Abstract
Insulin therapy plays an essential role in the treatment of diabetes mellitus. However, frequent injections required to effectively control the glycemic levels lead to substantial inconvenience and low patient compliance. In order to improve insulin delivery, many efforts have been made, such as developing the nanoparticles (NPs)-based release systems and oral insulin. Although some improvements have been achieved, the ultimate results are still unsatisfying and none of insulin-loaded NPs systems have been approved for clinical use so far. Recently, nano‒protein interactions and protein corona formation have drawn much attention due to their negative influence on the in vivo fate of NPs systems. As the other side of a coin, such interactions can also be used for constructing advanced drug delivery systems. Herein, we aim to provide an insight into the advance and flaws of various NPs-based insulin delivery systems. Particularly, an interesting discussion on nano‒protein interactions and its potentials for developing novel insulin delivery systems is initiated.
KEY WORDS: Insulin, Diabetic, Nanomaterials, Absorption, Controlled release, Protein adsorption
Graphical abstract
Nanoparticles-based systems have shown great potentials in delivering insulin but still have some limitations. Nano‒protein interactions, including the interaction of insulin with nanomaterials and between insulin-loaded nanoparticles and other proteins, may provide a future direction for advanced insulin delivery.
Highlights
-
•
Insulin therapy plays essential roles in treating diabetes.
-
•
Optimizing insulin delivery enhances insulin therapy.
-
•
Nanoparticles are promising systems for delivery of insulin.
-
•
Nano-protein interactions influence the delivery of nanoparticles.
-
•
Nano-protein interactions can be used for advanced delivery of insulin.
1. Introduction
Diabetes mellitus (DM), a kind of metabolic disorder disease, is characterized by the high glycemic levels due to the loss of functions to produce or use insulin1, 2, 3. According to statistics, the global diabetic patient number is over 280 million in 2010 and expected to be over 400 million by 20304. With the rapid increase in patient number, the associated costs for treatment of DM and its complications are extremely high, which are estimated to be up to US$500 billion per year all over the world5. Undoubtedly, efficient prevention and therapy of DM is quite meaningful for global economy and human health.
The therapeutic regimen for DM varies according to its different types. DM is generally categorized as two types: type I and type II. Patients suffering from type I DM lose the ability to produce insulin, a blood glucose level-regulating polypeptide which is secreted by β cells of pancreas islets, due to the destruction of β cells6,7. Hence, an insulin replacement therapy is prescribed for type I DM throughout the whole life of patients. In contrast to type I DM, type II is featured with a relative deficiency of insulin or insulin resistance mainly caused by obesity of the patients8. Patients with type II DM can produce insulin but the sensitivity of their liver and muscle cells to insulin is low. Reducing fat intake and increasing physical activity are recommended for early-stage type II DM patients to delay the disease progression9, 10, 11. Taking oral hypoglycemic drugs to enhance the production and function of insulin is another important way for type II diabetic patients to control glucose level12,13. In most cases, insulin therapy is ultimately required for treating type II DM and physicians and experts prefer to applying insulin therapy as early as possible14,15.
Because of the intrinsic instability of insulin under the physiological conditions, the delivery of insulin, especially via the oral route, is extremely challenging. It is well known that the rapid inactivation and degradation of insulin in the gastrointestinal (GI) tract and the low permeation through the intestinal epithelia barrier make the oral delivery of insulin very difficult16, 17, 18. Intranasal administration is another possible route for insulin delivery but many concerns have to be addressed before it can be practically used19,20. In addition, microneedle-based insulin delivery systems have also attracted much attention21. Nowadays, subcutaneous injection is still the dominating route for delivering insulin in clinic, and frequent injections are required to properly control the glycemic level due to the short-acting property of native insulin. The inconvenience and pains caused by the frequent injections lead to another problem that is the low patient compliance.
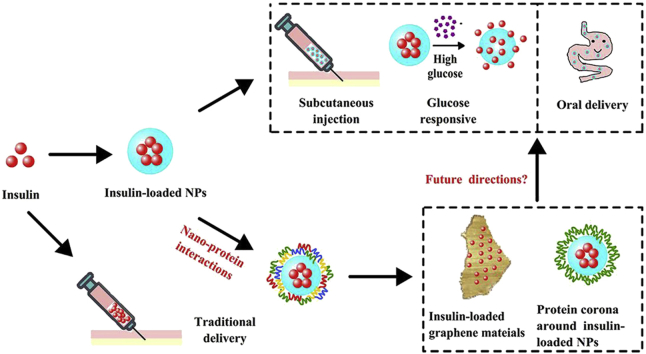
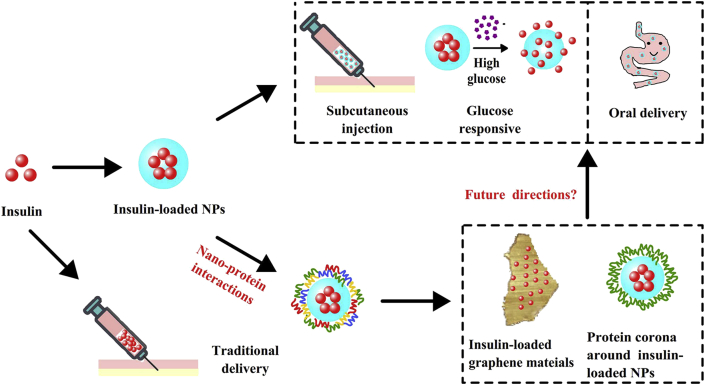
In order to improve the delivery efficiency as well as the patient compliance, nanoparticles (NPs) have been used in both subcutaneous and oral formulations to protect insulin from degradation, enhance its epithelial permeation and/or prolong its acting time17,22, 23, 24, 25. In the past decade, the nano‒protein interactions and the formation of protein corona have been considered as a challenge for NPs-based drug delivery26, 27, 28, 29, 30. Meanwhile, such interactions can also be used to decorate NPs for advanced drug delivery31, 32, 33. It is foreseeing that nano‒protein interactions may also play a role in insulin delivery. Herein, a comprehensive review of NPs-based insulin delivery is summarized, and a discussion on the nano‒protein interactions and the potentials of using such interactions for developing novel insulin delivery systems is initiated (Fig. 1).
Figure 1.
Schematics of the applications of nanoparticles (NPs) and nano-protein interactions in insulin delivery. Nano-protein interactions include the interaction between nanocarriers (such as graphene materials) and insulin and the one between insulin-loaded NPs and the other endogenous proteins.
2. Traditional insulin delivery and microneedles
Insulin, a protein produced by pancreatic β-cells, is the only blood glucose lowering hormone in organism, which is essential for DM. Generally, oral administration is the most favorable route for patients to take medicine. Unfortunately, insulin cannot be administered orally due partially to its instability and degradation in the GI tract34,35. Besides, the molecular weight of insulin is about 5800 Da, leading to a low drug absorption by intestinal epithelial cells. These above factors together result in an extremely low oral bioavailability of insulin.
Once upon a time, pulmonary delivery of insulin was of great interests. Exubera, the first insulin inhaled formulation, was successfully introduced to the market by Pfizer in 2006 but finally failed in the market because of the high variation in insulin absorption, the complex inhalation technique and the low patient acceptance17,36.
In addition, microneedle-based insulin delivery systems have also attracted much attention of researchers21. Unlike insulin injections, insulin in microneedles can be delivered across the skin to systemic circulation by minimally invasive patches, leading to greatly enhanced patients’ compliance. There are diverse structures of microneedles, such as solid, hollow, dissolved, degradable and phase transition microneedles. Although some microneedle-based insulin delivery systems (mainly the hollow systems) had entered the clinical trials, none of them has become commercially available so far37. Some concerns, including the safety of materials, the precise insulin deliver and the complicated fabrication process of microneedles, should be further studied and improved until its application in clinic.
Hitherto, subcutaneous injection is still the dominating route for administering insulin in clinic. However, the short half-life of insulin requires repeated injections in one day to efficiently control the glycemic level, which causes inconvenience to patients. To decrease the injection frequency of insulin and enhance the patient compliance, great endeavors have been made to develop advanced insulin delivery systems, in which NPs-based insulin delivery systems are the major research fields.
3. Nanoparticles-based insulin delivery systems
NPs have shown great potentials in sustained and controlled delivery of various theranostic agents34,36. To develop injectable long-acting insulin formulations and oral insulin systems, NPs are commonly utilized due to its capacity to control the release of insulin, protect insulin from degradation and enhance its bioavailability34,38,39. Herein, the potential applications of insulin-loaded NPs via subcutaneous or oral delivery route are summarized with emphasis on the latter since oral insulin is always of the greatest importance and interest. In addition, a newly emerging nanoparticle-based glucose-responsive insulin delivery system will also be discussed.
3.1. Subcutaneous delivery systems
3.1.1. Insulin-loaded long-term delivery systems
Subcutaneous injection is the only practical route for insulin administration since the failure of Exubera, an insulin formulation delivered by inhalation. Basically, frequent repeated injections are required for diabetic patients to effectively control the glycemic level. However, frequent injections cause pain and inconvenience and result in the low patient compliance. The drug delivery systems that can provide a long-term release of insulin have attracted much attention. NPs-based insulin delivery systems have been considered as the promising formulations for sustained and controlled delivery of insulin40, 41, 42. For instance, Shilo et al.38 coated insulin onto gold NPs (INS-GNPs) for controlled and prolonged glucose control. After subcutaneous injection into BALB/c mice, INS-GNPs with the size of 50 nm showed longer and stronger hypoglycemic effect than that of INS-GNPs of 20 and 70 nm. In the pharmacodynamics study on diabetic mice, the hypoglycemic effect of INS-GNPs lasted 3 times longer than that of insulin solution. The mechanism of the prolonged effect was proved to be that the insulin conjugation onto GNPs prevented its enzymatic degradation. Similarly, Tomar et al.43 developed insulin-loaded PLA-PEG NPs which showed a prolonged hypoglycemic effect of more than 7 days, and the NPs were proved to be nontoxic.
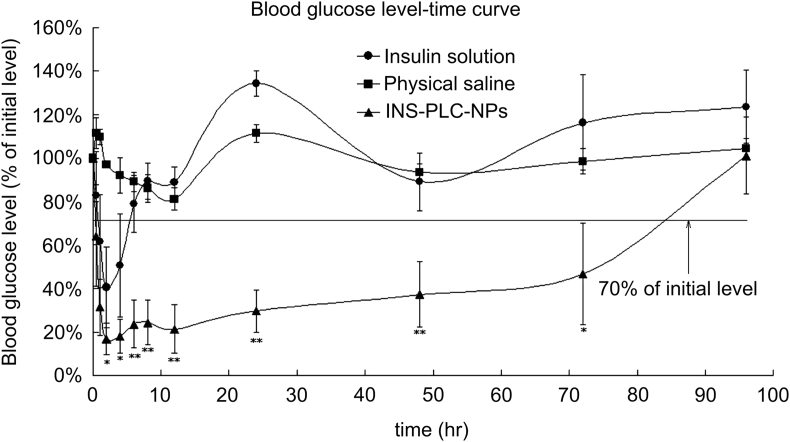
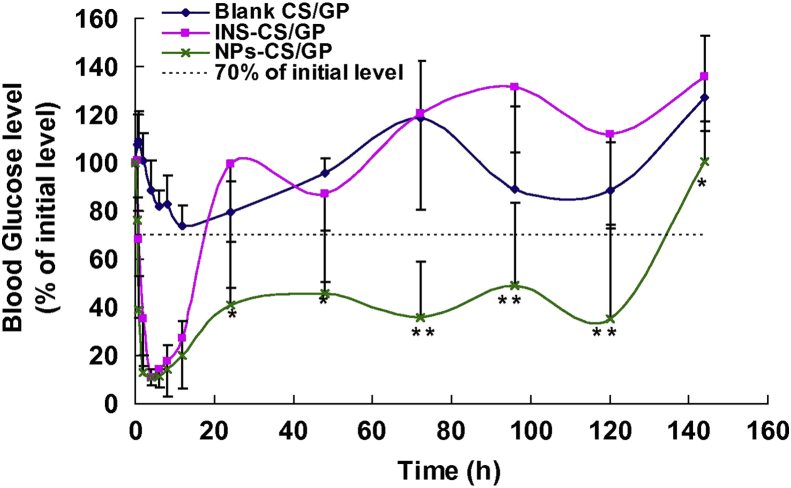
Our group has also demonstrated the great potentials of NPs in the long-term delivery of insulin. We constructed the insulin-phospholipid complex-loaded PHBHHx NPs (INS-PLC-NPs) which showed a long-term insulin release in vitro and very high insulin encapsulation efficiency (89.7%) due to the formation of INS-PLC44. Most importantly, upon a single subcutaneous injection into the diabetic rats, INS-PLC-NPs showed a rapid, stable, effective and lasting hypoglycemic effect for more than 3 days (Fig. 2). Such a sustained hypoglycemic effect conferred INS-PLC-NPs a greatly enhanced pharmacological bioavailability relative to free insulin (350%)44. In a further study, INS-PLC-NPs were loaded into a β-glycerophosphate disodium salt/chitosan-based thermo-sensitive hydrogel (NP-CS/GP) to further extend insulin release. We found NP-CS/GP has a hypoglycemic effect for more than 5 days and showed a pharmacological availability of 379.85% relative to the free insulin-loaded hydrogel (INS-CS/GP), and higher (Fig. 3)22.
Figure 2.
Blood glucose level-time profiles of insulin solution (1 IU/kg), physical saline and INS-PLC-NPs (4 IU/kg) in the diabetic rats model upon s.c. administration (n = 5). Significance of INS-PLC-NPs group vs. insulin solution group: ∗P < 0.05, ∗∗P < 0.001. Reprinted with the permission from Ref. 44 Copyright © 2012 Elsevier BV.
Figure 3.
Blood glucose level–time profiles of blank CS/GP, INS-CS/GP (4 IU/kg) and NP-CS/GP (6 IU/kg) in the diabetic rats model upon subcutaneous injection (n = 5). Significance of INS-CS/GP group vs. NP-CS/GP group: ∗P < 0.05, ∗∗P < 0.01. Reprinted with the permission from Ref. 22 Copyright © 2013 Elsevier BV.
3.1.2. Glucose-responsive insulin delivery systems
Glucose-responsive insulin delivery system is another newly and major category of subcutaneously injected insulin vehicles with intelligent control of insulin release. As we know, both of hyperglycemic and hypoglycemic are detrimental for diabetic patients45,46. However, because of the terribly narrow therapeutic index of insulin, monitoring and proper controlling of blood glucose all the time are very important but difficult. Accordingly, an intelligent system, glucose-responsive insulin delivery system emerged, which aims at emulating the function of pancreatic β-cells to release demanded insulin in response to high glycemic level and achieving accurate glycemic control seamlessly. In the smart glucose-responsive system, various glucose-responsive materials or strategies have been adopted for bio-inspired reaction47. Basically, insulin is embedded in a matrix containing glucose-responsive materials, which changes its structure to launch or stop insulin release in response to the blood glucose level.
In the past years, significant progress in glucose-responsive insulin delivery system has been achieved, which will be discussed according to different-responsive mechanisms in this section: (1) glucose oxidase-based system; (2) glucose binding protein-based system; (3) glucose binding molecule-based system; (4) modified insulin-based system.
3.1.2.1. Glucose oxidase-based systems
Typically, the glucose-responsive systems are based on glucose oxidase (GOD)48, and the first GOD-based pH sensitive hydrogel for glucose-responsive delivery of insulin was reported in 198449. GOD is widely used for the quantification of blood glucose due to its high sensitivity in reaction with glucose. Under the catalysis of GOD, glucose is transformed into gluconic acid and byproduct hydrogen peroxide (H2O2) as shown in Eq. (1). The hydrogen ion dissociated from gluconic acid will reduce the local pH as shown in Eq. (2) 50. The protonization and H2O2 can serve as activators for the collapsing or swelling of hydrogel51 or dissociating of nanoparticle matrix, subsequently leading to the release of loaded insulin52, 53, 54, 55.
| (1) |
| (2) |
In the GOD-based glucose-responsive nano-carriers for insulin, the mechanism is usually based on the dissembling/degradation of the matrix39,56,57. For instance, Qi et al.39 developed a glucose sensitive microcapsules using hemoglobin (Hb) and GOD cross-linked by glutaraldehyde (GA) in the form of layer by layer. With the increase of glucose level, GOD catalyzed glucose into gluconic acid and H2O2. When the local pH was decreased by the production of gluconic acid, the permeability of Hb/GOD capsule wall was enhanced and the structure of the multilayer loosed, which affiliated the release of loaded insulin.
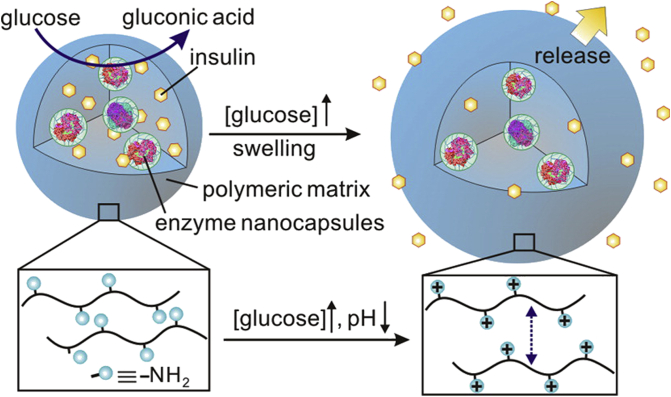
Besides, mesoporous silica was also used for GOD-based glucose-responsive systems58, 59, 60. Aznar et al.58 prepared a mesoporous silica nano-device capped with cyclodextrin-modified-glucose oxidase (CD-GOD), which could release the loaded cargo in response to the high glucose level. With the increase and accumulation of H2O2, the activity of GOD can be inhibited and inflammation may happen. Therefore, catalase (CAT) was often adopted into GOD-based system61. Interestingly, the production and consumption of H2O2 were also combined with GOD-based glucose-responsive system. Gu and colleagues52,62,63 have done much work in this field. For example, they developed a glucose-responsive system62, in which GOD and CAT were dispersed to form nanocapsules and then encapsulated into insulin-loaded pH-responsive chitosan microgels. The nanocapsules protected enzymes from denaturation and immunogenicity (Fig. 4). This was a self-regulating valve system. Under normal glycemic condition, microgels released insulin at a basal rate. While under hyperglycemic condition, glucose was catalyzed into gluconic acid and subsequently the amine groups in polymer chains were protonated to increase the charge in gel matrix, leading to microgels swelling and subsequently insulin release. Moreover, glucose sensitive nano-carriers were also combined with microneedle-array patches55,64.
Figure 4.
Schematics of the insulin- and enzyme nanocapsules-loaded microgels. Glucose is catalyzed by enzyme into the gluconic acid which in turn leads to the protonation of –NH2 on the polymer chains. Insulin is ultimately released as a result of the protonation-caused swelling of the microgels. Reprinted with the permission from Ref. 62 Copyright © 2013 American Chemical Society.
3.1.2.2. Glucose binding protein-based systems
Glucose binding proteins (GBP), usually lectins, can also be used in glucose-responsive systems due to their competitively combination with glucose. Concanavalin A (Con A) is the most widely studied GBP, which is in the form of tetramers exposing four glycoprotein binding sites in a neutral condition65. The first Con A-based glucose-responsive insulin delivery system was reported by Che and colleagues in 197966. Under the high glucose conditions, glucose competed for the binding sites on Con A with glycosylated insulin, resulting in the release of insulin in proportion to the glucose level. In further studies, the formulation was optimized with Con A derivatives (e.g., PEGylation Con A67), glycosylated insulin (e.g., succinyl amidophenyl mannopyranoside insulin68,69) or glycosylpoly(ethylene glycol) insulin70 to improve the stability and insulin release.
GBP-based mechanism is generally used for developing glucose-responsive hydrogels71, 72, 73. Meanwhile, novel materials were synthesized and adopted to formulate GBP-based glucose-responsive nano-carriers74, 75, 76. You et al.75 prepared a glucose-sensitive aggregates with Poly(ethylene oxide)-block-poly(2-glucosyloxyethyl acrylate) and Con A. Wu et al.76 prepared glucose- and pH-responsive mesoporous silica insulin-loaded nanocontainers with Con A gated and mannose functionalized. The insulin release could be triggered by the decrease of pH (<5.5) and increase of glucose concentration.
3.1.2.3. Phenylboronic acid-based systems
Phenylboronic acid (PBA) and its derivatives have been used as glucose binding agents to design the closed-loop insulin delivery systems since 199477 and have achieved great advances so far78. PBA and derivatives can form the reversible covalent complexes with glucose containing polyhydroxyl groups and thus can be used as the glucose sensor in glucose-responsive systems. PBA moieties have been widely implanted in crosslinked polymeric matrix loaded with insulin. With the increase of glucose concentration, glucose‒PBA complex forms and the hydrophilic phenylborate level increases, leading to the swelling of the matrix and insulin release53,79, 80, 81.
In addition, in PBA moieties-contained layer-by-layer particles82,83 or core–shell particles84, 85, 86, 87, 88, glucose competitively binds to PBA and triggers the release of insulin due to the particle disassembling and degradation. Shi et al.83 fabricated a poly(γ-glutamic acid) (γ-PGA) and chitosan oligosaccharide (CS) polyelectrolyte-based glucose-responsive capsule system. At the high glucose level, the competitive reaction of aminophenylboronic acid (APBA) with glucose caused the polymer capsules swelling and broke the cross-linking, ultimately resulting in capsules dissociation and insulin release. Meanwhile, decoration of the (γ-PGA-g-APBA)5 capsules with lactobionic acid (LA) led to the on-off regulation of insulin release.
Also, PBA-modified polymers were combined with glycosylated insulin to formulate glucose-responsive system89. Zhao et al.89 developed glucose-responsive mesoporous silica NPs (MSN) co-loaded with insulin and cyclic AMP. The boronic acid-functionalized MSNs were loaded with cyclic AMP inside and decorated with glycosylated insulin (G-Ins) on the exterior surface via interactions with PBA. Thus, the competitive binding of glucose with PBA triggered the release of G-Ins and cAMP which could stimulate the secretion of insulin by β-islet cells.
3.2. Oral delivery systems
Oral administration is widely recognized as the most convenient and tolerated drug delivery route for patients. However, as a protein drug, insulin is mainly administered by subcutaneous injection due to the harsh environment in the GI tract. To solve this problem, various functional NPs-based systems have been developed.
3.2.1. Barriers for oral absorption of insulin
The oral absorption barriers that insulin has to overcome in the GI tract mainly include chemical, enzymatic and physical barriers90. Basically, most oral drugs transit through the GI tract, adhere to the mucus layer, cross the intestinal epithelium, and finally enter the systemic circulation34. However, the oral bioavailability of insulin is quite low (less than 1%) due to its instability in the GI tract and low epithelial permeation91.
The remarkable change of pH values from 1.0 to 2.5 in the stomach to ∼7.5 in the terminal ileum constructs the chemical barrier to the orally administered insulin92. The low pH in the stomach is one of the harsh conditions leading to the degradation and low absorption of insulin. The proteases secreted into the GI tract act as the enzymatic barrier, which destruct the orally administered insulin into the inactive small amino acids or peptides. The enzymes involved in the degradation of proteins in the GI tract mainly include the pepsin in the stomach, the pancreatin in the small intestine (such as trypsin, chymotrypsin and elastase), the aminopeptidases in the brush border membrane, and the specific enzymes in the cytosol93, 94, 95. Moreover, even if a small amount of insulin can survive from the above proteases, it will be probably degraded by the liver enzymes, known as the first-pass effect.
The physical barriers in the GI tract are another challenge for the oral absorption of insulin. The mucus layer secreted on the intestinal epithelium is semipermeable and only allows the permeation of nutrients, water and small molecules. In this regard, insulin, a polypeptide with a molecular weight of ∼5800 Da, is hard to cross the mucus layer. In addition, the negative charge of insulin molecules (isoelectric point is ∼4.5) in the small intestine (pH ∼6.8) is also unfavorable for their transport across the mucus layer which is known to be negatively charged. The intestinal epithelial cells constitute another physical barrier for the oral absorption of insulin. It has been reported that only the hydrophilic drugs with a molecular weight of below 200 Da can pass through the intestinal epithelium by the paracellular route, and that only the lipophilic small molecules smaller than 700 Da are allowed to pass through the intestinal epithelium by the transcellular route34,96. Undoubtedly, insulin is difficult to be taken up by the epithelial cells due to its large molecular weight. The above-mentioned barriers work together and ultimately lead to the extremely low oral bioavailability of insulin. Therefore, many researchers have been working to solve this problem recent years.
3.2.2. Nanotechnology for enhancing oral absorption of insulin
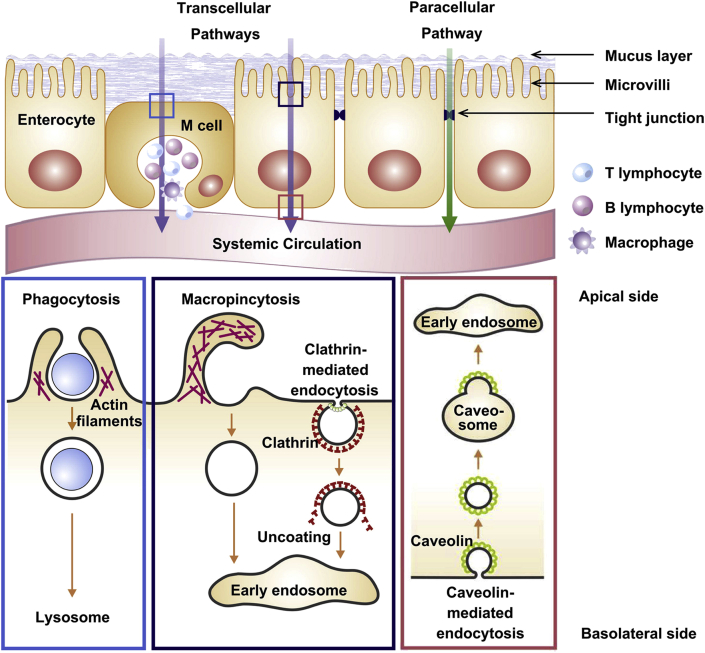
The past decades have witnessed the progress of NPs-based insulin delivery systems which showed great potentials to bypass the absorption barriers and enhance the oral bioavailability of insulin97, 98, 99. Encapsulated into the NPs, insulin can be protected from the chemical and enzymatic degradation100,101. Besides, polymeric NPs can significantly improve the uptake by small intestinal epithelial cells and promote the absorption via paracellular pathway34. Furthermore, the insulin-loaded NPs can also be taken up by M-cells, a kind of special enterocytes in the GI tract102. Generally, NPs facilitate the transport of insulin across the intestinal epithelium by both paracellular and transcellular pathways (Fig. 5)35,102. Paracellular pathway is characterized as an approach to get across the tight junctions between adjacent cells. In contrast, transcellular pathway is an approach to directly get across the epithelial cells via phagocytosis, macropinocytosis or endocytosis.
Figure 5.
Schematics of the main mechanisms for NPs uptake by intestinal epithelium, including paracellular pathway and transcellular pathway. Inserts show the simplified processes of varied transcellular transport, including phagocytosis, macropinocytosis and clathrin-/caveolae-mediated endocytosis. Reprinted with the permission from Ref. 34 Copyright © 2011 Elsevier BV.
3.2.2.1. Nanoparticles overcoming chemical and enzymatic degradation
At the early stage, efforts were focused on enhancing insulin resistance to chemical and enzymatic degradation with the help of NPs encapsulation. Ramachandran et al.103 prepared the insulin-loaded PEGylated calcium phosphate NPs which showed a negligible release of insulin under the gastric acidic condition (6.5%) and a complete release in the intestinal neutral pH. But only the high resistance to acidity is far from enough since the released insulin in the intestinal site will still encounter the enzymatic degradation and epithelial uptake limitation. Yu et al.104 constructed a more complicated system, in which the insulin-loaded polymer-lipid hybrid NPs (INS-PLGA-lipid-PEG NPs) were transformed into microparticles by spray freeze-drying and loaded in enteric capsules. The enteric capsules could prevent insulin from the gastric harsh conditions, and the NPs released in the intestine could increase the cellular permeability and uptake. Consequently, the insulin absorption was greatly enhanced and the oral bioavailability of the NPs system in diabetic rats was about 12% relative to the subcutaneously injected insulin. Some other pH sensitive materials were also used for oral delivery of insulin, such as poly(N-isopropylacrylamide) (NIPAAm) polymer derivatives105 and hydroxypropylmethyl cellulose phthalate (HPMCP)106.
NPs co-loaded with insulin and enzyme inhibitor is another strategy for overcoming the enzymatic barrier and enhancing oral absorption of proteins107,108. Su et al.107 prepared a functional NPs system by mixing CS with anionic γPGA-DTPA. The γPGA-DTPA could inhibit the proteases significantly and enhance the paracellular permeability of NPs by disrupting the intestinal tight junctions. Additionally, the CS/γPGA-DTPA NPs were also pH-responsive and in favor of intestinal absorption of insulin. Based on the above strategies, the insulin relative oral bioavailability of the functional NPs was increased to approximately 20%.
3.2.2.2. Nanoparticles overcoming the physical barriers
The mucus layer together with the intestinal epithelial cells constructs the physical barriers for insulin oral absorption. In order to overcome this issue, NPs systems decorated with mucoadhesive or absorption enhancers are developed. Damge et al.109 prepared Ins-NPs with hybrid polyester poly(ε-caprolactone) and polycationic acrylic polymer Eudragit® RS. With an insulin entrapment efficiency of 96% and the mucoadhesive property, the polymeric NPs preserved insulin bioactivity and showed a significant intestinal uptake.
Cell penetrating peptides (CPP) have been widely used to improve protein permeability across the intestinal mucus layer and epithelia23,110, 111, 112. Low molecular weight protamine (LMWP) is such a CPP113. Sheng et al.23 conjugated insulin with LMWP and encapsulated the conjugate into the N-trimethyl chitosan chloride-coated PLGA NPs (MNPs). MNPs showed the mucoadhesive property in the intestine, which shortened the distance between insulin and the epithelium and protected insulin from enzymatic degradation. After released from MNPs, the conjugate showed a high permeation across the mucus layer and epithelium. When orally administered to diabetic rats, the MNPs showed a fast onset, a long-lasting hypoglycemia effect and a relative bioavailability of 17.98 ± 5.61%. Particularly, the oral delivery of insulin or the conjugate (50 IU/kg) did not show any hypoglycemic effect, demonstrating the great protection effect of MNPs on insulin23. In another study, N-(2-hydroxypropyl) methacrylamide copolymer (pHPMA) derivative was used as a dissociable “mucus-inert” hydrophilic coating to encapsulate N-trimethylated chitosan (TMC)-based insulin core114. The NPs exhibited remarkable permeability across the mucus layer and epithelia barriers, and the co-loaded TMC could open the tight junctions between epithelial cells. After orally administered to the diabetic rats, the pHPMA-coated TMC-based NPs showed a relative bioavailability of 8.56%, 2.8-fold higher than that of the uncoated TMC-based NPs. CPP was also grafted onto chitosan, forming a novel material for insulin encapsulation and oral delivery (CPP-g-chitosan)115.
Ligand decoration of NPs is another strategy for enhancing insulin absorption in GI tract. For example, it was reported that some plant lectins showed different carbohydrate specificity and mucoadhesive property, facilitating NPs transport across the cellular barriers116. Wheat germ agglutinin (WGA) is reported to be greatly specific to intestinal cell lines117,118. Accordingly, WGA can be decorated onto drug carriers, mediate the adhesion interactions between WGA grafted drug carrier and WGA receptor, thus generating improved drug absorption in GI tract119,120. Compared with the undecorated nano-sized carriers, the WGA-decorated insulin carriers showed a much greater relative pharmacological bioavailability after oral administration121,122. Another work demonstrated that the vitamin B12-NPs conjugate had different in vitro and in vivo properties and hypoglycemic effect depended on the molecular weight of dextran in NPs (Conjugate 70 kD > Conjugate 10 kD > Conjugate 200 kD)123. The efficacy of vitamin B12‒NPs conjugates were further optimized using different degree of cross-linking and different linkage. It was reported that NPs with the low cross-linking (4.0%) and carbamate linkage were superior carriers which showed a relative pharmacological availability up to 29.4%124.
3.2.2.3. Multi-functional nanoparticles for oral delivery of insulin
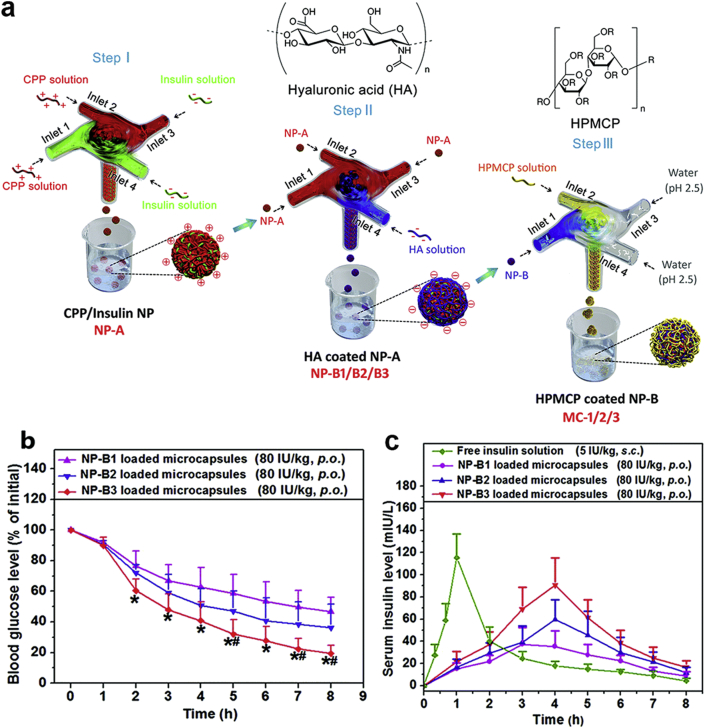
As discussed above, various strategies have been used to overcome oral absorption barriers for insulin. When combining different strategies together, a multi-functional nano-carrier is expected to achieve the highly enhanced oral drug delivery. For instance, He et al.106 developed a core–shell NPs-based insulin delivery system with an extremely high encapsulation efficiency (97%) and drug loading capacity (67%). The NPs, consisting of an insulin/L-penetratin (CPP) complex core and a hyaluronic acid (HA) coating, were protected by enteric microcapsules (MCs, Fig. 6a). In this system, the enteric microcapsules offered protection for insulin against the acidic stomach environment and allowed fast drug release in small intestinal. Moreover, the HA coating on the NPs could enhance the permeation of NPs across the intestinal epithelium, resulting in the significantly improved insulin absorption in the intestine. The in vivo results showed that this multifunctional system had a high relative oral bioavailability (11%) and thus could reduce the blood glucose level efficiently (Fig. 6b and c). Some typical literatures reporting the NPs-based insulin delivery systems are summarized in Table 1.
Figure 6.
(a) Schematics of the procedures for preparing CPP/insulin nanoparticle (NP-A) core, HA-coated NPs (NP-B1/B2/B3) and HPMCP coated NP-B (MC-1/2/3). (b) Blood glucose level-time curves of diabetic rats after oral administration of MC-1/2/3 (80 IU/kg). ∗P < 0.05 vs. NP-B1, #P < 0.05 vs. NP-B2. (c) Serum insulin level of diabetic rats following oral administration of MC-1/2/3 (80 IU/kg). Subcutaneous injection of insulin solution (5 IU/kg) is the control. Data are means ± SD (n = 6). Reprinted with the permission from Ref. 106 Copyright © 2018 Royal Society of Chemistry.
Table 1.
Examples of NPs-based insulin delivery systems.
| Nano-carrier | Size; Zeta potential | Entrapment efficiency (%) | Drug loading | Dose | Efficacy | Ref. |
|---|---|---|---|---|---|---|
| Insulin-coated gold NPs | 20−70 nm; – | – | – | s.c.: 6 mg/mouse | Effect lasting for 6 h | 38 |
| Poly(ethylene glycol) capped poly(lactic-co-glycolic) acid NPs | 140−170 nm; −14.5 mV | 66 | – | s.c.: 2 IU/kg | Effect lasting for ∼6 h | 42 |
| Poly(ethylene glycol) (PEG) and polylactic acid (PLA)-based copolymeric NPs | 181.9 nm; – | 58.5 | 313.4 IU/g | s.c.: 25, 50 IU/kg | Effect lasting for more than 7 days | 43 |
| Insulin phospholipid complex-loaded biodegradable PHBHHx NPs | 182.4 nm; −36.93 mV | 87.19 | – | s.c.: 4 IU/kg | Pharmacological availability (PA) 350.29%; effect lasting for ∼83.5 h | 44 |
| NPs loading insulin and glucose enzymes coated by chitosan or alginate | 340 nm/293 nm; 10.6 mV/−11.5 mV | 54.1/77.9 | 7.9%/11.4% | s.c.:− | Effect lasting for 10 days without peaks of hyperglycemia or hypoglycemia | 52 |
| Enzyme (GOx and CAT) nanocapsules loaded in chitosan microgels | 12 nm; – | 59.7 | 44.6% | s.c.: 40 mg/kg | Effect lasting for 24 h | 62 |
| Microparticles composed of chitosan, Con A and dextran | 2.5 μm; – | 92.2 | 9.1% | – | In vitro: glucose responsive insulin release | 74 |
| Poly(ethylene glycol)-b-Poly(acrylic acid-co-acrylamidophenylboronic acid) (PEG-b-(PAA-co-PAAPBA)) micelles | 130 nm; – | – | 29% | – | In vitro: glucose responsive insulin release | 85 |
| p(3-acrylamidophenylboronic acid-b-diethylene glycol methyl ether methacrylate) NPs | Submicron−sized; ‒37.2 mV | ∼70 | ∼15% | s.c.: 0.4 mg/kg |
In vitro: glucose- and temperature-sensitive insulin release In vivo: effect lasting for 48 h |
86 |
| Cetyl palmitate-based solid lipid NPs | 361 nm; ‒3.4 mV | 43 | – |
p.o.: 50 IU/kg s.c.: 2.5 IU/kg |
PA 1.6%; effect lasting for 24 h | 99 |
| INS-loaded polymer–lipid hybrid NPs | 176 nm; ‒31.1 mV | 92.30 | 2.40% |
p.o.: 40 IU/kg s.c.: 5 IU/kg |
RBA 12.42%; effect lasted for 24 h | 104 |
| Insulin/L-penetratin complex NPs coated with hyaluronic acid | 103.7 nm; ‒19.7 mV | 97 | 67% |
p.o.: 80 IU/kg s.c.: 5 IU/kg |
BAR 11%; PA 3.7%; blood glucose level (BGL) reduced by 60% in 8 h | 106 |
| CS/γPGA-DTPA NPs (CS: chitosan; γPGA: poly(γ-glutamic acid); DTPA: diethylene triamine pentaacetic acid) | 246.6 nm; +37 mV | 75.70 | 16.30% |
p.o.: 30 IU/kg s.c.: 5 IU/kg |
BAR 19.7%; prolonged reduction in BGL | 107 |
| Polyester poly(-ε-caprolactone) and a polycationic non-biodegradable acrylic NPs | 358 nm; +41.8 mV | 96 | – | p.o.: 50 IU/kg (PK); 100IU/kg (PD)s.c.: 10 IU/kg | BAR 13.21%; reducing the glythemia–time curve area by 38% | 109 |
| TMC-based NPs coated with pHPMA (TMC: trimethyl chitosan; pHPMA: N-(2-hydroxypropyl) methacrylamide copolymer) | 163 nm; ‒3.35 mV | 54.10 | 24.50% |
p.o.: 50 IU/kg s.c.: 5 IU/kg |
BAR 8.56%; maximal BGL reduced 36% at 4 h; effect lasting for 10 h | 114 |
| Cell-penetrating peptide (CPP) grafted chitosan NPs | 316 nm; +42 mV | – | – |
p.o.: 30 IU/kg s.c.: 5 IU/kg |
BAR 19.6%; BGL reduced 30% in 4 h and the effect lasted for 12 h | 115 |
| Lectin-modified solid lipid NPs | 75.3 nm; ‒13.1 mV | 40.18 | – |
p.o.: 50 IU/kg s.c.: 2 IU/kg |
BAR 7.11%; PA 6.08% | 121 |
| Vitamin B12‒nanosphere conjugate | 160–250 nm; – | 45–70 | 2%–4% |
p.o.: 20 IU/kg s.c.: 0.4 IU/kg |
PA 11.4–26.5%; effect prolonged for many hours | 123 |
| Vitamin B12 coated dextran NPs | 192 nm; – | 63.50 | 3% |
p.o.: 20 IU/kg s.c.: 0.4 IU/kg |
PA 29.4%; BGL reduced 70%–75% and effect lasting for 54 h | 124 |
| PEGylation and Con A-based targeted NPs | 196.3 nm; ‒25.6 mV | 44.60 | – |
p.o.: 20 IU/kg s.c.: 0.4 IU/kg |
A delayed (2–4 h) reduction of BGL and effect lasting for 24 h | 125 |
| FA-PEG-PLGA NPs | 260 nm; – | 87 | 6.50% |
p.o.: 50 IU/kg s.c.: 5 IU/kg |
BAR 19.62%; PA 20.40%; effect lasting for 24 h | 126 |
| FA-CS NPs | 288 nm; +21.90 mV | >80 | – |
p.o.: 50 IU/kg s.c.: 5 IU/kg |
BAR: 17.04%; significant decrease in BGL in 6 h and down to baseline at 24 h | 127 |
| Cp1-11 peptide/insulin-loaded NPs | 237.2 nm;– | 90.43 | 28.06% |
p.o.: 50 IU/kg s.c.: 4 IU/kg |
PA 9.83%; BAR 15.62%; BGL reduced to 55.1% in 2 h and maintained 60% for 8 h | 128 |
| Zein-carboxymethylated short-chain amylase/CS nanocomposites | 311.32 nm; 43.77 mV | 89.60 | 6.80% |
p.o.: 50 IU/kg s.c.: 5 IU/kg |
PA 14.12%; BAR 15.19%; effects lasting for up to 8 h | 129 |
‒Not applicable.
4. Nano‒protein interactions-based insulin delivery systems
4.1. Nano‒protein interactions and its negative impact on drug delivery
NPs have shown great potentials in improving insulin delivery, but a recently proposed issue of nano‒protein interactions stands like an obstacle for the practical use of NPs. It has been clearly demonstrated that when NPs are in contact with protein-containing biological fluids, such as serum, the proteins will rapidly interact with NPs forming the protein corona (Fig. 7)26,31,130, 131, 132. Basically, the physicochemical properties of NPs, such as size and morphology, will be changed upon the formation of protein corona. The significant size increase of NPs is a common result of protein corona formation and the size of NP-protein complex will become stable ultimately32,133. Maiorano et al.134 found that, the size of AuNPs of different original sizes quickly increased in cell culture medium with 10% fetal bovine serum (FBS) and reached a plateau at about 48 h, and different culture medium (PRIM and DMEM) showed different effect on the protein corona formation. Due to protein adsorption, the NPs surface is replaced with protein corona leading the substantial change of NPs surface morphology. Generally, the protein corona surrounding the NPs can be observed by transmission electron microscopy (TEM)26, scanning electron microscopy (SEM), and atomic force microscopy (AFM)32,135.
Figure 7.
A nanoparticle surrounded with protein corona upon its dynamic interactions with biological fluids. Reprinted with the permission from Ref. 132 Copyright © 2017 American Chemical Society.
Most importantly, the in vivo fate of NPs is substantially changed due to nano‒protein interactions30,133,135, 136, 137. Usually, after entering into systemic circulation, the proteins adsorbed on the surfaces of NPs are non-specific. According to previous reports, the major negative effects of the non-specific nano‒protein interactions on NPs delivery are mainly shown in pharmacokinetics and safety132,138. Firstly, the opsonization effect caused by opsonins (e.g., complements, fibrinogen and immunoglobulins) adsorption onto NPs leads to the fast clearance of drug-loaded NPs from systemic circulation, resulted in the rapid loss of therapeutic effects139. This is a big challenge for NPs drug delivery systems. Secondly, the protein corona covers the ligands or functional groups on the surface of NPs which are designed for targeted drug delivery, consequently leading to the loss of their targeting ability30,140. Thirdly, the increased size of NPs may enhance the risk of capillary blockage. For example, in our previous study, the size of GO/rGO increased to about 2 μm after incubated with the diluted serum136. Last but not the least, the potential toxicity induced by nano‒protein interactions cannot be ignored26,138,141,142.
4.2. Potentials of using nano‒protein interactions
Despite the undesired negative effects discussed above, nano‒protein interactions and protein corona formation can also be used in developing advanced NPs-based delivery systems31.
Firstly, pre-coating NPs with endogenous proteins or antibodies is a strategy for endowing NPs with targeting property143, 144, 145. For example, Tonigold et al.144 pre-coated NPs with anti-CD63 by adsorption method which kept the targeting property by resisting the uptake of monocyte derived dendritic cells. In another study, Au NPs pre-conjugated with albumin showed increased bio-distribution in brain and lung compared to Au NPs decorated with citrate and apolipoprotein E146. Besides, low-density lipoproteins (LDL)-decorated NPs showed a specific affinity for tumors, which was applied for targeted drug delivery for cancer therapy147.
In addition, it has been demonstrated that the specific albumin corona preformed surrounding NPs can serve as a protective coating for NPs system, prolonging the blood circulation time and improving the biostability32,33,148. Coating NPs with BSA corona, the drug release from NPs was greatly slowed and the stability of NPs in mice liver homogenate was enhanced compared with that of naked NPs. These results could be attributed to the physicochemical barrier effect of the protein corona32. Furthermore, the in vivo pharmacokinetics study showed that, the half-life of albumin corona-coated NPs was 6-fold higher than that of naked NPs after intravenously injected into rats, and reversely the clearance rate was declined by 2.5-fold33. Therefore, protein corona has the potential for constructing prolonged drug delivery systems.
Besides, protein corona can also be used for improving drug loading by self-assembly. In the study of Kah et al.149 showed that the gold NPs precoated with serum proteins had the higher drug loading capacity (∼5‒10-fold) for DNA and doxorubicin than covalently conjugated NPs did. Their further study found that150 the amount of proteins in the corona could regulate the payload release behavior. The DNA release was triggered with the amount increase of human serum or human serum albumin, which could be attributed to the enhanced degree of protein exchange in corona. Protein corona can also be used in other fields, such as antibacterial therapy and tissue engineering151, 152, 153. Therefore, nano‒protein interactions could be a potential strategy for improving drug delivery in different directions.
4.3. Potential insulin delivery systems based on nano‒protein interactions
Similarly, for insulin delivery, nano‒protein interactions may provide unique approaches. Herein, two potential strategies to use nano‒protein interactions for advanced insulin delivery will be discussed below, including adsorption of insulin on nanographene sheets and formation of protein corona on insulin-loaded NPs.
4.3.1. Interactions of graphene-based nanomaterials with insulin
Graphene-based nanomaterials (GBNs) have great potential applications in drug delivery due to their unique structure and properties154, 155, 156. The most typical GBNs include graphene oxide (GO) and its reduced form rGO. The huge specific area of GBNs confer them a great capacity to efficiently load many therapeutic molecules via various interactions, such as hydrophobic force, hydrogen bonding, π‒π conjugation and electrostatic interactions157, 158, 159, 160. Insulin can also be loaded onto GBNs via nano‒protein interactions between GBNs and insulin, forming the sustained and controlled insulin release systems158,161. Unlike the encapsulation of insulin by the traditional NPs, the loading of insulin on GBNs is largely dependent on the physical interactions between GBNs and insulin, such as π‒π conjugation and electrostatic interaction. In the case of GO, the electrostatic interaction plays essential roles in insulin loading. It has been shown that the insulin loading on GO is high at pH < 5.4 based on the strong electrostatic forces between positively charged insulin (PI = 5.4) and negatively charged GO162. In the case of rGO, the insulin loading is shown as pH-independent and largely dependent on the π–π stacking interaction between rGO and insulin158,161.
Insulin is susceptible to low pH and GBNs are found to be able to enhance its stability in low pH medium. Turcheniuk group162 prepared GO and GO-magnetic particles (GO-MPdop) to load insulin. In their study on Xenopus laevis oocytes model, after incubated at pH 2.0 for 5 h, GO–insulin and GO–MPdop–insulin showed almost unchanged meiotic resumption rate while insulin group showed greatly decreased rate, indicating the protection effect of GO on insulin. In addition, the photothermal conversion or electrochemical properties of GBNs confer the insulin-loaded GBNs a pulsatile release property triggered by NIR irradiation or pH change161,162.
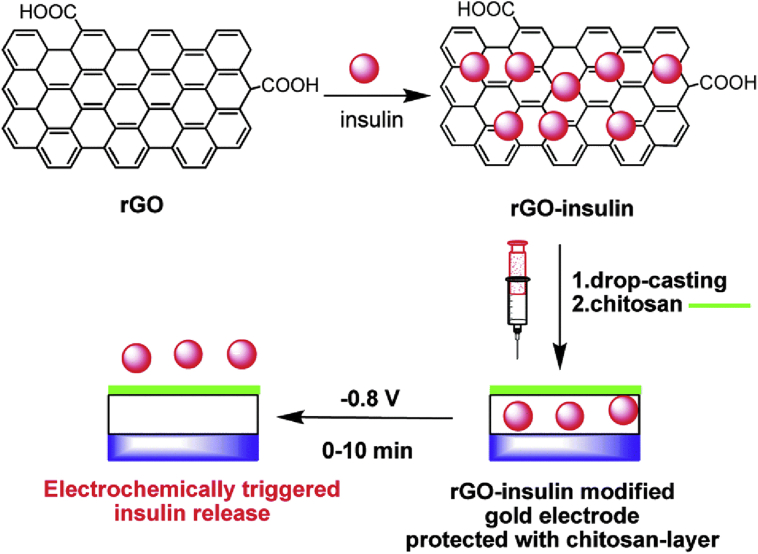
Teodorescu et al.161 took advantages of the electrochemical or photothermal conversion properties of rGO to develop an advanced insulin delivery system. They prepared a gold electrode modified with insulin-impregnated rGO, which was designed to combine with flexible skin patches for insulin transdermal delivery. In this system, insulin release could be triggered via electrochemical action. Under the potential pulse of 0.8 V, 70 ± 4% of human insulin was released within 30 min (the time of normal insulin secretion in vivo), and the bioactivity of released insulin was proved to be well preserved in this process (Fig. 8). Besides, rGO has been proved to be a great near infrared (rNIR)-absorbing photothermal agent which possesses rapid light-to-heat conversion ability under NIR irradiation and has been used as photothermally triggered drug delivery vehicles163, 164, 165. In another work, Teodorescu et al.158 developed a photothermally triggered insulin delivery system with rGO-modified hydrogels which were designed as a patch for transdermal insulin delivery. The hydrogels were made of poly(ethylene glycol) dimethacrylate (PEGDMA) and impregnated with rGO and finally loaded with insulin via physical adsorption overnight. The insulin loading capacity was enhanced with the increase of rGO content in PEGDMA-rGO hydrogels and saturated at 80% when rGO contents reached 0.8 mg. Under the laser intensity of 0.7 W/cm2 (980 nm), the hydrogel could reach the temperature of 70 °C within 10 min, which was high enough to interfere with the interaction between insulin and rGO, leading to insulin dissociation from the hydrogel. Moreover, under such high temperature, the hydrogels remained stable and the released insulin kept its bioactivity.
Figure 8.
Schematic representation of the formation of insulin-loaded rGO (rGO-insulin), gold electrode modified with rGO-insulin and protected with chitosan-layer, and electrochemically triggered insulin release. Reprinted with the permission from Ref. 161 Copyright © 2015 Royal Society of Chemistry.
GO has also been used as a drug carrier for proteins (including BSA and insulin)159,162,166. For instance, Zhou et al.166 loaded an insulin-derived peptide (EALYLV) onto PEG-modified graphene oxide to inhibit the aggregation of human islet amyloid polypeptide (hIAPP) which accumulated in the pancreatic islets of diabetic patients. In another study, insulin was loaded onto GO or GO decorated with 2-nitrodopamine-coated magnetic particle matrices (GO–MPdop), which showed high loading capacity of 100 ± 3% and 88 ± 3% in pH < 5.4, respectively162. The insulin loaded on GO matrices was proved to be stable under the acidic condition of pH 2, but insulin was released upon exposed to the media with higher pH values. At pH 7, more than 20% of insulin was released in 90 min. This study demonstrated the potential of applying GO for insulin delivery via oral route in diabetic treatment. Some other studies have also demonstrated that the photothermal conversion property of GBNs could be utilized in formulating controlled drug release systems167, 168, 169, 170. Therefore, GBNs-insulin interaction provides a potential approach for the controlled insulin delivery triggered by the changes of temperature or pH.
4.3.2. Formation of protein corona on insulin-loaded NPs
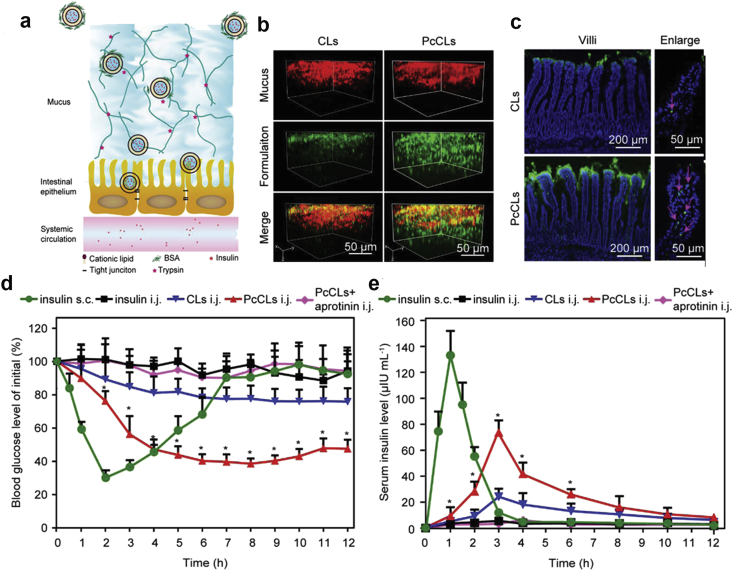
The formation of protein corona may also be used for developing novel oral insulin formulations. In an interesting work, Wand et al.171 developed the insulin-loaded cationic liposomes (CLs) which were coated with BSA corona (PcCLs, Fig. 9a). The PcCLs had a neutral charge and a hydrophilic surface and thus showed the strong penetration into the mucus layer (Fig. 9b). Moreover, the positively charged CLs could be exposed and directly interacted with the epithelium since the BSA corona would be hydrolyzed by enzymes within mucus layer (Fig. 9c). The in vivo data showed that the hypoglycemic effect of PcCLs was efficient and could last for 12 h after intrajejunal (i.j.) administration (Fig. 9d). Accordingly, the insulin bioavailability of PcCLs was up to 11.9% (Fig. 9e). This work provides an interesting idea of how to use protein corona for enhancing the oral absorption of insulin, although some key issues still need to be addressed, such as the stability of protein corona against various digestive enzymes in the upper area of small intestine. In our previous work, we showed that the digestive enzyme corona could rapidly form around the insulin-loaded cationic NPs (CNPs) in vitro135. More importantly, the CNPs could protect the loaded insulin from enzymatic degradation and the epithelial cell uptake of CNPs was significantly inhibited in the presence of the digestive enzyme corona. This finding implies that digestive enzyme corona can reduce the degradation and absorption of CNPs in the upper GI tract, leading to a large number of CNPs delivered to the lower area of GI tract where the CNPs have a great chance to be absorbed due to the presence of M-cells and the released insulin can also be absorbed in the intact form due to the extremely low enzyme activity135,172. This is another potential approach of using protein corona for oral delivery of insulin-loaded NPs. The discussions above indicate that protein corona has great potentials in enhancing insulin oral delivery, which may be the new direction of oral insulin research and development in the future.
Figure 9.
(a) Schematics of the process of PcCLs transport through the mucus layer and epithelium. (b) 3D images of CLs and PcCLs penetration into mucus (green: formulations; red: mucus). (c) Distribution of CLs and PcCLs in small intestinal villi (green: formulations; blue: intestinal villi nuclei). Red arrows indicate the absorption points. (d) Blood glucose level-time curves of diabetic rats after administration of insulin solution (s.c. 5 IU/kg and i.j. 75 IU/kg), CLs, PcCLs and PcCLs mixed with aprotinin (i.j. 75 IU/kg; s.c.: subcutaneous; i.j.: intrajejunal). (e) Corresponding serum insulin level-time curves. Data are mean ± SD, n = 6, ∗P < 0.05 vs. CLs group. Reprinted with the permission from Ref. 171 Copyright © 2019 John Wiley and Sons Ltd.
5. Problems and possible solutions
Despite the great advances of NPs-based insulin delivery systems over the traditional ones, no nano-formulations have been approved so far since many problems remain with regard to NPs. First, the potential systemic toxicity of NPs, especially the synthetic ones, is still unclear. Second, the oral bioavailability of insulin-loaded NPs is still far from enough although it has been significantly enhanced compared to insulin solution. Third, the complexity and the delayed response to the change of blood glucose level make the glucose-responsive NPs hard for commercialization. In addition, the nano‒protein interaction and corona formation after NPs exposure to biological fluids is an uncertain and complicated factor influencing the in vivo fate of NPs.
As the other side of a coin, nano‒protein interactions may provide potential approaches for solving these problems. The insulin-loaded GBNs have shown the potentials to provide both the basic insulin release and the desired burst release triggered by the photothermal effect. But the use of GBNs brings new concerns on their toxicity and degradation. Another nano‒protein interactions-based possible approach is the formation of protein corona surrounding the insulin-loaded NPs to enhance their oral absorption. The specific protein corona, if stable in the GI tract, can improve the mucus penetration and epithelium adhesion of NPs and ultimately enhance the absorption of NPs in the small intestine. Alternatively, the non-specific digestive enzyme corona is not beneficial for the absorption of NPs in the upper GI tract but may enhance the absorption of the intact NPs or the released insulin in the lower area of the GI tract. Utilization of nano‒protein interactions may be the new direction of oral insulin research and development in the future.
6. Conclusions and perspectives
Insulin therapy is of great importance for diabetic patients to control their blood glucose level. However, the frequent injections bring not only inconvenience but also pains. For many years, great efforts have been made to improve the insulin delivery. Although certain improvements are achieved, the current therapy is still unsatisfying enough. In this regard, various functional NPs loaded with insulin are developed and have shown significant advance over the traditional formulations. Unfortunately, none of them can be approved for clinical use due to the substantial concerns, such as the unclear toxicity, the still low oral bioavailability and the interference from the nano‒protein interactions. Recently, scientists have started to consider how to use the nano‒protein interactions for developing advanced insulin delivery systems. The insulin-loaded GBNs for photothermally triggered on-demand insulin release and the protein corona formation surrounding the insulin-loaded NPs for enhanced oral bioavailability have shown the potentials to improve the insulin delivery. It is undoubted that there is still a long way to go to achieve the goal of biomimic and/or oral insulin delivery. On the other hand, the efforts to achieve the goal will not be stopped despite of the great challenges. Utilizing the nano‒protein interactions may be a potential approach for designing novel insulin delivery systems in the future.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81973261 and 81700538), the Foundation of West China Hospital of Stomatology (No. RD-02-201903, China) and the Research Funding for Talents Developing, West China Hospital of Stomatology, Sichuan University (No. RCDWJS2020-7, China).
Qiang Peng and Ting Zhang designed the research and obtained funding. James Zhenggui Tang, Xiaofan Fei, Yanping Li, Yi Song, and Zhiyong Qian contributed to the discussion and review. Ting Zhang and Qiang Peng wrote the manuscript. Qiang Peng and James Zhenggui Tang revised the manuscript. All of the authors have read and approved the final manuscript.
The authors have no conflicts of interest to declare.
References
- 1.Sharma G., Sharma A.R., Nam J.S., Doss G.P., Lee S.S., Chakraborty C. Nanoparticle based insulin delivery system: the next generation efficient therapy for type 1 diabetes. J Nanobiotechnol. 2015;13:74. doi: 10.1186/s12951-015-0136-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Veiseh O., Tang B.C., Whitehead K.A., Anderson D.G., Langer R. Managing diabetes with nanomedicine: challenges and opportunities. Nat Rev Drug Discov. 2015;14:45–57. doi: 10.1038/nrd4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petersmann A., Nauck M., Muller-Wieland D., Kerner W., Muller U.A., Landgraf R. Definition, classification and diagnosis of diabetes mellitus. Exp Clin Endocrinol Diabetes. 2018;126:406–410. doi: 10.1055/a-0584-6223. [DOI] [PubMed] [Google Scholar]
- 4.Shaw J.E., Sicree R.A., Zimmet P.Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Zhang P., Zhang X.Z., Brown J., Vistisen D., Sicree R., Shaw J. Global healthcare expenditure on diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:293–301. doi: 10.1016/j.diabres.2010.01.026. [DOI] [PubMed] [Google Scholar]
- 6.Juntti-Berggren L., Refai E., Appelskog I., Andersson M., Imreh G., Dekki N. Apolipoprotein CIII promotes Ca2+-dependent beta cell death in type 1 diabetes. Proc Natl Acad Sci U S A. 2004;101:10090–10094. doi: 10.1073/pnas.0403551101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Regnell S.E., Lernmark A. Early prediction of autoimmune (type 1) diabetes. Diabetologia. 2017;60:1370–1381. doi: 10.1007/s00125-017-4308-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Devarshi P.P., McNabney S.M., Henagan T.M. Skeletal muscle nucleo-mitochondrial crosstalk in obesity and type 2 diabetes. Int J Mol Sci. 2017;18:831. doi: 10.3390/ijms18040831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tuomilehto J., Lindstrom J., Eriksson J.G., Valle T.T., Hamalainen H., Ilanne-Parikka P. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 10.Wadden T.A., Webb V.L., Moran C.H., Bailer B.A. Lifestyle modification for obesity: new developments in diet, physical activity, and behavior therapy. Circulation. 2012;125:1157–1170. doi: 10.1161/CIRCULATIONAHA.111.039453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American Diabetes A Standards of medical care in diabetes—2014. Diabetes Care. 2014;37(Suppl 1):S14–S80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 12.Umpierrez G.E., Gonzalez A., Umpierrez D., Pimentel D. Diabetes mellitus in the Hispanic/Latino population: an increasing health care challenge in the United States. Am J Med Sci. 2007;334:274–282. doi: 10.1097/MAJ.0b013e3180a6efe3. [DOI] [PubMed] [Google Scholar]
- 13.Ismail-Beigi F. Clinical practice. Glycemic management of type 2 diabetes mellitus. N Engl J Med. 2012;366:1319–1327. doi: 10.1056/NEJMcp1013127. [DOI] [PubMed] [Google Scholar]
- 14.Haas L.B. Optimizing insulin use in type 2 diabetes: role of basal and prandial insulin in long-term care facilities. J Am Med Dir Assoc. 2007;8:502–510. doi: 10.1016/j.jamda.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Bray G.A. Potential health risks from beverages containing fructose found in sugar or high-fructose corn syrup. Diabetes Care. 2013;36:11–12. doi: 10.2337/dc12-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moroz E., Matoori S., Leroux J.C. Oral delivery of macromolecular drugs: where we are after almost 100 years of attempts. Adv Drug Deliv Rev. 2016;101:108–121. doi: 10.1016/j.addr.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 17.Wong C.Y., Al-Salami H., Dass C.R. Potential of insulin nanoparticle formulations for oral delivery and diabetes treatment. J Control Release. 2017;264:247–275. doi: 10.1016/j.jconrel.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Wong C.Y., Al-Salami H., Dass C.R. Microparticles, microcapsules and microspheres: a review of recent developments and prospects for oral delivery of insulin. Int J Pharm. 2018;537:223–244. doi: 10.1016/j.ijpharm.2017.12.036. [DOI] [PubMed] [Google Scholar]
- 19.Picone P., Sabatino M.A., Ditta L.A., Amato A., San Biagio P.L., Mule F. Nose-to-brain delivery of insulin enhanced by a nanogel carrier. J Control Release. 2018;270:23–36. doi: 10.1016/j.jconrel.2017.11.040. [DOI] [PubMed] [Google Scholar]
- 20.Kim N.A., Thapa R., Jeong S.H., Bae H.D., Maeng J., Lee K. Enhanced intranasal insulin delivery by formulations and tumor protein-derived protein transduction domain as an absorption enhancer. J Control Release. 2019;294:226–236. doi: 10.1016/j.jconrel.2018.12.023. [DOI] [PubMed] [Google Scholar]
- 21.Chen X., Wang L., Yu H.J., Li C.J., Feng J.Y., Haq F. Preparation, properties and challenges of the microneedles-based insulin delivery system. J Control Release. 2018;288:173–188. doi: 10.1016/j.jconrel.2018.08.042. [DOI] [PubMed] [Google Scholar]
- 22.Peng Q., Sun X., Gong T., Wu C.Y., Zhang T., Tan J. Injectable and biodegradable thermosensitive hydrogels loaded with PHBHHx nanoparticles for the sustained and controlled release of insulin. Acta Biomater. 2013;9:5063–5069. doi: 10.1016/j.actbio.2012.09.034. [DOI] [PubMed] [Google Scholar]
- 23.Sheng J.Y., He H.N., Han L.M., Qin J., Chen S.H., Ru G. Enhancing insulin oral absorption by using mucoadhesive nanoparticles loaded with LMWP-linked insulin conjugates. J Control Release. 2016;233:181–190. doi: 10.1016/j.jconrel.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 24.Lee K.C., Chen W.J., Chen Y.C. Using dextran-encapsulated gold nanoparticles as insulin carriers to prolong insulin activity. Nanomedicine. 2017;12:1823–1834. doi: 10.2217/nnm-2017-0019. [DOI] [PubMed] [Google Scholar]
- 25.Sun L., Liu Z., Tian H., Le Z., Liu L., Leong K.W. Scalable manufacturing of enteric encapsulation systems for site-Specific oral insulin delivery. Biomacromolecules. 2018;20:528–538. doi: 10.1021/acs.biomac.8b01530. [DOI] [PubMed] [Google Scholar]
- 26.Liu J., Peng Q. Protein-gold nanoparticle interactions and their possible impact on biomedical applications. Acta Biomater. 2017;55:13–27. doi: 10.1016/j.actbio.2017.03.055. [DOI] [PubMed] [Google Scholar]
- 27.Charbgoo F., Nejabat M., Abnous K., Soltani F., Taghdisi S.M., Alibolandi M. Gold nanoparticle should understand protein corona for being a clinical nanomaterial. J Control Release. 2018;272:39–53. doi: 10.1016/j.jconrel.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Lundqvist M., Stigler J., Elia G., Lynch I., Cedervall T., Dawson K.A. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc Natl Acad Sci U S A. 2008;105:14265–14270. doi: 10.1073/pnas.0805135105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mahmoudi M., Lynch I., Ejtehadi M.R., Monopoli M.P., Bombelli F.B., Laurent S. Protein-nanoparticle interactions: opportunities and challenges. Chem Rev. 2011;111:5610–5637. doi: 10.1021/cr100440g. [DOI] [PubMed] [Google Scholar]
- 30.Salvati A., Pitek A.S., Monopoli M.P., Prapainop K., Bombelli F.B., Hristov D.R. Transferrin-functionalized nanoparticles lose their targeting capabilities when a biomolecule corona adsorbs on the surface. Nat Nanotechnol. 2013;8:137–143. doi: 10.1038/nnano.2012.237. [DOI] [PubMed] [Google Scholar]
- 31.Peng Q., Mu H.L. The potential of protein-nanomaterial interaction for advanced drug delivery. J Control Release. 2016;225:121–132. doi: 10.1016/j.jconrel.2016.01.041. [DOI] [PubMed] [Google Scholar]
- 32.Peng Q., Zhang S., Yang Q., Zhang T., Wei X.Q., Jiang L. Preformed albumin corona, a protective coating for nanoparticles based drug delivery system. Biomaterials. 2013;34:8521–8530. doi: 10.1016/j.biomaterials.2013.07.102. [DOI] [PubMed] [Google Scholar]
- 33.Peng Q., Wei X.Q., Yang Q., Zhang S., Zhang T., Shao X.R. Enhanced biostability of nanoparticle-based drug delivery systems by albumin corona. Nanomedicine. 2015;10:205–214. doi: 10.2217/nnm.14.86. [DOI] [PubMed] [Google Scholar]
- 34.Chen M.C., Sonaje K., Chen K.J., Sung H.W. A review of the prospects for polymeric nanoparticle platforms in oral insulin delivery. Biomaterials. 2011;32:9826–9838. doi: 10.1016/j.biomaterials.2011.08.087. [DOI] [PubMed] [Google Scholar]
- 35.Kaklotar D., Agrawal P., Abdulla A., Singh R.P., Mehata A.K., Singh S. Transition from passive to active targeting of oral insulin nanomedicines: enhancement in bioavailability and glycemic control in diabetes. Nanomedicine. 2016;11:1465–1486. doi: 10.2217/nnm.16.43. (London) [DOI] [PubMed] [Google Scholar]
- 36.Naidu P.S.R., Norret M., Dunlop S.A., Fitzgerald M., Clemons T.D., Iyer K.S. Novel hydrophilic copolymer-based nanoparticle enhances the therapeutic efficiency of doxorubicin in cultured MCF-7 cells. ACS Omega. 2019;4:17083–17089. doi: 10.1021/acsomega.8b02894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Daneman D. Type 1 diabetes. Lancet. 2006;367:847–858. doi: 10.1016/S0140-6736(06)68341-4. [DOI] [PubMed] [Google Scholar]
- 38.Shilo M., Berenstein P., Dreifuss T., Nash Y., Goldsmith G., Kazimirsky G. Insulin-coated gold nanoparticles as a new concept for personalized and adjustable glucose regulation. Nanoscale. 2015;7:20489–20496. doi: 10.1039/c5nr04881h. [DOI] [PubMed] [Google Scholar]
- 39.Qi W., Yan X., Duan L., Cui Y., Yang Y., Li J. Glucose-sensitive microcapsules from glutaraldehyde cross-linked hemoglobin and glucose oxidase. Biomacromolecules. 2009;10:1212–1216. doi: 10.1021/bm801502r. [DOI] [PubMed] [Google Scholar]
- 40.Tan Y.F., Lao L.L., Xiong G.M., Venkatraman S. Controlled-release nanotherapeutics: state of translation. J Control Release. 2018;284:39–48. doi: 10.1016/j.jconrel.2018.06.014. [DOI] [PubMed] [Google Scholar]
- 41.Finotelli P.V., Da Silva D., Sola-Penna M., Rossi A.M., Farina M., Andrade L.R. Microcapsules of alginate/chitosan containing magnetic nanoparticles for controlled release of insulin. Colloids Surf B Biointerfaces. 2010;81:206–211. doi: 10.1016/j.colsurfb.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 42.Saravanan S., Malathi S., Sesh P.S.L., Selvasubramanianc S., Balasubramanian S., Pandiyan V. Hydrophilic poly (ethylene glycol) capped poly (lactic-co-glycolic) acid nanoparticles for subcutaneous delivery of insulin in diabetic rats. Int J Biol Macromol. 2017;95:1190–1198. doi: 10.1016/j.ijbiomac.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 43.Tomar L., Tyagi C., Kumar M., Kumar P., Singh H., Choonara Y.E. In vivo evaluation of a conjugated poly(lactide-ethylene glycol) nanoparticle depot formulation for prolonged insulin delivery in the diabetic rabbit model. Int J Nanomed. 2013;8:505–520. doi: 10.2147/IJN.S38011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peng Q., Zhang Z.R., Gong T., Chen G.Q., Sun X. A rapid-acting, long-acting insulin formulation based on a phospholipid complex loaded PHBHHx nanoparticles. Biomaterials. 2012;33:1583–1588. doi: 10.1016/j.biomaterials.2011.10.072. [DOI] [PubMed] [Google Scholar]
- 45.Muggeo M., Zoppini G., Bonora E., Brun E., Bonadonna R.C., Moghetti P. Fasting plasma glucose variability predicts 10-year survival of type 2 diabetic patients: the verona diabetes study. Diabetes Care. 2000;23:45–50. doi: 10.2337/diacare.23.1.45. [DOI] [PubMed] [Google Scholar]
- 46.McCoy R.G., Van Houten H.K., Ziegenfuss J.Y., Shah N.D., Wermers R.A., Smith S.A. Increased mortality of patients with diabetes reporting severe hypoglycemia. Diabetes Care. 2012;35:1897–1901. doi: 10.2337/dc11-2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bratlie K.M., York R.L., Invernale M.A., Langer R., Anderson D.G. Materials for diabetes therapeutics. Adv Healthc Mater. 2012;1:267–284. doi: 10.1002/adhm.201200037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.VandenBerg M.A., Webber M.J. Biologically inspired and chemically derived methods for glucose-responsive insulin therapy. Adv Healthc Mater. 2019;8:1801466. doi: 10.1002/adhm.201801466. [DOI] [PubMed] [Google Scholar]
- 49.Kazuhiko Ishihara M.K., Ishimaru Naoshi, Shinohara Isao. Glucose induced permeation control of insulin through a complex membrane consisting of immobilized glucose oxidase and a poly(amine) Polym J. 1984;16:625–631. [Google Scholar]
- 50.Bankar S.B., Bule M.V., Singhal R.S., Ananthanarayan L. Glucose oxidase—an overview. Biotechnol Adv. 2009;27:489–501. doi: 10.1016/j.biotechadv.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 51.Farahani B.V., Ghasemzaheh H., Afraz S. Intelligent semi-IPN chitosan-PEG-PAAm hydrogel for closed-loop insulin delivery and kinetic modeling. RSC Adv. 2016;6:26590–26598. [Google Scholar]
- 52.Gu Z., Aimetti A., Wang Q., Dang T.T., Zhang Y., Veiseh O. Injectable nano-network for glucose-mediated insulin delivery. ACS Nano. 2013;7:4194–4201. doi: 10.1021/nn400630x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Siegel R.A., Gu Y., Lei M., Baldi A., Nuxoll E.E., Ziaie B. Hard and soft micro- and nanofabrication: an integrated approach to hydrogel-based biosensing and drug delivery. J Control Release. 2010;141:303–313. doi: 10.1016/j.jconrel.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yoshida K., Hasebe Y., Takahashi S., Sato K., Anzai J. Layer-by-layer deposited nano- and micro-assemblies for insulin delivery: a review. Mater Sci Eng C Mater Biol Appl. 2014;34:384–392. doi: 10.1016/j.msec.2013.09.045. [DOI] [PubMed] [Google Scholar]
- 55.Tong Z.Z., Zhou J.Y., Zhong J.X., Tang Q.J., Lei Z.T., Luo H.P. Glucose- and H2O2-responsive polymeric vesicles integrated with microneedle patches for glucose-sensitive transcutaneous delivery of insulin in diabetic rats. ACS Appl Mater Interfaces. 2018;10:20014–20024. doi: 10.1021/acsami.8b04484. [DOI] [PubMed] [Google Scholar]
- 56.Duan Y., Ye F.G., Huang Y.L., Qin Y.M., He C.M., Zhao S.L. One-pot synthesis of a metal-organic framework-based drug carrier for intelligent glucose-responsive insulin delivery. Chem Commun. 2018;54:5377–5380. doi: 10.1039/c8cc02708k. [DOI] [PubMed] [Google Scholar]
- 57.Jamwal S., Ram B., Ranote S., Dharela R., Chauhan G.S. New glucose oxidase-immobilized stimuli-responsive dextran nanoparticles for insulin delivery. Int J Biol Macromol. 2018;123:968–978. doi: 10.1016/j.ijbiomac.2018.11.147. [DOI] [PubMed] [Google Scholar]
- 58.Aznar E., Villalonga R., Gimenez C., Sancenon F., Marcos M.D., Martinez-Manez R. Glucose-triggered release using enzyme-gated mesoporous silica nanoparticles. Chem Commun. 2013;49:6391–6393. doi: 10.1039/c3cc42210k. [DOI] [PubMed] [Google Scholar]
- 59.Chen M.J., Huang C.S., He C.S., Zhu W.P., Xu Y.F., Lu Y.F. A glucose-responsive controlled release system using glucose oxidase-gated mesoporous silica nanocontainers. Chem Commun. 2012;48:9522–9524. doi: 10.1039/c2cc34290a. [DOI] [PubMed] [Google Scholar]
- 60.Diez P., Sanchez A., Gamella M., Martinez-Ruiz P., Aznar E., de la Torre C. Toward the design of smart delivery systems controlled by integrated enzyme-based biocomputing ensembles. J Am Chem Soc. 2014;136:9116–9123. doi: 10.1021/ja503578b. [DOI] [PubMed] [Google Scholar]
- 61.Ravaine V., Ancla C., Catargi B. Chemically controlled closed-loop insulin delivery. J Control Release. 2008;132:2–11. doi: 10.1016/j.jconrel.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 62.Gu Z., Dang T.T., Ma M., Tang B.C., Cheng H., Jiang S. Glucose-responsive microgels integrated with enzyme nanocapsules for closed-loop insulin delivery. ACS Nano. 2013;7:6758–6766. doi: 10.1021/nn401617u. [DOI] [PubMed] [Google Scholar]
- 63.Tai W., Mo R., Di J., Subramanian V., Gu X., Buse J.B. Bio-inspired synthetic nanovesicles for glucose-responsive release of insulin. Biomacromolecules. 2014;15:3495–3502. doi: 10.1021/bm500364a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yu J.C., Zhang Y.Q., Ye Y.Q., DiSanto R., Sun W.J., Ranson D. Microneedle-array patches loaded with hypoxia-sensitive vesicles provide fast glucose-responsive insulin delivery. Proc Natl Acad Sci U S A. 2015;112:8260–8265. doi: 10.1073/pnas.1505405112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sharon N., Lis H. Lectins: cell-agglutinating and sugar-specific proteins. Science. 1972;177:949–959. doi: 10.1126/science.177.4053.949. [DOI] [PubMed] [Google Scholar]
- 66.Brownlee M., Cerami A. A glucose-controlled insulin-delivery system: semisynthetic insulin bound to lectin. Science. 1979;206:1190–1191. doi: 10.1126/science.505005. [DOI] [PubMed] [Google Scholar]
- 67.Kim J.J., Park K. Glucose-binding property of pegylated concanavalin A. Pharm Res. 2001;18:794–799. doi: 10.1023/a:1011084312134. [DOI] [PubMed] [Google Scholar]
- 68.Sung Wan K., Chaul Min P., Kimiko M., Seminoff L.A., Holmberg D.L., Gleeson J.M. Self-regulated glycosylated insulin delivery. J Control Release. 1990;11:193–201. [Google Scholar]
- 69.Makino K., Mack E.J., Okano T., Sung Wan K. A microcapsule self-regulating delivery system for insulin. J Control Release. 1990;12:235–239. [Google Scholar]
- 70.Liu F., Song S.C., Mix D., Baudys M., Kim S.W. Glucose-induced release of glycosylpoly(ethylene glycol) insulin bound to a soluble conjugate of concanavalin A. Bioconjugate Chem. 1997;8:664–672. doi: 10.1021/bc970128e. [DOI] [PubMed] [Google Scholar]
- 71.Taylor M.J., Tanna S., Sahota T. In vivo study of a polymeric glucose-sensitive insulin delivery system using a rat model. J Pharmaceut Sci. 2010;99:4215–4227. doi: 10.1002/jps.22138. [DOI] [PubMed] [Google Scholar]
- 72.Kim J.J., Park K. Modulated insulin delivery from glucose-sensitive hydrogel dosage forms. J Control Release. 2001;77:39–47. doi: 10.1016/s0168-3659(01)00447-3. [DOI] [PubMed] [Google Scholar]
- 73.Yin R.X., Bai M.R., He J., Nie J., Zhang W.J. Concanavalin A-sugar affinity based system: binding interactions, principle of glucose-responsiveness, and modulated insulin release for diabetes care. Int J Biol Macromol. 2019;124:724–732. doi: 10.1016/j.ijbiomac.2018.11.261. [DOI] [PubMed] [Google Scholar]
- 74.Yin R.X., Han J., Zhang J.F., Nie J. Glucose-responsive composite microparticles based on chitosan, concanavalin A and dextran for insulin delivery. Colloids Surf B Biointerfaces. 2010;76:483–488. doi: 10.1016/j.colsurfb.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 75.You L.C., Lu F.Z., Li Z.C., Zhang W., Li F.M. Glucose-sensitive aggregates formed by poly(ethylene oxide) block poly(2-glucosyl-oxyethyl acrylate) with concanavalin A in dilute aqueous medium. Macromolecules. 2003;36:1–4. [Google Scholar]
- 76.Wu S.S., Huang X., Du X.Z. Glucose- and pH-responsive controlled release of cargo from protein-gated carbohydrate-functionalized mesoporous silica nanocontainers. Angew Chem Int Ed Engl. 2013;52:5580–5584. doi: 10.1002/anie.201300958. [DOI] [PubMed] [Google Scholar]
- 77.Kataoka K., Miyazaki H., Okano T., Sakurai Y. Sensitive glucose-induced change of the lower critical solution temperature of poly[N,N-(dimethylacrylamide)-co-3-(acrylamido)-phenylboronic acid] in physiological saline. Macromolecules. 1994;27:1061–1062. [Google Scholar]
- 78.Huang Q., Wang L., Yu H.J., Ur-Rahman K. Advances in phenylboronic acid-based closed-loop smart drug delivery system for diabetic therapy. J Control Release. 2019;305:50–64. doi: 10.1016/j.jconrel.2019.05.029. [DOI] [PubMed] [Google Scholar]
- 79.Wu W.T., Mitra N., Yan E.C., Zhou S.Q. Multifunctional hybrid nanogel for integration of optical glucose sensing and self-regulated insulin release at physiological pH. ACS Nano. 2010;4:4831–4839. doi: 10.1021/nn1008319. [DOI] [PubMed] [Google Scholar]
- 80.Matsumoto A., Yamamoto K., Yoshida R., Kataoka K., Aoyagi T., Miyahara Y. A totally synthetic glucose-responsive gel operating in physiological aqueous conditions. Chem Commun. 2010;46:2203–2205. doi: 10.1039/b920319b. [DOI] [PubMed] [Google Scholar]
- 81.Gaballa H., Theato P. Glucose-responsive polymeric micelles via boronic acid-diol complexation for insulin delivery at neutral pH. Biomacromolecules. 2019;20:871–881. doi: 10.1021/acs.biomac.8b01508. [DOI] [PubMed] [Google Scholar]
- 82.Wu J.Z., Williams G.R., Li H.Y., Wang D.X., Li S.D., Zhu L.M. Insulin-loaded PLGA microspheres for glucose-responsive release. Drug Deliv. 2017;24:1513–1525. doi: 10.1080/10717544.2017.1381200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shi D.J., Ran M.S., Zhang L., Huang H., Li X.J., Chen M.Q. Fabrication of biobased polyelectrolyte capsules and their application for glucose-triggered insulin delivery. ACS Appl Mater Interfaces. 2016;8:13688–13697. doi: 10.1021/acsami.6b02121. [DOI] [PubMed] [Google Scholar]
- 84.Yao Y., Zhao L.Y., Yang J.J., Yang J. Glucose-responsive vehicles containing phenylborate ester for controlled insulin release at neutral pH. Biomacromolecules. 2012;13:1837–1844. doi: 10.1021/bm3003286. [DOI] [PubMed] [Google Scholar]
- 85.Wang B.L., Ma R.J., Liu G., Li Y., Liu X.J., An Y.L. Glucose-responsive micelles from self-assembly of poly(ethylene glycol)-b-poly(acrylic acid-co-acrylamidophenylboronic acid) and the controlled release of insulin. Langmuir. 2009;25:12522–12528. doi: 10.1021/la901776a. [DOI] [PubMed] [Google Scholar]
- 86.Wu J.Z., Williams G.R., Li H.Y., Wang D., Wu H., Li S.D. Glucose- and temperature-sensitive nanoparticles for insulin delivery. Int J Nanomed. 2017;12:4037–4057. doi: 10.2147/IJN.S132984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gao L., Wang T.T., Jia K.K., Wu X., Yao C.H., Shao W. Glucose-responsive supramolecular vesicles based on water-soluble pillar[5]arene and pyridylboronic acid derivatives for controlled insulin delivery. Chemistry. 2017;23:6605–6614. doi: 10.1002/chem.201700345. [DOI] [PubMed] [Google Scholar]
- 88.Zeng Z.Y., Qi D.M., Yang L., Liu J., Tang Y.Q., Chen H. Stimuli-responsive self-assembled dendrimers for oral protein delivery. J Control Release. 2019;315:206–213. doi: 10.1016/j.jconrel.2019.10.049. [DOI] [PubMed] [Google Scholar]
- 89.Zhao Y., Trewyn B.G., Slowing I.I., Lin V.S. Mesoporous silica nanoparticle-based double drug delivery system for glucose-responsive controlled release of insulin and cyclic AMP. J Am Chem Soc. 2009;131:8398–8400. doi: 10.1021/ja901831u. [DOI] [PubMed] [Google Scholar]
- 90.He H.S., Lu Y., Qi J.P., Zhao W.L., Dong X.C., Wu W. Biomimetic thiamine- and niacin-decorated liposomes for enhanced oral delivery of insulin. Acta Pharm Sin B. 2018;8:97–105. doi: 10.1016/j.apsb.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kanzarkar M., Pathak P.P., Vaidya M., Brumlik C., Choudhury A. Oral insulin-delivery system for diabetes mellitus. Pharm Pat Anal. 2015;4:29–36. doi: 10.4155/ppa.14.53. [DOI] [PubMed] [Google Scholar]
- 92.Evans D.F., Pye G., Bramley R., Clark A.G., Dyson T.J., Hardcastle J.D. Measurement of gastrointestinal pH profiles in normal ambulant human subjects. Gut. 1988;29:1035–1041. doi: 10.1136/gut.29.8.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Alai M.S., Lin W.J., Pingale S.S. Application of polymeric nanoparticles and micelles in insulin oral delivery. J Food Drug Anal. 2015;23:351–358. doi: 10.1016/j.jfda.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Langguth P., Bohner V., Heizmann J., Merkle H.P., Wolffram S., Amidon G.L. The challenge of proteolytic enzymes in intestinal peptide delivery. J Control Release. 1997;46:39–57. [Google Scholar]
- 95.Bernkop-Schnurch A. The use of inhibitory agents to overcome the enzymatic barrier to perorally administered therapeutic peptides and proteins. J Control Release. 1998;52:1–16. doi: 10.1016/s0168-3659(97)00204-6. [DOI] [PubMed] [Google Scholar]
- 96.Andrews C.W., Bennett L., Yu L.X. Predicting human oral bioavailability of a compound: development of a novel quantitative structure-bioavailability relationship. Pharm Res. 2000;17:639–644. doi: 10.1023/a:1007556711109. [DOI] [PubMed] [Google Scholar]
- 97.Damgé C., Michel C., Aprahamian M., Couvreur P. New approach for oral administration of insulin with polyalkylcyanoacrylate nanocapsules as drug carrier. Diabetes. 1988;37:246–251. doi: 10.2337/diab.37.2.246. [DOI] [PubMed] [Google Scholar]
- 98.Mesiha M.S., Sidhom M.B., Fasipe B. Oral and subcutaneous absorption of insulin poly(isobutylcyanoacrylate) nanoparticles. Int J Pharm. 2005;288:289–293. doi: 10.1016/j.ijpharm.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 99.Sarmento B., Martins S., Ferreira D., Souto E.B. Oral insulin delivery by means of solid lipid nanoparticles. Int J Nanomed. 2007;2:743–749. [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang Y., Zhang L., Ban Q., Li J., Li C.H., Guan Y.Q. Preparation and characterization of hydroxyapatite nanoparticles carrying insulin and gallic acid for insulin oral delivery. Nanomedicine. 2018;14:353–364. doi: 10.1016/j.nano.2017.11.012. [DOI] [PubMed] [Google Scholar]
- 101.Presas E., McCartney F., Sultan E., Hunger C., Nellen S., Alvarez C.V. Physicochemical, pharmacokinetic and pharmacodynamic analyses of amphiphilic cyclodextrin-based nanoparticles designed to enhance intestinal delivery of insulin. J Control Release. 2018;286:402–414. doi: 10.1016/j.jconrel.2018.07.045. [DOI] [PubMed] [Google Scholar]
- 102.Lopes M.A., Abrahim B.A., Cabral L.M., Rodrigues C.R., Seica R.M., de Baptista Veiga F.J. Intestinal absorption of insulin nanoparticles: contribution of M cells. Nanomedicine. 2014;10:1139–1151. doi: 10.1016/j.nano.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 103.Ramachandran R., Paul W., Sharma C.P. Synthesis and characterization of PEGylated calcium phosphate nanoparticles for oral insulin delivery. J Biomed Mater Res, Part B. 2009;88:41–48. doi: 10.1002/jbm.b.31241. [DOI] [PubMed] [Google Scholar]
- 104.Yu F., Li Y., Liu C.S., Chen Q., Wang G.H., Guo W. Enteric-coated capsules filled with mono-disperse micro-particles containing PLGA-lipid-PEG nanoparticles for oral delivery of insulin. Int J Pharm. 2015;484:181–191. doi: 10.1016/j.ijpharm.2015.02.055. [DOI] [PubMed] [Google Scholar]
- 105.Karnoosh-Yamchi J., Mobasseri M., Akbarzadeh A., Davaran S., Ostad-Rahimi A.R., Hamishehkar H. Preparation of pH sensitive insulin-loaded nano hydrogels and evaluation of insulin releasing in different pH conditions. Mol Biol Rep. 2014;41:6705–6712. doi: 10.1007/s11033-014-3553-3. [DOI] [PubMed] [Google Scholar]
- 106.He Z.Y., Liu Z.J., Tian H.K., Hu Y.Z., Liu L.X., Leong K.W. Scalable production of core-shell nanoparticles by flash nanocomplexation to enhance mucosal transport for oral delivery of insulin. Nanoscale. 2018;10:3307–3319. doi: 10.1039/c7nr08047f. [DOI] [PubMed] [Google Scholar]
- 107.Su F.Y., Lin K.J., Sonaje K., Wey S.P., Yen T.C., Ho Y.C. Protease inhibition and absorption enhancement by functional nanoparticles for effective oral insulin delivery. Biomaterials. 2012;33:2801–2811. doi: 10.1016/j.biomaterials.2011.12.038. [DOI] [PubMed] [Google Scholar]
- 108.Yamamoto A., Taniguchi T., Rikyuu K., Tsuji T., Fujita T., Murakami M. Effects of various protease inhibitors on the intestinal absorption and degradation of insulin in rats. Pharm Res. 1994;11:1496–1500. doi: 10.1023/a:1018968611962. [DOI] [PubMed] [Google Scholar]
- 109.Damge C., Maincent P., Ubrich N. Oral delivery of insulin associated to polymeric nanoparticles in diabetic rats. J Control Release. 2007;117:163–170. doi: 10.1016/j.jconrel.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 110.He H., Sheng J., David A.E., Kwon Y.M., Zhang J., Huang Y. The use of low molecular weight protamine chemical chimera to enhance monomeric insulin intestinal absorption. Biomaterials. 2013;34:7733–7743. doi: 10.1016/j.biomaterials.2013.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ye J., Shin M.C., Liang Q., He H., Yang V.C. 15 years of ATTEMPTS: a macromolecular drug delivery system based on the CPP-mediated intracellular drug delivery and antibody targeting. J Control Release. 2015;205:58–69. doi: 10.1016/j.jconrel.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 112.Barbari G.R., Dorkoosh F.A., Amini M., Sharifzadeh M., Atyabi F., Balalaie S. A novel nanoemulsion-based method to produce ultrasmall, water-dispersible nanoparticles from chitosan, surface modified with cell-penetrating peptide for oral delivery of proteins and peptides. Int J Nanomed. 2017;12:3471–3483. doi: 10.2147/IJN.S116063. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 113.Park Y.J., Chang L.C., Liang J.F., Moon C., Chung C.P., Yang V.C. Nontoxic membrane translocation peptide from protamine, low molecular weight protamine (LMWP), for enhanced intracellular protein delivery: in vitro and in vivo study. Faseb J. 2005;19:1555–1557. doi: 10.1096/fj.04-2322fje. [DOI] [PubMed] [Google Scholar]
- 114.Liu M., Zhang J., Zhu X., Shan W., Li L., Zhong J.J. Efficient mucus permeation and tight junction opening by dissociable "mucus-inert" agent coated trimethyl chitosan nanoparticles for oral insulin delivery. J Control Release. 2016;222:67–77. doi: 10.1016/j.jconrel.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 115.Barbari G.R., Dorkoosh F., Amini M., Bahari Javan N., Sharifzadeh M., Atyabi F. Synthesis and characterization of a novel peptide-grafted Cs and evaluation of its nanoparticles for the oral delivery of insulin, in vitro, and in vivo study. Int J Nanomed. 2018;13:5127–5138. doi: 10.2147/IJN.S161240. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 116.Bies C., Lehr C.M., Woodley J.F. Lectin-mediated drug targeting: history and applications. Adv Drug Deliv Rev. 2004;56:425–435. doi: 10.1016/j.addr.2003.10.030. [DOI] [PubMed] [Google Scholar]
- 117.Ertl B., Heigl F., Wirth M., Gabor F. Lectin-mediated bioadhesion: preparation, stability and Caco-2 binding of wheat germ agglutinin-functionalized poly(d,l-lactic-co-glycolic acid)-microspheres. J Drug Target. 2000;8:173–184. doi: 10.3109/10611860008996863. [DOI] [PubMed] [Google Scholar]
- 118.Gabor F., Stangl M., Wirth M. Lectin-mediated bioadhesion: binding characteristics of plant lectins on the enterocyte-like cell lines Caco-2, HT-29 and HCT-8. J Control Release. 1998;55:131–142. doi: 10.1016/s0168-3659(98)00043-1. [DOI] [PubMed] [Google Scholar]
- 119.Lehr C.M., Gabor F. Lectins and glycoconjugates in drug delivery and targeting. Adv Drug Deliv Rev. 2004;56:419–420. doi: 10.1016/j.addr.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 120.Gabor F., Bogner E., Weissenboeck A., Wirth M. The lectin-cell interaction and its implications to intestinal lectin-mediated drug delivery. Adv Drug Deliv Rev. 2004;56:459–480. doi: 10.1016/j.addr.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 121.Zhang N., Ping Q.N., Huang G.H., Xu W.F., Cheng Y.N., Han X.Z. Lectin-modified solid lipid nanoparticles as carriers for oral administration of insulin. Int J Pharm. 2006;327:153–159. doi: 10.1016/j.ijpharm.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 122.Zhang N., Ping Q.N., Huang G.H., Xu W.F. Investigation of lectin-modified insulin liposomes as carriers for oral administration. Int J Pharm. 2005;294:247–259. doi: 10.1016/j.ijpharm.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 123.Chalasani K.B., Russell-Jones G.J., Yandrapu S.K., Diwan P.V., Jain S.K. A novel vitamin B12-nanosphere conjugate carrier system for peroral delivery of insulin. J Control Release. 2007;117:421–429. doi: 10.1016/j.jconrel.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 124.Chalasani K.B., Russell-Jones G.J., Jain A.K., Diwan P.V., Jain S.K. Effective oral delivery of insulin in animal models using vitamin B12-coated dextran nanoparticles. J Control Release. 2007;122:141–150. doi: 10.1016/j.jconrel.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 125.Sharma R., Gupta U., Garg N.K., Tyagi R.K., Jain N.K. Surface engineered and ligand anchored nanobioconjugate: an effective therapeutic approach for oral insulin delivery in experimental diabetic rats. Colloids Surf B Biointerfaces. 2015;127:172–181. doi: 10.1016/j.colsurfb.2015.01.035. [DOI] [PubMed] [Google Scholar]
- 126.Sanyog J., Rathi V.V., Jain A.K., Das M., Godugu C. Folate-decorated PLGA nanoparticles as a rationally designed vehicle for the oral delivery of insulin. Nanomedicine. 2012;7:1311–1337. doi: 10.2217/nnm.12.31. [DOI] [PubMed] [Google Scholar]
- 127.El Leithy E.S., Abdel-Bar H.M., Ali R.A.-M. Folate-chitosan nanoparticles triggered insulin cellular uptake and improved in vivo hypoglycemic activity. Int J Nanomed. 2019;571:118708. doi: 10.1016/j.ijpharm.2019.118708. [DOI] [PubMed] [Google Scholar]
- 128.Chen X.Y., Ren Y.S., Feng Y., Xu X.Y., Tan H., Li J.S. Cp1-11 peptide/insulin complex loaded pH-responsive nanoparticles with enhanced oral bioactivity. Int J Nanomed. 2019;562:23–30. doi: 10.1016/j.ijpharm.2019.03.020. [DOI] [PubMed] [Google Scholar]
- 129.Ji N., Hong Y., Gu Z.B., Cheng L., Li Z.F., Li C.M. Chitosan coating of zein-carboxymethylated short-chain amylose nanocomposites improves oral bioavailability of insulin in vitro and in vivo. J Control Release. 2019;313:1–13. doi: 10.1016/j.jconrel.2019.10.006. [DOI] [PubMed] [Google Scholar]
- 130.Wang Y.L., Cai R., Chen C.Y. The nano-bio interactions of nanomedicines: understanding the biochemical driving forces and redox reactions. Acc Chem Res. 2019;52:1507–1518. doi: 10.1021/acs.accounts.9b00126. [DOI] [PubMed] [Google Scholar]
- 131.Baimanov D., Cai R., Chen C.Y. Understanding the chemical nature of nanoparticle–protein interactions. Bioconjugate Chem. 2019;30:1923–1937. doi: 10.1021/acs.bioconjchem.9b00348. [DOI] [PubMed] [Google Scholar]
- 132.Ke P.C., Lin S., Parak W.J., Davis T.P., Caruso F. A decade of the protein corona. ACS Nano. 2017;11:11773–11776. doi: 10.1021/acsnano.7b08008. [DOI] [PubMed] [Google Scholar]
- 133.Zhang T.X., Zhu G.Y., Lu B.Y., Zhang C.L., Peng Q. Concentration-dependent protein adsorption at the nano-bio interfaces of polymeric nanoparticles and serum proteins. Nanomedicine. 2017;12:2757–2769. doi: 10.2217/nnm-2017-0238. [DOI] [PubMed] [Google Scholar]
- 134.Maiorano G., Sabella S., Sorce B., Brunetti V., Malvindi M., Cingolani R. Effects of cell culture media on the dynamic formation of protein–nanoparticle complexes and influence on the cellular response. ACS Nano. 2010;4:7481–7491. doi: 10.1021/nn101557e. [DOI] [PubMed] [Google Scholar]
- 135.Peng Q., Liu J., Zhang T., Zhang T.X., Zhang C.L., Mu H. Digestive enzyme corona formed in the gastrointestinal tract and its impact on epithelial cell uptake of nanoparticles. Biomacromolecules. 2019;20:1789–1797. doi: 10.1021/acs.biomac.9b00175. [DOI] [PubMed] [Google Scholar]
- 136.Wei X.Q., Hao L.Y., Shao X.R., Zhang Q., Jia X.Q., Zhang Z.R. Insight into the interaction of graphene oxide with serum proteins and the impact of the degree of reduction and concentration. ACS Appl Mater Interfaces. 2015;7:13367–13374. doi: 10.1021/acsami.5b01874. [DOI] [PubMed] [Google Scholar]
- 137.Ma Y., Hong J., Ding Y. Biological behavior regulation of gold nanoparticles via the protein corona. Adv Healthc Mater. 2020;9 doi: 10.1002/adhm.201901448. [DOI] [PubMed] [Google Scholar]
- 138.Cai R., Chen C.Y. The crown and the scepter: roles of the protein corona in nanomedicine. Adv Mater. 2019;31:1805740. doi: 10.1002/adma.201805740. [DOI] [PubMed] [Google Scholar]
- 139.Pozzi D., Colapicchioni V., Caracciolo G., Piovesana S., Capriotti A., Palchetti S. Effect of polyethyleneglycol (PEG) chain length on the bio-nano-interactions between PEGylated lipid nanoparticles and biological fluids: from nanostructure to uptake in cancer cells. Nanoscale. 2014;6:2782–2792. doi: 10.1039/c3nr05559k. [DOI] [PubMed] [Google Scholar]
- 140.Digiacomo L., Pozzi D., Palchetti S., Zingoni A., Caracciolo G. Impact of the protein corona on nanomaterial immune response and targeting ability. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2020:e1615. doi: 10.1002/wnan.1615. [DOI] [PubMed] [Google Scholar]
- 141.Shemetov A., Nabiev I., Sukhanova A. Molecular interaction of proteins and peptides with nanoparticles. ACS Nano. 2012;6:4585–4602. doi: 10.1021/nn300415x. [DOI] [PubMed] [Google Scholar]
- 142.Liu N., Tang M., Ding J.D. The interaction between nanoparticles-protein corona complex and cells and its toxic effect on cells. Chemosphere. 2020;245:125624. doi: 10.1016/j.chemosphere.2019.125624. [DOI] [PubMed] [Google Scholar]
- 143.Elechalawar C.K., Hossen M.N., McNally L., Bhattacharya R., Mukherjee P. Analysing the nanoparticle-protein corona for potential molecular target identification. J Control Release. 2020;322:122–136. doi: 10.1016/j.jconrel.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tonigold M., Simon J., Estupiñán D., Kokkinopoulou M., Reinholz J., Kintzel U. Pre-adsorption of antibodies enables targeting of nanocarriers despite a biomolecular corona. Nat Nanotechnol. 2018;13:862–869. doi: 10.1038/s41565-018-0171-6. [DOI] [PubMed] [Google Scholar]
- 145.Barran-Berdon A.L., Pozzi D., Caracciolo G., Capriotti A.L., Caruso G., Cavaliere C. Time evolution of nanoparticle-protein corona in human plasma: relevance for targeted drug delivery. Langmuir. 2013;29:6485–6494. doi: 10.1021/la401192x. [DOI] [PubMed] [Google Scholar]
- 146.Schäffler M., Sousa F., Wenk A., Sitia L., Hirn S., Schleh C. Blood protein coating of gold nanoparticles as potential tool for organ targeting. Biomaterials. 2014;35:3455–3466. doi: 10.1016/j.biomaterials.2013.12.100. [DOI] [PubMed] [Google Scholar]
- 147.Ng K.K., Lovell J.F., Zheng G. Lipoprotein-inspired nanoparticles for cancer theranostics. Acc Chem Res. 2011;44:1105–1113. doi: 10.1021/ar200017e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Dai Q., Yan Y., Ang C.S., Kempe K., Kamphuis M.M., Dodds S.J. Monoclonal antibody-functionalized multilayered particles: targeting cancer cells in the presence of protein coronas. ACS Nano. 2015;9:2876–2885. doi: 10.1021/nn506929e. [DOI] [PubMed] [Google Scholar]
- 149.Kah J.C., Chen J., Zubieta A., Hamad-Schifferli K. Exploiting the protein corona around gold nanorods for loading and triggered release. ACS Nano. 2012;6:6730–6740. doi: 10.1021/nn301389c. [DOI] [PubMed] [Google Scholar]
- 150.Cifuentes-Rius A., de Puig H., Kah J.C., Borros S., Hamad-Schifferli K. Optimizing the properties of the protein corona surrounding nanoparticles for tuning payload release. ACS Nano. 2013;7:10066–10074. doi: 10.1021/nn404166q. [DOI] [PubMed] [Google Scholar]
- 151.Ban D.K., Paul S. Protein corona over silver nanoparticles triggers conformational change of proteins and drop in bactericidal potential of nanoparticles: polyethylene glycol capping as preventive strategy. Colloids Surf B Biointerfaces. 2016;146:577–584. doi: 10.1016/j.colsurfb.2016.06.050. [DOI] [PubMed] [Google Scholar]
- 152.Ritz S., Schöttler S., Kotman N., Baier G., Musyanovych A., Kuharev J. Protein corona of nanoparticles: distinct proteins regulate the cellular uptake. Biomacromolecules. 2015;16:1311–1321. doi: 10.1021/acs.biomac.5b00108. [DOI] [PubMed] [Google Scholar]
- 153.Serpooshan V., Mahmoudi M., Zhao M.M., Wei K., Sivanesan S., Motamedchaboki K. Protein corona influences cell-biomaterial interactions in nanostructured tissue engineering scaffolds. Adv Funct Mater. 2015;25:4379–4389. doi: 10.1002/adfm.201500875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Liu J.Z., Dong J., Zhang T., Peng Q. Graphene-based nanomaterials and their potentials in advanced drug delivery and cancer therapy. J Control Release. 2018;286:64–73. doi: 10.1016/j.jconrel.2018.07.034. [DOI] [PubMed] [Google Scholar]
- 155.Xia M.Y., Xie Y., Yu C.H., Chen G.Y., Li Y.H., Zhang T. Graphene-based nanomaterials: the promising active agents for antibiotics-independent antibacterial applications. J Control Release. 2019;307:16–31. doi: 10.1016/j.jconrel.2019.06.011. [DOI] [PubMed] [Google Scholar]
- 156.Zhang T., Zhu G.Y., Yu C.H., Xie Y., Xia M.Y., Lu B.Y. The UV absorption of graphene oxide is size-dependent: possible calibration pitfalls. Microchim Acta. 2019;186:207. doi: 10.1007/s00604-019-3329-5. [DOI] [PubMed] [Google Scholar]
- 157.Park S., An J., Jung I., Piner R.D., An S.J., Li X. Colloidal suspensions of highly reduced graphene oxide in a wide variety of organic solvents. Nano Lett. 2009;9:1593–1597. doi: 10.1021/nl803798y. [DOI] [PubMed] [Google Scholar]
- 158.Teodorescu F., Oz Y., Queniat G., Abderrahmani A., Foulon C., Lecoeur M. Photothermally triggered on-demand insulin release from reduced graphene oxide modified hydrogels. J Control Release. 2017;246:164–173. doi: 10.1016/j.jconrel.2016.10.028. [DOI] [PubMed] [Google Scholar]
- 159.Zhao X.W., Zou X., Ye L. Controlled pH- and glucose-responsive drug release behavior of cationic chitosan based nano-composite hydrogels by using graphene oxide as drug nanocarrier. J Ind Eng Chem. 2017;49:36–45. [Google Scholar]
- 160.Liu Z., Robinson J.T., Sun X., Dai H. PEGylated nanographene oxide for delivery of water-insoluble cancer drugs. J Am Chem Soc. 2008;130:10876–10877. doi: 10.1021/ja803688x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Teodorescu F., Rolland L., Ramarao V., Abderrahmani A., Mandler D., Boukherroub R. Electrochemically triggered release of human insulin from an insulin-impregnated reduced graphene oxide modified electrode. Chem Commun. 2015;51:14167–14170. doi: 10.1039/c5cc05539c. [DOI] [PubMed] [Google Scholar]
- 162.Turcheniuk K., Khanal M., Motorina A., Subramanian P., Barras A., Zaitsev V. Insulin loaded iron magnetic nanoparticle–graphene oxide composites: synthesis, characterization and application for in vivo delivery of insulin. RSC Adv. 2014;4:865–875. [Google Scholar]
- 163.Wang C., Mallela J., Garapati U.S., Ravi S., Chinnasamy V., Girard Y. A chitosan-modified graphene nanogel for noninvasive controlled drug release. Nanomedicine. 2013;9:903–911. doi: 10.1016/j.nano.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kim H., Kim W.J. Photothermally controlled gene delivery by reduced graphene oxide-polyethylenimine nanocomposite. Small. 2014;10:117–126. doi: 10.1002/smll.201202636. [DOI] [PubMed] [Google Scholar]
- 165.Lim D.K., Barhoumi A., Wylie R.G., Reznor G., Langer R.S., Kohane D.S. Enhanced photothermal effect of plasmonic nanoparticles coated with reduced graphene oxide. Nano Lett. 2013;13:4075–4079. doi: 10.1021/nl4014315. [DOI] [PubMed] [Google Scholar]
- 166.Zhou X.B., Cao C.W., Chen Q.C., Yu Q.Q., Liu Y.N., Yin T.T. PEG modified graphene oxide loaded with EALYLV peptides for inhibiting the aggregation of hIAPP associated with type-2 diabetes. J Mater Chem B. 2015;3:7055–7067. doi: 10.1039/c5tb00487j. [DOI] [PubMed] [Google Scholar]
- 167.Matteini P., Tatini F., Cavigli L., Ottaviano S., Ghini G., Pini R. Graphene as a photothermal switch for controlled drug release. Nanoscale. 2014;6:7947–7953. doi: 10.1039/c4nr01622j. [DOI] [PubMed] [Google Scholar]
- 168.Fiorica C., Mauro N., Pitarresi G., Scialabba C., Palumbo F.S., Giammona G. Double-network-structured graphene oxide-containing nanogels as photothermal agents for the treatment of colorectal cancer. Biomacromolecules. 2017;18:1010–1018. doi: 10.1021/acs.biomac.6b01897. [DOI] [PubMed] [Google Scholar]
- 169.Yin F., Hu K., Chen Y.Z., Yu M.Y., Wang D.Y., Wang Q.Q. SiRNA delivery with PEGylated graphene oxide nanosheets for combined photothermal and genetherapy for pancreatic cancer. Theranostics. 2017;7:1133–1148. doi: 10.7150/thno.17841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Huang Y.Y., Chen C.Y., Li H.T., Xiao A.X., Guo T., Guan B.O. Insight into the local near-infrared photothermal dynamics of graphene oxide functionalized polymers through optical microfibers. Phys Chem Chem Phys. 2018;20:5256–5263. doi: 10.1039/c7cp07915j. [DOI] [PubMed] [Google Scholar]
- 171.Wang A.H., Yang T.T., Fan W.W., Yang Y.W., Zhu Q.L., Guo S.Y. Protein corona liposomes achieve efficient oral insulin delivery by overcoming mucus and epithelial Barriers. Adv Healthc Mater. 2019;8:1801123. doi: 10.1002/adhm.201801123. [DOI] [PubMed] [Google Scholar]
- 172.Saffran M., Kumar G.S., Savariar C., Burnham J.C., Williams F., Neckers D.C. A new approach to the oral administration of insulin and other peptide drugs. Science. 1986;233:1081–1084. doi: 10.1126/science.3526553. [DOI] [PubMed] [Google Scholar]