Abstract
Several therapeutic options have been developed to address the obesity epidemic and treat associated metabolic diseases. Despite the beneficial effects of surgery and drugs, effective therapeutic solutions have been held back by the poor long-term efficiency and detrimental side effects. The development of alternative approaches is thus urgently required. Fat transplantation is common practice in many surgical procedures, including aesthetic and reconstructive surgery, and is a budding future direction for treating obesity-related metabolic defects. This review focuses on adipose tissue transplantation and the recent development of cell-based therapies to boost the mass of energy-expenditure cells. Brown adipocyte transplantation is a promising novel therapy to manage obesity and associated metabolic disorders, but the need to have an abundant and relevant source of brown fat tissue or brown adipocytes for transplantation is a major hurdle to overcome. Current studies have focused on the rodent model to obtain a proof of concept of a tissue-transplantation strategy able to achieve effective long-term effects to reverse metabolic defects in obese patients. Future perspectives and opportunities to develop innovative human fat tissue models and 3D engineered hiPSC-adipocytes are discussed.
Keywords: Adipose tissues, iPSC-derived adipocytes, Transplantation, Obesity, Metabolic disorders
Introduction
Obesity is a major health issue and results from a physiological imbalance whereby energy intake exceeds its expenditure. Excessive fat storage in adipose and non-adipose tissues such as liver and skeletal muscles leads to metabolic dysfunctions. Obese patients are subject to many metabolic disorders with increased risk of insulin resistance, type 2 diabetes, dyslipidemia, nonalcoholic fatty liver, hypertension, cardiovascular diseases and respiratory problems. Obese patients were also identified as being at increased risk of developing cancer and critical COVID-19 [1].
At least three types of adipocytes coexist in the body, namely white, brown and beige/brite adipocytes. White adipose tissue (WAT) is dispersed throughout the body and is mainly involved in energy storage in triglyceride form. Excess WAT present in obese patients acts as a storage depot, but it is also an endocrine organ with effects on whole-body metabolism homeostasis (reviewed in [2]). The two largest WAT depots in humans are subcutaneous and visceral. These two WAT depots—distinguished by their localization and metabolic activity—differ in major ways during obesity. Whereas increased subcutaneous fat depots present little or no cardiovascular risk, increased visceral fat depots is correlated with adverse metabolic outcomes of obesity. Subcutaneous WAT (scWAT) is composed of adipocytes displaying insulin sensitivity and high lipid sequestration, which prevents ectopic lipid storage in other tissue [3], whereas visceral adipose tissue surrounding internal organs is associated with insulin resistance. Visceral fat also secretes a range of adipokines associated with systemic inflammation. In contrast to WAT, brown adipose tissue (BAT) has an abundance of mitochondria and when activated utilizes stored energy to produce heat by uncoupling oxidative phosphorylation in mitochondria through the uncoupling protein 1 (UCP1) [4, 5]. BAT represents a minor fraction of adipose tissue in human adults and disappears from most areas with age, persisting only in the anterior neck, in thoracic paravertebral locations and around deeper organs. The quantity of BAT in adults is negatively correlated with age and BMI, especially in obese humans where BAT is rare [6]. However, obese patients having high BAT activity were found to have high glucose tolerance [7]. Beige/brite adipocytes, the third type of adipocytes identified more recently, are brown-like adipocytes (BAs) that reside in clusters scattered within subcutaneous WAT depots. Beige/brite adipocytes can, upon cold or drug stimulation for instance, be recruited in WAT during a so-called beiging process.
Several therapeutic options have been developed to address the obesity epidemic and treat the associated metabolic diseases, including changes in lifestyle, pharmaceutical drug treatment for reducing the WAT mass or for activating BAT, as well as bariatric surgery. Despite the beneficial effects of surgery and drugs, poor long-term efficiency and detrimental side effects have held back effective therapeutic solutions. The development of alternative approaches is thus urgently required. This review is focused on fat transplantation and on the recent development of cell-based therapies for increasing the mass of energy-expenditure cells. Brown adipocyte transplantation is promising novel therapy to manage obesity and associated metabolic disorders, but the need to have an abundant and relevant source of brown fat tissue or brown adipocytes for transplantation is a major hurdle to overcome. Future perspectives and opportunities with regard to developing innovative human fat tissue models and 3D engineered human induced Pluripotent Stem Cell-derived adipocytes are discussed.
Fat tissue transplantation therapies
Fat transplantation is commonly used in many surgical procedures, including aesthetic and reconstructive surgery [8, 9], and is a promising future direction for treating metabolic defects in obesity. Due to safety regulations for this new approach, current studies have focused on the rodent model to obtain a proof of concept of a tissue-transplantation strategy able to achieve effective long-term effects to reverse metabolic defects in obese patients.
WAT transplantation
WAT tissue has beneficial metabolic functions via lipogenesis, fatty acid oxidation, adipokine secretion and its high lipid sequestration capacity, thereby preventing ectopic lipid storage in other tissues unequipped to neutralize fatty acid cytotoxicity (see for review [10]). The beneficial metabolic effects of WAT transplantation have been demonstrated in lipodystrophic mice. Indeed, transplantation of subcutaneous WAT from normal to lipodystrophic mice improved their metabolism, with decreased food intake, reduced glucose and insulin levels and decreased hepatic steatosis, as well as increased insulin sensitivity and glucose uptake into muscles. The mechanism by which the transplantation of this fat improved the metabolism of the target mice required adipocyte leptin secretion, and the fat transplant could be replaced by leptin administration [11]. However, most obese patients present with leptin resistance, thus reducing the interest of performing WAT transplantation to counteract obesity and metabolic disorders.
More recently, it has been shown that glucose tolerance was improved in mice after scWAT transplantation, and this effect was not attributable to plasma leptin concentrations. Mice receiving subcutaneous fat grafts displayed a marked reduction in plasma concentrations of several proinflammatory cytokines, such as TNF-α, IL-17 and macrophage inflammatory protein-1β, compared to sham-operated mice. In contrast, mice receiving visceral fat transplants were glucose intolerant, with increased hepatic triacylglycerol content and elevated plasma IL-6 concentrations [12]. Studies involving syngeneic adipose tissue transplantation in mice have consistently shown that intra-abdominal transplantation of subcutaneous (i.e. inguinal depot), but not visceral i.e. epididymal depot) improves glucose tolerance and reduces hepatic steatosis and adiposity [13]. These data suggest that scWAT fat is intrinsically different from visceral fat and produces substances that can act systemically to improve glucose metabolism. Other authors recently challenged the hypothesis that human visceral adipose tissue impairs glucose homeostasis and insulin action, whereas subcutaneous glutealfemoral WAT provides protection. They performed xenografting of human visceral or subcutaneous WATs in immune-deficient high-fat-fed mice and assessed glycemic control and insulin action. They proposed that the intrinsic properties of human visceral WAT and subcutaneous glutealfemoral WAT do not necessarily explain the negative and positive effects of these adipose tissue depots on glycemic control [14]. The underlying mechanisms explaining how transplantation of WAT protects against glucose intolerance and insulin resistance have yet to be identified.
BAT transplantation
Several studies have been conducted on rodents in recent decades to determine the beneficial effects of BAT transplantation. In most of these studies, the BAT grafts originated from healthy adult mice were transplanted in obese or diabetic mice in an attempt to reverse metabolic disorders and weight. BAT from C57BL/6 J mice transplanted in diet induced obesity (DIO) recipient mice or leptin deficient Ob/Ob mice [15, 16] increased the whole-body energy metabolism and in turn prompted a decrease in body fat. Another set of BAT transplantation studies conducted in Ob/Ob mice [15] and BAT-deficient obese mice [17] also revealed an active metabolic benefit of BAT transplantation by increasing energy expenditure and improving insulin sensitivity, while increasing adiponectin levels. The beneficial effects of transplanted BAT could be mediated via several different mechanisms, including batokine release [18, 19]. Diabetic type 1 non-obese mice transplanted with embryonic BAT showed a reduction in adipose tissue weight and inflammation markers. BAT transplants resulted in complete reversal of diabetes associated with rapid and long-lasting euglycemia. The authors also observed an increase in adiponectin and leptin levels from the surrounding white adipose tissue [20, 21]. These authors designated IGF-1, whose expression was elevated in BAT transplants, as a primary mechanism involved in the anti-diabetic effects of the graft in this model. The main limitation of this approach to clinical translation is the lack of IGF-1 expression in adult adipose tissue transplant compared to high expression in embryonic BAT [22]. However, the authors showed that a combination of adult BAT transplant with IGF-1 injection enabled correction of insulin levels, thus paving the way for mainstreaming this strategy in humans. Overall, these data suggest that adipokines secreted by BAT transplants could give rise to a new therapeutic strategy to control chronic glycemic defects in diabetic patients (for recent review see [23].
In a study on a more global approach for treating metabolic disorders in non-diabetic obese rodent models, Stanford and collaborators challenged the beneficial effects of adult BAT transplants with regard to energy expenditure and metabolic parameters. They investigated the effects of BAT transplants on glucose homeostasis in high fat diet (HFD)-induced obese mice [24]. Recipient mice were transplanted with 0.1–0.4 g of donor BAT tissues in the visceral cavity. After 8–12 weeks, the transplanted mice showed enhanced glucose tolerance, increased insulin sensitivity, lower body weight, decreased fat mass, and a complete reversal of HFD–induced insulin resistance. A positive correlation between the quantity of BAT transplanted and metabolic effects was also noted. Metabolic benefits of BAT transplantation were lost when BAT originated from IL6-knockout mice, thus demonstrating the crucial role of this cytokine in glucose homeostasis and insulin sensitivity. Liu and collaborators investigated the effects of BAT transplantation in wild-type recipient mice further submitted to HFD for 20 weeks. The transplanted mice showed reduced weight gain compared to HFD sham-operated mice [25]. Interestingly, BAT transplantation also led to an increase in the expression of fatty acid oxidation-related genes such as MCAD, PPARα, PGC1α, CPT1β and UCP1, but this only concerned endogenous BAT. BAT transplantation increases glucose uptake in endogenous BAT and WAT, but not in skeletal muscle. Effect of wild-type BAT transplantation in obese Ob/Ob mice could also trigger an endocrine reaction by increasing beta 3-adrenergic receptor and fatty acid oxidation related gene expression in subcutaneous and epididymal white adipose tissue [15].
Overall, BAT transplantation studies have demonstrated that increasing BAT mass improves glucose metabolism, increases insulin sensitivity, while reducing body mass. The beneficial effects of BAT transplantation have been demonstrated in several studies, but the underlying mechanisms have yet to be clarified. Increased energy expenditure and secretion of batokines seem to play a critical role in the mechanisms through which BAT transplantation improves metabolic health. UCP1-independent energy expenditure pathways have recently been identified (see for review[26]) and their contribution to beneficial effects of BAT transplantation should also be taken into account. Interestingly, some of the metabolic effects observed in transplanted mice may be more associated with the activation of endogenous brown and white fat browning than with the BAT transplant itself.
The accessibility of BAT is challenging due to its sparced distribution in human adults, and BAT is rare in obese patients. An abundant accessible source of autologous BAT therefore has to be identified for tissue-based therapy of obesity and associated metabolic disorders. We discuss this issue in the Future Perspectives section.
Induced pluripotent stem cell-based therapy
Besides tissue grafting, adipose stem cells (ASCs) are a promising source for cell based-therapy of obesity because of the potential of WAT-derived ASCs for ex vivo expansion and differentiation into beige adipocytes. The ASC-based therapy strategy is not within the scope of this review, and has been described by many authors [27, 28]. However, these studies have also highlighted the current limitations of this strategy for human clinical applications. This includes the potential of proliferation and further differentiation of ASCs in vivo, which has been shown to be negatively correlated within age [29]. Primary human ASCs can only be studied for short periods before they reach senescence and undergo differentiation failure, thereby precluding their extensive expansion for cell-based therapy. In addition, ASCs isolated from an obese environment have impaired differentiation, migration and angiogenic properties [30]. These findings highlight the low potential of autologous ASC-based therapy for obesity. Another physiological limitation is the poor engraftment success of transplanted cells, likely because the lack of vascularization.
Induced pluripotent stem cells (iPSCs) overcome some of these limitations and appear an interesting option for treating metabolic disorders. iPSC technology was introduced in 2007 by Yamanaka’s group [31]. iPSCs are defined as embryonic-like pluripotent stem cells obtained from somatic cells by ectopic expression of pluripotency transcription factors. Human iPSCs sidestep ethical problems caused by the use of human embryonic stem cells (hESCs). Human iPSCs can be obtained in unlimited quantities, cultivated for long time and differentiated into cell types of therapeutic interest. iPSCs have been widely used for disease modelling, drug discovery and cell therapy development (for recent review see [32]). Clinical use of iPSC-derived cells has been approved by the US Food and Drug Administration for the treatment of at least two diseases, i.e. iPSC-myogenic progenitors were approved for treating muscular dystrophic patients and, in 2014, the first clinical trial using human iPSC products was conducted, whereby retinal pigment epithelial sheets derived from the patient’s own iPSCs were transplanted to treat macular degeneration.
The potential use iPSC in the treatment of obesity and metabolic disorders has been investigated more recently. Some papers in the early 2000s reported the adipocyte-generation potential of human ES cells, and then Taura et al. demonstrated that human iPSCs have an adipogenic potential comparable to that of hES cells [33]. However, these authors did not assess the adipogenesis efficacy of human iPSCs nor the white or brown phenotype of the adipocytes generated. Nishio and colleagues reported, for the first time, the capacity of human iPSCs for generating brown adipocytes at high levels. Both lipid and glucose metabolisms were improved in BALB/c mice transplanted with hiPSC-derived brown adipocytes [34]. Ahfeldt et al. purified human iPSC-derived fibroblasts that were able to undergo differentiation into white or brown adipocytes following forced expression of adipogenic master genes [35]. This strategy allowed large-scale generation of human white and brown adipocytes, which could represent a powerful tool for drug discovery. However, the capacity of these reprogrammed fibroblasts to counteract metabolic disorders in rodents has not been addressed. More recently, two teams (including ours) described a procedure to isolate expandable brown/beige adipose progenitors from hiPSCs and to generate high levels of functional brown/beige adipocytes with no gene transfer [36–38] thereby paving the way for taking advantage of hiPSC features for anti-obesity drug testing and cell-based therapy with the aim of boosting the BA mass in patients. Adipogenic genes, including UCP1, Dio2, PGC1a and PRDM16, have been detected in differentiated cultures, indicating that cells having a brown-like adipocyte gene program are spontaneously generated during differentiation. Aaron Brown’s team described the generation of human beige adipocytes from hiPSCs of defined origin using a developmental approach. At the molecular level, progenitors expressed beige/brite markers such as CD137 and TMEM26 but not the brown-specific marker Zic1 [36]. Our group underlined the weak capacity of human iPSCs to generate brown/beige adipocytes compared to adult adipose progenitors and identified critical factors to switch on the commitment of pluripotent stem cells towards adipogenic lineages [37]. As mentioned above, ASCs from old subjects or obese patients lack beige adipogenic potential. Very interestingly, Su and colleagues were able to isolate ASCs from adipose tissue or from iPSCs, both of which were derived from a 76-year-old patient with type 2 diabetes, and then compared their beiging potential. In contrast to patient-derived ASCs, hiPSCs-adipose progenitors generated from the patient displayed a high thermogenic capacity and insulin sensitivity [36] suggesting that ASCs could have a high rejuvenation potential when derived from iPSCs. Further functional analyses and transplantation assays are now needed to fully evaluate the potential of these beige adipocytes for counteracting obesity and associated metabolic disorders. Very recently, Zhang et al. described a system for the generation of human classical brown adipocytes from iPSCs that had an impact on the metabolic activity of transplanted mice. Indeed, transplanted mice showed increased oxygen consumption, respiratory rates and whole-body energy expenditure. Finally, the authors demonstrated that transplanted hiPSC-derived brown adipocytes had the capacity to clear blood glucose [39].
There are still several obstacles associated with iPSC-based therapy that will need to be overcome. The main concern is the risk of tumorigenicity from iPSCs. Pluripotent cells—since are maintained in culture for a long time—can accumulate karyotypic abnormalities, yet differentiated cells derived from iPSCs have not been shown to generate teratomas (for recent review see [40]. Another concern is that autologous patient specific-iPSC therapy may not be practical for managing large numbers of patients given the high cost and lengthy period of time needed for iPSC generation, differentiation and quality control. An alternative approach may be the application of HLA-matched allogeneic iPSC lines from public biobanks, in particular from HLA-homozygous donors, which appears feasible especially in countries with a restricted HLA repertoire. Encapsulation of energy expenditure adipocytes, derived from hiPSCs or from adipose tissue models, in an immune tolerant scaffolds [41, 42] is an alternative approach for allogenic cell-based therapy of obesity. However, it remains to be determined whether allotransplantation could ensure successful long-term engraftment with very limited pharmacological immunosuppression.
Future perspectives for BAT and iPSC-derived adipocyte transplantation
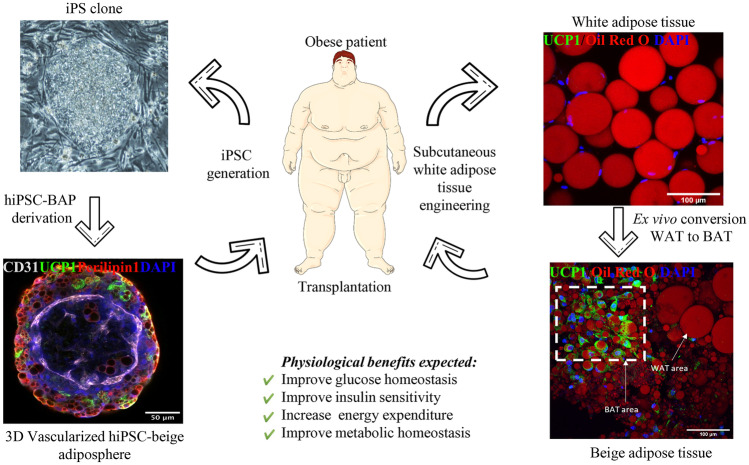
The accessibility of BAT is challenging due to its distribution in adult humans, and BAT is rare in obese patients. An abundant and accessible source of autologous BAT therefore has to be identified for tissue-based therapy of obesity and its associated metabolic disorders. Two sources of energy expenditure adipocytes for autologous transplantation in obese patients are illustrated in Fig. 1.
Fig. 1.
Sources of energy expenditure adipocytes and expected effects of their transplantation on metabolic health. Left part: iPSCs can be generated from patients. An iPSC colony and an iPSC-derived 3D vascularized adiposphere expressing perilipin 1 (red), UCP1 (green), and CD31 (white) are shown. Right part: A biopsy of subcutaneous white adipose tissue can be converted ex vivo in beige adipose tissue expressing UCP1 (green). Lipid droplets were staining with Oil Red O. Images showed in the figure originate from projects developed in Dani’s group
The most abundant source would be white adipose tissue which is present in excessive amounts in obese patients. WAT browning can be induced by several pathways, and this has been extensively reviewed [43]. In 2015, Stanford et al. transplanted scWAT—previously turned into beige fat via exercise training—into the visceral cavity of HFD mice [44]. They showed that transplantation of trained WAT in sedentary recipient mice enhanced skeletal muscle glucose uptake and whole-body metabolic homeostasis. Blumenfeld et al. proposed a direct tissue transplantation approach involving ex vivo beiging of mouse scWAT, or so-called ExBAT [45]. In this study, mouse WAT was cut into small pieces with a scalpel, then maintained 3 weeks ex vivo for browning before transplantation. After implantation, beige adipose tissue fragments still expressed UCP1 protein and vascularization patterns were observed. However, ExBAT transplantation in a diet-induced obesity mouse model did not generate significant changes in terms of weight loss or heat expenditure after cold exposure compared to WAT transplantation. Direct tissue grafting is a simple approach to increase the BAT mass. However, the beneficial effects of ExBAT on obesity and metabolic disorders have yet to be determined. Another challenge is the browning of human obese adipose tissues. The impact of a recipient obese microenvironment on transplanted tissues also remains to be investigated as it is a critical issue to determine the translational relevance of BAT transplantation in obese patients. The development of a procedure to turn obese white adipose tissue into beige tissue ex vivo, while maintaining the native adipose tissue structure as well as the different adipose tissue resident cell types, is thus required to design a relevant in vitro preclinical model to evaluate the efficacy and safety of BAT therapy. This could also be a powerful model for studying crosstalk between the graft and the recipient site ex vivo. Efforts are developed to establish a such procedure (see Fig. 1).
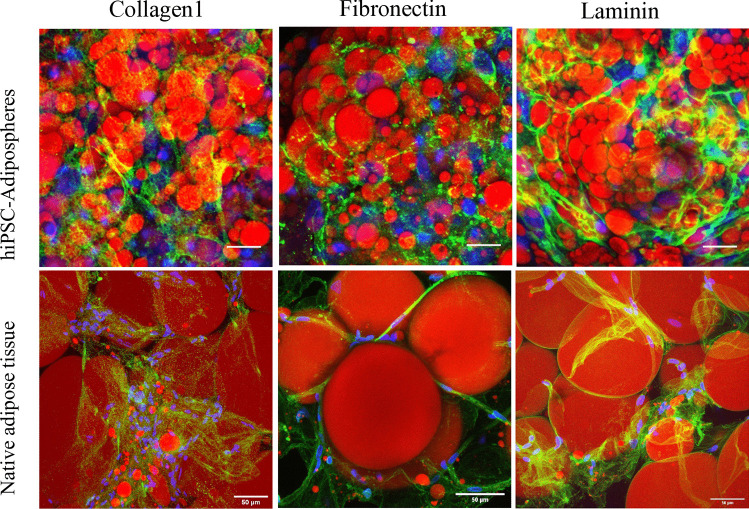
Further studies are needed to improve the physiological relevance of in vitro models proposed for preclinical studies in the obesity field. In conventional cell culture models, ASCs derived from adipose tissue or from iPSCs are conventionally grown as 2D monolayers, which does not reflect the in vivo situation. In this context, 3D culture technologies and bioengineering methods for seeding different cell types in an organoid-like structure are highly promising [46–48]. Three-dimensional cultures represent a bridge between traditional cell culture and live tissue. Therefore, a future challenge will be to generate 3D hiPSC-brown-like adipospheres resembling human adipose tissue. This should include enriching hiPSC-brown-like adipospheres with endothelial cells and macrophages embedded in an extracellular matrix to enable functional bidirectional interactions between the microenvironment and adipocytes. As a first step towards the generation of brown-like adipose tissue-like organoids, our laboratory has formed 3D spheroids of hiPSC brown-like adipose progenitors enriched with endothelial cells that are able to differentiate into adipospheres expressing UCP-1 (Fig. 2). Interestingly, hiPSC-adipospheres display an extracellular matrix structure resembling human subcutaneous adipose tissue (Fig. 3, unpublished work). Overall, these observations highlight the physiological relevance of the in vitro 3D adiposphere model for anti-obesity drug screening and cell-based therapy of obesity. The combination of the human iPSC platform with 3D organoid technologies could make human iPSCs an even more powerful cellular resource for stem cell-based cell therapy development.
Fig. 2.
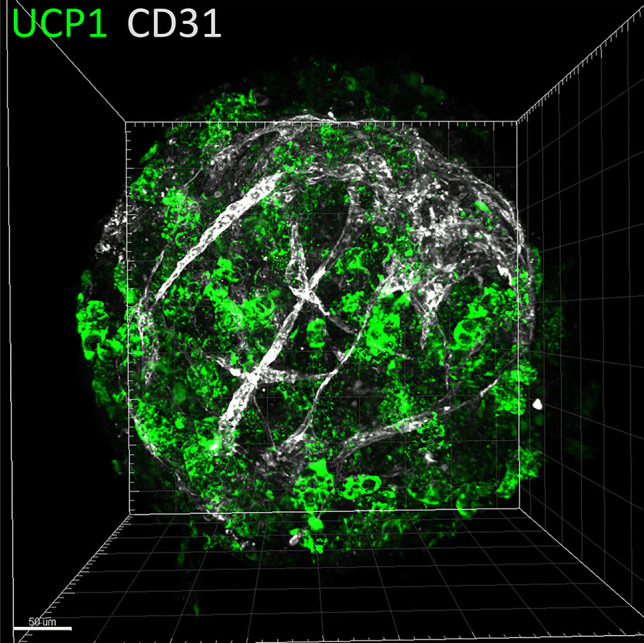
Generation of prevascularized hiPSC-3D adipospheres. Adipose progenitors were derived from hiPSCs and induced to differentiate in 3D adipospheres in the presence of endothelial cells. 3D adipospheres were stained for CD31 endothelial cells (white), and UCP1 (green) and then transparized as described by Yao Xi and Dani Christian (Methods in Molecular Biology, iPS cells: Methods and Protocols, in press). Images were acquired on a LSM780 confocal microscope and the 3D reconstruction was generated by the Imaris software. The scale bar is 50 μm
Fig. 3.
Comparison of the extra cellular matrix profile of hiPSC-3D adipospheres and human adipose tissue. 3D adipospheres and human adipose tissue were fixed and stained for lipid droplets (red) and proteins of the extra cellular matrix indicated
Most of cited studies investigating transplantation of adipose tissue or hiPSCs have been performed in mice housed under thermoneutrality, i.e. at a temperature activating thermogenic cells [49]. Thermoneutrality negatively regulates the thermogenic program, strongly suggesting that in human further interventions will be required to activate transplants. Warming transplanted mice might enable more predictive modelling cell based therapy of obesity.
As discussed earlier regarding BAT transplants, the impact of an obese microenvironment on hiPSC-derived adipocytes is also a critical issue. It has been well documented that temperature and Western diet induced whitening of BAT [50]. Therefore, one of the obese microenvironment impact, combined with the fact that transplanted patients will be at thermoneutrality, would likely be to whiten the transplant. A negative effect of the obese microenvironment could be overcome by engineering hiPSCs-derived adipocytes to maintain UCP1 expression. Very interestingly, Yu-Hua Tseng’s group has recently reported the possibility to activate endogenous UCP1 expression in immortalized white preadipocytes (HUMBLE cells) through clustered regularly interspaced short palindromic repeat-associated Cas9 (CRISPR/Cas9) technology [51]. In contrast to primary adipose progenitors, CRISPR-mediated genome editing of hiPSCs is practicable [52]. Combining the hiPSC technology to provide adipose progenitors from individual patients with recent advances in CRISPR system may open the opportunity to generate transplanted cells resistant to the obese microenvironment to achieve the best cell-based therapy outcome against obesity and associated diabetes.
Abbreviations
- hiPSCs
Human induced pluripotent Stem Cells
- WAT
White Adipose Tissue
- scWAT
Subcutaneous WAT
- BAT
Brown adipose tissue
- Bas
Brown-like adipocytes
- HFD
High fat diet
- ASCs
Adipose stem cells
- hESCs
Human embryonic stem cells
Funding
The work has been supported by the French National Research Agency (ANR) through the “Investments for the Future” program LABEX SIGNALIFE ANR-11-LABX-0028–01, grant number ANR-18-CE18-0006–01, and a grant from the SATT Sud Est.
Declarations
Conflicts of interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Vincent Dani and Xi Yao equal contribution to this review.
References
- 1.Yang J, Hu J, Zhu C. Obesity aggravates COVID-19: A systematic review and meta-analysis. J Med Virol. 2021;93:257–261. doi: 10.1002/jmv.26237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wozniak SE, Gee LL, Wachtel MS, Frezza EE. Adipose Tissue: The New Endocrine Organ? A Review Article Dig Dis Sci. 2009;54:1847–1856. doi: 10.1007/s10620-008-0585-3. [DOI] [PubMed] [Google Scholar]
- 3.Trayhurn P, Beattie JH. Physiological role of adipose tissue: white adipose tissue as an endocrine and secretory organ. Proc Nutr Soc Cambridge University Press. 2001;60:329–339. doi: 10.1079/PNS200194. [DOI] [PubMed] [Google Scholar]
- 4.Aquila H, Link TA, Klingenberg M. The uncoupling protein from brown fat mitochondria is related to the mitochondrial ADP/ATP carrier. Analysis of sequence homologies and of folding of the protein in the membrane. EMBO J. John Wiley & Sons, Ltd; 1985;4:2369–76. [DOI] [PMC free article] [PubMed]
- 5.Ricquier D, Bouillaud F. Mitochondrial uncoupling proteins: from mitochondria to the regulation of energy balance. J Physiol. 2000;529:3–10. doi: 10.1111/j.1469-7793.2000.00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Timmons JA, Pedersen BK. The importance of brown adipose tissue. N Engl J Med. 2009;361:415–6; author reply 418–421. [DOI] [PubMed]
- 8.Simonacci F, Bertozzi N, Grieco MP, Grignaffini E, Raposio E. Autologous fat transplantation for breast reconstruction: A literature review. Ann Med Surg. 2016;12:94–100. doi: 10.1016/j.amsu.2016.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kasem A, Wazir U, Headon H, Mokbel K. Breast Lipofilling: A Review of Current Practice. Arch Plast Surg. 2015;42:126–130. doi: 10.5999/aps.2015.42.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carobbio S, Pellegrinelli V, Vidal-Puig A. Adipose Tissue Function and Expandability as Determinants of Lipotoxicity and the Metabolic Syndrome. Adv Exp Med Biol. 2017;960:161–196. doi: 10.1007/978-3-319-48382-5_7. [DOI] [PubMed] [Google Scholar]
- 11.Tran TT, Kahn CR. Transplantation of adipose tissue and stem cells: role in metabolism and disease. Nat Rev Endocrinol Nature Publishing Group. 2010;6:195–213. doi: 10.1038/nrendo.2010.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hocking SL, Stewart RL, Brandon AE, Suryana E, Stuart E, Baldwin EM, et al. Subcutaneous fat transplantation alleviates diet-induced glucose intolerance and inflammation in mice. Diabetologia. 2015;58:1587–1600. doi: 10.1007/s00125-015-3583-y. [DOI] [PubMed] [Google Scholar]
- 13.Tran TT, Yamamoto Y, Gesta S, Kahn CR. Beneficial Effects of Subcutaneous Fat Transplantation on Metabolism. Cell Metab. 2008;7:410–420. doi: 10.1016/j.cmet.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsiloulis T, Raajendiran A, Keenan SN, Ooi G, Taylor RA, Burton P, et al. Impact of human visceral and glutealfemoral adipose tissue transplant on glycemic control in a mouse model of diet-induced obesity. Am J Physiol Endocrinol Metab. 2020;319:E519–E528. doi: 10.1152/ajpendo.00373.2019. [DOI] [PubMed] [Google Scholar]
- 15.Liu X, Wang S, You Y, Meng M, Zheng Z, Dong M, et al. Brown Adipose Tissue Transplantation Reverses Obesity in Ob/Ob Mice. Endocrinology. 2015;156:2461–2469. doi: 10.1210/en.2014-1598. [DOI] [PubMed] [Google Scholar]
- 16.Zhu Z, Spicer EG, Gavini CK, Goudjo-Ako AJ, Novak CM, Shi H. Enhanced sympathetic activity in mice with brown adipose tissue transplantation (transBATation) Physiol Behav. 2014;125:21–29. doi: 10.1016/j.physbeh.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oguri Y, Fujita Y, Abudukadier A, Ohashi A, Goto T, Furuya F, et al. Tetrahydrobiopterin activates brown adipose tissue and regulates systemic energy metabolism. JCI Insight. 2017;2:e91981. doi: 10.1172/jci.insight.91981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Villarroya F, Cereijo R, Villarroya J, Giralt M. Brown adipose tissue as a secretory organ. Nat Rev Endocrinol Nature Publishing Group. 2017;13:26–35. doi: 10.1038/nrendo.2016.136. [DOI] [PubMed] [Google Scholar]
- 19.Villarroya F, Gavaldà-Navarro A, Peyrou M, Villarroya J, Giralt M. The Lives and Times of Brown Adipokines. Trends Endocrinol Metab TEM. 2017;28:855–867. doi: 10.1016/j.tem.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Gunawardana SC, Piston DW. Insulin-independent reversal of type 1 diabetes in nonobese diabetic mice with brown adipose tissue transplant. Am J Physiol-Endocrinol Metab. American Physiological Society; 2015;308:E1043–55. [DOI] [PMC free article] [PubMed]
- 21.Gunawardana SC, Piston DW. Reversal of Type 1 Diabetes in Mice by Brown Adipose Tissue Transplant. Diabetes American Diabetes Association. 2012;61:674–682. doi: 10.2337/db11-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gunawardana SC, Piston DW. Insulin-Independent Reversal of Type-1 Diabetes Following Transplantation of Adult Brown Adipose Tissue Supplemented With IGF-1: Transplant Direct. 2019;5:e500. [DOI] [PMC free article] [PubMed]
- 23.White JD, Dewal RS, Stanford KI. The beneficial effects of brown adipose tissue transplantation. Mol Aspects Med. 2019;68:74–81. doi: 10.1016/j.mam.2019.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stanford KI, Middelbeek RJW, Townsend KL, An D, Nygaard EB, Hitchcox KM, et al. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest. 2013;123:215–223. doi: 10.1172/JCI62308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu X, Zheng Z, Zhu X, Meng M, Li L, Shen Y, et al. Brown adipose tissue transplantation improves whole-body energy metabolism. Cell Res Nature Publishing Group. 2013;23:851–854. doi: 10.1038/cr.2013.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roesler A, Kazak L. UCP1-independent thermogenesis. Biochem J. 2020;477:709–725. doi: 10.1042/BCJ20190463. [DOI] [PubMed] [Google Scholar]
- 27.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 28.Soler-Vázquez MC, Mera P, Zagmutt S, Serra D, Herrero L. New approaches targeting brown adipose tissue transplantation as a therapy in obesity. Biochem Pharmacol. 2018;155:346–355. doi: 10.1016/j.bcp.2018.07.022. [DOI] [PubMed] [Google Scholar]
- 29.Kirkland JL, Dobson DE. Preadipocyte Function and Aging: Links Between Age-Related Changes in Cell Dynamics and Altered Fat Tissue Function. J Am Geriatr Soc. 1997;45:959–967. doi: 10.1111/j.1532-5415.1997.tb02967.x. [DOI] [PubMed] [Google Scholar]
- 30.Pérez LM, Bernal A, San Martín N, Gálvez BG. Obese-derived ASCs show impaired migration and angiogenesis properties. Arch Physiol Biochem. 2013;119:195–201. doi: 10.3109/13813455.2013.784339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 32.Shi Y, Inoue H, Wu JC, Yamanaka S. Induced pluripotent stem cell technology: a decade of progress. Nat Rev Drug Discov. 2017;16:115–130. doi: 10.1038/nrd.2016.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taura D, Noguchi M, Sone M, Hosoda K, Mori E, Okada Y, et al. Adipogenic differentiation of human induced pluripotent stem cells: comparison with that of human embryonic stem cells. FEBS Lett. 2009;583:1029–1033. doi: 10.1016/j.febslet.2009.02.031. [DOI] [PubMed] [Google Scholar]
- 34.Nishio M, Yoneshiro T, Nakahara M, Suzuki S, Saeki K, Hasegawa M, et al. Production of functional classical brown adipocytes from human pluripotent stem cells using specific hemopoietin cocktail without gene transfer. Cell Metab. 2012;16:394–406. doi: 10.1016/j.cmet.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 35.Ahfeldt T, Schinzel RT, Lee Y-K, Hendrickson D, Kaplan A, Lum DH, et al. Programming human pluripotent stem cells into white and brown adipocytes. Nat Cell Biol. 2012;14:209–219. doi: 10.1038/ncb2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Su S, Guntur AR, Nguyen DC, Fakory SS, Doucette CC, Leech C, et al. A Renewable Source of Human Beige Adipocytes for Development of Therapies to Treat Metabolic Syndrome. Cell Rep. 2018;25(3215–3228):e9. doi: 10.1016/j.celrep.2018.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hafner A-L, Contet J, Ravaud C, Yao X, Villageois P, Suknuntha K, et al. Brown-like adipose progenitors derived from human induced pluripotent stem cells: Identification of critical pathways governing their adipogenic capacity. Sci Rep. 2016;6:32490. doi: 10.1038/srep32490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mohsen-Kanson T, Hafner A-L, Wdziekonski B, Takashima Y, Villageois P, Carrière A, et al. Differentiation of human induced pluripotent stem cells into brown and white adipocytes: role of Pax3. Stem Cells Dayt Ohio. 2014;32:1459–1467. doi: 10.1002/stem.1607. [DOI] [PubMed] [Google Scholar]
- 39.Zhang L, Avery J, Yin A, Singh AM, Cliff TS, Yin H, et al. Generation of Functional Brown Adipocytes from Human Pluripotent Stem Cells via Progression through a Paraxial Mesoderm State. Cell Stem Cell. 2020;27(784–797):e11. doi: 10.1016/j.stem.2020.07.013. [DOI] [PubMed] [Google Scholar]
- 40.Yamanaka S. Pluripotent Stem Cell-Based Cell Therapy-Promise and Challenges. Cell Stem Cell. 2020;27:523–531. doi: 10.1016/j.stem.2020.09.014. [DOI] [PubMed] [Google Scholar]
- 41.Vegas AJ, Veiseh O, Gürtler M, Millman JR, Pagliuca FW, Bader AR, et al. Long-term glycemic control using polymer-encapsulated human stem cell-derived beta cells in immune-competent mice. Nat Med. 2016;22:306–311. doi: 10.1038/nm.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Agulnick AD, Ambruzs DM, Moorman MA, Bhoumik A, Cesario RM, Payne JK, et al. Insulin-Producing Endocrine Cells Differentiated In Vitro From Human Embryonic Stem Cells Function in Macroencapsulation Devices In Vivo. Stem Cells Transl Med. 2015;4:1214–1222. doi: 10.5966/sctm.2015-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nedergaard J, Cannon B. The Browning of White Adipose Tissue: Some Burning Issues. Cell Metab. 2014;20:396–407. doi: 10.1016/j.cmet.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 44.Stanford KI, Middelbeek RJW, Goodyear LJ. Exercise Effects on White Adipose Tissue: Beiging and Metabolic Adaptations. Diabetes American Diabetes Association. 2015;64:2361–2368. doi: 10.2337/db15-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blumenfeld NR, Kang HJ, Fenzl A, Song Z, Chung JJ, Singh R, et al. A direct tissue-grafting approach to increasing endogenous brown fat. Sci Rep. 2018;8:7957. doi: 10.1038/s41598-018-25866-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Langhans SA. Three-Dimensional in Vitro Cell Culture Models in Drug Discovery and Drug Repositioning. Front Pharmacol. 2018;9:6. doi: 10.3389/fphar.2018.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu C, Oikonomopoulos A, Sayed N, Wu JC. Modeling human diseases with induced pluripotent stem cells: from 2D to 3D and beyond. Development. 2018;145. [DOI] [PMC free article] [PubMed]
- 48.Takebe T, Zhang B, Radisic M. Synergistic Engineering: Organoids Meet Organs-on-a-Chip. Cell Stem Cell. 2017;21:297–300. doi: 10.1016/j.stem.2017.08.016. [DOI] [PubMed] [Google Scholar]
- 49.de Jong JMA, Sun W, Pires ND, Frontini A, Balaz M, Jespersen NZ, et al. Human brown adipose tissue is phenocopied by classical brown adipose tissue in physiologically humanized mice. Nat Metab. 2019;1:830–843. doi: 10.1038/s42255-019-0101-4. [DOI] [PubMed] [Google Scholar]
- 50.Winn NC, Acin-Perez R, Woodford ML, Hansen SA, Haney MM, Ayedun LA, et al. A Thermogenic-Like Brown Adipose Tissue Phenotype Is Dispensable for Enhanced Glucose Tolerance in Female Mice. Diabetes. 2019;68:1717–1729. doi: 10.2337/db18-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang C-H, Lundh M, Fu A, Kriszt R, Huang TL, Lynes MD, et al. CRISPR-engineered human brown-like adipocytes prevent diet-induced obesity and ameliorate metabolic syndrome in mice. Sci Transl Med. 2020;12:eaaz8664. [DOI] [PMC free article] [PubMed]
- 52.Giacalone JC, Sharma TP, Burnight ER, Fingert JF, Mullins RF, Stone EM, et al. CRISPR-Cas9-Based Genome Editing of Human Induced Pluripotent Stem Cells. Curr Protoc Stem Cell Biol. 2018;44:5B.7.1–5B.7.22. [DOI] [PMC free article] [PubMed]