Abstract
The Convention on Biological Diversity (CBD) aims to end the loss of biodiversity, which is one of the greatest ecological challenges of our time. The lack of success in biodiversity policy implementation is partly related to gaps in biodiversity monitoring. Our overall objective is to contribute to the preparation of the upcoming post 2020 period by a review of biodiversity indicator choices in European CBD reports and hence in national monitoring systems. Negative binary generalized models and poisson generalized linear models prove that through free indicator choice in CBD reporting, countries do not choose biodiversity indicators according to their national geographic and socioeconomic characteristics. Moreover, species and ecosystem diversity indicators were chosen with a disproportionate frequency compared to that of genetic diversity indicators. Consequently, trends derived from national CBD reports and monitoring systems in Europe are not reliable, which should be an alarming signal concerning biodiversity policy implementation. Finally, a flow chart to revise national biodiversity monitoring systems is proposed.
Electronic supplementary material
The online version of this article 10.1007/s13280-020-01415-8 contains supplementary material, which is available to authorized users.
Keywords: Biodiversity indicators, Biodiversity monitoring, Biodiversity policy implementation, European species diversity
Introduction
With global extinction rates being one hundred to one thousand times greater than the natural baseline (Ceballos et al. 2010 and 2015), the loss of biodiversity is one of the greatest and most serious ecological challenges of our time (CBD 2006; Rockström et al. 2009). Biodiversity loss threatens the provision of ecosystem services at an accelerating rate and erodes the foundation of humanity (IPBES 2019). Nonetheless, the main drivers of extinction are of anthropogenic origin (Sala et al. 2000; Newbold et al. 2015).
Convention on biological diversity
Therefore, two hundred countries committed themselves to halt the loss of biodiversity by signing the UN Convention on Biological Diversity (CBD) in 1992. Thus, 14.4 billion USD was spent globally from 1992 to 2003 to slow down biodiversity loss. This effort reduced the expected species decline in that period by 29% (Waldron et al. 2017). Nonetheless, strategic CBD targets were not achieved until 2010 (CBD 2014), and Aichi targets for the successive period 2011 to 2020 will not be accomplished (CBD 2014; Tittensor et al. 2014). Why do the member countries fail to reach the targets even though numerous financial efforts have been made? Actually, there is no internal mechanism in the CBD body established to monitor national-level compliance and the implementation of biodiversity policies (Morgera and Tsioumami 2011; Vordermayer-Riemer 2019). Therefore, scientific evaluations of implementation deficits and the reasons for these deficits may be particularly valuable.
In addition to lacking capacity in terms of coordination, science, administration and legislation, the lack of success in biodiversity policy implementation is related to gaps in biodiversity monitoring (Pareira et al. 2012; CBD 2018). Scanning the Aichi targets (CBD 2012), we believe that establishing effective national biodiversity monitoring (target 19) is the basis for reaching eight out of twenty Aichi targets (targets 2, 5, 7, 8, 9, 11, 12, and 14). Improving biodiversity monitoring and reporting may greatly improve the ability to reach future strategic CBD goals.
Therefore, this paper focuses on the biodiversity indicator choice and biodiversity monitoring as important factors in biodiversity policy implementation. Biodiversity cannot be quantified directly, and thus, assessments are highly complex. Indicators are needed that are based on achievable, quantitative data, are policy and ecosystem relevant, assessable to monitoring, sensitive to pressures on biodiversity, have an indicative value and stable properties (CBD 1997). Actually, these ambitious requirements are very difficult to fulfill. Sometimes also value judgments have to be made, which makes biodiversity indicator choice neither entirely objective nor easy (CBD 1997). However, as the CBD reporting guidelines allow for the freedom of indicator choice, European indicator choice in national CBD reports may also display the conception of the complex term of biodiversity.
CBD post-2020 period
According to the definition of biodiversity used by the CBD body, biodiversity has three components of equal value (ecosystem diversity, species diversity and genetic diversity). However, freedom of indicator choice may allow for the use of conceptions of biodiversity that differ from the CBD definition, which would be disadvantageous for halting biodiversity loss. Genetic diversity is frequently neglected (Pareira et al. 2012), although genetic diversity is the foundation of all biological diversity and enhances persistence and the evolutionary potential of all species (Allendorf et al. 2012). In certain ecosystems, genetic diversity may provide biological functions similar to that of species diversity (Cook-Patton et al. 2011). However, monitoring genetic diversity requires complex laboratory analyses, whereas assessing ecosystem diversity and species diversity may be less challenging.
In this paper, we attempt to determine whether European biodiversity reporting and monitoring is reliable and whether the biodiversity conception is in line with the CBD definition. Gaps in biodiversity policy implementation may be closed by adapting institutional CBD reporting requirements and through the efforts of CBD member countries. Considering 42 European national CBD reports, we tried to answer the following questions: (1) Which kinds of biodiversity indicators are particularly prevalent? (2) Are ecosystem diversity, species diversity, and genetic diversity considered adequately? In addition, considering the CBD institutional point of view, we wanted to determine whether (3) the freedom of biodiversity indicator choice in CBD reporting guidelines is beneficial for biodiversity monitoring and reporting quality. Our scientific objective is to support the implementation of the CBD by systematically reviewing national reports and to contribute to preparing for the upcoming post-2020 period.
Materials and methods
Prevalent biodiversity indicators
First, the fifth national reports (strategic period 2011–2020) of all European CBD members (n = 43) were downloaded from the CBD homepage (https://www.cbd.int/countries/). Only 42 of these could be considered for further analysis, as a linguistic barrier impeded the evaluation of Belarus’ national report.
Due to the amount of data, analyzing a subset of five randomly chosen CBD reports was necessary to prepare a list of the biodiversity indicators reported most frequently in European national reports. In the following, these biodiversity indicators were grouped into three main categories: (1) ecosystem indicators, (2) species indicators, and (3) genetic indicators. Moreover, these categories were further divided into four subcategories (Table 1). Then, two additional reports not included in the subset were used to pretest the indicator list. Finally, all 42 national reports were systematically evaluated using the approved indicator list.
Table 1.
Biodiversity indicator categories and subcategories
| (1) Ecosystem indicators | (2) Species indicators | (3) Genetic indicators |
|---|---|---|
| (1a) land use | (2a) aquatic species | (3a) domesticated plants |
| (1b) forest structure | (2b) semiaquatic-terrestrial species | (3b) wild plants |
| (1c) nature protection | (2c) terrestrial flora | (3c) domesticated animals |
| (1d) human pressure | (2d) species of particular interest | (3d) wild animals |
Biodiversity reporting reliability
To evaluate reporting reliability in a second step, country characteristics (concerning geography and socioeconomy) were obtained from online platforms (Electronic Supplementary Material S1). Additionally, geographic isolation was measures as the ratio of country coastline to total country border length.
Indicator choice evaluation
Generally, all countries in our analysis prepared their national reports in accordance with the CBD reporting guidelines by answering the predetermined questions. However, the reports differ greatly in terms of elaborateness and information density, which impedes a direct comparison. To tackle this problem, we evaluated the biodiversity indicator choice by using a binary-coded indicator list (valid vs. not valid). For (1) ecosystem indicators and (2) species indicators, one measurable, quantitative value appearing in the national CBD report validated the indicator. For instance, the wording “The population of the bird species Eurasian bittern (Botaurus stellaris) is now 320 breeding pairs” in a national CBD report would be a valid bird species diversity/subcategory 2b count. For (3) genetic indicators, however, qualitative declarations about existing national programs or activities were rated as being sufficient (e.g., “The country established a gene bank for wild crop relatives” would result in a valid wild crop relative ex situ/subcategory 3b count). To treat all indicator categories in the evaluation equally would have been more logical. However, the frequency of genetic indicators would have been close to zero, and de facto, these indicators would have been disregarded. Detailed outcomes of the evaluation of the 42 national CBD reports can be found in Electronic Supplementary Material (S2).
Biodiversity reporting and monitoring reliability
To assess the reliability of national CBD reporting and monitoring, two assumptions had to be made: (1) National CBD reports reflect the indicator choice for national biodiversity monitoring and (2) reliable monitoring accurately reflects the geographic and socioeconomic characteristics of a country.
The first assumption could not be addressed by this study, but it seems very unlikely that member countries put large financial efforts in biodiversity monitoring without reporting to the CBD. The second assumption is a logical conclusion, as natural biodiversity levels are primarily determined by geographic factors (e.g., mean latitude, area size, biogeographic regions). However, the biodiversity present may differ from natural levels, mainly due to an anthropogenic impact (Sala et al. 2000; Newbold et al. 2015). Therefore, the second assumption is in line with the pressure-state-response framework underlying the CBD body, which states that “CBD indicators should monitor and assess status and trends of biodiversity and its components (CBD Articles 7(b) and 25 (2a)) and the causes of biodiversity loss or effects of processes which are likely to have an adverse impact on biodiversity (CBD Articles 7(b), 14(a)) and the effectiveness of measures taken (CBD Articles 25 (b) and 26)” (Vordermayer-Riemer 2019).
To check the reliability of biodiversity reporting, 12 hypotheses were elaborated based on well-established scientific findings. These hypotheses address the relationships between national characteristics (geography and socioeconomy) and their importance for a specific biodiversity indicator category.
For hypotheses 1–6, we chose the geographic variables land area size, coastline length, geographic isolation, mean geographic latitude, and number of biogeographic regions as important factors describing the national biodiversity status. Additionally, for hypotheses 7–9, agricultural area, forest cover, and human population density were used as variables of human pressure reflecting the extent to which landscapes have been modified. For hypotheses 10–11, the gross domestic product was chosen as a proxy for per-capita income to reflect the nation’s economy. For hypothesis 12, the duration of EU membership was employed as a variable for biodiversity funding and policy.
Countries can benefit from the freedom of indicator choice by adapting their reporting and monitoring according to national state and pressure on biodiversity. We examined this by assessing whether national geographic and socioeconomic characteristics impacted the number of biodiversity indicators chosen from a particular indicator category.
If the majority of the hypotheses are verified, then indicator choice is strongly affected by state and pressure on national biodiversity. In such cases, the freedom of indicator choice would be beneficial for national reporting quality. In contrast, if the hypotheses are not verified, then the CBD reporting guidelines need to be scrutinized.
Negative binary generalized models and poisson generalized linear models
Twelve hypotheses (Table 2) address the statistical association between country characteristics (independent variables with continuous values (x), see Electronic Supplementary Material S1) and the biodiversity indicator choice in the national CBD reports (dependent variables with nonnegative integer values (y), see Electronic Supplementary Material S2).
Table 2.
Hypotheses established to test the association between country’s characteristics (x) and the biodiversity indicator choice (y) and their rationales
| Nr | Hypotheses | Rationale |
|---|---|---|
| 1 | The country´s total land area correlates with the total number of biodiversity indicators in the national monitoring systems |
Species–area relationship: Rosenzweig (1995) Niche differentiation: Connell (1980) |
| 2 | The country´s total land area correlates with endemic species as biodiversity indicators |
Endemics-area relationship: Storch et al. (2012) Minimum viable population size: Shaffer (1981) |
| 3 | The country´s total coastline correlates with the number of aquatic species indicators |
About 80% of marine species diversity occurs in the coastal zones: Ray (1991) In oceans, microbial diversity of coastal waters is about a magnitude higher than in open water: Glöckner et al. (2012) |
| 4 | The geographical isolation of a country correlates with endemic species as biodiversity indicators | Island biogeography: McArthur and Wilson (1967) |
| 5 | The mean latitude of a country correlates with the number of species indicators | Latitudinal diversity gradient: MacArthur (1972) |
| 6 | The number of Biogeographical Regions in a country correlates with the number of species indicators |
Niche differentiation: Connell (1980) Biogeographical processes influence local species composition: Ricklefs (1987), Wiens and Donoghue (2004) |
| 7 | The country´s forest cover correlates with forest structural indicators and indicators of terrestrial flora diversity |
Structural diversity is a very important group of indicators to assess forest biodiversity: McElhinny et al. (2005), Dieler (2013) Forests provide habitat for 80% of all terrestrial species: FAO (2010) |
| 8 | The country´s agricultural area correlates with the number of genetic indicators of domesticated plants and animals |
Domestication is another important facet of biodiversity. Of 5000 vertebrate species described, 30–40 birds and mammals were domesticated: Dirzo and Raven (2003) About 30% of 500 families of flowering plants contain at least one crop species: Hammond (1995) |
| 9 | The country´s population density correlates with the number of indicators related to human pressure |
Biodiversity loss is driven by human socioeconomic pressures: Naidoo and Adamowicz (2001) Biodiversity changes can be predicted by human development pressures: Waldron et al. (2017) |
| 10 | The country´s GDP (gross domestic product) correlates with genetic indicators as well as with the total number of biodiversity indicators | The GDP correlates significantly positively with number of published scientific conservation and ecological articles and research expenditure: Doi and Takahara (2016) |
| 11 | The country´s GDP correlates with endemic species as biodiversity indicators | Strong, positive correlation between number country-endemic freshwater species and GDP can be found globally: Collen et al. (2004) |
| 12 | The duration of EU membership correlates with the total number of biodiversity indicators in the national monitoring systems |
European membership requires to adopt international commitments and the expansion of conservation areas: Grodzinska-jurcazak and Cent (2010) EU Nature Directives had positive impact on EU´s biodiversity: Beresford et al. (2016) |
Following the approach of Naidoo and Adamowicz (2001), the count data derived from the national CBD reports were tested by performing regression analysis; thus, twelve hypotheses were merged into eight models sharing the same dependent variable. Nonlinear regression analysis helps predict the value of the dependent variable based on the covariates (Naidoo and Adamowicz 2001). The distribution of discrete response variables places probability mass at nonnegative integer values only. To avoid inconsistent parameter estimation through incorrect distribution expectations, we developed two types of models: Negative binary generalized models (NBGM) and a generalized linear model with poisson error structure (Poisson GLM). The assumption of NBGM, i.e., variance is a quadratic function of the mean that differs from the assumption of Poisson GLM, i.e., variance equals the mean concerning variable distribution (Naidoo and Adamowicz 2001). Thus, the model fit was rated using null deviance and theta parameter values and was compared using the likelihood ratio Chi squared distribution test (Venables and Ripley 2002). For this purpose, the R packages “foreign”, “ggplot2” and “MASS” were used. The “MASS”-package uses a system modification to include the additional parameter theta and an alternating iteration process for the NBGM calculation. Hence, the means are fixed while the parameters are computed by using score and information iterations (Ripley et al. 2019). The model fit of the data is shown by the theta parameter for NBGM and the deviation value and overdispersion parameter for Poisson GLM.
The regressions calculated using the Poisson GLM were checked for overdispersion. Overdispersion frequently occurs when using count data, i.e., the variance of the response variable exceeds the variance of the mean and may lead to an underestimation of the standard errors and therefore overestimation of the significance of the regression parameters (Cox 1983). The Poisson GLMs were tested using the R package “AER”. Approved overdispersion implies that the poisson model assumptions were not met, and the model output is not confidential (Dormann 2016). For theta as well as for the overdispersion parameter, a small parameter outcome is favorable.
Results
To determine whether European biodiversity reporting and monitoring is reliable and biodiversity conception is in line with the CBD definition, we approached the following questions.
Which kinds of biodiversity indicators are particularly prevalent?
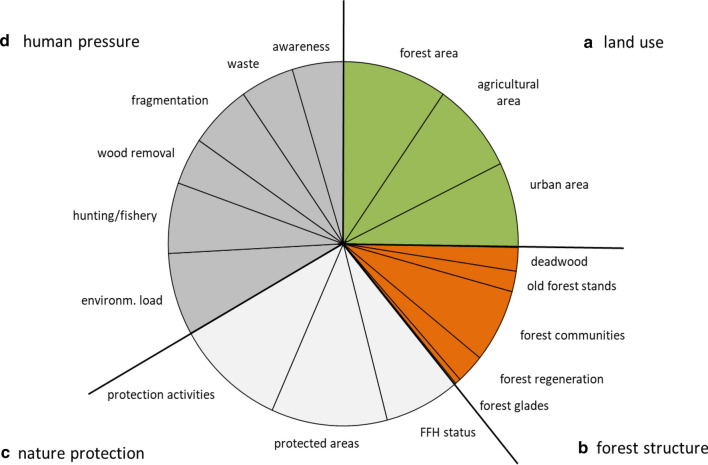
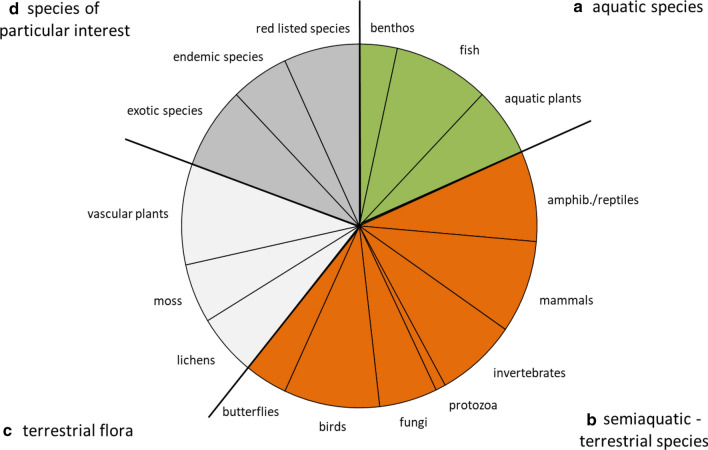
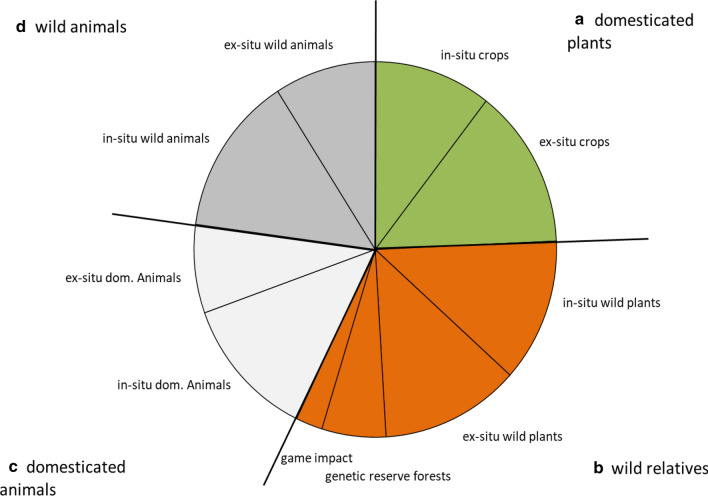
Among ecosystem indicators, “nature protection (1c)” and “human pressure (1d)” were mentioned most frequently, whereas subcategory “forest structure (1b)” indicated a low importance (Fig. 1). For species indicators, the high choice frequency of subcategory “semi aquatic-terrestrial species (2b)” was obvious, whereas “aquatic species (2a)” was of lowest importance (Fig. 2). Among genetic indicators, choice frequency was almost balanced between indicators for plant and animal genetic diversity (Fig. 3).
Fig. 1.
Indicators choice frequency for (1) ecosystem diversity indicators in European national CBD reports. Subcategories are (1a) land use, (1b) forest structure, (1c) nature protection, and (1d) human pressures
Fig. 2.
Indicators choice frequency for (2) species diversity indicators in European national CBD reports. Subcategories are (2a) aquatic species, (2b) semiaquatic-terrestrial species, (2c) terrestrial flora, and (2d) species of particular interest
Fig. 3.
Indicators choice frequency for (3) genetic diversity in European national CBD reports. Subcategories are (3a) domesticated plants, (3b) wild plants, (3c) domesticated animals, and (3d) wild animals
Are ecosystem diversity, species diversity, and genetic diversity considered adequately?
Following the CBD definition, biodiversity has three components of equal value (ecosystem diversity, species diversity, and genetic diversity). Therefore, we expected national CBD reports to mirror a quantitative balance in choice frequency among these three components.
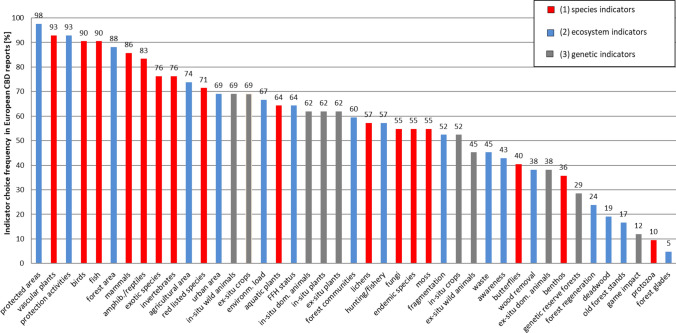
On the level of single indicators (Fig. 4), prevalent ecosystem indicators found were “protected areas” (97.6% of all reports) and “protection activities” (92.9%). In contrast, “old forest stands” (17%) and forest glades (5%) were seldom chosen.
Fig. 4.
Indicator choice frequency between (1) species indicators, (2) ecosystem indicators, and (3) genetic indicators in European CBD reports
For species indicators, namely, “vascular plant” (92.9%), “bird” (90.5%) and “fish” species diversity (90.5%) were commonly used. However, “benthos” (36%) and “protozoa” (10%) were rarely reported.
Among genetic indicators, “ex situ actions for domesticated plants” (69.1%) and “in situ actions for wild animals” (69.1%) were prevalently mentioned. Scarcely, the reports referred to “genetic reserve forests” (29%) and “game impact” (12%).
Species indicators and ecosystem indicators are generally chosen with a disproportionate frequency in comparison to genetic indicators. Our results underline that the current indicator choice in European CBD reports and national biodiversity monitoring systems consequently favors ecosystem and species diversity conservation.
Is freedom of biodiversity indicator choice in CBD reporting guidelines beneficial for biodiversity monitoring and reporting quality?
Free indicator choice may give flexibility to members to report and monitor biodiversity according to their knowledge, institutional capacities, financial abilities, and geographic and socioeconomic characteristics.
The results of NBGM and Poisson GLM are shown in Table 3. Although all hypotheses were scientifically backstopped, the variables poorly explained indicator choice. Of all models, only the Poisson GLM screening for statistical association between country size and total number of biodiversity indicators, was significant (p < 0.01). Moreover, the overdispersion parameter value of the two models could potentially have led to an overestimation of significance. As no Poisson GLM showed both significant association and overdispersion at the same time, this was of least concern.
Table 3.
Results of the negative binary generalized models (NBGM) and poisson generalized linear models (Poisson GLM)
| Dependent variable | Independent variable | NBGM | Poisson GLM | ||
|---|---|---|---|---|---|
| Significant variable | Model fit (θ) | Significant variable | Overdispersion | ||
| Total number of biodiversity indicators | Country size, gdp, access to EU | Country size (p < 0.1) | 20.9 | Country size (p < 0.01) | |
| Endemic species indicators | Country size, land vs. coast line border lenght, gdp | 22 441 | 1 | ||
| Nr. species indicators | Mean country latitude, nr. biogeographical regions | 61 361 | 0.5 | ||
| Nr. aquatic species indicators | Coast line border length | 86 722 | 0 | ||
| Nr. flora diversity plus forest structural indicators | Land use forest | Land use forest (p < 0.1) | 41 749 | Land use forest (p < 0.1) | 0.74 |
| Genetics indicators of domesticated plants and animals | Land use agriculture | Land use agriculture (p < 0.1) | 33 321 | Land use agriculture (p < 0.1) | 0.75 |
| Nr. genetics indicators | Gdp | 6.9 | |||
| Nr. indicators of human pressure on ecosystem | Population density | 27 367 | 0.6 | ||
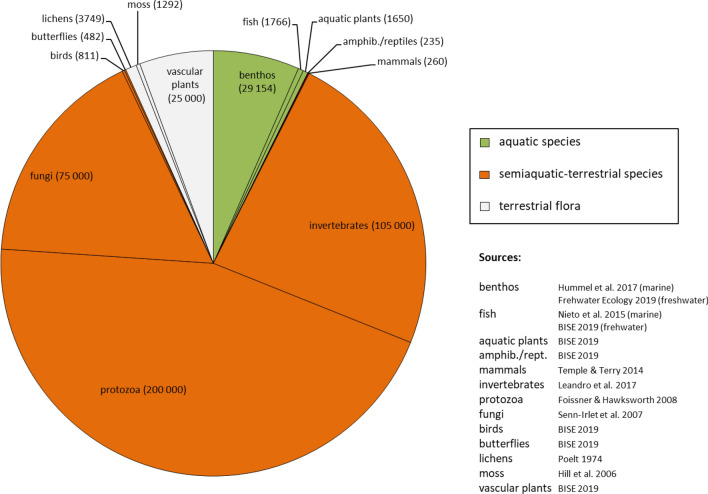
Based on these results, the reporting and monitoring are biased. Assessing the magnitude of bias for overall biodiversity was impossible in our study. Solely species diversity could be considered to roughly estimate the deviation between reporting and European species richness (Compare Fig. 2 excluding subcategory “species of particular interest” and Fig. 5). Apparently, the bulk of European species richness is not well represented through the free indicator choice. In fact, “protozoa species diversity” was the indicator chosen most rarely in our analysis (4.8%), although protozoa are the most diverse taxonomic group, with approximately 200 000 species in Europe. Concerning species of particular interest, there are 10 810 species red listed in Europe (IUCN 2019) as well as 12 221 nonnative species (Daisie 2009). The number of endemic species can be estimated to be at least 6300 species in Europe, i.e., approximately 5600 endemic vascular plant species, 436 freshwater fish, 142 butterfly, 59 mammal, 46 amphibian, and 18 dragonfly species (Bise 2019). However, the most endangered taxonomic groups are cycads (63% of species globally threatened) and amphibians (41%), whereas birds (13%) are the taxonomic group least threatened (Pareira et al. 2012).
Fig. 5.
Taxonomic species richness of Europe
Overall, biodiversity indicators reported in Europe are not statistically related to important geographic and socioeconomic characteristics. Hence, biodiversity monitoring fails to report the real status of European biodiversity. Freedom of biodiversity indicator choice in the CBD reporting guidelines is disadvantageous for European monitoring and reporting quality.
Discussion
We found the following key findings: Ecosystem diversity, species diversity, and genetic diversity are reported in an unbalanced manner. Freedom of indicator choice negatively affects the quality of biodiversity monitoring and reporting. Species diversity reporting deviates from European species richness. These results point to major gaps in CBD implementation.
Deficits in biodiversity policy implementation
International agreements and policies can only have a positive impact on combating biodiversity loss if implemented (Williams et al. 2012). Lacking robust evaluation of international conservation policies has been heavily criticized (Ferraro and Pattanayak 2006; Morgera and Tsioumani 2011). Actually, there is no internal mechanism established to monitor national-level compliance and implementation of biodiversity policies in the CBD body (Morgera and Tsioumani 2011; Vordermayer-Riemer 2019). On the one hand, missing supervisory mechanisms are a well-known problem of international legal systems, especially concerning multilateral environmental agreements (Morgera and Tsioumani 2011). On the other hand, the evaluation of biodiversity policy implementation is valuable to improve policy design and raise conservation impacts (Siebenhüner 2007).
Zisenis (2009) claimed that there are still serious CBD implementation deficits on the global, European, and national levels. For instance, national CBD reports were delivered increasingly with delay and were even not revised in the last period (CBD 2018) due to unwillingness or lacking resources (Raustiala 2000). However, steps towards further harmonization of national reports in the past were not successful (CBD 1997). On this account, the CBD now works as a pilot project on voluntary individual peer-review processes of national CBD reports in an informal working group (Ulloa et al. 2018).
Our paper contributes to the CBD post-2020 period by analyzing biodiversity indicator choices in European CBD reports and national biodiversity monitoring systems. The most prevalent biodiversity indicators were “protected areas”, “protection activities” and species diversity in vascular plants, birds, and fish. The diversity of ecosystems and species is overrepresented, whereas genetic diversity tends to be neglected.
Key functions of genetic diversity are recognized and anchored in the CBD definition of biodiversity. Our findings are in line with Laikre et al. (2010), who stated that genetic diversity on the national level is still not being monitored and indicators and thresholds are missing. CBD policies concerning genetic diversity lag behind implementation for other levels of biodiversity, although knowledge of conservation genetics, molecular genetics and statistical tools is available (Fussi et al. 2016; Hunter et al. 2018).
Reasons for this discrepancy between ecosystem and species diversity vs. genetic diversity could be easier data access (data already compiled, e.g., Natura 2000, European Environmental Agency), higher public and media interest (flagship species, lighthouse projects) or higher economic interest in specific data (national forest inventory, species relevant to hunting and fishery, exotic species). Lower public, governmental and media interest may also explain why monitoring systems for genetic diversity are not yet established in most countries. Additionally, for the genetic level of biodiversity, reporting obligations of the European Union and public funding are probably lower while at the same time demanding scientific expertise for developing and supervising such programs are needed. Overall, the joint European conception of the term biodiversity and even the focus of national conservation policies may differ from the CBD definition.
Our evaluation supports the finding that some species groups and certain ecosystems seem to be arbitrarily preferred in European nature conservation policies (Zisenis 2009; Cardoso et al. 2011). For species diversity, we demonstrate that the way species are actually monitored does not reflect the majority of European taxonomic species richness. This is an alarming finding indicating that the status and trends in European species diversity reported are not reliable. Instead, the majority of the funding for monitoring and conservation actions is probably invested in gaining knowledge about a small number of species showing comparably low taxonomic diversity (vascular plants, bird, and fish species diversity). In contrast, major parts of species diversity in Europe (fungi, protozoa, and invertebrates) are neither monitored nor directly protected. In fact, protozoan species diversity was the indicator chosen most rarely in our analysis, even if it is the most diverse taxonomic group in Europe. This may not only be a European issue. Merely 20–30% of global soil protozoa diversity has been scientifically described (Foissner 1997). Limited knowledge about species hinders adequate management to halt the ongoing loss of this level of biodiversity.
Towards closing the gap between science and policy
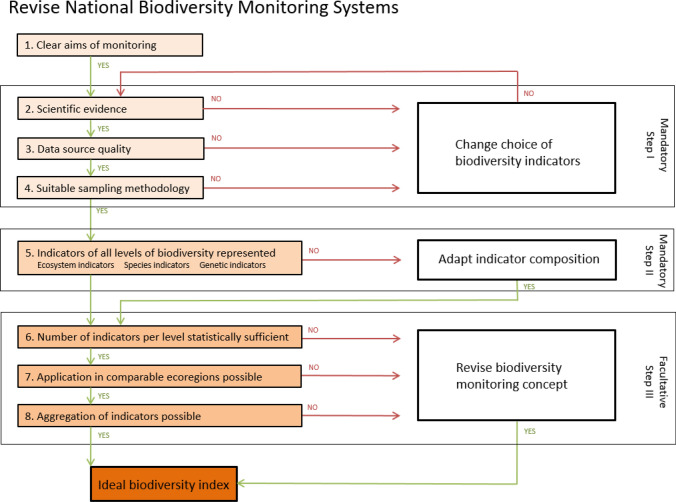
A need for a clear framework containing a limited number of criteria to assess biodiversity has often been claimed (e.g., Newton and Kapos 2002), as indicators are essential for effective management (CBD 1997). Countries differ strongly in geography, size, natural landscapes, and climate, which impedes a common indicator set. Nevertheless, as a first step, we would like to propose a comprehensive flow chart to revise national biodiversity monitoring systems in accordance with the CBD guidelines (Fig. 6). Step I and Step II are the minimum requirements, which should be fulfilled mandatorily by all CBD signatory countries. To further enhance the comparability and quality of the national monitoring systems, Step III could be used in a facultative manner.
Fig. 6.
Flow chart guiding to the revision of national biodiversity monitoring systems according to the CBD. Step I and step II should mandatory be fulfilled by all countries, whereas step III could be applied facultatively to further enhance quality of biodiversity monitoring and reporting
Clearly defined aims are the most elemental attribute of biodiversity monitoring (Pocock et al. 2015). Next, the first preliminary step includes identifying biodiversity indicators in line with CBD requirements (CBD 1997). Suitable indicators need scientific evidence for their indicative value, stable properties, and ecosystem and policy relevance; (2), are quantitatively provided by reliable data sources (3) and are sensitive, achievable, and assessable based on their field sampling methodology (4).
Step II ensures that, according to the CBD definition, (5) all levels of biodiversity (ecosystem, species, and genetic diversity) are considered in the national monitoring.
Step III reveals options of harmonization of national monitoring systems to effectively halt the loss of biodiversity. The number of indicators per level of biodiversity should be statistically large enough (6), so random deviation of one indicator may not lead to major misinterpretations. The monitoring system should be transferable to comparable ecoregions, e.g., whole biogeographic regions (7). Applying an aggregation scheme for indicators used (8), decision makers would gain an ideal biodiversity index as a reliable basis for biodiversity policy implementation.
Conclusion
European national reports differ heavily in elaborateness and are often of limited informative value. While information about status and trends in biodiversity in a country remains very vague, overall biodiversity indicator choice is misleading. For the international community, it is impossible to compare country performance and advancements towards the CBD based on national reports. Resources spent on CBD reporting and monitoring could be used more efficiently. Originating from the analysis of all European CBD reports, we would like to recommend harmonization of national reports through a core set of indictors per biogeographical region. They may be reported long term in a table format to easily detect trends in biodiversity based on high scientific evidence. Species indicators should align to domestic taxonomic richness, whereas ecosystem indicators should reflect major landscape elements and determinants for biodiversity. Moreover, applicable quantitative genetic indicators for all member states need to be defined.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Electronic supplementary material 1 (PDF 303 kb)
Acknowledgements
We highly appreciate the funding for our research by the European Union and the work of two anonymous reviewers.
Biographies
Jana-Sophie Ette
is doctoral candidate at the Austrian Federal Research Centre for Forests, Natural Hazards and Landscape. Her research interest includes biodiversity assessment, biodiversity indicators, and biodiversity management.
Thomas Geburek
is head of the Department of Forest Genetics at the Austrian Federal Research Centre for Forests, Natural Hazards and Landscape and professor at the University of Natural Resources and Life Sciences Vienna. His research interest includes applied genetic research and genetic diversity management in forest ecosystems.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jana-Sophie Ette, Email: sophie.ette@bfw.gv.at.
Thomas Geburek, Email: thomas.geburek@bfw.gv.at.
References
- Allendorf FW, Luikart GH, Aitken SN. Conservation and the genetics of populations. Hoboken: Wiley-Blackwell; 2012. [Google Scholar]
- Beresford AE, Graeme M, Buchanan F, Sanderson J, Jefferson R, Donald PF. The contributions of the EU nature directives to the CBD and other multilateral environmental agreements. Conservation Letters. 2016;9:479–488. [Google Scholar]
- BISE. 2019. Online database. Retrieved 27 August, 2020, from https://biodiversity.europa.eu/topics/species.
- Bruchmann, I. 2011. Plant endemism in Europe: Spatial distribution and habitat affinities of endemic vascular plants. PhD thesis. Flensburg, Germany: Flensburg University.
- Cardoso P, Erwin TL, Borges PAV, New TR. The seven impediments in invertebrate conservation and how to overcome them. Biological Conservation. 2011;144:2647–2655. [Google Scholar]
- CBD. 1992. The convention on biological diversity. https://www.cbd.int/convention/. Accessed 27 August, 2020
- CBD. 1997. Recommendations for a core set of indicators of biological diversity-Background paper. Subsidiary body on scientific, technical and technological advice, liaison group on indicators of biological diversity. UNEP/CBD/SBSTTA/3/Inf.13, Montreal, Canada.
- CBD. 2006. Global biodiversity outlook 2. A report by the secretariat of the convention on biological diversity, Montréal, Canada. https://www.cbd.int/doc/gbo/gbo2/cbd-gbo2-en.pdf. Accessed 28 August 2020.
- CBD. 2012. Strategic plan 2011-2020: Target 19. https://www.cbd.int/sp/targets/rationale/target-19/. Accessed 27 August 2020.
- CBD. 2014. Global biodiversity outlook 4. A report by the secretariat of the convention on biological diversity, Montréal, Canada. https://www.cbd.int/gbo/gbo4/publication/gbo4-en.pdf. Accessed 27 August 2020
- CBD. 2018. Update on progress in revising/updating and implementing national biodiversity strategies and action plans, including national targets. Subsidiary body on implementation, Note by the Executive Secretary. CBD/SBI/2/2/Add.1, Montreal, Canada.
- Ceballos G, Garcia A, Ehrlich PR. The sixth extinction crisis: Loss of animal populations and species. Journal of Cosmology. 2010;8:1821–1831. [Google Scholar]
- Ceballos G, Ehrlich PR, Barnosky AD, García A, Pringle RM, Palmer TM. Accelerated modern human–induced species losses: Entering the sixth mass extinction. Science Advances. 2015;1:5p. doi: 10.1126/sciadv.1400253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collen B, Whitton F, Dyer EE, Baillie JEM, Cumberlidge N, Darwall WRT, Pollock C, Richman NI, et al. Global patterns of freshwater species diversity, threat and endemism. Global Ecology and Biogeography. 2014;23:40–51. doi: 10.1111/geb.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connell JH. Diversity and the coevolution of competitors, or the ghost of competition past. Oikos. 1980;35:131–138. [Google Scholar]
- Cook-Patton SC, McArt SH, Parachnowitsch HL, Thaler JS, Agrawal AA. A direct comparison of the consequences of plant genotypic and species diversity on communities and ecosystem function. Ecology. 2011;92:915–923. doi: 10.1890/10-0999.1. [DOI] [PubMed] [Google Scholar]
- Cox DR. Some remarks on overdispersion. Biometrika. 1983;70:269–274. [Google Scholar]
- DAISIE . Handbook of alien species in Europe. Dordrecht: Springer; 2009. [Google Scholar]
- Dieler J. 2013. Biodiversity and forest management: Effects on species diversity, structural diversity and productivity. DVFFA, Sektion Ertragskunde: Beiträge zur Jahrestagung 2013, Rychnov nad Kneznou, Czech Republic (in German).
- Dirzo R, Raven PH. Global state of biodiversity and loss. Annual Review of Environment and Resources. 2003;28:137–167. [Google Scholar]
- Doi H, Takahara T. Global patterns of conservation research importance in different countries of the world. PeerJ. 2016;4:e2173. doi: 10.7717/peerj.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dormann, C.F. 2016. Overdispersion and how to deal with it in R and JAGS. https://biometry.github.io/APES/LectureNotes/2016-JAGS/Overdispersion/OverdispersionJAGS.pdf. Accessed 27 August 2020
- FAO. 2010. Global forest resources assessment 2010: Main report. Food and Agriculture Organization of the United Nations, FAO Forestry Paper 163, Rome, Italy.
- Ferraro PJ, Pattanayak SK. Money for nothing? A call for empirical evaluation of biodiversity conservation investments. PLoS Biology. 2006;4:482–488. doi: 10.1371/journal.pbio.0040105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foissner W. Global soil ciliate (Protozoa, Ciliophora) diversity: A probability-based approach using large sample collections from Africa, Australia and Antarctica. Biodiversity and Conservation. 1997;6:1627–1638. [Google Scholar]
- Foissner W, Hawksworth D. Protist diversity and geographical distribution: Topics in biodiversity and conservation, Book VIII. Heidelberg: Springer; 2008. [Google Scholar]
- Freshwater Ecology. 2019. Online database. https://www.freshwaterecology.info/.
- Fussi B, Westergren M, Aravanopoulos F, Baier R, Kavaliauskas D, Finzgar D, Alizoti P, Bozic G, et al. Forest genetic monitoring: An overview of concepts and definitions. Environmental Monitoring and Assessment. 2016;188:12. doi: 10.1007/s10661-016-5489-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glöckner, F.O., L.J. Stal, R.A. Sandaa, J.M. Gasol, F. O’Gara, F. Hernandez, M. Labrenz, and E. Stoica. 2012. Marine microbial diversity and its role in ecosystem functioning and environmental change. Marine Board Position Paper 17, ed. Calewaert, J.B., and McDonough N. Marine Board-ESF, Ostend, Belgium.
- Grodzinska-Jurczak M, Cent J. Expansion of nature conservation areas: Problems with natura 2000 implementation in Poland? Environmental Management. 2010;47:11–27. doi: 10.1007/s00267-010-9583-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond PM. The current magnitude of biodiversity. In: Heywood VH, editor. Global biodiversity assessment. Cambridge: Cambridge University Press; 1995. pp. 113–138. [Google Scholar]
- Hill MO, Bell N, Bruggeman-Nannenga MA, Brugués M, Cano MJ, Enroth J, Flatberg KI, Frahm J-P, et al. Bryological monograph: An annotated checklist of the mosses of Europe and Macaronesia. Journal of Bryology. 2006;28:198–267. [Google Scholar]
- Hummel, H., P. Van Avesaath, S. Wijnhoven, L. Kleine-Schaars, S. Degraer, F. Kerckhof, N. Bojanic, S. Skejic, et al. 2016. Geographic patterns of biodiversity in European coastal marine benthos. Journal of the Marine Biological Association of the United Kingdom.
- Hunter ME, Hoban SM, Bruford MW, Segelbacher G, Bernatchez L. Next-generation conservation genetics and biodiversity monitoring. Evolutionary Applications. 2018;11:1029–1034. doi: 10.1111/eva.12661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IUCN. 2019. https://www.iucn.org/regions/europe/our-work/species/european-red-list-threatened-species. Accessed 27 August 2020.
- IPBES. 2019. Global assessment report on biodiversity and ecosystem services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services. ed. Brondizio, E.S., J. Settele, S. Díaz, and H.T. Ngo. IPBES Secretariat, Bonn, Germany.
- Laikre L, Allendorf FW, Aroner LC, Baker CS, Gregovich DP, Hansen MM, Jackson JA, Kendall KC, et al. Neglect of genetic diversity in implementation of the convention on biological diversity. Conservation Biology. 2010;24:86–88. doi: 10.1111/j.1523-1739.2009.01425.x. [DOI] [PubMed] [Google Scholar]
- Leandro C, Jay-Robert P, Vergnes A. Bias and perspectives in insect conservation: A European scale analysis. Biological Conservation. 2017;215:213–224. [Google Scholar]
- MacArthur RH, Wilson EO. The theory of island biogeography. Princeton: Princeton University Press; 1967. [Google Scholar]
- MacArthur RH. Geographical ecology: Patterns in the distribution of species. Princeton: Princeton University Press; 1972. [Google Scholar]
- McElhinny C, Gibbons P, Brack C, Bauhus J. Forest and woodland stand structural complexity: Its definition and measurement. Forest Ecology and Management. 2005;218:1–24. [Google Scholar]
- Morgera E, Tsioumani E. Yesterday, today and tomorrow: Looking afresh at the convention on biological diversity. Yearbook of International Environmental Law. 2011;21:3–40. [Google Scholar]
- Naidoo R, Adamowicz WL. Effects of economic prosperity on numbers of threatened species. Conservation Biology. 2001;15:1021–1029. [Google Scholar]
- Newbold T, Hudson LN, Hill SLL, Contu S, Lysenko I, Senior RA, Börger L, Bennett DJ, et al. Global effects of land use on local terrestrial biodiversity. Nature. 2015;520:45–50. doi: 10.1038/nature14324. [DOI] [PubMed] [Google Scholar]
- Newton AC, Kapos V. Biodiversity indicators in national forest inventories. Unasylva. 2002;210:56–75. [Google Scholar]
- Nieto A, Ralph GM, Comeros-Raynal MT, Kemp J, Garcia Criado M, Allen DJ, Dulvy NK, Walls RHL, et al. European Red List of marine fishes. Luxembourg: Publications Office of the European Union; 2015. [Google Scholar]
- Pareira HM, Navarro LM, Santos Martins I. Global biodiversity change: The bad, the good, and the unknown. Annual Review of Environment and Resources. 2012;37:25–50. [Google Scholar]
- Pocock MJO, Newson SE, Henderson IG, Peyton J, Sutherland WJ, Noble DG, et al. Developing and enhancing biodiversity monitoring programmes: A collaborative assessment of priorities. Journal of Applied Ecology. 2015;52:686–695. doi: 10.1111/1365-2664.12423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poelt J. Identification key of European lichens. Vaduz: Cramer Publishers; 1974. [Google Scholar]
- Ricklefs RE. Community diversity: Relative roles of local and regional processes. Science. 1987;235:167–171. doi: 10.1126/science.235.4785.167. [DOI] [PubMed] [Google Scholar]
- Ripley, B., B. Venables, D.M. Bates, K. Hornik, A. Gebhardt, and D. Firth. 2019. The MASS-package: Functions and datasets to support Venables and Ripley, ``Modern Applied Statistics with S’’. https://cran.r-project.org/web/packages/MASS/MASS.pdf. Accessed 27 August 2020.
- Raustiala K. Compliance & effectiveness in international regulatory cooperation. Case Western Reserve Journal of International Law. 2000;32:387–440. [Google Scholar]
- Ray GC. Coastal zone biodiversity patterns. BioScience. 1991;41:490–498. [Google Scholar]
- Rockström J, Steffen W, Noone K, Persson Å, Chapin FS, Lambin E, Lenton TM, Scheffer M, et al. Planetary boundaries: Exploring the safe operating space for humanity. Ecology and Society. 2009;14:32. doi: 10.1038/461472a. [DOI] [PubMed] [Google Scholar]
- Rosenzweig ML. Species diversity in space and time. Cambridge: Cambridge University Press; 1995. [Google Scholar]
- Sala OE, Chapin FS, Armesto JJ, Berlow E, Bloomfield J, Dirzo R, Huber-Sanwald E, et al. Global biodiversity scenarios for the year 2100. Science. 2000;287:1770–1774. doi: 10.1126/science.287.5459.1770. [DOI] [PubMed] [Google Scholar]
- Senn-Irlet B, Heilmann-Clausen J, Genney D, Dahlberg A. Guidance for conservation of macrofungi in Europe. Strasbourg: Report for the European Council for Conservation of Fungi; 2007. [Google Scholar]
- Shaffer ML. Minimum population sizes for species conservation. BioScience. 1981;31:131–134. [Google Scholar]
- Siebenhüner B. Administrator of global biodiversity: the secretariat of the convention on biological diversity. Biodiversity Conservation. 2007;16:259–274. [Google Scholar]
- Storch D, Keil P, Jetz W. Universal species-area and endemics-area relationships at continental scales. Nature. 2012;488:78–81. doi: 10.1038/nature11226. [DOI] [PubMed] [Google Scholar]
- Temple HJ, Terry A. The status and distribution of European mammals. Luxembourg: Office for Official Publications of the European Communities; 2007. [Google Scholar]
- Tittensor DP, Walpole M, Hill SLL, Boyce DG, Britten GL, Burgess ND, Butchart SH, Leadley PW, et al. A mid-term analysis of progress toward international biodiversity targets. Science. 2014;346:241–244. doi: 10.1126/science.1257484. [DOI] [PubMed] [Google Scholar]
- Ulloa AM, Jax K, Karlsson-Vinkhuyzen SI. Enhancing implementation of the convention on biological diversity: A novel peer-review mechanism aims to promote accountability and mutual learning. Biological Conservation. 2018;217:371–376. [Google Scholar]
- Vordermayer-Riemer M. The (missing) monitoring mechanism of the biodiversity convention: The CBD in the context of international environmental law. BfN-Skripten. 2019;527:113–118. [Google Scholar]
- Venables WN, Ripley BD. Modern applied statistics with S: Statistics and computing. New York: Springer; 2002. [Google Scholar]
- Waldron A, Miller DC, Redding D, Mooers A, Kuhn TS, Nibbelink N, Roberts JT, Tobias JA, et al. Reductions in global biodiversity loss predicted from conservation spending. Nature. 2017;551:364–367. doi: 10.1038/nature24295. [DOI] [PubMed] [Google Scholar]
- Wiens JJ, Donoghue MJ. Historical biogeography, ecology, and species richness. Trends in Ecology & Evolution. 2004;19:639–644. doi: 10.1016/j.tree.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Williams SJ, Jones JPG, Clubbe C, Sharrock S, Gibbons JM. Why are some biodiversity policies implemented and others ignored? Lessons from the uptake of the Global Strategy for Plant Conservation by botanic gardens. Biodiversity Conservation. 2012;21:175–187. [Google Scholar]
- Zisenis M. To which extent is the interdisciplinary evaluation approach of the CBD reflected in European and international biodiversity-related regulations? Biodiversity Conservation. 2009;18:639–648. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Electronic supplementary material 1 (PDF 303 kb)