Abstract
Urbanization has rapidly increased in recent decades and the negative effects on biodiversity have been widely reported. Urban green areas can contribute to improving human well-being, maintaining biodiversity, and ecosystem services (e.g. pollination). Here we examine the evolution of studies on plant–pollinator interactions in urban ecosystems worldwide, reviewing also research funding and policy actions. We documented a significant increase in the scientific production on the theme in recent years, especially in the temperate region; tropical urban ecosystems are still neglected. Plant–pollinator interactions are threatened by urbanization in complex ways, depending on the studied group (plant or pollinator [generalist or specialist]) and landscape characteristics. Several research opportunities emerge from our review. Research funding and policy actions to pollination/pollinator in urban ecosystems are still scarce and concentrated in developed countries/temperate regions. To make urban green spaces contribute to the maintenance of biodiversity and the provision of ecosystem services, transdisciplinary approaches (ecological–social–economic–cultural) are needed.
Electronic supplementary material
The online version of this article (10.1007/s13280-020-01410-z) contains supplementary material, which is available to authorized users.
Keywords: Ecosystem service, Pollination, Scientometrics, Urban ecology, Urban green areas, Urbanization
Introduction
Pollination is directly related to the persistence and maintenance of biodiversity for both plant and associated animals, thus representing an important ecosystem function (e.g. Potts et al. 2010; Ollerton et al. 2011; IPBES 2016). In addition, pollination is a key ecosystem service for agricultural production, as about 35% of world crops rely on animal pollination (IPBES 2016; Potts et al. 2016), and about three-quarters of major food crops in the world depend to some extent on animal pollination services, either to ensure volume or quality of production (Klein et al. 2007; IPBES 2016).
Currently, cities are growing about twice as fast as the urban population itself (Seto et al. 2013). This growth may be due to four synergistic factors: natural growth, migration from rural to urban areas, migration due to extreme events (e.g. drought) and redefinition of administrative boundaries (Seto et al. 2013). Many cities around the world are usually surrounded by a green belt, with varying lengths, retaining agricultural or wild lands. Urbanization has negatively influenced biodiversity (McKinney 2002, 2006, 2008), through the fragmentation and destruction of natural habitats, introduction of non-native species, modification of natural events and changes in ecosystem processes (Müller et al. 2013). These effects occur due to rapid changes in landcover which lead to the functional impoverishment of the ecosystem (Tratalos et al. 2006). On the other hand, cities create social, economic and biological conservation opportunities, and promote the development of culture, art and education (e.g. McKinney 2002; Müller et al. 2013). When managed properly, urban green areas offer, thus, opportunities for maintaining biodiversity and act, therefore, as source areas for ecosystem services.
Urbanization has altered the dynamics of interactions between plants and pollinators around the world (Verboven et al. 2012; Harrison and Winfree 2015). While some studies in urban green areas document decreases in pollinator richness and abundance (e.g. Bergerot et al. 2010; Bates et al. 2011; Hamblin et al. 2018), others indicate beneficial effects of urbanization, such as contributing to increase in richness of plant and animal species (e.g. McKinney 2008; Baldock et al. 2015; Hall et al. 2016). This increase in pollinator richness has been reported exclusively for urban green spaces in temperate regions and is directly associated with the use of non-native plant species and species in which the flowering period overlaps in urban green spaces, especially in gardens and green roofs (e.g. Fetridge et al. 2008; Tonietto et al. 2011). However, even in temperate regions, urbanization can negatively affect plant–pollinator interactions by decreasing: (a) the number and diversity of interactions, (b) the frequency of visits by specialist pollinators, such as coleopterans, Sirphidae (Geslin et al. 2013) and solitary bees (Tonietto et al. 2011), (c) and consequently, the reproductive success of plant species (Verboven et al. 2012; Geslin et al. 2013). Thus, it is possible that the effects of urbanization on plant communities and pollinators may differ between regions and climates and depend on local species composition.
Urban green areas/spaces consist of any type of vegetation (public or private) inserted in an urban matrix and comprise a range of formats and sizes including squares, parks, public walkways, green roofs, and gardens (Kabisch and Haase 2012; Boulton et al. 2018). Urban green areas can contribute to human well-being (Cox et al. 2018), benefiting quality of life (e.g. air quality improvement, temperature reduction, humidity regulation, which contribute to reduction of diseases) (e.g. Zhang et al. 2017) and improve biodiversity, since they can act as ecological corridors by connecting natural vegetation remnants (e.g. Peng et al. 2017). In this context, urban ecosystems can sustain plant and animal populations (e.g. Barton and Pretty 2010; Elmqvist et al. 2015), representing a source of resources for pollinators (Aleixo et al. 2014; Baldock et al. 2015; Siemaszko and Zych 2017), frugivores (Pufal and Klein 2015), herbivores (e.g. Morón et al. 2017), scavengers (Bonnington et al. 2013) and omnivores (François et al. 2008). In addition, these areas also help in the educational process, through the understanding of nature (Cox et al. 2018). Thus, considering the rapid expansion of cities, urban green areas are elements that can, to a certain extent, mitigate the effects of the conversion of natural ecosystems to human modified landscapes, and therefore have great importance as a source area for the provision of ecosystem services (Wang et al. 2019) and global biodiversity conservation (e.g. Frankie et al. 2005).
Much of what urban green spaces provide is mainly due to trees that offer environmental, economic, social, and cultural benefits. These benefits involve: support climate change mitigation and adaptation (they can reduce local air temperature by between 2 and 8 °C), filter for urban pollutants, help protect water sources and contribute to the treatment of wastewater, play an important role in local biodiversity conservation, by providing habitat to plants and animals (environmental benefits), contribute to food and nutrition security, mainly due to urban agriculture, increase cities’ resilience to severe weather events, alleviate poverty (e.g. creating job opportunities such as in gardening, agriculture), increase property value by 20% and attract tourists (economic and livelihood benefits), create recreational, cultural and social opportunities, and positively impact physical and mental health (social and cultural benefits) (FAO 2014, 2018).
Plant–pollinator interactions in urban ecosystems was theme of only three literature reviews, which discusses the strengths and weaknesses of garden plant lists (in the United Kingdom, North America and Western Europe) to help pollinators (Garbuzov and Ratnieks 2014), review the mechanistic pathways through which urban drivers modify plant–pollinator interactions, basically in the temperate regions (Harrison and Winfree 2015) and, more recently, review how urbanization is driving insect pollinator diversity and pollination (Wenzel et al. 2020). These three reviews are distinct but none of them addresses: (1) a robust analysis of the evolution of the topic (scientometrics); (2) plant–pollinator interactions as ecological process, (3) in which type of urban green spaces the plants and pollinators are found, (4) other pollinator group than insects, (5) research funding/grants and (6) policy documents on the topic.
Here we examine the evolution of studies on plant–pollinator interactions in urban ecosystems worldwide, present the state of the art, knowledge gaps, opportunities and future directions, as well as the research funding/grants and policy documents related to plant–pollinator interactions in urban green spaces. In synthesis, we evaluate the main impacts of urbanization on plant–pollinator interactions and emphasize how urban green spaces act as opportunities for conservation/maintenance of biodiversity, seeking to answer some key questions: What is the global status of studies on plant–pollinator interactions in urban ecosystems? Which is the most studied region (temperate or tropical)? What are the most studied pollination vectors? What are the effects of urbanization on the studied organisms? What do we know about plant–pollinator interactions in tropical urban ecosystems? What is the global status of research funding and what are the policy actions on plant–pollinator interactions to urban ecosystems?
Methods
Scientometrics of studies on plant–pollinator interactions in urban ecosystems
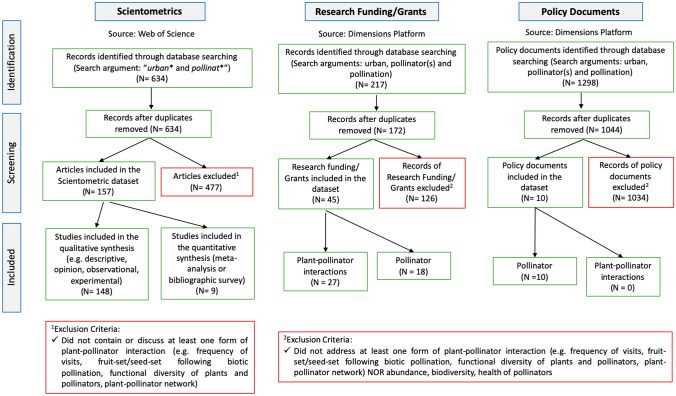
A bibliographical search was carried out in the ISI Web of Science—Science Citation Index Expanded platform (www.webofknowledge.com) (last accessed on February 28, 2020) with the following search argument: "urban* and pollinat*" appearing in the title, keywords and/or abstract. After searching and sorting, we kept articles dealing with plant–pollinator interactions in urban ecosystems, including diverse urban green spaces in temperate and tropical regions, such as: pollinators and plants they visited, frequency of pollinator visits to flowers, functional diversity of plants and pollinators, reproductive success of plants following biotic pollination, and plant–pollinator network (see Fig. 1 for details). We excluded from our database articles that dealt exclusively with subjects or aspects indirectly associated with pollination, such as: (a) genetic and/or physiological approaches, (b) other interactions such as herbivory, dispersion, ant protection, parasitism, (c) pollen-related allergy, (d) pollen "rain", (e) nesting sites, (f) plant lists, (g) animal lists (even if it was a checklist of bees or other pollinator agent), (h) phenology of plants or animals, (i) not conducted in urban areas and (j) conducted in natural forest remnants located in the urban perimeter (see Fig. 1 for details). Figure 1 is based on The PRISMA Statement: Preferred Reporting Items for Systematic Reviews and Meta-Analyses (sensu Moher et al. 2009; www.prisma-statement.org.).
Fig. 1.
Flow diagram representing the body of literature reviewed, research funding/grants and policy documents on plant–pollinator interactions in urban ecosystems, which were considered in this study (including counts of sources and exclusion criteria used to filter the body of literature, research funding/grants and policy documents into the final dataset reviewed). Search argument for the scientometric analysis in the Web of Science: “urban* and pollinat*”; search arguments for Dimensions Platform: urban, pollinator(s) and pollination. This diagram is based on The PRISMA Statement: Preferred Reporting Items for Systematic Reviews and Meta-Analyses (sensu Moher et al. 2009; www.prisma-statement.org.)
Our final database included 13 scientometric categories to evaluate the progress of the topic. These categories refer to different items in each article, such as: publication year, authors, journal, methodology, effects of urbanization, further detailed in Table 1. The scientometric analyses were summarized in graphs to identify the global scientific development of the theme and trends in publications.
Table 1.
Information and categories selected for the scientometric analysis
(Adapted from Viana et al. 2012)
| Items for analysis | Categories |
|---|---|
| 1. Publication year | – |
| 2. Corresponding author name | – |
| 3. Corresponding author country | – |
| 4. Co-author names | – |
| 5. Article title | – |
| 6. Journal title | – |
| 7. Country of the study area | – |
| 8. Ecoregion/Climatic zone where the study was carried out | 1. tropical, 2. subtropical, 3. temperate, 4. Mediterranean, 5. not applicable |
| 9. Nature of the method | 1. descriptive, 2. review, 3. observational (sampling), 4. experimental, 5. meta-analysis, 6. opinion |
| 10. Type of urban green space | 1. park, 2. garden (public and/or private), 3. road and/or sidewalk and/or flowerbed, 4. green roof, 5. university campus. 6. cemetery, 7. more than one category, 8. urbanization gradient, 9. others |
| 11. Plant origin | 1. native, 2. exotic, 3. invasive, 4. endemic, 5. not specified |
| 12. Pollination vector | 1.bees, 2. other insects, 3. bats, 4. birds, 5. several groups of pollinators, 6. not specified |
| 13. Effect of urbanization | 1. positive, 2. negative, 3. neutral, 4. more than one effect*, 5. not applicable |
Articles were surveyed in the ISI Web of Science—Science Citation Index Expanded platform (www.webofknowledge.com) with the following search argument: "urban* and pollinat*" appearing in the title, keywords and/or abstract
*For example: an increase in pollinator visits to flowers, but a decrease in reproductive success (fruit-set), in a plant species with genetic self-incompatibility
Review of studies on plant–pollinator interactions in urban ecosystems
The focus of the review was to evaluate the influence of urbanization on the pollination of plant species (i.e. plant–pollinator interactions) occurring in urban green areas. Urban green areas were classified in: university campus, gardens (public and/or private), parks, green roofs, and roads and/or public walkways (Table 1). In addition, information on the study focus (plant, pollinator or plant–pollinator interactions), plant origin (when reported in the study), pollination vector and effect of urbanization (see Table 1 for more information) were verified.
Research funding/grants and policy documents related to plant–pollinator interactions or to pollination and pollinators in urban ecosystems
In order to review research funding and policy documents related to plant–pollinator interactions or to pollination and pollinators in urban ecosystem worldwide, we used a database provided by Dimensions Platform (https://www.dimensions.ai/). The Dimensions platform is an inter-linked research information system provided by Digital Science, which focuses on the broader set of use cases, that goes beyond the standard publication-citation (research articles and their citations, books). This platform includes publication (more than 106 million), alternative metrics, grants, patents, clinical trials and policy documents with a standard set of research classifications via machine-learning techniques (Hook et al. 2018). Dimensions has gathered, cleaned and rendered unambiguous a global database, which check all sources on grant data for new data monthly.
Data on research funding/grants and policy documents were searched by combining the keywords: pollinator and urban, pollination and urban. The search was conducted in March 2020 and included all research funded and policy documents available in the platform (from 1962 for policy documents and from 1994 for research funding). All research funding records and policy documents were individually checked for duplicates. We expanded the inclusion criteria and considered research funding and policy documents that addressed pollinator (e.g. abundance, diversity and pollinator health) and plant–pollinator interactions (including pollination process as proxy to interaction) in urban ecosystems as the main subject of the funded project. For policy documents we considered documents that addressed conservation, maintenance and improvement of plant–pollinator interactions or pollinator in urban ecosystems (see Fig. 1 for details). The inclusion and exclusion criteria are described in Fig. 1. For the construction of maps with scientific publications, research funding and policy documents we grouped these data into two categories represented by countries with developed and developing economies according to the United Nations (2019).
Results and discussion
Scientometrics of studies on plant–pollinator interactions in urban ecosystems
Our initial bibliographical search returned 634 articles, published from January 1945 to February 2020, of which 157 were selected for analyzing the scientometrics of studies on plant–pollinator interactions in urban ecosystems (Supplementary Material Table S1). After sorting and excluding studies that were not directly related to the topic of our study, we observed that the first article investigating plant–pollinator interactions in urban areas was published in 1997. Articles on this topic were published in 19 of the 24 years since then, with a five-year gap without any publications (1998–2002) consecutive to the first publication. In total, 75.8% of the analyzed articles were published between 2012 and 2019, indicating a recent increase trend in publications on this topic worldwide. This increase contains three peaks of publications, in 2014, 2018 and 2019, in which 21, 21 and 20 articles were published, respectively. In the period between 1997 and 2011, we observed an average of 3.5 published articles/year (Fig. 2).
Fig. 2.
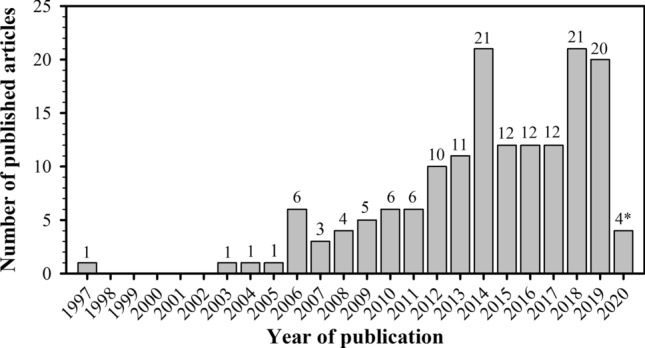
Growth of research effort through time on plant–pollinator interactions in urban ecosystems. Search was carried out in the Web of Science with the argument “urban* and pollinat*”, returning 634 articles, 157 of which were related to this topic and included in our database. *Number of articles for 2020 refers only to January and February
During these 24 years, articles documenting plant–pollinator interactions in urban ecosystems worldwide were published in 79 scientific journals (Supplementary Material Table S1); 53 of these scientific journals published only one article related to the topic, these journals are not restricted to urban ecology (Supplementary Material Table S2). The scientific journals that published more on plant–pollinator interactions in urban ecosystems were Urban Ecosystems (15 articles), Landscape and Urban Planning and Biological Conservation (10 articles each) (Fig. S1). However, only the journals Urban Ecosystems and Landscape and Urban Planning focus exclusively on urban green spaces dynamics and together accounted for 31.6% (N = 25) of the registered articles. These findings reveal that scientific production on plant–pollinator interactions in urban ecosystems is recent and still largely neglected. This pattern is reinforced by the fact that most scientific publications are published in journals not primarily focused in urban ecology.
The three most cited articles on plant–pollinator interactions ecology in urban ecosystem [1. Winfree et al. (2007), published in Conservation Biology, 2. Parker (1997), Ecology, and 3. McFrederick and LeBuhn (2006), Biological Conservation; Supplementary Material Table S1] investigated several aspects of plant–pollinator interactions, all of them in temperate urban ecosystems, such as pollination outcomes, pollinator behavior and the role of temperate urban green spaces in maintaining species of plants and pollinators. These articles also reveal the main branches on which the current knowledge on plant–pollinator interactions in urban systems has been developed, as discussed below.
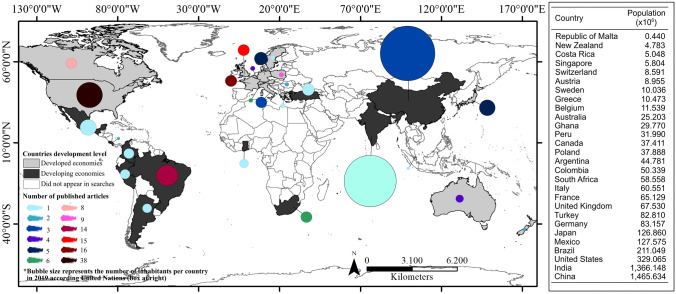
The authors of the 157 selected publications are affiliated to institutions in 26 countries. These authors are mainly affiliated with institutions in the United States (45 articles), United Kingdom (17 articles), France (16 articles) and Brazil (13) that together authored 59.8% of the selected articles (Figs. 3 and S2A). Similarly, studies developed in these countries account for 59.2% of the published articles (Figs. 3 and S2B).
Fig. 3.
Distribution of articles related to plant–pollinator interactions in urban ecosystems in countries with developed (light grey) (80.89%; N = 127) and developing economies (19.11%, N = 30) (dark grey). For the inclusion and exclusion criteria when searching articles at Web of Science (2020) (www.webofknowledge.com), please see the Flow diagram in Fig. 1. Bubble color represents the number of published articles; Bubble size represents the number of inhabitants (population) in each country, which are informed in the box at right (Data
source: The United Nations 2019)
Regarding climatic regions, 77.7% of the analyzed articles were conducted in temperate regions, followed by tropical regions (14.6%). The subtropical and Mediterranean regions represented less than 3.2% of the articles each (Fig. 4a). Our data revealed that the number of published articles carried out in temperate urban ecosystems is disproportionately high when compared to the amount conducted in tropical ecosystems, a fact corroborated by the nationality of the corresponding authors. Research on urban ecology in the tropics is limited by lack of investments (Anderson et al. 2013; Pauchard and Barbosa 2013), which is quite worrying. The tropical region is recognized for its high species richness, diversity and endemism (e.g. Brown 2014), and is where most biodiversity hotspots are located (Myers et al. 2000). In addition, these regions have cities that are growing at an alarming rate.
Fig. 4.
Number of articles on plant–pollinator interactions in urban ecosystems a per the climatic zones: tropical, subtropical, temperate, Mediterranean, of the country of the study; b categories of nature of the study method; and c type of urban green space where the studies were developed: park, garden (public and/or private), road and/or public walkways and/or flowerbed, green roofs, university campus, cemetery, more than one category, urbanization gradient and others. Our search in the Web of Science with the argument “urban* and pollinat*” returned 634 articles, 157 of which were related to this topic and included in our database
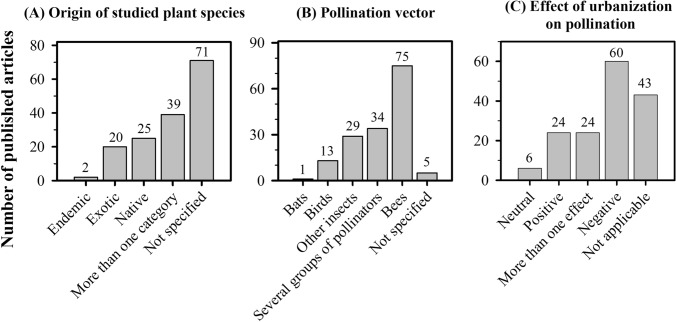
Observational articles represented 84.0% of our sampling, followed by experimental articles (8.9%) and meta-analyses (3.9%), respectively. Literature reviews, descriptive and opinion articles were the least represented, with less than 1.3% each (Fig. 4b). Observational approaches were mostly local and/or regional, therefore making data collection less complex and costly.
From the two literature reviews that returned from our bibliographical search, one discusses the strengths and weaknesses of lists of garden plants to help pollinators, documenting that although the choice of species used in urban green spaces in the United Kingdom, North America and Western Europe was mostly based on non-empirical information, those lists were considered useful and a starting point for future research (Garbuzov and Ratnieks 2014). The second article reviews the mechanistic pathways through which urban drivers modify plant–pollinator interactions and concludes there are knowledge gaps, including understanding the effects of plant and pollinator trait filtering on plant–pollinator interactions and the need for more studies on temperate European-American regions and bee pollinators (Harrison and Winfree 2015). Considering the low representativeness of literature reviews (only two articles, Fig. 4b) and the approach chosen by the authors, it is notorious that plant–pollinator interactions in urban ecosystems is still poorly explored in both temperate and tropical regions. A third review on the topic, not yet indexed in the ISI Web of Science until the end of February 2020, Wenzel et al. (2020), aimed to present a systematic review of literature to identify drivers of urban pollinator populations and pollination, but they reviewed publications on insect pollination exclusively. The authors did not to mention the existence of studies with other groups of pollinators, such as vertebrates (see for example French et al. 2005; Mendonça and Anjos 2006; Arizmendi et al. 2007; Pauw and Louw 2012; Previatto et al. 2012; Coetzee et al. 2018, for birds and Kobayashi et al. 2018 for bats) or the neglect of studies with these vectors as urban pollinators. In our review we found that studies involving vertebrate pollinating vectors accounted for almost 10% of the articles (Fig. 5b; Supplementary Material Table S1) on the plant pollinator interactions in urban ecosystems as will be detailed below.
Fig. 5.
Number of articles on plant–pollinator interactions in urban ecosystems regarding the a origin of the plant(s) involved in the interaction with the study site; b pollination vector; and c categories of urbanization effect: neutral, positive, more than one effect, negative and not applicable in studies on this topic Our search in the Web of Science with the argument “urban* and pollinat*” returned 634 articles, 157 of which were related to this theme and included in our database
In total, at least six types of urban green spaces were mentioned in the 157 analyzed articles (Fig. 4c). Studies carried out exclusively in gardens represented 23.5% of our sampling. These studies were followed by articles conducted across urbanization gradients or in university campi, which account for 17.8% and 5.7% respectively. Articles investigating urban plant–pollinator interactions exclusively in parks, green roofs, and roads and/or public walkways are scarce, representing less than 3.3% each. Despite these differences, articles carried out in more than one type of urban green space are dominant and accounted for 33.7% of all articles. In urban ecosystems, parks can have similar species composition to surrounding natural forest remnants (EIP Associates 2002), at least in cities in temperate regions. Due to native biodiversity, urban parks may favor migration and gene flow across urban green areas, increasing the connection of these areas with natural forests remnants close to cities (Florgard 2007). Thus, we emphasize that parks seem to represent a great opportunity to understand changes in pollination mechanisms caused by the intensification of urbanization in both temperate and tropical regions.
Surprisingly, we have identified only two articles dealing with plant–pollinator interactions and urban agriculture (Frankie et al. 2009; Potter and Lebuhn 2015). In both papers residential gardens are used for the development of urban agriculture. Urban agriculture can be defined as the plant cultivation and/or raising of animals in/or around cities for subsistence and/or commercialization, practiced in any type of urban green space (e.g. Smit et al. 1996; van Veenhuizen 2006). This activity has grown impressively in the world (Orsini et al 2013), and is strongly practiced by low-income population of developed and developing economies (e.g. van Veenhuizen 2006; de Zeeuw et al. 2011; Orsini et al 2013). Urban agriculture can provide a variety of ecosystem services, including pollination (Potter and Lebuhn 2015; Aerts et al. 2016; Zhao et al. 2019). However, little is known about pollination services for urban agriculture (Matteson and Langellotto 2009). Nonetheless, some studies indicate that urban agriculture in residential gardens can play a fundamental role in maintaining pollinators, especially wild bees, regardless of landscape context (garden size, proximity to a natural area, surface permeability) (Smith et al. 2006; Frankie et al. 2009; Potter and Lebuhn 2015; Bennett and Lovell 2019; Zhao et al. 2019). Although the provision of pollination services for urban agriculture has been investigated only for some groups of insects, especially bees (e.g. Smith et al 2006; Frankie et al. 2009; Potter and Lebuhn 2015; Bennett and Lovell 2019; Zhao et al. 2019), urban agriculture has the potential to promote richness, abundance and diversity of other pollinator groups in urban ecosystems.
Unexpectedly, a total of 45.2% of the studies did not inform the origin of the plants. Among the ones that reported origin, 16% studied pollination of native plants, 12.7% evaluated exotic plants and only 1.3% mentioned investigation of endemic plants (Fig. 5a). The occurrence, and in some instances, predominance of exotic plants in urban ecosystems, mainly those located in tropical regions, is due to the ornamental function associated to urban green areas (Moro et al. 2014; Moro and Castro 2015; Silva et al. 2020). These urban green spaces dominated by exotic plants may be detrimental to the maintenance of local biodiversity (McKinney 2002, 2006). On the other hand, native plant species may improve the ecological quality of urban green spaces by supporting the native plant and animal community (Moro and Castro 2015) and are strongly recommended for future urban forestry initiatives (Courtney et al. 2014; Silva et al. 2020).
Regarding pollination vectors, bees were the most studied, representing 47.8% of the analyzed articles, followed by studies that included several groups of pollinators, which represented 21.6% of the articles. Articles focusing on other insect groups, birds and bats accounted for 18.5% (25 articles), 8.3% (13) and 0.6% (2), respectively (Fig. 5b). In Wenzel et al. (2020), which only address pollinating insects, bees also appear as the main vector in urban green areas. Indeed, bees are the most common biotic pollinator and are associated with the maintenance of most plant species in many communities, both in temperate and tropical regions, and in urban and natural ecosystems as well (e.g. Potts et al. 2010; Hennig and Ghazoul 2011; Aleixo et al. 2014; IPBES 2016, and this study). Additionally, bees are the main pollinators of agricultural crops, pollinating about 90% of the crops for which the degree of dependence on pollinators has already been estimated (Klein et al. 2007). Bees are critical to ensure not only increased production (e.g. Gallai and Vaissière 2009), but also the quality of the fruit produced (Garratt et al. 2014; Klatt et al. 2014). Finally, the ease of data collection (observation and specimen collection) also drives researchers to study bees.
Alternatively, based on our findings, other pollinator groups, mainly vertebrates, seem to be neglected in studies on plant–pollinator interactions of urban ecosystem. Vertebrate pollinators (e.g. birds, bats, rodents and lizards) are also essential for crops of economic interest (see IPBES 2016; Ratto et al. 2018). As the dependence on pollination by vertebrates is higher in the tropics than in temperate regions [since the exclusion of vertebrate pollinators negatively affects up to 71% the reproductive success of plant species and caused a reduction in seed set of almost 58% in the tropics (Ratto et al. 2018)], it is expected that these groups may be also relevant in tropical urban ecosystems. Analyzing bats separately, which are considered the most specialized vertebrates, the decline of pollinating bats may result in reduced fruit and seed formation of 83% of bat pollinated plants worldwide (Ratto et al. 2018). This is also relevant to urban ecosystems, as bats commonly occur in urban green spaces (Jung and Threfall 2015; Russo and Ancillotto 2015). Due to the sensitivity of specialist and vertebrate pollinators to the effects of urbanization (Melles et al. 2003; Pauw and Louw 2012; Jung and Threlfall 2015), the occurrence of these groups can act as bioindicators of the integrity of ecological functions and services in urban green spaces (e.g. Russo and Ancillotto 2015).
Main impacts of urbanization on plant–pollinator interactions
The effect of urbanization on plant–pollinator interactions was mentioned in 114 (72.6%) of the 157 analyzed articles. Among the 114 studies that mentioned an effect of urbanization on plant–pollinator interactions, 52.6% reported negative effects, 21.05% registered positive effects, and 5.3% did not detected any impact of urbanization on plant–pollinator interactions (neutral); urbanization may also have distinct and complex effects (more than one effect) on plant–pollinator interactions, which have been observed in 21.05% of the analyzed articles (Fig. 5c). An example of a complex effect would be an increase in pollinator visits to flowers, but a decrease in reproductive success (fruit-set), in a plant species with genetic self-incompatibility. The impacts of urbanization on plant–pollinator interactions are mainly related to the maintenance of plant and pollinator species, to pollinator behavior, and to plant reproductive success in urban green spaces.
Plants and pollinators in urban green areas were investigated in isolation or in combination with other aspects of plant–pollinator interactions in 82 out of the 157 analyzed articles. In general, urban ecosystems in both temperate and tropical regions fail in preserving the whole diversity of plant species and functional traits (e.g. Bergerot et al. 2010; Geslin et al. 2013; Matteson et al. 2013; Anderson et al. 2014; Garbuzov and Ratnieks 2014; Desaegher et al. 2019). The diversity of pollinators, specifically those with specialized functional traits (e.g. Bergerot et al. 2010), is also reduced in urban green spaces in temperate regions (e.g. Basteri and Benvenuti 2010; Bates et al. 2011; Matteson and Langellotto 2011; Tonietto et al. 2011; Glaum et al. 2017). Similarly, reductions in the diversity of bees, wasps and beetles (e.g. Guenat et al. 2018; Oliveira et al. 2019a), butterflies (e.g. Jain et al. 2016), and specialized birds (e.g. Coetzee et al. 2017; Maruyama et al. 2019) were documented in tropical urban green spaces associated to urbanization or to natural areas. Articles comparing the diversity of species of other pollinator groups, such as hawkmoths, bats and non-flying mammals between natural areas and urban green spaces in tropical regions were not present in our database, indicating that the impacts of urbanization on specialized plant–pollinator interactions in the tropics are still poorly known.
In temperate regions, the distribution of the reduced diversity of plant and pollinators retained in urban green areas seems to follow the characteristics of the surrounding habitat. The proximity of temperate urban green spaces to forest remnants and agricultural areas, together with an increased diversity of flowering plants and floral resources, are positively related to the diversity of bees, flies, hoverflies, butterflies, beetles and wasps among other insect pollinators (e.g. Winfree et al. 2007; Sattler et al. 2010; Bates et al. 2011; Tonietto et al. 2011; Pellissier et al. 2013; Shwartz et al. 2013; Pardee and Philpott 2014; Kratschmer et al. 2018; Luder et al. 2018). This biodiversity is also mainly positively correlated with the proximity to gardens and allotments (e.g. Foster et al. 2016; Baldock et al. 2019). In the tropics, in addition to these aspects, urban green spaces that present a high number of plant species that continuously offer floral resources may represent a refuge to many groups of pollinators, mainly bees (e.g. Aleixo et al. 2014). Independently of habitat characteristics, plants and pollinators with generalist functional traits tend to benefit from urban ecosystems in both temperate (Hamblin et al. 2018; Kratschmer et al. 2018; Desaegher et al. 2019) and tropical regions (Zotarelli et al. 2014; Coetzee et al. 2018; Guenat et al. 2018).
The impacts of urbanization in pollinator behavior were mentioned, separately or in combination with other aspects of plant–pollinator interactions, in 92 out of the 157 analyzed articles. In general, urbanization has complex effects on pollinator behavior, mainly by changing foraging routes, frequency and duration of floral visits, and by promoting changes in floral resource selection. Reduced number of visits and interactions among flowers and pollinators are frequently observed in urban green areas in temperate regions (e.g. Liu and Koptur 2003; Andrieu et al. 2009; Geslin et al. 2013; Matteson et al. 2013) and in the few studies carried out in the tropics (e.g. Maruyama et al. 2019; Oliveira et al. 2019a). Despite the reduction in visitation rate, increase in the number of flowers, larger floral displays and higher production of pollen and nectar had positive effects on floral visits by bees (e.g. Leveau 2008; Hennig and Ghazoul 2011; Coetzee et al. 2017; Irwin et al. 2018; Jusselme et al. 2019) and by hummingbirds (Calviño-Cancela 2006) in temperate urban green spaces. In addition, pollinators may preferably visit native (e.g. French et al. 2005) or exotic plant species depending on the intensity of urban stressors (Buchholz and Kowarik 2019).
Increased intensity of anthropogenic disturbances such as habitat loss, fragmentation, heat island and soil pollution, can act as filters and pave the way for the introduction and even invasion of exotic plant species in urban green areas (e.g. Harrison and Winfree 2015). Exotic species can offer a greater amount of resources in places where native plants are absent or with reduced abundance. However, exotic plants can (a) represent a risk to the health of pollinators (since the floral resources offered may not have nutritional values similar to those of native plants) (Stout and Morales 2009; (b) compete with native plants for native pollinators, (Totland et al. 2006; see references cited in Kovács-Hostyánski et al. 2016); in addition to (c) facilitating the invasion of non-native floral visitors (Morales and Aizen 2002, 2006; Traveset et al. 2013). Consequently, the frequency of pollinator visits to exotic species can be increased (Totland et al. 2006), as well as the reproductive success (Silva et al. 2020). Silva et al. (2020) analyzed tree fruiting in 19 urban green spaces (parks and squares) in a tropical city (Recife), northeastern Brazil, and documented that all exotic species (which represents ~ 50% of the total) set fruits. It is worth mentioning that, from a landscape perspective, the introduction of exotic plant species in urban areas can threaten urban and peri-urban/peri-rural agriculture in both temperate and tropical countries by changing pollinator foraging route in those areas. Thus, food crops that depend on animal pollinators (about 35%) to guarantee the volume or quality of production (Klein et al 2007) may have their production threatened due to pollinator decline. Reductions in visitation rates in urban green spaces may be related to the combination of reduced pollinator diversity, reduction in area of foraging routes, as previously observed for Euglossini bees in tropical regions (Lopez-Uribe et al. 2008), disruption of plant–pollinator networks (Lowenstein et al. 2019), and exclusion of less competitive pollinator groups (Brizola-Bonacina et al. 2012) in many urban green areas. Regarding the duration of floral visits and foraging routes, they may be increased in urban ecosystems, as observed for butterflies in temperate regions (Andrieu et al. 2009) and for hummingbirds in the Neotropical region (Calviño-Cancela 2006). We emphasize that, as floral resources are more sparsely distributed and scarce in urban green spaces (Bergerot et al. 2010; Desaegher et al. 2019), pollinators may travel longer distances and spend more time collecting floral resources to supply their energy demands.
Impacts of urbanization on plant phenology and reproductive success were mentioned, in isolation or in combination with other aspects of plant–pollinator interactions, in 40 of the 157 analyzed articles. In total, seven articles informed the flowering period of the plants, three studies carried out in tropical regions, three in temperate regions and one in areas in both climatic zones. In temperate urban green areas, mass flowering and production of floral resources are positively associated with attraction of numerous insects, such as bumblebees and honeybees, flies, moths, and butterflies (Denisow et al. 2013; Benvenuti 2014; Wray and Elle 2015). Similarly, continuous flowering may provide pollen, nectar and oil that support bee communities in tropical urban ecosystems (Aleixo et al. 2014). Alternatively, urbanization can be associated with increased flowering duration in tropical urban green spaces, contrasting with shorter flowering (3–4 months) in forest remnants, as observed for a legume tree (e.g. Oliveira et al. 2019a, b). Articles investigating the flowering phenology of plants pollinated by nocturnal animals in temperate and tropical regions were not registered in our database.
Among the articles investigating the relationship between urbanization and plant reproductive success, 21 analyzed the female component, six the male component and five both. Female reproductive success was measured as the proportion of flowers that originated viable fruits and number of seeds per fruit in most of the analyzed articles, while male reproductive success was mainly measured by pollen load observed in pollinators and pollen dispersion. In general, both male and female components of plant reproductive success are negatively affected by urbanization processes in temperate areas (e.g. Parker 1997; Jędrzejewska-Szmek and Zych 2013; Leong et al. 2014; Bennet et al. 2018; Hou et al. 2019) and in the few studies in the tropics (Oliveira et al. 2019a, b).
In the case of female reproductive success, distinct levels of fruit- (Parker 1997; Pellissier et al. 2012; Pellegrino and Bellusci 2014; Oliveira et al. 2019a) and seed-set decreases have been extensively documented in urban green areas, sometimes reaching up to 80% (e.g. Cheptou and Andevaño 2006). Female reproductive success may also change across distinct types of urban green spaces in temperate regions (e.g. Pellissier et al. 2013), in which green roofs may have higher levels of seed set in comparison to plants on the ground level (e.g. Ksiazek et al. 2012). Reductions in female plant reproductive success may be strongly affected by the quality and quantity of pollen grains deposited in the flower stigma. Besides bees, flies, butterflies and moths also collect pollen from very diverse plant groups in temperate urban ecosystems, and may contribute to dispersion of pollen grains over short distances (e.g. Jędrzejewska-Szmek and Zych 2013; Bennet et al. 2018). We emphasize that limitations in pollen movement and pollen deposition in receptive stigmas may be directly related to reductions in the reproductive output of plants in urban green spaces worldwide. Studies investigating other aspects of reproductive success, such as pollen removal by pollinators in both temperate and tropical regions were not observed in our database.
There are countless factors (landscape, local, abiotic, and social) responsible for driving plant–pollinator interaction in urban ecosystems. The reviews by Harrison and Winfree (2015) and Wenzel et al. (2020) highlight landscape, local, and abiotic factors. The landscape factors considered are the composition of the landscape, which includes the proportion of impervious surface, and the configuration of landscape, such as the spatial distribution and size of urban green spaces (resulting from fragmentation and habitat loss), in addition drives related to connectivity between urban areas and urban areas and natural or semi-natural areas. The local drives include temporal distribution of floral resources, referring to the beginning and duration of flowering and nesting sites, for example. Abiotic factors, on the other hand, are related to establishment of heat islands (higher temperature in cities than in the surrounding areas), which has been amplified due to climate change; air pollution, through the high emission of greenhouse gases and soil pollution, where pollutants can be concentrated in the nectar and pollen produced by plants; use of pesticides for weed control, for example; and the presence of native and exotic species of plants and animals, which may have been managed or not (Harrison and Winfree 2015; Wenzel et al. 2020). In addition to these main and well-discussed factors, we must emphasize some social factors that can also act on the plant–pollinator interaction in urban green areas, such as the level of education, income and lifestyle of the population (including ethnicity/culture). These factors are capable of influencing the management of domestic gardens, parks, flower beds and green roofs, for example, changing the local floristic composition (through the planting of non-native plant species) and consequently the supply of resources to pollinators (Kendal et al. 2012). This is particularly important for the management of urban agriculture in the different types of green spaces (Zhao et al. 2019).
In synthesis, we identified a pattern of impairment or disruption of plant–pollinator interactions in urban ecosystems worldwide, although there are conflicting evidences suggesting that cities could retain high diversity of pollinators (Tommasi et al. 2004; Sattler et al. 2010) thus adequately contributing to plant reproductive success (e.g. Rossum 2010). Plant–pollinator interactions in urban green areas may be negatively impacted in distinct levels. Firstly, reduced diversity of flowering plants and nesting sites in urban green spaces may impair the establishment of pollinators. Indeed, many groups of pollinators, such as bees, flies, hoverflies, wasps, birds and hummingbirds, had reduced diversity of species or functional traits in urban green areas according to our review. In a second level, urbanization is associated with negative changes in pollinator behavior, such as decreases in foraging routes (e.g. Lopes-Uribe et al. 2008), which may be particularly detrimental for plant–pollinator interactions involving sparsely distributed urban green spaces. Specifically, many plant species in urban green areas are not visited by pollinators (Lowenstein et al. 2019), as a response to limitation in foraging routes, scattered distribution and impervious surfaces surrounding urban ecosystems. Finally, by reducing pollinator movement, the frequency of floral visits and the quantity of pollen grains that may be deposited in the flower stigmas are decreased, thus harming the female reproductive success of many plants in urban ecosystems.
Research funding/grants and policy documents related to pollination and pollinators in urban ecosystem
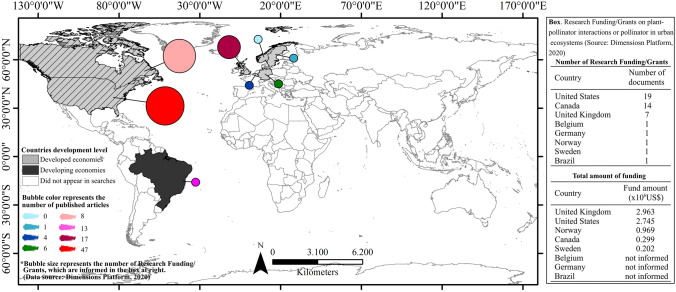
Our initial search returned 217 research funding/grants, published from 1994 to 2020. After sorting and excluding duplicates, 172 records remained; of which 45 were selected because they were destined to researches that addressed pollination, pollinator or plant–pollinator interactions in urban ecosystems; 27 of these 45 were directed to plant–pollinator interactions in urban ecosystems (Supplementary Material Table S3). The first research funding/grant on the theme occurred only in 2005 (Supplementary Material Table S3). Developed economies concentrated practically all funding (97.8%) related to plant–pollinator interactions or pollinators in urban ecosystems, while only a single research funding was granted and applied in a country with developing economy (2.2%) (Fig. 6). The United States, Canada and the United Kingdom accounted, together, for 89% of the research funding/grants; while countries such as Brazil, Belgium, Germany, Norway and Sweden had only one research funding/grant on the topic (Supplementary Materia Table S3). Research funding on plant–pollinator interactions or pollinators in urban ecosystems was only approximately US$7.1 million in the 25 years since the first funding. It is worth mentioning that: (1) 62% of the research funding/grants were addressed to researches on pollinating insects, and of these 85.7% were specifically for bees; (2) 29% had some information at the website to which the project was linked but failed to inform the pollinator group of the study; (3) 4.5% focused on birds and bats (2 research funding/grants only); (4) 4.5% of the research funding/grants did not have any information at the website to which the project was linked (Supplementary Material, Table S3 for details).
Fig. 6.
Research Funding/Grants for projects related to plant–pollinator interactions or to pollinator (e.g. abundance, diversity, healthy) in urban ecosystems in countries with developed (light grey) (97.78%; N = 44) and developing economies (2.22%, N = 1) (dark grey). In light grey and hatch pattern Grants/Research Funding for Canada (US$ 299,370.00 million), the United States (US$ 2,745,636.00) and the United Kingdom (US$ 2,963,526.00)—together they accounted for 83.67% of the investments (N = 40). For the inclusion and exclusion criteria when searching Research Funding/Grants at Dimensions Platform (2020), please see the Flow diagram in Fig. 1. Bubble color represents the number of published articles; Bubble size represents the number of Research Funding/Grants, which are informed in the box at right. This box also details the total amount of funding per country (Data
source: Dimensions Platform 2020; https://www.dimensions.ai/)
We did not find any policy documents related to plant–pollinator interactions in urban ecosystem. However, 10 policy documents addressed, in general, the conservation, maintenance and improvement of pollinators in the United Kingdom (N = 8), Italy (N = 1) and Australia (N = 1). None of the policy documents are specific to urban ecosystems, although they mention urban green areas. All these policy documents were established by government agencies of developed countries (Supplementary Material Table S4).
In the last decade, there has been a growing concern about the decline of pollinators worldwide (e.g. Potts et al. 2010, 2016; Dicks et al. 2016). But the alerts and efforts to protect and conserve pollinators are mainly related to food production and security (e.g. Gallai et al. 2009; Bauer and Wing 2010; Novais et al. 2016; Sluijs and Vaage 2016). In a recent review, for instance, Hall and Steiner (2019) examined relevant policies for insect pollinators in the United States, and reported 109 laws related to agriculture, pesticide use, habitat improvement and awareness. Despite this, our findings clearly indicate that research funding and the development of government policies aimed at plant–pollinator interactions or pollinators in urban ecosystems are still insufficient and/or scarce, especially in countries with developing economy. Pollination in urban green spaces represents an ecosystem service that contributes to human well-being (e.g. Cox et al. 2018). Therefore, we must pay attention to the need for greater technical and scientific funding, local recommendations, particularities of each location, and public awareness, aiming to protect plants and pollinators in urban green areas to promote healthier urban ecosystems. In particular, public initiatives are needed to encourage and support the development of urban agriculture, with the establishment of clear recommendations and with planning and management techniques that maximize biodiversity. Thus, urban green spaces, in turn, may help in the maintenance of biodiversity, ecosystem services and improve the quality of life of humans in cities.
Conclusions
Our scientometric approach reveals that plant–pollinator interactions in urban ecosystems is a recent research field that is mainly explored in temperate regions by researchers affiliated to institutions in the United States, United Kingdom and France. Plant–pollinator interactions in tropical urban ecosystems is still neglected. The most studied pollination vectors are insects, mainly bees, in both tropical and temperate regions. In general, urbanization may exert negative and complex effects on plant–pollinator interactions. Generalist plants and pollinators predominate in urban ecosystems. Despite the recent increase in the number of published articles, several research opportunities emerge from our review. Specifically, studies investigating more detailed aspects of pollen removal by distinct groups of pollinators were not registered in our database. Similarly, studies investigating the pollination of nocturnal plants and maintenance and behavior of nocturnal vectors in urban areas, such as bats, are scarce or even nonexistent, when considering other nocturnal pollinators, such as non-flying mammals, lizards and hawkmoths. These studies would be particularly relevant for the tropics, which harbor the most biodiverse and populated regions of the world, but where plant–pollinator interactions in urban ecosystem is still neglected. Additionally, research funding and policy actions for pollination and pollinators in urban ecosystems are still scarce and concentrated in developed economy countries and in temperate region. To make urban green areas contribute to the maintenance of biodiversity and provision of ecosystem services, transdisciplinary approaches that integrates ecological, social, economic and cultural factors are needed.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank Fundação de Amparo à Ciência e Tecnologia do Estado de Pernambuco-FACEPE, which funded this study via grants to JLSS (Grant No. IBPG-0774-2.03/13), MTPO (grant number IBPG-0420-2.03/14) and OCN (Grant No. APQ-0789-2.05/16 and BCT-0208-2.05/17). The study was also funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico-CNPq (Grants: 481755/2013-6 and 309505/20186 to AVL) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-CAPES (Grant 001 to MTPO, JLSS, OCN, MT and AVL). Data on Research funding/Grants and Policy documents were kindly provided by Digital Science’s Dimensions Platform (2020), an inter-linked research information system (https://app.dimensions.ai).
Biographies
Jéssica Luiza S. Silva
is a postdoc at the Department of Botany of the Universidade Federal de Pernambuco. Her research interests include plant ecology and pollination biology in urban and natural ecosystems.
Marcela Tomaz Pontes de Oliveira
has got her PhD at the Department of Botany of the Universidade Federal de Pernambuco. Her research interests include pollination in urban ecosystems.
Oswaldo Cruz-Neto
is a postdoc at the Department of Botany of the Federal University of Pernambuco. His research interests include plant ecology, with an emphasis on tropical pollination ecology.
Marcelo Tabarelli
is a Professor at the Universidade Federal de Pernambuco (UFPE). His research work includes diverse aspects of tropical environmental degradation and conservation ecology. MT is a leading Brazilian ecologist who recently won an award from The Humboldt Foundation for his scientific contribution to tropical ecology and conservation. He has coordinated and collaborated in diverse projects funded by the Brazilian Government.
Ariadna Valentina Lopes
is a Professor at the Universidade Federal de Pernambuco. AVL is Member and Leading Author of the IPBES (Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services) Thematic assessment on pollinators, pollination and food production. Her recent research includes analyses of how the diversity of plant reproductive strategies influences the diversity of biological communities; landscape ecology; effects of human disturbance on the reproduction of tropical plants; pollination service. She is a leading Brazilian author who has coordinated and collaborated in diverse projects funded by the Brazilian Government.
Author contributions
JLSS, MTPO, and OCN have contributed equally to this work. AVL, JLSS and OCN conceived the ideas; JLSS, MTPO and OCN contributed to data collection; JLSS, MTPO and OCN analyzed the data; JLSS and OCN wrote first draft; JLSS, OCN, MT and AVL contributed to writing, review and editing; all author contributed to funding acquisition.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jéssica Luiza S. Silva, Email: jessicaluizassilva@gmail.com
Marcela Tomaz Pontes de Oliveira, Email: marcelatomaz@gmail.com.
Oswaldo Cruz-Neto, Email: ocruznt@gmail.com.
Marcelo Tabarelli, Email: mtrelli@ufpe.br.
Ariadna Valentina Lopes, Email: avflopes@ufpe.br.
References
- Aerts R, Dewaelheyns V, Achten WMJ. Potential ecosystem services of urban agriculture: A review. PeerJ. 2016;4:e2286vl. doi: 10.7287/peerj.preprints.2286v1. [DOI] [Google Scholar]
- Aleixo KP, Faria LB, Groppo M, Castro MMN, Silva CI. Spatiotemporal distribution of floral resources in a Brazilian city: Implications for the maintenance of pollinators, especially bees. Urban Forestry & Urban Greening. 2014;13:689–696. doi: 10.1016/j.ufug.2014.08.002. [DOI] [Google Scholar]
- Anderson, P.M.L., C. Okereke, A. Rudd, and S. Parnell. 2013. Regional assessment of Africa. In Urbanization, biodiversity and ecosystem services: Challenges and opportunities, A global assessment, ed. T. Elmqvist, M. Fragkias, J. Goodness, B. Güneralp, P.J. Marcotullio, R.I. McDonald, S. Parnell, M. Scheweniius, et al., 453–560. London: Springer.
- Anderson PML, Avlonitis G, Ernstson H. Ecological outcomes of civic and expert-led urban greening projects using indigenous plant species in Cape Town, South Africa. Landscape and Urban Planning. 2014;127:104–113. doi: 10.1016/j.landurbplan.2014.03.007. [DOI] [Google Scholar]
- Andrieu E, Dornier A, Rouifed S, Schatz B, Cheptou PO. The town Crepis and the country Crepis: How does fragmentation affect a plant–pollinator interaction? Acta Oecologia. 2009;35:1–7. doi: 10.1016/j.actao.2008.07.002. [DOI] [Google Scholar]
- Arizmendi MC, Constanza MS, Lourdes J, Ivonne FM, Edgar LS. Effect of nectar feeders over diversity and abundance of hummingbirds and breeding success of two plant species in a sub-urban park next to Mexico City. Biological Conservation. 2007;136:155–158. doi: 10.1016/j.biocon.2006.11.016. [DOI] [Google Scholar]
- Baldock KCR, Goddard MA, Hicks DM, Kunin WE, Mitschunas N, Osgathorpe LM, Vaughan PI, Memmott J. Where is the UK’s pollinators biodiversity? The importance of urban areas for flower-visiting insects. Proceedings of the Royal Society B. 2015;282:20142849. doi: 10.1098/rspb.2014.2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldock KCR, Goddard MA, Hicks DM, Kunin WE, Mitschunas N, Morse H, Osgathorpe LM, Potts SG, et al. A systems approach reveals urban pollinator hotspots and conservation opportunities. Nature Ecology & Evolution. 2019;3:363–373. doi: 10.1038/s41559-018-0769-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton J, Pretty J. What is the best dose of nature and green exercise for improving mental health: A multi-study analysis? Environmental Science and Technology. 2010;44:3947–3955. doi: 10.1021/es903183r. [DOI] [PubMed] [Google Scholar]
- Basteri G, Benvenuti S. Wildflowers pollinators-attractivity in the urban ecosystem. Acta Horticulturae. 2010;881:585–590. doi: 10.17660/ActaHortic.2010.881.98. [DOI] [Google Scholar]
- Bates AJ, Sadler JP, Fairbrass AJ, Falk SJ, Hale JD, Matthews TJ. Changing bee and hoverfly pollinators assemblages along an urban-rural gradient. PLoS ONE. 2011;6:e23459. doi: 10.1371/journal.pone.0023459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer DM, Wing IS. Economic consequences of pollinator declines: A synthesis. Agricultural and Resource Economics Review. 2010;39:368–383. doi: 10.1017/S1068280500007371. [DOI] [Google Scholar]
- Bennett AB, Lovell S. Landscape and local site variables differentially influence pollinators and pollination services in urban agricultural sites. PLoS ONE. 2019;14:e0212034. doi: 10.1371/journal.pone.0212034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennet DG, Kelly D, Clemens J. Food plants and foraging distances for the native bee Lasioglossum sordidumin Christchurch Botanic Gardens. New Zealand Journal of Ecology. 2018;42:40–47. doi: 10.20417/nzjecol.42.1. [DOI] [Google Scholar]
- Benvenuti S. Wildflower green roofs for urban landscaping, ecological sustainability and biodiversity. Landscape and Urban Planning. 2014;124:151–161. doi: 10.1016/j.landurbplan.2014.01.004. [DOI] [Google Scholar]
- Bergerot B, Fontaine B, Renard M, Cadi A, Julliard R. Preferences for exotics flowers do not promote urban life in butterflies. Landscape and Urban Planning. 2010;96:98–107. doi: 10.1016/j.landurbplan.2010.02.007. [DOI] [Google Scholar]
- Bonnington C, Gaston KJ, Evans KL. Squirrels in suburbia: Influence of urbanization on the occurrence and distribution of a common exotic mammal. Urban Ecosystem. 2013;17:533–546. doi: 10.1007/s11252-013-0331-2. [DOI] [Google Scholar]
- Boulton C, Dedekorkut-Howes A, Byrne J. Factors shaping urban greenspace provision: A systematic review of the literature. Landscape and Urban Planning. 2018;178:82–101. doi: 10.1016/j.landurbplan.2018.05.029. [DOI] [Google Scholar]
- Brizola-Bonacina AK, Arruda VM, Alves-Junior VV, Chaud-Netto J, Polatto LP. Bee visitors of quaresmeira flowers (Tibouchina granulosa Cogn.) in the Region of Dourados (MS-Brasil) Sociobiology. 2012;59:1253–1267. [Google Scholar]
- Brown JH. Why are there so many species in the tropics? Journal of Biogeography. 2014;41:8–22. doi: 10.1111/jbi.12228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchholz S, Kowarik I. Urbanisation modulates plant–pollinator interactions in invasive vs. native plant species. Scientific Reports. 2019;9:6375. doi: 10.1038/s41598-019-42884-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calviño-Cancela M. Time-activity budgets and behaviour of the Amazilia hummingbird, Amazilia amazilia (Apodiformes: Trochilidae) in an urban environment. Revista de Biología Tropical. 2006;54:873–878. doi: 10.15517/rbt.v54i3.13684. [DOI] [PubMed] [Google Scholar]
- Cheptou PO, Andevaño VLG. Pollination processes and the Allee effect in highly fragmented populations: Consequences for the mating system in urban environments. New Phytologist. 2006;172:774–783. doi: 10.1111/j.1469-8137.2006.01880.x. [DOI] [PubMed] [Google Scholar]
- Coetzee A, Barnard P, Pauw A. Indigenous plants and artificial nectar sources facilitate urban adjustment of avian pollinators in Cape Town. South African Journal of Botany. 2017;109:330. doi: 10.1016/j.sajb.2017.01.039. [DOI] [Google Scholar]
- Coetzee A, Barnard P, Pauw A. Urban nectarivorous bird communities in Cape Town, South Africa, are structured by ecological generalisation and resource distribution. Journal of Avian Biology. 2018;49:e01526. doi: 10.1111/jav.01526. [DOI] [Google Scholar]
- Courtney EG, Potts BM, Schweitzer JA, Bailey JK. Shifts in species interactions due to the evolution of functional differences between endemics and non-endemics: An endemic syndrome hypothesis. PLoS ONE. 2014;9:e111190. doi: 10.1371/journal.pone.0111190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox DTC, Shanahan DF, Hudson HL, Fuller RA, Gaston KJ. The impact of urbanization on nature dose and the implications for human health. Landscape and Urban Planning. 2018;179:72–80. doi: 10.1016/j.landurbplan.2018.07.013. [DOI] [Google Scholar]
- Denisow B, Antón S, Szymczak G. The flowering, pollen production, and insect visitors in the ornamental shrub Potentilla fruticose L. (Rosaceae) Journal of Apicultural Science. 2013;57:95–105. doi: 10.2478/jas-2013-0011. [DOI] [Google Scholar]
- Desaegher J, Nadot S, Machon N, Colas B. How does urbanization affect the reproductive characteristics and ecological affinities of street plant communities? Ecology and Evolution. 2019;9:9977–9989. doi: 10.1002/ece3.5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Zeeuw H, van Veenhuizen R, Dubbeling M. The role of urban agriculture in building resilient cities in developing countries. Journal of Agricultural Science. 2011;149:153–163. doi: 10.1017/S0021859610001279. [DOI] [Google Scholar]
- Dicks LV, Viana B, Bommarco R, Brosi B, Arizmendi MD, Cunningham SA, Galetto L, Hill R, et al. Ten policies for pollinators. Science. 2016;354:975–976. doi: 10.1126/science.aai9226. [DOI] [PubMed] [Google Scholar]
- Dimensions Platform. 2020. https://app.dimensions.ai. Accessed on March 13, 2020, under licence agreement.
- EIP Associates . Significant Natural Resource Areas Management Plan. San Francisco: E.I.P Associates; 2002. [Google Scholar]
- Elmqvist T, Setälä H, Handel SN, van der Ploeg S, Aronson J, Blignaut JN, Gómez-Baggethun E, Nowak DJ, et al. Benefits of restoring ecosystem services in urban areas. Current Opinion in Environmental Sustainability. 2015;14:101–108. doi: 10.1016/j.cosust.2015.05.001. [DOI] [Google Scholar]
- FAO. 2014. Benefits of urban and peri-urban forestry. https://www.fao.org/forestry/urbanforestry/87029/en/.
- FAO. 2018. Forests and sustainable cities: Inspiring stories from around the world. 92p.
- Fetridge ED, Ascher JS, Langellotto GA. The bee fauna of residential gardens in a suburb of New York City (Hymenoptera: Apoidea) Annals of the Entomological Society of America. 2008;101:1067–1077. doi: 10.1603/0013-8746-101.6.1067. [DOI] [Google Scholar]
- Flogard C. Preserved and remnant natural vegetation in cities: A geographically divided field of research. Landscape Research. 2007;32:79–94. doi: 10.1080/01426390601097750. [DOI] [Google Scholar]
- Foster G, Bennett J, Sparks T. An assessment of bumblebee (Bombus spp.) land use and floral preference in UK gardens and allotments cultivated for food. Urban Ecosystems. 2016;20:425–434. doi: 10.1007/s11252-016-0604-7. [DOI] [Google Scholar]
- François C, Alexandre L, Julliard R. Effects of landscape urbanization on magpie occupancy dynamics in France. Landscape Ecology. 2008;23:527–538. doi: 10.1007/s10980-008-9211-1. [DOI] [Google Scholar]
- Frankie GW, Thorp RW, Schindler M, Hernandez J, Ertter B, Rizzardi M. Ecological patterns of bees and their host ornamental flowers in two northern California cities. Journal of Kansas Entomological Society. 2005;78:227–246. doi: 10.2317/0407.08.1. [DOI] [Google Scholar]
- Frankie GW, Thorp RW, Hernandez J, Rizzardi M, Ertter B, Pawelek JC, Witt SL, Schindler M, et al. Native bees are a rich natural resource in urban California gardens. California Agriculture. 2009;63:113–120. doi: 10.3733/ca.v063n03p113. [DOI] [Google Scholar]
- French K, Major R, Hely K. Use of native and exotic garden plants by suburban nectarivorous birds. Biological Conservation. 2005;121:545–559. doi: 10.1016/j.biocon.2004.06.004. [DOI] [Google Scholar]
- Gallai N, Vaissière BE. Guidelines for the economic valuation of pollination services at a national scale. Rome: FAO; 2009. [Google Scholar]
- Gallai N, Salles J, Settele J, Vaissière BE. Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecological Economics. 2009;68:810–821. doi: 10.1016/j.ecolecon.2008.06.014. [DOI] [Google Scholar]
- Garbuzov M, Ratnieks FLW. Listamania: The strengths and weaknesses of lists of garden plants to help pollinators. BioScience. 2014;64:1019–1026. doi: 10.1093/biosci/biu150. [DOI] [Google Scholar]
- Garratt MPD, Breeze TD, Jenner N, Polce C, Biesmeijer JC, Potts SG. Avoiding a bad apple: Insect pollination enhances fruit quality and economic value. Agriculture, Ecosystems and Environment. 2014;184:34–40. doi: 10.1016/j.agee.2013.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geslin B, Gauzens B, Thébault E, Dajoz I. Plant pollinator networks along a gradient of urbanization. PLoS ONE. 2013;8:e63421. doi: 10.1371/journal.pone.0063421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaum P, Simao MC, Vaidya C, Fitch G, Iulinao B. Big city Bombus: Using natural history and land-use history to find significant environmental drivers in bumble-bee declines in urban development. Royal Society Open Science. 2017;4:170156. doi: 10.1098/rsos.170156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenat S, Kunin WE, Dougill AJ, Dallimer M. Effects of urbanisation and management practices on pollinators in tropical Africa. Journal of Applied Ecology. 2018;56:214–224. doi: 10.1111/1365-2664.13270. [DOI] [Google Scholar]
- Hall D, Camilo GR, Tonietto RK, Ollerton J, Ahrné K, Arduser M, Ascher JS, Baldock KC, et al. The city as a refuge for insect pollinators. Conservation Biology. 2016;31:24–29. doi: 10.1111/cobi.12840. [DOI] [PubMed] [Google Scholar]
- Hall DM, Steiner R. Insect pollinator conservation policy innovations: Lessons for lawmakers. Environmental Science and Policy. 2019;93:118–128. doi: 10.1016/j.envsci.2018.12.026. [DOI] [Google Scholar]
- Hamblin AL, Youngsteadt E, Frank SD. Wild bee abundance declines with urban warming, regardless of floral density. Urban Ecosystems. 2018;21:419–428. doi: 10.1007/s11252-018-0731-4. [DOI] [Google Scholar]
- Harrison T, Winfree R. Urban drivers of plant–pollinator interactions. Functional Ecology. 2015;29:879–888. doi: 10.1111/1365-2435.12486. [DOI] [Google Scholar]
- Hennig EI, Ghazoul J. Pollinating animals in the urban environment. Urban Ecosystems. 2011;15:149–166. doi: 10.1007/s11252-011-0202-7. [DOI] [Google Scholar]
- Hook DW, Porter SJ, Herzog C. Dimensions: Building context for search and evaluation. Frontiers in Research Metrics and Analytics. 2018;3:1–11. doi: 10.3389/frma.2018.00023. [DOI] [Google Scholar]
- Hou QZ, Pang X, Wang YP, Sun K, Jia LY, Zhang SH, Li QX. Urbanization threaten the pollination of Gentiana dahurica. Scientific Reports. 2019;9:583. doi: 10.1038/s41598-018-36773-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IPBES - Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services. 2016. The assessment report of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services on pollinators, pollination and food production. Potts SG, Imperatriz-Fonseca VL, Ngo HT (Eds). Secretariat of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services, Bonn, Germany.
- Irwin RE, Galen C, Rabenold JJ, Kaczorowski R, McCutcheon ML. Mechanisms of tolerance to floral larceny in two wildflower species. Ecology. 2018;889:3093–3104. doi: 10.1890/08-0081.1. [DOI] [PubMed] [Google Scholar]
- Jain A, Kunte K, Webb EL. Flower specialization of butterflies and impacts of non-native flower use in a transformed tropical landscape. Biological Conservation. 2016;201:184–191. doi: 10.1016/j.biocon.2016.06.034. [DOI] [Google Scholar]
- Jędrzejewska-Szmek K, Zych M. Flower-visitor and pollen transport networks in a large city: Structure and properties. Arthropod–Plant Interactions. 2013;7:503–516. doi: 10.1007/s11829-013-9274-z. [DOI] [Google Scholar]
- Jung K, Threlfall CG. Urbanisation and its effects: a global meta-analysis. In: Voigt CC, Kingston T, editors. Bats in the Anthropocene: Conservation of bats in changing world. Berlin: Springer; 2015. pp. 13–34. [Google Scholar]
- Jusselme MD, Pruvost C, Motard E, Giusti-Miller S, Frechault S, Alphonse V, Balland-Bolou-Bi C, Dajoz I, et al. Increasing the ability of a green roof to provide ecosystem services by adding organic matter and earthworms. Applied Soil Ecology. 2019;143:61–69. doi: 10.1016/j.apsoil.2019.05.028. [DOI] [Google Scholar]
- Kabisch N, Haase D. Green spaces of European cities revisited for 1990–2006. Landscape and Urban Planning. 2012;110:113–122. doi: 10.1016/j.landurbplan.2012.10.017. [DOI] [Google Scholar]
- Kendal D, Williams NSG, Williams KJH. Drivers of diversity and tree cover in gardens, parks and streetscapes in an Australian city. Urban Forestry and Urban Greening. 2012;11:257–265. doi: 10.1016/j.ufug.2012.03.005. [DOI] [Google Scholar]
- Klatt BK, Holzschuh A, Westphal C, Clough Y, Smit I, Pawelzik E, Tscharntke T. Bee pollination improves crop quality, shelf life and commercial value. Proceedings of the Royal Society B: Biological Sciences. 2014;281:20132440. doi: 10.1098/rspb.2013.2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein AM, Vaissière BE, Cane JH, Steffan-Dewenter I, Cunningham SA, Kremen C, Tscharntke T. Importance of pollinators in changing landscapes for world crops. Proceedings in Biological Sciences/ The Royal Society. 2007;274:303–313. doi: 10.1098/rspb.2006.3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S, Denda T, Liao C-C, Lin Y-H, Liu W-T, Izawa M. Comparison of visitors and pollinators of Mucuna macrocarpa between urban and forest environments. BioOne. 2018;43:219–228. doi: 10.3106/ms2018-0029. [DOI] [Google Scholar]
- Kovács-Hostyánski A, Li JL, Pettis J, Settele J, Aneni T, Espíndola A, Kahono S, Szentgyorgyi H, et al. Drivers of change of pollinators, pollination networks and pollination. In: Potts SG, Imperatriz-Fonseca VL, Ngo HT, et al., editors. IBPES: The assessment report of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services on pollinators, pollination and food production. Bonn: IBPES; 2016. pp. 27–149. [Google Scholar]
- Kratschmer S, Kriechbaum M, Pachinger B. Buzzing on top: Linking wild bee diversity, abundance and traits with green roof qualities. Urban Ecosystems. 2018;21:429–446. doi: 10.1007/s11252-017-0726-6. [DOI] [Google Scholar]
- Ksiazek K, Fant J, Skogen K. An assessment of pollen limitation on Chicago green roofs. Landscape and Urban Planning. 2012;4:401–408. doi: 10.1016/j.landurbplan.2012.07.008. [DOI] [Google Scholar]
- Leong M, Kremen C, Roderick GK. Pollinator interactions with Yellow Starthistle (Cantaurea solstitialis) across urban, agricultural and natural landscapes. PLoS ONE. 2014;9:e86357. doi: 10.1371/journal.pone.0086357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leveau LM. Dynamics of nectarivore in the house sparrow in an urban environment. Ornitologia Tropical. 2008;19:275–281. [Google Scholar]
- Liu H, Koptur S. Breeding system and pollination on a narrowly endemic herb of the Lower Florida Keys: Impacts of the urban-wildland interface. American Journal of Botany. 2003;90:1180–1187. doi: 10.3732/ajb.90.8.1180. [DOI] [PubMed] [Google Scholar]
- Lopez-Uribe MM, Oi CA, Del Lama MA. Nectar-foraging behavior of Euglossini bees (Hymenoptera: Apidae) in urban areas. Apidologie. 2008;39:410–418. doi: 10.1051/apido:2008023. [DOI] [Google Scholar]
- Lowenstein DM, Matteson KC, Minor ES. Evaluating the dependence of urban pollinators on ornamental, non-native, and ‘weedy’ floral resources. Urban Ecosystems. 2019;22:293–302. doi: 10.1007/s11252-018-0817-z. [DOI] [Google Scholar]
- Luder K, Knop E, Menz MHM. Contrasting responses in community structure and phenology of migratory and non-migratory pollinators to urbanization. Diversity and Distribution. 2018;24:919–927. doi: 10.1111/ddi.12735. [DOI] [Google Scholar]
- Maruyama PK, Bonizário C, Marcon AP, D'Angelo G, Silva MM, Neto ENS, Oliveira PE, Sazima I, et al. Plant-hummingbird interaction networks in urban areas: Generalization and the importance of trees with specialized flowers as a nectar resource for pollinator conservation. Biological Conservation. 2019;230:187–194. doi: 10.1016/j.biocon.2018.12.012. [DOI] [Google Scholar]
- Matteson KC, Langellotto GA. Bumble bee abundance in New York City community gardens: Implications for urban agriculture. Cities and Environment. 2009;2:5–12. doi: 10.15365/cate.2152009. [DOI] [Google Scholar]
- Matteson KC, Langellotto GA. Small scale additions of native plants fail to increase beneficial insect richness in urban gardens. Insect Conservation and Diversity. 2011;4:89–98. doi: 10.1111/j.1752-4598.2010.00103.x. [DOI] [Google Scholar]
- Matteson KC, Grace JB, Minor ES. Direct and indirect effects of land use on floral resources and flower-visiting insects across an urban landscape. Oikos. 2013;122:682–694. doi: 10.1111/j.1600-0706.2012.20229.x. [DOI] [Google Scholar]
- McFrederick QS, LeBuhn G. Are urban parks refuges for bumble bees Bombus spp. (Hymenoptera: Apidae)? Biological Conservation. 2006;129:372–382. doi: 10.1016/j.biocon.2005.11.004. [DOI] [Google Scholar]
- McKinney ML. Urbanization, biodiversity and conservation. BioScience. 2002;52:883–890. doi: 10.1641/0006-3568(2002)052[0883:UBAC]2.0.CO;2. [DOI] [Google Scholar]
- McKinney ML. Urbanization as a major cause of biotic homogenization. Biological Conservation. 2006;127:247–260. doi: 10.1016/j.biocon.2005.09.005. [DOI] [Google Scholar]
- McKinney ML. Effects of urbanization on species richness: A review of plants and animals. Urban Ecosystems. 2008;11:161–176. doi: 10.1007/s11252-007-0045-4. [DOI] [Google Scholar]
- Melles S, Glenn S, Martin K. Urban bird diversity and landscape complexity: Species-environment association a long a multiscale habitat gradient. Ecology and Society. 2003;7:5. [Google Scholar]
- Mendonça LB, Anjos L. Feeding behavior of hummingbirds and perching birds on Erythrina speciosa Andrews (Fabaceae) flowers in an urban area, Londrina, Paraná, Brazil. Revista Brasileira de Zoologia. 2006;23:42–49. doi: 10.1590/S0101-81752006000100002. [DOI] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. PLoS Medicine. 2009;6(7):e1000097. doi: 10.1371/journal.pmed1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales CL, Aizen MA. Does invasion of exotic plants promote invasion of exotic flower visitors? A case study from the temperate forests of the southern Andes. Biological Invasions. 2002;4:87–100. doi: 10.1023/A:1020513012689. [DOI] [Google Scholar]
- Morales CL, Aizen MA. Invasive mutualisms and the structure of plant-pollinator interactions in the temperate forests of north-west Patagonia, Argentina. Journal of Ecology. 2006;94:171–180. doi: 10.1111/j.1365-2745.2005.01069.x. [DOI] [Google Scholar]
- Moro MF, Westerkamp C, Araújo FS. How much importance is given to native plants in cities’ treescape? A case study in Fortaleza, Brazil. Urban Forestry & Urban Greening. 2014;13:365–374. doi: 10.1016/j.ufug.2014.01.005. [DOI] [Google Scholar]
- Moro MF, Castro ASF. A check list of plant species in the urban forestry of Fortaleza, Brazil: Where are the native species in the country of megadiversity? Urban Ecosystems. 2015;18:47–71. doi: 10.1007/s11252-014-0380-1. [DOI] [Google Scholar]
- Morón D, Przylowiccs L, Nobis M, Nobis A, Klichowska E, Lenda M, Skórka P, Tryjanowski P. Do levees support diversity and affects spatial turnover of communities in plant-herbivore systems in an urban landscape? Ecological Engineering. 2017;105:198–204. doi: 10.1016/j.ecoleng.2017.04.052. [DOI] [Google Scholar]
- Müller, N., M. Ignatieva, C.H. Nilon, P. Werner, and W.C. Zipperer. 2013. Patterns and trends in urban biodiversity and landscape design. In Urbanization, biodiversity and ecosystem services: Challenges and opportunities, A global assessment, ed. T. Elmqvist, M. Fragkias, J. Goodness, B. Güneralp, P.J. Marcotullio, R.I. McDonald, S. Parnell, M. Scheweniius, et al., 123–174. Springer: London.
- Myers N, Mittermeier RA, Mittermeier CG, Fonseca GAB, Kent J. Biodiversity hotspots for conservation priorities. Nature. 2000;403:853–858. doi: 10.1038/35002501. [DOI] [PubMed] [Google Scholar]
- Novais SMA, Nunes CA, Santos NB, D’Amico AR, Fernandes GW, Quesada M, Braga RF, Neves ACO. Effects of a possible pollinator crisis on food crop production in Brazil. PLoS ONE. 2016;11:e0167292. doi: 10.1371/journal.pone.0167292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollerton J, Winfree R, Tarrant S. How many flowering plants are pollinated by animals? Oikos. 2011;120:321–326. doi: 10.1111/j.1600-0706.2010.18644.x. [DOI] [Google Scholar]
- Oliveira W, Silva JLS, Oliveira MTP, Cruz-Neto O, Silva LAP, Borges LA, Sobrinho MS, Lopes AV. Reduced reproductive success of the endangered tree brazilwood (Paubrasilia echinata, Leguminosae) in urban ecosystem compared to Atlantic forest remnant: Lessons for tropical urban ecology. Urban Forestry & Urban Greening. 2019;41:303–312. doi: 10.1016/j.ufug.2019.04.020. [DOI] [Google Scholar]
- Oliveira W, Silva JLS, Oliveira MTP, Cruz-Neto O, Silva LAP, Borges LA, Sobrinho MS, Lopes AV. Flowering and fruiting synchronization, pollen number, floral visitors and reproductive success of Paubrasilia echinata (brazilwood; Leguminosae) in tropical urban ecosystem in comparison to Atlantic forest remnant: A dataset description. Data in Brief. 2019;25:104177. doi: 10.1016/j.dib.2019.104177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsini F, Kahane R, nono-Womdim, R., Giaquinto, G. Urban agriculture in the developing world: A review. Agronomy for Sustainable Development. 2013;33:695–720. doi: 10.1007/s13593-013-0143-z. [DOI] [Google Scholar]
- Pauchard, A., and O. Barbosa. 2013. Regional assessment of Latin America: Rapid urban development and social economic inequity threaten biodiversity hotspots. In Urbanization, biodiversity and ecosystem services: Challenges and opportunities A global assessment, ed. T. Elmqvist, M. Fragkias, J. Goodness, B. Güneralp, P.J. Marcotullio, R.I. McDonald, S. Parnell, M. Scheweniius, et al., 580–607. London: Springer.
- Pardee GL, Philpott SM. Native plants are the bee’s knees: Local and landscape predictors of bee richness and abundance in backyard gardens. Urban Ecosystems. 2014;17:641–659. doi: 10.1007/s11252-014-0349-0. [DOI] [Google Scholar]
- Parker IM. Pollinator limitation of Cytisus scoparius (Scotch Broom), an invasive exotic shrub. Ecology. 1997;78:1457–1470. doi: 10.1890/0012-9658(1997)078[1457:PLOCSS]2.0.CO;2. [DOI] [Google Scholar]
- Pauw A, Louw K. Urbanization drives a reduction in functional diversity in a guild of nectar-feeding birds. Ecology and Society. 2012;17:27. doi: 10.5751/ES-04758-170227. [DOI] [Google Scholar]
- Pellegrino G, Bellusci F. Effects of human disturbance on reproductive success and population viability of Serapias cordigera. Botanical Journal of the Linnean Society. 2014;176:408–420. doi: 10.1111/boj.12204. [DOI] [Google Scholar]
- Pellissier V, Muratet A, Verfaillie F, Machon N. Pollination success of Lotus corniculatus (L.) in an urban context. Acta Oecologia. 2012;39:94–100. doi: 10.1016/j.actao.2012.01.008. [DOI] [Google Scholar]
- Pellissier V, Maurel N, Machon N. Multi-scale assessment of pollination of Lotus corniculatus (L.) in a peri-urban fringe. Plant Ecology & Diversity. 2013;6:195–203. doi: 10.1080/17550874.2012.755227. [DOI] [Google Scholar]
- Peng J, Zhao H, Liu Y. Urban ecological corridors construction: A review. Acta Ecologica Sinica. 2017;37:23–30. doi: 10.1016/j.chnaes.2016.12.002. [DOI] [Google Scholar]
- Previatto, D.M., R.S. Mizove, and S.R. Posso. 2012. Birds as potential pollinators of the Spatodea nilotica (Bignoniaceae) in the urban environment. Brazilian Journal of Biology 73: 737–741. [DOI] [PubMed]
- Potter A, LeBuhn G. Pollination service to urban agriculture in San Francisco, CA. Urban Ecosystems. 2015;18:885–893. doi: 10.1007/s11252-015-0435-y. [DOI] [Google Scholar]
- Potts SG, Biesmejier JC, Kremen C, Neumann P, Schweiger O, Kunin WE. Global pollinator declines: Trends, impacts and drivers. Trends in Ecology and Evolution. 2010;25:345–353. doi: 10.1016/j.tree.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Potts SG, Imperatriz-Fonseca V, Ngo HT, Biesmeijer JC, Breeze TD, Dicks LV, Garibaldi LA, Hill R, et al. Summary for policymakers of the thematic assessment on pollinators, pollination and food production Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Key messages Values of pollinators and pollination. Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services. 2016;3:1–24. [Google Scholar]
- Pufal G, Klein AM. Spatial scale affects seed predation and dispersal in contrasting anthropogenic landscapes. Basic and Applied Ecology. 2015;16:726–736. doi: 10.1016/j.baae.2015.07.003. [DOI] [Google Scholar]
- Ratto F, Simmons BI, Spake R, Zamora-Gutierrez V, MacDonald MA, Merriman JC, Tremlett CJ, Poppy GM, et al. Global importance of vertebrate pollinators for plant reproductive success: A meta-analysis. Frontiers in Ecology and the Environment. 2018;16:82–90. doi: 10.1002/fee.1763. [DOI] [Google Scholar]
- Rossum FV. Reproductive success and pollen dispersal in urban populations of an insect-pollinated hay-meadow herb. Perspectives in Plant, Ecology, Evolution and Systematics. 2010;12:21–29. doi: 10.1016/j.ppees.2009.08.002. [DOI] [Google Scholar]
- Russo D, Ancillotto L. Sensitivity of bats to urbanization: A review. Mammalian Biology. 2015;80:205–2012. doi: 10.1016/j.mambio.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler T, Duelli P, Obrist MK, Arlettaz R, Moretti M. Response of arthropod species richness and functional groups to urban habitat structure and management. Landscape Ecology. 2010;25:941–954. doi: 10.1007/s10980-010-9473-2. [DOI] [Google Scholar]
- Shwartz A, Turbé A, Simon L, Julliard R. Enhancing urban diversity and its influence on city-dwellers: An experiment. Biological Conservation. 2013;171:82–90. doi: 10.1016/j.biocon.2014.01.009. [DOI] [Google Scholar]
- Seto, K.C., S. Parnell, and T. Elmqvist. 2013. A global outlook on urbanization. In Urbanization, biodiversity and ecosystem services: Challenges and opportunities, A global assessment, ed. T. Elmqvist, M. Fragkias, J. Goodness, B. Güneralp, P.J. Marcotullio, R.I. McDonald, S. Parnell, M. Scheweniius, et al. London: Springer.
- Siemszko M, Zych M. Urban ecosystems—a place for pollinators? A mini-review and social implications. Miestųželdynų Formavimas. 2017;1:193–201. [Google Scholar]
- Silva JLS, Oliveira MTP, Oliveira W, Borges LA, Cruz-Neto O, Lopes AV. High richness of exotic species in tropical urban green spaces: Reproductive systems, fruiting and associated risks to native species. Urban Forestry and Urban Greening. 2020;50:126659. doi: 10.1016/j.ufug.2020.126659. [DOI] [Google Scholar]
- Sluijs JP, Vaage NS. Pollinators and global food security: The need for holistic global stewardship. Food Ethics. 2016;1:75–91. doi: 10.1007/s41055-016-0003-z. [DOI] [Google Scholar]
- Smit J, Ratta A, Nasr J. Urban agriculture: Food, jobs and sustainable cities. New York: UNDP; 1996. [Google Scholar]
- Smith RM, Warren PH, Thompson K, Gaston KJ. Urban domestic gardens (VI): Environmental correlates of invertebrate species richness. Biodiversity and Conservation. 2006;15:2415–2538. doi: 10.1007/s10531-004-5014-0. [DOI] [Google Scholar]
- Stout JC, Morales CL. Ecological impacts of invasive alien species on bee. Apidologie. 2009;40:388–409. doi: 10.1051/apido/2009023. [DOI] [Google Scholar]
- Tommasi D, Miro A, Higo HA, Winston ML. Bee diversity and abundance in an urban setting. The Canadian Entomologist. 2004;136:851–869. doi: 10.4039/n04-010. [DOI] [Google Scholar]
- Tonietto R, Fant J, Ascher J, Ellis K, Larkin D. A comparison of bee communities of Chicago green roofs, parks and prairies. Landscape and Urban Planning. 2011;103:102–108. doi: 10.1016/j.landurbplan.2011.07.004. [DOI] [Google Scholar]
- Totland O, Nielsen A, Bjerknes AL, Ohlson M. Effects of an exotic plant and habitat disturbance on pollinator visitation and reproduction in a boreal forest herb. American Journal of Botany. 2006;93:868–873. doi: 10.3732/ajb.93.6.868. [DOI] [PubMed] [Google Scholar]
- Tratalos J, Fuller RA, Warren PH, Davies RG, Gaston KJ. Urban form, biodiversity potential and ecosystem services. Landscape and Urban Planning. 2006;83:308–317. doi: 10.1016/j.landurbplan.2007.05.003. [DOI] [Google Scholar]
- Traveset, A., R. Heleno, S. Chamorro, P. Vargas, C.K. McMullen, R. Castro-Urgal, M. Nogales, H.W. Herrera, et al. 2013. Invaders of pollination networks in the Galápagos Islands: emergence of novel communities. Proceedings of the Royal Society 280: 20123040. 10.1098/rspb.2012.3040. [DOI] [PMC free article] [PubMed]
- United Nations. 2019. World economic situation prospects 2019. Department of Economic and Social Affairs, United Nation publication, Sales No.E.19.II.C.1. 218pp.
- van Veenhuizen, R. 2006. Cities farming for the future, urban agriculture for green and productive cities. RUAF Foundation, IDRC and IIRR, Phillipines
- Verboven HAS, Brys R, Hermy M. Sex in the city: Reproductive success of Digitalis purpurea in a gradient from urban to rural sites. Landscape and Urban Planning. 2012;106:158–164. doi: 10.1016/j.landurbplan.2012.02.015. [DOI] [Google Scholar]
- Viana BF, Boscolo DN, Mariano-Neto E, Lopes LE, Lopes AV, Ferreira PA, Pigozzo CM, Primo LM. How well do we understand landscape effects on pollinators and pollination services? Journal of Pollination Ecology. 2012;7:31–40. doi: 10.26786/1920-7603(2012)2. [DOI] [Google Scholar]
- Wang D, Chen J, Zhang L, Sun Z, Wang X, Zhang X, Zhang W. Establishing an ecological security pattern for urban agglomeration, taking ecosystem services and human interferences factors into consideration. PeerJ. 2019;7:e7306. doi: 10.7717/peerj.7306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel A, Grass I, Belavadi VV, Tscharntke T. How urbanization is driving pollinator diversity and pollination: A systematic review. Biological Conservation. 2020;241:108321. doi: 10.1016/j.biocon.2019.108321. [DOI] [Google Scholar]
- Winfree R, Griswold T, Kremen C. Effect of human disturbance on bee communities in a forested ecosystem. Conservation Biology. 2007;21:213–223. doi: 10.1111/j.1523-1739.2006.00574.x. [DOI] [PubMed] [Google Scholar]
- Wray JC, Elle E. Flowering phenology and nesting resources influence pollinator community composition in a fragmented ecosystem. Landscape Ecology. 2015;30:261–271. doi: 10.1007/s10980-014-0121-0. [DOI] [Google Scholar]
- Zhang L, Tan PY, Diehl J. A conceptual framework for studying urban green spaces effects on health. Journal of Urban Ecology. 2017;3:1–13. doi: 10.1093/jue/jux015. [DOI] [Google Scholar]
- Zhao C, Sander HA, Hendrix SD. Wild bees and urban agriculture: Assessing pollinator supply and demand across urban landscapes. Urban Ecosystems. 2019;22:455–470. doi: 10.1007/s11252-019-0826-6. [DOI] [Google Scholar]
- Zotarelli HGS, Evans DM, Bego LR, Sofia SH. A comparison of social bee-plant networks between two urban areas. Neotropical Entomology. 2014;43:399–408. doi: 10.1007/s13744-014-0227-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.