Abstract
Pain remains a very pervasive problem throughout medicine. Classical pain management is achieved through the use of opiates belonging to the mu opioid receptor (MOR) class, which have significant side effects that hinder their utility. Pharmacologists have been trying to develop opioids devoid of side effects since the isolation of morphine from papaver somniferum, more commonly known as opium by Sertürner in 1804. The natural products salvinorin A, mitragynine, and collybolide represent three nonmorphinan natural product-based targets, which are potent selective agonists of opioid receptors, and emerging next-generation analgesics. In this work, we review the phytochemistry and medicinal chemistry efforts on these templates and their effects on affinity, selectivity, analgesic actions, and a myriad of other opioid-receptor-related behavioral effects.
Graphical Abstract
More than 100 000 adults in the US are affected by chronic pain, with estimated costs up to $635 billion per year in medical treatment and lost productivity.1 Nonsteroidal anti-inflammatory drugs (NSAIDS), acetaminophen, selective serotonin reuptake inhibitors (SSRIs), tricyclic antidepressants (TCAs), gamma amino butyric acid (GABA) analogues, and opioids are routinely used to treat pain. However, although NSAIDS work for inflammatory pain, they are associated with cardiovascular side effects, gastrointestinal (GI) bleeding, and renal disease, while acetaminophen can be hepatotoxic. On the other hand, SSRIs, TCAs, and GABA analogues show limited analgesic efficacy.2 Morphine and other clinically used opioid agonists that target the MOR remain the preferred treatment of moderate to severe pain, which is attributed to MOR-mediated hyperpolarization of nociceptive pathways and CNS pain processing centers.3 Drugs targeting MOR are effective analgesics when used appropriately but are also highly addictive and are associated with serious side effects such as tolerance or respiratory depression.4–6 As the use of opioid painkillers has increased so has the diversion, misuse, and transition to illicit opioids, with about 80% of addicts reported initiating their habit through prescription opioids. The epidemic of opioid abuse has caused more than 47 600 deaths in 2017 alone, making drug overdose the leading cause of accidental death in the US.7 As effective analgesics are essential to minimize pain and suffering of many diseases, the identification of safer analgesic molecular targets with diminished side effects and abuse potential is critical for breaking the vicious cycle fueling the current problem. Accordingly, the generation of safe and abuse-free opioid analgesics represents a long-standing scientific challenge of major health and societal importance, with added urgency due to the ongoing opioid crisis epidemic.
Some approaches in the opioid field have targeted other opioid subtypes like the kappa opioid receptor (KOR). Early compounds targeting KOR8 can cause aversion, dysphoria, and hallucinations, although targeting this receptor may eventually become a viable option in the near future. Delta opioid receptor (DOR) agonists9 have been investigated; early analogues precipitated seizures, and the more recently developed derivatives have not yet been validated clinically.4 Other approaches have been taken over the years, starting with the development of partial agonists10 and mixed agonist/antagonists.11,12 Another is to take advantage of the biased agonism, in which distinct downstream pathways can be activated by different molecules working through the same receptor.13,14 It has been proposed that biased ligands not recruiting β-arrestin215 or showing a preference for activating specific G-protein-mediated signal transduction pathways against β-arrestin2 might have diminished side effects.16,17
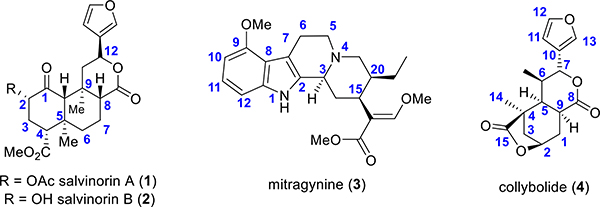
In search of novel analgesic targets, opioid chemists have looked into natural products to develop safer analgesic agents. Natural products have traditionally provided numerous leads, which has led to the design of new pharmaceuticals. Together natural products and their analogues account for approximately 34% of approved drugs.18,19 Morphine, the most commonly employed opioid and the baine, the structure on which the vast majority of semisynthetic opiates is based, are natural alkaloids found in the poppy plant, Papaver somniferum. While opioid chemistry has traditionally been dominated by the bainederived alkaloids isolated from poppy,20 there is a growing number of opioid natural products derived from structures other than the traditional morphinan scaffold and thus structurally not closely related to morphine. These include analogues of salvinorin A (1, Figure 1)-, mitragynine (3)-, and collybolide (4)-based alkaloids derived from kratom; each of these will be thoroughly discussed in this review.
Figure 1.
Structures of the natural products covered by this review.
SALVINORIN A
Salvinorin A (1), a neoclerodane diterpenoid, is the main active compound isolated from the leaves of Salvia divinorum.21,22 Salvia divinorum is a member of the mint family23 and has been traditionally ingested as a quid or smoked in spiritual practices by the Mazatec people of Oaxaca (Mexico), for many centuries. It was uncovered to be a potent and selective KOR agonist.24,25 Its hallucinogenic activity in humans is similar to the potency of other known compounds such as tetrahydrocannabinol (THC) or lysergic acid diethylamide (LSD), respectively, which target 5HT2A.26–28 The KOR agonistic activity of 1 was interesting because of its lack of structural similarity to the other psychotomimetic substances. A different mechanism was anticipated for its activity, and its congeners were expected to be the lead compounds for the development of drugs and for the treatment of pain, obesity, and other pathologies. Analogues of salvinorin A are usually potent selective KOR agonists over MOR/DOR, while some other C2-substituted aroyl ring analogues are MOR G-protein-biased agonists, and these features have been described in the literature along with salvinorin A pharmacology.29–32
Phytochemistry.
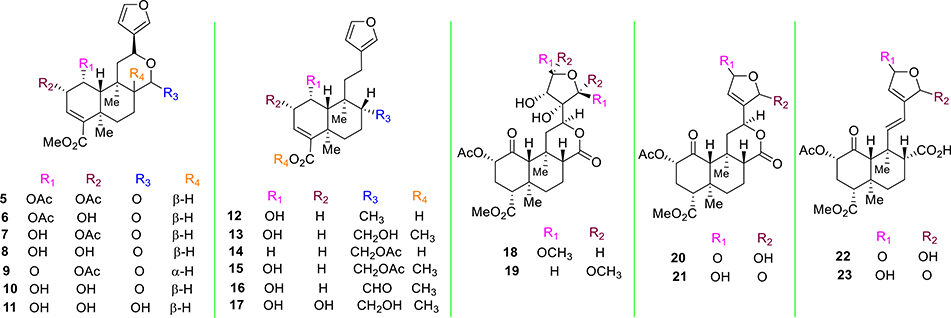
The major terpenes present in Salvia divinorum responsible for its biological activity isolated from the plant include salvinorin A (1) and salvinorin B (2, Figure 1).21,22 Valdés et al. described the isolation of the terpenoid divinorin A and its congener divinorin B.22 Structural comparison of divinorin A with 1 isolated by Ortega et al.21 found structures to be quite identical. Therefore, divinorins A and B were named as salvinorin A and B, respectively. Further studies by Valdés et al. on Salvia divinorum isolated a different neoclerodane diterpene, namely, salvinorin C (5, Figure 2).33 Additional phytochemical investigations on that plant led to the isolation of salvinorins D–I (6–11, Figure 2),34–37 divinatorins A–F (12–17, Figure 2),35,36,38 salvinicins A and B (18 and 19),39 and salvidivins A–D (20–23, Figure 2).36
Figure 2.
Structures of naturally occurring analogues from Salvia divinorum.
KOR affinity has been evaluated for most of the naturally occurring analogues of 1.35,40,41 Most of them displayed poor binding activities toward the KOR with Ki values >10 000 nM.35,40,41 Though there are a few exceptions, the affinity (Ki = 1022 nM) of salvinorin C (5) was found to be 250-fold lower compared with the affinity of 1 (Ki = 4 nM).40 Both divinatorin D (15) and divinatorin E (16) exhibited a reduced affinity for KOR (Ki = 230 nM and 418 nM respectively), compared to 1.35 Among other natural products, salvinicin A (18, KOR Ki = 390 nM) and salvidivin A (20, (KOR Ki = 440 nM) have been reported in the literature to have KOR affinity. Compound 20 (KOR Ke = 440 nM) was also recognized as the first naturally occurring neoclerodane with KOR antagonist activity, albeit its potency is very weak.41
Pharmacology of Salvinorin A (SalA).
SalA is a KOR selective ligand.42 In radioligand binding assays, its affinity at KOR is 18 nM, while showing >10 000 nM affinity at both MOR and DOR. In functional assays, it showed an EC50 of 7 nM and full agonism at KOR, while showing >10 000 nM potency at both MOR and DOR and recruited β-arrestin-2 similar to the classical KOR agonists.43
SalA has been shown to exhibit short-lasting hallucinations with a potency similar to LSD.44 A smoked dose of 200–500 μg of SalA produces hallucinations with a peak effect lasting between 5 and 10 min up to an hour.27,28 In primate studies, SalA crosses the blood–brain barrier (BBB) efficiently within the 40s, while lasting in the brain for 8 min mimicking the peak hallucinating effects in humans.45 Human psychopharmacology and dose-related effects of salvinorin A in humans were also reported by Griffiths46,47 and Ranganathan groups.48 The peak effect of inhaled Sal A was observed at 2 min, and actions lasted up to 20 min. Sal A behaved like a classical hallucinogen in these studies, and dose-related memory impairment and dissociative effects were also reported. No euphoria but psychomimetic effects along with an increase in prolactin and cortisol levels consistent with SalA’s KOR effects were observed. In general, Sal A was well tolerated, and no adverse effects were observed.
SalA has several putative metabolic sites like C2 acetyl, C4 ester, and a lactone ring. Ester hydrolysis of the C2 acetate results in the formation of salvinorin B,45,49 which is less active at KOR.33,50,51 The C4 ester has been found to be enzymatically stable, while the lactone group is also reported to be labile.52 Taken together, no active metabolites are believed to be responsible for SalA actions.
SalA produces KOR-mediated short-acting analgesia in mice (consistent with the molecule’s lability and short-acting hallucinogenic effects in humans), which was blocked by both KOR antagonists53 and was lost in KOR KO mice showing that its effect is mainly mediated through the KOR.54 It produced conditioned place aversion (CPA) and other side effects like depression, anxiety, and hypolocomotion similar to most KOR agonists.31,55 Medicinal chemistry efforts in the opioid field have focused on the syntheses of probes with higher metabolic stability, which dissociate the hallucinations from the analgesic actions mediated by this template. A special emphasis has been made toward increasing the half-life of this agent.
Total Synthesis.
The most attractive structural feature of the molecule is having seven asymmetric centers and five oxygenated functionalities. Also, there are issues of epimerization at C8 under acidic or basic reaction conditions.29 The significant biological activity and the inherent novel architecture have prompted scientists to investigate the total synthesis of salvinorin A.
Synthetic studies are quiet limited,56–58 possibly due to difficulties related to the construction of the complex core of SalA. There are few strategies in the literature for the synthesis of SalA.59–61 The first synthesis of the molecule was reported by Evans et al. in 29 steps based on the transannular sequential Michael strategy of 14-membered macrocyclic lactones.62
Asymmetric Synthesis of Salvinorin A by Using a Transannular Reaction Cascade.
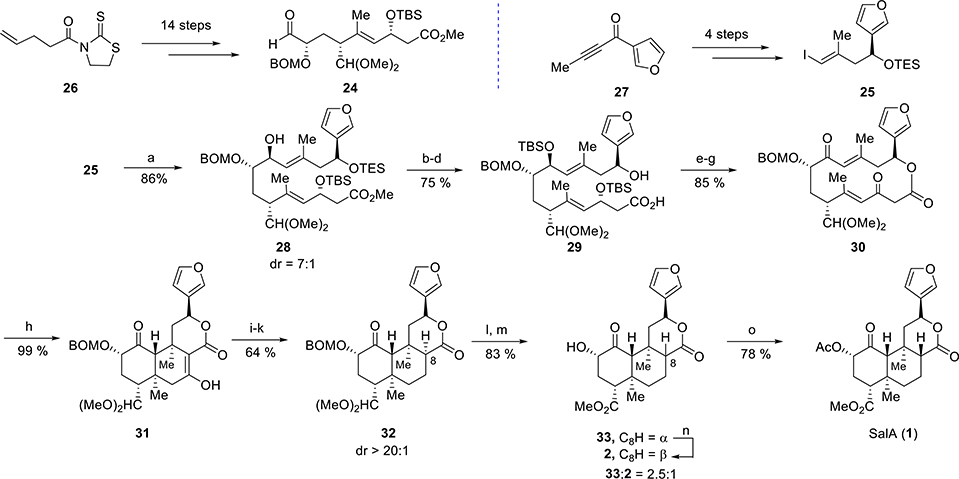
In this approach by Evans et al., the two main precursors for the construction of the SalA core were aldehyde 24 and vinyl iodide 25 (Scheme 1). Aldehyde 24 was synthesized from thiazolidinethione 26 in 14 steps, and vinyl iodide 25 was made from ketone 27 in just 4 steps. Chelate controlled addition of the Grignard reagent prepared from iodide 25 was reacted with the aldehyde 24 to produce allylic alcohol 28 as a 7:1 mixture of diastereoisomers. Several protecting group manipulations and hydrolysis of the ester group afforded acid 29. Macrolactonization of acid 29 using the Shiina protocol,63,64 followed by desilylation and oxidation, resulted in macrocycle 30. A selective transannular reaction cascade was induced for the construction of tricycle 31. Treatment of lactone 30 with TBAF furnished tricycle 31 as a single diastereomer. A deoxygenation sequence including enol triflate formation, palladium-mediated triflate reduction, and sequential conjugate reduction was employed to produce ketone 32, epimeric at C8. Deprotection of both the acetals in 32 followed by oxidation and esterification resulted in 33. Epimerization of 33 at C8 by K2CO3 to give 2 (salvinorin B), followed by acylation using Ac2O, finally completed the total synthesis of salvinorin A (1).
Scheme 1. Asymmetric Synthesis of Salvinorin A by Evans et al.a.
aReagents and conditions: (a) n-BuLi, MgBr2·OEt2, −78 °C, then 24, DCM, −78 °C to 0 °C; (b) TBSOTf, 2,6-lutidine; (c) PPTS, MeOH; (d) LiOH, i-PrOH, H2O; (e) MNBA, DMAP [0.0015 M]; (f) TBAF; (g) Dess–Martin periodinane; (h) TBAF, −78 °C to 5 °C; (i) NaH, Comins reagent; (j) Pd(OAc)2, dppf, Et3SiH; (k) L-Selectride, t-BuOH, −78 °C to −55 °C; (l) LiBF4, MeCN/H2O; (m) NaClO2, TMSCHN2; (n) K2CO3, MeOH, quant. mass recovery; (o) Ac2O, py, DMAP.
Ten-Step Synthesis of 20-Nor-salvinorin A.
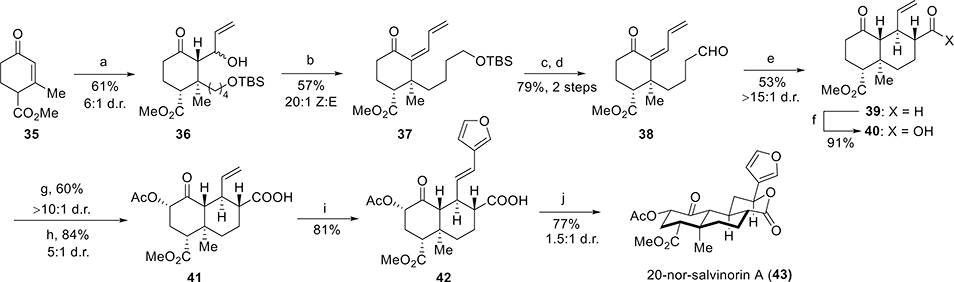
Both semisynthesis and total synthesis of SalA come across the configurational lability of the C8 carbon. Epimerization takes place easily at C8 to a lower affinity isomer, 8-epi-SalA.65 In order to stabilize the scaffold and to prevent the epimerization, C20 deletion was carried out, which simultaneously stabilized the SalA skeleton, made the synthesis simpler, and retained its high affinity and selectivity for the KOR. The synthesis of 20-norsalvinorin A was achieved in 10 steps66 from Hagemann’s ester, a commercially available building block for terpene synthesis.67
Commercially available tert-butyl (4-chlorobutoxy) dimethylsilane was converted to corresponding Grignard reagent 34 and was treated with ester 35 (Scheme 2). The enolates resulting from the conjugate addition were trapped immediately by the addition of acrolein in the presence of zinc chloride to produce 36 as a 6:1 mixture of allylic alcohols. Mesylation followed by the elimination of alcohol 36 by the addition of DBU afforded 37 as a 20:1 mixture of (E) and (Z) dienones. tert-Butyldimethylsilyl removal of 37 with 2 M HCl, followed by Swern oxidation of the deprotected alcohol furnished aldehyde 38. The next step was intramolecular Michael addition of the corresponding pyrrolidine enamine in THF/MeOH. The reaction was quenched by K2CO3 to shift the equilibrium for the formation of predominantly one isomer, 39. Pinnick oxidation of aldehyde 39 resulted in acid 40. Deprotection of 40 with LDA followed by Davis oxaziridine68 addition generated the axial α-hydroxy-decalone. By modification of reaction conditions, the hydroxy group was selectively acetylated to give acid 41. Carboxylic acid 41 was found to undergo very efficient Heck arylation as their alkali salts: the potassium carboxylate resulted in the highest yield of 42. The final step required the lactonization of the carboxylic acid to an electron-rich conjugate double bond with maintaining Markonikov regioselectivity and equatorial stereoselectivity. This was done by the application of hexafluoroisopropanol (HFIP), and 20-nor-salvinorin A (43) was obtained in a 63% yield as an easily separable 4:1 diastereomeric ratio.
Scheme 2. Synthesis of 20-Nor-salvinorin A by Roach et al.a.
aReagents and conditions: (a) ClMg(C4H8)OTBS (34), CuBr·DMS, THF, HMPA, −78 °C to 0 °C, acrolein, ZnCl2, −78 °C; (b) MsCl, Et3N, DCM, 0 °C, DBU, 22 °C; (c) 2 M HCl (aq), THF, 0 °C; (d) (COCl)2, DMSO, Et3N, −78 °C to 22 °C; (e) pyrrolidine, AcOH, THF/MeOH, 65 °C, K2CO3; (f) NaClO2, t-BuOH, NaH2PO4, C5H10; (g) LDA, −78 °C, Davis oxaziridine; (h) Ac2O, DMAP, DBU, 22–80 °C; (i) 3-bromofuran, Pd(OAc)2, XPhos, K2CO3, DMF, 80 °C; (j) (CF3)2CHOH, 100 °C, 1 h.
Total Synthesis of Salvinorin A via the Diels–Alder Strategy.
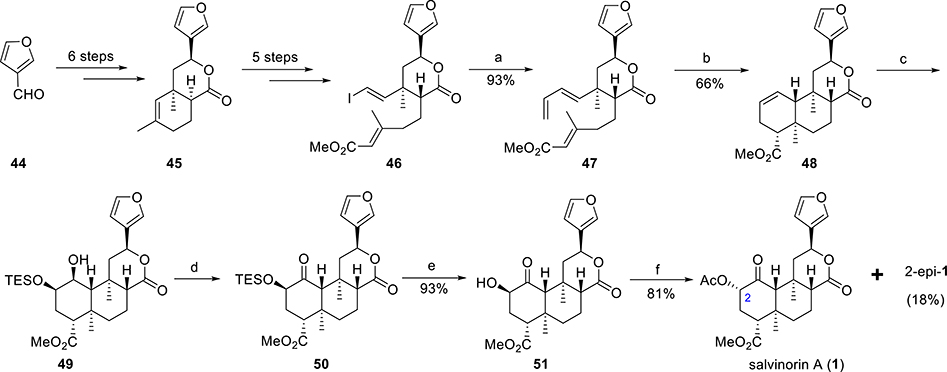
A concise total synthesis of salvinorin A starting from 3-furaldehyde (44, Scheme 3) was reported by Metz et. al.69 They used highly diastereoselective intramolecular Diels–Alder reactions (IMDA) as the key steps.
Scheme 3. Total Synthesis of Salvinorin A by Metz et al.a.
aReagents and conditions: (a) tributyl(vinyl)tin, Pd(MeCN)2Cl2, NMP, 0 °C; (b) PhCl, 1.2 equiv of BHT, 200 °C; (c) (i) OsO4, 3,5-lutidine, THF, toluene, 0 °C to rt, (95%); (ii) TESCl, imidazole, DCM, 38–40 °C; (d) 10 mol % TPAP, NMO, 4 Å sieves, DCM, rt; (e) TBAF, HOAc, THF, 0 °C to rt, (99%); (f) 6 equiv of HOAc, 3 equiv of PPh3, 3 equiv of DBAD, THF, 60 °C.
3-Furaldehyde (44) was converted to cycloadduct 45 in 6 steps (Scheme 3). The synthesis of vinyl iodide 46 was achieved from bicyclic lactone 45 in 5 steps. Stille coupling of vinyl iodide 46 with vinylstannane afforded triene 47, the precursor for IMDA. Triene 47 was heated at 200 °C in chlorobenzene for 3.5 days in a sealed tube with 1.2 equiv of BHT to furnish the desired diastereoisomer 48 in a 66% isolated yield along with a minor amount of other isomers. OsO4-mediated dihydroxylation (proceeded from sterically less hindered face) of cyclohexene 48 followed by silylation with TESCl predominantly (86:14) gave regioisomer 49. Ley–Griffith oxidation of compound 49 afforded ketone 50 as a single regio- and diastereoisomer. TBAF-mediated desilylation of ketone 50 gave 2-epi-salvinorin B (51) in a quantitative yield. Finally, the Mitsunobu inversion of 51 with acetic acid produced salvinorin A (1) in 81% yield along with 18% of 2-epi-1.
Structure–Activity Relationships of Salvinorin A Analogues.
Elucidation of Salvinorin A SAR from Total Synthesis.
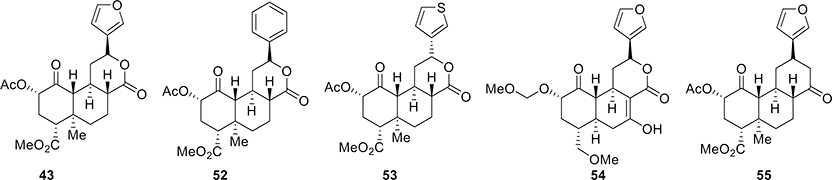
Though several total synthetic approaches have been described, only approaches used by Sherwood et al.58 and Roach et al.66 have aided in analogue synthesis and allowed accessing positions on a template not easily derivatizable using semisynthesis. 20-nor-SalA 43 (Figure 3), for example, had similar activity as SalA (a 7-fold decrease in both affinity and potency).66 Purely unsubstituted phenyl analogues of 20-norSalA, 52, retained the same binding affinity as their furyl counterparts.
Figure 3.
Selective synthetic analogues of salvinorin A.
Phenyl analogue 52 had a 3-fold lower affinity than SalA and showed an 18-fold loss in activity.66 The thiophene compound 53 also showed a high binding affinity.66 Some other analogues were also synthesized to evaluate SAR in this scaffold.66 Analogue MOM ether 54 had significant structural divergence from SalA, while still retaining high activity at the KOR.58 The replacement of C20 with H and a cyclohexanone in place of the C ring lactone stabilized the SalA scaffold relative to its C8 epimer. This new compound, O6C-20-nor-SalA (55), retained high potency for agonism of KOR (equipotent to U69,693 and 4–10-fold less potent over Sal A).70 Thus, using the novel total synthesis, the oxidatively labile C7 furan ring and metabolically unstable lactone ring can be substituted with more stable entities. In vivo characterization of these more stable analogues in rodent models of analgesia is not reported, though with the chemistry established this template may be utilized for future drug development.
Modification at C2.
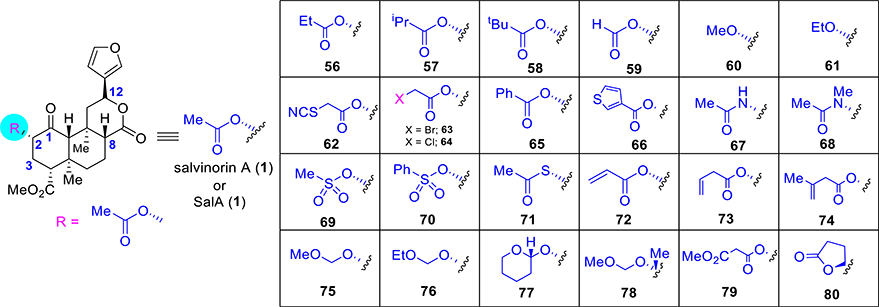
The most common modification of SalA is the replacement of the C2 acetate group, which is believed to be metabolically labile and contributes to the short time action of salvinorin A.29,49 Efforts thereby have been to substitute this group with substituents that enhance stability while retaining KOR activity of the parent. Reaction conditions for hydrolysis of the acetate group at C2 have been developed, which has afforded easy entry to a broad range of substitution patterns.71 Carbonates, carbamates, different ester groups, amines, amides, ethers, sulfonic esters, sulfonamides, and thioesters have all been made and evaluated (Figure 4) for their activity. Some of the initial analogues comprise alkyl esters. Propionate 56 (Figure 4) exhibited up to a 5-fold drop in affinity and a 4–8-fold loss in activity.65,72,51,73 Increasing the bulk of the ester moiety resulted in further loss: isopropyl ester 57 lost affinity 10-fold,73 and tert-butyl ester 58 was inactive.51 On the contrary, the reduced bulk of formic ester 59 resulted in about a 5-fold drop in affinity and a 7–11-fold loss in activity.40,74,75 Replacing the acetate ester with alkyl ethers lowered KOR activity. Methyl ether 60 lost 120–170-fold affinity,65,72 whereas ethyl ether 61 bound KOR 6–23-fold less efficiently.65,72,76 Analogues designed like thiocyanate 62 (pharmacology described in the next section) or bromoacetate 63 improved on SalA’s affinity (3-fold and 1.2-fold better, respectively) and potency (250-fold and 2-fold enhanced, respectively).77 Chloroacetate 64 exhibited comparable G-protein potency (pharmacology described in the next section) compared to SalA.77 Aryl esters with a bulkier group like 65 (discussed in next section) and 66 resulted in a loss of affinity and potency at KOR but, in several cases, led to high MOR activity.73,78–80 Incorporation of H-bond donors at C2 of SalA generally led to the loss of binding. Acetamide 67 bound to KOR 16–110-fold less over SalA,65,73 while the corresponding N-methylacetamide 68 led to the retention of SalA’s affinity and potency.65,76 Analogues with H-bond donors like having methyl sulfonic ester 69 (pharmacology in discussion in the next section) was comparable in affinity and potency to SalA.73 The phenyl sulfonic ester 70 showed a 32-fold reduction in KOR affinity.79 Other acetate replacements such as thioacetate 71 lost its affinity and potency by 3–25-fold and 2-fold, respectively.81,82 Various unsaturated esters were also investigated such as ester 72 with α–β unsaturation, which showed reduced affinity (6-fold), whereas β–γ-unsaturated esters were more potent, as in 73 (3-fold worse binding) and 74 (2-fold less binding).80
Figure 4.
Selective synthetic analogues at C2 of salvinorin A.
Alkoxy methyl ethers (75–78, pharmacology discussed in the next section) showed improved affinities and potencies compared to the parent agent SalA. Ether 75 bound 2–4-fold more efficiently and was 5–8-fold more potent than SalA.75,83–85 Among the others alkoxyl methyl ethers, compound 76 (13–63-fold more potent) had higher affinities and potencies over SalA.75,84 Tetrahydropyran 77, where the rotation was restricted by introducing a six-membered ring, did not significantly aid with respect to either binding or potency at KOR.75,84 Additional substitutions at C2 as in 78 exhibited a 4-fold loss in affinity relative to SalA and around a 10-fold loss relative to the parent ether 75.85 The presence of an H-bond acceptor in the malonate 79 appeared to be beneficial, demonstrating a 3-fold enhancement in affinity relative to SalA, but a 27-fold drop in potency compared to SalA.86 Finally, restriction of bond rotation of the acetate as in spirolactone 80 resulted in a 3-fold loss in potency.87 Microsomal stability studies showed that 80 was more metabolically resistant over SalA.87
Pharmacology of Few C2-Substituted Salvinorin A Analogues. RB64 and RB48.
These two compounds were initially designed77 to covalently couple KOR to map out the binding site of KOR. Both compounds hold an electrophilic handle, thereby one with a thiocyanate moiety (as in compound 62, RB64, Figure 4) and the other with a chloromethyl group (compound 64, RB48, Figure 4). RB48 showed comparable G-protein potency (EC50 = 8.8 nM, Emax = 101%) compared to SalA (EC50 = 5.2 nM, Emax = 100%) and, however, showed reduced potency as well as efficacy in the β-arrestin2 assay50 (EC50 = 143 nM, Emax = 63%) compared to SalA (EC50 = 5.8 nM, Emax = 100%). Similarly, RB64 showed a bias for G-protein signaling (EC50 = 5.2 nM, Emax = 101%) over the β-arrestin2 pathway (EC50 = 391 nM, Emax = 104%). RB64 was evaluated in vivo in mice and found to be analgesic without triggering sedation and anhedonia but was associated with CPA. The analgesic effect of RB64 was lost in KOR KO mice, while CPA induced by balanced agonists U50,488h, SalA, and RB64 was retained in both WT as well as β-arrestin2 KO mice. The Roth group hypothesized that p38MAPK implicated in KOR-induced dysphoria88,89 can possibly be activated by other transducing pathways independent of β-arrestin2 or aversion arises from signaling distinct from the p38MAPK pathway. Studies with this probe also suggest that the G-protein pathway may mitigate motor coordination from analgesia, but dysphoria may also be dependent on the G-protein pathway. The results are comparable to a diphenylethylamine-based analogue HS666,90 another KOR biased agonist, which shows CPA in addition to analgesia and attenuated locomotor behavior, and in contrast to triazole 1.191,92 and HS665, which show a more complete dissociation of KOR-induced dysphoria and sedation in mice models arguing for more KOR-biased ligands to be synthesized and investigated.
Mesyl Salvinorin B.
Replacement of the acetyl group by the H-bond donor mesylate group resulted in the design of analogue 69 (Figure 4), which was comparable at KOR affinity (Ki = 2.3 nM vs Ki = 1.9 nM) and potency (EC50 = 30 nM vs EC50 = 40 nM) to SalA.73 Mesylate 69 was found to be a full agonist at KOR and showed less β-arrestin-2 recruitment than other balanced agonists.73 In the antinociception assays, mesyl SalB was not as potent compared to SalA in reducing pain, though the analgesic time course of action93 was more consistent with other C2-substituted analogues, which replace the labile acetyl group.94 Compound 69 did not produce sedation, aversion, or anxiety in rats; although, in the forced swim test, increased immobility was detected, indicating prodepressive effects.94
MOM Salvinorin B.
Replacement of the acetoxy group at C2 by methoxymethyl ether72,83 (75, Figure 4) improved both the affinity and potency for KOR. In comparison to SalA, 75 exhibited higher binding affinity (Ki = 0.4 nM vs 1.3 nM for U50,488h, and 1.4 nM over SalA) for KOR. In [35S]GTPγS functional assays, compound 75 showed a potency (EC50 = 0.6 nM) nearly 7 times greater than SalA (1) (EC50 = 4.5 nM), while also being a full agonist of the KOR.95 Noteworthy, this compound was found to be a balanced agonist with β-arrestin-2 recruitment similar to U50 and 488h. The substitution of the C2 acetyl group with the MOM group led to enhanced potency as well as the enhanced analgesic duration of action (120 min vs 20 min54 for SalA), possibly due to decreased metabolism. The antinociceptive effect was blocked by the KOR antagonist norbinaltorphimine (norBNI). Typical KOR side effects like motor coordination and CPA were still detected with this molecule.96 Interestingly, analogue 76 (Figure 4) showed potent analgesic activity with reduced KOR side effects like anxiety, depression, and locomotor activity at the highest doses tested.97
β-Tetrahydropyranyl Ether Salvinorin B.
Prisinzano and co-workers hypothesized that, on the SalA scaffold, the presence of more flexible groups at C2 can lead to different structural conformations, while interacting with KORs.75 In order to explore KOR affinity and potency, the concept of conformational restriction was applied for the development of a new analogue, β-tetrahydropyranyl ether of salvinorin B (77, Figure 4). This strategy did not significantly affect the binding affinities toward the KOR. The new analogue tetrahydropyran 77 showed slightly higher affinity (Ki = 6.2 nM) over SalA (Ki = 7.4 nM) at KOR. In the [35S]GTPγS functional assay, compound 77 showed a potency (EC50 = 60 nM) almost similar to that of SalA (1) (EC50 = 40 nM). Also, exchange with this tetrahydropyran group at C2 led to potent anti-inflammatory (reducing both phase 1 as well as phase 2 inflammatory pain in formalin test) analgesic effects along with a reduction in paclitaxel-induced neuropathic pain.98 This compound was additionally 5-fold more potent than U50,488h and equipotent to SalA in acute thermal pain assays. Taken together, this particular analogue exhibited potent analgesic actions in both acute as well as chronic pain models while also showing some separation of KOR-induced side effects. 77 showed classical CPA associated with KOR agonists but interestingly showed attenuated prodepressive phenotype, hypolocomotion, and anxiety compared to typical KOR agonists.97
Herkinorin.
The introduction of a benzoyl group (65, Figure 4) in SalB core resulted in a 47-fold loss of KOR affinity (Ki = 90 nM vs Ki = 1.9 nM) compared to Sal A (1).73 This modification also led to a 25-fold increase (Ki = 12 nM vs Ki > 1000 nM) in MOR affinity compared to 1. In [35S]GTPγS functional assays, herkinorin was found to exhibit 30-fold less potency (EC50 = 1320 nM vs EC50 = 40 nM) as a KOR agonist compared to 1, and also displayed agonism at MOR (EC50 = 500 nM and Emax = 130%).73 This compound is one of the very few agents with a non-nitrogenous chemical scaffold, which can act as a MOR ligand. Subsequent in vitro assays showed that this compound does not recruit β-arrestin-2 and showed no internalization of MOR81 though this observation has been challenged recently.99 However, the lack of central analgesic actions100 has prevented detailed characterization of its in vivo pharmacology. Swapping the benzoyl group with benzamide led to the synthesis of herkamide (67, Figure 4), a molecule that retained high potency and selectivity at MOR over KOR. Finally, 67 robustly recruited β-arrestin-2 and showed MOR internalization, suggesting that small changes at C2 can lead to differential G vs arrestin signaling.
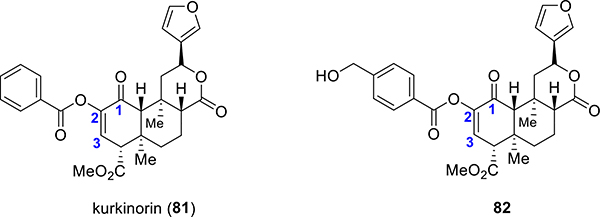
Kurkinorin.
A new analogue kurkinorin (81, Figure 5) was synthesized by the introduction of an additional degree of unsaturation between C-2 and C-3 in herkinorin (65).78 In cAMP assays, the additional unsaturation to the scaffold of herkinorin led to a potent MOR agonist with EC50 = 1.2 nM.78 This analogue was extremely selective for MORs (>8000-fold selectivity over KOR) compared to morphine (66-fold selectivity over KOR) and herkinorin (4-fold selectivity over KOR). Additionally, kurkinorin has similar potency compared to prototypic MOR agonist DAMGO, while also showing a bias for the G-protein pathway.78 In the tail-flick assays, 81 produced a significant antinociceptive effect with potency and peak analgesic effects similar to morphine.78 Compound 81 also displayed reduced tolerance, sedation, and rewarding properties in comparison to morphine.78 A more recent study on the same template was recently reported where a p-CH2OH101 (compound 82, Figure 5) substituent was placed on the phenyl ring of kurkinorin. This particular compound retained the MOR over KOR selectivity and G-protein-biased activity of kurkinorin while showing higher MOR potency (100× over morphine). The analgesic actions in thermal pain assays were MOR-dependent, and similarly to kurkinorin, this agent showed reduced tolerance in vivo. The respiratory depression potential of C2-substituted aroyl G-biased analogues has not been investigated, though the reduced tolerance potential shows many promises and indicates that further optimization may lead to more molecular probes in order to study MOR signaling.
Figure 5.
Kurkinorin and p-CH2OH kurkinorin
Modification at C4.
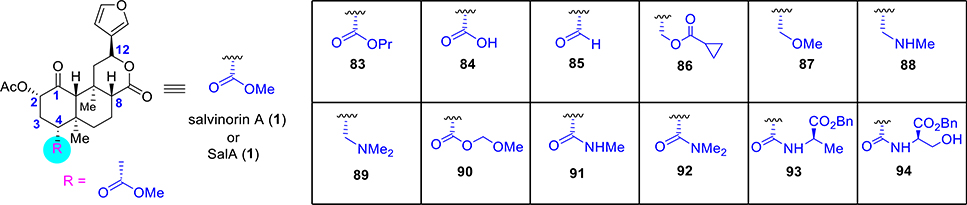
Epimerization of the C8 position is very common during the selective cleavage of the methyl ester at C4 and requires the separation of these diastereomers during chemical synthesis. Analogues that hold a bulkier substituent in this position generally display poor binding affinities for KORs. Transformation of the methyl ester of SalA (1) to bulkier alkyl esters like propyl ester 83 (Figure 6) demonstrated a total loss of affinity (Ki > 1000 nM).102 On the other hand, other related functional groups like a carboxylic acid (84)103,102,104 or an aldehyde (85)104 also resulted in a complete loss of affinity. The cyclopropyl ester 86 exhibited a 170-fold loss in affinity and 80-fold drop in potency relative to SalA.104 Substituting the ester to ethers (87)104 or amines (88, 89)65 led to a complete loss of affinity. Although alkyl esters with a bulkier moiety almost consistently caused complete loss of binding, incorporation of polar groups led to less drastic changes in affinity. MOM ether 90 exhibited moderate affinity (77-fold drop from SalA) and activity (13-fold drop from SalA).102 Instead of the methyl ester, the presence of an H-bond donor such as with the amide 91 demonstrated a significant loss (540-fold) of affinity.65 Dimethylamide 92, which is not an H-bond donor, did not show any KOR binding.65 Increased steric bulk with an amino acid as in the alanine derivative 93 exhibited a 21-fold drop in affinity and a 10-fold loss in potency. On the other hand, serine derivative 94 was completely inactive.102 In conclusion, the C4 position is less amenable to modifications and appears critical for KOR affinity and function though there are exceptions like, for instance, with the compound 54 (Figure 3).
Figure 6.
Selective synthetic analogues at C4 of salvinorin A.
Modification at C12.
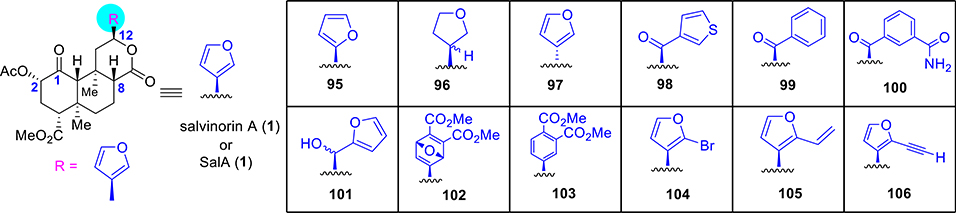
Additional work has focused on the role of the furan ring. Alteration of the regiochemistry of the furan ring as in 95 (Figure 7) retained an affinity for KOR and decreased potency by 4-fold relative to the parent compound SalA.105 Hydrogenation of the furan ring as in 96 retained the high affinity and activity at KOR similar to the parent template. The R epimer had a similar affinity for KOR as 1 but was 17-fold less potent over 1.40,41 Connecting the furan with the opposite stereochemistry at C12, as in 97 (12-epi-1), led to a loss of affinity (2–16-fold).106–108 Thiophene analogue 98 was found to exhibit 4-fold less affinity and 16-fold less activity.105 Replacement of the thiophene ring (98) by a phenyl as in 99 resulted in a 10-fold loss in affinity and a 57-fold loss in potency. Additional testing found the meta-carboxamide analogue 100 to demonstrate 5-fold less affinity and 18-fold less activity.105 Reduction of the ketone is well tolerated as alcohol 101 retained affinity and activity at KOR (8-fold less affinity, 17-fold less activity).106 The oxanorbornadiene derivative 102 has also been made by the Diels–Alder reaction with alkynes. This sterically demanding oxanorbornadienes showed only an 8-fold loss in affinity relative to SalA, while the corresponding substituted benzene analogue 103 was found to have a 39-fold loss in affinity.109 Grafting of a bromine in the furan ring as in 104 displayed affinity and potency comparable to SalA.41,106,110,111 Bromide 104 was also used as a coupling partner to further substitute the furan ring. Substitution with a vinyl group as in compound 105, was found to decrease potency by 2–32-fold,106,110 while the related alkyne analogue 106 retained potency similar to the parent SalA.110,111
Figure 7.
Selective synthetic analogues at C12 of salvinorin A.
Future Directions.
The SalA template has been investigated in detail over the years by medicinal chemists. One of the key questions that remain unanswered is the binding mode of SalA. SalA has no structural similarity to arylacetamides or dynorphins (endogenous KOR modulators) and, most importantly, lacks a basic nitrogen. How does a lipophilic terpene bind and activate a receptor that usually interacts with alkaloids? The active state KOR structure,112 solved by the Roth group using MP1104,113 a molecule designed by the Majumdar group, provides some evidence that the salt bridge between MP1104 tertiary nitrogen, and D1383.32 may not be necessary for binding to KOR. Mutation of this residue to the alanine A leads to a total loss of activity for dynorphins (endogenous KOR modulator) but retains binding of MP1104 as well as SalA, and the loss of activity for both MP1104 and SalA is similar, ~15-fold. An active state structure of SalA is required to map out the binding pose of SalA and to elucidate how it activates KOR specifically.
On the chemistry side, efforts have primarily focused on regions readily accessible through semisynthesis, and further structural diversification with the syntheses of novel analogues might shed light on the interactions with the opioid receptors. Similarly, the concept of partial agonism10 on this template needs to be evaluated in cell lines, not overexpressing the receptor. KOR antagonists, ligands with DOR affinity, are virtually unknown on this template, and KOR-biased agonists are rare. In vivo pharmacology of furan ring replacements is currently understudied, and the effects of MOR-biased ligands in this template on respiration are currently not known.
MITRAGYNINE
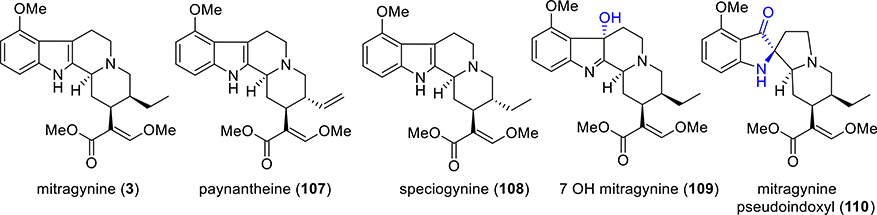
The psychoactive plant Mitragyna speciosa has been traditionally used for many years by people in Southeast Asia to treat a wide variety of illnesses. This plant is known as “kratom” in Thailand and “biak biak” in Malaysia. The plant material is either chewed directly or consumed as a tea. More than 30 different alkaloids with indole moiety have been identified and isolated from this plant.114–117 The major alkaloid mitragynine (3, Figure 8) has been found up to 66% by mass of crude alkaloids. Paynantheine, speciogynine, and speciociliatine have been found to be the other major alkaloids in the plant. Depending on the age of the plant and different geographical varieties, the quantities of these major alkaloids can considerably change. A wide variety of minor alkaloids are also found in this plant.114
Figure 8.
Structures of major kratom alkaloids.
Recent studies show that kratom118 and its alkaloids mitragynine (3), 7-OH mitragynine (7-OH, 109), and mitragynine pseudoindoxyl (MP, 110)119,120 (a spirocyclic compound that can be obtained by a skeletal rearrangement of 7-OH under Lewis acidic conditions), are MOR modulators exhibiting bias toward G-protein signaling.119,121 Orally administered mitragynine in mice metabolizes to 7-OH mitragynine via a CYP3A-mediated mechanism.122 An in vivo study of kratom and its alkaloids showed that they are analgesic,119 block alcohol intake in mice,118 and also prevent heroin self-administration in rats.123 Studies from McCurdy and co-workers124 also show that intravenous (i.v.) mitragynine is not self-administered.123 Taken together, mitragynine and its analogues represent promising starting points toward the development of therapeutics for the treatment of pain.
Total Synthesis.
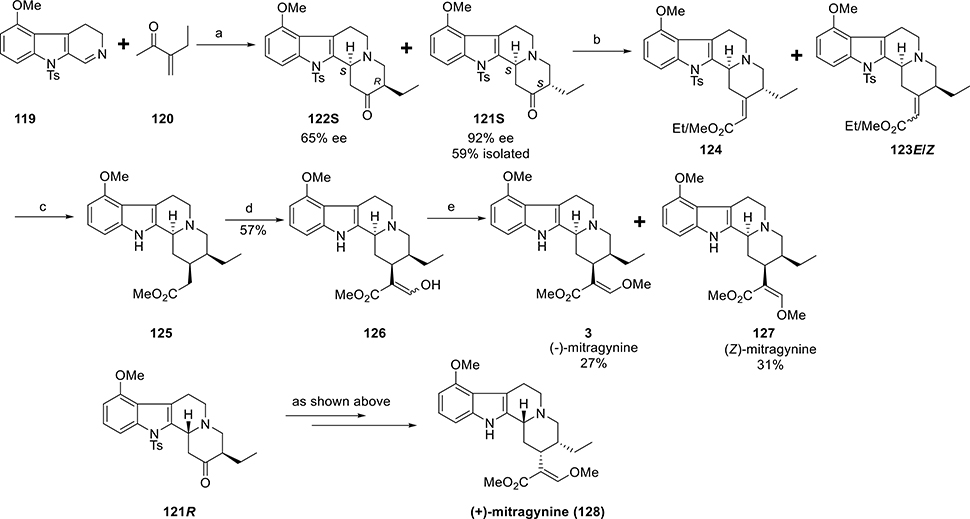
Both total synthesis and partial synthesis have been explored on the mitragynine scaffold.125–127,121,128,129 In this review, we describe the first asymmetric total synthesis of mitragynine by Takayama and co-workers129 and will also detail the enantioselective total synthesis of both (−)-mitragynine and its unnatural enantiomer, (+)-mitragynine by Sames and co-workers.121
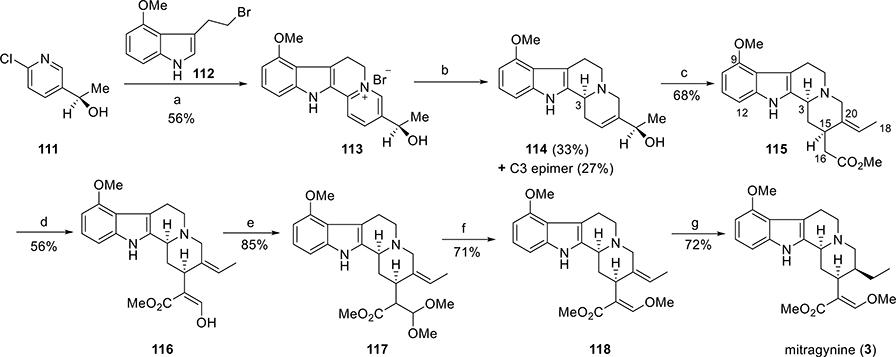
Asymmetric Total Synthesis of Mitragynine by Takayama.
The total synthesis of mitragynine by Takayama was initiated from the synthesis of the optically pure alcohol (R)-111 (Scheme 4).129 The other counterpart was 4-methoxytryptophyl bromide (112). It was prepared from 4-hydroxyindole via a five-step sequence. Optically pure pyridine derivative (R)-111 and bromide 112 were condensed in refluxing benzene in the presence of catalytic NaI. The resulting pyridinium salt 113 was then reduced by using sodium borohydride to furnish two diastereomers (114 and its C3 epimer) in 33% and 27% isolated yields, respectively. Allylic alcohol 114 was then subjected to Claisen rearrangement to incorporate an acetic acid residue at the C15 position. Treatment of alcohol 114 with trimethyl orthoacetate in refluxing o-xylene in the presence of a catalytic amount of benzoic acid resulted in acetate 115 as a single product. From the CD spectra, the absolute configuration at C3 in 115 was determined. At C3 and C15 positions, compound 115 had the appropriate absolute configuration for further transformation into mitragynine. A formyl group was next introduced at C16 in 115 by using the conventional (LDA, HCO2Me) method to give compound 116. The formyl group in 116 was then converted to the dimethyl acetal derivative 117. Treatment of the acetal 117 with KOtBu in DMF furnished the methyl enol ether 118 in a 71% yield. Stereoselective reduction of the double bond of 118 at the C19–20 positions finally produced the target compound, mitragynine (3), with the natural absolute configuration.
Scheme 4. First Asymmetric Total Synthesis of Mitragyninea.
aReagents and conditions: (a) cat. Nal, PhH, Δ; (b) NaBH4; (c) CH3C(OMe)3, cat. PhCOOH, o-xylene; (d) LDA, HCOOMe, THF, −78 °C, 30 min; (e) HCl in MeOH; (f) t-BuOK, DMF; (g) PtO2/H2, EtOH.
Total Synthesis of Both Enantiomeric Forms of Mitragynine.
The synthesis by the Sames group121 started from 3,4-dihydro-β-carboline (119, Scheme 5), which was successfully synthesized in six steps starting from commercially available 4-methoxyindole. The required enone (120) was prepared from methyl 2-ethyl-3- oxobutanoate adopting literature procedures. A proline-catalyzed Manich–Michael-type cyclization was performed to form ring D. In the presence of proline, β-carboline 119 was reacted with an excess of enone 120 to afford the desired ketone isomer 121S in 59% (along with 122S) isolated yield and with excellent enantiomeric excess. Carbanion derived from methyl diethylphosphonoacetate was reacted with ketone 121S to furnish the desired eneester (with axial ethyl group) as a mixture of E and Z isomers, 123E and 123Z in 37% (along with another stereoisomer 124) isolated yield. Simultaneous reduction and detosylation of the mixed ene-esters 123E/Z with magnesium followed by transesterification produced ester 125. By using the conventional (LDA, HCO2Me) method on ester 125, a formyl group was incorporated to give enol-ester 126 in a 57% yield. Finally, O-methylation of the enol-ester intermediate 126 provided (−)-mitragynine (3) in 27% yield along with the isomeric analogue (Z)-mitragynine (127) in 31% yield. Following the same reaction sequence, the unnatural enantiomer, (+)-mitragynine (128), was also synthesized starting from ketone 121R.
Scheme 5. Total Synthesis of Both (−)-Mitragynine and (+)-Mitragninea.
aReagents and conditions: (a) D-proline (100 mol %), DMSO, rt, 5 days; (b) (EtO)2P(O)CH2CO2Me, NaH, 1,2-DME, 0 °C to rt, 3 h; (c) (i) Mg, NH4Cl, MeOH, rt, 1 h, yield 51% (ii) NaOMe, MeOH, rt, 1 h, quantitative (d) LDA, HCOOMe, THF, −78 °C to 0 °C; (e) (i) NaOMe, MeOH/Et2O, rt (ii) (MeO)2SO2, benzene, rt, 20 h.
Pharmacology and Structure–Activity Relationships of Mitragynine Analogues.
Elucidation of Mitragynine SAR from Total Synthesis.
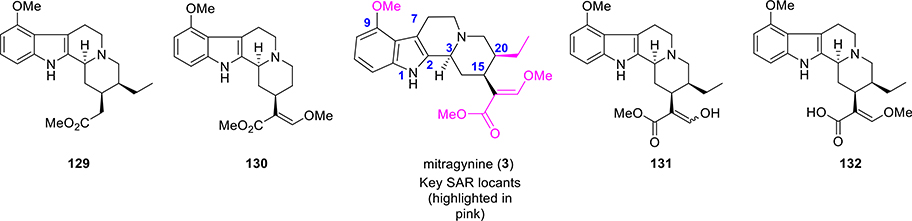
The total synthetic approach from Sames’s group has allowed access to positions in the natural product not readily available by semisynthesis and elucidate the molecular determinants of binding and function at the opioid receptors. Some of the key synthetic modifications were carried out at the β-methoxyacrylate moiety and at the ethyl group on ring D (Figure 9, SAR exploration). The importance of the absolute stereochemistry at C3, C15, and C20 were also investigated.121
Figure 9.
SAR locants and selective analogues of mitragynine from a total synthetic approach.
In terms of both efficacy and potency, the unnatural enantiomer (+)-mitragynine (128, Scheme 5) was found to be a far weaker agonist at human MOR (hMOR).121 It was found to be a partial agonist with low potency at human KOR (hKOR) in comparison to naturally occurring (−)-mitragynine (3). Switch from antagonistic to agonistic activity at hKOR was identified by the inversion of the stereochemistry in this scaffold. The stereochemical inversion of the β-methoxyacrylate moiety from E to Z (as in Z-mitragynine, 127, Scheme 5) was found to exhibit almost similar activity compared to the natural product. However, complete removal of the enol ether as in compound 129 (Figure 9) completely abolished the activity at hMOR (both agonist and antagonist). The desethylmitragynine analogue 130 retained agonistic activity at hMOR, but with lower potency. As such, the substituent at C20 is critical both in terms of efficacy (agonist vs antagonist) and potency. Most of the synthetic derivatives (127, 128, 129, and 130) were also found to be inactive at hKOR and hDOR.121 The enol derivative 131 and acid derivative 132 were also synthesized and studied for their activity. Compound 131 was found to be more efficacious than mitragynine (3) for activation of hMOR but ~3-fold less potent, while compound 132 was completely inactive as an agonist at concentrations up to 100 μM.
Pharmacology and SAR of Mitragynine Analogues.
In CHO cells expressing transfected MOR, mitragynine was found to have moderate affinity (Ki = 230 nM vs 3.3 nM for DAMGO) and potency (EC50 = 203 nM vs 19 nM for DAMGO).119 Mitragynine was also found not to recruit β-arrestin2 up to a 10 μM concentration. Similar affinity, potency compared to prototypic opioids, and biased activity have been reported by other groups independently.121,128,130,131 Antinociceptive properties exhibited by mitragynine (3) were most extensively investigated by Macko et al. in rodents and dogs initially in 1972.132 Mitragynine was active as an analgesic (comparable potency with codeine) after oral (o.p.) or intraperitoneal (i.p.) administration in all species, but when administered subcutaneously (s.c.) in both mice and rats, mitragynine was mostly inactive. Recent reports, however, contradict these findings.122,133
Matsumoto and co-workers in 1996 studied the analgesic mechanism of action caused by mitragynine (3) with a different approach.134 Investigations of antinociceptive activity after i.p. and intracerebroventricular (i.c.v.) injections were performed using the tail-pinch and hot-plate tests. A dose-dependent antinociceptive activity was observed for mitragynine (5.0—30 mg/kg, i.p. and 1.0—10m g/mouse, i.c.v.) with a peak effect at 15—45 min after injection. The antinociceptive activity of i.p. mitragynine was completely eliminated by both s.c. and i.c.v. administered naloxone. Naloxone administered i.c.v. also antagonized the analgesia of i.c.v. mitragynine. These results indicate that supraspinal analgesic actions of mitragynine in mice are typically MOR-mediated. In more recent studies, s.c. mitragynine was found to be analgesic but was found to exhibit weaker potency (analgesic ED50 > 100 mg/kg) in CD1119 and 129S1 mice.122,134
In addition to the MOR, it has been found that other nonopioid receptors play a role in the analgesic actions of mitragynine. Most notably, studies by Matsumoto135 et al., using the tail-pinch and hot-plate tests in mice, show that, with mechanical noxious stimulation, antinociception of mitragynine involves both descending noradrenergic and serotonergic systems; however, upon thermal noxious stimulation, the activity of mitragynine comes from the predominant contribution of the descending noradrenergic system.135 A more recent study from McCurdy and co-workers133 suggests that mitragynine antinociceptive effects at larger doses disrupts learned behavior. These disruptive effects of mitragynine on learned behavior did not appear to be mediated by opioid receptors but by adrenergic receptors. Thus, mitragynine pharmacology comprises substantial nonopioid mechanisms and suggests that the major alkaloid in kratom, mitragynine, has a pharmacological mechanism that differs from that of classical opioids.
Further investigations from Javitch/Majumdar/Sames groups found that an active metabolite is responsible for the analgesic effects of mitragynine.122 It was found that mitragynine is converted to the much more potent MOR agonist 7-OH (pharmacology discussed in a later section) in mouse and human liver preparations by cytochrome P450 3A isoforms. Mitragynine was converted to 7-OH in mice, and the concentration of this metabolite in the brain is sufficient to explain all opioid-receptor-mediated analgesic activity.122 Conversion of mitragynine to 7-OH was also reported in vitro by the McCurdy group.136
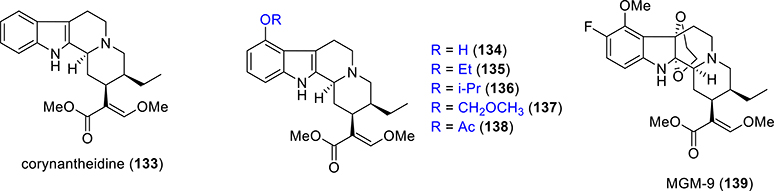
The molecular scaffold of mitragynine has been explored through semisynthetic approaches. In comparison to common corynanthe-type alkaloids, the presence of a C9 methoxy group on the indole ring of mitragynine (3) is a structural characteristic of Mitragyna alkaloids. Corynantheidine (133), a naturally occurring kratom alkaloid,137 also known as 9-demethoxymitragynine (Figure 10), was devoid of opioid agonistic activity in guinea pig ileum preparation.128 From these findings, it appeared clearly that the C9 methoxy group in 3 is essential for producing the analgesic activity. No opioid agonistic activity was observed with corynantheidine, but the compound was able to reverse the morphine-inhibited twitch contraction in the guinea pig ileum.128 It also showed an interesting concentration-dependent antagonistic effect. A very recent study130 concluded that mitragynine had a higher affinity at opioid receptors than at adrenergic receptors, while the exact opposite was observed for corynantheidine.
Figure 10.
Selective synthetic analogues of mitragynine.
Based on these results, the chemical diversification of the C9 function in mitragynine (3) has been reported. The 9-demethylation of mitragynine afforded 9-hydroxycorynantheidine (134), which binds to MOR with moderate affinity, while functional assays revealed it was a partial agonist at opioid receptors.128,138 Thus, the transformation of the C9 substituent of mitragynine, from OCH3 to OH to H, led to a change of activity from full agonism to partial agonist and then ultimately to antagonism at MOR.
Among other analogues, compounds 135 and 136, with an elongated carbon chain on the C9 position instead of the methyl group induced naloxone-insensitive inhibition of twitch contraction, suggesting an inhibitory effect via mechanisms distinct from those of the stimulation of opioid receptors.128 No opioid agonistic activity was observed for compound 137, the MOM-ether analogue of mitragynine. The grafting of an acetoxy group at C9 of mitragynine (compound 138) showed a marked reduction of intrinsic activity as well as potency compared to parent mitragynine (3). Thus, the C9 position acts as a functional switch in controlling receptor intrinsic activity at MOR and a C9 methoxy group is the most optimal substituent for pharmacophore binding to opioid receptors.
Takayama and co-workers also synthesized an ethylene glycol-bridged C10-fluorinated mitragynine (MGM-9, 139, Figure 10).139,140 MGM-9 showed very high affinity for both MOR and KOR. MGM-9 exhibited high affinity with Ki = 7.3 nM for MOR (compared to DAMGO Ki = 1.2 nM). The KOR affinity of this analogue was also measured with Ki = 18 nM (compared to U69,593 Ki = 0.66 nM). It showed potent orally active antinociceptive effects in thermal antinociception assays (7–22-fold higher than morphine), while producing less reward in the condition place preference paradigm and tolerance, possibly due to dual agonism at MOR and KOR.140 Taken together, these results suggest MGM-9 is a promising innovative analgesic with a robust analgesic effect and fewer adverse effects over morphine.
7-Hydroxymitragynine (7-OH).
The oxidation product of mitragynine, 7-hydroxymitragynine, also known as 7-OH (109, Figure 8), which was isolated as a minor constituent from Mitragyna speciosa, is a potent opioid analgesic alkaloid.141 7-OH acts as a full or partial agonist on the opioid receptors depending on the assays or cell line used in vitro.119,121,131 In comparison to mitragynine (3), the introduction of a hydroxy group at C7 led to a higher affinity and potency for MOR. Compound 109 has a moderate affinity (37 nM vs 3.3 nM for DAMGO and 230 nM for mitragynine) and selectivity for MOR over KOR/DOR in radioligand binding assays.119,122,142 Compound 105 like other mitragynine template-based derivatives shows G-protein-biased activity at MOR.119,121,131 When administered subcutaneously, 109 exhibited a potent antinociceptive effect through activation of MOR in several thermal antinociception assays.119,122,143 In mice models of tolerance, dependence,143 GI transit,144 place preference,131 and self-administratione,124 it behaves similarly to classical opiates. The effect of respiration with 7-OH mitragynine has not been reported yet in the literature.
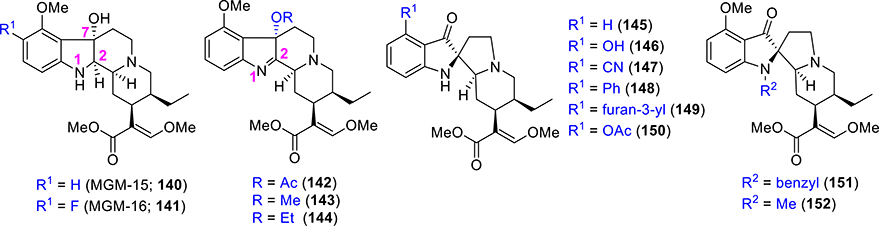
The Takayama group also reported two analogues of 7-OH where the imine was reduced (C=N reduction at C1–2, Figure 11), namely, MGM 15 and MGM-16 (compound 140 and 141, respectively, in Figure 11). MGM-15 and MGM-16 acted as dual agonists at MOR/DOR.145 In vitro and in vivo assays showed that the potency of MGM-16 was higher in comparison to MGM-15 and 7-OH. For both MOR and DOR, MGM-16 showed high affinity (Ki = 2.1 nM compared to DAMGO Ki = 1.2 nM) and (Ki = 7 nM compared to DPDPE Ki = 1.2 nM) for DOR. In GTPγS assays, full agonistic effects were observed with MGM-16 for both MOR and DOR. MGM16 was active in acute pain models such as the tail-flick and chronic pain antiallodynia models as well. MGM-16 was approximately 240 times more antinociceptive than morphine, and this effect was MOR-mediated as well as DOR-mediated.145 Last but not least, compound MGM-16 has potential therapeutic utility for the treatment of neuropathic pain suggestive that the mitragynine template-based analogues may find utility in treating both acute as well as chronic pain. Effects of this compound on other opioid-induced side effects were not reported yet and need to be assessed in several models in order to conclude on the potential impact of such agent.
Figure 11.
Selective analogues of 7-hydroxymitragynine and mitragynine pseudoindoxyl.
SAR was also explored for the 7-OH scaffold by introducing different functionality at C7 instead of the hydroxy group.128 Installation of an acetoxy group at C7 (compound 142) reduced the intrinsic activity over mitragynine (3), but potency remains almost equal to mitragynine (3).128 Significant reduction was observed in both intrinsic activity and potency when a methoxy or an ethoxy group was incorporated at C7 as in compounds 143 and 144, respectively.128 It can be concluded that a C7 hydroxy group is necessary for the improved potency of this scaffold to opioid receptors, and this hydroxyl group may H-bond with residues in the MOR pocket.119
Mitragynine Pseudoindoxyl (MP).
An indole alkaloid related to mitragynine, mitragynine pseudoindoxyl (MP, 110, Figure 8), was first isolated in 1974 by Zarembo et al. as a microbial fermentation product of mitragynine (3) from the fungus Helminthosporum sp.146 This is an oxidative rearrangement product of 7-OH with a spirocyclic core. In later reports by Yamamoto and co-workers, this compound acted non-selectively on MOR and DOR, whereas its affinity on KOR was negligible.147 Later on, the in vivo supraspinal analgesic activities of 110 were briefly discussed by Takayama et al.128
SAR of the MP scaffold, detailed investigations, and analgesic actions were reported by the Majumdar group in 2016.119 Modifications at both C9 and N1 positions were explored. C9 modifications (compounds 145–152, Figure 11) did not significantly affect receptor affinities, while the incorporation of OAc at C9 slightly decreased MOR and DOR affinities.119 None of the derivatives of MP were associated with β-arrestin-2 activation, suggesting that compounds in this template are totally G-protein-biased. Various substituents at C9 were found to maintain nanomolar binding activities (1–3 nM compared to DAMGO 3 nM) and full MOR agonism (EC50 = 1–4 nM compared to DAMGO 19 nM). Efficacies at MOR were not affected either by the removal of the methoxy group (145) or by C-9 O-demethylation (146). Compounds 145 and 146 were full agonists at MOR compared to DAMGO. However, by changing the substituents, the activity at DOR receptors was affected differentially. DOR antagonism was retained for compounds 145–152, but the C9-phenyl analogue, 148, appeared to be a DOR agonist. Compound 148 exhibited dual MOR/DOR agonism with comparable intrinsic activity and potency at both receptors. The introduction of bulky groups at N-1 as in N-benzyl analogue (151) and N-methyl analogue (152) showed reduced affinities at all of the three opioid receptors compared to parent pseudoindoxyl (110), suggesting a free NH is required for binding to opioid receptors.119 The equipotent binding with C9 analogues for MOR also suggests that space around this position in the MOR pocket.
In vivo, the 9-OH analogue (146) was more potent than 110. Analgesic potency was found to increase by the removal of the methoxy group (compound 145). Potencies of analogues with C9-cyano (147), C9-phenyl (148), and C9-furan-3′-yl (149) were almost similar to 110. A slightly negative effect was observed with the C9-acetate (150).119
The in vivo analgesic actions of MP have also been characterized in detail. MP was analgesic in tail-flick and hot-plate assays administered subcutaneously with potency similar or 3–5-fold greater than morphine using the same route. MP was, however, short-acting compared to morphine lasting 60–90 min compared to 150 min at equianalgesic ED80 doses. Supraspinally, it was equipotent to DAMGO and morphine but appeared to have a ceiling effect compared to morphine and DAMGO which where full agonists in the tail-flick assay through the same route. The analgesic actions were MOR opioid-dependent in vivo consistent with in vitro functional assays in which it was agonist at MOR, while being an antagonist at DOR and KOR. In contrast to classical MOR agonists, MP showed no reinforcing properties at 10× analgesic ED50 doses and showed reduced respiratory depression. A ceiling effect was seen in GI transit assays compared to morphine where the drug had reduced constipation. Among its most promising feature, the drug showed far less analgesic tolerance and physical dependence compared to morphine. Tolerance appeared with morphine within 5 days, while it took 29 days for MP to develop tolerance on acute administration. Reduced tolerance was also seen on chronic administration. The promising opioid functional selectivity is possibly rooted in two mechanisms its G-protein bias at MOR in conjunction with DOR/KOR antagonism.
Future Directions.
The mitragynine template provides chemists and pharmacologists unique opportunities to develop novel pain therapeutics as well as identify mechanisms which separate analgesia from other opioid-induced side effects. The ability of i.v. mitragynine to block opioid self-administration while not being addictive on its own suggests a metabolically stable mitragynine analogue not converting to 7-OH mitragynine and retaining the pharmacological properties of the parent may be useful toward next-generation opioid modulators. The role of MOR partial agonism and adrenergic actions toward mitragynine pharmacology needs to be better understood. Similarly the mode of binding and functional activation by a template lacking the classical phenolic group seen in enkephalins and other morphinans needs to be investigated. At present, computational studies from the Filizola group121 suggests that the β-methoxyacrylate moiety mimics the H2976.52 interaction seen with phenol of Bu72148 and DAMGO149 in MOR. Among other analogues, MP with its dual properties of G-bias at MOR and KOR/DOR antagonism is of interest. The roles of G-protein bias150 as well as DOR antagonism151–153 in reducing MOR-mediated tolerance and dependence is well established in the field. It is however less clear if MP is addictive in self-administration models unlike other kratom alkaloids like mitragynine (administered sc),123,124 and 7-OH mitragynine.124 Similarly beyond the C9 and C10 positions of the aromatic ring of mitragynine, no SAR is known at other positions including the C20 position, which is possibly critical for receptor efficacy. It is hoped that future investigations will aim at delving into mapping the other positions combining total synthesis with semisynthesis in order to understand the molecular mechanisms that lead to MOR activation with this template.
COLLYBOLIDE
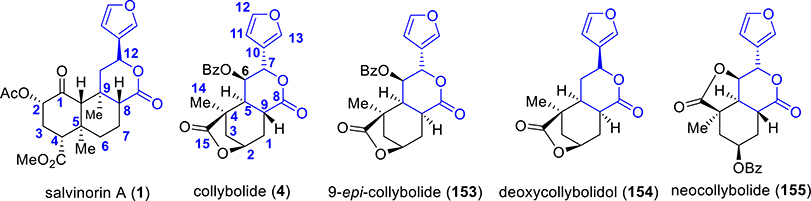
Dr. Pierre Potier’s research group in 1974 first extracted the natural product collybolide (4, Figure 1) from the fungus Collybia maculata.154 Collybolides represent the first examples of sesquiterpene structures with the furyl-δ-lactone motif. Collybolide shares structural similarity, particularly a familiar furyl-δ-lactone core, with SalA (shown in blue in Figure 12).155 Other natural products isolated in this series include 9-epicollybolide, isocollybolide, and neocollybolide. The structure of 9-epicollybolide (153) was wrongly elucidated in the original article154 but was later corrected in a subsequent paper.155 A few more collybolide-like sesquiterpenes (Figure 12) were isolated, and their structures have been assigned.156
Figure 12.
Structure of naturally occurring analogues from Collybia maculata.
In 2016, the Devi group described the KOR activity of two terpenes, collybolide and 9-epicollybolide. In the same fashion like SalA, collybolide is a highly potent and selective KOR agonist (nM affinity and agonism), while 9-epicollybolide was less active, suggesting that the stereochemistry at the 9-position plays a key role over in KOR activity.155 In competitive radioligand binding assays in KOR transfected cell lines, collybolide showed partial inhibition (24–40%) of binding when either of the agonist (3H–U69,693) or antagonist (3H-diprenorphine/3H-naloxone) was used as the radioligand compared to Sal A, which fully competed all KOR binding sites. These results showed differences between structurally similar KOR templates, where collybolide is either a partial agonist and/or showed affinity for a subset of kappa binding sites or receptor complexes, while SalA labels all kappa sites. In functional assays (GTPγS and adenyl cyclase inhibition), collybolide was uncovered to be a KOR agonist and is less efficacious than SalA. In other downstream signaling assays, collybolide was found to internalize KOR,108 similar to Sal A, suggesting that collybolide is either not a G-protein KOR agonist or that others mechanisms are involved in receptor internalization. β-Arrestin2 recruitment was not reported with this agonist, which would be of importance in order to shed light on the pharmacology of this agent. Treatment of KOR cells with agonists usually leads to ERK1/2 phosphorylation. SalA and collybolide showed differences in this assay with SalA showing a sigmoidal curve and collybolide showing an inverted-shaped curve. Robust ERK 1/2 phosphorylation is seen initially at low doses, but levels of phosphorylation dip at higher doses, and collybolide was more potent by 100-fold compared to SalA in this assay. In another phosphorylation assay, namely, Akt at S473 and T308, collybolide and SalA were similar, both ligands showing sigmoidal curves, and collybolide was more efficacious than SalA.
In mice, collybolide was analgesic, and the time course of action was similar to SalA, which is not surprising given the number of metabolically labile groups present in both molecules. In other KOR actions in vivo, collybolide was active in inhibiting itch similar to the nitrogenous KOR agonists157 and showed CPA,158 while acting as an antidepressant and anxiogenic in mice models like the forced swim test and open field instead of being a prodepressant159 and anxiolytic like classical KOR agonists in the nitrogenous class and non-nitrogenous class like SalA.
KOR selectivity for collybolide was then probed in binding assays and GTPγS assays, and in vivo for blocking chloroquine induced itching in KOR KO mice. In all of these assays, the effects were attenuated, suggesting KOR actions both in vitro and in vivo.
Taken together, these data show that collybolide is a novel non-nitrogenous KOR agonist analgesic with subtle but important differences compared to classical nitrogenous KOR ligands in the arylacetamide class like U50,488h and non-nitrogenous ligands like SalA. In rodents, it retained the aversive actions, which have limited the usage of KOR agonists as analgesics. However, given its antidepressant properties and unique binding mode, distinct signaling properties in the ERK1/2, and AKT phosphorylation assays compared to SalA, analogues of collybolide might be of use in the development of novel and safer kappa analgesics.
Exploration of the SARs on this template are missing, and the syntheses of metabolically more stable analogues (especially on the lactone group) are required. Total synthesis on this template would help in accessing other critical positions of this scaffold (which are not readily accessible by semisynthesis) and as such would be of tremendous interest. In particular, the oxidatively labile furan group must be substituted. The molecular structure binding mechanism and activation of collybolide on KOR is required to probe this agent, which could even bring more information if compared to the MP1104 KOR solved structure.43 Other natural products like isocollybolide and neocollybolide may provide additional avenues to probe KOR function. Analogues which retain signaling (ERK 1/2 and Akt) seen with the parent template and potentially with less β-arrestin-2 recruitment88,160 and/or leading to less internalization of KOR may be necessary to separate aversive actions from analgesia.
ROLE OF BIASED AGONISM IN OPIOID FUNCTIONAL SELECTIVITY
Several analogues of salvinorin A, mitragynine, and collybollide (itself) are G-protein-biased opioid agonists. The role of G-protein-biased signaling is fiercely debated in the opioid field.161 The role of MOR opioid-induced respiratory depression linked to recruitment of β-arrestin2162 has been questioned. Biased agonists like PZM2199 still show respiratory depression,163 while three recent reports in β-arrestin2 KO mice164,165 and mice with C-tail mutations166 incapable of recruiting β-arrestin2 show persistence of MOR-mediated respiratory depression. However, the reports corroborate previous findings that analgesic efficacy of opioids is limited by β-arrestin2 recruitment.16,166–168
The recent approval of the first-generation biased agonist, i.e., TRV130/oligoceridine,169 allows the field to test the hypothesis in humans. Findings may allow the field to either call it a day on biased agonism or design of better probes to better delineate this pathway.
The role of biased agonism at DOR13,170–172 in separating analgesia from seizures (associated with classical agonists) is more promising. Recent studies with PN6047173 show effectiveness in preclinical models of chronic pain while lacking proconvulsive activity or analgesic tolerance. It is possible that knowledge gained from the evaluation of biased agonists at MOR may eventually lead to safer analgesics at other subtypes.
CONCLUSIONS
Natural products based upon kratom and salvia have been used in traditional medicine for more than two centuries, while much less is known about collybia maculata. Salvinorin A, mitragynine, and collybolide show unique receptor binding, signaling, and opioid analgesic profiles in rodents. Diversification of these templates has led to the development of a wide variety of probes aiming at dissociating opioid-receptor-induced analgesia from its physiological adverse effects, understanding polypharmacology and biased G-protein signaling, but also aimed at subtype selectivity. We hope that the next generation of probe molecules will delve on G-protein subtype bias,174 allosterism,175 as well as investigate the roles of endogenous peptide ligands176,177 in pain relief and addiction; three emerging themes in the opioid field in current times.
Acknowledgments
Funding
S.M. is supported by funds from NIH grants DA045884, DA046487, and DA048379 and start-up funds from the Center for Clinical Pharmacology, St. Louis College of Pharmacy, and Washington University.
ABBREVIATIONS
- CHO
Chinese hamster ovary
- CPA
conditioned place aversion
- CPP
conditioned place preference
- DAMGO
[D-Ala2, N-MePhe4, Gly-ol5]-enkephalin
- DCM
dichloromethane
- THF
tetrahydrofuran
- LDA
lithium diisopropylamide
- TBAF
tetrabutylammonium fluoride
- DBU
1,8-diazabicyclo-[5.4.0]undec-7-ene
- DPDPE
[DPen2, D-Pen5]Enkephalin
- KO
knockout
Footnotes
Notes
The authors declare the following competing financial interest(s): S.M. is the co-founder of Sparian Inc and is an inventor on patent applications related to mitragynine analogues, which may lead to royalties or other licensing revenues from future commercial products.
Contributor Information
Soumen Chakraborty, Center for Clinical Pharmacology, St. Louis College of Pharmacy and Washington University School of Medicine, St. Louis, Missouri 63110, United States; Department of Anesthesiology, Washington University School of Medicine, St. Louis, Missouri 63110, United States.
Susruta Majumdar, Center for Clinical Pharmacology, St. Louis College of Pharmacy and Washington University School of Medicine, St. Louis, Missouri 63110, United States; Department of Anesthesiology, Washington University School of Medicine, St. Louis, Missouri 63110, United States.
REFERENCES
- (1).Simon LS (2012) Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, And Research. J. Pain Palliat. Care Pharmacother 26 (2), 197–198. [Google Scholar]
- (2).Catalani B, Hamilton CS, Herron EW, Urman RD, Fox CJ, and Kaye AD (2014) Psychiatric Agents and Implications for Perioperative Analgesia. Bailliere’s Best Pract. Res., Clin. Anaesthesiol 28, 167–181. [DOI] [PubMed] [Google Scholar]
- (3).Pasternak GW, Pasternak G, and Sloan-Kettering M (2014) Opiate Pharmacology and Relief of Pain. J. Clin. Oncol 32, 1655–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Pasternak GW, and Pan Y-X (2013) Mu Opioids and Their Receptors: Evolution of a Concept. Pharmacol. Rev 65 (4), 1257–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Corbett AD, Henderson G, Mcknight AT, and Paterson SJ (2009) 75 Years of Opioid Research: The Exciting but Vain Quest for the Holy Grail 147, S153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Compton WM, Jones CM, and Baldwin GT (2016) The Authors Reply. In New England Journal of Medicine, p 1296, Massachusetts Medical Society. [Google Scholar]
- (7).Overdose Death Rates | National Institute on Drug Abuse (NIDA) https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates (accessed Sep 23, 2019).
- (8).Kivell B, and Prisinzano TE (2010) Kappa Opioids and the Modulation of Pain. Psychopharmacology., 109–119. [DOI] [PubMed] [Google Scholar]
- (9).Dripps IJ, and Jutkiewicz EM (2017) Delta Opioid Receptors and Modulation of Mood and Emotion. Handb. Exp. Pharmacol 247, 179–197. [DOI] [PubMed] [Google Scholar]
- (10).Kivell B, and Prisinzano TE (2010) Kappa Opioids and the Modulation of Pain. Psychopharmacology. 210, 109–119. [DOI] [PubMed] [Google Scholar]
- (11).Ananthan S (2006) Opioid Ligands with Mixed μ/δ Opioid Receptor Interactions: An Emerging Approach to Novel Analgesics. AAPS J. 8, E118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Cunningham CW, Elballa WM, and Vold SU (2019) Bifunctional Opioid Receptor Ligands as Novel Analgesics. Neuropharmacology 151, 195–207. [DOI] [PubMed] [Google Scholar]
- (13).Pradhan AA, Smith ML, Kieffer BL, and Evans CJ (2012) Ligand-Directed Signalling within the Opioid Receptor Family. Br. J. Pharmacol 167, 960–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Violin JD, and Lefkowitz RJ (2007) β-Arrestin-Biased Ligands at Seven-Transmembrane Receptors. Trends Pharmacol. Sci 28, 416–422. [DOI] [PubMed] [Google Scholar]
- (15).Majumdar S, and Devi LA (2018) Strategy for Making Safer Opioids Bolstered. Nature 553 (7688), 286–288. [DOI] [PubMed] [Google Scholar]
- (16).Raehal KM, and Bohn LM (2011) The Role of Beta-Arrestin2 in the Severity of Antinociceptive Tolerance and Physical Dependence Induced by Different Opioid Pain Therapeutics. Neuropharmacology 60 (1), 58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Bohn LM, Lefkowitz RJ, and Caron MG (2002) Differential Mechanisms of Morphine Antinociceptive Tolerance Revealed in BArrestin-2 Knock-Out Mice. J. Neurosci 22 (23), 10494–10500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Li JWH, and Vederas JC (2009) Drug Discovery and Natural Products: End of an Era or an Endless Frontier? Science 325, 161–165. [DOI] [PubMed] [Google Scholar]
- (19).Newman DJ, and Cragg GM (2016) Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod 79, 629–661. [DOI] [PubMed] [Google Scholar]
- (20).Furst S, Hosztafi S, and Friedmann T (1995) Structure-Activity Relationships of Synthetic and Semisynthetic Opioid Agonists and Antagonists. Curr. Med. Chem 1 (6), 423–440. [PubMed] [Google Scholar]
- (21).Ortega A, Blount JF, and Manchand PS (1982) Salvinorin, a New Trans-Neoclerodane Diterpene from Salvia Divinorum (Labiatae). J. Chem. Soc., Perkin Trans 1, 2505–2508. [Google Scholar]
- (22).Valdes LJ, Butler WM, Hatfield GM, Paul AG, and Koreeda M (1984) Divinorin A, a psychotropic terpenoid, and divinorin B from the hallucinogenic Mexican mint, Salvia divinorum. J. Org. Chem 49 (24), 4716–4720. [Google Scholar]
- (23).Wasson RG, and Wasson RG (1962) A New Mexican Psychotropic Drug from the Mint Family. Bot. Museum Leafl. Harvard Univ 20, 77–84. [Google Scholar]
- (24).Roth BL, Baner K, Westkaemper R, Siebert D, Rice KC, Steinberg SA, Ernsberger P, and Rothman RB (2002) Salvinorin A: A potent naturally occurring nonnitrogenous opioid selective agonist. Proc. Natl. Acad. Sci. U. S. A 99 (18), 11934–11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Yan F, Mosier PD, Westkaemper RB, Stewart J, Zjawiony JK, Vortherms TA, Sheffler DJ, and Roth BL (2005) Identification of the Molecular Mechanisms by Which the Diterpenoid Salvinorin A Binds to κ-Opioid Receptors. Biochemistry 44 (24), 8643–8651. [DOI] [PubMed] [Google Scholar]
- (26).Schultes RE The Botany and Chemistry of Hallucinogens. https://pdfs.semanticscholar.org/cbc1/e8f5571dae08a5c9d97fe2eccca649412f7b.pdf
- (27).Siebert DJ (1994) Salvia Divinorum and Salvinorin A: New Pharmacologic Findings. J. Ethnopharmacol 43 (1), 53–56. [DOI] [PubMed] [Google Scholar]
- (28).Valdés LJ (1994) Salvia Divinorum and the Unique Diterpene Hallucinogen, Salvinorin (Divinorin) A. J. Psychoact. Drugs 26 (3), 277–283. [DOI] [PubMed] [Google Scholar]
- (29).Roach JJ, and Shenvi RA (2018) A Review of Salvinorin Analogues and Their Kappa-Opioid Receptor Activity. Bioorg. Med. Chem. Lett 28, 1436–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Zjawiony JK, Machado AS, Menegatti R, Ghedini PC, Costa EA, Pedrino GR, Lukas SE, Franco OL, Silva ON, and Fajemiroye JO (2019) Cutting-Edge Search for Safer Opioid Pain Relief: Retrospective Review of Salvinorin A and Its Analogues. Frontiers in Psychiatry 10, 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Butelman ER, and Kreek MJ (2015) Salvinorin A, a Kappa-Opioid Receptor Agonist Hallucinogen: Pharmacology and Potential Template for Novel Pharmacotherapeutic Agents in Neuropsychiatric Disorders. Front. Pharmacol 6 (SEP), 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Cunningham CW, Rothman RB, and Prisinzano TE (2011) Neuropharmacology of the Naturally Occurring κ-Opioid Hallucinogen Salvinorin A. Pharmacol. Rev 63 (2), 316–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Valdés LJ, Chang HM, Visger DC, and Koreeda M (2001) Salvinorin C, a New Neoclerodane Diterpene from a Bioactive Fraction of the Hallucinogenic Mexican Mint Salvia Divinorum. Org. Lett 3 (24), 3935–3937. [DOI] [PubMed] [Google Scholar]
- (34).Munro TA, and Rizzacasa MA (2003) Salvinorins D–F, New Neoclerodane Diterpenoids from Salvia Divinorum, and an Improved Method for the Isolation of Salvinorin A. J. Nat. Prod 66 (5), 703–705. [DOI] [PubMed] [Google Scholar]
- (35).Lee DYW, Ma Z, Liu-Chen LY, Wang Y, Chen Y, Carlezon WA, and Cohen B (2005) New Neoclerodane Diterpenoids Isolated from the Leaves of Salvia Divinorum and Their Binding Affinities for Human κ Opioid Receptors. Bioorganic. Bioorg. Med. Chem 13 (19), 5635–5639. [DOI] [PubMed] [Google Scholar]
- (36).Shirota O, Nagamatsu K, and Sekita S (2006) Neo-Clerodane Diterpenes from the Hallucinogenic Sage Salvia Divinorum. J. Nat. Prod 69 (12), 1782–1786. [DOI] [PubMed] [Google Scholar]
- (37).Ma Z, and Lee DYW (2007) Revised Structure of Deacetyl-1,10-Didehydrosalvinorin G. Tetrahedron Lett. 48 (31), 5461–5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Bigham AK, Munro TA, Rizzacasa MA, and Robins-Browne RM (2003) Divinatorins A-C, New Neoclerodane Diterpenoids from the Controlled Sage Salvia Divinorum. J. Nat. Prod 66 (9), 1242–1244. [DOI] [PubMed] [Google Scholar]
- (39).Harding WW, Tidgewell K, Schmidt M, Shah K, Dersch CM, Snyder J, Parrish D, Deschamps JR, Rothman RB, and Prisinzano TE (2005) Salvinicins A and B, New Neoclerodane Diterpenes from Salvia Divinorum. Org. Lett 7 (14), 3017–3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Munro TA, Rizzacasa MA, Roth BL, Toth BA, and Yan F (2005) Studies toward the Pharmacophore of Salvinorin A, a Potent κ Opioid Receptor Agonist. J. Med. Chem 48 (2), 345–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Simpson DS, Katavic PL, Lozama A, Harding WW, Parrish D, Deschamps JR, Dersch CM, Partilla JS, Rothman RB, Navarro H, and Prisinzano TE (2007) Synthetic Studies of Neoclerodane Diterpenes from Salvia Divinorum: Preparation and Opioid Receptor Activity of Salvinicin Analogueues. J. Med. Chem 50 (15), 3596–3603. [DOI] [PubMed] [Google Scholar]
- (42).Listos J, Merska A, and Fidecka S (2011) Pharmacological Activity of Salvinorin A, the Major Component of Salvia Divinorum. Pharmacol. Rep 63, 1305–1309. [DOI] [PubMed] [Google Scholar]
- (43).Che T, Majumdar S, Zaidi SA, Ondachi P, McCorvy JD, Wang S, Mosier PD, Uprety R, Vardy E, Krumm BE, Han GW, Lee M-Y, Pardon E, Steyaert J, Huang X-P, Strachan RT, Tribo AR, Pasternak GW, Carroll FI, Stevens RC, Cherezov V, Katritch V, Wacker D, and Roth BL (2018) Structure of the Nanobody-Stabilized Active State of the Kappa Opioid Receptor. Cell 172 (1–2), 55–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Sheffler DJ, and Roth BL (2003) The “magic Mint” Hallucinogen Finds a Molecular Target in the Kappa Opioid Receptor. Trends Pharmacol. Sci 24, 107–109. [DOI] [PubMed] [Google Scholar]
- (45).Hooker JM, Xu Y, Schiffer W, Shea C, Carter P, and Fowler JS (2008) Pharmacokinetics of the Potent Hallucinogen, Salvinorin A in Primates Parallels the Rapid Onset and Short Duration of Effects in Humans. NeuroImage 41 (3), 1044–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).MacLean KA, Johnson MW, Reissig CJ, Prisinzano TE, and Griffiths RR (2013) Dose-Related Effects of Salvinorin A in Humans: Dissociative, Hallucinogenic, and Memory Effects. Psychopharmacology (Berl) 226 (2), 381–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Johnson MW, MacLean KA, Reissig CJ, Prisinzano TE, and Griffiths RR (2011) Human Psychopharmacology and Dose-Effects of Salvinorin A, a Kappa Opioid Agonist Hallucinogen Present in the Plant Salvia Divinorum. Drug Alcohol Depend. 115 (1–2), 150–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Ranganathan M, Schnakenberg A, Skosnik PD, Cohen BM, Pittman B, Sewell RA, and D’Souza DC (2012) Dose-Related Behavioral, Subjective, Endocrine, and Psychophysiological Effects of the κ Opioid Agonist Salvinorin A in Humans. Biol. Psychiatry 72 (10), 871–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Schmidt MS, Prisinzano TE, Tidgewell K, Harding W, Butelman ER, Kreek MJ, and Murry DJ (2005) Determination of Salvinorin A in Body Fluids by High Performance Liquid Chromatography-Atmospheric Pressure Chemical Ionization. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci 818 (2), 221–225. [DOI] [PubMed] [Google Scholar]
- (50).White KL, Scopton AP, Rives M-L, Bikbulatov RV, Polepally PR, Brown PJ, Kenakin T, Javitch JA, Zjawiony JK, and Roth BL (2014) Identification of Novel Functionally Selective K-Opioid Receptor Scaffolds S. Mol. Pharmacol. Mol. Pharmacol 85, 83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Chavkin C, Sud S, Jin W, Stewart J, Zjawiony JK, Siebert DJ, Toth BA, Hufeisen SJ, and Roth BL (2004) Salvinorin A, an Active Component of the Hallucinogenic Sage Salvia Divinorum Is a Highly Efficacious κ-Opioid Receptor Agonist: Structural and Functional Considerations. J. Pharmacol. Exp. Ther 308 (3), 1197–1203. [DOI] [PubMed] [Google Scholar]
- (52).Tsujikawa K, Kuwayama K, Miyaguchi H, Kanamori T, Iwata YT, and Inoue H (2009) In Vitro Stability and Metabolism of Salvinorin A in Rat Plasma. Xenobiotica 39 (5), 391–398. [DOI] [PubMed] [Google Scholar]
- (53).John TF, French LG, and Erlichman JS (2006) The Antinociceptive Effect of Salvinorin A in Mice. Eur. J. Pharmacol 545 (2–3), 129–133. [DOI] [PubMed] [Google Scholar]
- (54).McCurdy CR, Sufka KJ, Smith GH, Warnick JE, and Nieto MJ (2006) Antinociceptive Profile of Salvinorin A, a Structurally Unique Kappa Opioid Receptor Agonist. Pharmacol., Biochem. Behav 83 (1), 109–113. [DOI] [PubMed] [Google Scholar]
- (55).Kivell B, Uzelac Z, Sundaramurthy S, Rajamanickam J, Ewald A, Chefer V, Jaligam V, Bolan E, Simonson B, Annamalai B, Mannangatti P, Prisinzano TE, Gomes I, Devi LA, Jayanthi LD, Sitte HH, Ramamoorthy S, and Shippenberg TS (2014) Salvinorin A Regulates Dopamine Transporter Function via a Kappa Opioid Receptor and ERK1/2-Dependent Mechanism. Neuropharmacology 86, 228–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Lingham AR, Hügel HM, and Rook TJ (2006) Studies Towards the Synthesis of Salvinorin A. Aust. J. Chem 59 (5), 340. [Google Scholar]
- (57).Burns AC, and Forsyth CJ (2008) Intramolecular Diels-Alder/Tsuji Allylation Assembly of the Functionalized Trans-Decalin of Salvinorin A. Org. Lett 10 (1), 97–100. [DOI] [PubMed] [Google Scholar]
- (58).Sherwood AM, Williamson SE, Crowley RS, Abbott LM, Day VW, and Prisinzano TE (2017) Modular Approach to Pseudo-Neoclerodanes as Designer κ-Opioid Ligands. Org. Lett 19 (19), 5414–5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Nozawa M, Suka Y, Hoshi T, Suzuki T, and Hagiwara H (2008) Total Synthesis of the Hallucinogenic Neoclerodane Diterpenoid Salvinorin A. Org. Lett 10 (7), 1365–1368. [DOI] [PubMed] [Google Scholar]
- (60).Line NJ, Burns AC, Butler SC, Casbohm J, and Forsyth CJ (2016) Total Synthesis of (−)-Salvinorin A. Chem. - Eur. J 22 (50), 17983–17986. [DOI] [PubMed] [Google Scholar]
- (61).Hagiwara H, Suka Y, Nojima T, Hoshi T, and Suzuki T (2009) Second-Generation Synthesis of Salvinorin A. Tetrahedron 65 (25), 4820–4825. [Google Scholar]
- (62).Scheerer JR, Lawrence JF, Wang GC, and Evans DA (2007) Asymmetric Synthesis of Salvinorin A, a Potent κ Opioid Receptor Agonist. J. Am. Chem. Soc 129 (29), 8968–8969. [DOI] [PubMed] [Google Scholar]
- (63).Shiina I, Kubota M, and Ibuka R (2002) A Novel and Efficient Macrolactonization of ω-Hydroxycarboxylic Acids Using 2-Methyl-6-Nitrobenzoic Anhydride (MNBA). Tetrahedron Lett. 43 (42), 7535–7539. [Google Scholar]
- (64).Shiina I, Kubota M, Oshiumi H, and Hashizume M (2004) An Effective Use of Benzoic Anhydride and Its Derivatives for the Synthesis of Carboxylic Esters and Lactones: A Powerful and Convenient Mixed Anhydride Method Promoted by Basic Catalysts. J. Org. Chem 69 (6), 1822–1830. [DOI] [PubMed] [Google Scholar]
- (65).Béguin C, Richards MR, Li JG, Wang Y, Xu W, Liu-Chen LY, Carlezon WA, and Cohen BM (2006) Synthesis and in Vitro Evaluation of Salvinorin A Analogueues: Effect of Configuration at C(2) and Substitution at C(18). Bioorg. Med. Chem. Lett 16 (17), 4679–4685. [DOI] [PubMed] [Google Scholar]
- (66).Roach JJ, Sasano Y, Schmid CL, Zaidi S, Katritch V, Stevens RC, Bohn LM, and Shenvi RA (2017) Dynamic Strategic Bond Analysis Yields a Ten-Step Synthesis of 20-norSalvinorin A, a Potent κ-OR Agonist. ACS Cent. Sci 3 (12), 1329–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Pollini GP, Benetti S, De Risi C, and Zanirato V (2010) Hagemann’s Ester: A Timeless Building Block for Natural Product Synthesis. Tetrahedron 66, 2775–2802. [Google Scholar]
- (68).Davis FA, Vishwakarma LC, Billmers JM, and Finn J (1984) Synthesis of α-Hydroxy Carbonyl Compounds (Acyloins): Direct Oxidation of Enolates Using 2-Sulfonyloxaziridines. J. Org. Chem 49 (17), 3241–3243. [Google Scholar]
- (69).Wang Y, and Metz P (2018) Total Synthesis of the Neoclerodane Diterpene Salvinorin A via an Intramolecular Diels-Alder Strategy. Org. Lett 20 (11), 3418–3421. [DOI] [PubMed] [Google Scholar]
- (70).Hirasawa S, Cho M, Brust TF, Roach JJ, Bohn LM, and Shenvi RA (2018) O6C-20-nor-Salvinorin A Is a Stable and Potent KOR Agonist. Bioorg. Med. Chem. Lett 28 (16), 2770–2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Tidgewell K, Harding WW, Schmidt M, Holden KG, Murry DJ, and Prisinzano TE (2004) A Facile Method for the Preparation of Deuterium Labeled Salvinorin A: Synthesis of [2,2,2–2H 3]-Salvinorin A. Bioorg. Med. Chem. Lett 14 (20), 5099–5102. [DOI] [PubMed] [Google Scholar]
- (72).Béguin C, Richards MR, Wang Y, Chen Y, Liu-Chen LY, Ma Z, Lee DYW, Carlezon WA, and Cohen BM (2005) Synthesis and in Vitro Pharmacological Evaluation of Salvinorin A Analogueues Modified at C(2). Bioorg. Med. Chem. Lett 15 (11), 2761–2765. [DOI] [PubMed] [Google Scholar]
- (73).Harding WW, Tidgewell K, Byrd N, Cobb H, Dersch CM, Butelman ER, Rothman RB, and Prisinzano TE (2005) Neoclerodane Diterpenes as a Novel Scaffold for μ Opioid Receptor Ligands. J. Med. Chem 48, 4765–4771. [DOI] [PubMed] [Google Scholar]
- (74).Lee DYW, Yang L, Xu W, Deng G, Guo L, and Liu-Chen LY (2010) Synthesis and Biological Evaluation of C-2 Halogenated Analogues of Salvinorin A. Bioorg. Med. Chem. Lett 20 (19), 5749–5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Prevatt-Smith KM, Lovell KM, Simpson DS, Day VW, Douglas JT, Bosch P, Dersch CM, Rothman RB, Kivell B, and Prisinzano TE (2011) Potential Drug Abuse Therapeutics Derived from the Hallucinogenic Natural Product Salvinorin A. MedChemComm 2 (12), 1217–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Béguin C, Potter DN, Dinieri JA, Munro TA, Richards MR, Paine TA, Berry L, Zhao Z, Roth BL, Xu W, Liu-Chen LY, Carlezon WA, and Cohen BM (2008) N-Methylacetamide Analogue of Salvinorin A: A Highly Potent and Selective κ-Opioid Receptor Agonist with Oral Efficacy. J. Pharmacol. Exp. Ther 324 (1), 188–195. [DOI] [PubMed] [Google Scholar]
- (77).Yan F, Bikbulatov RV, Mocanu V, Dicheva N, Parker CE, Wetsel WC, Mosier PD, Westkaemper RB, Allen JA, Zjawiony JK, and Roth BL (2009) Structure-Based Design, Synthesis, and Biochemical and Pharmacological Characterization of Novel Salvinorin A Analogueues as Active State Probes of the κ-Opioid Receptor. Biochemistry 48 (29), 6898–6908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Crowley RS, Riley AP, Sherwood AM, Groer CE, Shivaperumal N, Biscaia M, Paton K, Schneider S, Provasi D, Kivell BM, Filizola M, and Prisinzano TE (2016) Synthetic Studies of Neoclerodane Diterpenes from Salvia Divinorum: Identification of a Potent and Centrally Acting μ Opioid Analgesic with Reduced Abuse Liability. J. Med. Chem 59 (24), 11027–11038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (79).Tidgewell K, Harding WW, Lozama A, Cobb H, Shah K, Kannan P, Dersch CM, Parrish D, Deschamps JR, Rothman RB, and Prisinzano TE (2006) Synthesis of Salvinorin A Analogueues as Opioid Receptor Probes. J. Nat. Prod 69 (6), 914–918. [DOI] [PubMed] [Google Scholar]
- (80).Polepally PR, Huben K, Vardy E, Setola V, Mosier PD, Roth BL, and Zjawiony JK (2014) Michael Acceptor Approach to the Design of New Salvinorin A-Based High Affinity Ligands for the Kappa-Opioid Receptor. Eur. J. Med. Chem 85, 818–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (81).Tidgewell K, Groer CK, Harding WW, Lozama A, Schmidt M, Marquam A, Hiemstra J, Partilla JS, Dersch CM, Rothman RB, Bohn LM, and Prisinzano TE (2008) Herkinorin Analogueues with Differential β-Arrestin-2 Interactions. J. Med. Chem 51 (8), 2421–2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (82).Bikbulatov RV, Yan F, Roth BL, and Zjawiony JK (2007) Convenient Synthesis and in Vitro Pharmacological Activity of 2-Thioanalogues of Salvinorins A and B. Bioorg. Med. Chem. Lett 17 (8), 2229–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (83).Lee DYW, Karnati VVR, He M, Liu-Chen LY, Kondaveti L, Ma Z, Wang Y, Chen Y, Beguin C, Carlezon WA, and Cohen B (2005) Synthesis and in Vitro Pharmacological Studies of New C(2) Modified Salvinorin A Analogueues. Bioorg. Med. Chem. Lett 15 (16), 3744–3747. [DOI] [PubMed] [Google Scholar]
- (84).Munro TA, Duncan KK, Xu W, Wang Y, Liu-Chen LY, Carlezon WA, Cohen BM, and Béguin C (2008) Standard Protecting Groups Create Potent and Selective κ Opioids: Salvinorin B Alkoxymethyl Ethers. Bioorg. Med. Chem 16 (3), 1279–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (85).Lee DYW, Deng G, Ma Z, Xu W, Yang L, Liu J, Dai R, and Liu-Chen LY (2015) Synthesis and Biological Evaluation of 2-Alkyl-2-Methoxymethyl-Salvinorin Ethers as Selective κ-Opioid Receptor Agonists. Bioorg. Med. Chem. Lett 25 (20), 4689–4692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (86).Polepally PR, White K, Vardy E, Roth BL, Ferreira D, and Zjawiony JK (2013) Kappa-Opioid Receptor-Selective Dicarboxylic Ester-Derived Salvinorin A Ligands. Bioorg. Med. Chem. Lett 23 (10), 2860–2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (87).Sherwood AM, Crowley RS, Paton KF, Biggerstaff A, Neuenswander B, Day VW, Kivell BM, and Prisinzano TE (2017) Addressing Structural Flexibility at the A-Ring on Salvinorin A: Discovery of a Potent Kappa-Opioid Agonist with Enhanced Metabolic Stability. J. Med. Chem 60 (9), 3866–3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (88).Bruchas MR, and Chavkin C (2010) Kinase Cascades and Ligand-Directed Signaling at the Kappa Opioid Receptor. Psychopharmacology (Berl) 210 (2), 137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (89).Bruchas MR, Land BB, Aita M, Xu M, Barot SK, Li S, and Chavkin C (2007) Stress-Induced P38 Mitogen-Activated Protein Kinase Activation Mediates-Opioid-Dependent Dysphoria. J. Neurosci 27 (43), 11614–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (90).Spetea M, Eans SO, Ganno ML, Lantero A, Mairegger M, Toll L, Schmidhammer H, and McLaughlin JP (2017) Selective κ Receptor Partial Agonist HS666 Produces Potent Antinociception without Inducing Aversion after i.c.v. Administration in Mice. Br. J. Pharmacol 174 (15), 2444–2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (91).Brust TF, Morgenweck J, Kim SA, Rose JH, Locke JL, Schmid CL, Zhou L, Stahl EL, Cameron MD, Scarry SM, Aubé J, Jones SR, Martin TJ, and Bohn LM (2016) Biased Agonists of the Kappa Opioid Receptor Suppress Pain and Itch without Causing Sedation or Dysphoria. Sci. Signaling 9 (456), ra117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (92).Huskinson SL, Platt DM, Brasfield M, Follett ME, Prisinzano TE, Blough BE, and Freeman KB (2020) Quantification of Observable Behaviors Induced by Typical and Atypical Kappa-Opioid Receptor Agonists in Male Rhesus Monkeys. Psychopharmacology (Berl) 237 (7), 2075–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (93).Simonson B, Morani AS, Ewald AWM, Walker L, Kumar N, Simpson D, Miller JH, Prisinzano TE, and Kivell BM (2015) Pharmacology and Anti-Addiction Effects of the Novel κ Opioid Receptor Agonist Mesyl Sal B, a Potent and Long-Acting Analogueue of Salvinorin A. Br. J. Pharmacol 172 (2), 515–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (94).Kivell B, Paton K, Kumar N, Morani A, Culverhouse A, Shepherd A, Welsh S, Biggerstaff A, Crowley R, and Prisinzano T (2018) Kappa Opioid Receptor Agonist Mesyl Sal B Attenuates Behavioral Sensitization to Cocaine with Fewer Aversive Side-Effects than Salvinorin A in Rodents. Molecules 23 (10), 2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (95).Wang Y, Chen Y, Xu W, Lee DYW, Ma Z, Rawls SM, Cowan A, and Liu-Chen LY (2008) 2-Methoxymethyl-Salvinorin B Is a Potent κ Opioid Receptor Agonist with Longer Lasting Action in Vivo than Salvinorin A. J. Pharmacol. Exp. Ther 324 (3), 1073–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (96).Liu JJ, Chiu YT, DiMattio KM, Chen C, Huang P, Gentile TA, Muschamp JW, Cowan A, Mann M, and Liu-Chen LY (2019) Phosphoproteomic Approach for Agonist-Specific Signaling in Mouse Brains: MTOR Pathway Is Involved in κ Opioid Aversion. Neuropsychopharmacology 44 (5), 939–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (97).Ewald AWM, Bosch PJ, Culverhouse A, Crowley RS, Neuenswander B, Prisinzano TE, and Kivell BM (2017) The C-2 Derivatives of Salvinorin A, Ethoxymethyl Ether Sal B and β-Tetrahydropyran Sal B, Have Anti-Cocaine Properties with Minimal Side Effects. Psychopharmacology (Berl) 234 (16), 2499–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (98).Paton KF, Kumar N, Crowley RS, Harper JL, Prisinzano TE, and Kivell BM (2017) The Analgesic and Anti-Inflammatory Effects of Salvinorin A Analogueue β-Tetrahydropyran Salvinorin B in Mice. Eur. J. Pain (United Kingdom) 21 (6), 1039–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (99).Manglik A, Lin H, Aryal DK, McCorvy JD, Dengler D, Corder G, Levit A, Kling RC, Bernat V, Hubner H, Huang X-P, Sassano MF, Giguere PM, Lober S, Duan Da, Scherrer G, Kobilka BK, Gmeiner P, Roth BL, and Shoichet BK (2016) Structure-Based Discovery of Opioid Analgesics with Reduced Side Effects. Nature 537 (7619), 185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (100).Lamb K, Tidgewell K, Simpson DS, Bohn LM, and Prisinzano TE (2012) Antinociceptive Effects of Herkinorin, a MOP Receptor Agonist Derived from Salvinorin A in the Formalin Test in Rats: New Concepts in Mu Opioid Receptor Pharmacology: From a Symposium on New Concepts in Mu-Opioid Pharmacology. Drug Alcohol Depend. 121 (3), 181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (101).Crowley RS, Riley AP, Alder AF, Anderson RJ, Luo D, Kaska S, Maynez P, Kivell BM, and Prisinzano TE (2020) Synthetic Studies of Neoclerodane Diterpenes from Salvia Divinorum: Design, Synthesis, and Evaluation of Analogueues with Improved Potency and G-Protein Activation Bias at the μ-Opioid Receptor. ACS Chem. Neurosci 11, 1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (102).Lee DYW, He M, Kondaveti L, Liu-Chen LY, Ma Z, Wang Y, Chen Y, Li JG, Beguin C, Carlezon WA, and Cohen B (2005) Synthesis and in Vitro Pharmacological Studies of C(4) Modified Salvinorin A Analogueues. Bioorg. Med. Chem. Lett 15 (19), 4169–4173. [DOI] [PubMed] [Google Scholar]
- (103).Munro TA, Duncan KK, Staples RJ, Xu W, Liu-Chen LY, Béguin C, Carlezon WA, and Cohen BM (2007) 8-Epi-Salvinorin B: Crystal Structure and Affinity at the κ Opioid Receptor. Beilstein J. Org. Chem 3, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (104).Lee DYW, He M, Liu-Chen LY, Wang Y, Li JG, Xu W, Ma Z, Carlezon WA, and Cohen B (2006) Synthesis and in Vitro Pharmacological Studies of New C(4)-Modified Salvinorin A Analogueues. Bioorg. Med. Chem. Lett 16 (21), 5498–5502. [DOI] [PubMed] [Google Scholar]
- (105).Lovell KM, Vasiljevik T, Araya JJ, Lozama A, Prevatt-Smith KM, Day VW, Dersch CM, Rothman RB, Butelman ER, Kreek MJ, and Prisinzano TE (2012) Semisynthetic Neoclerodanes as Kappa Opioid Receptor Probes. Bioorg. Med. Chem 20 (9), 3100–3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (106).Béguin C, Duncan KK, Munro TA, Ho DM, Xu W, Liu-Chen LY, Carlezon WA, and Cohen BM (2009) Modification of the Furan Ring of Salvinorin A: Identification of a Selective Partial Agonist at the Kappa Opioid Receptor. Bioorg. Med. Chem 17 (3), 1370–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (107).Béguin C, Potuzak J, Xu W, Liu-Chen LY, Streicher JM, Groer CE, Bohn LM, Carlezon WA, and Cohen BM (2012) Differential Signaling Properties at the Kappa Opioid Receptor of 12-Epi-Salvinorin A and Its Analogueues. Bioorg. Med. Chem. Lett 22 (2), 1023–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (108).Dimattio KM, Ehlert FJ, and Liu-Chen LY (2015) Intrinsic Relative Activities of κ Opioid Agonists in Activating Gα Proteins and Internalizing Receptor: Differences between Human and Mouse Receptors. Eur. J. Pharmacol 761, 235–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (109).Lozama A, Cunningham CW, Caspers MJ, Douglas JT, Dersch CM, Rothman RB, and Prisinzano TE (2011) Opioid Receptor Probes Derived from Cycloaddition of the Hallucinogen Natural Product Salvinorin A. J. Nat. Prod 74 (4), 718–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (110).Riley AP, Groer CE, Young D, Ewald AW, Kivell BM, and Prisinzano TE (2014) Synthesis and κ-Opioid Receptor Activity of Furan-Substituted Salvinorin A Analogueues. J. Med. Chem 57 (24), 10464–10475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (111).Paton KF, Biggerstaff A, Kaska S, Crowley RS, La Flamme AC, Prisinzano TE, and Kivell BM (2020) Evaluation of Biased and Balanced Salvinorin A Analogues in Preclinical Models of Pain. Front. Neurosci 14, 765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (112).Che T, Majumdar S, Zaidi SA, Ondachi P, McCorvy JD, Wang S, Mosier PD, Uprety R, Vardy E, Krumm BE, Han GW, Lee M-Y, Pardon E, Steyaert J, Huang X-P, Strachan RT, Tribo AR, Pasternak GW, Carroll FI, Stevens RC, Cherezov V, Katritch V, Wacker D, and Roth BL (2018) Structure of the Nanobody-Stabilized Active State of the Kappa Opioid Receptor. Cell 172 (1–2), 55–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (113).Váradi A, Marrone GF, Eans SO, Ganno ML, Subrath JJ, Le Rouzic V, Hunkele A, Pasternak GW, McLaughlin JP, and Majumdar S (2015) Synthesis and Characterization of a Dual Kappa-Delta Opioid Receptor Agonist Analgesic Blocking Cocaine Reward Behavior. ACS Chem. Neurosci 6 (11), 1813–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (114).Takayama H (2004) Chemistry and Pharmacology of Analgesic Indole Alkaloids from the Rubiaceous Plant, Mitragyna Speciosa. Chem. Pharm. Bull 52 (8), 916–28. [DOI] [PubMed] [Google Scholar]
- (115).Adkins JE, Boyer EW, and McCurdy CR (2011) Mitragyna Speciosa, A Psychoactive Tree from Southeast Asia with Opioid Activity. Curr. Top. Med. Chem 11 (9), 1165–1175. [DOI] [PubMed] [Google Scholar]
- (116).Raffa RB, Beckett JR, Brahmbhatt VN, Ebinger TM, Fabian CA, Nixon JR, Orlando ST, Rana CA, Tejani AH, and Tomazic RJ (2013) Orally Active Opioid Compounds from a Non-Poppy Source. J. Med. Chem 56, 4840–4848. [DOI] [PubMed] [Google Scholar]
- (117).León F, Habib E, Adkins JE, Furr EB, McCurdy CR, and Cutler SJ (2009) Phytochemical Characterization of the Leaves of Mitragyna Speciosa Grown in USA. Nat. Prod. Commun 4 (7), 907–910. [PMC free article] [PubMed] [Google Scholar]
- (118).Gutridge AM, Robins MT, Cassell RJ, Uprety R, Mores KL, Ko MJ, Pasternak GW, Majumdar S, and Rijn RM (2020) G Protein-Biased Kratom-Alkaloids and Synthetic Carfentanil-Amide Opioids as Potential Treatments for Alcohol Use Disorder. Br. J. Pharmacol 177, 1497–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (119).Varadi A, Marrone GF, Palmer TC, Narayan A, Szabo MR, Le Rouzic V, Grinnell SG, Subrath JJ, Warner E, Kalra S, Hunkele A, Pagirsky J, Eans SO, Medina JM, Xu J, Pan Y-X, Borics A, Pasternak GW, McLaughlin JP, and Majumdar S (2016) Mitragynine/Corynantheidine Pseudoindoxyls As Opioid Analgesics with Mu Agonism and Delta Antagonism, Which Do Not Recruit β-Arrestin-2. J. Med. Chem 59 (18), 8381–8397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (120).Stoeber M, Jullié D, Li J, Chakraborty S, Majumdar S, Lambert NA, Manglik A, and von Zastrow M (2020) Agonist-Selective Recruitment of Engineered Protein Probes and of GRK2 by Opioid Receptors in Living Cells. eLife 9, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (121).Kruegel AC, Gassaway MM, Kapoor A, Váradi A, Majumdar S, Filizola M, Javitch JA, and Sames D (2016) Synthetic and Receptor Signaling Explorations of the Mitragyna Alkaloids: Mitragynine as an Atypical Molecular Framework for Opioid Receptor Modulators. J. Am. Chem. Soc 138 (21), 6754–6764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (122).Kruegel AC, Uprety R, Grinnell SG, Langreck C, Pekarskaya EA, Le Rouzic V, Ansonoff M, Gassaway MM, Pintar JE, Pasternak GW, Javitch JA, Majumdar S, and Sames D (2019) 7-Hydroxymitragynine Is an Active Metabolite of Mitragynine and a Key Mediator of Its Analgesic Effects. ACS Cent. Sci 5 (6), 992–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (123).Yue K, Kopajtic TA, and Katz JL (2018) Abuse Liability of Mitragynine Assessed with a Self-Administration Procedure in Rats. Psychopharmacology (Berl) 235 (10), 2823–2829. [DOI] [PubMed] [Google Scholar]
- (124).Hemby SE, McIntosh S, Leon F, Cutler SJ, and McCurdy CR (2019) Abuse Liability and Therapeutic Potential of the Mitragyna Speciosa (Kratom) Alkaloids Mitragynine and 7-Hydroxymitragynine. Addict. Biol 24 (5), 874–885. [DOI] [PubMed] [Google Scholar]
- (125).Ma J, Yin W, Zhou H, and Cook JM (2007) Total Synthesis of the Opioid Agonistic Indole Alkaloid Mitragynine and the First Total Syntheses of 9-Methoxygeissoschizol and 9-Methoxy-N b-Methylgeissoschizol. Org. Lett 9 (18), 3491–3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (126).Ma J, Yin W, Zhou H, Liao X, and Cook JM (2009) General Approach to the Total Synthesis of 9-Methoxy-Substituted Indole Alkaloids: Synthesis of Mitragynine, as Well as 9-Methox-ygeissoschizol and 9-Methoxy-Nb-Methylgeissoschizol. J. Org. Chem 74 (1), 264–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (127).Kerschgens IP, Claveau E, Wanner MJ, Ingemann S, Maarseveen JHV, and Hiemstra H (2012) Total Syntheses of Mitragynine, Paynantheine and Speciogynine via an Enantioselective Thiourea-Catalysed Pictet-Spengler Reaction. Chem. Commun 48 (100), 12243–12245. [DOI] [PubMed] [Google Scholar]
- (128).Takayama H, Ishikawa H, Kurihara M, Kitajima M, Aimi N, Ponglux D, Koyama F, Matsumoto K, Moriyama T, Yamamoto LT, Watanabe K, Murayama T, and Horie S (2002) Studies on the Synthesis and Opioid Agonistic Activities of Mitragynine-Related Indole Alkaloids: Discovery of Opioid Agonists Structurally Different from Other Opioid Ligands. J. Med. Chem 45 (9), 1949–1956. [DOI] [PubMed] [Google Scholar]
- (129).Takayama H, Maeda M, Ohbayashi S, Kitajima M, Sakai S. ichiro, and Aimi N (1995) The First Total Synthesis of (−)-Mitragynine, an Analgesic Indole Alkaloid in Mitragyna Speciosa. Tetrahedron Lett. 36 (51), 9337–9340. [Google Scholar]
- (130).Obeng S, Kamble SH, Reeves ME, Restrepo LF, Patel A, Behnke M, Chear NJY, Ramanathan S, Sharma A, León F, Hiranita T, Avery BA, McMahon LR, and McCurdy CR (2020) Investigation of the Adrenergic and Opioid Binding Affinities, Metabolic Stability, Plasma Protein Binding Properties, and Functional Effects of Selected Indole-Based Kratom Alkaloids. J. Med. Chem 63 (1), 433–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (131).Gutridge AM, Robins MT, Cassell RJ, Uprety R, Mores KL, Ko MJ, Pasternak GW, Majumdar S, and Rijn RM (2020) G Protein-biased Kratom-alkaloids and Synthetic Carfentanil-amide Opioids as Potential Treatments for Alcohol Use Disorder. Br. J. Pharmacol 177 (7), 1497–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (132).Macko E, Weisbach JA, and Douglas B (1972) Some Observations on the Pharmacology of Mitragynine. Arch. Int. Pharmacodyn. Ther 198 (1), 145–161. [PubMed] [Google Scholar]
- (133).Hiranita T, Leon F, Felix JS, Restrepo LF, Reeves ME, Pennington AE, Obeng S, Avery BA, McCurdy CR, McMahon LR, and Wilkerson JL (2019) The Effects of Mitragynine and Morphine on Schedule-Controlled Responding and Antinociception in Rats. Psychopharmacology (Berl) 236 (9), 2725–2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (134).Matsumoto K, Mizowaki M, Suchitra T, Takayama H, Sakai SI, Aimi N, and Watanabe H (1996) Antinociceptive Action of Mitragynine in Mice: Evidence for the Involvement of Supraspinal Opioid Receptors. Life Sci. 59 (14), 1149–1155. [DOI] [PubMed] [Google Scholar]
- (135).Matsumoto K, Mizowaki M, Suchitra T, Murakami Y, Takayama H, Sakai SI, Aimi N, and Watanabe H (1996) Central Antinociceptive Effects of Mitragynine in Mice: Contribution of Descending Noradrenergic and Serotonergic Systems. Eur. J. Pharmacol 317 (1), 75–81. [DOI] [PubMed] [Google Scholar]
- (136).Kamble SH, Sharma A, King TI, León F, McCurdy CR, and Avery BA (2019) Metabolite Profiling and Identification of Enzymes Responsible for the Metabolism of Mitragynine, the Major Alkaloid of Mitragyna Speciosa (Kratom). Xenobiotica 49 (11), 1279–1288. [DOI] [PubMed] [Google Scholar]
- (137).Sharma A, Kamble SH, León F, Chear NJ-Y, King TI, Berthold EC, Ramanathan S, McCurdy CR, and Avery BA (2019) Simultaneous Quantification of Ten Key Kratom Alkaloids in Mitragyna Speciosa Leaf Extracts and Commercial Products by Ultra-performance Liquid Chromatography–tandem Mass Spectrometry. Drug Test. Anal 11 (8), 1162–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (138).Matsumoto K, Takayama H, Ishikawa H, Aimi N, Ponglux D, Watanabe K, and Horie S (2006) Partial Agonistic Effect of 9-Hydroxycorynantheidine on μ-Opioid Receptor in the Guinea-Pig Ileum. Life Sci. 78 (19), 2265–2271. [DOI] [PubMed] [Google Scholar]
- (139).Takayama H, Misawa K, Okada N, Ishikawa H, Kitajima M, Hatori Y, Murayama T, Wongseripipatana S, Tashima K, Matsumoto K, and Horie S (2006) New Procedure to Mask the 2,3-π Bond of the Indole Nucleus and Its Application to the Preparation of Potent Opioid Receptor Agonists with a Corynanthe Skeleton. Org. Lett 8 (25), 5705–5708. [DOI] [PubMed] [Google Scholar]
- (140).Matsumoto K, Takayama H, Narita M, Nakamura A, Suzuki M, Suzuki T, Murayama T, Wongseripipatana S, Misawa K, Kitajima M, Tashima K, and Horie S (2008) MGM-9 [(E)-Methyl 2-(3-Ethyl-7a, 12a-(Epoxyethanoxy)-9-Fluoro-1,2,3,4,6,7,12,12b-Octahydro-8-Methoxyindolo[2,3-a]Quinolizin-2-Yl)-3-Methoxyacrylate], a Derivative of the Indole Alkaloid Mitragynine: A Novel Dual-Acting μ- and κ-Opioid Agonist with Potent Antinociceptive and Weak Rewarding Effects in Mice. Neuropharmacology 55 (2), 154–165. [DOI] [PubMed] [Google Scholar]
- (141).Ponglux D, Wongseripipatana S, Takayama H, Kikuchi M, Kurihara M, Kitajima M, Aimi N, and Sakai S (1994) A New Indole Alkaloid, 7 α-Hydroxy-7 H -Mitragynine, from Mitragyna Speciosa in Thailand. Planta Med. 60 (06), 580–581. [DOI] [PubMed] [Google Scholar]
- (142).Matsumoto K, Horie S, Ishikawa H, Takayama H, Aimi N, Ponglux D, and Watanabe K (2004) Antinociceptive Effect of 7-Hydroxymitragynine in Mice: Discovery of an Orally Active Opioid Analgesic from the Thai Medicinal Herb Mitragyna Speciosa. Life Sci. 74 (17), 2143–2155. [DOI] [PubMed] [Google Scholar]
- (143).Matsumoto K, Horie S, Takayama H, Ishikawa H, Aimi N, Ponglux D, Murayama T, and Watanabe K (2005) Antinociception, Tolerance and Withdrawal Symptoms Induced by 7-Hydroxymitragynine, an Alkaloid from the Thai Medicinal Herb Mitragyna Speciosa. Life Sci. 78 (1), 2–7. [DOI] [PubMed] [Google Scholar]
- (144).Matsumoto K, Hatori Y, Murayama T, Tashima K, Wongseripipatana S, Misawa K, Kitajima M, Takayama H, and Horie S (2006) Involvement of μ-Opioid Receptors in Antinociception and Inhibition of Gastrointestinal Transit Induced by 7-Hydroxymitragynine, Isolated from Thai Herbal Medicine Mitragyna Speciosa. Eur. J. Pharmacol 549 (1–3), 63–70. [DOI] [PubMed] [Google Scholar]
- (145).Matsumoto K, Narita M, Muramatsu N, Nakayama T, Misawa K, Kitajima M, Tashima K, Devi LA, Suzuki T, Takayama H, and Horie S (2014) Orally Active Opioid μ/δ Dual Agonist MGM-16, a Derivative of the Indole Alkaloid Mitragynine, Exhibits Potent Antiallodynic Effect on Neuropathic Pain in Mices. J. Pharmacol. Exp. Ther 348 (3), 383–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (146).Zarembo JE, Douglas B, Valenta J, and Weisbach JA (1974) Metabolites of Mitragynine. J. Pharm. Sci 63 (9), 1407–1415. [DOI] [PubMed] [Google Scholar]
- (147).Yamamoto LT, Horie S, Takayama H, Aimi N, Sakai SI, Yano S, Shan J, Pang PKT, Ponglux D, and Watanabe K (1999) Opioid Receptor Agonistic Characteristics of Mitragynine Pseudoindoxyl in Comparison with Mitragynine Derived from Thai Medicinal Plant Mitragyna Speciosa. Gen. Pharmacol 33 (1), 73–81. [DOI] [PubMed] [Google Scholar]
- (148).Huang W, Manglik A, Venkatakrishnan AJ, Laeremans T, Feinberg EN, Sanborn AL, Kato HE, Livingston KE, Thorsen TS, Kling RC, Granier S, Gmeiner P, Husbands SM, Traynor JR, Weis WI, Steyaert J, Dror RO, and Kobilka BK (2015) Structural Insights into μ-Opioid Receptor Activation. Nature 524 (7565), 315–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (149).Koehl A, Hu H, Maeda S, Zhang Y, Qu Q, Paggi JM, Latorraca NR, Hilger D, Dawson R, Matile H, Schertler GFX, Granier S, Weis WI, Dror RO, Manglik A, Skiniotis G, and Kobilka BK (2018) Structure of the M-Opioid Receptor–Gi Protein Complex. Nature 558 (7711), 547–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (150).Grim TW, Schmid CL, Stahl EL, Pantouli F, Ho JH, Acevedo-Canabal A, Kennedy NM, Cameron MD, Bannister TD, and Bohn LM (2020) A G Protein Signaling-Biased Agonist at the μ-Opioid Receptor Reverses Morphine Tolerance While Preventing Morphine Withdrawal. Neuropsychopharmacology 45 (2), 416–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (151).Zhu Y, King MA, Schuller AGP, Nitsche JF, Reidl M, Elde RP, Unterwald E, Pasternak GW, and Pintar JE (1999) Retention of Supraspinal Delta-like Analgesia and Loss of Morphine Tolerance in δ Opioid Receptor Knockout Mice. Neuron 24 (1), 243–252. [DOI] [PubMed] [Google Scholar]
- (152).Abdelhamid EE, Sultana M, Portoghese PS, and Takemori AE Selective Blockage of Delta Opioid Receptors Prevents the Development of Morphine Tolerance and Dependence in Mice. J. Pharmacol. Exp. Ther 1991, 258 (1). [PubMed] [Google Scholar]
- (153).Anand JP, Kochan KE, Nastase AF, Montgomery D, Griggs NW, Traynor JR, Mosberg HI, and Jutkiewicz EM (2018) In Vivo Effects of μ-Opioid Receptor Agonist/δ-Opioid Receptor Antagonist Peptidomimetics Following Acute and Repeated Administration. Br. J. Pharmacol 175 (11), 2013–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (154).Bui AM, Cavé A, Janot MM, Parello J, Potier P, and Scheidegger U (1974) Isolement et Analyse Structurale Du Collybolide, Nouveau Sesquiterpene Extrait de Collybia Maculata Alb. et Sch. Ex Fries (Basidiomycetes). Tetrahedron 30 (11), 1327–1336. [Google Scholar]
- (155).Gupta A, Gomes I, Bobeck EN, Fakira AK, Massaro NP, Sharma I, Cave A, Hamm HE, Parello J, and Devi LA (2016) Collybolide Is a Novel Biased Agonist of Ê-Opioid Receptors with Potent Antipruritic Activity. Proc. Natl. Acad. Sci. U. S. A 113 (21), 6041–6046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (156).Castronovo F, Clericuzio M, Toma L, and Vidari G (2001) Fungal Metabolites. Part 45: The Sesquiterpenes of Collybia Maculata and Collybia Peronata. Tetrahedron 57 (14), 2791–2798. [Google Scholar]
- (157).Inan S, and Cowan A (2004) Kappa Opioid Agonists Suppress Chloroquine-Induced Scratching in Mice. Eur. J. Pharmacol 502 (3), 233–237. [DOI] [PubMed] [Google Scholar]
- (158).Liu-Chen LY (2004) Agonist-Induced Regulation and Trafficking of κ Opioid Receptors. Life Sci. 75, 511–536. [DOI] [PubMed] [Google Scholar]
- (159).Carlezon WA, Beguin C, DiNieri JA, Baumann MH, Richards MR, Todtenkopf MS, Rothman RB, Ma Z, Lee DY-W, and Cohen BM (2006) Depressive-like Effects of the κ-Opioid Receptor Agonist Salvinorin A on Behavior and Neurochemistry in Rats. J. Pharmacol. Exp. Ther 316 (1), 440–447. [DOI] [PubMed] [Google Scholar]
- (160).Land BB, Bruchas MR, Schattauer S, Giardino WJ, Aita M, Messinger D, Hnasko TS, Palmiter RD, and Chavkin C (2009) Activation of the Kappa Opioid Receptor in the Dorsal Raphe Nucleus Mediates the Aversive Effects of Stress and Reinstates Drug Seeking. Proc. Natl. Acad. Sci. U. S. A 106 (45), 19168–19173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (161).Conibear AE, and Kelly E (2019) MINIREVIEW-COMPUTATIONAL ANALYSIS OF GPCRS A Biased View of m-Opioid Receptors? Mol. Pharmacol 96, 542–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (162).Raehal KM, Walker JKL, and Bohn LM (2005) Morphine Side Effects in β-Arrestin 2 Knockout Mice. J. Pharmacol. Exp. Ther 314 (3), 1195–1201. [DOI] [PubMed] [Google Scholar]
- (163).Hill R, Disney A, Conibear A, Sutcliffe K, Dewey W, Husbands S, Bailey C, Kelly E, and Henderson G (2018) The Novel μ-Opioid Receptor Agonist PZM21 Depresses Respiration and Induces Tolerance to Antinociception. Br. J. Pharmacol 175, 2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (164).Bachmutsky I, Durand A, and Yackle K (2020) SS2-Arrestin Germline Knockout Does Not Attenuate Opioid Respiratory Depression 5. In bioRxiv, 2020.08.28.272575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (165).Kliewer A, Gillis A, Hill R, Schmiedel F, Bailey C, Kelly E, Henderson G, Christie MJ, and Schulz S (2020) Morphine-Induced Respiratory Depression Is Independent of β-Arrestin2 Signalling. Br. J. Pharmacol 177 (13), 2923–2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (166).Kliewer A, Schmiedel F, Sianati S, Bailey A, Bateman JT, Levitt ES, Williams JT, Christie MJ, and Schulz S (2019) Phosphorylation-Deficient G-Protein-Biased μ-Opioid Receptors Improve Analgesia and Diminish Tolerance but Worsen Opioid Side Effects. Nat. Commun 10 (1), 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (167).Raehal KM, Schmid CL, Groer CE, and Bohn LM (2011) Functional Selectivity at the μ-Opioid Receptor: Implications for Understanding Opioid Analgesia and Tolerance. Pharmacol. Rev 63 (4), 1001–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (168).Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, and Lin FT (1999) Enhanced Morphine Analgesia in Mice Lacking β-Arrestin 2. Science (Washington, DC, U. S.) 286 (5449), 2495–8. [DOI] [PubMed] [Google Scholar]
- (169).FDA Approves New Opioid for Intravenous Use in Hospitals, Other Controlled Clinical Settings | FDA; https://www.fda.gov/news-events/press-announcements/fda-approves-new-opioid-intravenous-use-hospitals-other-controlled-clinical-settings (accessed Sep 10, 2020). [Google Scholar]
- (170).Chiang T, Sansuk K, and Van Rijn RM (2016) β-Arrestin 2 Dependence of δ Opioid Receptor Agonists Is Correlated with Alcohol Intake. Br. J. Pharmacol 173 (2), 332–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (171).Vicente-Sanchez A, Dripps IJ, Tipton AF, Akbari H, Akbari A, Jutkiewicz EM, and Pradhan AA (2018) Tolerance to High-Internalizing δ Opioid Receptor Agonist Is Critically Mediated by Arrestin 2. Br. J. Pharmacol 175 (14), 3050–3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (172).Pradhan AA, Perroy J, Walwyn WM, Smith ML, Vicente-Sanchez A, Segura L, Bana A, Kieffer BL, and Evans CJ (2016) Agonist-Specific Recruitment of Arrestin Isoforms Differentially Modify Delta Opioid Receptor Function. J. Neurosci 36 (12), 3541–3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (173).Conibear AE, Asghar J, Hill R, Henderson G, Borbely E, Tekus V, Helyes Z, Palandri J, Bailey C, Starke I, von Mentzer B, Kendall D, and Kelly E (2020) A Novel G Protein-Biased Agonist at the d Opioid Receptor with Analgesic Efficacy in Models of Chronic Pain. J. Pharmacol. Exp. Ther 372 (2), 224–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (174).Olsen RHJ, DiBerto JF, English JG, Glaudin AM, Krumm BE, Slocum ST, Che T, Gavin AC, McCorvy JD, Roth BL, and Strachan RT (2020) TRUPATH, an Open-Source Biosensor Platform for Interrogating the GPCR Transducerome. Nat. Chem. Biol 16 (8), 841–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (175).Livingston KE, and Traynor JR (2018) Allostery at Opioid Receptors: Modulation with Small Molecule Ligands. Br. J. Pharmacol 175, 2846–2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (176).Fricker LD, Margolis EB, Gomes I, and Devi LA (2020) Five Decades of Research on Opioid Peptides: Current Knowledge and Unanswered Questions. Mol. Pharmacol 98 (2), 96–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (177).Gomes I, Sierra S, Lueptow L, Gupta A, Gouty S, Margolis EB, Cox BM, and Devi LA (2020) Biased Signaling by Endogenous Opioid Peptides. Proc. Natl. Acad. Sci. U. S. A 117 (21), 11820–11828. [DOI] [PMC free article] [PubMed] [Google Scholar]