively chronic inflammatory infiltrate, as opposed to a more frequent mixed inflammatory infiltrate (6 out of 10 cases) in each one of stage 2 and 3 groups. The distribution of the inflammatory infiltrate varied from focal to diffuse: the latter pattern was most frequent in all stages and even more so in stage 3 (accounting for 6 out of 10 cases for each one of stage 1 and 2 groups and 8 out of 10 cases for stage 3 group). Regarding severity (corresponding to the density of the inflammatory cell population), a tendency for more intense inflammation with higher stage was noticed: while stage 1 cases showed an almost equal distribution among mild, moderate and severe intensity, stage 2 cases were all classified as having moderate or severe inflammation (5 each) and stage 3 cases were almost uniformly characterized by higher intensity of inflammation.
Supplementary Table 2 summarizes the features of the inflammatory infiltrate in MRONJ cases, as well as among control groups (receiving or not antiresorptive medication). Noticeably, chronic inflammation was present in all control cases: focal distribution and mild intensity predominated among control cases not receiving antiresorptives, while an either focal or diffuse pattern and a more variable intensity was seen in control cases on antiresorptives.
Density of M1 and M2 macrophages across MRONJ and controls
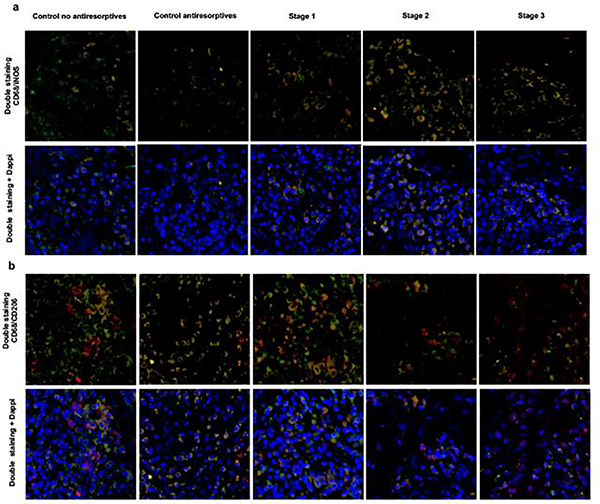
Immunofluorescence analysis was performed to quantify the density of M1 and M2 macrophages in mucosal tissues surrounding necrotic bone in patients with MRONJ stages 1–3 and controls. Cohen’s kappa for inter-rater agreement was 0.90, which represents an almost perfect level of agreement. In the MRONJ cohort (n = 30), the median density of CD68+/iNOS+ M1 macrophages was 18 cells/mm2 (IQR: 11–24), while a median of 12.5 cells/mm2 (IQR: 8–17) was counted for CD68+/CD206+ M2 macrophages. Representative examples of CD68+/iNOS+ and CD68+/CD206+ immunofluorescence staining are shown in Figure 1a–b.
Fig. 1.
a Representative case of CD68/iNOS immunofluorescence staining (x40) (CD68+:FITC, iNOS+:TRITC). b Representative case of CD68+/CD206+ immunofluorescence staining (x40) (CD68+:FITC, CD206+ :TRITC)
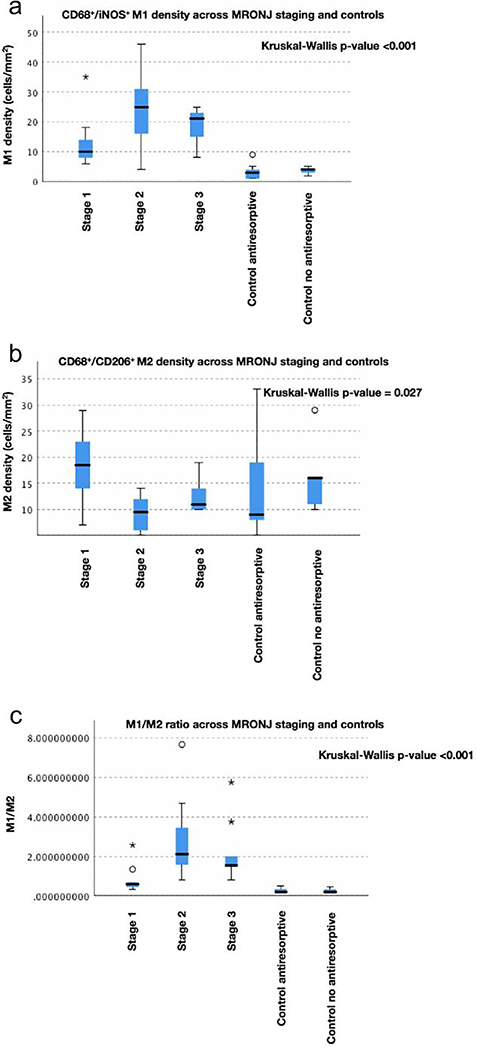
The density of CD68+/iNOS+ M1 macrophages was statistically significant different across MRONJ stages and controls (χ2(4) = 30.575, p <0.001, Kruskal-Wallis H test; Table 2), with a median CD68+/iNOS+ M1 macrophage density of 10 cells/mm2 for stage 1, 25 cells/mm2 for stage 2, 21 cells/mm2 for stage 3, 3 cells/mm2 for control group receiving antiresorptive therapy and 4 cells/mm2 for control group not receiving antiresorptive therapy. Pairwise comparison of M1 macrophage distribution across MRONJ stages and controls showed a statistically significant higher M1 macrophage density in: i) stage 2 compared to both control groups (antiresorptives: p <0.001, no antiresorptives: p = 0.006) and ii) stage 3 compared to both control groups (antiresorptives: p <0.001, no antiresorptives: p = 0.017). We also observed a higher M1 macrophage density in stage 1 compared to control group receiving antiresorptive therapy, however, this difference was not statistically significant (p = 0.069) (Table 3).
Table 2.
Comparison of M1 and M2 macrophage density across MRONJ staging and controls
| M1–M2 macrophage density |
|||
|---|---|---|---|
| Variable | M1a median (IQR) | M2b median (IQR) | M1/M2 median (IQR) |
| MRONJ stage 1 | 10 (8–14) | 18.5 (14–23) | 0.56 (0.44–0.68) |
| MRONJ stage 2 | 25 (16–31) | 9.5 (6–12) | 2.11 (1.57–3.45) |
| MRONJ stage 3 | 21 (15–23) | 11 (10–14) | 1.55 (1.47–2) |
| Control–antiresorptive | 3 (1–4) | 9 (8–19) | 0.2 (0.15–0.33) |
| Control–no antiresorptive | 4 (3–4) | 16 (11–16) | 0.19 (0.16–0.32) |
| p-valuec | <0.001** | 0.027* | <0.001** |
CD68+/iNOS+ M1 macrophage density (cells/mm2).
CD68+/CD206+ M2 macrophage density (cells/mm2).
Kruskal-Wallis H test.
p <0.05
p <0.01
MRONJ = Medication-Related Osteonecrosis of the Jaw; IQR= interquartile range.
Table 3.
Comparison of M1 and M2 macrophage density across MRONJ stages and controls pairwise
| Pairwise comparison | M1a p-valuec | M2b p-value | M1/M2 p-value |
|---|---|---|---|
| Control antiresorptive vs. Control_no antiresorptive | 1.000 | 1.000 | 1.000 |
| Control antiresorptive vs. Stage 1 | 0.069 | 0.478 | 0.732 |
| Control antiresorptive vs. Stage 3 | <0.001** | 1.000 | 0.002** |
| Control antiresorptive vs. Stage 2 | <0.001** | 1.000 | <0.001** |
| Control no antiresorptive vs. Stage 1 | 0.473 | 1.000 | 1.000 |
| Control no antiresorptive vs. Stage 3 | 0.017* | 1.000 | 0.016* |
| Control no antiresorptive vs. Stage 2 | 0.006** | 0.805 | 0.002** |
| Stage 1 vs. Stage 3 | 1.000 | 0.199 | 0.357 |
| Stage 1 vs. Stage 2 | 0.762 | 0.024* | 0.049* |
| Stage 3 vs. Stage 2 | 1.000 | 1.00 | 1.000 |
CD68+/iNOS+ M1 macrophage density (cells/mm2).
CD68+/CD206+ M2 macrophage density (cells/mm2).
Kruskal-Wallis Test, pairwise comparison; p-values have been adjusted by the Bonferroni correction for multiple tests.
p <0.05
p <0.01
MRONJ = Medication-Related Osteonecrosis of the Jaw.
The results showed a statistically significant difference in CD68+/CD206+ M2 macrophage density across MRONJ stages and controls (χ2(4) = 10.935, p = 0.027; Table 2), with a median CD68+/CD206+ M2 macrophage density of 18.5 cells/mm2 for stage 1, 9.5 cells/mm2 for stage 2, 11 cells/mm2 for stage 3, 9 cells/mm2 for control group receiving antiresorptive therapy and 16 cells/mm2 for control group not receiving antiresorptive therapy. Pairwise comparison of M2 macrophage distribution across MRONJ stages and controls revealed a statistically significant higher M2 macrophage density in stage 1 compared to stage 2 (p = 0.024) (Table 3).
The analysis demonstrated a statistically significant difference in the (CD68+/iNOS+) M1) / (CD68+/CD206+) M2 ratio across MRONJ stages and controls (χ2(4) = 29.817, p <0.001; Table 2). A M1/M2 ratio >1 indicates that there are relatively more M1- than M2-polarized macrophages and vice versa. The results showed a median M1/M2 ratio of 0.56 for stage 1, 2.11 for stage 2, 1.55 for stage 3, 0.2 for control group receiving antiresorptive therapy and 0.19 for control group not receiving antiresorptive therapy. Pairwise comparison of M1/M2 ratio across MRONJ stages and controls showed a statistically significant higher M1/M2 ratio in: i) stage 2 compared to both control groups (antiresorptives: p <0.001, no antiresorptives: p = 0.002) and ii) stage 3 compared to both control groups (antiresorptives: p = 0.002, no antiresorptives: p = 0.016). In contrast, a statistically significant lower M1/M2 ratio was found in stage 1 compared to stage 2 (p = 0.049) (Table 3). The boxplots in Figure 2a-c show the densities of CD68+/iNOS+ M1 macrophages, CD68+/CD206+ M2 macrophages and the M1/M2 ratio across patients with MRONJ stages 1–3 and controls.
Fig. 2.
Boxplots show densities for a CD68+/iNOS+ M1 macrophages b CD68+/CD206+ M2 macrophages c M1/M2 ratio, across MRONJ patients with stages 1–3 and controls. Extreme and mild outliers are marked with asterisks (*) and a circles (O) on the boxplots, respectively
Density of M1 and M2 macrophages according to clinical variables in MRONJ
The comparison of M1 and M2 macrophage density according to clinical variables in patients with MRONJ is shown in Table 4. The analysis showed that density of M1 and M2 macrophages was statistically significant higher in patients receiving bisphosphonates compared to those receiving denosumab (p = 0.005 and p = 0.002, respectively; Mann-Whitney U test). In particular, with regard to specific antiresorptive agents [zoledronic acid, alendronate, denosumab (Prolia), denosumab (Xgeva)] there was a statistically significant difference in the density of M1 and M2 macrophages (p = 0.018 and p = 0.016, respectively; Kruskal-Wallis H test). Pairwise comparison of M1 macrophage distribution across antiresorptive agents showed a statistically significant higher M1 macrophage density in: i) patients receiving zoledronic acid (Zometa) compared to patients receiving denosumab (Prolia) (p = 0.008) and ii) patients receiving alendronate (Fosamax) compared to patients receiving denosumab (Prolia) (p = 0.007). Pairwise comparison of M2 macrophage distribution across antiresorptive agents revealed a statistically significant higher M2 macrophage density in: i) patients receiving zoledronic acid (Zometa) compared to patients receiving denosumab (Xgeva) (p = 0.003) and ii) patients receiving alendronate (Fosamax) compared to patients receiving denosumab (Xgeva) (p = 0.033). M2 macrophage density was statistically significant higher in patients > 60 years and those receiving antiresorptive therapy for > 36 months (p = 0.021 and p = 0.033, respectively).
Table 4.
Comparison of M1 and M2 macrophage density according to clinical variables in patients with MRONJ
| M1–M2 macrophage density |
|||||||
|---|---|---|---|---|---|---|---|
| Variable | Category | M1a median (IQR) | p-valuec | M2b median (IQR) | p-value | M1/M2 median (IQR) | p-value |
| Sex | Male | 22 (16–29) | 0.226 | 13 (11–14) | 0.504 | 1.81 (1.26–2.21) | 0.625 |
| Female | 15 (9–22) | 12 (7–17) | 1.5 (0.68–2) | ||||
| Age | ≤ 60 years | 15 (11.5–20.5) | 0.606 | 7 (5.5–8.5) | 0.021* | 2 (1.15–4.16) | 0.291 |
| > 60 years | 20 (12–24.5) | 14 (11–17.5) | 1.5 (0.66–2) | ||||
| Primary disease | Osteoporosis | 18 (8–22) | 0.422 | 13 (11–17) | 0.625 | 1.34 (0.8–1.5) | 0.125 |
| Cancer | 18 (14–24) | 12 (7–17) | 1.81 (0.68–2.57) | ||||
| Antiresorptive class | Bisphosphonates | 22 (17–30) | 0.005** | 14 (12.5–20) | 0.002** | 1.53 (0.97–2.11) | 0.967 |
| Denosumab | 15 (8–18) | 10 (6.5–12.5) | 1.5 (0.72–2.28) | ||||
| Antiresorptive agents | Zoledronic acid (Zometa) | 22 (13.5–30) | 0.018*d | 14 (13–20) | 0.016*d | 1.57 (0.64–2.31) | 0.202d |
| Alendronate (Fosamax) | 23.5 (21–30) | 15 (12–21.5) | 1.5 (1.4–1.7) | ||||
| Denosumab (Prolia) | 8 (8–9) | 12 (10–14) | 0.8 (0.64–0.8) | ||||
| Denosumab (Xgeva) | 15.5 (14–20) | 7.5 (6–11) | 2 (1.5–3.7) | ||||
| Administration period | ≤ 36 months | 15 (10–21) | 0.111 | 10 (6.5–16) | 0.033* | 1.5 (0.6–3) | 0.703 |
| > 36 months | 22 (19–27) | 14 (12.5–17) | 1.53 (1.4–1.8) | ||||
| Site of MRONJ | Maxilla | 19 (10–22.5) | 0.832 | 12 (8.5–17) | 0.849 | 1.52 (0.66–2.2) | 0.849 |
| Mandible | 17 (13–28) | 12.5 (8–18) | 1.53 (0.68–2.2) | ||||
CD68+/iNOS+ M1 macrophage density (cells/mm2).
CD68+/CD206+ M2 macrophage density (cells/mm2).
Mann-Whitney U test, unless otherwise specified
Kruskal-Wallis H test.
p <0.05
p <0.01
MRONJ = Medication-Related Osteonecrosis of the Jaw; IQR= interquartile range.
Expression of IL-6 and IL-10 across MRONJ and controls
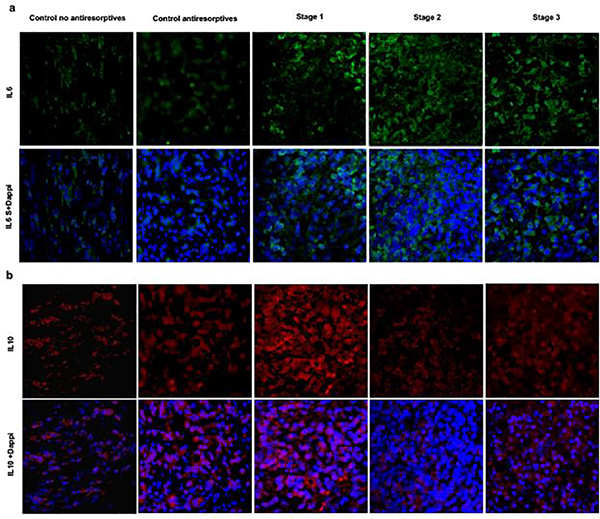
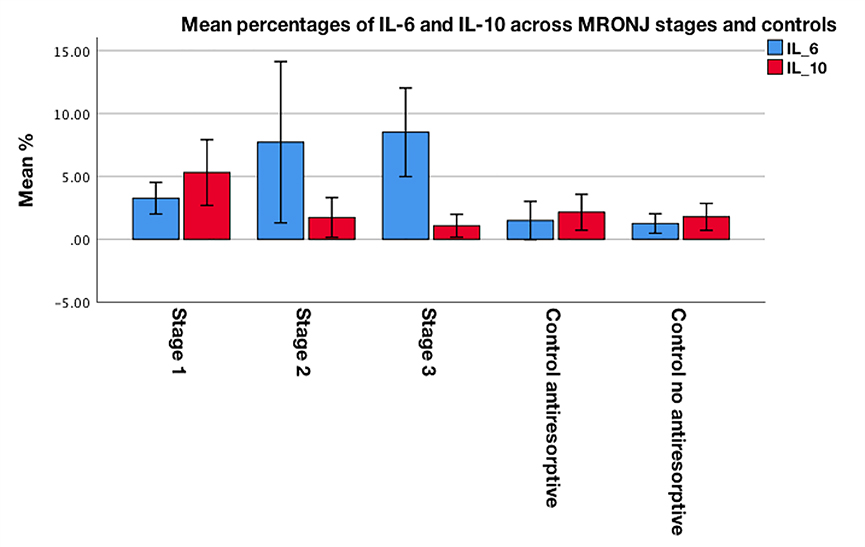
Figure 3a–b shows representative examples of IL-6 and IL-10 immunostaining. The mean percentages of positive IL-6 and IL-10 staining across patients with MRONJ stages 1–3 and controls are presented in Figure 4. There was a statistically significant difference in the mean percentages of positive IL-6 staining across MRONJ stages and controls (F(4,40) = 32.244, p <0.001; one-way ANOVA), with a mean percentage of positive IL-6 expression of 3.25 for stage 1, 7.71 for stage 2, 8.50 for stage 3, 1.47 for control group receiving antiresorptive therapy and 1.24 for control group not receiving antiresorptive therapy (Table 5). Pairwise comparison of IL-6 expression across MRONJ stages and controls showed a statistically significant higher IL-6 expression in: i) stage 2 compared to both control groups (antiresorptives: p <0.001, no antiresorptives: p <0.001), ii) stage 3 compared to both control groups (antiresorptives: p <0.001, no antiresorptives: p <0.001), and iii) stages 2 and 3 compared to stage 1 (both p<0.001) (Supplementary Table 3).
Fig. 3.
a Representative case of IL-6 immunofluorescence staining (x40) (IL6 :FITC). b Representative case of IL10 immunofluorescence staining (x40) (IL10 :AF568)
Fig. 4.
Clustered bar chart for mean percentages of positive IL-6 and IL-10 staining across MRONJ patients with stages 1–3 and controls
Table 5.
Comparison of mean percentages of positive IL-6 and IL-10 staining across MRONJ stages and controls
| Variable | IL-6 mean % (SD) | IL-10 mean % (SD) |
|---|---|---|
| MRONJ stage 1 | 3.25 (0.62) | 5.29 (1.31) |
| MRONJ stage 2 | 7.71 (3.21) | 1.73 (0.78) |
| MRONJ stage 3 | 8.5 (1.76) | 1.06 (0.45) |
| Control–antiresorptive | 1.47 (0.76) | 2.14 (0.71) |
| Control–no antiresorptive | 1.24 (0.38) | 1.77 (0.53) |
| p-valuea | <0.001** | 0.027* |
One-Way ANOVA.
p <0.05
p <0.01
MRONJ=Medication-Related Osteonecrosis of the Jaw; SD=Standard Deviation.
The analysis showed a statistically significant difference in the mean percentages of positive IL-10 staining among MRONJ stages and controls (F(4,40) = 37.975, p <0.027; one-way ANOVA), with a mean percentage of positive IL-10 expression of 5.29 for stage 1, 1.73 for stage 2, 1.06 for stage 3, 2.14 for control group receiving antiresorptive therapy and 1.77 for control group not receiving antiresorptive therapy (Table 5). Pairwise comparison of IL-10 expression across MRONJ stages and controls demonstrated a statistically significant higher IL-10 expression in: i) stage 1 compared to both control groups (antiresorptives: p <0.001, no antiresorptives: p <0.001) and ii) stage 1 compared to stages 2 and 3 (both p <0.001) (Supplementary Table 3).
Discussion
In this study, we aimed to investigate the relationship between M1–M2 macrophage polarization status in mucosal tissues adjacent to necrotic bone and clinical stage of MRONJ in patients who underwent treatment with bisphosphonates or denosumab. Our data suggest that early stage MRONJ patients without clinical evidence of infection show a predominantly M2 polarization, as indicated by the higher density of CD68+/CD206+ M2 macrophages and the decreased M1/M2 ratio compared to patients with advanced stage, as well as the significant overexpression of IL-10 compared to patients with advanced stage and controls. In contrast, late stage MRONJ patients who developed clinical infection demonstrate primarily M1-polarized macrophages, as revealed by the significantly higher density of CD68+/iNOS+ M1 macrophages, the increased M1/M2 ratio and the upregulation of IL-6 expression compared to controls. Furthermore, our results show a significantly higher density of both M1 and M2 phenotypes in MRONJ patients undergoing therapy with either zoledronic acid or alendronate compared to those receiving denosumab.
It is well-established that in response to changes in the local microenvironment, monocyte precursors differentiate to macrophages and polarize toward classically activated M1 or alternatively activated M2 phenotypes [32–34]. The molecular networks orchestrating M1–M2 macrophage reprogramming are yet not fully understood and include signaling pathways, such as toll-like receptors (TLR)/nuclear factor-κB (NF-κB), peroxisome proliferator-activated receptor-γ (PPAR-γ)/NF-κB and janus kinase (JAK)/signal transducers and activators of transcription (STAT), and post-transcriptional regulation by microRNAs (miRNAs) [35]. Staging of MRONJ is determined by the extension of exposed necrotic bone and the absence or presence of clinical signs of infection and inflammation in the maxillofacial region [4]. Given this background, we sought to investigate the relationship between macrophage polarization status in samples of patients with MRONJ and disease progression as determined by clinical stage. Our results suggest that early stage 1 MRONJ patients demonstrate a shift of macrophage polarization primarily toward the M2 population, as evidenced by the increased density of CD68+/CD206+ M2 macrophages. Although a higher CD68+/iNOS+ M1 macrophage density was also observed in stage 1 compared to controls, this difference did not reach statistical significance (p = 0.069). Nevertheless, a M1/M2 ratio <1 was found, suggesting relatively more M2- than M1-polarized macrophages in early phase of MRONJ. Considering that M2-polarized macrophages produce anti-inflammatory cytokines that are associated with tissue repair and homeostasis regulation, we next investigated the expression of IL-10 in tissues of MRONJ patients with early and advanced stages of disease. IL-10 is an anti-inflammatory cytokine that plays a central role in the regulation of immune responses by suppressing the production of proinflammatory cytokines, such as tumor necrosis factor (TNF)-α, IL-1, and IL-6, and downregulating the expression of major histocompatibility complex (MHC) class II on macrophage surface, thereby suppressing the ability of activated macrophages to stimulate antigen-specific CD4+ T cells [25]. IL-10 also has an inhibitory effect on osteoclastogenesis, directly by suppressing osteoclast formation and indirectly by upregulating the expression of osteoprotegerin (OPG) and downregulating the expression of RANKL [36]. Our data showed a significant upregulation of IL-10 in MRONJ patients with stage 1 compared to patients with advanced stages and controls.
Our analysis indicates that MRONJ patients with advanced stage show a shift of macrophage polarization toward the M1 phenotype, as evidenced by the increased density of CD68+/iNOS+ M1 macrophages and the M1/M2 ratio >1 indicating comparatively more M1- than M2-polarized macrophages. Macrophage transition toward the M1 population in late MRONJ stages may be facilitated by the positive modulatory effects of antiresorptive agents on proinflammatory signaling pathways or induced by the progression of bacterial infection in oral tissues [26,28,37]. Zhang et al. reported that zoledronic acid mediates enhanced expression of IL-17, which in turn promotes IFN-γ–induced M1 polarization in the mucosal tissues bordering extraction sockets of BRONJ patients [38]. Recent evidence suggests that bacterial infection of the oral mucosa, periodontium or alveolar bone may play a central role in the development and progression of MRONJ [28,39]. Pathogen-associated molecular pattern molecules (PAMPs), such as LPS, interact with IFN-γ to switch macrophages toward the M1 phenotype. Given that M1-polarized macrophages secrete proinflammatory cytokines, we subsequently assessed the expression of IL-6 in samples of MRONJ patients with early and advanced stages of disease. IL-6 is a pleiotropic cytokine that plays a critical role in the regulation of immune and proinflammatory responses and is involved in organ development and modulation of metabolism [40]. Il-6 is also actively involved in osteoclastogenesis by inducing osteoblasts to increase the expression of RANKL, a mediator of osteoclast formation and differentiation, thus leading to excessive bone resorption [41]. Our results suggest that MRONJ patients with advanced stages show a significantly higher expression of IL-6 compared to early stage 1 patients and controls, which may further exacerbate oral inflammation and impair bone tissue homeostasis, thus contributing to the progression of MRONJ.
Accumulating evidence indicates that in response to stimuli from the local microenvironment, macrophages show substantial plasticity and are capable of polarization changes from the M1- to the M2-phenotype and vice versa [35,42]. Our analysis suggests that the M1–M2 macrophage polarization status is associated with clinical staging and may determine progression of MRONJ. Thus, inhibition of the proinflammatory M1 phenotype and suppression of the IL-6, IL-1β and TNF-α signaling pathways might be beneficial strategies for patient with advanced stages of MRONJ. Furthermore, MRONJ patients who received antiresorptive treatment for benign diseases might benefit from modulatory agents inducing macrophage reprogramming to the anti-inflammatory M2 phenotype, including peroxisome proliferator-activated receptor-γ (PPAR-γ) agonists, Vitamin D and statins [43–45].
Evidence from in vitro studies and animal models suggests that bisphosphonates induce macrophage polarization toward the M1 phenotype [37,38]. Zhu et al. reported that zoledronic acid administration increases TLR-4 expression, which leads to activation of the NF-κB pathway, and subsequently enhanced M1 phenotype both in vitro and in vivo [46]. To date, no study has been conducted to assess the potential effects of denosumab on macrophage polarization. Nevertheless, we performed an exploratory subgroup analysis to investigate the relationship between M1–M2 macrophage phenotypes and progression of bisphosphonate-related osteonecrosis of the jaw (BRONJ) and denosumab-related osteonecrosis of the jaw (DRONJ). We found that patients with early stage BRONJ and DRONJ show a switch primarily toward M2-polarized macrophages, while advanced stage BRONJ and DRONJ patients demonstrate a shift toward the M1 phenotype (Supplementary Tables 4–7). The density, however, of both M1 and M2 populations was significantly enhanced in patients receiving bisphosphonates compared to those receiving denosumab.
This case-control study is limited by its relatively small sample size, which did not allow a statistically meaningful control for potential confounding factors, including co-morbidities and systemic risk factors. However, our eligibility criteria were strict and the investigation was based on a formally approved study protocol that was exactly followed. Moreover, we tried to avoid bias resulting from cutoff point determination, therefore marker expression was considered as continuous variable.
To the best of our knowledge, this is the first study investigating the correlation between M1–M2 macrophage polarization in mucosal tissues surrounding necrotic bone and disease progression in patients with MRONJ who underwent treatment with bisphosphonates or denosumab. We demonstrate that early stage MRONJ patients without evidence of clinical infection show a switch toward the M2 phenotype, as indicated by the higher density of M2 macrophages, the decreased M1/M2 ratio and the significant upregulation of IL-10. We also reveal that late stage MRONJ patients with established infection show a shift toward M1-polarized macrophages, as implied by the higher density of M1 macrophages, the increased M1/M2 ratio and the significant overexpression of IL-6. Thus, therapeutic molecules targeting the inflammatory microenvironment via the regulation of either M1 or M2 macrophage polarization may represent a novel strategy for treatment of MRONJ. Well-designed prospective studies are warranted to validate our findings and widen our understanding of the M1–M2 paradigm of macrophage polarization in MRONJ.
Supplementary Material
Suppl. Fig. 1 Histopathologic characteristics of inflammatory infiltrate in MRONJ patients (per stage) and control groups. (Hematoxylin and eosin stain; upper row: low magnification x20; lower row: high magnification x40).
Acknowledgments
Funding/ Acknowledgements: This study was supported by NIH/NIDCR grant R01 DE019465.
Footnotes
Compliance with Ethical Standards
Conflict of Interest: The authors declare that they have no conflict of interest.
Ethical approval: All procedures performed in study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the institutional ethics review board (429). The Strengthening the Reporting of Observational studies in Epidemiology (STROBE) guidelines were followed.
Informed consent: Informed consent was obtained from all individual participants included in the study.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Pazianas M (2011) Osteonecrosis of the jaw and the role of macrophages. J Natl Cancer Inst 103 (3):232–240. doi: 10.1093/jnci/djq516 [DOI] [PubMed] [Google Scholar]
- 2.Nonnenmuhlen N, Burnic A, Bartella A, Lethaus B, Gerhards F, Ristow O, Pautke C, Holzle F, Steiner T (2019) Comparison of mucosal and mucoperiosteal wound cover for the treatment of medication-related osteonecrosis of the jaw lesions: a retrospective cohort study. Clin Oral Investig 23 (1):351–359. doi: 10.1007/s00784-018-2443-9 [DOI] [PubMed] [Google Scholar]
- 3.Matsumoto A, Sasaki M, Schmelzeisen R, Oyama Y, Mori Y, Voss PJ (2017) Primary wound closure after tooth extraction for prevention of medication-related osteonecrosis of the jaw in patients under denosumab. Clin Oral Investig 21 (1):127–134. doi: 10.1007/s00784-016-1762-y [DOI] [PubMed] [Google Scholar]
- 4.Ruggiero SL, Dodson TB, Fantasia J, Goodday R, Aghaloo T, Mehrotra B, O’Ryan F, American Association of O, Maxillofacial S (2014) American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw−−2014 update. J Oral Maxillofac Surg 72 (10):1938–1956. doi: 10.1016/j.joms.2014.04.031 [DOI] [PubMed] [Google Scholar]
- 5.Marx RE (2003) Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg 61 (9):1115–1117. doi: 10.1016/s0278-2391(03)00720-1 [DOI] [PubMed] [Google Scholar]
- 6.Russell RG, Watts NB, Ebetino FH, Rogers MJ (2008) Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy. Osteoporos Int 19 (6):733–759. doi: 10.1007/s00198-007-0540-8 [DOI] [PubMed] [Google Scholar]
- 7.Russell RG, Xia Z, Dunford JE, Oppermann U, Kwaasi A, Hulley PA, Kavanagh KL, Triffitt JT, Lundy MW, Phipps RJ, Barnett BL, Coxon FP, Rogers MJ, Watts NB, Ebetino FH (2007) Bisphosphonates: an update on mechanisms of action and how these relate to clinical efficacy. Ann N Y Acad Sci 1117:209–257. doi: 10.1196/annals.1402.089 [DOI] [PubMed] [Google Scholar]
- 8.Sonis ST, Watkins BA, Lyng GD, Lerman MA, Anderson KC (2009) Bony changes in the jaws of rats treated with zoledronic acid and dexamethasone before dental extractions mimic bisphosphonate-related osteonecrosis in cancer patients. Oral Oncol 45 (2):164–172. doi: 10.1016/j.oraloncology.2008.04.013 [DOI] [PubMed] [Google Scholar]
- 9.Aghaloo TL, Kang B, Sung EC, Shoff M, Ronconi M, Gotcher JE, Bezouglaia O, Dry SM, Tetradis S (2011) Periodontal disease and bisphosphonates induce osteonecrosis of the jaws in the rat. J Bone Miner Res 26 (8):1871–1882. doi: 10.1002/jbmr.379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hadaya D, Soundia A, Gkouveris I, Dry SM, Aghaloo TL, Tetradis S (2019) Development of Medication-Related Osteonecrosis of the Jaw After Extraction of Teeth With Experimental Periapical Disease. J Oral Maxillofac Surg 77 (1):71–86. doi: 10.1016/j.joms.2018.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soundia A, Hadaya D, Esfandi N, de Molon RS, Bezouglaia O, Dry SM, Pirih FQ, Aghaloo T, Tetradis S (2016) Osteonecrosis of the jaws (ONJ) in mice after extraction of teeth with periradicular disease. Bone 90:133–141. doi: 10.1016/j.bone.2016.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khan A, Morrison A, Cheung A, Hashem W, Compston J (2016) Osteonecrosis of the jaw (ONJ): diagnosis and management in 2015. Osteoporos Int 27 (3):853–859. doi: 10.1007/s00198-015-3335-3 [DOI] [PubMed] [Google Scholar]
- 13.Marx RE, Sawatari Y, Fortin M, Broumand V (2005) Bisphosphonate-induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: risk factors, recognition, prevention, and treatment. J Oral Maxillofac Surg 63 (11):1567–1575. doi: 10.1016/j.joms.2005.07.010 [DOI] [PubMed] [Google Scholar]
- 14.Otto S, Schreyer C, Hafner S, Mast G, Ehrenfeld M, Sturzenbaum S, Pautke C (2012) Bisphosphonate-related osteonecrosis of the jaws - characteristics, risk factors, clinical features, localization and impact on oncological treatment. J Craniomaxillofac Surg 40 (4):303–309. doi: 10.1016/j.jcms.2011.05.003 [DOI] [PubMed] [Google Scholar]
- 15.Rasmusson L, Abtahi J (2014) Bisphosphonate associated osteonecrosis of the jaw: an update on pathophysiology, risk factors, and treatment. Int J Dent 2014:471035. doi: 10.1155/2014/471035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Otto S, Pautke C, Van den Wyngaert T, Niepel D, Schiodt M (2018) Medication-related osteonecrosis of the jaw: Prevention, diagnosis and management in patients with cancer and bone metastases. Cancer Treat Rev 69:177–187. doi: 10.1016/j.ctrv.2018.06.007 [DOI] [PubMed] [Google Scholar]
- 17.Silva LF, Curra C, Munerato MS, Deantoni CC, Matsumoto MA, Cardoso CL, Curi MM (2016) Surgical management of bisphosphonate-related osteonecrosis of the jaws: literature review. Oral Maxillofac Surg 20 (1):9–17. doi: 10.1007/s10006-015-0538-x [DOI] [PubMed] [Google Scholar]
- 18.Graziani F, Vescovi P, Campisi G, Favia G, Gabriele M, Gaeta GM, Gennai S, Goia F, Miccoli M, Peluso F, Scoletta M, Solazzo L, Colella G (2012) Resective surgical approach shows a high performance in the management of advanced cases of bisphosphonate-related osteonecrosis of the jaws: a retrospective survey of 347 cases. J Oral Maxillofac Surg 70 (11):2501–2507. doi: 10.1016/j.joms.2012.05.019 [DOI] [PubMed] [Google Scholar]
- 19.Davies LC, Jenkins SJ, Allen JE, Taylor PR (2013) Tissue-resident macrophages. Nat Immunol 14 (10):986–995. doi: 10.1038/ni.2705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinez FO, Gordon S (2014) The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep 6:13. doi: 10.12703/P6-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xue Q, Yan Y, Zhang R, Xiong H (2018) Regulation of iNOS on Immune Cells and Its Role in Diseases. Int J Mol Sci 19 (12). doi: 10.3390/ijms19123805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murakami M, Kamimura D, Hirano T (2019) Pleiotropy and Specificity: Insights from the Interleukin 6 Family of Cytokines. Immunity 50 (4):812–831. doi: 10.1016/j.immuni.2019.03.027 [DOI] [PubMed] [Google Scholar]
- 23.Ariel A, Maridonneau-Parini I, Rovere-Querini P, Levine JS, Muhl H (2012) Macrophages in inflammation and its resolution. Front Immunol 3:324. doi: 10.3389/fimmu.2012.00324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsuchiya K, Suzuki Y, Yoshimura K, Yasui H, Karayama M, Hozumi H, Furuhashi K, Enomoto N, Fujisawa T, Nakamura Y, Inui N, Yokomura K, Suda T (2019) Macrophage Mannose Receptor CD206 Predicts Prognosis in Community-acquired Pneumonia. Sci Rep 9 (1):18750. doi: 10.1038/s41598-019-55289-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ip WKE, Hoshi N, Shouval DS, Snapper S, Medzhitov R (2017) Anti-inflammatory effect of IL-10 mediated by metabolic reprogramming of macrophages. Science 356 (6337):513–519. doi: 10.1126/science.aal3535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wehrhan F, Moebius P, Amann K, Ries J, Preidl R, Neukam FW, Weber M (2017) Macrophage and osteoclast polarization in bisphosphonate associated necrosis and osteoradionecrosis. J Craniomaxillofac Surg 45 (6):944–953. doi: 10.1016/j.jcms.2017.02.023 [DOI] [PubMed] [Google Scholar]
- 27.Hoefert S, Schmitz I, Weichert F, Gaspar M, Eufinger H (2015) Macrophages and bisphosphonate-related osteonecrosis of the jaw (BRONJ): evidence of local immunosuppression of macrophages in contrast to other infectious jaw diseases. Clin Oral Investig 19 (2):497–508. doi: 10.1007/s00784-014-1273-7 [DOI] [PubMed] [Google Scholar]
- 28.Russmueller G, Seemann R, Weiss K, Stadler V, Speiss M, Perisanidis C, Fuereder T, Willinger B, Sulzbacher I, Steininger C (2016) The association of medication-related osteonecrosis of the jaw with Actinomyces spp. infection. Sci Rep 6:31604. doi: 10.1038/srep31604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruggiero SL, Kohn N (2015) Disease Stage and Mode of Therapy Are Important Determinants of Treatment Outcomes for Medication-Related Osteonecrosis of the Jaw. J Oral Maxillofac Surg 73 (12 Suppl):S94–S100. doi: 10.1016/j.joms.2015.09.024 [DOI] [PubMed] [Google Scholar]
- 30.Aljohani S, Gaudin R, Weiser J, Troltzsch M, Ehrenfeld M, Kaeppler G, Smeets R, Otto S (2018) Osteonecrosis of the jaw in patients treated with denosumab: A multicenter case series. J Craniomaxillofac Surg 46 (9):1515–1525. doi: 10.1016/j.jcms.2018.05.046 [DOI] [PubMed] [Google Scholar]
- 31.Bone HG, Wagman RB, Brandi ML, Brown JP, Chapurlat R, Cummings SR, Czerwinski E, Fahrleitner-Pammer A, Kendler DL, Lippuner K, Reginster JY, Roux C, Malouf J, Bradley MN, Daizadeh NS, Wang A, Dakin P, Pannacciulli N, Dempster DW, Papapoulos S (2017) 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol 5 (7):513–523. doi: 10.1016/S2213-8587(17)30138-9 [DOI] [PubMed] [Google Scholar]
- 32.Biswas SK, Mantovani A (2012) Orchestration of metabolism by macrophages. Cell Metab 15 (4):432–437. doi: 10.1016/j.cmet.2011.11.013 [DOI] [PubMed] [Google Scholar]
- 33.Mantovani A, Locati M (2013) Tumor-associated macrophages as a paradigm of macrophage plasticity, diversity, and polarization: lessons and open questions. Arterioscler Thromb Vasc Biol 33 (7):1478–1483. doi: 10.1161/ATVBAHA.113.300168 [DOI] [PubMed] [Google Scholar]
- 34.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K (2010) Development of monocytes, macrophages, and dendritic cells. Science 327 (5966):656–661. doi: 10.1126/science.1178331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang N, Liang H, Zen K (2014) Molecular mechanisms that influence the macrophage m1-m2 polarization balance. Front Immunol 5:614. doi: 10.3389/fimmu.2014.00614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Evans KE, Fox SW (2007) Interleukin-10 inhibits osteoclastogenesis by reducing NFATc1 expression and preventing its translocation to the nucleus. BMC Cell Biol 8:4. doi: 10.1186/1471-2121-8-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaneko J, Okinaga T, Hikiji H, Ariyoshi W, Yoshiga D, Habu M, Tominaga K, Nishihara T (2018) Zoledronic acid exacerbates inflammation through M1 macrophage polarization. Inflamm Regen 38:16. doi: 10.1186/s41232-018-0074-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Q, Atsuta I, Liu S, Chen C, Shi S, Shi S, Le AD (2013) IL-17-mediated M1/M2 macrophage alteration contributes to pathogenesis of bisphosphonate-related osteonecrosis of the jaws. Clin Cancer Res 19 (12):3176–3188. doi: 10.1158/1078-0432.CCR-13-0042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morita M, Iwasaki R, Sato Y, Kobayashi T, Watanabe R, Oike T, Nakamura S, Keneko Y, Miyamoto K, Ishihara K, Iwakura Y, Ishii K, Matsumoto M, Nakamura M, Kawana H, Nakagawa T, Miyamoto T (2017) Elevation of pro-inflammatory cytokine levels following anti-resorptive drug treatment is required for osteonecrosis development in infectious osteomyelitis. Sci Rep 7:46322. doi: 10.1038/srep46322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garbers C, Heink S, Korn T, Rose-John S (2018) Interleukin-6: designing specific therapeutics for a complex cytokine. Nat Rev Drug Discov 17 (6):395–412. doi: 10.1038/nrd.2018.45 [DOI] [PubMed] [Google Scholar]
- 41.Kang S, Tanaka T, Narazaki M, Kishimoto T (2019) Targeting Interleukin-6 Signaling in Clinic. Immunity 50 (4):1007–1023. doi: 10.1016/j.immuni.2019.03.026 [DOI] [PubMed] [Google Scholar]
- 42.Galli SJ, Borregaard N, Wynn TA (2011) Phenotypic and functional plasticity of cells of innate immunity: macrophages, mast cells and neutrophils. Nat Immunol 12 (11):1035–1044. doi: 10.1038/ni.2109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yao Q, Liu J, Zhang Z, Li F, Zhang C, Lai B, Xiao L, Wang N (2018) Peroxisome proliferator-activated receptor gamma (PPARgamma) induces the gene expression of integrin alphaVbeta5 to promote macrophage M2 polarization. J Biol Chem 293 (43):16572–16582. doi: 10.1074/jbc.RA118.003161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wasnik S, Rundle CH, Baylink DJ, Yazdi MS, Carreon EE, Xu Y, Qin X, Lau KW, Tang X (2018) 1,25-Dihydroxyvitamin D suppresses M1 macrophages and promotes M2 differentiation at bone injury sites. JCI Insight 3 (17). doi: 10.1172/jci.insight.98773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang T, Shao B, Liu GA (2017) Rosuvastatin promotes the differentiation of peripheral blood monocytes into M2 macrophages in patients with atherosclerosis by activating PPAR-gamma. Eur Rev Med Pharmacol Sci 21 (19):4464–4471 [PubMed] [Google Scholar]
- 46.Zhu W, Xu R, Du J, Fu Y, Li S, Zhang P, Liu L, Jiang H (2019) Zoledronic acid promotes TLR-4-mediated M1 macrophage polarization in bisphosphonate-related osteonecrosis of the jaw. FASEB J 33 (4):5208–5219. doi: 10.1096/fj.201801791RR [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Suppl. Fig. 1 Histopathologic characteristics of inflammatory infiltrate in MRONJ patients (per stage) and control groups. (Hematoxylin and eosin stain; upper row: low magnification x20; lower row: high magnification x40).