Abstract
Background
The role of myeloid-derived suppressor cells (MDSCs) in patients with severe tuberculosis who suffer from uncontrolled pulmonary inflammation caused by hypervirulent mycobacterial infection remains unclear.
Methods
This issue was addressed using C57BL/6 mice infected with highly virulent Mycobacterium bovis strain MP287/03.
Results
CD11b+GR1int population increased in the bone marrow, blood and lungs during advanced disease. Pulmonary CD11b+GR1int (Ly6GintLy6Cint) cells showed granularity similar to neutrophils and expressed immature myeloid cell markers. These immature neutrophils harbored intracellular bacilli and were preferentially located in the alveoli. T-cell suppression occurred concomitantly with CD11b+GR1int cell accumulation in the lungs. Furthermore, lung and bone marrow GR1+ cells suppressed both T-cell proliferation and interferon γ production in vitro. Anti-GR1 therapy given when MDSCs infiltrated the lungs prevented expansion and fusion of primary pulmonary lesions and the development of intragranulomatous caseous necrosis, along with increased mouse survival and partial recovery of T-cell function. Lung bacterial load was reduced by anti-GR1 treatment, but mycobacteria released from the depleted cells proliferated extracellularly in the alveoli, forming cords and clumps.
Conclusions
Granulocytic MDSCs massively infiltrate the lungs during infection with hypervirulent mycobacteria, promoting bacterial growth and the development of inflammatory and necrotic lesions, and are promising targets for host-directed therapies.
Keywords: tuberculosis, hypervirulent mycobacteria, myeloid-derived suppressor cells, lung damage, immunomodulation
Granulocytic myeloid-derived suppressor cells (MDSCs) act as a permissive niche for intracellular mycobacterial growth and spread bacilli throughout lung tissue in advanced MP287/03 infection. Massive death of infected MDSCs is apparently decisive for the development of pulmonary necrotic lesions.
Tuberculosis is among the top 10 causes of death worldwide, with approximately 1.3 million fatal cases annually [1]. Mycobacterium tuberculosis is primarily responsible for tuberculosis in humans, but other mycobacterial species belonging to M. tuberculosis complex such as Mycobacterium bovis, Mycobacterium africanum, and Mycobacterium canetti, can also cause the disease [2]. Host immune response is critical for eliminating or containing the pathogen within granulomas for long periods of time [3, 4]. Deficiencies in host defense promote the transition from latent to active tuberculosis and the rapid progression and dissemination of the disease in severe cases [5–7]. Treatment failure resulting from multiple drug resistance enhances the risk of developing aggressive forms of tuberculosis, which is characterized by uncontrolled pulmonary inflammation and widespread tissue necrosis [8, 9]. A better understanding of the immunopathogenesis of severe tuberculosis is essential to design new therapeutic interventions targeting the host immune response to reduce tissue injury.
Despite the important role of the innate immune response in controlling tuberculosis, recent studies indicate that immature myeloid cell subpopulations contribute to worsening the disease [10–12]. These subpopulations, collectively named myeloid-derived suppressor cells (MDSCs), were described first in cancer and then in other pathological conditions, such as infectious diseases, autoimmune diseases, obesity and pregnancy [13]. MDSCs are a heterogeneous group of immature myeloid CD11b+ cells with a potential ability to suppress T-cell responses [14]. According to relative expression of the surface markers Ly6C and Ly6G, murine MDSCs are subdivided into 2 major subsets, monocytic and granulocytic MDSCs (M-MDSCs and G-MDSCs, respectively). M-MDSCs express high levels of Ly6C and are negative for Ly6G, whereas G-MDSCs express low levels of Ly6C and intermediate levels of Ly6G [10, 15]. Interestingly, an early-stage MDSC population unable to suppress T-cell responses in humans and a small population of immature eosinophils with immunosuppressive activity in Staphylococcus aureus–infected mice were also identified [16, 17]. The immunosuppressive activity of MDSCs operates via several mechanisms, such as degradation of essential nutrients for T-cell function, induction of regulatory cells, expression of negative immune check-point molecules, generation of adenosine and production of anti-inflammatory cytokines, reactive oxygen species, and nitric oxide [18].
The role of MDSCs in tuberculosis pathogenesis has been reported in M. tuberculosis–susceptible mice, such as mice deficient in key immunoprotective molecules (inducible nitric oxide synthase [iNOS]−/− and recombination-activating gene [RAG]−/−) or with genetic polymorphisms that weaken natural immunity (I/St, 129S2, and C3HeB/FeJ) [10–12]. On infection with virulent H37Rv mycobacteria, tuberculosis-susceptible mice developed necrotizing pneumonia associated with early death. Depletion of GR1+ cells reduced lung inflammation and rescued 129S2 mice from lethal tuberculosis [11]. However, the role of MDSCs in immunocompetent hosts with severe tuberculosis is still unclear. This information is crucial to determine whether patients in the advanced stage of the disease can benefit from therapies directed against MDSCs [19, 20]. A balanced immune response is important for a favorable tuberculosis outcome, while uncontrolled inflammation promotes tissue necrosis, compromises lung function, and causes patient death [21]. MDSCs play a key role in dampening excessive inflammation in sepsis and pneumonia caused by Klebsiella pneumoniae [22, 23]. The accumulation of MDSCs in heavily inflamed and damaged tissues may represent an effort by the host to regulate exacerbated T-helper (Th) 1 responses [24, 25].
Similar to rapidly progressive forms of pulmonary tuberculosis in immunocompetent patients, a low dose of intratracheal infection with the hypervirulent M. bovis strain MP287/03 induces extensive areas of pneumonia and pulmonary necrosis in C57BL/6 mice [26, 27]. The present study used this experimental model to investigate the role of MDSCs in severe tuberculosis with extensive damage to the lung tissue. Massive infiltration of G-MDSCs into the lungs in the advanced stage of MP287/03 infection promoted mycobacterial growth and the development of inflammatory and necrotic pulmonary lesions. Our findings support the use of therapies targeting MDSCs to improve the outcome of severe tuberculosis associated with uncontrolled pulmonary inflammation.
MATERIALS AND METHODS
Mice
Specific pathogen-free C57BL/6 mice (6–8-week old) were bred at the isogenic mice facility of the Biomedical Science Institute, University of São Paulo, Brazil. After infection, mice were maintained in microisolator cages as described elsewhere [28]. All procedures were performed in accordance with national regulations of the ethical guidelines for mouse experimentation (permit no. 31/2016).
Mycobacteria and Mouse Infection
The hypervirulent MP287/03 M. bovis strain isolated from cattle was provided by José Soares Ferreira Neto (Full professor at Veterinary Medical Institute, University of São Paulo, Brazil). The H37Rv M. tuberculosis strain was obtained from the American Type Culture Collection. Frozen bacilli were thawed and cultured as described elsewhere [27]. Bacterial concentrations were measured using a spectrophotometer at 600 nm. Mice were anesthetized and infected intratracheally with approximately 100 bacilli [26]. Mycobacterial load was estimated using serial dilutions [27].
Cell Harvesting from Lung, Bone Marrow, and Blood
Lung cells were harvested as described elsewhere [27]. Bone marrow cells were harvested from femurs and tibias. Blood was collected via cardiac puncture in the presence of 0.03-mg/mL heparin (Merck). Erythrocytes in cell suspensions were lysed using ACK buffer (Thermo Fisher Scientific).
Phenotypic Cell Analysis
Lung, bone marrow, and blood cells were labeled with the appropriated combination of monoclonal antibodies listed in the Supplementary Methods. The sample processing, intracellular interferon (IFN) γ staining and flow cytometry analysis (conventional and t-distributed stochastic neighbor embedding) are detailed in the Supplementary Methods.
GR1+ Cell Enrichment and Functional Assays
GR1+ cells were obtained from the lungs and bone marrow using magnetic cell sorting, as described in the Supplementary Methods.
Cytokine Quantification
Lung cells were cultured for 48 hours, as described elsewhere [26]. Cytokine levels in cell supernatants were quantified using the Cytometric Bead Array (CBA) Mouse Th1/Th2/Th17 Cytokine kit (BD Bioscience).
Lung Macroscopic Analysis and Histopathology
The right upper lung lobe was preserved in 10% paraformaldehyde and subsequently photographed. Histopathological and immunohistochemical analyses are described in the Supplementary Methods.
Gene Expression Measurement Using RT-PCR
Total RNA was isolated from the right lung postcaval lobe using TRIzol (Thermo Fisher Scientific) and the RNeasy Mini kit (Qiagen), as detailed in the Supplementary Methods.
In Vivo GR1+ Cell Depletion
Mice were treated with rat anti-GR1 antibodies (RB6-8C5; immunoglobulin (Ig) G2b isotype) (0.2 mg per mouse) from day 21 to day 28 after infection, every 72 hours. The control group received rat serum IgG2b (0.2 mg per mouse) under similar conditions.
Blood Leukocyte Quantification
The blood leukocyte analysis was performed using a panoptical fast staining kit (Laborclin).
Statistical Analysis
Statistical analysis was performed using GraphPad Prism 7 software (GraphPad ), as described in the Supplementary Methods.
RESULTS
Migration of CD11b+GR1int Myeloid Cells Into Lungs During Severe Tuberculosis Caused by Hypervirulent MP287/03 Mycobacteria
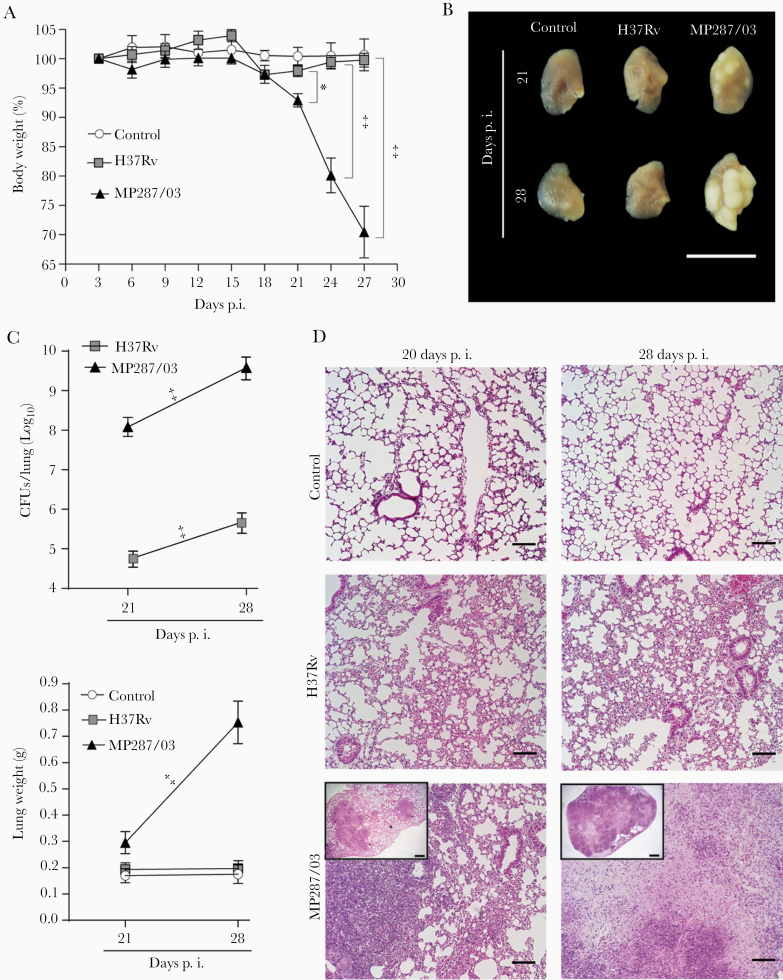
To evaluate the myeloid cell response during severe tuberculosis, lung-infiltrating CD11b+ cells were analyzed in C57BL/6 mice at days 21 and 28 after infection with approximately 100 bacilli of hypervirulent MP287/03 M. bovis strain or reference virulent H37Rv M. tuberculosis strain. Confirming our group’s previous findings [26–28], MP287/03-infected mice displayed accentuated body weight loss starting 21 days after infection (Figure 1A). Macroscopic observation revealed white nodules in the lungs at day 21 after infection, which were more prominent 28 days after infection (Figure 1B). Concomitant with bacterial growth, lung weights and relative masses also increased in MP287/03-infected mice (Figure 1C and Supplementary Figure 1A). During this period, focal granulomatous lesions containing bacilli progressed rapidly to extensive pneumonia with areas of necrosis (Figure 1D and Supplementary Figure 1B), significantly reducing aerated alveolar space and increasing lung-infiltrating cell numbers (Supplementary Figure 1C and 1D).
Figure 1.
Pulmonary tuberculosis progresses rapidly in C57BL/6 mice infected with MP287/03 mycobacteria. C57BL/6 mice were infected intratracheally with approximately 100 H37Rv or MP287/03 bacilli, and noninfected mice were used as control. A, Percentages of body weights in relation to time 0 are shown. B, Lung macroscopic images (scale bars represent 1 cm). C, Colony-forming units (CFUs) per lung and lung weights a. D, Representative lung sections stained with hematoxylin-eosin (×100 magnification; scale bars represent 100 µm in amplified images and 500 µm in inserts). Data represent 2 independent experiments with 3–5 mice each. *P < .05; ‡P < .001.
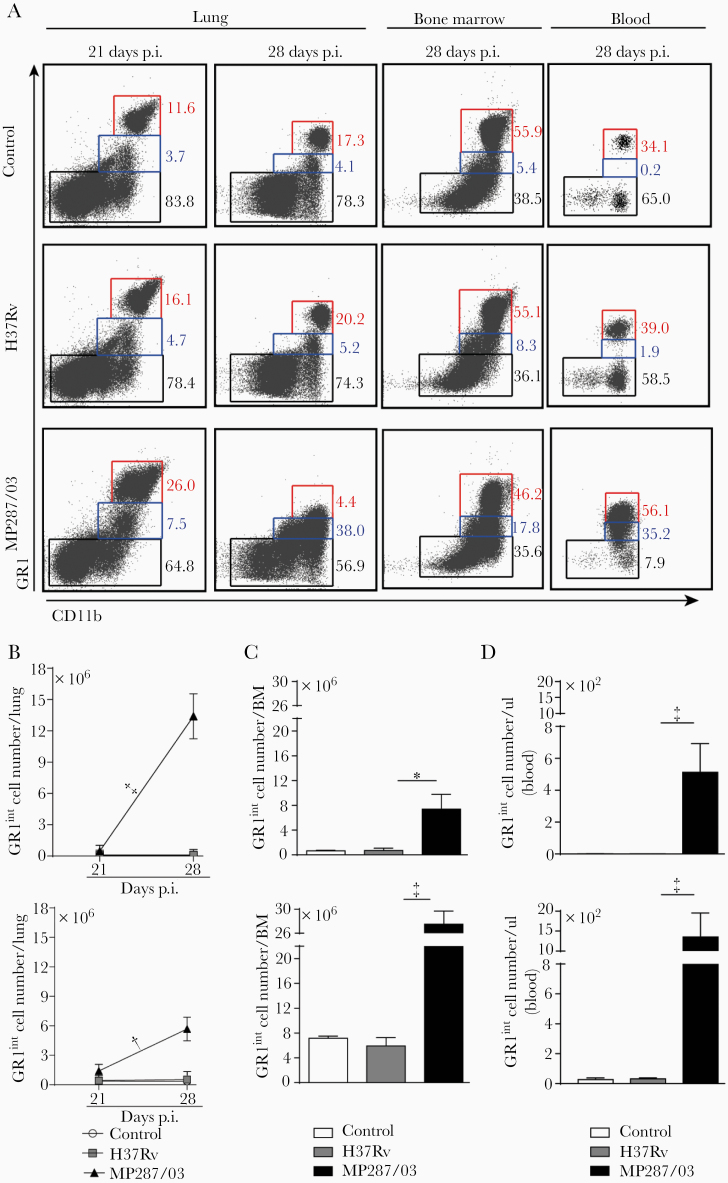
Bone marrow and blood cellularity also augmented in MP287/03 infection (Supplementary Figure 1E). Notably, an increase in the lung CD11b+ population expressing low levels of the granulocytic marker GR1 accompanied the worsening of the disease (Figure 2A and 2B, and Supplementary Figure 1F). This phenotype is a feature of monocytes and immature myeloid cells, including M-MDSCs and G-MDSCs [29, 30]. The GR1int population was also enlarged in the bone marrow and blood during severe tuberculosis, although less than the GR1hi population (Figure 2A, 2C, and 2D). In contrast, H37Rv infection caused mild pulmonary inflammation with no increase in the GR1int population (Figures 1D and 2A–2D). These data suggest that CD11b+GR1int cells are produced in the bone marrow and migrate via the bloodstream to the lungs in the advanced stage of severe tuberculosis, as demonstrated for other pathological conditions [31].
Figure 2.
CD11b+GR1int myeloid cells migrate into the lungs during severe tuberculosis. C57BL/6 mice were infected intratracheally with approximately 100 H37Rv or MP287/03 bacilli, and noninfected mice were used as control. A, Dot plots showing GR1 and CD11b expression in CD4−CD8−CD19−NK1.1−CD11c−CD45+ live cells from lungs, bone marrow (BM), and blood at 21 or 28 days after infection. B–D, GR1int and GR1hi cell counts in lungs, BM (2 femurs and 2 tibias, at 28 days after infection), and blood (at 28 days). Data represent 2 independent experiments with 3–5 mice each. *P < .05; †P < .01; ‡P < .001.
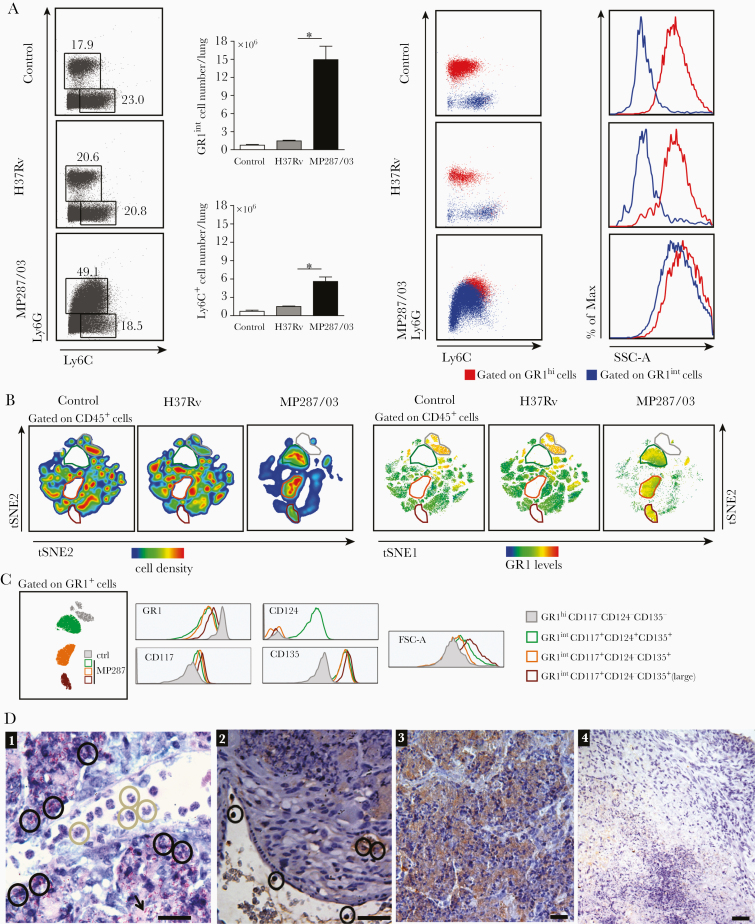
Massive Infiltration of Lungs and Harboring of Intracellular Bacilli by CD11b+GR1int Cells Expressing G-MDSC Phenotype
Experiments were next performed to elucidate the monocytic or granulocytic origin of GR1int cells that infiltrated the lungs during severe tuberculosis. On day 28 after infection, lung Ly6G+ and Ly6C+ populations were much larger in MP287/03-infected mice than in H37Rv-infected mice and noninfected controls, and the Ly6G+ population predominated only in mice with severe tuberculosis (Figure 3A and Supplementary Figure 1F). Lung GR1hi and GR1int cells were identified, respectively, as granulocytes (Ly6G+SSChi) and monocytes/macrophages (Ly6C+SSClow) in H37Rv-infected mice and noninfected controls. In contrast, these populations expressed both Ly6G and Ly6C markers and showed high granularity in MP287/03-infected mice.
Figure 3.
Immature granulocytic cells massively infiltrate the lungs during severe tuberculosis. A–C, Lung infiltrating cells were evaluated in C57BL/6 mice at day 28 after infection with approximately 100 H37Rv or MP287/03 bacilli. Noninfected mice were used as controls. A, Left, Dot plots showing Ly6G and Ly6C expression in CD4−CD8−CD19−NK1.1−CD11c−CD45+ live cells, and CD11b+Ly6G+ and CD11b+Ly6C+ cell counts per lung. Right, Dot plots show Ly6G and Ly6C expression in CD11b+GR1hi and CD11b+GR1int cells, and histograms display cell granularity. Abbreviation: SSC-A, side scatter area. B, The t-distributed stochastic neighbor embedding (t-SNE) maps show CD45+ cell clusters of concatenated lung cells from controls and MP287/03-infected mice in relation to cell density and GR1 levels. Four CD11b+GR1+ cell clusters are outlined in different colors (gray, green, orange, brown). C, Histograms show GR1, CD117, CD124, and CD135 expression and forward scatter area (FSC-A) in the CD11b+GR1+ cell clusters. D, Lung histological analyses of mice at day 28 after infection with approximately 100 MP287/03 bacilli are shown (scale bars represent 20 µm). D1, Granulocytes with unilobed or ring-shaped nucleus, consistent with the morphology of polymorphonuclear (PMN)-myeloid-derived suppressor cells (G-MDSCs) (gray circles), recruited into the lungs and infected soon after transmigration across the vascular endothelium (black circles). Many necrotic cells releasing intracellular bacteria stained in red by the Ziehl-Neelsen method can be seen in lung tissue (black arrow). D2, Immunohistochemical image shows transmigrating GR1+ cells (black circles). D3, Strong staining of intra-alveolar GR1+ cells can be seen in areas of alveolitis. Note intact and dying cells. D4, Weak staining for the GR1 marker was observed in necrotic area and surrounding tissue in a granulomatous lesion. Data represent 2 independent experiments with 3–5 mice each. *P < .05.
Pulmonary CD45+ leukocytes in infected and noninfected mice were concatenated into a single file for t-distributed stochastic neighbor embedding analysis of the hematopoietic progenitor markers CD117 (c-kit), CD124 (interleukin Rα [IL-4Rα]) and CD135 (Flt-3). A major cluster of GR1hi cells was identified in H37Rv-infected mice and noninfected controls (Figure 3B). In contrast, MP287/03-infected mice lacked the GR1hi cluster and presented 3 exclusive GR1int populations. Concatenated GR1+ clusters of infected and noninfected mice were analyzed for CD117, CD124, and CD135 expression and cell size (Figure 3C). All GR1int populations of MP287/03-infected mice showed higher levels of CD117 and CD135 than the GR1hi cluster of noninfected controls, but only one population expressed CD124. The 2 GR1+CD117+CD124−CD135+ populations primarily differed in cell size (forward scatter).
To investigate the behavior of immature myeloid cells during severe tuberculosis, their location in the lung tissue and ability to harbor intracellular bacilli were assessed by histopathological analysis on day 28 after infection with MP287/03 mycobacteria. Inflammatory cells, mainly represented by immature polymorphonuclear leukocytes exhibiting unilobed or ring-shaped nuclei, were recruited into the lungs and infected shortly after transmigration through the vascular endothelium (Figure 3D). The lung tissue presented many necrotic cells releasing intracellular bacteria. Immunohistochemical staining revealed transmigrating GR1+ cells and intra-alveolar GR1+ cell clusters in areas of alveolitis. Weak staining for the GR1 marker was observed surrounding necrotic lesions. Taken together, these results show that lung CD11b+GR1int cells generated during MP287/03 infection have phenotype and morphology consistent with G-MDSCs, are permissive to mycobacterial infection, and are located preferentially in the pulmonary alveoli.
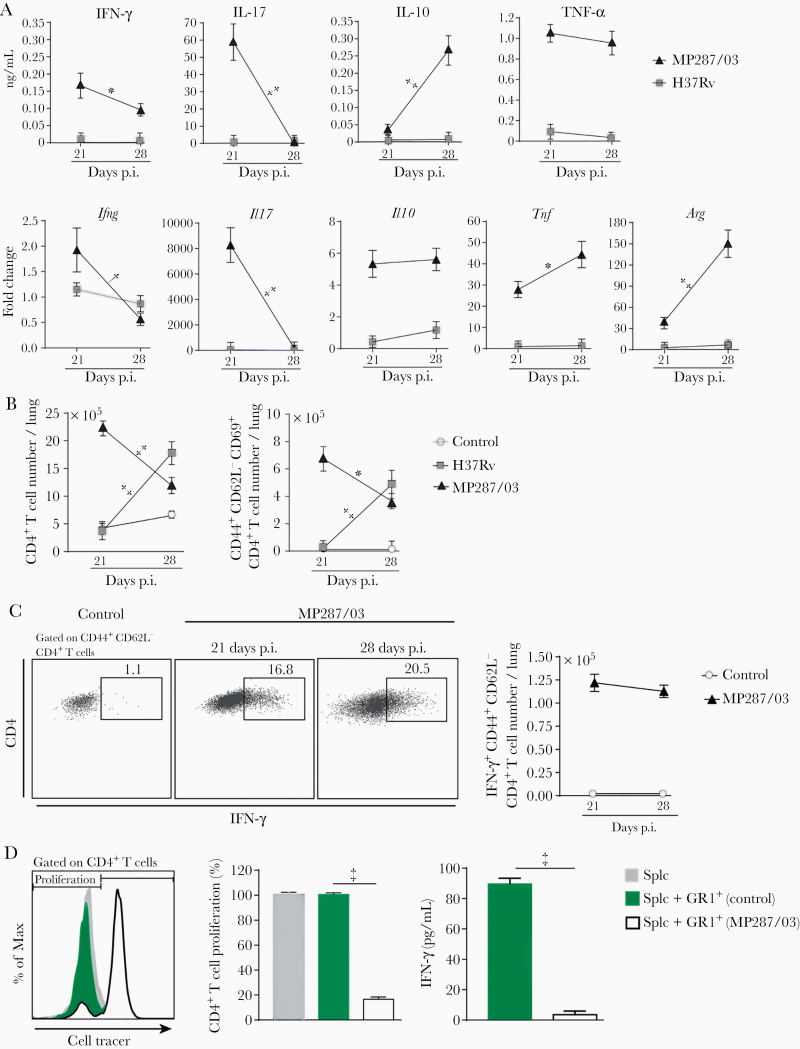
Suppression of T-Cell Responses by CD11b+GR1int Cells Generated During Severe Tuberculosis
An immunosuppressed environment was established in parallel with CD11b+GR1int cell accumulation in the lungs. Both messenger RNA and protein levels of IFN-γ and interleukin 17A in lung cell preparations decreased from day 21 to 28 after infection with MP287/03 mycobacteria (Figure 4A). Interleukin 10 protein levels were found enhanced only for MP287/03-infected mice at day 28 after infection, demonstrating predominance of an immunosuppressive environment in advanced disease. In addition, high tumor necrosis factor α production in severe tuberculosis was accompanied by the late expression of the arginase 1 (Arg1) gene, a hallmark of MDSCs [32]. The expression of these genes and cytokines was low in lung cell preparations of H37Rv-infected mice. In addition, total CD4+ and CD44+CD62L−CD69+CD4+ T-cell numbers per lung were reduced in MP287/03 infection and increased in H37Rv infection from day 21 to 28 after infection (Figure 4B and Supplementary Figure 2A and 2B).
Figure 4.
GR1+ cells suppress CD4+ T-cell responses. C57BL/6 mice were infected with approximately 100 H37Rv or MP287/03 bacilli, and noninfected mice were used as controls. A, Cytokine concentrations in 48-hour-culture supernatants of lung cells and the Ifng, Il17a, Il10, Tnfa, and Arg1 gene expression (fold change) in lung cell preparations are shown. Abbreviations: IFN, interferon; IL-10 and IL-17, interleukin 10 and 17; TNF, tumor necrosis factor. B, Graphs showing CD4+ and CD44+CD62L−CD69+CD4+ T-cell counts per lung. C, Dot plots showing intracellular IFN-γ in CD44+CD62L−CD4+ T cells and IFN-γ +CD44+CD62L−CD4+ T-cell counts per lung. D, Naive splenocytes were stimulated with anti-CD3 and anti-CD28 monoclonal antibodies in the presence of GR1+ lung cells from mice at day 28 after infection with MP287/03 mycobacteria. GR1+ lung cells from noninfected mice were used as controls. Histograms show CD4+ T-cell proliferation, assessed by cell tracer dilution assay. Graphs show percentages of proliferating CD4+ T cells and IFN-γ concentrations in cell supernatants. Data represent 2 independent experiments with 3–5 mice each. *P < .05; †P < .01; ‡P < .001.
Similar results were obtained for CD8+ T cells (Supplementary Figure 2A, 2C, and 2D). CD69 expression in CD44+CD62L− T cells is an indicative phenotype for cell activation and homing to lung parenchyma [33]. Notably, the numbers of IFN-γ–producing CD44+CD62L−CD4+ and CD44+CD62L−CD8+ T cells were comparable on days 21 and 28 after infection with MP287/03 mycobacteria, despite the increase in lung inflammatory response (Figure 4C and Supplementary Figure 3A). Furthermore, magnetically sorted GR1+ cells from lung and bone marrow cell preparations obtained at day 28 after infection with MP287/03 mycobacteria suppressed CD4+ and CD8+ T-cell proliferation and IFN-γ production (Figure 4D and Supplementary Figure 3B–3E). In contrast, lung and bone marrow GR1+ cells from noninfected controls had no suppressive effect on T-cell responses. Of note, GR1+ cell isolation performed for these experiments yielded >90% purity (Supplementary Figure 3F). These findings demonstrate that CD11b+GR1int cells generated during severe tuberculosis efficiently suppress T-cell responses.
Depletion of GR1+ Cells in Mice With Severe Tuberculosis: Effects on Disease and T-Cell Suppression
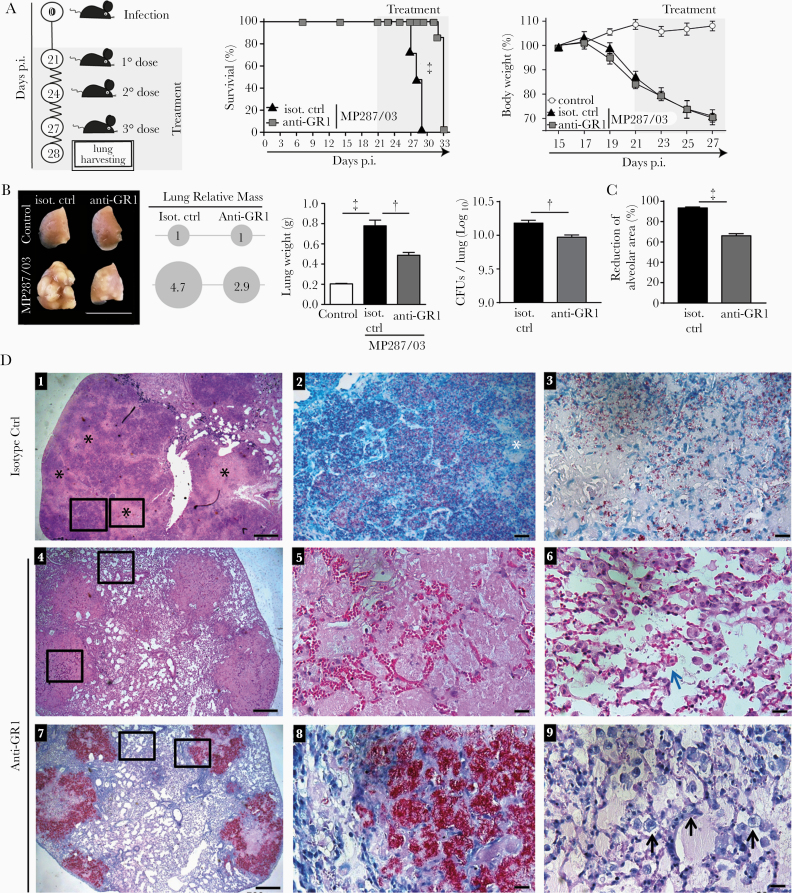
To assess whether CD11b+GR1int cells contribute to tuberculosis severity, MP287/03-infected mice were treated with anti-GR1 antibodies or IgG isotype control every 3 days starting 21 days after infection (Figure 5A). Anti-GR1 treatment significantly increased mouse survival but had no effect on body weight loss. Pneumonia and tissue damage were greatly reduced after anti-GR1 treatment as evidenced macroscopically by the decrease in lung white nodules, relative masses and weights (Figure 5B). Improved pathogen control was noted in the lower numbers of colony-forming units in the lungs and bone marrow of anti-GR1–treated mice (Figure 5B and Supplementary Figure 4A).
Figure 5.
Anti-GR1 treatment reduces tuberculosis severity in MP287/03-infected mice. C57BL/6 mice were infected intratracheally with approximately 100 MP287/03 bacilli and treated with anti-GR1 monoclonal antibodies or isotype control. A, Schematic illustration of the experimental protocol for anti-GR1 therapy, survival curves, and percentages of body weights in relation to time 0. B–D, Mouse lungs were evaluated at day 28 of infection. B, C, Lung macroscopic images, relative masses (circles), lung weights, colony-forming units (CFUs) per lung and morphometric quantifications of the alveolar space are shown. D, Representative lung sections stained using hematoxylin-eosin (D1, D4–D6) or Ziehl-Neelsen (D2, D3, D7–D9) methods. Scale bars correspond to 20 µm (D2, D3, D5, D6, D8, D9) or 500 µm (D1, D4, D7). D1, Extensive areas of pneumonia with numerous foci of caseous necrosis (black stars). D2, Magnified area from D1 (left square) showing alveolitis with numerous intracellular bacilli in the alveoli and extracellular bacilli in region of recent necrosis (white star). D3, Magnified area from D1 (right square) showing low numbers of bacilli in region of central necrosis. D4, Medium-sized lesions surrounded by relatively preserved lung tissue. D5, Magnified lesion from D4 (lower square) showing alveoli filled with homogeneous acellular liquid mass and numerous erythrocytes in alveolar walls. D6, Magnified area from D4 (upper square) showing relatively preserved lung tissue with macrophages phagocytizing erythrocytes (blue arrow). D7, Alveoli filled with extracellular bacilli in medium-sized lesions. D8, Magnified area from D7 (right square) showing numerous extracellular growing bacilli, forming cords and clumps. D9, Magnified area from D7 (left square) showing numerous alveolar macrophages with highly vacuolated cytoplasm (black arrows). Data represent 2 independent experiments with 3–5 mice each. †P < .01; ‡P < .001.
Lung histological analysis revealed a lower commitment of the aerated alveolar space after anti-GR1 therapy (Figure 5C). Mice treated with isotype control showed extensive areas of pneumonia with numerous foci of caseous necrosis (Figure 5D). Alveolitis with abundant intracellular bacteria and necrotic areas with many extracellular bacteria were observed throughout the lung tissue in isotype control–treated mice. In contrast, anti-GR1–treated mice developed medium-sized lung lesions composed of alveoli filled with homogeneous acellular liquid mass and numerous erythrocytes in the alveolar walls. Notably, the alveoli in these lesions contained large numbers of growing bacilli, forming cords and clumps. Alveolar macrophages with highly vacuolated cytoplasm and containing phagocytized erythrocytes were also observed in the preserved lung tissue.
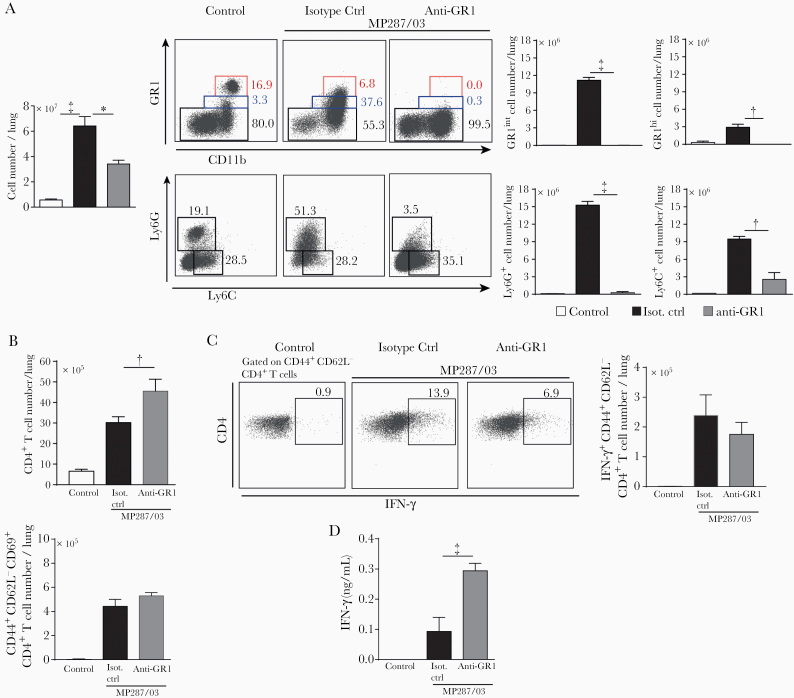
Next, the effects of antibody therapy on GR1+ cells were assessed in MP287/03-infected mice. The leukocyte population in the lungs was reduced after anti-GR1 treatment (Figure 6A). Phenotypical analysis of lung myeloid cells showed a complete elimination of GR1int, GR1hi and Ly6G+ populations. Depletion of neutrophils in the blood ranged from 25 to 50%, and the monocyte population was reduced by almost 75% (Supplementary Figure 4B). Because the presence of CD11b+GR1int cells in the lungs of MP287/03-infected mice was associated with T-cell suppression in vitro and in vivo, we investigated whether the elimination of GR1+ cells benefited T-cell responses.
Figure 6.
Anti-GR1 treatment efficiently depletes GR1+ cells and attenuates CD4+ T-cell suppression during severe tuberculosis. C57BL/6 mice were infected intratracheally with approximately 100 MP287/03 bacilli and treated with anti-GR1 monoclonal antibodies or isotype control. A, Left, Cell counts per lung. Dot plots show GR1, CD11b, Ly6G, and Ly6C expression in CD4−CD8−CD19−NK1.1−CD11c−CD45+ live cells. Right, GR1int, GR1hi, Ly6G+, and Ly6C+ cell numbers per lung. B, Total CD4+ and CD44+CD62L−CD69+CD4+ T-cell counts per lung. C, Dot plots of intracellular interferon (IFN) γ in CD44+CD62L−CD69+CD4+ T cells. The IFN-γ +CD44+CD62L−CD69+CD4+ T-cell counts per lung are shown. D, IFN-γ concentrations in 48-hour-culture supernatants of lung cells. Data represent 2 independent experiments with 3–5 mice each. *P < .05; †P < .01; ‡P < .001.
Anti-GR1 therapy increased CD4+ and CD8+ T-cell numbers per lung, but no effect was observed on CD44+CD62L−CD69+CD4+ and CD44+CD62L−CD69+CD8+ populations (Figure 6B and Supplementary Figure 4C). The numbers of IFN-γ–producing CD44+CD62L−CD4+ and CD44+CD62L−CD8+ T cells were also not affected by antibody therapy (Figure 6C and Supplementary Figure 4D). However, IFN-γ +CD44+CD62L− T-cell percentages in lung infiltrates were nearly 80% higher in anti-GR1–treated mice (0.78% ± 0.10%) than in isotype control–treated mice (0.44% ± 0.05%). In addition, a 3-fold higher IFN-γ production was observed in lung leukocyte supernatants of anti-GR1–treated mice compared with isotype control–treated mice (Figure 6D).
Taken together, these results show that GR1+ cell depletion during severe tuberculosis caused by MP287/03 mycobacteria prevents the expansion and fusion of primary pulmonary lesions, as well as the development of intragranulomatous caseous necrosis (Figure 7). An increase in mouse survival and partial recovery of T-cell function were also observed in infected mice depleted of GR1+ cells. In addition, anti-GR1 therapy reduced lung bacterial load, but mycobacteria released from the depleted cells proliferated extracellularly in the pulmonary alveoli.
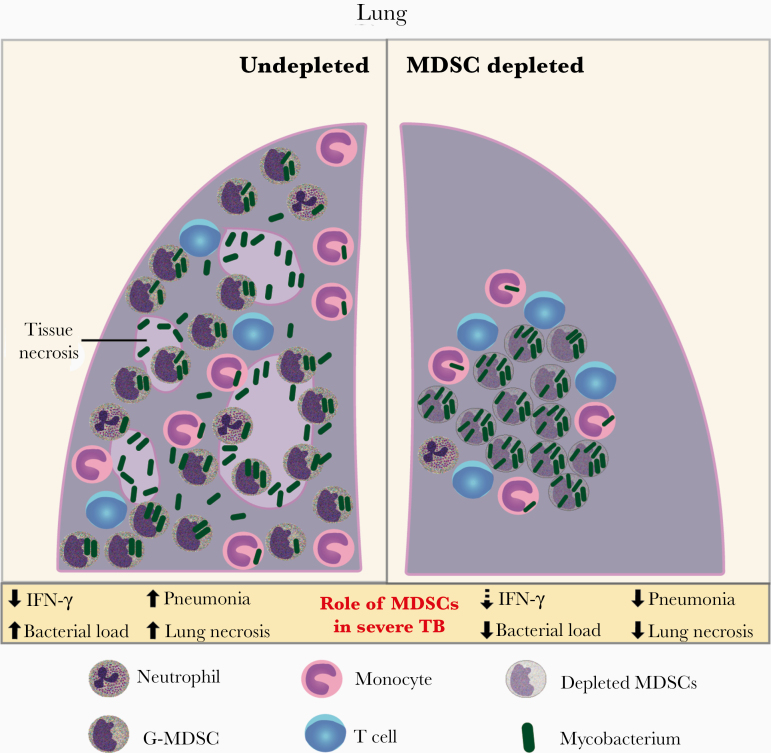
Figure 7.
Schematic illustration showing the role of granulocytic myeloid-derived suppressor cells (G-MDSCs) in severe tuberculosis caused by hypervirulent mycobacteria. In the advanced stage of tuberculosis caused by highly virulent mycobacteria, G-MDSCs produced in the bone marrow are massively recruited into the lungs. These cells are infected shortly after crossing the vascular endothelium and suppress T-cell proliferation and interferon (IFN) γ production. Located preferentially in the alveoli, G-MDSCs promote mycobacterial growth and the development of inflammatory and necrotic pulmonary lesions. Depletion of GR1+ cells results in marked improvement of pulmonary tuberculosis and increased mouse survival, along with partial recovery of T-cell function. Lung bacterial load is reduced in the absence of these cells, but mycobacteria released from the depleted cells proliferated extracellularly inside the alveoli. This scenario supports the use of therapies targeting MDSCs to improve the outcome of severe tuberculosis associated with uncontrolled pulmonary inflammation.
DISCUSSION
MDSCs have been recently detected in blood and lung compartments of children and adults with active pulmonary tuberculosis [34–37]. However, the role of these cells in tuberculosis pathogenesis, including possible positive or negative effects, is still under debate, and our findings may help clarify this issue. The accumulation of CD11b+GR1int cells in the lungs during advanced MP287/03 infection, along with the development of extensive inflammatory and necrotic lesions, supports their involvement in disease progression. Three large CD11b+GR1int subsets were identified based on immature myeloid cell markers and cell size. IL-4Rα expression characterized the largest subset, which also expressed the classic hematopoiesis-promoting cytokine receptors c-kit and Flt-3 [38, 39]. Positive regulation of IL-4Rα contributes to cell function and survival [40–42] and was observed in G-MDSC and M-MDSC subsets from tuberculosis-susceptible mice infected with H37Rv mycobacteria [11].
The other 2 subsets were positive only for c-kit and Flt-3, and their subdivision according to a small or large cell size may reflect different stages of the proliferative cycle. The presence of lung and bone marrow GR1+ cells in MP287/03-infected mice with immunosuppressive activity in vitro, together with T-cell suppression in vivo, allowed us to classify CD11b+GR1int cells as MDSCs [13]. The predominance of G-MDSCs in the lungs of MP287/03-infected mice was demonstrated by Ly6G expression, high cell granularity, and morphology compatible with immature neutrophils. G-MDSCs likely migrated to the lungs via the bloodstream with the worsening of the disease, as seen in cancer patients [31, 43]. These cells were infected by MP287/03 mycobacteria right after crossing the vascular endothelium and located preferentially in the alveoli.
Emergency hematopoiesis supplies the high demand for myeloid cells in response to excessive or chronic infections, and it plays a beneficial role for the host by helping to restrict the proliferation of pathogens and assisting tissue repair [44]. However, MDSCs may harbor intracellular microbial multiplication and suppress the immune response at the infection site due to their regulatory profile, which worsens disease outcomes. The first signal for the generation of MDSCs is a sustained production of factors that stimulate myelopoiesis [45, 46]. A second signal is required for the acquisition of immunosuppressive activity [46]. In the case of severe tuberculosis, mycobacterial recognition by the Toll-like receptor 2–NF-kB pathway and damage-associated molecules such as heat shock proteins and adenosine may provide the second signal [45, 47, 48]. MP287/03 bacilli were found not only in the lungs, but also in the bone marrow, providing the second signal for MDSCs locally. In milder infections, local and circulating cells are sufficient to contain the infection, and emergency hematopoiesis is not induced [44]. This view may explain why a low-dose infection with MP287/03 bacilli induced robust G-MDSC recruitment into the lungs, but the same did not occur with H37RV bacilli.
In this model of severe tuberculosis, G-MDSCs infiltrated the lungs during acute infection. In previous studies of our group and others using the slowly progressive H37Rv strain or moderately virulent M299 Beijing strain, the presence of G-MDSCs in the lungs was observed in the late chronic infection, coinciding with an increased inflammatory response [10, 49]. These findings support the concept that MDSC recruitment is associated with disease severity, and not with a particular mycobacterial strain. Accordingly, GR1 expression inversely correlated with weight loss in tuberculosis-susceptible mice infected with H37Rv mycobacteria [10]. Furthermore, P2X7-deficient mice that are resistant to MP287/03 infection showed moderate infiltrates of mononuclear leukocytes, but not MDSCs, when infected with this hypervirulent mycobacteria [27, 28].
In addition, our study and others corroborate the view that immature myeloid cells acquire immunosuppressive activity when tuberculosis becomes severe [10, 11]. Ly6Ghi and Ly6Gint cells from the lungs of tuberculosis-resistant C57BL/6 mice infected with H37Rv bacilli did not suppress T-cell function in vitro, but both cell populations showed immunosuppressive activity in tuberculosis-susceptible 129SvPas mice [50]. MDSCs isolated from the bone marrow of tuberculosis-susceptible I/St mice also suppressed T cells [10]. Although the intense migration of MDSCs to the lungs during acute infection seems to be harmful to the host, the presence of a limited MDSC population during the chronic disease can control inflammatory responses and prolong host survival, as previously suggested [49].
The effects of anti-GR1 therapy administered when the disease worsened and MDSCs infiltrated the lungs allow us to understand important aspects regarding the role of these cells in the pathogenesis of severe tuberculosis. The most striking observation in anti-GR1–treated mice was the presence of a large number of extracellular bacteria proliferating inside the pulmonary alveoli in medium-sized lesions, while the adjacent tissues were relatively preserved and apparently free of bacilli. This scenario contrasts with the extensive inflammatory and necrotic lesions with many intracellular and extracellular bacilli observed in isotype control–treated mice and suggests that GR1+ cells, mainly G-MDSCs in our experimental conditions, act as a permissive niche for intracellular mycobacterial growth [50] and spread the bacilli throughout the lung tissue. Furthermore, the massive death of infected G-MDSCs is apparently decisive for the development of pulmonary necrotic lesions in MP287/03 infection.
Although the immunosuppressive activity of G-MDSCs may contribute to increase the pulmonary bacterial load in MP287/03-infected mice, GR1+ cell depletion promoted a limited recovery in T-cell function, which does not seem to be the main reason for the marked improvement of the disease. Mycobacteria released from depleted cells proliferated extracellularly in the alveoli apparently free of immunological control. The increase in IFN-γ production ex vivo, but not in the number of IFN-γ + T cells per lung in vivo, can be explained by the higher percentage of this population among infiltrating leukocytes as a result of GR1+ cell depletion. A reduction in T-cell exhaustion leading to enhanced IFN-γ secretion is another feasible explanation. The efficacy of MDSC depletion in improving pulmonary tuberculosis already in its advanced stage expands the spectrum of possibilities for MDSC-targeted therapies [19, 20].
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We are grateful to José Soares Ferreira Neto for providing the MP287/03 Mycobacterium bovis strain. We also thank Maria Áurea de Alvarenga, Silvana Silva, and José Israel Lima for technical assistance.
Author contributions. C. C. B. B., E. P. A., and M. R. D. L. conceived and designed the experiments. C. C. B. B., I. S. C., G. A. S., E. M. S., A. H. R. d. N., F. M. A., T. L. B. V. S., and A. L. R. performed the experiments. C. C. B. B., G. A. S., E. M. S., and M. R. D. L. analyzed the data. C. C. B. B., M. H. H., R. A. F., J. M. A., E. B. L., and M. R. D. L. contributed reagents, materials, or analysis tools.
Financial support. This work was supported by Fundação de Amparo à Pesquisa do Estado de Paulo (FAPESP-Brazil; grants 2015/20432-8 to M. R. D. L. and 2014/22986-8 and 2014/22986-8 and 2017/09110-4 to C. C. B. B.) and by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq-Brazil; grants 408909/2018-8 and 303810/2018-1 to M. R. D. L.).
Potential conflicts of interest: All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Global tuberculosis report 2019. Geneva, Switzerland: World Health Organization, 2019.
- 2. Khan MK, Islam MN, Ferdous J, Alam MM. An overview on epidemiology of tuberculosis. Mymensingh Med J 2019; 28:259–66. [PubMed] [Google Scholar]
- 3. Scriba TJ, Penn-Nicholson A, Shankar S, et al. ; other members of the ACS cohort study team . Sequential inflammatory processes define human progression from M. tuberculosis infection to tuberculosis disease. PLoS Pathog 2017; 13:a018499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pagan AJ, Ramakrishnan L. Immunity and immunopathology in the tuberculous granuloma. Cold Spring Harb Perspect Med 2014; 5:e1006687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Keane J, Gershon S, Wise RP, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med 2001; 345:1098–104. [DOI] [PubMed] [Google Scholar]
- 6. Bell LCK, Noursadeghi M. Pathogenesis of HIV-1 and Mycobacterium tuberculosis co-infection. Nat Rev Microbiol 2018; 16:80–90. [DOI] [PubMed] [Google Scholar]
- 7. Goletti D, Petrone L, Ippolito G, Niccoli L, Nannini C, Cantini F. Preventive therapy for tuberculosis in rheumatological patients undergoing therapy with biological drugs. Expert Rev Anti Infect Ther 2018; 16: 501–12. [DOI] [PubMed] [Google Scholar]
- 8. Caws M, Thwaites G, Dunstan S, et al. The influence of host and bacterial genotype on the development of disseminated disease with Mycobacterium tuberculosis. PLoS Pathog 2008; 4:e1000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tram TTB, Nhung HN, Vijay S, et al. Virulence of Mycobacterium tuberculosis clinical isolates is associated with sputum pre-treatment bacterial load, lineage, survival in macrophages, and cytokine response. Front Cell Infect Microbiol 2018; 8:417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tsiganov EN, Verbina EM, Radaeva TV, et al. Gr-1dimCD11b+ immature myeloid-derived suppressor cells but not neutrophils are markers of lethal tuberculosis infection in mice. J Immunol 2014; 7:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Knaul JK, Jörg S, Oberbeck-Mueller D, et al. Lung-residing myeloid-derived suppressors display dual functionality in murine pulmonary tuberculosis. Am J Respir Crit Care Med 2014; 190:1053–66. [DOI] [PubMed] [Google Scholar]
- 12. Obregon-Henao A, Henao-Tamayo M, Orme IM, Ordway DJ. Gr1intCD11b+ myeloid-derived suppressor cells in Mycobacterium tuberculosis infection. PLoS One 2013; 8:e80669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Veglia F, Perego M, Gabrilovich D. Myeloid-derived suppressor cells coming of age. Nat Immunol 2018; 19: 108–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gabrilovich DI, Bronte V, Chen SH, et al. The terminology issue for myeloid-derived suppressor cells. Cancer Res 2007; 67:425–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol 2008; 181:5791–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bronte V, Brandau S, Chen SH, et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun 2016; 7:12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Goldmann O, Beineke A, Medina E. Identification of a novel subset of myeloid-derived suppressor cells during chronic staphylococcal infection that resembles immature eosinophils. J Infect Dis 2017; 216:1444–51. [DOI] [PubMed] [Google Scholar]
- 18. Principi E, Raffaghello L. The role of the P2X7 receptor in myeloid-derived suppressor cells and immunosuppression. Curr Opin Pharmacol 2019; 47:82–9. [DOI] [PubMed] [Google Scholar]
- 19. Young C, Walzl G, Du Plessis N. Therapeutic host-directed strategies to improve outcome in tuberculosis. Mucosal Immunol 2020; 13:190–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. du Plessis N, Kotze LA, Leukes V, Walzl G. translational potential of therapeutics targeting regulatory myeloid cells in tuberculosis. Front Cell Infect Microbiol 2018; 8:332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Orme IM, Robinson RT, Cooper AM. The balance between protective and pathogenic immune responses in the TB-infected lung. Nat Immunol 2015; 16:57–63. [DOI] [PubMed] [Google Scholar]
- 22. Sander LE, Sackett SD, Dierssen U, et al. Hepatic acute-phase proteins control innate immune responses during infection by promoting myeloid-derived suppressor cell function. J Exp Med 2010; 207:1453–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Poe SL, Arora M, Oriss TB, et al. STAT1-regulated lung MDSC-like cells produce IL-10 and efferocytose apoptotic neutrophils with relevance in resolution of bacterial pneumonia. Mucosal Immunol 2013; 6:189–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Medina-Echeverz J, Haile LA, Zhao F, et al. IFN-γ regulates survival and function of tumor-induced CD11b+ Gr-1high myeloid derived suppressor cells by modulating the anti-apoptotic molecule Bcl2a1. Eur J Immunol 2014; 44:2457–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Umansky V, Blattner C, Gebhardt C, Utikal J. The role of myeloid-derived suppressor cells (MDSC) in cancer progression. Vaccines 2016; 4:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Amaral EP, Machado de Salles É, Barbosa Bomfim CC, et al. Inhibiting adenosine receptor signaling promotes accumulation of effector CD4+ T cells in the lung parenchyma during severe tuberculosis. J Infect Dis 2019; 219: 964–74. [DOI] [PubMed] [Google Scholar]
- 27. Amaral EP, Ribeiro SC, Lanes VR, et al. Pulmonary infection with hypervirulent mycobacteria reveals a crucial role for the P2X7 receptor in aggressive forms of tuberculosis. PLoS Pathog 2014; 10:e1004188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bomfim CCB, Amaral EP, Cassado ADA, et al. P2X7 receptor in bone marrow-derived cells aggravates tuberculosis caused by hypervirulent Mycobacterium bovis. Front Immunol 2017; 8:435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fleming TJ, Fleming ML, Malek TR. Selective expression of Ly-6G on myeloid lineage cells in mouse bone marrow. RB6-8C5 mAb to granulocyte-differentiation antigen (Gr-1) detects members of the Ly-6 family. J Immunol 1993; 151:2399–408. [PubMed] [Google Scholar]
- 30. Hestdal K, Ruscetti FW, Ihle JN, et al. Characterization and regulation of RB6-8C5 antigen expression on murine bone marrow cells. J Immunol 1991; 147:22–8. [PubMed] [Google Scholar]
- 31. Hoffmann SHL, Reck DI, Maurer A, et al. Visualization and quantification of in vivo homing kinetics of myeloid-derived suppressor cells in primary and metastatic cancer. Theranostics 2019; 9:5869–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Youn JI, Collazo M, Shalova IN, Biswas SK, Gabrilovich DI. Characterization of the nature of granulocytic myeloid-derived suppressor cells in tumor-bearing mice. J Leukoc Biol 2012; 91:167–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bankovich AJ, Shiow LR, Cyster JG. CD69 suppresses sphingosine 1-phosophate receptor-1 (S1P1) function through interaction with membrane helix 4. J Biol Chem 2010; 285:22328–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. El Daker S, Sacchi A, Tempestilli M, et al. Granulocytic myeloid derived suppressor cells expansion during active pulmonary tuberculosis is associated with high nitric oxide plasma level. PLoS One 2015; 10:e0123772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. du Plessis N, Loebenberg L, Kriel M, et al. Increased frequency of myeloid-derived suppressor cells during active tuberculosis and after recent Mycobacterium tuberculosis infection suppresses T-cell function. Am J Respir Crit Care Med 2013; 188:724–32. [DOI] [PubMed] [Google Scholar]
- 36. Du Plessis N, Jacobs R, Gutschmidt A, et al. Phenotypically resembling myeloid derived suppressor cells are increased in children with HIV and exposed/infected with Mycobacterium tuberculosis. Eur J Immunol 2017; 47:107–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yang B, Wang X, Jiang J, Zhai F, Cheng X. Identification of CD244-expressing myeloid-derived suppressor cells in patients with active tuberculosis. Immunol Lett 2014; 158:66–72. [DOI] [PubMed] [Google Scholar]
- 38. Kao J, Ko EC, Eisenstein S, Sikora AG, Fu S, Chen SH. Targeting immune suppressing myeloid-derived suppressor cells in oncology. Crit Rev Oncol Hematol 2011; 77:12–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kazi JU, Rönnstrand L. FMS-like tyrosine kinase 3/FLT3: from basic science to clinical implications. Physiol Rev 2019; 99:1433–66. [DOI] [PubMed] [Google Scholar]
- 40. Roth F, De La Fuente AC, Vella JL, Zoso A, Inverardi L, Serafini P. Aptamer-mediated blockade of IL4Rα triggers apoptosis of MDSCs and limits tumor progression. Cancer Res 2012; 72:1373–83. [DOI] [PubMed] [Google Scholar]
- 41. Mandruzzato S, Solito S, Falisi E, et al. IL4Rα + myeloid-derived suppressor cell expansion in cancer patients. J Immunol 2009; 182:6562–8. [DOI] [PubMed] [Google Scholar]
- 42. Kohanbash G, McKaveney K, Sakaki M, et al. GM-CSF promotes the immunosuppressive activity of glioma-infiltrating myeloid cells through interleukin-4 receptor-α. Cancer Res 2013; 73:6413–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dubinski D, Wölfer J, Hasselblatt M, et al. CD4+ T effector memory cell dysfunction is associated with the accumulation of granulocytic myeloid-derived suppressor cells in glioblastoma patients. Neuro Oncol 2016; 18:807–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Boettcher S, Manz MG. Regulation of inflammation- and infection-driven hematopoiesis. Trends Immunol 2017; 38:345–57. [DOI] [PubMed] [Google Scholar]
- 45. Millrud CR, Bergenfelz C, Leandersson K. On the origin of myeloid-derived suppressor cells. Oncotarget 2017; 8:3649–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Condamine T, Mastio J, Gabrilovich DI. Transcriptional regulation of myeloid-derived suppressor cells. J Leukoc Biol 2015; 98:913–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chalmin F, Ladoire S, Mignot G, et al. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J Clin Invest 2010; 120:457–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ryzhov S, Novitskiy SV, Goldstein AE, et al. Adenosinergic regulation of the expansion and immunosuppressive activity of CD11b+Gr1+ cells. J Immunol 2011; 187:6120–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Almeida FM, Ventura TL, Amaral EP, et al. Hypervirulent Mycobacterium tuberculosis strain triggers necrotic lung pathology associated with enhanced recruitment of neutrophils in resistant C57BL/6 mice. PLoS One 2017; 12:e0173715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lovewell RR, Baer CE, Mishra BB, Smith CM, Sassetti CM. Granulocytes act as a niche for Mycobacterium tuberculosis growth. Mucosal Immunol 2020. doi: 10.1038/s41385-020-0300-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.