Abstract
The Hedgehog (HH) signaling pathway plays important roles in gastrointestinal carcinogenesis and the gastrointestinal tumor microenvironment (TME). Aberrant HH signaling activation may accelerate the growth of gastrointestinal tumors and lead to tumor immune tolerance and drug resistance. The interaction between HH signaling and the TME is intimately involved in these processes, for example, tumor growth, tumor immune tolerance, inflammation, and drug resistance. Evidence indicates that inflammatory factors in the TME, such as interleukin 6 (IL-6) and interferon-γ (IFN-γ), macrophages, and T cell-dependent immune responses, play a vital role in tumor growth by affecting the HH signaling pathway. Moreover, inhibition of proliferating cancer-associated fibroblasts (CAFs) and inflammatory factors can normalize the TME by suppressing HH signaling. Furthermore, aberrant HH signaling activation is favorable to both the proliferation of cancer stem cells (CSCs) and the drug resistance of gastrointestinal tumors. This review discusses the current understanding of the role and mechanism of aberrant HH signaling activation in gastrointestinal carcinogenesis, the gastrointestinal TME, tumor immune tolerance and drug resistance and highlights the underlying therapeutic opportunities.
Key words: Hedgehog, Carcinogenesis, Tumor microenvironment, Gastrointestinal cancer, Cancer stem cells, Drug resistance
Abbreviations: 5-Fu, 5-fluorouracil; ALK5, TGF-β receptor I kinase; ATO, arsenic trioxide; BCC, basal cell carcinoma; BCL-2, B cell lymphoma 2; BMI-1, B cell-specific moloney murine leukemia virus insertion region-1; CAFs, cancer-associated fibroblasts; ch5E1, chimeric monoclonal antibody 5E1; CSCs, cancer stem cells; DHH, Desert Hedgehog; EGF, epidermal growth factor; FOLFOX, oxaliplatin; and leucovorin, GLI; glioma-associated oncogene homologue, GRK2; G protein coupled receptor kinase 2, HH; Hedgehog, HIF-1α; hypoxia-inducible factor 1α, IFN-γ: interferon-γ; IHH, Indian Hedgehog; IL-10/6, interleukin 10/6; ITCH, itchy E3 ubiquitin ligase; MDSCs, myeloid-derived suppressor cells; NK, natural killer; NOX4, NADPH Oxidase 4; PD-1, programmed cell death-1; PD-L1, programmed cell death ligand-1; PKA, protein kinase A; PTCH, Patched; ROS, reactive oxygen species; SHH, Sonic Hedgehog; SMAD3, mothers against decapentaplegic homolog 3; SMO, Smoothened; SNF5, sucrose non-fermenting 5; STAT3, signal transducer and activator of transcription 3; SUFU, Suppressor of Fused; TAMs, tumor-related macrophages; TGF-β, transforming growth factor β; TME, tumor microenvironment; VEGF, vascular endothelial growth factor; WNT, Wingless/Integrated; βArr2, β-arrestin2
Graphical abstract
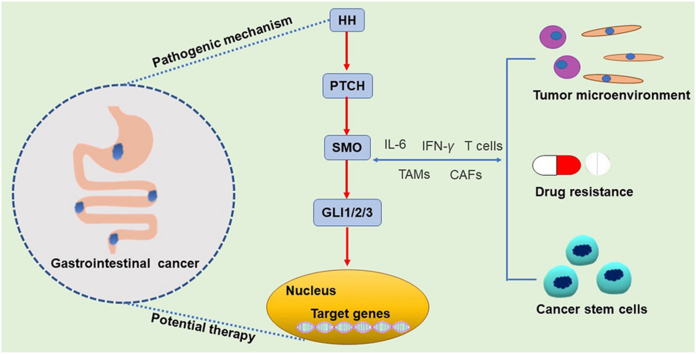
Hedgehog pathway (HH) is one of the most important modulators of gastrointestinal cancer. This review concludes the role of HH pathway in gastrointestinal carcinogenesis as well as the development of tumor microenvironment, which indicates the mechanisms of immune tolerance and drug resistance in gastrointestinal cancers and highlights the potential therapeutics.
1. Introduction
Hedgehog (HH) molecules are the key modulators that regulate diverse processes ranging from tissue patterning and cell differentiation to cancer initiation, progression, and metastasis1. In the gastrointestinal tract, HH signaling is extensively involved in gastrointestinal organogenesis and cancer development, especially in regulating the tumor microenvironment (TME)2, 3, 4. The gastrointestinal tract develops from the embryonic gut, which consists of an endoderm-derived epithelium surrounded by cells of mesodermal origin5. Normal activation of HH signaling during embryonic development has been documented to be related to the development of the gastrointestinal tract6. In the early stage, the widespread activation of Indian Hedgehog (IHH) and Sonic Hedgehog (SHH) in the endoderm is essential for the development of the gastrointestinal tract. In the late stage, changes in the expression of IHH and SHH promote the differentiation of gastrointestinal tract cells towards different lineages. In addition to its critical roles in normal gastrointestinal development, HH signaling, when aberrantly activated, is involved in the development, progression, metastasis, and drug resistance of gastrointestinal cancers7.
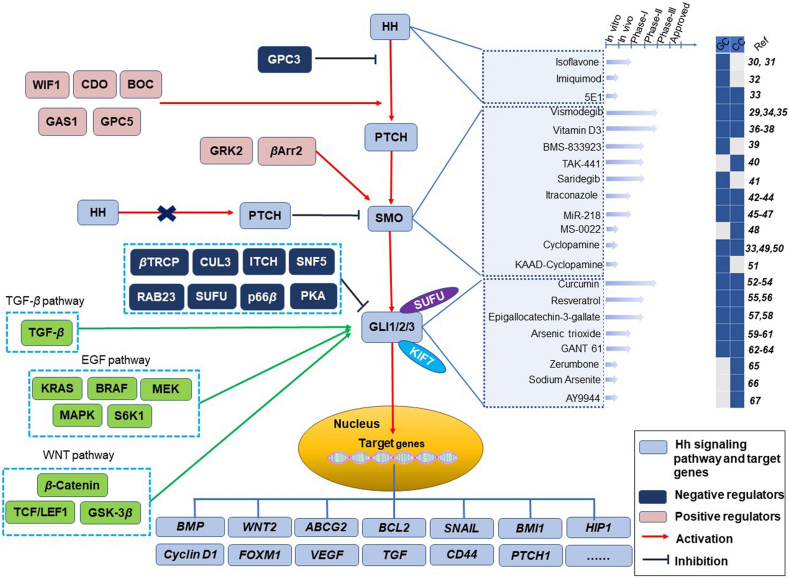
The canonical HH signaling pathway is tightly regulated by a complex signaling network (Fig. 1). Following the binding of HH ligands [including IHH, SHH, and Desert Hedgehog (DHH) molecules] to their receptor Patched (PTCH), Smoothened (SMO) is derepressed to activate glioma-associated oncogene homologue (GLI) signal transduction and promote the transcription of downstream HH-related genes8, 9, 10, 11, 12, 13, 14. Among the GLI family of transcription factors (GLI1, GLI2 and GLI3), GLI1 is well known as an exclusively full-length transcriptional activator. Its activity is regulated by the Suppressor of Fused (SUFU) protein, vital suppressor of the HH signaling pathway15,16. In the absence of ligand binding, SUFU directly prevents the translocation of GLI proteins from the cytoplasm to the nucleus and thus inhibits HH signal transduction2,17,18. In addition, kinesin-like protein KIF7 is involved in the processing of GLI molecules19. The function of SMO requires the presence of β-arrestin2 (βArr2) and G protein-coupled receptor kinase 2 (GRK2)20,21. Other negative regulators of GLI molecules include RAB23, protein kinase A (PKA), SUFU, sucrose non-fermenting 5 (SNF5), β-TRCP, the itchy E3 ubiquitin ligase (ITCH), and so on16,22, 23, 24, 25. Moreover, the HH pathway interacts with other signaling pathways, such as the transforming growth factor β (TGF-β) pathway, epidermal growth factor (EGF) pathway and Wingless/Integrated (WNT) pathway (Fig. 1)26,27.
Figure 1.
The Hedgehog (HH) signaling pathway and its related regulators, target genes and current therapeutic landscape. The coreceptors for HH include cell adhesion molecule-related/downregulated by oncogenes (CDO), brother of CDO (BOC), growth arrest-specific 1 (GAS1), glypican 3 (GPC3) and glypican 5 (GPC5). Wingless/Integrated (WNT) inhibitory factor-1 (WIF1) affects HH signaling via CDO, BOC or GPC5. The function of Smoothened (SMO) needs β-arrestin 2 (βArr2) and G protein-coupled receptor kinase 2 (GRK2). Suppressor of Fused (SUFU)/KIF7 is involved in the processing of glioma-associated oncogene homologue (GLI) molecules. Other negative regulators of GLI molecules include RAB23, protein kinase A (PKA), SUFU, sucrose non-fermenting 5 (SNF5), cullin-3 (CUL3), p66β, β-TRCP and Itch. Patched (PTCH) is shuttled out of the cilium and cannot inhibit SMO in the presence of HH, and HH binding promotes a conformational change in SMO. However, PTCH inhibits SMO signaling independent of HH ligand binding. The pathways interacting with the HH pathway, including the transforming growth factor β (TGF-β) pathway, epidermal growth factor (EGF) pathway and WNT pathway, are shown in green. HH inhibitors that have been effective in treating gastrointestinal tumors include isoflavone30,31, imiquimod32 and 5E133. SMO inhibitors that have been effective in treating gastrointestinal tumors include vismodegib29,34,35, vitamin D336, 37, 38, BMS-83392339, TAK-44140, saridegib41, itraconazole42, 43, 44, miR-21845, 46, 47, MS-002248, cyclopamine33,49,50 and KAAD-cyclopamine51. GLI inhibitors that have been effective in treating gastrointestinal tumors include curcumin52, 53, 54, resveratrol55,56, epigallocatechin-3-gallatel57,58, arsenic trioxide59, 60, 61, GANT 6162, 63, 64, zerumbone65, sodium arsenite66 and AY994467. Dark blue represents the type of tumor that the inhibitor is effective in. The arrows indicate progress. CC, colorectal cancer; GC, gastric cancer; BMP, bone morphogenetic protein; FOXM1, forkhead box protein M1; VEGF, vascular endothelial growth factor; MAPK, mitogen-activated protein kinase; TCF/LEF1, T-cell factor/lymphoid enhancer factor1; GSK-3β, glycogen synthase kinase-3β; HIP1, Huntingtin-interacting protein 1; BMI-1, B cell-specific Moloney murine leukemia virus insertion region-1; Ref, reference.
Recently, aberrant activation of the HH signaling pathway has been reported to play vital roles in both the carcinogenesis and pathogenesis of gastrointestinal tumors2,6,28. In addition, the role of HH activation in cancer stem cells (CSCs) is one of the mechanisms that lead to drug resistance in gastrointestinal tumors29. In this review, we aim to review the current understanding of the roles and mechanisms of aberrant HH signaling activation in gastrointestinal carcinogenesis and the gastrointestinal TME and to highlight the underlying therapeutic insights.
2. HH signaling in gastrointestinal tract carcinogenesis
In tumor tissues, aberrant activation of HH signaling can be observed in both cancer cells and the surrounding stroma. In cancer cells, HH ligand overexpression or mutations in HH signaling-related genes (SMO, PTCH1, GLI1 and GLI2) are involved in the pathogenesis of many epithelial cancers, including stomach, liver, esophageal, breast, and skin cancers68,69. In stromal cells, HH signaling activation favors the formation of an immunosuppressive environment to support tumor development and progression70,71. HH inhibitors can exert cytotoxic effects by directly targeting HH signaling activity in cancer cells and inhibit tumor growth72. The following sections discuss the role and mechanism of HH signaling activation in gastrointestinal cancers.
2.1. The role of HH signaling in gastrointestinal carcinogenesis
Aberrant HH signaling activation is associated with a variety of gastrointestinal diseases, including Pallister–Hall syndrome, gut malrotation, and gastric cancer6. Aberrant activation of HH signaling can promote the proliferation, metastasis, and drug resistance of gastric cancer cells and inhibit their apoptosis7. In gastritis, activation of HH signaling is required for the transformation of infiltrating myeloid cells to myeloid-derived suppressor cells (MDSCs), which help transformed cells evade immune surveillance, indicating the essential role of HH signaling activation in cancer development73,74. During Helicobacter pylori infection, the SHH ligand originating from gastric parietal cells is ectopically expressed to induce metaplasia, which can eventually lead to tumorigenesis75,76. In gastrointestinal cancer, the expression of SHH and IHH signaling pathway components and their target genes is upregulated77,78. Specifically, the expression of GLI1 is usually correlated with the degree of malignancy of gastric cancer, especially with lymph node metastasis79.
In addition, aberrantly activated HH signaling interacts with signaling in immune checkpoint pathways to favor gastric cancer development. A recent study indicated that during H. pylori infection, the bacteria induce programmed cell death ligand-1 (PD-L1) expression on gastric epithelial cells and that this process is dependent on the HH signaling pathway, suggesting an interaction between HH signaling and this immune checkpoint pathway during tumor development80. This interaction is also observed in cancer cells. HH signaling activation has been reported to induce the expression of PD-L1 on gastric cancer cells and thus help cancer cells evade immune surveillance, suggesting the potential immunosuppressive and the carcinogenic effects of aberrant HH signaling activation on gastrointestinal cancer63.
2.2. HH signaling and gastrointestinal CSCs
Aberrant activation of the HH signaling pathway is involved in the growth and regulation of CSCs in gastrointestinal cancer. Upregulation of SHH and GLI1 is crucial for CD44+ gastrointestinal CSCs to maintain their stem-like phenotype and malignant transformation ability, and the role of HH signaling activation in gastrointestinal CSCs has been observed in many studies29,81, 82, 83, 84, 85, 86. For example, genistein can inhibit the activity of highly migratory CD44+ cells by downregulating GLI1 expression. This study showed that GLI1 inhibition can attenuate the cancer stem-like properties of gastric cancer cells and thus reduce their invasive ability84. Upregulation of GLI2A, an activator of GLI2, rapidly accelerates gastric cancer development from Lgr5+ CSCs87. Activation of GLI1 through the integrin αvβ3/ERK1/2 pathway is reported to be crucial for maintaining the stem-like phenotype during gastric cancer metastasis79. In addition, GLI1 is co-expressed with CSC markers such as SOX9 and CD133 in tissues of colorectal adenocarcinoma patients, and inhibiting GLI1 expression can reduce the expression of these markers in gastric cancer cells, suggesting an important role of GLI1 in colorectal adenocarcinoma stemness88,89. Whole-transcriptome analysis revealed that the expression levels of both noncanonical PTCH1-dependent and SHH-dependent HH signaling components are upregulated in colorectal CSCs, and the expression levels of both PTCH1 and GLI1 are positively correlated with colorectal cancer stemness90. Moreover, other studies have shown that HH signaling activation affects the expression of CSC-related markers, such as B cell-specific Moloney murine leukemia virus insertion region-1 (BMI1), SNAIL, B cell lymphoma 2 (BCL-2), WNT2, and CD44 (Fig. 1)29,81,91,92.
In summary, it is reasonable to believe that upregulation of SHH, GLI1 and PTCH1 can maintain the stem-like phenotype and malignant transformation ability of gastrointestinal CSCs.
2.3. HH signaling and drug resistance in gastrointestinal cancer
Activation of HH signaling is intimately involved in drug resistance in gastrointestinal cancer. A series of studies demonstrated that inhibition of GLI using pharmacological inhibitors (GANT61 and GDC-0449) or knockdown of GLI1/SHH can induce apoptosis and enhance the antitumor effect of chemotherapy in xenograft models85. GLI can modulate ABCG2 expression through transcriptional regulation to maintain 5-fluorouracil (5-Fu) resistance92, 93, 94, 95. In addition, another recent study proposed that GLI2 is activated to promote chemoresistance via hypoxia-inducible factor (HIF-1α) and TGF-β2 in the hypoxic TME of colorectal cancer and that high expression levels of GLI2 are significantly related to post chemotherapy recurrence in colorectal cancer patients. These results suggest that GLI2 could be a biomarker for drug resistance in colorectal cancer96. Furthermore, evidence from a phase II clinical study indicates that vismodegib, an HH inhibitor, may reduce resistance to 5-Fu, oxaliplatin, and leucovorin (FOLFOX) therapy in advanced gastric cancers with high CD44 expression29. AY9944 and GANT61, two other HH inhibitors, can downregulate the expression of CSC markers (c-MYC, CD44, and Nanog) and significantly reduce resistance to 5-Fu and irinotecan in colorectal cancer67,97. Finally, the cooperative action of cyclopamine and paclitaxel has shown significant therapeutic effects on the enhancement of PD-1 checkpoint blockade treatment in solid tumors by augmenting CD8+ T cell infiltration, suggesting that HH inhibitors can overcome resistance to immunotherapeutic drugs98.
In conclusion, drug resistance resulting from HH signaling activation may be mediated by CSCs, and inhibiting HH signaling in CSCs can contribute to reducing drug resistance and enhancing the antitumor effect of chemotherapy or immunotherapy in gastrointestinal tumors.
3. HH signaling in the gastrointestinal TME
3.1. The gastrointestinal TME
The TME comprises various types of nontransformed cells (e.g., endothelial cells, fibroblasts, and immune cells) and extracellular components (e.g., inflammatory cytokines, various growth factors, and the extracellular matrix) that surround tumor cells and include a complex vascular network99, 100, 101. Accumulating evidence indicates that aberrant activation of the HH signaling pathway contributes to immune evasion and cancer development by modulating the TME. HIF-1α and TGF-β2 secreted by cancer-associated fibroblasts (CAFs), a main component of the TME, have been reported to upregulate the expression of GLI2 in CSCs, resulting in increased stemness/dedifferentiation and drug resistance96. Moreover, aberrant activation of HH signaling can affect many genes or cytokines, such as vascular endothelial growth factor (VEGF), angiogen-1, and angiogen-2 in endothelial cells; interleukin 6 (IL-6) in myofibroblasts; and BMI-1 and NANOG in CSCs. In fact, these genes or cytokines can promote tumor growth by maintaining an immunosuppressive environment102, 103, 104. Inhibition of HH pathway signaling reduces the solid tumor mass by eliminating tumor cells and stromal cells, indicating the important role of HH signaling in the TME105.
3.2. The interaction between HH signaling and CAFs promotes tumor proliferation
CAFs belong to the intermediate mesenchymal cell population in solid tumors, with morphological and some functional characteristics like those of fibroblasts in normal tissues106. However, CAFs in the TME are also significantly involved in tumor development and progression by promoting the proliferation of tumor cells and assisting theirs to escape from immune killing107. Aberrant activated HH signaling and CAFs interact with each other and cooperatively promote tumor development108. On the one hand, tumor cell-derived SHH has been confirmed to modulate CAFs via paracrine activation of HH signaling in solid cancers, and inhibition of HH signaling by vismodegib remodels the TME by reducing the proliferation of CAFs71,109. Moreover, studies have shown that overexpression of SMO in CAFs contributes to HH signal transduction and GLI1 activation, a possible mechanism underlying paracrine activation of HH signaling in solid cancers110. In addition, other studies have shown that the HH inhibitors cyclopamine and vismodegib can decrease the population of stroma-producing CAFs in tumors71,111. Therefore, inhibition of CAF proliferation in tumors by HH signaling antagonists contributes to normalizing the TME, which in turn helps to overcome gastric cancer.
On the other hand, CAFs, which are the major source of proinflammatory cytokines in the TME, can produce tumor-associated cytokines such as IL-6, HIF-1α and TGF-β2 in gastrointestinal cancer. These cytokines can regulate SHH expression during tumor transformation and ultimately accelerate tumor development96,112, 113, 114, 115, 116. IL-6 has been found to promote carcinogenic HH/GLI signaling through signal transducer and activator of transcription 3 (STAT3) activation117,118. Other studies have shown that exosomal SHH derived from CAFs facilitates the proliferation and malignant progression of tumor cells119. Thus, aberrant activation of HH signaling can further promote the inflammatory response and change the microenvironment by increasing the levels of tumor-related inflammatory factors. Furthermore, CAFs from colorectal tumors can inhibit the activity of natural killer (NK) cells and the secretion interferon-γ (IFN-γ), which is related to their proinflammatory response to immune tolerance in the TME120.
Therefore, it is reasonable to conclude that the crosstalk between CAFs and gastrointestinal tumors via aberrant activation of the HH signaling pathway is one of the factors that promote tumor development, and strategies targeting CAF-tumor crosstalk via the HH signaling pathway may provide alternative approaches for overcoming gastric cancer.
3.3. The interaction among HH signaling, tumor-associated macrophages (TAMs), and immune cells promotes tumor immune tolerance
TAMs are divided mainly into the proinflammatory M1 subtype and the anti-inflammatory M2 subtype. Studies show that tumors with high expression of HH exhibit upregulation of inflammation-related genes and increased infiltration of TAMs, which may promote tumor growth by affecting the immune response121. On the one hand, activation of HH signaling can mediate the interaction between cancer cells and macrophages and stimulate selective polarization of macrophages towards M2 subtype122. Among the TAM subtypes, M2 TAMs can activate HH signaling by secreting interleukin 10 (IL-10) and TGF-β1 to regulate the immune response and facilitate the malignant progression of gastric cancer cells, and TGF-β-mediated HH signaling is activated via the TGF-β receptor I kinase (ALK5)/mothers against decapentaplegic homolog 3 (SMAD3) pathway51,123. In addition, the activation of HH signaling can induce the recruitment of M2 subtype to upregulate FOXP3 expression and increase Treg cells124, 125, 126, 127.
On the other hand, HH-induced M2 polarization can inhibit the recruitment of CD8+ T cells and enhance the infiltration of suppressive regulatory T cells by inhibiting the production of CXCL9 and CXCL10 by TAMs, leading to TAM-mediated immunosuppression98,122. In addition, MDSCs, among other factors, contribute to tumor immunosuppression128. Activation of HH signaling can enhance the infiltration of MDSCs into the TME, which further leads to tumor immunosuppression129. Studies have shown that overexpression of SHH in H. pylori-infected mice accelerates the emergence of MDSCs in the gastric corpus73. This immunosuppressive effect was confirmed by upregulation of PD-L1, which inactivates effector T cell function and allows the proliferation of gastric cancer cells63,130. Furthermore, inhibition of HH signaling with vismodegib led to the accumulation of cytotoxic T cells and decreases in the population of immunosuppressive M2 macrophages and MDSCs in the TME70,131, 132, 133, 134.
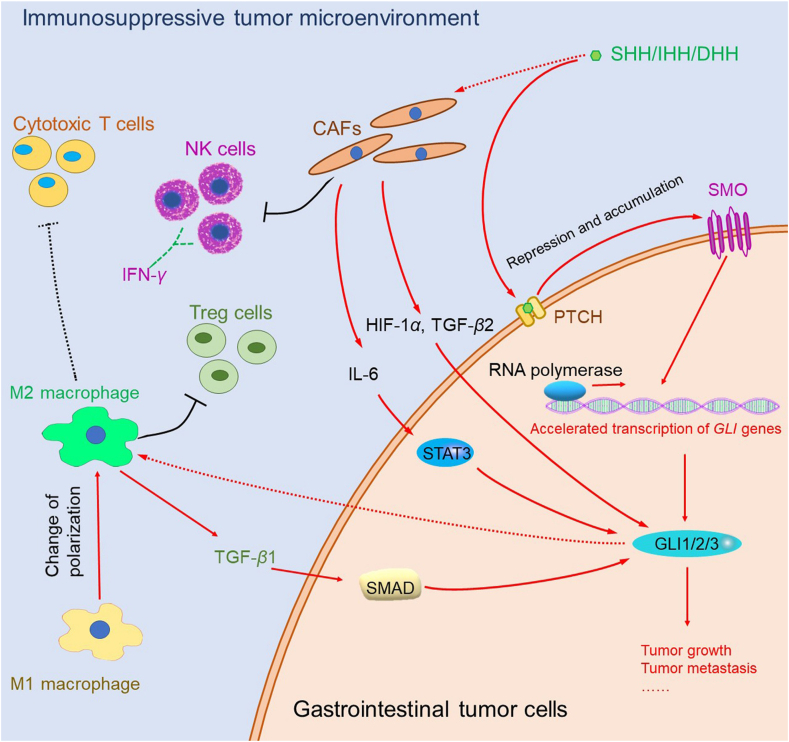
In summary, the above evidence indicates that TAMs affect the HH signaling pathway by secreting cytokines and that HH-induced M2 polarization can inhibit the accumulation of cytotoxic T cells and increase Treg cells. The overall effect of these events is the promotion of tumor growth via suppression of the immune response in gastrointestinal tumors. Inhibition of HH signaling can promote the infiltration of cytotoxic T cells, inhibit the accumulation of M2 macrophages and MDSCs, and then remodel the immunosuppressive state of the TME (Fig. 2).
Figure 2.
Interaction between Hedgehog (HH) signaling and the tumor microenvironment in gastrointestinal tumor cells. Red arrow: upregulation; black arrow: downregulation; green dashed arrow: secretion; solid line: direct interaction; dashed line: indirect interaction. CAFs, cancer-associated fibroblasts; DHH, Desert Hedgehog; IHH, Indian Hedgehog; SHH, Sonic Hedgehog; NK cells, natural killer cells; PTCH, Patched; SMO, Smoothened; TGF-β, Transforming growth factor β; IL-6, interleukin 6; IFN-γ, interferon γ; HIF-1α, hypoxia-inducible factor 1α; RNA, ribonucleic acid; STAT3, signal transducer and activator of transcription 3; SMAD, Drosophila mothers against decapentaplegic protein; GLI, glioma-associated oncogene homologue.
4. HH signaling as a potential clinical prognostic and therapeutic target in gastrointestinal cancer
4.1. The expression level of HH signaling components has important prognostic value in gastrointestinal tumors
Clinically, the level of HH signaling pathway components is associated with the prognosis of gastrointestinal cancer patients135. Clinicopathological analysis of gastrointestinal cancers revealed that the HH pathway is abnormally modulated. A series of molecules in the HH signaling pathway, such as SHH, GLI1, GLI2, SMO and PTCH, have been reported to be related to the prognosis of gastric cancer patients. First, overexpression of SHH is a biomarker for poor prognosis in gastrointestinal cancer. In a study including 117 patients who underwent radical gastrectomy, the survival time of gastric cancer patients with high expression of SHH was significantly shortened136. In 228 human colon cancer biopsies, overexpression of SHH, PTCH or GLI1 was found to be an indicator of poor prognosis, and analysis of a database containing information for 735 colon and rectal cancers showed that SMO overexpression might be associated with poor prognosis in colorectal cancer135,137. Second, positive GLI1 expression is a reliable indicator of poor prognosis in patients with highly aggressive gastric cancer. In addition, a meta-analysis of 886 patients with gastric cancer showed that positive GLI1 expression is correlated with poor prognosis in gastric cancer patients138. The poor prognosis of patients with high expression levels of GLI1 may be related to reactive oxygen species (ROS) generated by NADPH oxidase 4 (NOX4); increased production of ROS in hypoxia causes GLI1 upregulation139. Finally, some studies have shown that SUFU as a negative regulator of HH signaling pathway is downregulated and negatively associated with the tumor stage in gastric cancer, supporting the potential of SUFU expression as another diagnostic or prognostic marker140,141.
In addition to upregulation of the HH pathway, mutations in the key proteins in the HH pathway may also be important in gastrointestinal cancer development. An early study reported that 85% of basal cell carcinomas (BCCs) have HH pathway gene mutations, of which approximately 73%, 20% and 8% have PTCH1, SMO and SUFU driver mutations, respectively. This observation indicates that HH pathway gene mutations are the main driver mutations in BCC142. In a group of 39 gastrointestinal cancers, only three SMO mutations and one PTCH1 mutation were found. However, the frequencies of these mutations were low in these patients, and, due to the small sample size, the role of these mutations could not be inferred143. Therefore, large-scale profiling studies are needed to help us understand the role of those mutations in gastrointestinal cancer.
In conclusion, the levels of HH signaling pathway components have substantial prognostic value in gastrointestinal tumors. The role of SMO and PTCH1 mutations in gastrointestinal tumors and the clinical value of these mutations need to be analyzed in larger samples.
4.2. HH signaling pathway inhibitors
The HH signaling pathway is a promising therapeutic target in gastrointestinal tumors. Various molecules that inhibit this pathway have been evaluated in the treatment of gastrointestinal tumors. Most of these agents are designed to target the SHH, SMO and GLI proteins in the HH signaling pathway.
More inhibitors have been developed to target SMO than SHH or GLI. Cyclopamine, the first reported SMO inhibitor, exhibits inhibitory activity in vitro gastrointestinal tumors models33,49,50. Saridegib (IPI-926), a semisynthetic analog of cyclopamine, with improved chemical stability and biological activity, has entered a phase I clinical trial for the treatment of gastric cancer144. Vismodegib (GDC-0449), a potent inhibitor of SMO, exhibited antitumor efficacy in both preclinical studies and clinical trials145. Vismodegib and saridegib are currently approved in the United States and Europe for the treatment of adult patients with metastatic or locally advanced BCC146,147. Clinical studies showed high tumor control rates and low drug resistance in the designated BCC patient population148. Therefore, vismodegib and saridegib are effective and well-tolerated systemic treatments for specific BCC patients149. Other SMO inhibitors that have been effective in treating gastrointestinal tumors include miR-218, itraconazole, vitamin D3, BMS-833923 (XL139), TAK-441 and MS-0022 (Fig. 1).
Given the high tumor recurrence rate in clinical trials of SMO inhibitors, exploration of other components of the HH signaling pathway as therapeutic targets is urgently needed150. GLI proteins show advantages as new targets because they can be activated by both SHH ligand-dependent and SHH ligand-independent mechanisms151. GANT61, a GLI1 inhibitor, was designed to block the binding between GLI1 and DNA or to alter the conformation of the GLI1–DNA complex152. GANT61 has been observed to induce DNA damage and extensive cell death in human colon cancer cells in vivo64. Other inhibitors of GLI that have been studied in gastrointestinal tumors include curcumin, resveratrol, epigallocatechin-3-gallate, arsenic trioxide (ATO), zerumbone, AY9944 and sodium arsenite (Fig. 1).
The chimeric monoclonal antibody 5E1 (ch5E1) has been shown to inhibit the SHH signaling pathway by binding to SHH, resulting in inhibitory effects on gastrointestinal tumor cells in vitro34,153,154. Other inhibitors of SHH include imiquimod and isoflavones (genistein), which have been used in vitro and in vivo, respectively, in gastrointestinal tumor studies (Fig. 1).
In summary, only a few inhibitors have entered clinical trials (the most advanced are in phase II) for the treatment of gastrointestinal tumors. Research and development progress on HH-targeting drugs in gastrointestinal cancer lags behind that in other types of cancer. A deeper understanding of the role and mechanism of HH signaling and a comprehensive characterization of current HH inhibitors may provide novel insights for accelerated investigations targeting HH signaling as a therapeutic strategy in gastrointestinal cancer.
4.3. HH signaling and TME as potential therapeutic targets in gastrointestinal cancer
Due to its extensive involvement in carcinogenesis, tumor development, and progression, the HH signaling pathway has recently been intensively investigated in anticancer drug discovery pipelines, leading to two U.S. Food and Drug Administration (FDA)-approved small molecule drugs and dozens of clinical trial-stage inhibitors155, 156, 157. These drugs and inhibitors cover a wide range of indications, including BCC, medulloblastoma, pancreatic tumors, and non-small cell lung cancer157, 158, 159. The critical role of HH provides a promising therapeutic opportunity in gastrointestinal cancer. Unlike for BCC, no specific HH signaling-based clinical therapies for gastrointestinal cancer are currently available. These findings highlight the urgency and importance of discovering of HH signaling-based therapies for gastrointestinal cancer. Currently, 8 HH inhibitors have been evaluated in phase I or phase II clinical trials for gastrointestinal cancer (Table 1), although none have entered phase III clinical trials or have been approved. Unfortunately, the results from a phase II clinical trial (NCT00982592) show that the addition of vismodegib to FOLFOX chemotherapy failed to achieve significant improvement in progression-free survival in gastrointestinal cancer160. Therefore, other mechanisms may influence the therapeutic effect of HH pathway inhibitors in gastrointestinal tumors.
Table 1.
Clinical trials of Hedgehog pathway modulators discussed in gastrointestinal disease (data from ClinicalTrials. gov39).
| Drug | Target | Disease indication | Maximum developmental stage | ClinicalTrials.gov identifier |
|---|---|---|---|---|
| Vismodegib (GDC-0449) | SMO (antagonist) | Metastatic colorectal cancer | Phase II | NCT00636610 |
| Sonidegib (LDE225) | SMO (antagonist) | Certain cancers (including metastatic colorectal cancer, gastric cancer, gastroesophageal junction cancer) | Phase I | NCT01576666 |
| Vismodegib (GDC-0449) | SMO (antagonist) | Advanced stomach cancer or gastroesophageal junction cancer | Phase II | NCT00982592 |
| Vitamin D3 | SMO (antagonist) | Stage IV colorectal cancer | Phase II | NCT01074216 |
| TAK-441 | SMO (antagonist) | Advanced nonhematologic malignancies | Phase I | NCT01204073 |
| BMS-833923 | SMO (antagonist) | In inoperable, metastatic gastric, gastroesophageal, or esophageal adenocarcinomas | Phase I | NCT00909402 |
| Curcumin | GLI (antagonist) | Unresectable colorectal cancer | Phase II | NCT02439385 |
| Resveratrol | GLI (antagonist) | Colorectal cancer | Phase I | NCT00433576 |
| Epigallocatechin-3-gallate | GLI (antagonist) | Colorectal cancer | Phase I | NCT02891538 |
SMO, Smoothened; GLI, glioma-associated oncogene homologue.
Considering the role of HH signaling activation in the TME, adopting a more comprehensive consideration of the TME may provide a new opportunity for the design of effective HH-based therapies for gastrointestinal cancer161. In vivo, pharmacological inhibition of HH signaling can suppress tumor growth by modulating the TME, an approach that could be exploited in rational therapeutic design. For example, the SMO inhibitor vismodegib normalizes the TME in breast cancer by reducing the proliferation of CAFs and thus significantly improves the efficacy of Abraxane and Doxil in xenograft tumors71. In addition, vismodegib can ameliorate the immunosuppressive TME, which stems from M2 subtype TAMs and regulatory T cells134. Based on the modulatory effect of HH signaling activation on the TME, HH inhibitors can be combined with immunotherapies to improve therapeutic efficacy by increasing the infiltration of cytotoxic T cells into tumors. Recently, a nanodrug that carried the HH inhibitor cyclopamine and paclitaxel to alter the TME of pancreatic cancer was designed to enhance the efficacy of immune checkpoint blockade98. Moreover, inhibiting HH activity in the stroma has been reported to increase the density of the intratumoral vasculature and thus allow enhanced infiltration of cytotoxic CD8+ T cells, which finally increases the antitumor activity of anti-programmed cell death-1 (PD-1) antibodies. This evidence indicates that inhibiting HH signaling may be a strategy to balance or reboot the tumor immune status and therefore allow the effective use of immunotherapies such as PD-1 and PD-L1 inhibitors in gastrointestinal cancer.
In summary, owing to the important roles of HH signaling activation in the gastrointestinal TME, a combined approach of targeting the HH signaling pathway and modifying TME may present a promising therapeutic opportunity for gastrointestinal cancer. Additional clinical studies are anticipated.
5. Conclusions
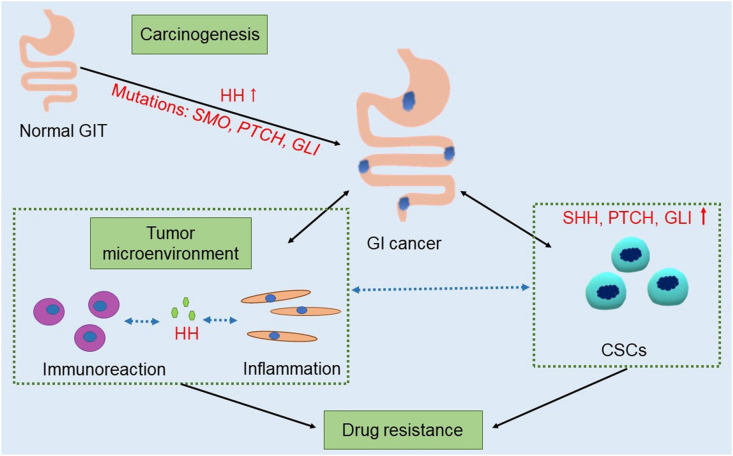
In conclusion, aberrant activation of the HH signaling pathway plays a central role in both gastrointestinal carcinogenesis and gastrointestinal TME (Fig. 3). In fact, abnormal HH signaling activation not only causes uncontrolled proliferation of tumor cells but also contributes to the establishment of an immunosuppressive TME by regulating macrophage and T cell-dependent immune responses as well as the expression of tumor-related inflammatory factors. Moreover, HH contributes to the proliferation of gastrointestinal CSCs, a mechanism underlying drug resistance. Therefore, the results of current studies demonstrate that HH is not only a promoter of CSC proliferation but also a critical immunosuppressive and inflammatory factor in the TME, an observation that highlights a potential therapeutic approach for gastrointestinal tumors through inhibition of HH signaling.
Figure 3.
The Hedgehog signaling pathway plays an important role in gastrointestinal carcinogenesis, the tumor microenvironment and cancer stem cells. GIT, gastrointestinal tract; GI, gastrointestinal; CSCs, cancer stem cells; HH, Hedgehog; SHH, Sonic Hedgehog; SMO, Smoothened; PTCH, Patched; GLI, glioma-associated oncogene homologue.
Acknowledgments
This work was supported by Scientific Research Project of Shanghai Health and Family Planning Committee (Grant No. 201640017, China), National Science and Technology Major Project (Grant No. 2017ZX09304030, China), and Shanghai Science and Technology Funds (Grant No. 19ZR1456100, China).
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Contributor Information
Dianwen Ju, Email: dianwenju@fudan.edu.cn.
Kai Yin, Email: kyin67@smmu.edu.cn.
Author contributions
Jinghui Zhang, Jiajun Fan, Xian Zeng, Kai Yin, and Dianwen Ju wrote the paper; Mingming Nie, Jingyun Luan, and Yichen Wang collected data and contributed to the literature search. Jinghui Zhang, Jiajun Fan, and Xian Zeng draw the illustrations. Kai Yin, and Dianwen Ju edited the paper.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Jiang J., Hui C.C. Hedgehog signaling in development and cancer. Dev Cell. 2008;15:801–812. doi: 10.1016/j.devcel.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Merchant J.L. Hedgehog signalling in gut development, physiology and cancer. J Physiol. 2012;590:421–432. doi: 10.1113/jphysiol.2011.220681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van den Brink G.R. Hedgehog signaling in development and homeostasis of the gastrointestinal tract. Physiol Rev. 2007;87:1343–1375. doi: 10.1152/physrev.00054.2006. [DOI] [PubMed] [Google Scholar]
- 4.Hanna A., Shevde L.A. Hedgehog signaling: modulation of cancer properies and tumor mircroenvironment. Mol Canc. 2016;15:24. doi: 10.1186/s12943-016-0509-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Noah T.K., Donahue B., Shroyer N.F. Intestinal development and differentiation. Exp Cell Res. 2011;317:2702–2710. doi: 10.1016/j.yexcr.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kolterud A., Grosse A.S., Zacharias W.J., Walton K.D., Kretovich K.E., Madison B.B. Paracrine Hedgehog signaling in stomach and intestine: new roles for hedgehog in gastrointestinal patterning. Gastroenterology. 2009;137:618–628. doi: 10.1053/j.gastro.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Konstantinou D., Bertaux-Skeirik N., Zavros Y. Hedgehog signaling in the stomach. Curr Opin Pharmacol. 2016;31:76–82. doi: 10.1016/j.coph.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waszak S.M., Robinson G.W., Gudenas B.L., Smith K.S., Forget A., Kojic M. Germline elongator mutations in Sonic Hedgehog medulloblastoma. Nature. 2020;580:396–401. doi: 10.1038/s41586-020-2164-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polizio A.H., Chinchilla P., Chen X., Manning D.R., Riobo N.A. Sonic Hedgehog activates the GTPases Rac1 and RhoA in a Gli-independent manner through coupling of smoothened to Gi proteins. Sci Signal. 2011;4:pt7. doi: 10.1126/scisignal.2002396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carballo G.B., Honorato J.R., de Lopes G.P.F., Spohr T. A highlight on Sonic hedgehog pathway. Cell Commun Signal. 2018;16:11. doi: 10.1186/s12964-018-0220-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Will A.J., Cova G., Osterwalder M., Chan W.L., Wittler L., Brieske N. Composition and dosage of a multipartite enhancer cluster control developmental expression of Ihh (Indian hedgehog) Nat Genet. 2017;49:1539–1545. doi: 10.1038/ng.3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gong X., Qian H., Cao P., Zhao X., Zhou Q., Lei J. Structural basis for the recognition of Sonic Hedgehog by human Patched1. Science. 2018;361 doi: 10.1126/science.aas8935. eaas8935. [DOI] [PubMed] [Google Scholar]
- 13.Hui C.C., Angers S. Gli proteins in development and disease. Annu Rev Cell Dev Biol. 2011;27:513–537. doi: 10.1146/annurev-cellbio-092910-154048. [DOI] [PubMed] [Google Scholar]
- 14.Huang P., Zheng S., Wierbowski B.M., Kim Y., Nedelcu D., Aravena L. Structural basis of smoothened activation in Hedgehog signaling. Cell. 2018;174:312–324 e16. doi: 10.1016/j.cell.2018.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y., Fu L., Qi X., Zhang Z., Xia Y., Jia J. Structural insight into the mutual recognition and regulation between Suppressor of Fused and Gli/Ci. Nat Commun. 2013;4:2608. doi: 10.1038/ncomms3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Infante P., Faedda R., Bernardi F., Bufalieri F., Lospinoso Severini L., Alfonsi R. Itch/beta-arrestin2-dependent non-proteolytic ubiquitylation of SuFu controls Hedgehog signalling and medulloblastoma tumorigenesis. Nat Commun. 2018;9:976. doi: 10.1038/s41467-018-03339-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lex R.K., Ji Z., Falkenstein K.N., Zhou W., Henry J.L., Ji H. GLI transcriptional repression regulates tissue-specific enhancer activity in response to Hedgehog signaling. Elife. 2020;9 doi: 10.7554/eLife.50670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Milenkovic L., Scott M.P. Not lost in space: trafficking in the hedgehog signaling pathway. Sci Signal. 2010;3:pe14. doi: 10.1126/scisignal.3117pe14. [DOI] [PubMed] [Google Scholar]
- 19.He M., Subramanian R., Bangs F., Omelchenko T., Liem K.F., Jr., Kapoor T.M. The kinesin-4 protein Kif7 regulates mammalian Hedgehog signalling by organizing the cilium tip compartment. Nat Cell Biol. 2014;16:663–672. doi: 10.1038/ncb2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kovacs J.J., Whalen E.J., Liu R., Xiao K., Kim J., Chen M. Beta-arrestin-mediated localization of smoothened to the primary cilium. Science. 2008;320:1777–1781. doi: 10.1126/science.1157983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao Z., Lee R.T., Pusapati G.V., Iyu A., Rohatgi R., Ingham P.W. An essential role for Grk2 in Hedgehog signalling downstream of Smoothened. EMBO Rep. 2016;17:739–752. doi: 10.15252/embr.201541532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang W., Yu F., Wang Y., Zhang Y., Meng L., Chi Y. Rab23 promotes the cisplatin resistance of ovarian cancer via the Shh-Gli-ABCG2 signaling pathway. Oncol Lett. 2018;15:5155–5160. doi: 10.3892/ol.2018.7949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moore B.S., Stepanchick A.N., Tewson P.H., Hartle C.M., Zhang J., Quinn A.M. Cilia have high cAMP levels that are inhibited by Sonic Hedgehog-regulated calcium dynamics. Proc Natl Acad Sci U S A. 2016;113:13069–13074. doi: 10.1073/pnas.1602393113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jagani Z., Mora-Blanco E.L., Sansam C.G., McKenna E.S., Wilson B., Chen D. Loss of the tumor suppressor Snf5 leads to aberrant activation of the Hedgehog–Gli pathway. Nat Med. 2010;16:1429–1433. doi: 10.1038/nm.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li N., Truong S., Nouri M., Moore J., Al Nakouzi N., Lubik A.A. Non-canonical activation of hedgehog in prostate cancer cells mediated by the interaction of transcriptionally active androgen receptor proteins with Gli3. Oncogene. 2018;37:2313–2325. doi: 10.1038/s41388-017-0098-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pelullo M., Zema S., Nardozza F., Checquolo S., Screpanti I., Bellavia D. Wnt, Notch, and TGF-beta pathways impinge on Hedgehog signaling complexity: an open window on cancer. Front Genet. 2019;10:711. doi: 10.3389/fgene.2019.00711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Della Corte C.M., Bellevicine C., Vicidomini G., Vitagliano D., Malapelle U., Accardo M. SMO gene amplification and activation of the Hedgehog pathway as novel mechanisms of resistance to anti-epidermal growth factor receptor drugs in human lung cancer. Clin Canc Res. 2015;21:4686–4697. doi: 10.1158/1078-0432.CCR-14-3319. [DOI] [PubMed] [Google Scholar]
- 28.Gerling M., Buller N.V., Kirn L.M., Joost S., Frings O., Englert B. Stromal Hedgehog signalling is downregulated in colon cancer and its restoration restrains tumour growth. Nat Commun. 2016;7:12321. doi: 10.1038/ncomms12321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoon C., Park D.J., Schmidt B., Thomas N.J., Lee H.J., Kim T.S. CD44 expression denotes a subpopulation of gastric cancer cells in which Hedgehog signaling promotes chemotherapy resistance. Clin Canc Res. 2014;20:3974–3988. doi: 10.1158/1078-0432.CCR-14-0011. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Cui M.L., Yang H.Y., He G.Q. Apoptosis induction of colorectal cancer cells HTL-9 in vitro by the transformed products of soybean isoflavones by Ganoderma lucidum. J Zhejiang Univ Sci B. 2017;18:1101–1112. doi: 10.1631/jzus.B1700189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang W., Wan C., Luo Q., Huang Z., Luo Q. Genistein-inhibited cancer stem cell-like properties and reduced chemoresistance of gastric cancer. Int J Mol Sci. 2014;15:3432–3443. doi: 10.3390/ijms15033432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang J., Dong L., Shi H.T., Guo X.Y., Qin B., Wang Y. Imiquimod inhibits the growth of SGC7901 cells in vitro through induction of autophagy and apoptosis. Mol Med Rep. 2016;13:393–397. doi: 10.3892/mmr.2015.4524. [DOI] [PubMed] [Google Scholar]
- 33.Song Z., Yue W., Wei B., Wang N., Li T., Guan L. Sonic hedgehog pathway is essential for maintenance of cancer stem-like cells in human gastric cancer. PLoS One. 2011;6 doi: 10.1371/journal.pone.0017687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Magistri P., Battistelli C., Strippoli R., Petrucciani N., Pellinen T., Rossi L. SMO inhibition modulates cellular plasticity and invasiveness in colorectal cancer. Front Pharmacol. 2017;8:956. doi: 10.3389/fphar.2017.00956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berlin J., Bendell J.C., Hart L.L., Firdaus I., Gore I., Hermann R.C. A randomized phase II trial of vismodegib versus placebo with FOLFOX or FOLFIRI and bevacizumab in patients with previously untreated metastatic colorectal cancer. Clin Canc Res. 2013;19:258–267. doi: 10.1158/1078-0432.CCR-12-1800. [DOI] [PubMed] [Google Scholar]
- 36.Baek S., Lee Y.S., Shim H.E., Yoon S., Baek S.Y., Kim B.S. Vitamin D3 regulates cell viability in gastric cancer and cholangiocarcinoma. Anat Cell Biol. 2011;44:204–209. doi: 10.5115/acb.2011.44.3.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bi X., Shi Q., Zhang H., Bao Y., Hu D., Pohl N. c-Jun NH2-teminal kinase 1 interacts with vitamin D receptor and affects vitamin D-mediated inhibition of cancer cell proliferation. J Steroid Biochem Mol Biol. 2016;163:164–172. doi: 10.1016/j.jsbmb.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 38.Milczarek M., Psurski M., Kutner A., Wietrzyk J. Vitamin D analogs enhance the anticancer activity of 5-fluorouracil in an in vivo mouse colon cancer model. BMC Canc. 2013;13:294. doi: 10.1186/1471-2407-13-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.ClinicalTrials.gov. Available from: https://clinicaltrials.gov/(accessed on 10 2 2020).
- 40.Goldman J., Eckhardt S.G., Borad M.J., Curtis K.K., Hidalgo M., Calvo E. Phase I dose-escalation trial of the oral investigational Hedgehog signaling pathway inhibitor TAK-441 in patients with advanced solid tumors. Clin Canc Res. 2015;21:1002–1009. doi: 10.1158/1078-0432.CCR-14-1234. [DOI] [PubMed] [Google Scholar]
- 41.Ma H., Tian Y., Yu X. Targeting smoothened sensitizes gastric cancer to chemotherapy in experimental models. Med Sci Monit. 2017;23:1493–1500. doi: 10.12659/MSM.903012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu Q., Hou Y.C., Huang J., Fang J.Y., Xiong H. Itraconazole induces apoptosis and cell cycle arrest via inhibiting Hedgehog signaling in gastric cancer cells. J Exp Clin Canc Res. 2017;36:50. doi: 10.1186/s13046-017-0526-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Popova S.A., Buczacki S.J.A. Itraconazole perturbs colorectal cancer dormancy through SUFU-mediated WNT inhibition. Mol Cell Oncol. 2018;5 doi: 10.1080/23723556.2018.1494950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buczacki S.J.A., Popova S., Biggs E., Koukorava C., Buzzelli J., Vermeulen L. Itraconazole targets cell cycle heterogeneity in colorectal cancer. J Exp Med. 2018;215:1891–1912. doi: 10.1084/jem.20171385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deng M., Zeng C., Lu X., He X., Zhang R., Qiu Q. miR-218 suppresses gastric cancer cell cycle progression through the CDK6/Cyclin D1/E2F1 axis in a feedback loop. Canc Lett. 2017;403:175–185. doi: 10.1016/j.canlet.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 46.He X., Dong Y., Wu C.W., Zhao Z., Ng S.S., Chan F.K. MicroRNA-218 inhibits cell cycle progression and promotes apoptosis in colon cancer by downregulating BMI1 polycomb ring finger oncogene. Mol Med. 2013;18:1491–1498. doi: 10.2119/molmed.2012.00304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lun W., Wu X., Deng Q., Zhi F. MiR-218 regulates epithelial-mesenchymal transition and angiogenesis in colorectal cancer via targeting CTGF. Canc Cell Int. 2018;18:83. doi: 10.1186/s12935-018-0575-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roberg-Larsen H., Strand M.F., Grimsmo A., Olsen P.A., Dembinski J.L., Rise F. High sensitivity measurements of active oxysterols with automated filtration/filter backflush-solid phase extraction–liquid chromatography–mass spectrometry. J Chromatogr A. 2012;1255:291–297. doi: 10.1016/j.chroma.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 49.Gu H., Li X.U., Zhou C., Wen Y., Shen Y., Zhou L. Effects and mechanisms of blocking the hedgehog signaling pathway in human gastric cancer cells. Oncol Lett. 2015;9:1997–2002. doi: 10.3892/ol.2015.3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qualtrough D., Rees P., Speight B., Williams A.C., Paraskeva C. The Hedgehog inhibitor cyclopamine reduces beta-catenin-Tcf transcriptional activity, induces E-cadherin expression, and reduces invasion in colorectal cancer cells. Cancers (Basel) 2015;7:1885–1899. doi: 10.3390/cancers7030867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yoo Y.A., Kang M.H., Kim J.S., Oh S.C. Sonic hedgehog signaling promotes motility and invasiveness of gastric cancer cells through TGF-beta-mediated activation of the ALK5-Smad 3 pathway. Carcinogenesis. 2008;29:480–490. doi: 10.1093/carcin/bgm281. [DOI] [PubMed] [Google Scholar]
- 52.He W., Xia Y., Cao P., Hong L., Zhang T., Shen X. Curcuminoid WZ35 synergize with cisplatin by inducing ROS production and inhibiting TrxR1 activity in gastric cancer cells. J Exp Clin Canc Res. 2019;38:207. doi: 10.1186/s13046-019-1215-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou X., Wang W., Li P., Zheng Z., Tu Y., Zhang Y. Curcumin enhances the effects of 5-fluorouracil and oxaliplatin in inducing gastric cancer cell apoptosis both in vitro and in vivo. Oncol Res. 2016;23:29–34. doi: 10.3727/096504015X14452563486011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li M., Yue G.G., Tsui S.K., Fung K.P., Lau C.B. Turmeric extract, with absorbable curcumin, has potent anti-metastatic effect in vitro and in vivo. Phytomedicine. 2018;46:131–141. doi: 10.1016/j.phymed.2018.03.065. [DOI] [PubMed] [Google Scholar]
- 55.Gao Q., Yuan Y., Gan H.Z., Peng Q. Resveratrol inhibits the hedgehog signaling pathway and epithelial-mesenchymal transition and suppresses gastric cancer invasion and metastasis. Oncol Lett. 2015;9:2381–2387. doi: 10.3892/ol.2015.2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang S., Li W., Sun H., Wu B., Ji F., Sun T. Resveratrol elicits anti-colorectal cancer effect by activating miR-34c-KITLG in vitro and in vivo. BMC Canc. 2015;15:969. doi: 10.1186/s12885-015-1958-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ran Z.H., Xu Q., Tong J.L., Xiao S.D. Apoptotic effect of Epigallocatechin-3-gallate on the human gastric cancer cell line MKN45 via activation of the mitochondrial pathway. World J Gastroenterol. 2007;13:4255–4259. doi: 10.3748/wjg.v13.i31.4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jin G., Yang Y., Liu K., Zhao J., Chen X., Liu H. Combination curcumin and (–)-epigallocatechin-3-gallate inhibits colorectal carcinoma microenvironment-induced angiogenesis by JAK/STAT3/IL-8 pathway. Oncogenesis. 2017;6:e384. doi: 10.1038/oncsis.2017.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen Z., Zhang H., Yang L., Jiang H., Guo S., Li Y. Construction of a metabolomics profile of arsenic trioxide effect in gastric carcinoma cell line SGC7901. Acta Biochim Biophys Sin (Shanghai) 2016;48:474–481. doi: 10.1093/abbs/gmw022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sun X.P., Zhang X., He C., Qiao H., Jiang X., Jiang H. ABT-737 synergizes with arsenic trioxide to induce apoptosis of gastric carcinoma cells in vitro and in vivo. J Int Med Res. 2012;40:1251–1264. doi: 10.1177/147323001204000404. [DOI] [PubMed] [Google Scholar]
- 61.Thomas-Schoemann A., Batteux F., Mongaret C., Nicco C., Chereau C., Annereau M. Arsenic trioxide exerts antitumor activity through regulatory T cell depletion mediated by oxidative stress in a murine model of colon cancer. J Immunol. 2012;189:5171–5177. doi: 10.4049/jimmunol.1103094. [DOI] [PubMed] [Google Scholar]
- 62.Yan R., Peng X., Yuan X., Huang D., Chen J., Lu Q. Suppression of growth and migration by blocking the Hedgehog signaling pathway in gastric cancer cells. Cell Oncol (Dordr) 2013;36:421–435. doi: 10.1007/s13402-013-0149-1. [DOI] [PubMed] [Google Scholar]
- 63.Chakrabarti J., Holokai L., Syu L., Steele N.G., Chang J., Wang J. Hedgehog signaling induces PD-L1 expression and tumor cell proliferation in gastric cancer. Oncotarget. 2018;9:37439–37457. doi: 10.18632/oncotarget.26473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mazumdar T., Devecchio J., Agyeman A., Shi T., Houghton J.A. Blocking Hedgehog survival signaling at the level of the GLI genes induces DNA damage and extensive cell death in human colon carcinoma cells. Canc Res. 2011;71:5904–5914. doi: 10.1158/0008-5472.CAN-10-4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sithara T., Dhanya B.P., Arun K.B., Sini S., Dan M., Kokkuvayil Vasu R. Zerumbone, a cyclic sesquiterpene from Zingiber zerumbet induces apoptosis, cell cycle arrest, and antimigratory effects in SW480 colorectal cancer cells. J Agric Food Chem. 2018;66:602–612. doi: 10.1021/acs.jafc.7b04472. [DOI] [PubMed] [Google Scholar]
- 66.Othumpangat S., Kashon M., Joseph P. Sodium arsenite-induced inhibition of eukaryotic translation initiation factor 4E (eIF4E) results in cytotoxicity and cell death. Mol Cell Biochem. 2005;279:123–131. doi: 10.1007/s11010-005-8284-2. [DOI] [PubMed] [Google Scholar]
- 67.Usui T., Sakurai M., Umata K., Elbadawy M., Ohama T., Yamawaki H. Hedgehog signals mediate anti-cancer drug resistance in three-dimensional primary colorectal cancer organoid culture. Int J Mol Sci. 2018;19:1098. doi: 10.3390/ijms19041098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xin M., Ji X., de La Cruz L.K., Thareja S., Wang B. Strategies to target the Hedgehog signaling pathway for cancer therapy. Med Res Rev. 2018;38:870–913. doi: 10.1002/med.21482. [DOI] [PubMed] [Google Scholar]
- 69.Bhateja P., Cherian M., Majumder S., Ramaswamy B. The Hedgehog signaling pathway: a viable target in breast cancer? Cancers (Basel) 2019;11 doi: 10.3390/cancers11081126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hanna A., Metge B.J., Bailey S.K., Chen D., Chandrashekar D.S., Varambally S. Inhibition of Hedgehog signaling reprograms the dysfunctional immune microenvironment in breast cancer. OncoImmunology. 2019;8:1548241. doi: 10.1080/2162402X.2018.1548241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mpekris F., Papageorgis P., Polydorou C., Voutouri C., Kalli M., Pirentis A.P. Sonic-hedgehog pathway inhibition normalizes desmoplastic tumor microenvironment to improve chemo- and nanotherapy. J Control Release. 2017;261:105–112. doi: 10.1016/j.jconrel.2017.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jeng K.S., Chang C.F., Lin S.S. Sonic Hedgehog signaling in organogenesis, tumors, and tumor microenvironments. Int J Mol Sci. 2020;21:758. doi: 10.3390/ijms21030758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ding L., Hayes M.M., Photenhauer A., Eaton K.A., Li Q., Ocadiz-Ruiz R. Schlafen 4-expressing myeloid-derived suppressor cells are induced during murine gastric metaplasia. J Clin Invest. 2016;126:2867–2880. doi: 10.1172/JCI82529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Merchant J.L., Ding L. Hedgehog signaling links chronic inflammation to gastric cancer precursor lesions. Cell Mol Gastroenterol Hepatol. 2017;3:201–210. doi: 10.1016/j.jcmgh.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schumacher M.A., Donnelly J.M., Engevik A.C., Xiao C., Yang L., Kenny S. Gastric sonic Hedgehog acts as a macrophage chemoattractant during the immune response to Helicobacter pylori. Gastroenterology. 2012;142:1150–1159 e6. doi: 10.1053/j.gastro.2012.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Echizen K., Horiuchi K., Aoki Y., Yamada Y., Minamoto T., Oshima H. NF-kappaB-induced NOX1 activation promotes gastric tumorigenesis through the expansion of SOX2-positive epithelial cells. Oncogene. 2019;38:4250–4263. doi: 10.1038/s41388-019-0702-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wessler S., Krisch L.M., Elmer D.P., Aberger F. From inflammation to gastric cancer—the importance of Hedgehog/GLI signaling in Helicobacter pylori-induced chronic inflammatory and neoplastic diseases. Cell Commun Signal. 2017;15:15. doi: 10.1186/s12964-017-0171-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Merchant J.L., Saqui-Salces M. Inhibition of Hedgehog signaling in the gastrointestinal tract: targeting the cancer microenvironment. Canc Treat Rev. 2014;40:12–21. doi: 10.1016/j.ctrv.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dong H., Liu H., Zhou W., Zhang F., Li C., Chen J. GLI1 activation by non-classical pathway integrin αvβ3/ERK1/2 maintains stem cell-like phenotype of multicellular aggregates in gastric cancer peritoneal metastasis. Cell Death Dis. 2019;10:574. doi: 10.1038/s41419-019-1776-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Holokai L., Chakrabarti J., Broda T., Chang J., Hawkins J.A., Sundaram N. Increased programmed death-ligand 1 is an early epithelial cell response to Helicobacter pylori infection. PLoS Pathog. 2019;15 doi: 10.1371/journal.ppat.1007468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Takebe N., Miele L., Harris P.J., Jeong W., Bando H., Kahn M. Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: clinical update. Nat Rev Clin Oncol. 2015;12:445–464. doi: 10.1038/nrclinonc.2015.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Batsaikhan B.E., Yoshikawa K., Kurita N., Iwata T., Takasu C., Kashihara H. Cyclopamine decreased the expression of Sonic Hedgehog and its downstream genes in colon cancer stem cells. Anticancer Res. 2014;34:6339–6344. [PubMed] [Google Scholar]
- 83.Zhou H., Xiong Y., Peng L., Wang R., Zhang H., Fu Z. LncRNA-cCSC1 modulates cancer stem cell properties in colorectal cancer via activation of the Hedgehog signaling pathway. J Cell Biochem. 2020;121:2510–2524. doi: 10.1002/jcb.29473. [DOI] [PubMed] [Google Scholar]
- 84.Yu D., Shin H.S., Lee Y.S., Lee D., Kim S., Lee Y.C. Genistein attenuates cancer stem cell characteristics in gastric cancer through the downregulation of Gli1. Oncol Rep. 2014;31:673–678. doi: 10.3892/or.2013.2893. [DOI] [PubMed] [Google Scholar]
- 85.Xu M., Gong A., Yang H., George S.K., Jiao Z., Huang H. Sonic hedgehog-glioma associated oncogene homolog 1 signaling enhances drug resistance in CD44+/Musashi-1+ gastric cancer stem cells. Canc Lett. 2015;369:124–133. doi: 10.1016/j.canlet.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 86.Chen J.H., Zhai E.T., Chen S.L., Wu H., Wu K.M., Zhang X.H. CD44, Sonic Hedgehog, and Gli1 expression are prognostic biomarkers in gastric cancer patients after radical resection. Gastroenterol Res Pract. 2016;2016:1013045. doi: 10.1155/2016/1013045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Syu L.J., Zhao X., Zhang Y., Grachtchouk M., Demitrack E., Ermilov A. Invasive mouse gastric adenocarcinomas arising from Lgr5+ stem cells are dependent on crosstalk between the Hedgehog/GLI2 and mTOR pathways. Oncotarget. 2016;7:10255–10270. doi: 10.18632/oncotarget.7182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yang Z., Zhang C., Qi W., Cui Y., Xuan Y. GLI1 promotes cancer stemness through intracellular signaling pathway PI3K/Akt/NFkappaB in colorectal adenocarcinoma. Exp Cell Res. 2018;373:145–154. doi: 10.1016/j.yexcr.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 89.Ruiz i Altaba A. Hedgehog signaling and the Gli code in stem cells, cancer, and metastases. Sci Signal. 2011;4:pt9. doi: 10.1126/scisignal.2002540. [DOI] [PubMed] [Google Scholar]
- 90.Regan J.L., Schumacher D., Staudte S., Steffen A., Haybaeck J., Keilholz U. Non-canonical Hedgehog signaling is a positive regulator of the WNT pathway and is required for the survival of colon cancer stem cells. Cell Rep. 2017;21:2813–2828. doi: 10.1016/j.celrep.2017.11.025. [DOI] [PubMed] [Google Scholar]
- 91.Yan G.N., Yang L., Lv Y.F., Shi Y., Shen L.L., Yao X.H. Endothelial cells promote stem-like phenotype of glioma cells through activating the Hedgehog pathway. J Pathol. 2014;234:11–22. doi: 10.1002/path.4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fu Y.Z., Yan Y.Y., He M., Xiao Q.H., Yao W.F., Zhao L. Salinomycin induces selective cytotoxicity to MCF-7 mammosphere cells through targeting the Hedgehog signaling pathway. Oncol Rep. 2016;35:912–922. doi: 10.3892/or.2015.4434. [DOI] [PubMed] [Google Scholar]
- 93.Yao Y., Zhou D., Shi D., Zhang H., Zhan S., Shao X. GLI1 overexpression promotes gastric cancer cell proliferation and migration and induces drug resistance by combining with the AKT-mTOR pathway. Biomed Pharmacother. 2019;111:993–1004. doi: 10.1016/j.biopha.2019.01.018. [DOI] [PubMed] [Google Scholar]
- 94.Yu B., Gu D., Zhang X., Liu B., Xie J. The role of GLI2-ABCG2 signaling axis for 5Fu resistance in gastric cancer. J Genet Genomics. 2017;44:375–383. doi: 10.1016/j.jgg.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zeng F., Wang F., Zheng Z., Chen Z., Wah To K.K., Zhang H. Rociletinib (CO-1686) enhanced the efficacy of chemotherapeutic agents in ABCG2-overexpressing cancer cells in vitro and in vivo. Acta Pharm Sin B. 2020;10:799–811. doi: 10.1016/j.apsb.2020.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tang Y.A., Chen Y.F., Bao Y., Mahara S., Yatim S., Oguz G. Hypoxic tumor microenvironment activates GLI2 via HIF-1alpha and TGF-beta2 to promote chemoresistance in colorectal cancer. Proc Natl Acad Sci U S A. 2018;115:E5990–E5999. doi: 10.1073/pnas.1801348115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang L., Song R., Gu D., Zhang X., Yu B., Liu B. The role of GLI1 for 5-Fu resistance in colorectal cancer. Cell Biosci. 2017;7:17. doi: 10.1186/s13578-017-0145-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhao J., Xiao Z., Li T., Chen H., Yuan Y., Wang Y.A. Stromal modulation reverses primary resistance to immune checkpoint blockade in pancreatic cancer. ACS Nano. 2018;12:9881–9893. doi: 10.1021/acsnano.8b02481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cheng H.S., Lee J.X.T., Wahli W., Tan N.S. Exploiting vulnerabilities of cancer by targeting nuclear receptors of stromal cells in tumor microenvironment. Mol Canc. 2019;18:51. doi: 10.1186/s12943-019-0971-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Balkwill F.R., Capasso M., Hagemann T. The tumor microenvironment at a glance. J Cell Sci. 2012;125:5591–5596. doi: 10.1242/jcs.116392. [DOI] [PubMed] [Google Scholar]
- 101.Guan X. Cancer metastases: challenges and opportunities. Acta Pharm Sin B. 2015;5:402–418. doi: 10.1016/j.apsb.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xiao C., Feng R., Engevik A.C., Martin J.R., Tritschler J.A., Schumacher M. Sonic Hedgehog contributes to gastric mucosal restitution after injury. Lab Invest. 2013;93:96–111. doi: 10.1038/labinvest.2012.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shaker A., Binkley J., Darwech I., Swietlicki E., McDonald K., Newberry R. Stromal cells participate in the murine esophageal mucosal injury response. Am J Physiol Gastrointest Liver Physiol. 2013;304:G662–G672. doi: 10.1152/ajpgi.00225.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shahi M.H., Rey J.A., Castresana J.S. The sonic hedgehog–GLI1 signaling pathway in brain tumor development. Expert Opin Ther Targets. 2012;16:1227–1238. doi: 10.1517/14728222.2012.720975. [DOI] [PubMed] [Google Scholar]
- 105.Heller E., Hurchla M.A., Xiang J., Su X., Chen S., Schneider J. Hedgehog signaling inhibition blocks growth of resistant tumors through effects on tumor microenvironment. Canc Res. 2012;72:897–907. doi: 10.1158/0008-5472.CAN-11-2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Arcucci A., Ruocco M.R., Granato G., Sacco A.M., Montagnani S. Cancer: an oxidative crosstalk between solid tumor cells and cancer associated fibroblasts. BioMed Res Int. 2016;2016:4502846. doi: 10.1155/2016/4502846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sahai E., Astsaturov I., Cukierman E., DeNardo D.G., Egeblad M., Evans R.M. A framework for advancing our understanding of cancer-associated fibroblasts. Nat Rev Canc. 2020;20:174–186. doi: 10.1038/s41568-019-0238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.von Ahrens D., Bhagat T.D., Nagrath D., Maitra A., Verma A. The role of stromal cancer-associated fibroblasts in pancreatic cancer. J Hematol Oncol. 2017;10:76. doi: 10.1186/s13045-017-0448-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Valenti G., Quinn H.M., Heynen G., Lan L., Holland J.D., Vogel R. Cancer stem cells regulate cancer-associated fibroblasts via activation of Hedgehog signaling in mammary gland tumors. Canc Res. 2017;77:2134–2147. doi: 10.1158/0008-5472.CAN-15-3490. [DOI] [PubMed] [Google Scholar]
- 110.Walter K., Omura N., Hong S.M., Griffith M., Vincent A., Borges M. Overexpression of smoothened activates the sonic hedgehog signaling pathway in pancreatic cancer-associated fibroblasts. Clin Canc Res. 2010;16:1781–1789. doi: 10.1158/1078-0432.CCR-09-1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhao J., Wang H., Hsiao C.H., Chow D.S., Koay E.J., Kang Y. Simultaneous inhibition of hedgehog signaling and tumor proliferation remodels stroma and enhances pancreatic cancer therapy. Biomaterials. 2018;159:215–228. doi: 10.1016/j.biomaterials.2018.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kinoshita H., Hirata Y., Nakagawa H., Sakamoto K., Hayakawa Y., Takahashi R. Interleukin-6 mediates epithelial–stromal interactions and promotes gastric tumorigenesis. PLoS One. 2013;8 doi: 10.1371/journal.pone.0060914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Parker K.H., Beury D.W., Ostrand-Rosenberg S. Myeloid-derived suppressor cells: critical cells driving immune suppression in the tumor microenvironment. Adv Canc Res. 2015;128:95–139. doi: 10.1016/bs.acr.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shintani Y., Fujiwara A., Kimura T., Kawamura T., Funaki S., Minami M. IL-6 secreted from cancer-associated fibroblasts mediates chemoresistance in NSCLC by increasing epithelial-mesenchymal transition signaling. J Thorac Oncol. 2016;11:1482–1492. doi: 10.1016/j.jtho.2016.05.025. [DOI] [PubMed] [Google Scholar]
- 115.Reyes-Ramos A.M., Ramos-Cruz K.P., Rodriguez-Merced N.J., Martinez-Montemayor M.M., Franqui-Rios N.D., Rios-Grant J.P. Mesenchymal cells support the oncogenicity and therapeutic response of the Hedgehog pathway in triple-negative breast cancer. Cancers (Basel) 2019;11:1522. doi: 10.3390/cancers11101522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu J., Chen S., Wang W., Ning B.F., Chen F., Shen W. Cancer-associated fibroblasts promote hepatocellular carcinoma metastasis through chemokine-activated hedgehog and TGF-beta pathways. Canc Lett. 2016;379:49–59. doi: 10.1016/j.canlet.2016.05.022. [DOI] [PubMed] [Google Scholar]
- 117.Zhu Q., Zhang X., Zhang L., Li W., Wu H., Yuan X. The IL-6–STAT3 axis mediates a reciprocal crosstalk between cancer-derived mesenchymal stem cells and neutrophils to synergistically prompt gastric cancer progression. Cell Death Dis. 2014;5:e1295. doi: 10.1038/cddis.2014.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sternberg C., Gruber W., Eberl M., Tesanovic S., Stadler M., Elmer D.P. Synergistic cross-talk of hedgehog and interleukin-6 signaling drives growth of basal cell carcinoma. Int J Canc. 2018;143:2943–2954. doi: 10.1002/ijc.31724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhao G., Li H., Guo Q., Zhou A., Wang X., Li P. Exosomal Sonic Hedgehog derived from cancer-associated fibroblasts promotes proliferation and migration of esophageal squamous cell carcinoma. Canc Med. 2020;9:2500–2513. doi: 10.1002/cam4.2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li T., Yi S., Liu W., Jia C., Wang G., Hua X. Colorectal carcinoma-derived fibroblasts modulate natural killer cell phenotype and antitumor cytotoxicity. Med Oncol. 2013;30:663. doi: 10.1007/s12032-013-0663-z. [DOI] [PubMed] [Google Scholar]
- 121.Margol A.S., Robison N.J., Gnanachandran J., Hung L.T., Kennedy R.J., Vali M. Tumor-associated macrophages in SHH subgroup of medulloblastomas. Clin Canc Res. 2015;21:1457–1465. doi: 10.1158/1078-0432.CCR-14-1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Petty A.J., Li A., Wang X., Dai R., Heyman B., Hsu D. Hedgehog signaling promotes tumor-associated macrophage polarization to suppress intratumoral CD8+ T cell recruitment. J Clin Invest. 2019;129:5151–5162. doi: 10.1172/JCI128644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gordon S., Martinez F.O. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 124.Jin Q., Gui L., Niu F., Yu B., Lauda N., Liu J. Macrophages in keloid are potent at promoting the differentiation and function of regulatory T cells. Exp Cell Res. 2018;362:472–476. doi: 10.1016/j.yexcr.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 125.Standing A.S.I., Yanez D.C., Ross R., Crompton T., Furmanski A.L. Frontline science: Shh production and Gli signaling is activated in vivo in lung, enhancing the Th2 response during a murine model of allergic asthma. J Leukoc Biol. 2017;102:965–976. doi: 10.1189/jlb.3HI1016-438RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ryzhakov G., West N.R., Franchini F., Clare S., Ilott N.E., Sansom S.N. Alpha kinase 1 controls intestinal inflammation by suppressing the IL-12/Th1 axis. Nat Commun. 2018;9:3797. doi: 10.1038/s41467-018-06085-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nizzoli G., Krietsch J., Weick A., Steinfelder S., Facciotti F., Gruarin P. Human CD1c+ dendritic cells secrete high levels of IL-12 and potently prime cytotoxic T-cell responses. Blood. 2013;122:932–942. doi: 10.1182/blood-2013-04-495424. [DOI] [PubMed] [Google Scholar]
- 128.Talmadge J.E., Gabrilovich D.I. History of myeloid-derived suppressor cells. Nat Rev Canc. 2013;13:739–752. doi: 10.1038/nrc3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Xie J. The hedgehog's trick for escaping immunosurveillance: the molecular mechanisms driving myeloid-derived suppressor cell recruitment in hedgehog signaling-dependent tumors. OncoImmunology. 2014;3 doi: 10.4161/onci.29180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chen X., Pan X., Zhang W., Guo H., Cheng S., He Q. Epigenetic strategies synergize with PD-L1/PD-1 targeted cancer immunotherapies to enhance antitumor responses. Acta Pharm Sin B. 2020;10:723–733. doi: 10.1016/j.apsb.2019.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fan F., Wang R., Boulbes D.R., Zhang H., Watowich S.S., Xia L. Macrophage conditioned medium promotes colorectal cancer stem cell phenotype via the hedgehog signaling pathway. PLoS One. 2018;13 doi: 10.1371/journal.pone.0190070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jinushi M., Chiba S., Yoshiyama H., Masutomi K., Kinoshita I., Dosaka-Akita H. Tumor-associated macrophages regulate tumorigenicity and anticancer drug responses of cancer stem/initiating cells. Proc Natl Acad Sci U S A. 2011;108:12425–12430. doi: 10.1073/pnas.1106645108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Otsuka A., Dreier J., Cheng P.F., Nageli M., Lehmann H., Felderer L. Hedgehog pathway inhibitors promote adaptive immune responses in basal cell carcinoma. Clin Canc Res. 2015;21:1289–1297. doi: 10.1158/1078-0432.CCR-14-2110. [DOI] [PubMed] [Google Scholar]
- 134.Katoh M. Genomic testing, tumor microenvironment and targeted therapy of Hedgehog-related human cancers. Clin Sci (Lond) 2019;133:953–970. doi: 10.1042/CS20180845. [DOI] [PubMed] [Google Scholar]
- 135.Xu M., Li X., Liu T., Leng A., Zhang G. Prognostic value of hedgehog signaling pathway in patients with colon cancer. Med Oncol. 2012;29:1010–1016. doi: 10.1007/s12032-011-9899-7. [DOI] [PubMed] [Google Scholar]
- 136.Ertao Z., Jianhui C., Chuangqi C., Changjiang Q., Sile C., Yulong H. Autocrine Sonic hedgehog signaling promotes gastric cancer proliferation through induction of phospholipase Cgamma1 and the ERK1/2 pathway. J Exp Clin Canc Res. 2016;35:63. doi: 10.1186/s13046-016-0336-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Li T., Liao X., Lochhead P., Morikawa T., Yamauchi M., Nishihara R. SMO expression in colorectal cancer: associations with clinical, pathological, and molecular features. Ann Surg Oncol. 2014;21:4164–4173. doi: 10.1245/s10434-014-3888-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lu L., Wu M., Zhao F., Fu W., Li W., Li X. Prognostic and clinicopathological value of Gli-1 expression in gastric cancer: a meta-analysis. Oncotarget. 2016;7:69087–69096. doi: 10.18632/oncotarget.12011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tang C.T., Lin X.L., Wu S., Liang Q., Yang L., Gao Y.J. NOX4-driven ROS formation regulates proliferation and apoptosis of gastric cancer cells through the GLI1 pathway. Cell Signal. 2018;46:52–63. doi: 10.1016/j.cellsig.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 140.Peng Y., Zhang X., Ma Q., Yan R., Qin Y., Zhao Y. MiRNA-194 activates the Wnt/beta-catenin signaling pathway in gastric cancer by targeting the negative Wnt regulator, SUFU. Canc Lett. 2017;385:117–127. doi: 10.1016/j.canlet.2016.10.035. [DOI] [PubMed] [Google Scholar]
- 141.Xu Y., Song S., Wang Z., Ajani J.A. The role of hedgehog signaling in gastric cancer: molecular mechanisms, clinical potential, and perspective. Cell Commun Signal. 2019;17:157. doi: 10.1186/s12964-019-0479-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bonilla X., Parmentier L., King B., Bezrukov F., Kaya G., Zoete V. Genomic analysis identifies new drivers and progression pathways in skin basal cell carcinoma. Nat Genet. 2016;48:398–406. doi: 10.1038/ng.3525. [DOI] [PubMed] [Google Scholar]
- 143.Wang X.D., Inzunza H., Chang H., Qi Z., Hu B., Malone D. Mutations in the hedgehog pathway genes SMO and PTCH1 in human gastric tumors. PLoS One. 2013;8 doi: 10.1371/journal.pone.0054415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pan S., Wu X., Jiang J., Gao W., Wan Y., Cheng D. Discovery of NVP-LDE225, a potent and selective smoothened antagonist. ACS Med Chem Lett. 2010;1:130–134. doi: 10.1021/ml1000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Frampton J.E., Basset-Seguin N. Vismodegib: a review in advanced basal cell carcinoma. Drugs. 2018;78:1145–1156. doi: 10.1007/s40265-018-0948-9. [DOI] [PubMed] [Google Scholar]
- 146.Basset-Seguin N., Hauschild A., Kunstfeld R., Grob J., Dreno B., Mortier L. Vismodegib in patients with advanced basal cell carcinoma: primary analysis of STEVIE, an international, open-label trial. Eur J Canc. 2017;86:334–348. doi: 10.1016/j.ejca.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 147.Basset-Seguin N., Hauschild A., Grob J.J., Kunstfeld R., Dreno B., Mortier L. Vismodegib in patients with advanced basal cell carcinoma (STEVIE): a pre-planned interim analysis of an international, open-label trial. Lancet Oncol. 2015;16:729–736. doi: 10.1016/S1470-2045(15)70198-1. [DOI] [PubMed] [Google Scholar]
- 148.Sekulic A., Migden M.R., Oro A.E., Dirix L., Lewis K.D., Hainsworth J.D. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171–2179. doi: 10.1056/NEJMoa1113713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Koelblinger P., Lang R. New developments in the treatment of basal cell carcinoma: update on current and emerging treatment options with a focus on vismodegib. OncoTargets Ther. 2018;11:8327–8340. doi: 10.2147/OTT.S135650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhang H., Sun Z., Liu Z., Song C. Overcoming the emerging drug resistance of smoothened: an overview of small-molecule SMO antagonists with antiresistance activity. Future Med Chem. 2018;10:2855–2875. doi: 10.4155/fmc-2018-0200. [DOI] [PubMed] [Google Scholar]
- 151.Rimkus T.K., Carpenter R.L., Qasem S., Chan M., Lo H.W. Targeting the Sonic Hedgehog signaling pathway: review of smoothened and GLI inhibitors. Cancers (Basel) 2016;8:22. doi: 10.3390/cancers8020022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lauth M., Bergstrom A., Shimokawa T., Toftgard R. Inhibition of GLI-mediated transcription and tumor cell growth by small-molecule antagonists. Proc Natl Acad Sci U S A. 2007;104:8455–8460. doi: 10.1073/pnas.0609699104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Maun H.R., Wen X., Lingel A., de Sauvage F.J., Lazarus R.A., Scales S.J. Hedgehog pathway antagonist 5E1 binds hedgehog at the pseudo-active site. J Biol Chem. 2010;285:26570–26580. doi: 10.1074/jbc.M110.112284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Xiao C., Ogle S.A., Schumacher M.A., Schilling N., Tokhunts R.A., Orr-Asman M.A. Hedgehog signaling regulates E-cadherin expression for the maintenance of the actin cytoskeleton and tight junctions. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1252–G1265. doi: 10.1152/ajpgi.00512.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Raleigh D.R., Reiter J.F. Misactivation of Hedgehog signaling causes inherited and sporadic cancers. J Clin Invest. 2019;129:465–475. doi: 10.1172/JCI120850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zeng X., Ju D. Hedgehog signaling pathway and autophagy in cancer. Int J Mol Sci. 2018;19:2279. doi: 10.3390/ijms19082279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wu F., Zhang Y., Sun B., McMahon A.P., Wang Y. Hedgehog signaling: from basic biology to cancer therapy. Cell Chem Biol. 2017;24:252–280. doi: 10.1016/j.chembiol.2017.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zeng X., Zhao H., Li Y., Fan J., Sun Y., Wang S. Targeting Hedgehog signaling pathway and autophagy overcomes drug resistance of BCR-ABL-positive chronic myeloid leukemia. Autophagy. 2015;11:355–372. doi: 10.4161/15548627.2014.994368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Fan J., Zhang X., Wang S., Chen W., Li Y., Zeng X. Regulating autophagy facilitated therapeutic efficacy of the sonic Hedgehog pathway inhibition on lung adenocarcinoma through GLI2 suppression and ROS production. Cell Death Dis. 2019;10:626. doi: 10.1038/s41419-019-1840-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Christos C.D., Paul J., Sparano J.A., Kindler H.L., Virgil D., Catenacci T. Vismodegib (V), a hedgehog (HH) pathway inhibitor, combined with FOLFOX for first-line therapy of patients (pts) with advanced gastric and gastroesophageal junction (GEJ) carcinoma: a New York Cancer Consortium led phase II randomized study. J Clin Oncol. 2013;31(Suppl):abs4011. [Google Scholar]
- 161.Wu X., Hu W., Lu L., Zhao Y., Zhou Y., Xiao Z. Repurposing vitamin D for treatment of human malignancies via targeting tumor microenvironment. Acta Pharm Sin B. 2019;9:203–219. doi: 10.1016/j.apsb.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]